Abstract
The second messenger c-di-GMP is implicated in regulation of various aspects of the lifestyles and virulence of Gram-negative bacteria. Cyclic di-GMP is formed by diguanylate cyclases with a GGDEF domain and degraded by phosphodiesterases with either an EAL or HD-GYP domain. Proteins with tandem GGDEF-EAL domains occur in many bacteria, where they may be involved in c-di-GMP turnover or act as enzymatically-inactive c-di-GMP effectors. Here, we report a systematic study of the regulatory action of the eleven GGDEF-EAL proteins in Xanthomonas oryzae pv. oryzicola, an important rice pathogen causing bacterial leaf streak. Mutational analysis revealed that XOC_2335 and XOC_2393 positively regulate bacterial swimming motility, while XOC_2102, XOC_2393 and XOC_4190 negatively control sliding motility. The ΔXOC_2335/XOC_2393 mutant that had a higher intracellular c-di-GMP level than the wild type and the ΔXOC_4190 mutant exhibited reduced virulence to rice after pressure inoculation. In vitro purified XOC_4190 and XOC_2102 have little or no diguanylate cyclase or phosphodiesterase activity, which is consistent with unaltered c-di-GMP concentration in ΔXOC_4190. Nevertheless, both proteins can bind to c-di-GMP with high affinity, indicating a potential role as c-di-GMP effectors. Overall our findings advance understanding of c-di-GMP signaling and its links to virulence in an important rice pathogen.
Cyclic diguanylate (c-di-GMP) was initially discovered as an allosteric activator of cellulose synthesis in Gluconacetobacter xylinus1,2. The molecule is now recognized as an universal second messenger in bacteria that regulates a wide range of functions including cell differentiation, bacterial adhesion and biofilm formation, bacterial motility, colonization of host tissues and virulence3,4. The c-di-GMP-mediated signaling network is complex and regulation can occur at multiple levels to include transcription, by binding to transcription factors such as FleQ5, post-transcriptional, such as binding to GEMM RNAs6, and at the posttranslational level, such as in the regulation of Pel polysaccharide synthesis7,8. Cyclic di-GMP is formed from two GTP molecules by diguanylate cyclases (DGCs) that have a GGDEF domain and is broken into pGpG or GMP by phosphodiesterases (PDEs) containing either an EAL or HD-GYP domain4. These domains involved in c-di-GMP metabolism are widely present in Gram-negative bacterial proteins. For example, the Escherichia coli K-12 strain contains 29 GGDEF/EAL domain proteins, whereas Vibrio cholerae and Pseudomonas aeruginosa has 53 and 38 such proteins, respectively9,10. By contrast, the HD-GYP proteins are less common and even absent in some bacterial species11. These c-di-GMP metabolism proteins precisely modulate intracellular concentrations of c-di-GMP, and thus alter phenotypes through regulating different signaling pathways12.
A major sub-group of proteins involved in c-di-GMP signaling contain both GGDEF and EAL domains arranged in tandem13. Several such proteins have been demonstrated to have both DGC and PDE enzymatic activities; for example MsDGC-1 in Mycobacterium smegmatis14, Lpl0329 in Legionella pneumophila15, and ScrC in Vibrio parahemeolyticus16. In many cases however, one of the two domains in the GGDEF-EAL proteins is catalytically inactive. For example, AxDGC2 in G. xylinus only displays the DGC activity17, whereas CC3396, a GGDEF-EAL protein from Caulobacter crescentus, has only the PDE activity18. The inactive GGDEF or EAL domains of these proteins may act in a regulatory capacity. The DGC-inactive GEDEF domain of CC3396 is able to bind GTP and activates the PDE activity in the neighboring EAL domain18. The third situation is that both domains are enzymatically inactive but instead function as c-di-GMP effectors. For example, LapD from Pseudomonas fluorescens serves as a high affinity c-di-GMP receptor via a degenerate EAL domain and controls biofilm formation through regulating localization of the large cell surface adhesin LapA19,20. Another GGDEF-EAL protein Filp in Xanthomonas oryzae pv. oryzae (Xoo) also acts as the receptor of c-di-GMP that binds to the degenerate EAL domain with high affinity21. Mutation of the filp gene in Xoo attenuates bacterial virulence21. Recently, it was demonstrated in Xylella fastidiosa that a Tn5 insertion mutant of PD1671, which encodes a putative GGDEF-EAL protein, has a hypervirulent phenotype in grapevines. This negative effect of PD1671 on virulence was attributed to enhanced expression of gum genes leading to increased production of the fastidian exopolysaccharide and associated biofilm formation22.
X. oryzae pv. oryzicola (Xoc) causes bacterial leaf streak (BLS) in rice, one of the most important bacterial diseases in subtropical Asia. BLS has expanded rapidly in South China and South-East Asia; no resistance genes to this disease are available23. Although BLS disease symptoms are very similar to those of another important rice disease, bacterial leaf blight caused by Xoo, the two causal pathogens have different infection processes and styles. Xoc initially enters leaf tissues of rice through stomata or wounds, and then colonizes the intercellular space of mesophyll while Xoo infects leaf through water pores and causes a systemic vascular disease23,24. Genome-wide mutational analyses have revealed multiple factors that contribute to Xoc virulence. These factors include type III secretion, lipopolysaccharide synthesis, type IV pilus and twitching motility, carbohydrate synthesis and two-component regulation25,26. As in other xanthomonads, c-di-GMP associated signalling pathways are also implicated in Xoc virulence. In X. campestris pv. campestris, multiple GGDEF/EAL/HD-GYP domain proteins have shown to contribute to virulence and environmental adaptation27. The HD-GYP domain regulator RpfG acts together with the sensor kinase RpfC in a two-component system to regulate the synthesis of particular virulence factors in response to the diffusible signal factor DSF28,29,30. Similarly, deletion of rpfG in Xoc results in reduced virulence31, suggesting an important role for c-di-GMP signaling. The Xoc BLS256 genome encodes 32 GGDEF/EAL/HD-GYP proteins with a potential role in c-di-GMP metabolism and perception31. No functional study on GGDEF and/or EAL domain-containing proteins in Xoc has been reported so far however.
In the present study, we constructed a panel of strains each with a deletion of one of the eleven genes that encode GGDEF-EAL proteins in Xoc. The effects of these mutations on virulence-associated phenotypes and virulence were systematically investigated. Four of these proteins (XOC_2102, XOC_2335, XOC_2393, and XOC_4190) influenced motility and one of them, XOC_4190, influenced virulence. We further demonstrated that in vitro purified XOC_4190 and XOC_2102 were enzymatically inactive, but were able to bind to c-di-GMP with high affinity. The findings add to an understanding of c-di-GMP signaling and its links to virulence in this important rice pathogen.
Results
A panel of deletion mutants for eleven genes encoding GGDEF-EAL domain-containing proteins in Xoc
The Xoc BLS256 genome encodes eleven tandem GGDEF-EAL domain-containing proteins32 (see Supplementary Fig. S1). Most of these proteins have additional sensory and signal transduction domains. Accordingly, XOC_1633, XOC_2102, XOC_2277 and XOC_2395 contain PAS domains that have been shown to sense diverse changes in environmental or cellular cues, such as light, redox state and oxygen33; XOC_2179, XOC_2277 and XOC_2466 carry GAF domains that in other proteins have been implicated in small ligand binding and protein-protein interactions34; XOC_2120 and XOC_2944 contain HAMP domains that may be associated with plasma membrane localization and signaling; XOC_2335 contains three novel conserved MHYT domains with a likely signaling function35; and XOC_2102, XOC_2393 and XOC_4190 have REC domains and may function as regulators in two-component systems36,37. These functional domains in the proteins indicate that their activities in cyclic di-GMP turnover or perception are responsive to environmental cues9,12. Deletion mutants were constructed for the eleven genes encoding these proteins following the strategy described in the Materials and Methods section and were listed in Supplementary Table S1. All mutants used for phenotypic studies were confirmed by Southern blot analyses (see Supplementary Fig. S2).
XOC_2335 and XOC_ 2393 positively regulate swimming motility
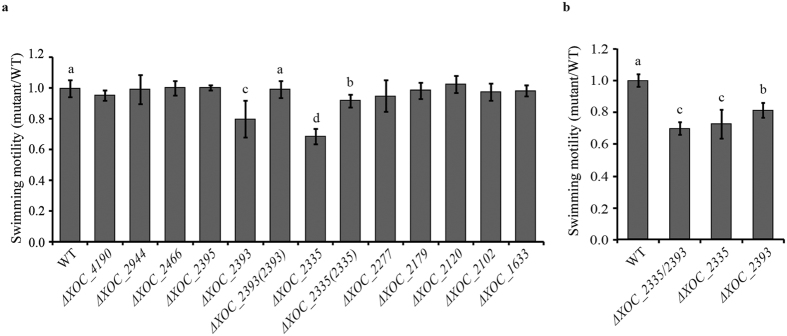
Swimming motility is a major survival mechanism of most Gram-negative bacteria. In bacteria, high level of c-di-GMP often suppresses swimming motility11. Therefore, the panel of mutants was first tested for swimming motility on semisolid (0.2% agar) medium plates. The results showed that the swimming motility of ΔXOC_2335 and ΔXOC_2393 was attenuated by ~30% and ~20% compared with the wild-type strain (Fig. 1a). By contrast, other mutants displayed swimming motility similar to the wild type (Fig. 1a). The complementation of ΔXOC_2335 and ΔXOC_2393 strains with plasmid-borne full-length genes restored swimming motility to wild type or near wild-type level (Fig. 1a). Since both XOC_2335 and XOC_2393 regulate swimming motility, a double mutant ΔXOC_2335/XOC_2393 was constructed to investigate genetically the relationship between the two proteins in the control of swimming motility. As shown in Fig. 1b, the swimming ability of ΔXOC_2335/XOC_2393 was similar to that of the mutant ΔXOC_2335 and was lower than that of ΔXOC_2393 (Fig. 1b). The results demonstrated that XOC_2335 and XOC_2393 positively regulate the swimming motility of Xoc, with XOC_2335 having the predominant effect.
