Abstract
Previous in vitro and in vivo studies have demonstrated the potential of using cysteinyl leukotriene receptor antagonists (LTRAs) for chemoprevention, but this has not been investigated in any clinical setting. We therefore investigated the chemopreventive effect of LTRAs in a nationwide population-based study. From the Taiwan National Health Insurance Research Database, we enrolled adults with newly-diagnosed asthma between 2001 and 2011. Among these patients, each LTRA user was matched with five randomly-selected LTRA non-users by sex, age, asthma diagnostic year and modified Charlson Comorbidity Index score. We considered the development of cancer as the outcome. Totally, 4185 LTRA users and 20925 LTRA non-users were identified. LTRA users had a significantly lower cancer incidence rate than LTRA non-users did. Multivariable Cox regression analyses adjusting for baseline characteristics and comorbidities showed LTRA use was an independent protecting factor (hazard ratio = 0.31 [95% CI: 0.24–0.39]), and cancer risk decreased progressively with higher cumulative dose of LTRAs. In conclusion, this study revealed that the LTRA use decreased cancer risk in a dose-dependent manner in asthma patients. The chemopreventive effect of LTRAs deserves further study.
Cancer is a leading cause of death worldwide and has become the most common cause of death in Taiwan for more than 25 years1. Although much improvement has been made in anti-cancer treatment, the therapeutic outcome remained unsatisfying. Developing preventive strategies to reduce cancer incidence is therefore as important as improving anti-cancer strategies2,3. Chemoprevention is the use of a specific agent to reverse, suppress, or prevent the process of carcinogenesis2,3,4. Because limited effective and potent chemopreventive strategies are available to date, the cancer incidence remained high. Taking lung cancer, the most common cause of cancer death, for example, no specific agents have been recommended for primary, secondary, or tertiary chemoprevention although much effort has been made in the field of chemoprevention research4.
Cysteinyl leukotriene receptor antagonists (LTRAs), such as montelukast and zafirlukast, are widely used drugs for treating allergic asthma5,6. In addition to its well-known role in asthma, the leukotriene pathway is also responsible for carcinogenesis and tumour-mediated immunosuppression7. Overexpression of a cysteinyl leukotriene receptor, CysLT1R, has been shown in colorectal cancer, prostate cancer, renal cell carcinoma, transitional cell carcinoma and testicular cancer, and montelukast induces apoptosis of these cancer cells8,9,10,11,12,13,14. Only few in vivo studies to date have reported the chemopreventive effect of leukotriene pathway inhibitors14,15,16, while the chemopreventive effect of LTRAs has not been investigated in clinical setting.
Because some in vitro and in vivo studies had demonstrated the potential of using LTRAs for chemoprevention, we therefore conducted a nationwide population-based study to investigate the chemopreventive effect of LTRAs. Using a retrospective cohort study design, we found that LTRA use was associated with a decreased cancer risk in a dose-dependent manner.
Methods
Data Source
The Taiwan National Health Insurance (NHI) has covered ambulatory care, inpatient care and prescription drugs in Taiwan since 1996. The NHI coverage rate was 96.2% of whole population in 2000 and increased to >99% by 20052,17,18,19,20,21. The NHI Research Database therefore comprises comprehensive health care information from nearly the entire population of 23.72 million in Taiwan, becoming one of the largest insurance databases in the world17,19,20,21,22,23. The database used for this study is a cohort of two million subjects randomly sampled from NHI beneficiaries in 2000, and has been verified to be representative of the overall population of beneficiaries in terms of age, sex, geographic distribution and healthcare costs. The database includes information on medical reimbursement claims (such as ambulatory care claims, inpatient care claims, prescriptions, and registration entries) as well as information from Catastrophic Illness Registry, National Cancer Registry and National Register of Deaths. The database is managed by the Collaboration Center of Health Information Application (CCHIA), Ministry of Health and Welfare. For protection of confidentiality, patient identification has been already encrypted, and the authorized researchers are only permitted to perform data linkage, processing and statistical analyses with a specified computer in a closely monitored room. Using the scrambled personal identifier for each subject, the researchers are able to link the files to obtain socio-demographic information, longitudinal medical history and other information. Only statistical results were allowed to be brought out.
Study population
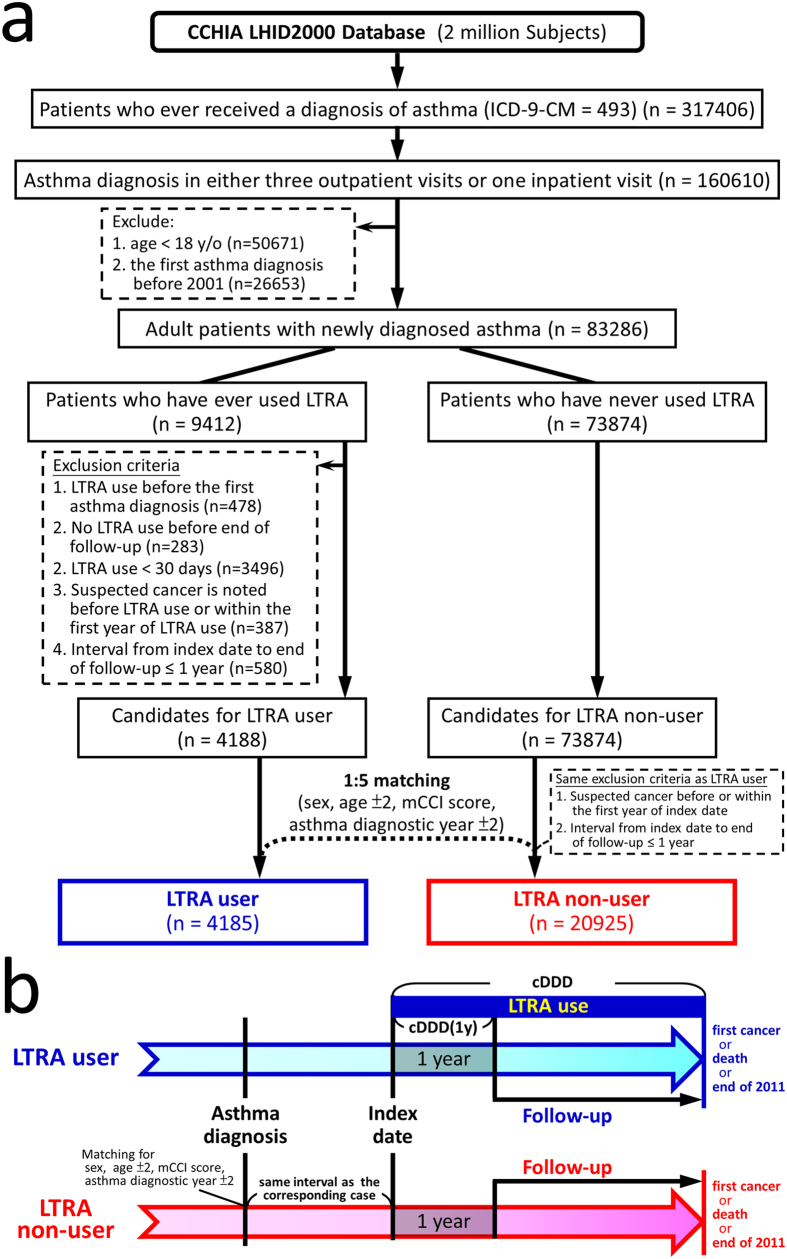
From the dataset, patients with newly diagnosed asthma were identified by the algorithm showed in Fig. 1. Patients with asthma diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification code [ICD-9-CM] of 493) in the ambulatory or inpatient claim database were identified, and only those with asthma diagnosis in at least three ambulatory claims or one inpatient claim were enrolled18. To ensure newly diagnosed adult asthma, those having asthma diagnosis before 2001 or those younger than 18 years old on their first asthma diagnosis were excluded.
