Polyploidy (multiple copies of whole genomes) is over-represented in invasive plants and thought to promote their success in novel environments. Understanding functional traits supporting colonization can provide a foundation for development of effective management strategies. We compared how two aquatic invasive congeners differing in ploidy (diploid, decaploid) respond to resource availability (light, nutrients). Counter to our predictions, the diploid congener out-performed the decaploid with nutrient enrichment. Our results suggest the congeners have alternate colonization strategies, and trait responses underlying their success may change with ontogeny. Management strategies for invasive Ludwigia species should therefore be tailored for specific cytotypes and unique characteristics of their life stages.
Keywords: Aquatic plants, functional plant traits, invasion ecology, invasive plants, Ludwigia, polyploidy, reproductive allocation
Abstract
Understanding traits underlying colonization and niche breadth of invasive plants is key to developing sustainable management solutions to curtail invasions at the establishment phase, when efforts are often most effective. The aim of this study was to evaluate how two invasive congeners differing in ploidy respond to high and lowresource availability following establishment from asexual fragments. Because polyploids are expected to have wider niche breadths than diploid ancestors, we predicted that a decaploid species would have superior ability to maximize resource uptake and use, and outperform a diploid congener when colonizing environments with contrasting light and nutrient availability. A mesocosm experiment was designed to test the main and interactive effects of ploidy (diploid and decaploid) and soil nutrient availability (low and high) nested within light environments (shade and sun) of two invasive aquatic plant congeners. Counter to our predictions, the diploid congener outperformed the decaploid in the early stage of growth. Although growth was similar and low in the cytotypes at low nutrient availability, the diploid species had much higher growth rate and biomass accumulation than the polyploid with nutrient enrichment, irrespective of light environment. Our results also revealed extreme differences in time to anthesis between the cytotypes. The rapid growth and earlier flowering of the diploid congener relative to the decaploid congener represent alternate strategies for establishment and success.
Introduction
Polyploidization, a key process in the evolution of vascular plants, and angiosperms in particular (Levin 2002; Soltis et al. 2004, 2009), appears to be over-represented in invasive plant species (Thébault et al. 2011), suggesting that polyploids have acquired evolutionary advantages over their diploid ancestors that underlie their success as invaders. Whole genome duplication and increased genetic diversity within polyploids are thought to promote higher flexibility and increased potential for rapid evolution toward new or improved traits that may contribute to their success (Soltis and Soltis 2000). Polyploidization can affect a suite of morphological, physiological and ecological traits that may enable plants to grow larger (Levin 2002), increase fitness (Hufbauer and Torchin 2007) and exploit novel climate niches (Manzaneda et al. 2012). For example, experimental studies of tetraploid and diploid cytotypes of invasive Centaurea stoebe have demonstrated that polyploid invaders (unlike diploid conspecifics) can have pre-adapted traits that act in concert with rapid adaptive change to enhance their invasive success (Henery et al. 2010).
In polyploids, phenotypic plasticity is thought to increase niche breadth and promote their success as invasive species (Pandit et al. 2011; te Beest et al. 2012). It is predicted that enhanced phenological plasticity is also adaptive and may lead to rapid evolution of novel traits that can increase invasiveness in changing environments (Drenovsky et al. 2012). Experimental evidence provides support for increased phenotypic plasticity associated with polyploid cytotypes (i.e. Hahn et al. 2012). However, results of other studies suggest that this ability is not universal among polyploid invaders (Münzbergová 2007a, b; Černá and Münzbergová 2013; Godsoe et al. 2013), and in a few instances, diploids tolerated a wider range of ecological conditions than closely related polyploids (Buggs and Pannell 2007; Černá and Münzbergová 2013). Studies comparing the ecological responses of diploid and polyploid congeners to contrasting environments are rare (Soltis et al. 2010). Clearly, further empirical research is needed to provide more insight regarding the role of polyploidization in plant invasions.