Figure 1. Effects of mutation of individual genes encoding GGDEF-EAL proteins on the swimming motility of Xoc.
(a) The swimming motility of ΔXOC_2335 and ΔXOC_2393 was significantly reduced compared with that of the wild-type and other gene-deletion strains. The complementation strains ΔXOC_2335(2335) and ΔXOC_2393(2393) with plasmid-borne full-length genes restored the swimming motility nearly to or completely to the wild-type level, respectively. (b) The double-gene deletion mutant ΔXOC_2335/XOC_2393 exhibited a similar swimming ability to the ΔXOC_2335 mutant. The swimming motility of different Xoc strains was evaluated on semisolid plates with 0.2% noble agar after incubating at 28 °C for 4 d. The ratios of colony diameter of the mutant strains to the wild type were shown. Bars are means ± standard error (SE). The letters (a–d) indicate significant difference (P < 0.05) by Duncan’s multiple range test.
XOC_2102, XOC_2393 and XOC_4190 negatively regulate sliding motility
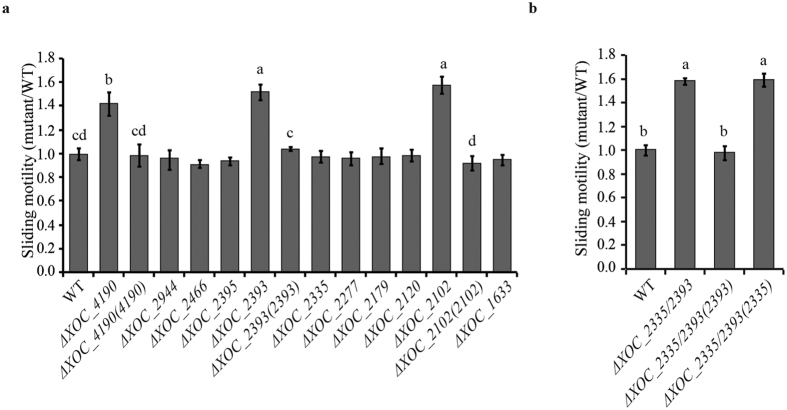
C-di-GMP signaling has been also demonstrated to be involved in control of type IV pili (T4P)-dependent motility in bacteria12. T4P is a thin filamentous structure present on outer surfaces of many bacteria. T4P has been shown to participate in twitching, sliding and several other important physiological processes such as adherence to surfaces38. The panel of mutants was tested for sliding motility on 0.6% agar SB medium plates. The ΔXOC_2102, ΔXOC_2393 and ΔXOC_4190 mutants had enhanced motility with colony diameters ~58%, ~52% and ~42% larger than the wild-type strain. Other mutants had no significant alteration in sliding motility. Complementation with the full-length XOC_2102, XOC_2393 or XOC_4190 gene restored the sliding motility of ΔXOC_2102, ΔXOC_2393 and ΔXOC_4190 to the wild-type level, respectively (Fig. 2a). Similarly, the ΔXOC_2393/XOC_2335 mutant had a larger colony diameter on SB medium plates than the wild-type strain. The full-length XOC_2393 gene, but not XOC_2335, restored the sliding motility of the double-gene deletion mutant (Fig. 2b). The results indicate that XOC_2102, XOC_2393 and XOC_4190 negatively regulate the sliding motility of Xoc.
Figure 2. Effects of mutations of GGDEF-EAL protein-encoding genes on sliding motility of Xoc.
The sliding motility of ΔXOC_2102, ΔXOC_2393 and ΔXOC_4190 was significantly increased compared with that of the wild-type strain. The complementation strains ΔXOC_2102(2102), ΔXOC_2393(2393) and ΔXOC_4190(4190) restored sliding motility to the wild-type level. (b) The sliding motility of ΔXOC_2335/XOC_2393 was significantly increased compared with the wild type and was restored by the full-length XOC_2393 gene, but not by the full-length XOC_2335 gene. The sliding motility of different strains was evaluated on SB medium plates with 0.6% noble agar after incubating at 28 °C for 4 d. The ratios of colony diameter of the mutant strains to the wild type were shown. Means ± SE are shown. The letters (a–d) indicate significant difference (P < 0.05) by Duncan’s multiple range test.
GGDEF-EAL domain proteins do not significantly affect EPS production, protease secretion and biofilm formation
Bacterial biofilm formation, EPS production and secretion of proteases are all important virulence factors in Xanthomonas spp.39,40. When the panel of single gene-deletion mutants was tested for these virulence-associated phenotypes, all strains produced similar amounts of EPS and biofilm biomass as the wild-type strain (see Supplementary Fig. S3). Similarly, the ability to synthesize and secrete proteases was not apparently altered in these mutants (see Supplementary Fig. S3). In addition, the double ΔXOC_2335/XOC_2393 mutant exhibited similar phenotypes in EPS production, biofilm formation and protease secretion to the wild-type and single gene-deletion mutant strains (see Supplementary Fig. S3).
XOC_2335, XOC_2393 and XOC_4190 are involved in regulating virulence to rice
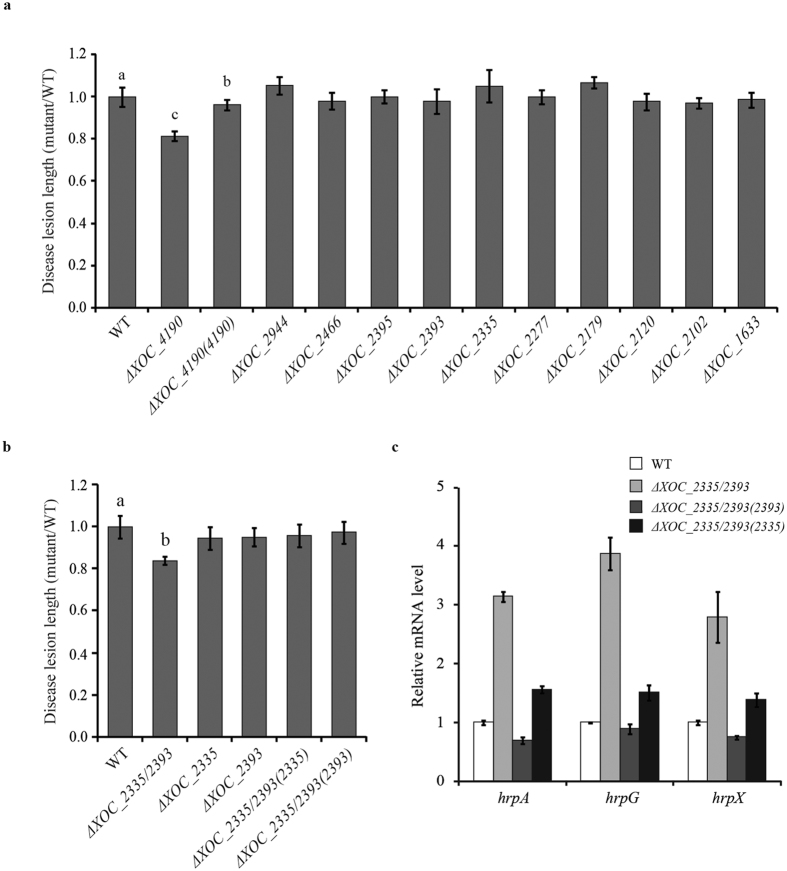
To investigate the roles of GGDEF-EAL proteins in Xoc virulence, the panel of mutants was pressure-inoculated into the leaves of rice plants. The length of disease lesions formed on the inoculated leaves was measured to evaluate bacterial virulence. The ΔXOC_4190 mutant was the only single-gene deletion strain with altered virulence, causing disease lesions shorter than the wild type (Fig. 3a). The ΔXOC_2335 and ΔXOC_2393 strains, which did show alteration in swimming ability (see above), produced disease lesions of similar sizes to the wild type. These experiments were extended to test the virulence of the ΔXOC_2335/XOC_2393 double mutant. The disease lesions caused by this strain were significantly shorter than those by the wild-type strain (Fig. 3b, see Supplementary Fig. S4). Complementation of the ΔXOC_2335/XOC_2393 mutant with either XOC_2335 or XOC_2393 restored virulence of the mutant to the wild-type level (Fig. 3b, see Supplementary Fig. S4). Collectively, the results indicate that XOC_4190, XOC_2335 and XOC_2393 all contribute to virulence of X. oryzae pv. oryzicola to rice. Our previous study demonstrated that the Xoc ΔrpfG mutant had an altered expression of hrp regulon31. Therefore, we further determined the expression level of hrpA, hrpG and hrpX in the ΔXOC_2335/XOC_2393 mutant using quantitative real-time reverse transcription PCR (qRT-PCR). The results showed that simultaneous deletion of XOC_2335 and XOC_2393 significantly increased the expression of hrpA, hrpG and hrpX under type III secretion-inducing conditions (Fig. 3c). Complementation of the double mutant by expression of XOC_2335 and XOC_2393 in trans restored the expression of hrpA, hrpG and hrpX to the wild type or near to wild-type level (Fig. 3c). These results imply that XOC_2335 and XOC_2393 act synergistically and negatively control the expression of the type III secretion system (T3SS).