Figure 1.
(a) Algorism for identifying the study cohorts. (b) Study design. From the dataset, adult patients with newly diagnosed asthma were identified. Through the algorism, subjects using LTRA for more than a month (30 days) before the end of follow-up were identified as candidates for LTRA user cohort. The subjects who had never used LTRA were identified as candidates for LTRA non-user cohort. Each LTRA user was matched with five randomly-selected LTRA non-users by sex, age (±2), asthma diagnostic year (±2) and mCCI score. The index date was defined as the date of first LTRA prescription for LTRA users; the LTRA non-users were given the index date with the same interval from their first asthma diagnosis as their corresponding LTRA users. During the matching process, the same exclusion criteria for the LTRA users were also applied while selecting LTRA non-users to ensure enough follow-up time and absence of any cancer diagnosis before the end of the first year after index date. The subjects were followed from a year after the index date to either development of cancer, death or the end of 2011, whichever came first. The cumulative defined daily doses of LTRA were calculated from the index date to the end of follow-up (cDDD) and to a year after the index date [cDDD(1y)]. Abbreviations: CCHIA = Collaboration Center of Health Information Application; LHID = Longitudinal Health Insurance Database; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification code; mCCI = modified Charlson Comorbidity Index.
The subjects who had ever used either montelukast or zafirlukast, the LTRAs available in Taiwan, after their asthma diagnoses were identified. After excluding those with neoplasm diagnosis (ICD-9-CM of 140-239 in any claims) before the end of the first year of LTRA use and those with the interval between first LTRA prescription and end of follow-up ≤1 year, subjects using LTRA for ≥30 days before the end of follow-up were identified as candidates for LTRA user cohort. The subjects who had never used LTRA were identified as candidates for LTRA non-user cohort.
Definitions of variables
The endpoint of this study was the development of cancer, defined by the appearance of cancer diagnosis in Catastrophic Illness Registry or National Cancer Registry. Pathological confirmation is generally required for reporting a cancer diagnosis to these registries. The date of death was obtained from the National Register of Deaths.
The presence of comorbidity was identified by the presence of any corresponding diagnostic codes before the index date in the claim databases and confirmed by the presence of the codes for at least three times in the ambulatory claim database or at least once in the inpatient claim database. Based on the comorbidities, modified Charlson Comorbidity Index (mCCI) score was calculated by subtracting chronic pulmonary disease from the original Charlson Comorbidity Index score24.
Study cohorts
Each LTRA user was matched with five randomly-selected LTRA non-users by sex, age (±2), asthma diagnostic year (±2) and mCCI score. The index date was defined as the date of first LTRA prescription for LTRA users; the LTRA non-users were given the index date with the same interval from their first asthma diagnosis as their corresponding LTRA users. During the matching process, the same exclusion criteria for the LTRA users were also applied while selecting LTRA non-users to ensure enough follow-up time and absence of any cancer diagnosis before the end of the first year after index date.
To minimize immortal time bias, the follow-up period was calculated from a year after the index date. The subjects were followed from a year after the index date to either development of cancer, death or the end of 2011, whichever came first. The defined daily doses (DDD) were 10 mg and 40 mg for montelukast and zafirlukast, respectively. To quantify individual’s exposure to LTRA, the cumulative defined daily doses of LTRA from the index date to the end of follow-up (cDDD) and to a year after the index date [cDDD(1y)] were calculated.
Statistical analysis
The demographic data and comorbidities were compared between LTRA users and non-users using Pearson’s χ2 test for categorical variables or Student’s t test for continuous variables, as appropriate. The cancer incidence rate (IR) was calculated as the number of cancer developed during the follow-up period divided by the total person-year. The cancer IRs in LTRA users and non-users were compared by estimating the incidence rate ratio (IRR) using Poisson regression and adjusted IRR (aIRR) using multivariable Poisson regression after adjusting for age, residency, income level, marriage status, education level and the presence of various comorbidities. Cumulative incidence of cancer was calculated and compared with Kaplan-Meier method and log-rank test. To further assess the effect of LTRA, multivariable Cox proportional hazards regression analyses were performed with adjustment of the same covariates as in Poisson regression. In addition, stratified analyses were also performed for Poisson and Cox regression in subgroups of covariates. To determine the effect of LTRA on the risk of different cancers, we also calculated the hazard ratios of LTRA use for several major cancers in Taiwan.
Extraction and computation of data, data linkage, processing and sampling and statistical analyses were performed using SAS system (version 9.3 for Windows, SAS Institute Inc., Cary, NC). The statistical significance level was set at a two-sided p value of <0.05.
Results
From the database, 317406 asthma patients were identified. Through the algorithm (Fig. 1), 4185 LTRA users and 20925 matched LTRA non-users were identified. The mean (±SD) age was 47.3 (±16.5) years, and 59% of the subjects were female (Table 1). LTRA users had significantly higher income and higher education level as compared with LTRA non-users, and more LTRA users lived in northern Taiwan. In the LTRA users, 3975 (95%) and 366 (9%) subjects had ever used montelukast and zafirlukast, respectively; the median (IQR) of cDDD and cDDD(1y) were 101 (56–235) and 77 (42–145), respectively.