The aim of this study was to evaluate how two invasive Ludwigia congeners differing in ploidy levels respond to high and lowresource availability during their early establishment and growth. We focussed on the mechanisms underlying colonization and niche breadth of these congeners, as it is during early establishment and growth that management strategies are most effective (Richardson and Pyšek 2006). A large body of literature suggests that polyploid species have evolved wider niche breadths than their diploid ancestors. Therefore, we predicted that the decaploid species Ludwigia hexapetala would perform better than the diploid species L. peploides subsp. montevidensis during initial vegetative growth. We hypothesized that L. hexapetala (decaploid) would outperform L. p. subsp. montevidensis (diploid) in response to different light and soil nutrient conditions when established from asexual stolon fragments.
Methods
Study taxa
Originating in South America, Ludwigia sect. Jussiaea is composed of highly invasive, perennial aquatic taxa varying in their morphology and cytological characters. Ludwigia peploides subsp. montevidensis and L. hexapetala have become problematic across Europe and are the most invasive aquatic plant taxa in France (Thouvenot et al. 2013). In the USA, these same two Ludwigia congeners are aggressive invaders of wetland and riparian habitats in Pacific, Atlantic and Gulf Coast states [see Supporting Information]. Ludwigia peploides is the ancestral diploid cytotype of the section. Natural hybrids occur within sect. Jussiaea, and L. hexapetala is thought to be an allodecaploid with chromosomes derived from hybridization, though confirmation is needed (Zardini et al. 1991). Both cytotypes produce woody capsules with uniseriate seeds (Hoch et al. 2015) and form persistent seed banks (B. Grewell, unpubl. data). Both taxa are vigorously aquatic, floating-leaved emergent macrophytes that have recently become invasive in wetlands, rivers and lakes of California (Wagner et al. 2007; Hoch and Grewell 2012; Hoch et al. 2015). With seasonal hydrologic drawdown, L. hexapetala tolerates dry surface soil conditions in transitional upland areas, while dry conditions accelerate seasonal senescence of L. p. subsp. montevidensis.
Molecular analyses revealed very limited genotypic and genet variation in populations of L. hexapetala from California watersheds (Okada et al. 2009). Therefore, invasive spread appears to be primarily clonal through hydrochorous dispersal of stolon fragments (Okada et al. 2009; Ruaux et al. 2009). These stolon fragments have periodic rooting nodes that allow them to produce floating roots in water, or as water recedes, these roots penetrate into moist soil, and the taxa persist and spread as emergent macrophytes. Local recruitment by sexual reproduction and seed bank emergence also occurs, and hydrochorous dispersal of buoyant seed capsules from both taxa can support the spread of invasions (Ruaux et al. 2009). Seeds of aquatic Ludwigia spp. germinate readily following passage through waterbird guts (García-Álvarez et al. 2015), which may support long-distance range expansion of the species.
Experimental design
A full factorial experiment arranged in a blocked, split plot design was designed to test the effects of ploidy (diploid and decaploid) and soil nutrient availability (low and high), nested within light environments (shade and sun) and their interactions on trait responses of two invasive Ludwigia congeners. Apical shoot fragments of L. p. subsp. montevidensis and L. hexapetala were collected in early summer (June) 2014 from two adjacent watersheds with established invasions in northern California. Source material of L. p. subsp. montevidensis was collected from a Napa River tributary (Sage Creek, 38°29′24.6″N, 122°20′49.9″W), and L. hexapetala was collected from the Russian River near Duncans Mills (38°27′53.2″N, 123°02′49.2″W).