Figure 3. The effects of mutation of GGDEF-EAL protein-encoding genes on virulence of X. oryzae pv. oryzicola to rice.
(a) The lesion length on the ΔXOC_4190-inoculated leaves of rice cv. Shanyou 63 was significantly shorter than that caused by the wild-type strain. Virulence of the ΔXOC_4190 mutant was restored by the plasmid-borne full-length XOC_4190 gene. Other single-gene deletion mutants have no altered virulence to rice compared with the wild type. (b) The lesion length caused by the ΔXOC_2335/XOC_2393 double mutant was significantly shorter than that caused by the wild-type strain. Both XOC_2335 and XOC_2393 can restore virulence of the double-gene deletion mutant to the wild-type level. The length of disease lesions was measured at 14 d after pressure inoculation of the wild-type, mutant and complemented strains, respectively. The ratios of disease lesion length caused by the mutant strains to that caused by the wild-type strain were shown. Data are presented as means ± SE. The letters (a,b) indicate significant difference (P < 0.05) by Duncan’s multiple range test. (c) The effect of double-gene deletion of XOC_2335 and XOC_2393 on the expression of hrp genes in Xoc. Expression of hrpA, hrpG and hrpX in the wild-type (WT), ΔXOC_2335/2393, ΔXOC_2335/2393(2393), ΔXOC_2335/2393(2335) strains was detected by qRT-PCR. 16S RNA was used as an internal control for data analyses. A significant increase of hrpA, hrpG and hrpX mRNA expression was detected in ΔXOC_2335/2393 compared with the wild-type and complementation strains.
Both GGDEF and EAL motifs of XOC_2335 are required for regulation of swimming motility
The phenotypic analyses indicate a role for XOC_4190, XOC_2335, XOC_2393 and XOC_2102 in virulence or motility of Xoc. As outlined above, proteins that contain tandem GGDEF and EAL domains can have DGC and/or PDE enzymatic activities. In some cases, both domains are enzymatically inactive but instead function as c-di-GMP effectors and/or are involved in protein-protein interactions. We used bioinformatic, genetic, biochemical and functional analyses (to include c-di-GMP binding) to reveal the possible mechanisms by which regulation by these different Xoc proteins may occur.
In silico analysis revealed that the GGDEF motif of XOC_2335 is degenerate, with the non-canonical GADEF motif at the active site. In contrast, all of the active site residues in the EAL domain12 were conserved, suggesting that overall XOC_2335 has a PDE activity. To determine the importance of the EAL motif for XOC_2335 function, a construct expressing a variant with EAA rather than EAL was made via site-directed mutagenesis. Complementation studies with this construct demonstrated that the XOC_2335EAL-EAA variant was unable to restore swimming motility of ΔXOC_2335 to the wild-type level (Fig. 4a). Another construct with the GADEF motif replaced by GAAAF in XOC_2335 was used to investigate the importance of the GGDEF motif. Similarly, ΔXOC_2335 expressing the XOC_2335GADEF-GAAAF variant had a similar swimming ability to the gene-deletion mutant (Fig. 4a). To confirm expression, constructs expressing variant proteins carrying HA tags were also made, allowing detection via immunoblotting. The results showed that the variant proteins with point mutations and the wild-type protein were all well expressed in Xoc (Fig. 4b). Furthermore, we demonstrated that expression of truncated XOC_2335-HA proteins, including XOC_2335ΔE without EAL domain or XOC_2335ΔG lacking GGDEF domain, in ΔXOC_2335 did not restore swimming motility of the mutant (see Supplementary Fig. S5). Complementation analyses using the XOC_2335 variants demonstrated that both non-canonical GADEF and conserved EAL motifs are essential for XOC_2335 function in vivo. Unfortunately, multiple attempts to express and purify XOC_2335 in E. coli failed because the protein was insoluble, precluding further studies.
Figure 4. Point mutations in the GGDEF or EAL motifs of XOC_2335 abolish its function in the regulation of swimming motility.
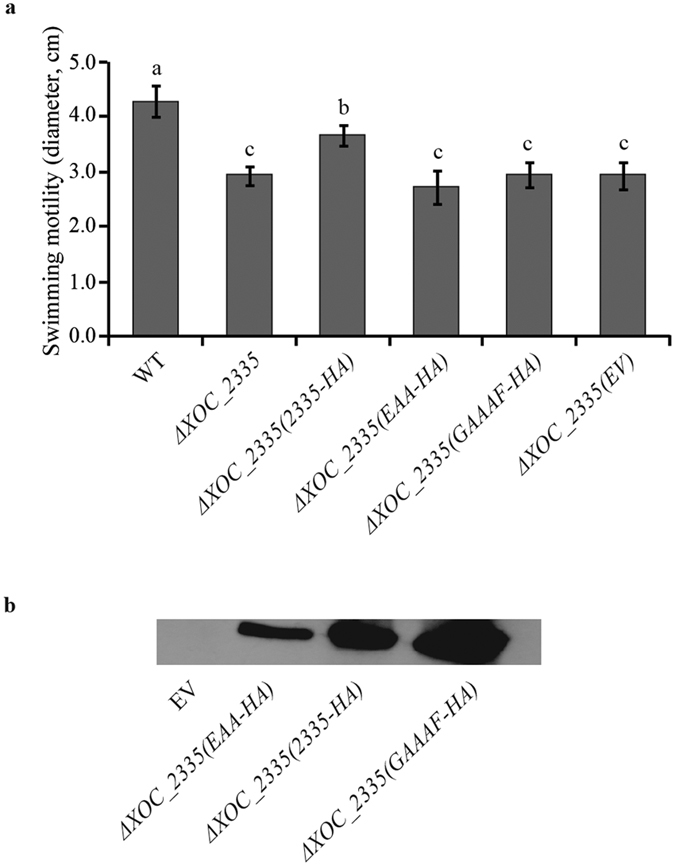
(a) The gene variant constructs with point mutations encoding XOC_2335GADEF-GAAAF-HA or XOC_2335EAL-EAA-HA variant proteins did not complement the swimming motility of ΔXOC_2335 while the XOC_2335-HA construct partially restores the swimming motility of ΔXOC_2335. The colony diameters of different Xoc strains cultured on semisolid plates with 0.2% noble agar were shown. WT, the wild-type strain. (b) Expression of HA-tagged XOC_2335 and its variants XOC_2335GADEF-GAAAF or XOC_2335EAL-EAA in Xoc was detected by western blot analysis with an anti-HA antibody. EV indicates the wild-type strain transformed with the empty vector pVSP61 as negative control. The letters (a–c) indicate significant difference (P < 0.05) by Duncan’s multiple range test.