Table 1. Baseline characteristics of the study population.
| All patients (n = 25110) | LTRA non-users (n = 20925) | LTRA users (n = 4185) | P value | |
|---|---|---|---|---|
| Sex, n (%)# | ||||
| Female | 14934 (59%) | 12445 (59%) | 2489 (59%) | |
| Male | 10176 (41%) | 8480 (41%) | 1696 (41%) | |
| Age (year), mean ± SD# | 47.3 ± 16.5 | 47.3 ± 16.5 | 47.2 ± 16.7 | 0.6696 |
| Age (year), n (%)# | 0.5387 | |||
| Age ≤40 | 9559 (38%) | 7936 (38%) | 1623 (39%) | |
| 40 <Age ≤65 | 11061 (44%) | 9247 (44%) | 1814 (43%) | |
| Age >65 | 4490 (18%) | 3742 (18%) | 748 (18%) | |
| Interval between asthma diagnosis to index date (year), median (IQR)# | 0.8 (0–3.4) | 0.8 (0–3.4) | 0.8 (0–3.4) | |
| Residency, n (%) | <0.0001 | |||
| Northern Taiwan | 13836 (55%) | 11191 (53%) | 2645 (63%) | |
| Other areas | 11274 (45%) | 9734 (47%) | 1540 (37%) | |
| Monthly income (NT$), median (IQR) | 25200 (21900–42000) | 25200 (21900–42000) | 27600 (21900–43900) | <0.0001 |
| Monthly income (NT$), n (%) | <0.0001 | |||
| ≤24000 | 12290 (49%) | 10412 (50%) | 1878 (45%) | |
| >24000 | 12820 (51%) | 10513 (50%) | 2307 (55%) | |
| Marriage status, n (%) | 0.2194 | |||
| Married | 16049 (64%) | 13409 (64%) | 2640 (63%) | |
| Not married | 9061 (36%) | 7516 (36%) | 1545 (37%) | |
| Education level, n (%) | <0.0001 | |||
| Elementary school or lower | 9616 (38%) | 8131 (39%) | 1485 (35%) | |
| High school | 11136 (44%) | 9310 (44%) | 1826 (44%) | |
| College or higher | 4358 (17%) | 3484 (17%) | 874 (21%) | |
| With comorbidity, n (%)# | ||||
| No (mCCI score = 0) | 21630 (86%) | 18025 (86%) | 3605 (86%) | |
| Yes (mCCI score ≥1) | 3480 (14%) | 2900 (14%) | 580 (14%) | |
| Comorbidity, n (%) | ||||
| Heart disease | 979 (4%) | 814 (4%) | 165 (4%) | 0.8726 |
| Myocardial infarction | 145 (1%) | 123 (1%) | 22 (1%) | 0.6282 |
| Congestive heart failure | 878 (3%) | 726 (3%) | 152 (4%) | 0.6014 |
| Peripheral vascular disease | 185 (1%) | 144 (1%) | 41 (1%) | 0.0441 |
| Major neurological disorder | 1582 (6%) | 1342 (6%) | 240 (6%) | 0.0991 |
| Cerebral vascular disease | 1520 (6%) | 1295 (6%) | 225 (5%) | 0.0442 |
| Dementia | 160 (1%) | 130 (1%) | 30 (1%) | 0.4781 |
| Hemiplegia | 118 (0%) | 100 (0%) | 18 (0%) | 0.6799 |
| Connective tissue disease | 393 (2%) | 319 (2%) | 74 (2%) | 0.2462 |
| Peptic ulcer disease | 4845 (19%) | 4015 (19%) | 830 (20%) | 0.3343 |
| Liver disease | 2449 (10%) | 2006 (10%) | 443 (11%) | 0.0468 |
| Diabetes mellitus | 2018 (8%) | 1698 (8%) | 320 (8%) | 0.3090 |
| Renal disease | 479 (2%) | 401 (2%) | 78 (2%) | 0.8205 |
Categorical variables and continuous variables were compared using χ2 test and Student’s t-test, respectively.
Abbreviation: LTRA = cysteinyl leukotriene receptor antagonist; SD = standard deviation; IQR = interquartile range; NT = New Taiwan Dollar; mCCI = modified Charlson Comorbidity Index.
#matched factors.
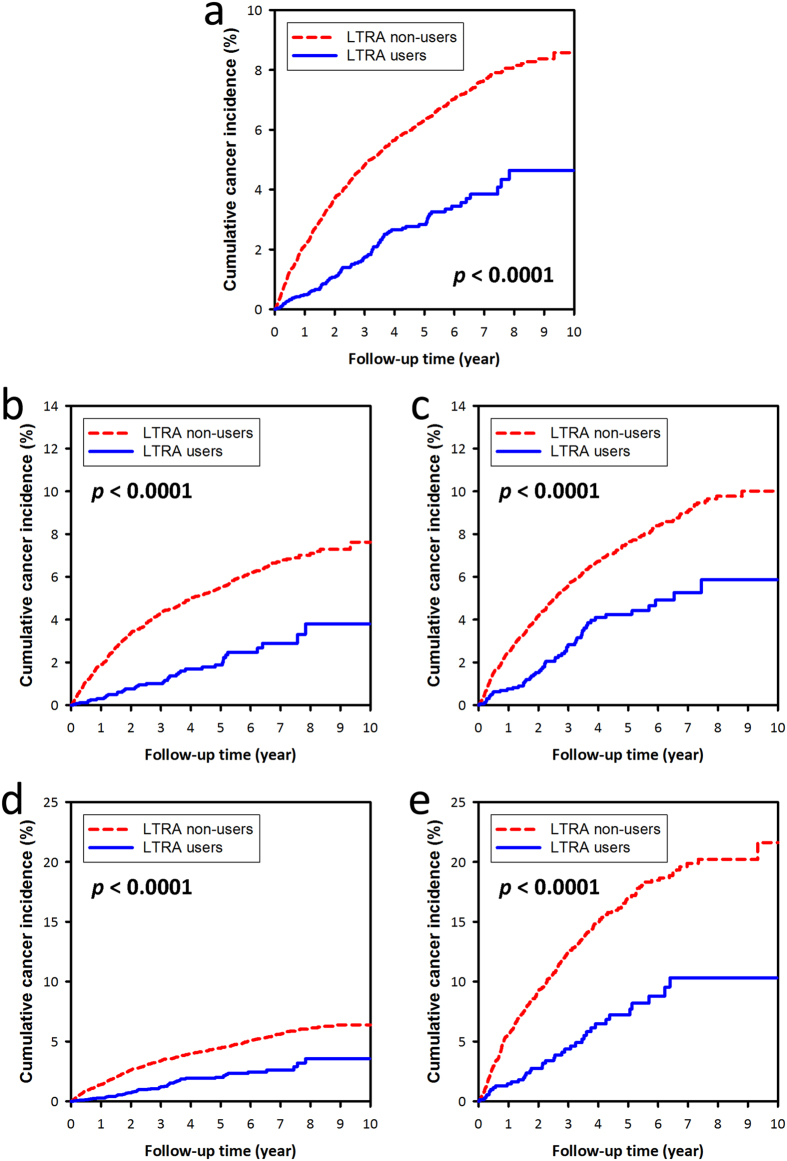
LTRA users had a significantly lower cancer IR than LTRA non-users did (5.8 vs. 13.1 per 1000 patient-years; aIRR = 0.41 [95% CI: 0.36–0.47], p < 0.0001) (Table 2), and all stratified analyses showed consistent findings. The cumulative cancer incidence was significantly lower in LTRA users than in LTRA non-users (p < 0.0001) (Fig. 2a). On stratified analyses, the LTRA users had a significantly lower cumulative cancer incidence as compared with LTRA non-users in strata of female, male, younger and elder subjects (all p < 0.0001) (Fig. 2b–e).