For each cytotype by treatment combination, six replicate shoot fragments (50 cm length) were weighed, and then planted in pots (19.5 cm height × 14 cm diameter) with random assignment to one of two soil nutrient treatments (low and high) and one of two light treatments (shade and sun). Soil nutrient and light treatments were manipulated to represent the range of measured resources present at invaded sites in the donor watersheds. The low nutrient soil contained a 90 : 10 ratio of sand to topsoil and contained 2.9 p.p.m. carbon (C), 0.63 p.p.m. nitrogen (N) and 16 p.p.b. extractable phosphorous (P). The high nutrient soil contained a 90 : 10 ratio of topsoil to sand with 25 p.p.m. C, 2.1 p.p.m. N and 165 p.p.b. extractable P. Potted experimental plants were then transferred from the greenhouse and placed in six fibreglass aquatic mesocosms (9500 L volume; 0.9 m depth × 3.7 m diameter). Pots with experimental plants were all placed on concrete blocks within the mesocosms so that the water depth above the pot surface was 30 cm. Each of the six mesocosms contained eight plants assigned to the sun or shade treatment. Three mesocosms had no cover for the full sun treatment, and three were covered with 80 % shade cloth secured to 3.9 × 3.9 m square canopy frames. Following treatment initiation, light levels were measured in both treatments using a Licor LI-250A light meter (LI-COR Inc., Lincoln, NE, USA) at solar noon. The average photosynthetically active radiation (PAR) values in full sun were 1839.7 ± 14.3 µmol m−2 s−1 (mean ± SE), while the average PAR values in the shade treatment were 295.0 ± 6.7 µmol m−2 s−1 (mean ± SE). Therefore, the 80 % shade cloth reduced light levels to ∼15 % of ambient. Water temperature was recorded hourly throughout the duration of the experiment using HOBO Water Temperature Pro v2 data loggers (U22-001, Onset Computer Corp., Bourne, MA, USA). Experimental conditions included a mean water temperature difference of 3.38 °C between treatments (mean water temperature—sun mesocosms: 27.1 °C; shade mesocosms: 23.7 °C). Plants were grown in treatments for 6 weeks, to represent the establishment and early growth phase of invading shoot fragments that have dispersed to a novel habitat.
Pre-harvest response measurements
Prior to harvest in August, numbers of branches emerging above the water surface were counted, and the height above the water surface of the tallest emergent branch was measured (hereafter called emergent canopy height). The tallest emergent shoot of each plant was identified, and the lengths of each of three stem internodes closest to the midpoint were measured. Six fully expanded leaves from the tallest emergent branch were removed and individually weighed, photographed for leaf morphology characterization and frozen for chlorophyll analysis. The date of the first observed flower was recorded and the number of days to anthesis was calculated for each plant. We used this information to score each plant for presence or absence of flowers or flower buds prior to harvest.
Post-harvest response measurements
At harvest, the longest primary stem length was measured on each plant, as well as the number of primary and secondary branches and the number of rooting nodes along the primary stem for each replicate. The lengths of three internodes closest to the midpoint of the primary floating stem were measured. For dry mass and biomass allocation, shoots were separated from roots at the soil surface, and the soil was sieved through a No. 20 mesh sieve to obtain fine belowground roots. Mass data were used to calculate relative growth rate (RGR = final mass − initial mass/days), leaf mass ratio (LMR, leaf mass/total biomass), specific leaf area (leaf area per unit leaf mass) and root mass ratio (RMR, total root mass/total biomass). Total leaf area and average leaf length and width were analysed using image analysis (WinFOLIA 2009a, Regent Instruments, Quebec, Canada).
Leaf chemical analyses
Leaves were analysed for total N concentration using a Perkin Elmer 2400 CHNS/O analyser (Perkin Elmer, Waltham, MA, USA). Leaf total P was measured using the colorimetric molybdenum–ascorbic acid method (Murphy and Riley 1962) following tissue combustion for 4 h at 550 °C and subsequent acid digestion. Photosynthetic pigments were extracted using 0.1 g of frozen leaf tissue in 20 mL of 80 % aqueous acetone. Chlorophyll a (Chl a) and chlorophyll b (Chl b) concentrations were determined in a spectrophotometer (Beckman Coulter DU-730, Beckman Coulter, Inc., Brea, CA, USA), using three wavelengths (663, 647 and 470 nm). Concentrations of pigments (mg g−1 DW) were obtained through calculation (Lichtenthaler 1987).