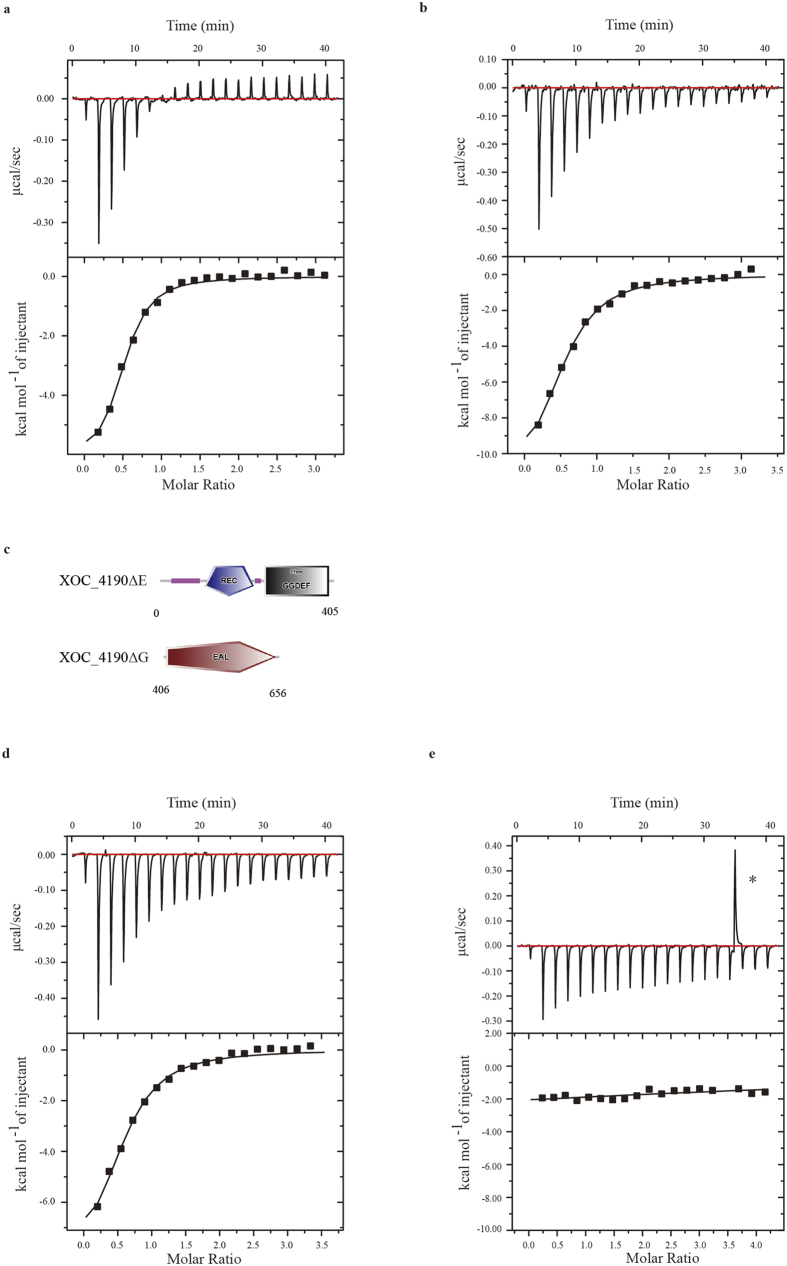
The GGDEF and EAL domains of XOC_ 2102 and XOC_4190 are degenerate and enzymatically inactive
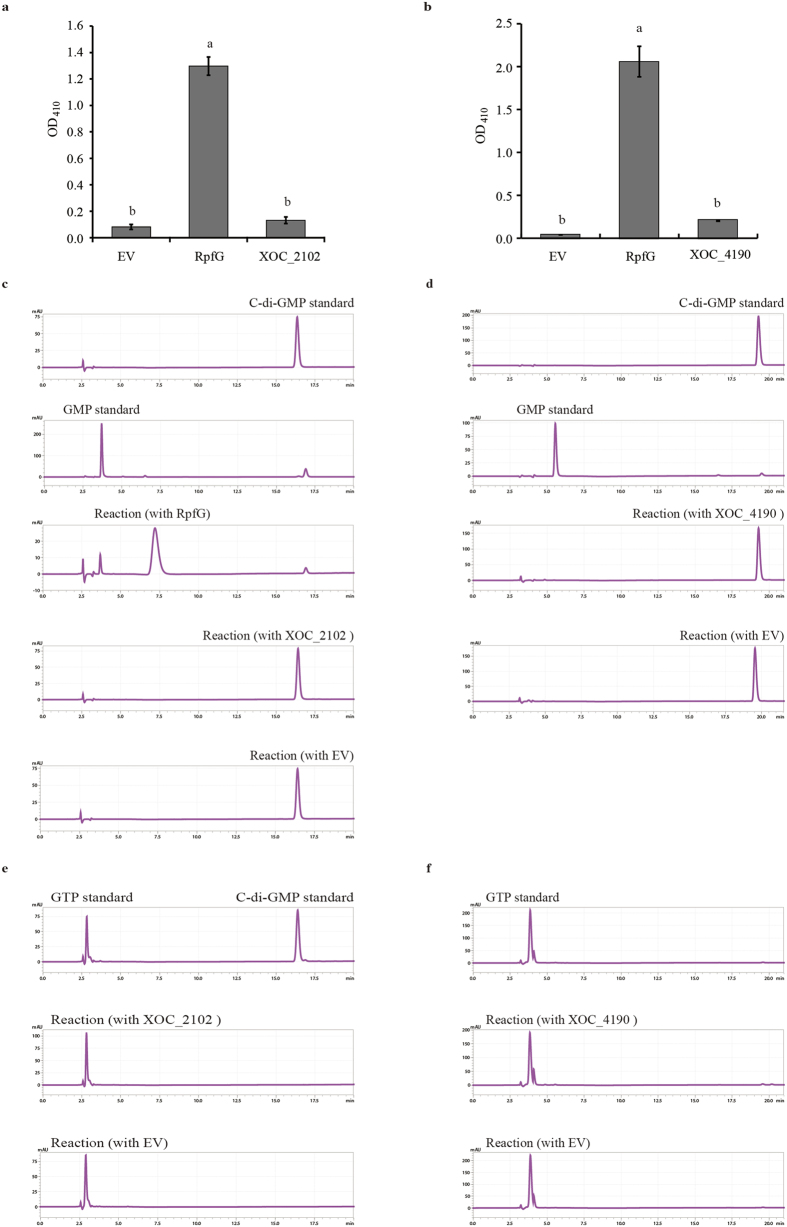
Bioinformatic analysis indicates that the GGDEF and EAL domains of both XOC_2102 and XOC_4190 are degenerate, with the variant motifs NDNST and QVL respectively in XOC_4190 and GEHSF and QAF respectively in XOC_2102. To experimentally verify these predictions, XOC_2102 and XOC_4190 with N-terminal His6-tags were expressed in E. coli and the recombinant proteins were purified as described in Materials and Methods. The purified proteins were first tested for the PDE activity through colorimetric assay. In this assay, the PDE activity was evaluated by the hydrolysis of the colorless substrate, bis(p-nitrophenyl) phosphate, into yellow p-nitrophenol that can be detected spectrophotometrically at 410 nm. Incubation of His6-XOC_2102 or His6-XOC_4190 with bis(p-nitrophenyl) phosphate did not give production of p-nitrophenol that was not significantly different from the mock control (no enzyme) (Fig. 5a,b). As a positive control, RpfG efficiently converted bis(p-nitrophenyl) phosphate into p-nitrophenol (Fig. 5a,b). The DGC or PDE activity of His6-XOC_2102 and His6-XOC_4190 was also tested by reverse phase high-performance liquid chromatography (HPLC) separation of reaction mixtures in which the purified proteins were incubated with GTP or c-di-GMP. No synthesis or degradation of c-di-GMP was detected under our experimental conditions (Fig. 5c–f). By contrast, a significant amount of the degraded product pGpG was produced when RpfG was incubated with c-di-GMP (Fig. 5c)31. Taken together, these results indicate that XOC_2102 and XOC_4190 with degenerate GGDEF and EAL domains are enzymatically inactive.
Figure 5. XOC_2102 and XOC_4190 are phosphodiesterase- and diguanylate cyclase-inactive.
The PDE activity of the purified XOC_2102 (a) and XOC_4190 (b) was detected by colorimetric assays. No yellow degradation product p-nitrophenol was detected when the purified XOC_2102 (a) or XOC_4190 (b) was incubated with the colorless phosphodiesterase substrate bis(p-nitrophenyl) phosphate. As a positive control, the known PDE RpfG degraded the substrate into the yellow product that was detected spectrophotometrically at 410 nm. The PDE activity of the purified XOC_2102 (c) and XOC_4190 (d) was detected by HPLC assays. No degraded product was detected when XOC_2102 and XOC_4190 were incubated with c-di-GMP. In the same reaction buffer, two hydrolytic products pGpG and GMP were detected after RpfG was incubated with c-di-GMP for 6 h (c). The DGC activity of XOC_2102 (e) and XOC_4190 (f) was assayed by HPLC. No synthetic c-di-GMP was detected when the purified XOC_2102 (e) and XOC_4190 (f) were incubated with GTP in the assay buffer for 6 h. GTP and c-di-GMP were loaded and detected as standards. The eluant from empty vector (EV)-transformed E. coli cells through nickel column was used as a negative control.
XOC_2102 and XOC_4190 bind to c-di-GMP in vitro
As pointed out above, proteins containing degenerate GGDEF and EAL domains can serve as c-di-GMP receptors19,41. Isothermal titration calorimetry (ITC) was performed to assess the binding of c-di-GMP to XOC_2102 and XOC_4190. In this assay, the purified recombinant His6-XOC_2102 and His6-XOC_4190 were titrated with c-di-GMP at room temperature. The dissociation constants (Kd) were determined after analysis of the normalized ITC curve by the Origin software42. The data indicate that XOC_2102 binds to c-di-GMP with a Kd of 2.90 ± 0.13 μM, and that XOC_4190 binds to c-di-GMP with a Kd of 4.59 ± 0.36 μM (Fig. 6a,b).
Figure 6. XOC_2102 and XOC_4190 bind to c-di-GMP in vitro.