Table 2. Incidence rates of cancer in LTRA users and non-users.
| All patients | LTRA non-users | IR | LTRA users | IRR [95% CI] | aIRR [95% CI] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Cancer | PY | IR | N | Cancer | PY | N | Cancer | PY | IR | ||||
| Whole study population Stratified analyses | 25110 | 1197 | 100593.2 | 11.9 | 20925 | 1104 | 84593.3 | 13.1 | 4185 | 93 | 15999.9 | 5.8 | 0.45 [0.39–0.51]*** | 0.41 [0.36–0.47]*** |
| Sex | ||||||||||||||
| Female | 14934 | 625 | 61015.7 | 10.2 | 12445 | 585 | 51404.0 | 11.4 | 2489 | 40 | 9611.7 | 4.2 | 0.37 [0.30–0.44]*** | 0.34 [0.28–0.41]*** |
| Male | 10176 | 572 | 39577.5 | 14.5 | 8480 | 519 | 33189.3 | 15.6 | 1696 | 53 | 6388.2 | 8.3 | 0.53 [0.44–0.64]*** | 0.49 [0.41–0.58]*** |
| Age | ||||||||||||||
| Age ≤40 | 9559 | 109 | 43348.8 | 2.5 | 7936 | 104 | 36702.5 | 2.8 | 1623 | 5 | 6646.3 | 0.8 | 0.27 [0.19–0.36]*** | 0.27 [0.20–0.37]*** |
| 40< Age ≤65 | 11061 | 620 | 43523.3 | 14.2 | 9247 | 570 | 36702.9 | 15.5 | 1814 | 50 | 6820.4 | 7.3 | 0.47 [0.39–0.57]*** | 0.45 [0.37–0.55]*** |
| Age >65 | 4490 | 468 | 13721.1 | 34.1 | 3742 | 430 | 11187.9 | 38.4 | 748 | 38 | 2533.2 | 15.0 | 0.39 [0.30–0.51]*** | 0.38 [0.29–0.50]*** |
| Residency | ||||||||||||||
| Northern Taiwan | 13836 | 589 | 56174.4 | 10.5 | 11191 | 530 | 46127.2 | 11.5 | 2645 | 59 | 10047.2 | 5.9 | 0.51 [0.43–0.60]*** | 0.43 [0.37–0.50]*** |
| Other areas | 11274 | 608 | 44418.8 | 13.7 | 9734 | 574 | 38466.1 | 14.9 | 1540 | 34 | 5952.7 | 5.7 | 0.38 [0.30–0.48]*** | 0.38 [0.30–0.47]*** |
| Monthly income | ||||||||||||||
| ≤NT$24000 | 12290 | 759 | 47025.8 | 16.1 | 10412 | 708 | 39915.9 | 17.7 | 1878 | 51 | 7109.9 | 7.2 | 0.40 [0.33–0.49]*** | 0.36 [0.30–0.44]*** |
| >NT$24000 | 12820 | 438 | 53567.4 | 8.2 | 10513 | 396 | 44677.4 | 8.9 | 2307 | 42 | 8890.0 | 4.7 | 0.53 [0.45–0.64]*** | 0.48 [0.41–0.57]*** |
| Marriage status | ||||||||||||||
| Married | 16049 | 921 | 64873.1 | 14.2 | 13409 | 845 | 54554.7 | 15.5 | 2640 | 76 | 10318.4 | 7.4 | 0.48 [0.41–0.56]*** | 0.44 [0.38–0.51]*** |
| Not married | 9061 | 276 | 35720.1 | 7.7 | 7516 | 259 | 30038.6 | 8.6 | 1545 | 17 | 5681.5 | 3.0 | 0.35 [0.27–0.45]*** | 0.31 [0.24–0.39]*** |
| Education level | ||||||||||||||
| Elementary school or lower | 9616 | 731 | 34513.9 | 21.2 | 8131 | 680 | 29182.4 | 23.3 | 1485 | 51 | 5331.6 | 9.6 | 0.41 [0.33–0.51]*** | 0.39 [0.32–0.48]*** |
| High school | 11136 | 348 | 47369.8 | 7.3 | 9310 | 319 | 40247.0 | 7.9 | 1826 | 29 | 7122.8 | 4.1 | 0.51 [0.42–0.63]*** | 0.43 [0.35–0.52]*** |
| College or higher | 4358 | 118 | 18709.5 | 6.3 | 3484 | 105 | 15163.9 | 6.9 | 874 | 13 | 3545.6 | 3.7 | 0.53 [0.39–0.71]*** | 0.42 [0.32–0.55]*** |
| With comorbidity | ||||||||||||||
| No (mCCI score = 0) | 16500 | 685 | 71023.6 | 9.6 | 13750 | 636 | 59945.8 | 10.6 | 2750 | 49 | 11077.8 | 4.4 | 0.42 [0.35–0.50]*** | 0.38 [0.32–0.44]*** |
| Yes (mCCI score ≥ 1) | 8610 | 512 | 29569.6 | 17.3 | 7175 | 468 | 24647.5 | 19.0 | 1435 | 44 | 4922.1 | 8.9 | 0.47 [0.38–0.58]*** | 0.45 [0.37–0.55]*** |
The subjects were followed from a year after the index date to either development of cancer, death or the end of 2011, whichever came first.
The incidence rate (IR) is expressed as incident cancer per 1000 patient-years. The IRs of cancer in LTRA users and non-users were compared by estimating the incidence rate ratio (IRR) using Poisson regression and adjusted IRR (aIRR) using multivariable Poisson regression after adjusting for age, residency, income level, marriage status, education level and the presence of various comorbidities (except for the variable used for stratification).
*P < 0.05; **P < 0.01; ***P < 0.0001.
Abbreviation: N = number of patients; Cancer = number of patients with incident cancer; PY = total patient-years of follow-up; CI = confidence interval.
Figure 2.
The cumulative cancer incidence of (a) the whole study population, (b) female patients, (c) male patients, (d) subjects ≤65 years old, and (e) subjects >65 years old. The red dashed lines and blue continuous lines show the cumulative cancer incidence of LTRA non-users and LTRA users, respectively. LTRA users had a significantly lower cumulative cancer incidence than LTRA non-users did (p < 0.0001).
On multivariable Cox proportional hazards regression analyses adjusting for age, residency, income level, marriage status, education level and comorbidities, LTRA use was associated with a decreased cancer risk (hazard ratio = 0.31 [95% CI: 0.24–0.39], p < 0.0001) (Table 3, model 1). The cancer risk decreased progressively with higher cumulative dose of LTRA use as compared with LTRA non-users. LTRA users with lower and higher cDDD of LTRA had 60% and 78% cancer risk reduction, respectively (Table 3, model 2). Similarly, LTRA users with lower and higher cDDD(1y) of LTRA had a 66% and 72% cancer risk reduction, respectively (Table 3, model 3). On stratified analyses, LTRA use was associated with a significantly lower cancer risk in all strata (Fig. 3a). LTRA users with higher cDDD or cDDD(1y) use had lower cancer risk than those with lower cDDD or cDDD(1y) did in nearly all strata (Fig. 3b,c). The significant effect of LTRA on cancer risk reduction was observed mainly in lung, colorectal, liver and breast cancer (Table 4).