Statistical analyses
All response variables were evaluated for compliance with model assumptions. Shapiro–Wilks and O'Brien's tests were performed, and variables were transformed for normality and equal variance (x1/2: RGR, leaf biomass, root and rhizome biomass, reproductive biomass, total leaf area; ln(x): total biomass; x1/3: leaf width; 1/x: specific leaf area). Response variables were grouped by functional trait types, and all response traits were evaluated with a correlation analysis. Redundant, highly correlated variables (r > 0.95) were removed prior to analysis with multivariate analysis of variance (MANOVA) using a general linear model (GLM) and Pillai's trace test. Trait groups included (i) biomass and allocation, (ii) plant architecture, (iii) leaf growth and (iv) leaf tissue chemistry. The MANOVAs tested the main effect of light because it is the least powerful, statistically and, therefore, most conservative test of multi-trait significance in this study design. Multivariate analysis of variance results were significant [P < 0.05; see Supporting Information], so we proceeded to run ‘protected’ univariate tests using GLMs (Schiener 2001). Error terms were defined following a split plot with main plots in a randomized complete block design (for example, light level error was defined as block × light level). Post hoc tests on least square means (Tukey–Kramer multiple comparisons test) were performed with Bonferroni-corrected α level P = 0.00625 (0.05/n, n= 8). All models were run with SAS v.9.4 (SAS Institute Inc., Cary, NC, 2012).
Results
Biomass and allocation
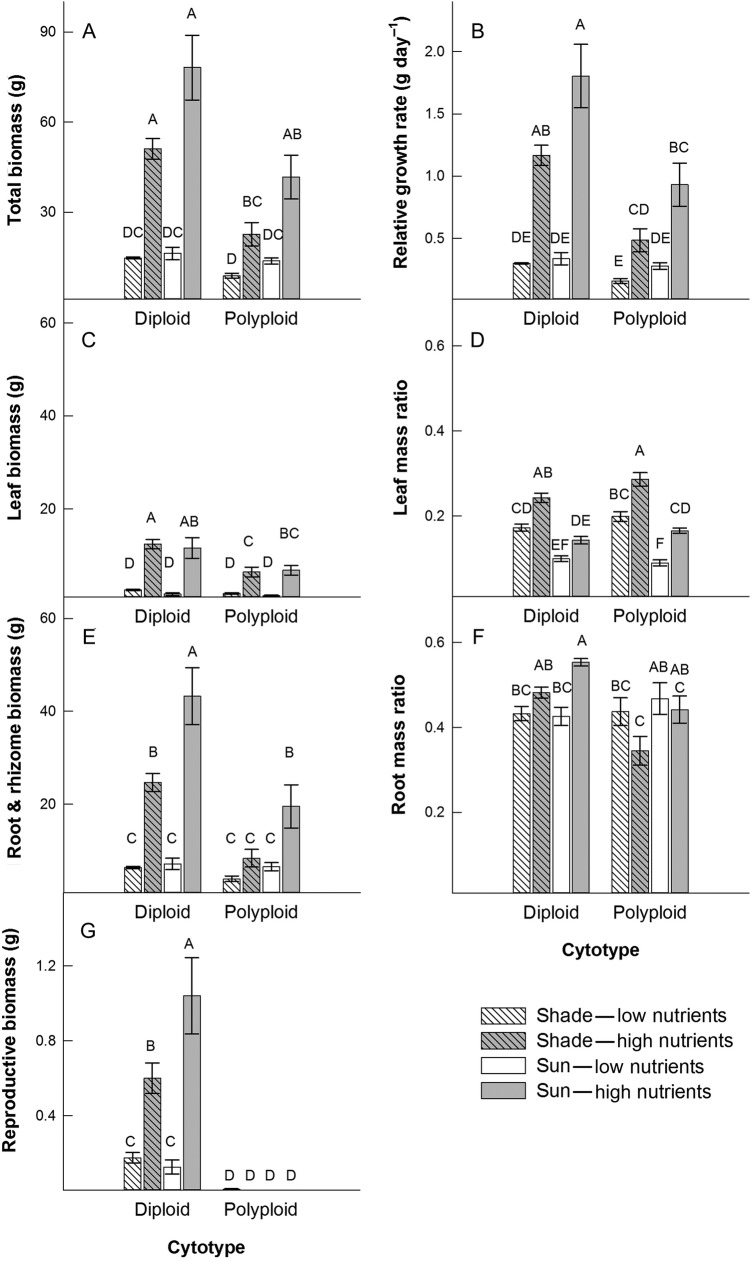
Both cytotypes produced 3.6-fold more total biomass under higher than under low soil nutrient conditions (F1,2= 280.62, P = 0.0035; Fig. 1A), and the diploid cytotype produced 84 % more total biomass than the polyploid (F1,2 = 47.27, P = 0.0205). Irrespective of light availability, RGR of experimental plants increased with elevated nutrient availability, and under high nutrient availability, the diploid cytotype's RGR was 4.7-fold higher than the polyploid (nutrient × cytotype interaction, F1,2 = 22.64, P = 0.0414; Fig. 1).