Isothermal titration calorimetry (ITC) measurement for the interaction of c-di-GMP and XOC_2102 (a) or XOC_4190 (b). The data indicate that the dissociation constants (Kd) are 2.90 ± 0.13 μM or 4.59 ± 0.36 μM, respectively. (c) Schematic representation of the two truncated XOC_4190 proteins. XOC_4190ΔE, the truncated XOC_4190 without EAL domain; XOC_4190ΔG, the truncated XOC_4190 with the only EAL domain. ITC measurement for the interaction between c-di-GMP and XOC_4190ΔG (d) or XOC_4190ΔE (e). The data indicate that the Kd is 4.46 ± 0.28 μM for interaction between c-di-GMP and XOC_4190ΔG. Top panels, the titration calorimetry of the proteins with c-di-GMP at room temperature; lower panels, normalized ITC data for titrations versus molar ratio of c-di-GMP and the proteins.
To further investigate c-di-GMP binding to XOC_4190, two truncated proteins, XOC_4190∆G lacking the GGDEF domain and XOC_4190∆E without the EAL domain, were expressed and purified (Fig. 6c). Both truncated proteins were investigated for c-di-GMP binding via ITC assays. It was shown that XOC_4190∆G still bound to c-di-GMP with a Kd of 4.46 ± 0.28 μM, while XOC_4190∆E completely lost the c-di-GMP binding ability (Fig. 6d,e). The results indicate that the EAL domain, but not the GGDEF domain is required for binding of c-di-GMP by XOC_4190.
Xoc ΔXOC_2335, ΔXOC_2393 and ΔXOC_2335/XOC_2393 mutants, but not ΔXOC_4190, have elevated intracellular c-di-GMP levels
To determine if mutation of these GGDEF-EAL proteins affects the intracellular level of c-di-GMP, we quantified the c-di-GMP concentration in the wild type and multiple gene-deletion mutants using liquid chromatography-mass spectrometry. As shown in Fig. 7, ΔrpfG has the highest c-di-GMP concentration among the wild-type and tested mutant strains, consistent with previous findings that RpfG functions as a PDE and the ΔrpfG mutant showed more drastic changes phenotypically28,31. Our results also showed an elevated c-di-GMP concentration in ΔXOC_2335, ΔXOC_2393 and ΔXOC_2335/XOC_2393 mutants compared with the wild-type strain. Meanwhile, the double mutant had a higher level of c-di-GMP than the single gene deletion mutants (Fig. 7). By contrast, no change in the c-di-GMP level was detected in the ΔXOC_4190 mutant, consistent with the results from PDE and DGC activity assays. These results suggest that XOC_2335 and XOC_2393, but not XOC_4190, function to degrade c-di-GMP and are indeed PDEs.
Figure 7. Measurement of intracellular c-di-GMP levels in wild-type or mutant Xoc strains.
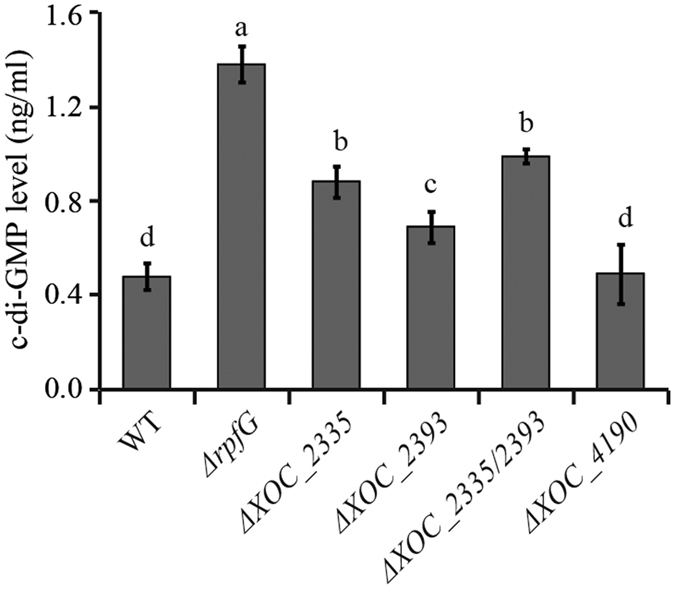
Assays were performed as described in the Methods. The c-di-GMP concentrations were quantified in the wild type (WT), ΔrpfG, ΔXOC_2335, ΔXOC_2393, ΔXOC_2335/2393 and ΔXOC_4190 mutants. An increased c-di-GMP concentration was revealed in ΔrpfG, ΔXOC_2335/2393, ΔXOC_2335, and ΔXOC_2393 in comparison with the wild-type strain. The c-di-GMP level in ΔXOC_4190 was similar to that in the wild-type strain. Data are presented as means ± SE. The letters (a–d) indicate significant difference (P < 0.05) by Duncan’s multiple range test.
Discussion
C-di-GMP is an important secondary messenger in phytopathogenic bacteria that has pleiotropic effects on virulence-associated cellular processes3. In the present study, we have addressed one facet of c-di-GMP regulation in Xoc by assessing the contribution of eleven tandem GGDEF-EAL proteins to bacterial virulence and virulence-associated traits. Three of these proteins (XOC_4190, XOC_2335 and XOC_2393) were implicated in the regulation of Xoc virulence and together with XOC_2102 were shown to control bacterial swimming and sliding motilities, but did not significantly affect other virulence-related functions such as biofilm formation, EPS production and protease secretion. The action of these four proteins in regulation appears to be different. XOC_2102 and XOC_4190 do not have detectable DGC or PDE activity but bind to cyclic di-GMP with high affinity, suggesting that they may act as effector proteins for the nucleotide. In contrast, XOC_2335 and XOC_2393 may be PDEs that can influence intracellular levels of c-di-GMP.
Virulence was assessed following direct inoculation of bacteria into rice leaves. This assay bypasses the earlier phases of the disease cycle where bacteria have an epiphytic lifestyle before entry into the host extracellular spaces through stomata and wounds. It may well be that other GGDEF-EAL domain proteins control factors that are important in this early phase of disease. In this context it should be noted that many GGDEF-EAL proteins carry other sensory and signal transduction domains and may regulate virulence-associated traits in response to environmental cues only found in naturally infected plants. The absence of an effect of mutation on known virulence factors when assayed in vitro may not reflect what occurs in planta during disease. For example, the PAS-GGDEF-EAL domain protein XC2324 of X. campestris pv. campestris may sense molecular oxygen through binding to the PAS domain, only causes a significant reduction in the synthesis of the virulence factors endoglucanase and endomannanase under low oxygen condition27.
Quantification of the intracellular c-di-GMP concentration in ΔXOC_2335, ΔXOC_2393 and ΔXOC_2393/XOC_2335 mutants showed that these mutants all had higher c-di-GMP levels than the wild type (Fig. 7), indicating that XOC_2335 and XOC_2393 are indeed PDEs. Interestingly, the double ΔXOC_2393/XOC_2335 mutant was attenuated in virulence to rice, although ΔXOC_2393 and ΔXOC_2335 exhibited no significant alteration in virulence. These results suggest that XOC_2335 and XOC_2393 might function redundantly or additively, consistent with the finding that the double mutant has a higher c-di-GMP level than the single gene mutants. In a previous study, we demonstrated that deletion of rpfG abolished Xoc virulence to rice and RpfG negatively regulated the expression of T3SS31. Consistently, hrp operon was shown to be significantly up-regulated in the ΔXOC_2393/XOC_2335 mutant (Fig. 3c). Together with the finding that the c-di-GMP level is dramatically elevated in the ΔrpfG mutant, these results suggest that higher concentration of c-di-GMP is involved in positive regulation of T3SS expression in Xoc. In contrast, PdeR, a homolog of XOC_2393, with PDE activity, was required for the expression of the T3SS that is essential for bacterial virulence in Xoo19. It warrants to be further investigated why these PDEs might regulate T3SS expression in different ways in Xoo and Xoc.
The GGDEF-EAL domain proteins affecting virulence in Xoc have homologs in Xoo, but the effects of mutation of the encoding genes are different. As noted above, XOC_2393 is a homolog of PdeR in Xoo PXO99A. The pdeR mutant is attenuated in virulence to rice and produces much less exopolysaccharide than the wild type19. By contrast, under our experimental conditions the ΔXOC_2393 mutant exhibited no significant difference from the wild type in phenotypes other than motility. XOC_4190 and XOC_2102 are homologous to PXO_02944 and Filp of Xoo, respectively. In contrast to ΔXOC_4190 that has attenuated virulence to rice, ΔPXO_02944 exhibits increased EPS production, biofilm formation and also elevated virulence to rice43. ΔXOC_2102 and Δfilp strains showed similar phenotypes, with altered motility, but no effects on EPS production or biofilm formation. However, the filp mutant is attenuated in virulence to rice unlike the ΔXOC_2102 strain. Different phenotypes caused by mutation of homologous genes in xanthomonads have been reported previously27,44. Several reports suggest that DSF signaling regulates virulence-associated traits in a completely different pattern in Xoo and Xcc45,46,47. The difference might be due to genetic divergence and different infection styles among the Xanthomonas species.