Table 3. Multivariable Cox regression analyses of the related factors for developing cancer in asthma patients.
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||
| lower | upper | lower | upper | lower | upper | |||||||
| Age: (vs. age ≤40) | ||||||||||||
| 40< Age ≤65 | 0.74 | 0.30 | 1.80 | 0.5049 | 0.76 | 0.31 | 1.85 | 0.5460 | 0.74 | 0.30 | 1.80 | 0.5046 |
| Age >65 | 1.24 | 0.44 | 3.50 | 0.6847 | 1.29 | 0.46 | 3.60 | 0.6325 | 1.24 | 0.44 | 3.50 | 0.6840 |
| Residency (northern Taiwan vs. other areas) | 1.02 | 0.88 | 1.17 | 0.8385 | 1.02 | 0.89 | 1.17 | 0.7706 | 1.02 | 0.88 | 1.17 | 0.8216 |
| Monthly income (>NT$24000 vs. ≤NT$24000) | 0.91 | 0.78 | 1.07 | 0.2731 | 0.92 | 0.78 | 1.08 | 0.2876 | 0.92 | 0.78 | 1.07 | 0.2780 |
| Marriage status (married vs. not married) | 1.06 | 0.90 | 1.25 | 0.4926 | 1.06 | 0.90 | 1.25 | 0.4873 | 1.06 | 0.90 | 1.25 | 0.4979 |
| Education level: (vs. elementary school or lower) | ||||||||||||
| High school | 1.00 | 0.84 | 1.19 | 0.9767 | 1.00 | 0.84 | 1.18 | 0.9561 | 1.00 | 0.84 | 1.19 | 0.9714 |
| College or higher | 1.25 | 0.96 | 1.62 | 0.0934 | 1.25 | 0.96 | 1.62 | 0.0941 | 1.25 | 0.96 | 1.62 | 0.0957 |
| Presence of comorbidity: | ||||||||||||
| Heart disease | 1.05 | 0.68 | 1.63 | 0.8126 | 1.06 | 0.68 | 1.64 | 0.7996 | 1.06 | 0.68 | 1.63 | 0.8113 |
| Peripheral vascular disease | 0.90 | 0.41 | 1.99 | 0.7972 | 0.89 | 0.41 | 1.96 | 0.7779 | 0.90 | 0.41 | 1.99 | 0.8027 |
| Major neurological disorder | 0.94 | 0.62 | 1.43 | 0.7683 | 0.94 | 0.62 | 1.44 | 0.7776 | 0.94 | 0.62 | 1.44 | 0.7778 |
| Connective tissue disease | 0.86 | 0.47 | 1.57 | 0.6234 | 0.85 | 0.47 | 1.56 | 0.6092 | 0.86 | 0.47 | 1.57 | 0.6225 |
| Peptic ulcer disease | 1.12 | 0.78 | 1.59 | 0.5491 | 1.12 | 0.78 | 1.60 | 0.5354 | 1.12 | 0.78 | 1.60 | 0.5441 |
| Liver disease | 1.56 | 1.08 | 2.25 | 0.0180 | 1.58 | 1.09 | 2.27 | 0.0153 | 1.57 | 1.08 | 2.26 | 0.0171 |
| Diabetes mellitus | 1.03 | 0.67 | 1.57 | 0.9106 | 1.02 | 0.67 | 1.57 | 0.9215 | 1.02 | 0.67 | 1.57 | 0.9162 |
| Renal disease | 1.09 | 0.56 | 2.13 | 0.8017 | 1.09 | 0.56 | 2.12 | 0.8001 | 1.09 | 0.56 | 2.12 | 0.8013 |
| LTRA users (vs. LTRA non-users) | 0.31 | 0.24 | 0.39 | <0.0001 | ||||||||
| cDDD of LTRA (vs. LTRA non-users) | ||||||||||||
| cDDD ≤112 | 0.40 | 0.30 | 0.54 | <0.0001 | ||||||||
| cDDD >112 | 0.22 | 0.16 | 0.32 | <0.0001 | ||||||||
| cDDD(1y) of LTRA (vs. LTRA non-users) | ||||||||||||
| cDDD(1y) ≤84 | 0.34 | 0.25 | 0.45 | <0.0001 | ||||||||
| cDDD(1y) >84 | 0.28 | 0.20 | 0.39 | <0.0001 | ||||||||
The follow-up time was calculated from a year after the index date to either development of cancer, death or the end of 2011, whichever came first. The cumulative defined daily doses of LTRA were calculated from the index date to the end of follow-up (cDDD) and to a year after the index date [cDDD(1y)].
Using LTRA non-users as reference, the adjusted HRs of LTRA use (model 1), lower and higher cDDD (model 2) and lower and higher cDDD(1y) were calculated by the multivariable Cox proportional hazards regression analyses adjusted for age, residency, income level, marriage status, education level and the presence of various comorbidities.
Abbreviations: HR = hazard ratio; CI = confidence interval.
Figure 3.