Figure 1.
Means (±1 SE) for biomass production, RGRs and allocation of biomass in two cytotypes, L. peploides subsp. montevidensis (diploid) and L. hexapetala (decaploid), in response to light (shade and sun) and soil nutrient (low and high) treatments. Letters above bars indicate significance in Tukey–Kramer multiple comparisons tests (α level is P = 0.00625).
Leaf biomass production was similar between the two cytotypes in response to contrasting light environments but was over five times greater under high than low soil nutrient conditions (F1,2 = 121.13, P = 0.0082; Fig. 1B). Overall, LMR was 81 % greater in the shade compared with LMR of plants grown in full sunlight (F1,2 = 57.13, P = 0.0171; Fig. 1C) and 50 % greater in high nutrient sediment than low nutrient sediment conditions (F1,2 = 730.00, P = 0.0014). Although both the diploid and decaploid increased root biomass and root biomass allocation in response to nutrient amendment, the response was stronger in the diploid cytotype (nutrient × cytotype interaction, F1,2 = 64.49, P = 0.0152; Fig. 1D). As a result, RMR was five times greater in the diploid under high soil nutrient conditions, but only 2.7 times greater in the polyploid with elevated nutrient availability (nutrient × cytotype interaction, F1,2 = 731.64, P = 0.0014; Fig. 1E).
The diploid cytotype allocated 5.5-fold greater biomass to reproductive structures (buds and flowers) with high nutrient availability compared with low nutrient conditions. In contrast, only three individuals of the polyploid cytotype had transitioned to flowering, thus producing very little reproductive biomass (nutrient × cytotype interaction, F1,2 = 251.79, P = 0.0039; Fig. 1F). Reproductive biomass was greatest in high light and high nutrient conditions (light × nutrient interaction, F1,2 = 131.17, P = 0.0075).
Plant architecture
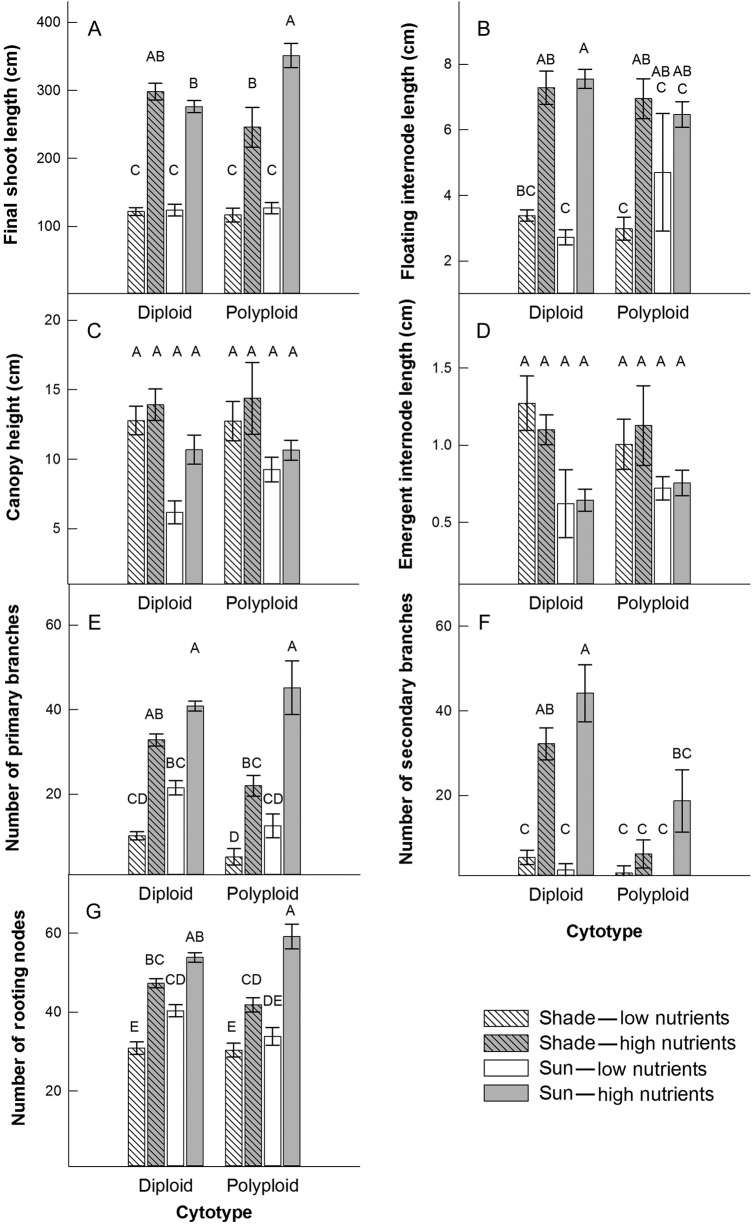
Nutrient and light availability greatly affected traits pertaining to the structure of plant growth. On average, final shoot length was 2.5-fold greater in high light and high soil nutrient conditions compared with shoot lengths where resource availability was low (light × nutrient interaction, F1,2 = 101.13, P = 0.0097; Fig. 2A). However, the polyploid, but not the diploid, grew longer shoots in high light compared with low light conditions (light × cytotype interaction, F1,2 = 47.10, P = 0.0206; Fig. 2A).