Bacteria exhibit several types of motilities, such as swimming, twitching and sliding motilities under various conditions12. A body of work has implicated GGDEF-EAL domain proteins in the regulation of these different modes of motility. For example, mutation of XC2161 in X. campestris pv. campestris reduces pilus-dependent motility, while loss of another GGDEF-EAL protein XC2226 causes an opposite phenotype27. FimX of P. aeruginosa is reported to be involved in type IV pilus-based motility12. In general, higher levels of c-di-GMP suppresses swimming motility12. Our finding that deletion of the genes encoding the putative PDEs XOC_2335 and XOC_2393 caused the elevated intracellular c-di-GMP level and reduced swimming motility is consistent with this contention. Bioinformatic analysis of XOC_2393 indicates that it has a degenerate GGDEF domain, with a GSDEM motif, but that key residues in the EAL domain required for active PDEs are all conserved. XOC_2393 shares 95.2% amino acid sequence similarity to PdeR in X. oryzae pv. oryzae PXO99A and the EAL domains of the two proteins are identical. Intriguingly, PdeR has been shown to have PDE activity in vitro44. Complementation experiments with point mutations in GGDEF or EAL motifs demonstrated that both motifs were required for XOC_2335 function in regulating swimming motility. In C. crescentus, the GGDEF-EAL protein CC3396 is able to bind GTP through degenerate GGDEF domain and then activates the PDE activity in the neighboring EAL domain18. Therefore, it is interesting to further investigate whether XOC_2335 and XOC_2393 function in swimming motility with a similar mechanism to CC3396. Unfortunately, multiple attempts to express and purify XOC_2335 and XOC_2393 in E. coli failed because the proteins were insoluble.
Interestingly, we demonstrated that XOC_2335 positively controlled swimming ability, but did not affect sliding motility. By contrast, XOC_2393 regulated both swimming and sliding motilities in the opposite way. Therefore, the effect of XOC_2335 and XOC_2393 on phenotypes was not completely redundant. Swimming motility is mediated by flagella, while sliding motility is mediated by the type IV pili. Speculatively, XOC_2393 might regulate the pili-mediated sliding motility in a c-di-GMP-independent manner. These findings indicate a complexity in regulation that might reflect an emerging concept of the multi-functionality of c-di-GMP signaling proteins, which can have a regulatory action exerted through protein-protein interactions that is independent of their enzymatic activity in cyclic di-GMP turnover.
Bioinformatic analysis indicates that XOC_2102 and XOC_4190 with degenerate GGDEF and EAL domains might not function as a DGC or PDE, which is consistent with the DGC and PDE enzymatic assays (Fig. 5). The hypothesis is also supported by the fact that the c-di-GMP level is not altered in ΔXOC_4190 mutant (Fig. 7). Previous studies showed that some enzymatically inactive GGDEF-EAL domain proteins, such as FimX from P. aeruginosa, LapD from P. fluorescens, and Filp from X. oryzae pv. oryzae PXO99A can act as a major class of c-di-GMP receptors in different bacteria. These proteins bind to c-di-GMP with high-affinity through the degenerate and enzymatically inactive C-terminal EAL domains and serve as the receptors of this signal molecule19,21,37. In this study, ITC assays clearly showed that XOC_4190 and XOC_2102 bound to c-di-GMP. Furthermore, we further revealed that the only EAL domain of XOC_4190 can bind to c-di-GMP, while the GGDEF domain cannot. Therefore, several of these Xoc proteins with degenerate GGDEF-EAL domains might function in c-di-GMP signaling through serving as c-di-GMP effectors.
In this study, we systemically analyzed the functions of GGDEF-EAL proteins in Xoc. XOC_4190 was shown to be an essential regulator of bacterial virulence likely through functioning as a c-di-GMP receptor. Both XOC_2335 and XOC_2393 individually regulate bacterial motility and together control Xoc virulence. These novel findings suggest new questions for further research. How do c-di-GMP signaling proteins regulate various types of bacterial motility in opposite ways? How is c-di-GMP signaling involved in virulence regulation? Identification of protein-protein interactions involved in signaling and determination of the crystal structure of the putative effectors in complex with c-di-GMP will help to elucidate the molecular mechanisms underlying the diverse regulatory functions of GGDEF-EAL proteins.
Methods
Bacterial strains and culture conditions
The X. oryzae pv. oryzicola strain RS105 and its mutants were cultured in nutrient broth (NB) medium (3 g/L beef extract, 1 g/L yeast extract, 5 g/L tryptone, 10 g/L sucrose) at 28 °C. Yeast cultures were grown in YPDA medium (20 g/L BactoTM Peptone, 10 g/L yeast extract, 20 g/L glucose, 30 mg/L adenine) at 28 °C. Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; rifampin, 25 μg/ml. All gene constructs were confirmed by sequencing.
Mutant construction and complementation of Xoc mutant strains
Non-marker homologous recombination was used to construct gene-deletion mutants of Xoc as described previously31. Briefly, ~1 kb long flanking regions upstream and downstream of open reading frames (ORFs) of the target genes were amplified by PCR with specifically designed primers (see Supplementary Table S2). PCR products were gel purified and added together into a fusion PCR. The resultant PCR fragments were cloned into pUFR80, which carries the sacB suicide gene48. The constructed plasmids were then conjugated into Xoc RS105 through triparental mating. The recombinant conjugants resulted from double cross-over events were screened on nutrient agar (NB medium with 1.5% agar) plates with 5% sucrose. The gene-deletion genotypes of sucrose-insensitive Xoc colonies were identified through colony PCR and were then subjected to confirmation via Southern blot analyses.
To construct complementation strains of Xoc mutants, full-length genes including native promoters were amplified by PCR using designed primers (see Supplementary Table S2). The amplified products were cloned into the wide host-range vector pVSP6149. After being confirmed by sequencing, the constructs were individually conjugated into the corresponding mutants and successful conjugants were then selected on kanamycin-containing NA plates.
Southern blot analysis
Southern blot analysis was performed according to standard protocols in molecular biology50. Briefly, genomic DNA was isolated from the wild-type and mutant strains of Xoc using a genomic DNA isolation kit (New Industry Company, Beijing, China) and was then digested with appropriate restriction enzymes overnight. After separated by agarose gel, digested genomic DNA was transferred onto Hybond-N nylon membrane (GE Healthcare) and was then hybridized with the 32P-labeled gene fragments that were amplified by PCR using the primers listed in the Supplementary Table S2.
Biofilm quantification
Biofilm masses of Xoc cultures were quantified as described previously31. Briefly, bacterial strains were cultured in 5 ml L medium (10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl and 1 g/L glucose) in the borosilicate glass tubes. After 7 d of incubation at quiescent state at 28 °C, cultured cells were stained with 10% crystal violet (CV) for 15 min. The tubes were rinsed with H2O carefully to remove the surplus dye. The CV-stained biofilm attached to the tubes was solubilized in 90% ethanol and quantified by spectrophotometry at 590 nm.
Exopolysaccharide production
Exopolysaccharide (EPS) production in Xoc cultures was determined as described previously31,51. Overnight cultures of Xoc were collected and re-suspended in sterile water to an OD600 of ~1.0. The cells were diluted at 1:1, 000 with M210 medium (8 g/L casein enzymatic hydrolysates, 4 g/L yeast extract, 5 g/L sucrose, 3 g/L KH2PO4, 0.3 g/L MgSO4·7H2O) and cultured further for 30 h. The supernatants (10 ml) were collected after centrifugation at 12,000 rpm for 5 min. EPS was precipitated by mixing the supernatant with two volumes of absolute ethanol and incubating at −20 °C overnight. The precipitate was collected by centrifugation at 10,000 rpm for 5 min and fully dried at 55 °C before weighing.
Protease secretion assay
The secretion of extracellular proteases of Xoc strains was assayed as described elsewhere52. Briefly, bacteria were cultured on the water agar plates containing 1% (w/v) skimmed milk at 28 °C for 4 d. The diameter of clearing zones around the colonies formed by the proteolytic degradation of skimmed milk was measured to assess the activity of secreted proteases.
Bacterial motility assays
The swimming motility of Xoc strains was determined on semisolid medium plates containing 0.2% noble agar, 0.03% yeast extract and 0.03% bacto peptone53. Xoc strains were inoculated into the center of the plates by pipetting. After culturing at 28 °C for 4 d, the diameter of bacterial colonies was measured. The sliding motility of Xoc was evaluated by the diameter of bacterial colonies after Xoc strains were incubated on SB medium plates (5 g/L bacto peptone, 5 g/L yeast extract, 1 g/L L-glutamic acid, and 0.6% noble agar) for 4 d at 28 °C38.
Virulence assay of Xoc to rice
Virulence assays were performed as described previously25. Briefly, overnight grown cells were collected and re-suspended in sterile distilled water to cell density of OD600 = 0.3. Bacterial suspensions were pressure-infiltrated into the leaves of 6-week-old rice plants using needleless syringes. The length of disease lesions was measured at 14 d after inoculation. At least 10 inoculated leaves were measured for each of the tested Xoc strains.
RNA isolation and quantitative real-time RT-PCR
Overnight Xoc cultures were diluted in the hrp-inducing XOM3 medium54 to an OD600 of 0.08 and grew further till OD600 = 0.6. The cells were then harvested to isolate RNA using TransZol Up Plus RNA Kit (Transgen, Beijing, China) following the manufacturer’s instructions. Quantitative real-time RT-PCR (qRT-PCR) was performed as described previously with minor modifications31. Bestar® SYBRGreen qPCR Mastermix (DBI® Bioscience, Shanghai, China) was used here in qRT-PCR reactions to quantify the transcript levels. Expression of 16S rRNA was used as the internal reference for data analysis.