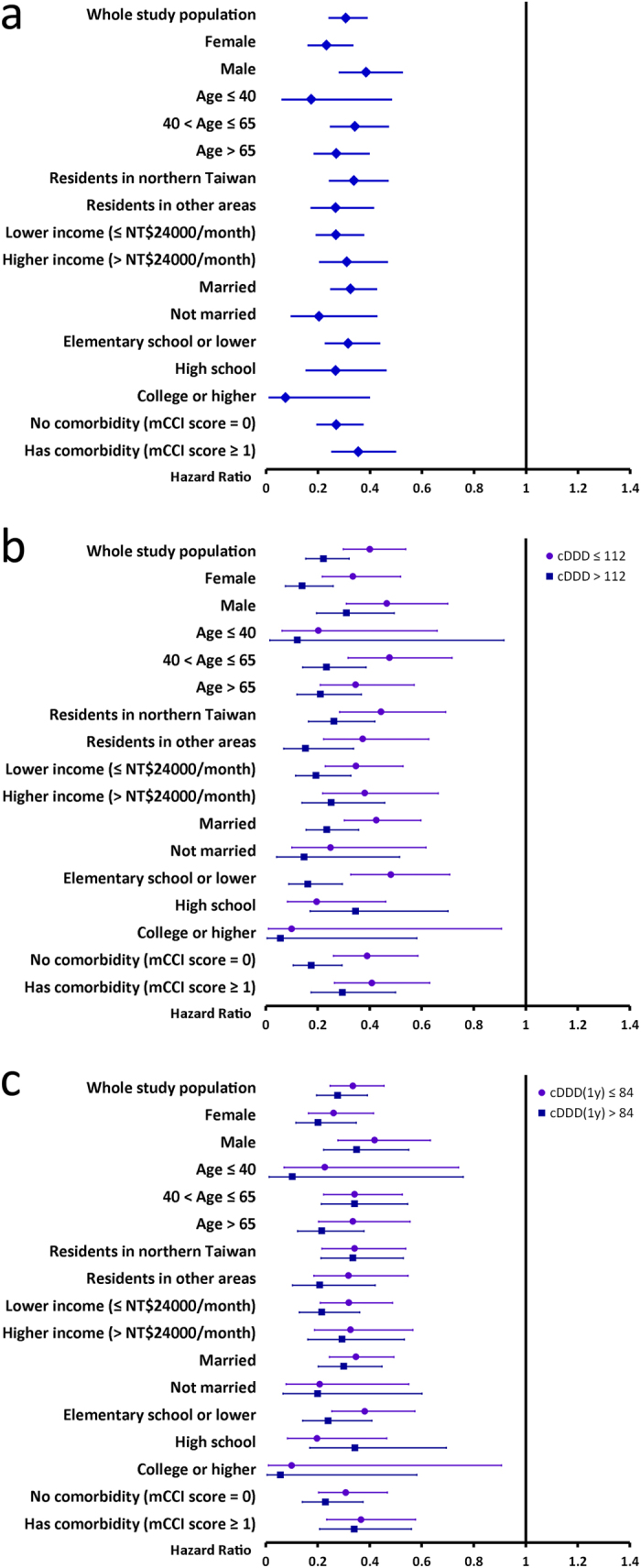
Stratified analyses of the multivariable Cox proportional hazards regression analyses showing adjusted hazard ratios (HRs) of (a) LTRA use and (b,c) lower and higher doses of LTRA use. The results are presented with adjusted HRs (95% confidence interval) of either (a) LTRA use or (b,c) lower and higher doses of LTRA use, which are adjusted for age, residency, income level, marriage status, education level and the presence of various comorbidities (except for the variable used for stratification). The follow-up time was calculated from a year after the index date to either development of cancer, death or the end of 2011, whichever came first. The cumulative defined daily doses of LTRA were calculated from the index date to the end of follow-up (cDDD) and to a year after the index date [cDDD(1y)].
Table 4. Multivariable Cox regression analyses of the related factors for developing various cancers in asthma patients.
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LTRA users | cDDD ≤112 | cDDD >112 | cDDD(1y) ≤84 | cDDD(1y) >84 | ||||||
| HR [95% CI] | P value | HR [95% CI] | P value | HR [95% CI] | P value | HR [95% CI] | P value | HR [95% CI] | P value | |
| Lung cancer | 0.34 [0.20–0.60] | 0.0002 | 0.43 [0.21–0.90] | 0.0256 | 0.27 [0.12–0.62] | 0.0019 | 0.32 [0.14–0.72] | 0.0057 | 0.37 [0.18–0.78] | 0.0094 |
| Colorectal cancer | 0.35 [0.20–0.62] | 0.0004 | 0.43 [0.20–0.93] | 0.0324 | 0.28 [0.12–0.66] | 0.0037 | 0.42 [0.19–0.91] | 0.0275 | 0.29 [0.12–0.68] | 0.0045 |
| Gastric cancer | 0.30 [0.09–0.99] | 0.0486 | 0.37 [0.08–1.71] | 0.2040 | 0.21 [0.03–1.66] | 0.1400 | 0.38 [0.08–1.72] | 0.2087 | 0.21 [0.03–1.62] | 0.1328 |
| Liver cancer | 0.34 [0.17–0.69] | 0.0027 | 0.44 [0.18–1.08] | 0.0738 | 0.24 [0.08–0.76] | 0.0147 | 0.47 [0.20–1.10] | 0.0806 | 0.19 [0.05–0.70] | 0.0129 |
| Pancreatic cancer | 0.26 [0.05–1.44] | 0.1220 | 0.24 [0.02–3.13] | 0.2742 | 0.27 [0.03–2.42] | 0.2426 | 0.20 [0.02–2.42] | 0.2068 | 0.33 [0.03–3.50] | 0.3553 |
| Oral cancer | 0.35 [0.12–1.01] | 0.0519 | 0.32 [0.07–1.43] | 0.1343 | 0.38 [0.08–1.72] | 0.2093 | 0.32 [0.07–1.43] | 0.1345 | 0.38 [0.08–1.72] | 0.2100 |
| Nasopharyngeal carcinoma | 0.26 [0.03–2.51] | 0.2470 | ‡ | ‡ | ‡ | ‡ | ||||
| Brain cancer | 0.26 [0.03–2.51] | 0.9974 | ‡ | ‡ | ‡ | ‡ | ||||
| Thyroid cancer | 0.30 [0.06–1.55] | 0.1504 | ‡ | ‡ | ‡ | ‡ | ||||
| Skin cancer | 0.61 [0.15–2.53] | 0.4964 | 0.67 [0.10–4.53] | 0.6855 | 0.54 [0.06–4.79] | 0.5797 | 0.71 [0.10–4.82] | 0.7259 | 0.51 [0.06–4.49] | 0.5453 |
| Urinary cancer | 0.78 [0.33–1.88] | 0.5839 | 0.94 [0.32–2.77] | 0.9112 | 0.55 [0.11–2.82] | 0.4752 | 0.71 [0.22–2.24] | 0.5550 | 0.92 [0.23–3.70] | 0.9049 |
| Breast cancer | 0.09 [0.03–0.26] | <0.0001 | 0.15 [0.04–0.49] | 0.0019 | 0.05 [0.01–0.34] | 0.0025 | 0.09 [0.02–0.36] | 0.0008 | 0.10 [0.02–0.44] | 0.0022 |
| Cervical cancer | 0.48 [0.18–1.26] | 0.1341 | 0.44 [0.12–1.60] | 0.2129 | 0.52 [0.13–2.09] | 0.3608 | 0.53 [0.17–1.64] | 0.2718 | 0.38 [0.07–2.05] | 0.2584 |
| Prostate cancer | 0.16 [0.03–0.94] | 0.0419 | 0.19 [0.02–1.7] | 0.1372 | 0.14 [0.01–1.74] | 0.1265 | 0.16 [0.02–1.53] | 0.1106 | 0.17 [0.01–2.34] | 0.1873 |
The results are presented with adjusted hazard ratios (HRs) (95% confidence interval) of LTRA users (model 1) or lower (cDDD ≤ 112 in model 2 and cDDD(1y) ≤ 84 in model 3) and higher (cDDD > 112 in model 2 and cDDD(1y) >84 in model 3) doses of LTRA use, using LTRA non-users as reference, which are adjusted for age, residency, income level, marriage status, education level and the presence of various comorbidities.
The follow-up time was calculated from a year after the index date to either development of the specific cancer, death or the end of 2011, whichever came first.