Figure 2.
Means (±1 SE) for plant architecture traits in two cytotypes, L. peploides subsp. montevidensis (diploid) and L. hexapetala (polyploid), in response to light (shade and sun) and soil nutrient (low and high) treatments. Letters above bars indicate significance in Tukey–Kramer multiple comparisons tests (α level is P = 0.00625).
Floating stem internode length increased more in the high nutrient, low light environment than in the high nutrient, high light environment (light × nutrient interaction, F1,2 = 45.73, P = 0.0212; Fig. 2B). However, canopy height did not differ among cytotypes or respond to resource conditions (e.g. canopy height; Fig. 2C and see Supporting Information—Table S2). Emergent stem internode lengths were 1.6 times greater in low light conditions regardless of soil nutrient level or cytotype (F1,2 = 19.85, P = 0.0469; Fig. 2D).
Overall, primary branching increased 71 % in high light compared with low light conditions (F1,2 = 309.35, P = 0.0032) and was 185 % greater in high nutrient soil compared with low nutrient soil conditions (F1,2 = 113.78, P = 0.0087; Fig. 2E). The tremendous increase in number of secondary branches due to high nutrient conditions was much greater in high light (26.9-fold) than low light (5.5-fold; light × nutrient interaction, F1,2 = 34.45, P = 0.0278; Fig. 2F). The polyploid developed proportionally more secondary branches under high soil nutrient conditions (14.9-fold) than the diploid (10-fold; nutrient × cytotype interaction, F1,2 = 45.98, P = 0.0211; Fig. 2F), although the absolute number of secondary branches was greatest in the diploid relative to the polyploid. This difference in secondary branch development influenced the overall shape of the individual plants, with the polyploid producing a longer, less branched floating stolon compared with the diploid.
The number of rooting nodes was influenced by an interaction of cytotype, light and soil nutrient conditions (light × nutrient × cytotype interaction, F1,2 = 29.23, P = 0.0326; Fig. 2G). In general, the number of rooting nodes was greater in high soil nutrient conditions, especially for the polyploid cytotype (Fig. 2G), in which the polyploid plants experienced a 41 % increase in rooting nodes due to higher nutrient and light availability.