Site-directed mutagenesis and gene constructs encoding truncated proteins
Site-directed mutagenesis was performed through circular PCR following the manufacturer’s instructions (Stratagene). Briefly, PCR was performed with Pfu Ultra DNA polymerase using the pUC19-XOC_2335 plasmid DNA as template. After the methylated template DNA was digested with Dpn I for 3 h at 37 °C, PCR products were transformed into E. coli DH5α cells. The plasmid DNA with point mutations were isolated and subject to confirmation by sequencing. The mutated gene fragments were released from the pUC19 constructs with BamH I and Sac I and were then re-ligated into pVSP61. The native promoter and coding sequences for GGDEF domain of XOC_2335 were amplified with the primer set 2335-Sac I-HA-F/2335-∆E-Hind III-HA-R (see Supplementary Table S2). The promoter and coding sequences for EAL domain of XOC_2335 were amplified with the primer sets 2335-Sac I-HA-F/2335-∆G-R and 2335-∆G-F/2335-HindIII-HA-R, respectively (see Supplementary Table S2). Two fragments were fused by overlap extension PCR and the resultant PCR products were cloned into pVSP6149.
Protein expression and purification
The ORFs of XOC_4190 and XOC_2102 were amplified from Xoc RS105 genome by PCR using primer sets listed in the Supplementary Table S2. The amplified fragments were inserted into pQE30 (Qiagen) after the digestion with BamH I and Hind III. The truncated coding sequences for GGDEF and EAL domains of XOC_4190 (1–405 and 406–656 amino acid residues, respectively) were amplified with the primer sets XOC_4190∆E-F/XOC_4190∆E-R and XOC_4190∆G-F/XOC_4190∆G-R, respectively (see Supplementary Table S2). The PCR fragments were sub-cloned into the expression vector pET28a (Novagen, Madison, WI) after gel purification and digestion. The pQE30 and pET28a constructs were transformed into the E. coli strains XL1-blue and BL21(DE3), respectively. To induce expression of the target proteins, isopropyl β-D-thiogalactopyranoside (IPTG, 0.1 mM) was added into cell cultures when OD600 reached 0.5 ~ 0.7. After 4 ~ 5 h of induction at room temperature, cultured cells were collected by centrifugation. For protein purification, cell pellets were re-suspended in lysis buffer (20 mM Tris-Cl, 50 mM NaCl, and 10 mM imidazole, pH 8.0) and then subject to sonication. The supernatant was prepared by centrifugation at 10,000 g for 20 min and loaded onto nickel-nitrilotriacetic acid agarose superflow columns (Qiagen), which were thoroughly rinsed with washing buffer (20 mM Tris-Cl, 50 mM NaCl, and 20 mM imidazole, pH 8.0). The bound His6-tagged protein was eluted with elution buffer (20 mM Tris-Cl, 50 mM NaCl, and 250 mM imidazole, pH 8.0) and dialyzed extensively in 10 mM Tris-Cl (pH 8.0). The concentration of purified proteins was determined using PierceTM BCA protein assay kit (Thermo Scientific, IL, USA).
Immunoblotting
Total protein extracts were separated by a 12% polyacrylamide gel after boiling for 10 min. The proteins were electrophoretically transferred onto Immobilon-P membrane (Millipore) for immunoblotting with a horseradish peroxidase-conjugated anti-HA antibody (Roche). The membranes were then incubated with the eECL Western Blot chemiluminescent substrate (CWBio, China) for 5 min, and then exposed to X-ray films.
Phosphodiesterase colorimetric assay
In vitro purified proteins were assayed for the PDE activity using colorimetric assays55. Briefly, purified proteins (20 μg) were incubated with the PDE substrate, 5 mM bis(p-nitrophenyl) phosphate, at 37 °C for 1.5 h in assay buffer (50 mM Tris-Cl, 1 mM MnCl2, pH 8.5). The yellow product was quantified using spectrophotometer at OD410.
Enzymatic assays by high-performance liquid chromatography
The DGC and PDE activities were also assayed using high-performance liquid chromatography (HPLC) as described31,55. The enzyme activities were investigated using 20 μg of purified proteins in a buffer containing 25 mM Tris-Cl, pH 7.9, 250 mM NaCl, and 10 mM MgCl2. The PDE activity was tested using 100 μM of c-di-GMP as substrates, while the DGC activity was determined by replacing c-di-GMP with GTP (100 μM). The reaction mixture was incubated at 37 °C for 6 h and then stopped by boiling for 3 min. After centrifugation at 15,000 g for 2 min, the supernatant was filtered through a 0.22 μm membrane. Each sample (20 μl) was injected into a reverse phase C18 column (250 × 4.60 mm, 5 μm; Phenomenex, USA) with a Shimadzu 10AT system (Shimadzu Co., Ltd, Japan). The material was eluted at a flow rate of 1 ml/min with a 1%/min linear gradient of 0 ~ 20% methanol in 20 mM potassium phosphate buffer (pH 5.8). The products were visualized under 254-nm UV light.
Isothermal titration calorimetry assay
Isothermal titration calorimetry (ITC) assays were performed on a MicroCal iTC200 colorimeter (MicroCal, Northampton, MA) to investigate the binding of c-di-GMP to in vitro purified proteins42. Titration calorimetry was performed at 25 °C in the assay buffer containing 20 mM Tris-Cl (pH 8.0), 500 mM NaCl and 250 mM imidazole. In brief, c-di-GMP solution (100 ~ 500μM, 2 μl aliquots) was injected at 2 min intervals via a 40 μl syringe into the sample cell containing the purified proteins (10 ~ 50 μM). ITC data were analyzed by integrating heat effects after being normalized to the amount of injected c-di-GMP. Curve fitting was performed based on a single-site binding model to determine the dissociation constants (Kd) using the MicroCal ORIGIN version 7.0 software.
Determination of intracellular c-di-GMP concentration
Intracellular c-di-GMP levels in the wild-type and mutant strains were determined by liquid chromatography tandem mass spectrometry (LC-MS/MS) as described previously56. Briefly, overnight bacterial cultures were diluted to 1:100 into 90 ml LB media in a flask and grew further till OD600 ~ 0.8. The cultures were collected by centrifugation and were then re-suspended with 100 μl of extraction buffer (40% methanol and 40% acetonitrile in 0.1 N formic acid) per 48 mg of wet cell weight. The slurries were incubated 30 min at −20 °C and insoluble material was removed by centrifugation at 4 °C. The supernatants were neutralized by the addition of 4 μl of 15% NH4 HCO3 per 100 μl of sample. Each sample (100 μl) was analyzed using LC-MS/MS.
Statistical Analysis
Significant differences in various phenotypes among different strains (P < 0.05) were determined by Duncan’s multiple range test using SAS program.
Additional Information
How to cite this article: Wei, C. et al. A systematic analysis of the role of GGDEF-EAL domain proteins in virulence and motility in Xanthomonas oryzae pv. oryzicola. Sci. Rep. 6, 23769; doi: 10.1038/srep23769 (2016).
Supplementary Material
Acknowledgments
We thank Fengquan Liu and Guoliang Qian at Nanjing Agricultural University for providing materials. We also thank Yu Tian and Weihua Wang at Drug Discovery Facility, Center of Biomedical Analysis, Tsinghua University for help in LC-MS/MS analysis. The work is supported by the Special Fund for Agro-Scientific Research in the Public Interest of China (201303015), National Natural Science Foundation of China grant 31272007, the National High Technology Research and Development program of China 2012AA100703, and the 111 project B13006 to W.S.
Footnotes
Author Contributions W.J., C.W., M.Z., J.L., X.Z., J.D. and D.J. conducted experiments. W.J., C.W., J.M.D. and W.S. designed experiments and analyzed data. W.J., C.W., J.M.D. and W.S. wrote the manuscript.