The cumulative defined daily doses of LTRA were calculated from the index date to the end of follow-up (cDDD) and to a year after the index date [cDDD(1y)].
‡The HR of some cancer types could not be estimated due to small sample size.
Discussion
This large population-based study revealed that LTRA use was associated with a decreased cancer risk in asthma patients. Particularly, the chemopreventive effect appeared larger with a higher cumulative dose, indicating a dose-dependent manner of LTRA in this issue. The strengths of this study are its population-based sampling, avoidance of selection bias, adjustment for confounders, and, most importantly, the demonstration of dose-dependent protection effect. To the best of our knowledge, we are not only the first to report the chemopreventive effect of LTRAs in the clinical setting but also the first to demonstrate a dose-response relationship between the use of LTRAs and reduced risk of cancer. Further clinical studies are required to confirm our findings, and further in vivo and in vitro studies should be taken to investigate the chemopreventive mechanisms of LTRAs.
As inflammation is a major contributor for carcinogenesis and cancer progression, immune responses are the most important mechanisms running in tumour microenvironment. Indeed, the interaction between cancer cells and the surrounding immune cells have been noted to form a milieu which is suitable for carcinogenesis, as well as proliferation and migration of cancer cells25. Eicosanoids involve in a variety of inflammatory and immune responses throughout the body, and are also important regulators in the immune responses in tumour microenvironment7. Using selective cyclooxygenase-2 (COX-2) inhibitors for chemoprevention is therefore widely discussed. Our previous population-based study indicated that selective COX-2 inhibitors reduced development of colorectal cancer by at least 10%3.
In recent years, the role of leukotriene pathway in carcinogenesis and tumour-mediated immunosuppression has been increasingly recognized7,26. While much effort has been made in identifying the role of LTB4 pathway in cancer, the tumour-promoting role of cysteinyl leukotrienes, including LTC4, LTD4 and LTE4, is less studied. Cysteinyl leukotrienes are originally recognized for their effect to promote bronchoconstriction, inflammation, microvascular permeability and mucus secretion5. Since more than a decade ago, LTD4 has been shown to reduce apoptosis, enhance proliferation, induce transcriptional activity of potentially oncogenic genes and induce migration of intestinal epithelial cells27. Clinically, increased expression of CysLT1R was noted in specimens from colorectal, gastric and breast cancers, and the elevated CysLT1R expression correlated to poorer survival13,28,29,30. The circulating LTD4 level was significantly higher in patients with hepatocellular carcinoma than in healthy subjects31. Over-expression of CysLT1R has also been shown in prostate cancer, renal cell carcinoma, transitional cell carcinoma and testicular cancer, and montelukast induces early apoptosis of these cancer cells8,9,10,11,12,14.
In addition to the pro-apoptotic effect of montelukast on few cancer cell lines, however, only few in vivo studies have reported chemoprevention effect of leukotriene pathway inhibitors in the literature while no clinical study is available currently. An early study demonstrated chemopreventive effect of leukotriene pathway inhibitors, accolate, zileuton and MK-866, in vinyl carbamate-induced lung tumours in mice15. In an in vivo LLC cells metastasis model, pranlukast and montelukast prevented tumour metastasis through peripheral capillaries16. A recent study using nude mice demonstrated that an LTRA, ZM198,615 or montelukast, inhibited the growth of colon cancer xenografts14.
In contrast to our previous study showing about 50% cancer risk reduction in users of selective COX-2 inhibitor, the present study showed an impressive 60–78% cancer risk reduction with using LTRAs3. Many adverse effects of selective COX-2 inhibitors, especially renal failure and cardiovascular complications, prevent their wide application. LTRAs used in current clinical practice are generally so safe that can be used in paediatric asthma patients6. After our findings are further validated in other clinical studies, using LTRAs for chemoprevention might be much easier due to their satisfying safety profiles.
There are several limitations in our studies. First, some well-known potentially important clinical covariates, such as smoking history and environmental exposure, are not available in the database. Interpreting of our results must be careful to account for possible impacts of these risk factors. Nevertheless, LTRA users and LTRA non-users are matched by sex, age, asthma diagnostic year and mCCI score, and Cox regression analyses were adjusted for age, residency, income level, marriage status, education level and comorbidities. To address the potential issues caused by the administrative database, we also conducted various stratified analyses and found consistent results. Because smoking rate was much lower in female (4.2%) than in male (46.8%) in Taiwan2,32, the results of stratified analyses in female subjects might be taken as a proxy for the effect of LTRAs in non-smokers. In this study, the chemopreventive effect of LTRAs seemed more pronounced in the female and younger subjects, as compared to male subjects and elder subjects, respectively. These findings suggested that the chemopreventive effect of LTRAs might be more pronounced in non-smokers than in smokers, and the detailed mechanisms deserves further study. Second, because our studies enrolled only asthma patients, whether the results can be applied to patients without asthma needs further study. However, because LTRAs are mainly used for allergic asthma, choosing subjects from asthma patients are therefore required to homogenize the case and control cohorts. Third, time-related biases were always a concern as in many observational studies33. Our study design inherently avoided time-lagging bias by unifying the interval between the initial asthma diagnosis and the index date of an LTRA non-user with that of the corresponding LTRA user. Although immortal time bias and time-window bias were not totally avoided in this study, our study design substantially minimized the impact of these biases. Besides, asthma was not associated with significantly increased cancer incidence17. Furthermore, the dose-dependent effect shown in multivariable Cox regression analyses increased the reliability of our results. Finally, this study was conducted in patients of Han Chinese ethnicity. Whether the findings are also applicable to other ethnic population require further evaluation.
In summary, our study reveals that the use of LTRA in asthma patients is associated with a decreased risk of cancer in a dose-dependent manner. The utility of LTRAs as chemopreventive agents deserves further study in depth.
Additional Information
How to cite this article: Tsai, M.-J. et al. Cysteinyl Leukotriene Receptor Antagonists Decrease Cancer Risk in Asthma Patients. Sci. Rep. 6, 23979; doi: 10.1038/srep23979 (2016).
Acknowledgments
The authors thank the help from the Statistical Analysis Laboratory, Department of Internal Medicine and the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital. This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by the Collaboration Center of Health Information Application (CCHIA), Ministry of Health and Welfare, Executive Yuan, Taiwan. The interpretation and conclusions contained herein do not represent those of Ministry of Health and Welfare. This work was supported by grants from the Ministry of Science and Technology [MOST 104-2314-B-037-005 and MOST 104-2314-B-037-034-MY3]; and the Aim for the Top Journals Grant, Kaohsiung Medical University Research Foundation [KMU-DT103008].
The authors declare no competing financial interests.