References
- Ross P. et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325, 279–281 (1987). [DOI] [PubMed] [Google Scholar]
- Ross P., Mayer R. & Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 55, 35–58 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E., Pultz I. S., Kulasekara H. D. & Miller S. I. The bacterial second messenger c-di-GMP: mechanisms of signalling. Cell. Microbiol. 13, 1122–1129 (2011). [DOI] [PubMed] [Google Scholar]
- Sondermann H., Shikuma N. J. & Yildiz F. H. You’ve come a long way: c-di-GMP signaling. Curr. Opin. Microbiol. 15, 140–146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraquet C. & Harwood C. S. Cyclic diguanosine monophosphate represses bacterial flagella synthesis by interacting with the Walker a motif of the enhancer-binding protein FleQ. Proc. Natl. Acad. Sci. USA 110, 18478–18483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsan N. et al. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 321, 411–413 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. T. et al. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 65, 1474–1484 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. C. et al. Structure of the cytoplasmic region of PelD, a degenerate diguanylate cyclase receptor that regulates exopolysaccharide production in Pseudomonas aeruginosa. J. Biol. Chem. 287, 23582–23593 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povolotsky T. L. & Hengge R. ‘Life-style’ control networks in Escherichia coli: Signaling by the second messenger c-di-GMP. J. Biotechnol. 160, 10–16 (2012). [DOI] [PubMed] [Google Scholar]
- Waters C. M., Lu W., Rabinowitz J. D. & Bassler B. L. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 190, 2527–2536 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7, 263–273 (2009). [DOI] [PubMed] [Google Scholar]
- Romling U., Galperin M. Y. & Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 77, 51–52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshasayee A. S., Fraser G. M. & Luscombe N. M. Comparative genomics of cyclic-di-GMP signalling in bacteria: post-translational regulation and catalytic activity. Nucleic Acids Res. 38, 5970–5981 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharati B. K. et al. A full-length bifunctional protein involved in c-di-GMP turnover is required for long-term survival under nutrient starvation in Mycobacterium smegmatis. Microbiology 158, 1415–1427 (2012). [DOI] [PubMed] [Google Scholar]
- Levet-Paulo M. et al. The atypical two-component sensor kinase Lpl0330 from Legionella pneumophila controls the bifunctional diguanylate cyclase-phosphodiesterase Lpl0329 to modulate bis-(3′-5′)-cyclic dimeric GMP synthesis. J. Biol. Chem. 286, 31136–31144 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R. B., Antunes L. C., Greenberg E. P. & McCarter L. L. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. J. Bacteriol. 190, 851–860 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Rao F., Luo Z. & Liang Z. X. A flavin cofactor-binding PAS domain regulates c-di-GMP synthesis in AxDGC2 from Acetobacter xylinum. Biochemistry 48, 10275–10285 (2009). [DOI] [PubMed] [Google Scholar]
- Christen M., Christen B., Folcher M., Schauerte A. & Jenal U. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 280, 30829–30837 (2005). [DOI] [PubMed] [Google Scholar]
- Newell P. D., Monds R. D. & O’Toole G. A. LapD is a bis-(3′,5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc. Natl. Acad. Sci. USA 106, 3461–3466 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell P. D., Yoshioka S., Hvorecny K. L., Monds R. D. & O’Toole G. A. Systematic analysis of diguanylate cyclases that promote biofilm formation by Pseudomonas fluorescens Pf0-1. J. Bacteriol. 193, 4685–4698 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F. et al. The degenerate EAL-GGDEF domain protein Filp functions as a cyclic di-GMP receptor and specifically interacts with the PilZ-domain protein PXO_02715 to regulate virulence in Xanthomonas oryzae pv. oryzae. Mol. Plant Microbe Interact. 27, 578–589 (2014). [DOI] [PubMed] [Google Scholar]
- Cursino L. et al. Characterization of the Xylella fastidiosa PD1671 gene encoding degenerate c-di-GMP GGDEF/EAL domains, and its role in the development of Pierce’s disease. PLoS One 10, e0121851 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIÑO-LIU D. O., Ronald P. C. & Bogdanove A. J. Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol. Plant Pathol. 7, 303–324 (2006). [DOI] [PubMed] [Google Scholar]
- Mew T. W., Alvarez A. M., Leach J. E. & Swings J. Focus on bacterial-blight of rice. Plant Dis. 77, 5–12 (1993). [Google Scholar]
- Wang L., Makino S., Subedee A. & Bogdanove A. J. Novel candidate virulence factors in rice pathogen Xanthomonas oryzae pv. oryzicola as revealed by mutational analysis. Appl. Environ. Microbiol. 73, 8023–8027 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H. et al. Construction of a Tn5-tagged mutant library of Xanthomonas oryzae pv. oryzicola as an invaluable resource for functional genomics. Curr. Microbiol. 62, 908–916 (2011). [DOI] [PubMed] [Google Scholar]
- Ryan R. P. et al. Cyclic di-GMP signalling in the virulence and environmental adaptation of Xanthomonas campestris. Mol. Microbiol. 63, 429–442 (2007). [DOI] [PubMed] [Google Scholar]
- Ryan R. P. et al. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc. Natl. Acad. Sci. USA 103, 6712–6717 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Poplawsky A. R., Walters D. M., Rouviere P. E. & Chun W. A gene for a dioxygenase-like protein determines the production of the DF signal in Xanthomonas campestris pv. campestris. Mol. Plant Pathol. 6, 653–657 (2005). [DOI] [PubMed] [Google Scholar]
- Slater H., Alvarez-Morales A., Barber C., Daniels M. & Dow J. A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol. Microbiol. 38, 986–1003 (2000). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. The HD-GYP domain protein RpfG of Xanthomonas oryzae pv. oryzicola regulates synthesis of extracellular polysaccharides that contribute to biofilm formation and virulence on rice. PLoS One 8, e59428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove A. J. et al. Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J. Bacteriol. 193, 5450–5464 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. L. et al. Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor. Biochemistry 40, 3420–3426 (2001). [DOI] [PubMed] [Google Scholar]
- Hurley J. H. GAF domains: Cyclic nucleotides come full circle. Sci. STKE. 164, pe1 (2003). [DOI] [PubMed] [Google Scholar]
- Galperin M. Y., Gaidenko T. A., Mulkidjanian A. Y., Nakano M. & Price C. W. MHYT, a new integral membrane sensor domain. FEMS Microbiol. Lett. 205, 17–23 (2001). [DOI] [PubMed] [Google Scholar]
- Gao R. & Stock A. M. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 63, 133 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y. et al. Binding of cyclic diguanylate in the non-catalytic EAL domain of FimX induces a long-range conformational change. J. Biol. Chem. 286, 2910–2917 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo C. R., Salinas R. K., Andrade M. O. & Farah C. S. PILZ protein structure and interactions with PILB and the FIMX EAL domain: Implications for control of type IV pilus biogenesis. J. Mol. Biol. 393, 848–866 (2009). [DOI] [PubMed] [Google Scholar]
- Stoodley P., Sauer K., Davies D. G. & Costerton J. W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56, 187–209 (2002). [DOI] [PubMed] [Google Scholar]
- Buttner D. & Bonas U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 34, 107–133 (2010). [DOI] [PubMed] [Google Scholar]
- Navarro M. V. et al. Structural basis for c-di-GMP-mediated inside-out signaling controlling periplasmic proteolysis. PLoS Biol. 9, e1000588 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y. et al. Functional divergence of FimX in PilZ binding and type IV pilus regulation. J. Bacteriol. 194, 5922–5931 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. T. et al. Molecular characterization of the negative regulator PXO_02944 in virulence, extracellular polysaccharide production and biofilm formation in Xanthomonas oryzae pv. oryzae. Sci. Agric. Sin. 47, 2563–2570 (2014). [Google Scholar]
- Yang F. H. et al. A novel two-component system PdeK/PdeR regulates c-di-GMP turnover and virulence of Xanthomonas oryzae pv. oryzae. Mol. Plant Microbe Interact. 25, 1361–1369 (2012). [DOI] [PubMed] [Google Scholar]
- Ryan R. P. Cyclic di-GMP signalling and the regulation of bacterial virulence. Microbiology 159, 1286–1297 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R. P., An S. Q., Allan J. H., McCarthy Y. & Dow J. M. The DSF family of cell-cell signals: An expanding class of bacterial virulence regulators. PLoS Pathog. 11, e1004986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. W., Wu J., Cha J. S. & Zhang L. H. Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol. 10, 187 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried J. L. & Collmer A. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57, 239–246 (1987). [DOI] [PubMed] [Google Scholar]
- Loper J. E. & Lindow S. E. Lack of evidence for the in situ fluorescent pigment production by Pseudomonas syringae pv. syringae on bean leaf surfaces. Phytopathology 77, 1449–1454 (1987). [Google Scholar]
- Cannon F. C., Riedel G. E. & Ausubel F. M. Overlapping sequences of Klebsiella pneumoniae nif DNA cloned and characterized. Mol. Gen. Genet. 174, 59–66 (1979). [DOI] [PubMed] [Google Scholar]
- O’Toole G. A. & Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30, 295–304 (1998). [DOI] [PubMed] [Google Scholar]
- Tang J. L. et al. Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris. Mol. Gen. Genet. 226, 409–417 (1991). [DOI] [PubMed] [Google Scholar]
- DiLuzio W. R. et al. Escherichia coli swim on the right-hand side. Nature 435, 1271–1274 (2005). [DOI] [PubMed] [Google Scholar]
- Jia J. et al. Expression of the hrcC, hrpE and hpa3 genes is not regulated by the hrpG and hrpX genes in a rice pathogen Xanthomonas oryzae pv. oryzicola. Acta Microbiol. Sin. 49, 1018–1025 (2009). [PubMed] [Google Scholar]
- Yi X., Yamazaki A., Biddle E., Zeng Q. & Yang C. H. Genetic analysis of two phosphodiesterases reveals cyclic diguanylate regulation of virulence factors in Dickeya dadantii. Mol. Microbiol. 77, 787–800 (2010). [DOI] [PubMed] [Google Scholar]
- Bobrov A. G. et al. Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol. Microbiol. 79, 533–551 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.