Author Contributions M.J.T., P.L.K. and M.S.H. conceived and designed the study. M.J.T., Y.H.Y. and M.S.H. directed the study, had full access to all the data in the study, takes responsibility for the integrity of the data, and the accuracy of the data analyses. P.H.W., C.C.S., Y.L.H., W.A.C., J.Y.H., C.J.Y. and P.L.K. gave important intellectual content in all phases of the study. M.J.T. and Y.H.Y. did the statistical analyses. M.J.T. wrote the first draft of the manuscript, and all authors contributed to the revision and final approval of manuscript.
05/12/2016
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
- Ministry of Health and Welfare. Cause of deaths statistics. (2014) Available at: http://www.mohw.gov.tw/EN/Ministry/Statistic.aspx?f_list_no=474&fod_list_no=3443. (Accessed: 25th December 2015).
- Tsai M. J. et al. Metformin decreases lung cancer risk in diabetic patients in a dose-dependent manner. Lung Cancer 86, 137–143 (2014). [DOI] [PubMed] [Google Scholar]
- Yang Y. H., Yang Y. H., Cheng C. L., Ho P. S. & Ko Y. C. The role of chemoprevention by selective cyclooxygenase-2 inhibitors in colorectal cancer patients-a population-based study. BMC Cancer 12, 582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E., Mao J. T., Lam S., Reid M. E. & Keith R. L. Chemoprevention of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 143, e40S–60S (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. P. & Peters-Golden M. Antileukotriene agents for the treatment of lung disease. Am J Respir Crit Care Med 188, 538–544 (2013). [DOI] [PubMed] [Google Scholar]
- Dahlen S. E., Dahlen B. & Drazen J. M. Asthma treatment guidelines meet the real world. N Engl J Med 364, 1769–1770 (2011). [DOI] [PubMed] [Google Scholar]
- Wang D. & Dubois R. N. Eicosanoids and cancer. Nat Rev Cancer 10, 181–193 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama M. et al. Overexpression of cysteinyl lt1 receptor in prostate cancer and cyslt1r antagonist inhibits prostate cancer cell growth through apoptosis. Oncol Rep 18, 99–104 (2007). [PubMed] [Google Scholar]
- Funao K. et al. The cysteinyllt1 receptor in human renal cell carcinoma. Mol Med Rep 1, 185–189 (2008). [PubMed] [Google Scholar]
- Matsuyama M. et al. Relationship between cysteinyl-leukotriene-1 receptor and human transitional cell carcinoma in bladder. Urology 73, 916–921 (2009). [DOI] [PubMed] [Google Scholar]
- Matsuyama M. et al. Expression of cysteinyllt1 receptor in human testicular cancer and growth reduction by its antagonist through apoptosis. Mol Med Rep 2, 163–167 (2009). [DOI] [PubMed] [Google Scholar]
- Matsuyama M. & Yoshimura R. Cysteinyl-leukotriene1 receptor is a potent target for the prevention and treatment of human urological cancer. Mol Med Rep 3, 245–251 (2010). [DOI] [PubMed] [Google Scholar]
- Nielsen C. K. et al. The leukotriene receptor cyslt1 and 5-lipoxygenase are upregulated in colon cancer. Adv Exp Med Biol 525, 201–204 (2003). [DOI] [PubMed] [Google Scholar]
- Savari S., Liu M., Zhang Y., Sime W. & Sjolander A. Cyslt(1)r antagonists inhibit tumor growth in a xenograft model of colon cancer. PLos One 8, e73466 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning W. T., Kramer P. M., Steele V. E. & Pereira M. A. Chemoprevention by lipoxygenase and leukotriene pathway inhibitors of vinyl carbamate-induced lung tumors in mice. Cancer Res 62, 4199–4201 (2002). [PubMed] [Google Scholar]
- Nozaki M. et al. Cysteinyl leukotriene receptor antagonists inhibit tumor metastasis by inhibiting capillary permeability. Keio J Med 59, 10–18 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C. Y. et al. Cancer risk in patients with allergic rhinitis, asthma and atopic dermatitis: A nationwide cohort study in taiwan. Int J Cancer 130, 1160–1167 (2012). [DOI] [PubMed] [Google Scholar]
- Chung W. S. et al. Asthma increases pulmonary thromboembolism risk: A nationwide population cohort study. Eur Respir J 43, 801–807 (2014). [DOI] [PubMed] [Google Scholar]
- Lin Y. T. et al. Comparison of dementia risk between end stage renal disease patients with hemodialysis and peritoneal dialysis–a population based study. Sci Rep 5, 8224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. T., Arnold Chan K., Chen H. M., Lai C. L. & Lai M. S. Class effect of beta-blockers in survivors of st-elevation myocardial infarction: A nationwide cohort study using an insurance claims database. Sci Rep 5, 13692 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. F. et al. Longitudinal risk of herpes zoster in patients with non-hodgkin lymphoma receiving chemotherapy: A nationwide population-based study. Sci Rep 5, 14008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Y. et al. Effective reduction of gastric cancer risk with regular use of nonsteroidal anti-inflammatory drugs in helicobacter pylori-infected patients. J Clin Oncol 28, 2952–2957 (2010). [DOI] [PubMed] [Google Scholar]
- Wu C. Y. et al. Association between nucleoside analogues and risk of hepatitis b virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 308, 1906–1914 (2012). [DOI] [PubMed] [Google Scholar]
- Deyo R. A., Cherkin D. C. & Ciol M. A. Adapting a clinical comorbidity index for use with icd-9-cm administrative databases. J Clin Epidemiol 45, 613–619 (1992). [DOI] [PubMed] [Google Scholar]
- Kuo P. L. et al. Synergistic effect of lung tumor-associated dendritic cell-derived hb-egf and cxcl5 on cancer progression. Int J Cancer 135, 96–108 (2014). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. Clin Cancer Res 10, 6703–6709 (2004). [DOI] [PubMed] [Google Scholar]
- Massoumi R. & Sjolander A. The role of leukotriene receptor signaling in inflammation and cancer. ScientificWorldJournal 7, 1413–1421 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson C. et al. Low expression of cyslt1r and high expression of cyslt2r mediate good prognosis in colorectal cancer. Eur J Cancer 46, 826–835 (2010). [DOI] [PubMed] [Google Scholar]
- Magnusson C. et al. Cysteinyl leukotriene receptor expression pattern affects migration of breast cancer cells and survival of breast cancer patients. Int J Cancer 129, 9–22 (2011). [DOI] [PubMed] [Google Scholar]
- Venerito M. et al. Upregulation of leukotriene receptors in gastric cancer. Cancers (Basel) 3, 3156–3168 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Guo D., Li H. & Jie S. Circulating ltd4 in patients with hepatocellular carcinoma. Tumour Biol 32, 139–144 (2011). [DOI] [PubMed] [Google Scholar]
- Wen C. P., Levy D. T., Cheng T. Y., Hsu C. C. & Tsai S. P. Smoking behaviour in taiwan, 2001. Tob Control 14 Suppl 1, i51–55 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa S. & Azoulay L. Metformin and the risk of cancer: Time-related biases in observational studies. Diabetes Care 35, 2665–2673 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]