Leaf growth
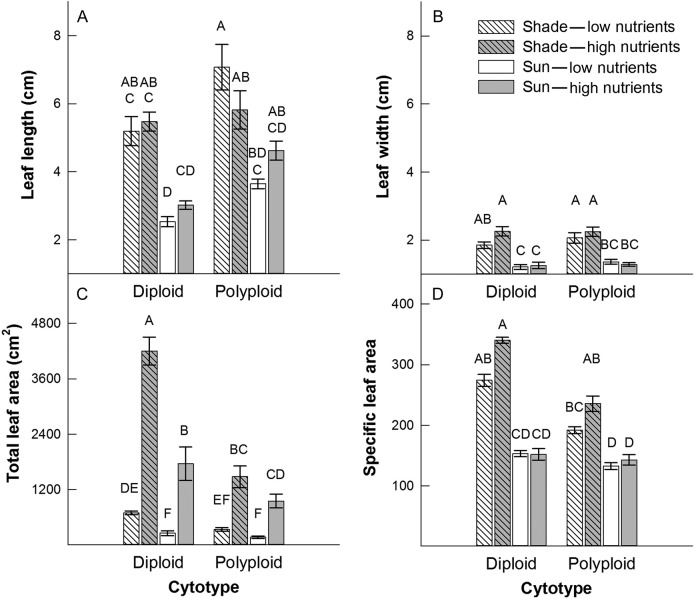
Leaf length was 70 % greater in low light compared with high light conditions (F1,2 = 306.12, P = 0.0033; Fig. 3A), and 30 % greater in the polyploid than the diploid cytotype (F1,2 = 34.37, P = 0.0279; Fig. 3A). Leaf width was 1.6 times greater (F1,2 = 112.23, P = 0.0088; Fig. 3B) and total leaf area was 2.1 times greater in low light conditions (F1,2 = 27.45, P = 0.0345; Fig. 3C). Total leaf area increased 4.8- and 6.3-fold for the polyploid and diploid congeners, respectively, in response to increased soil nutrient availability (nutrient × cytotype interaction, F1,2 = 21.08, P = 0.0443; Fig. 3C). Specific leaf area was 31 % greater in the diploid cytotype (F1,2 = 139.59, P = 0.0071), and while nutrient availability did not have a substantial impact on specific leaf area in high light conditions, an increase in nutrient availability under low light conditions led to a 23 % increase in specific leaf area (light × nutrient interaction, F1,2 = 20.92, P = 0.0446; Fig. 3D).
Figure 3.
Means (±1 SE) for leaf growth traits in two cytotypes, L. peploides subsp. montevidensis (diploid) and L. hexapetala (polyploid), in response to light (shade and sun) and soil nutrient (low and high) treatments. Letters above bars indicate significance in Tukey–Kramer multiple comparisons tests (α level is P = 0.00625).
Leaf tissue chemistry
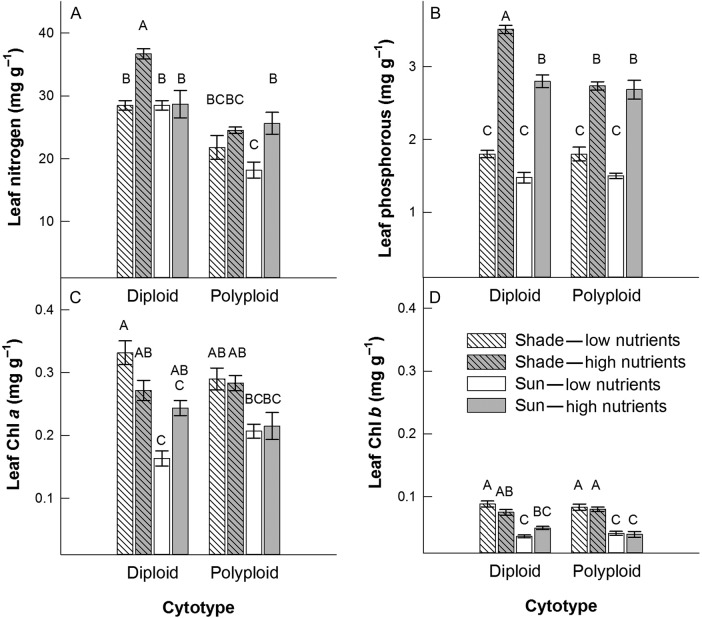
Leaf N was similar across all treatments, but was highest for the diploid in the shaded high nutrient environment and lowest for the polyploid in the high light, low nutrient environment (light × nutrient × cytotype interaction, F1,2 = 132.91, P = 0.0074; Fig. 4A). Leaf P was 16 % greater in all plants grown in low light conditions (F1,2 = 73.80, P = 0.0133; Fig. 4B). The proportional increase in leaf P was greater in the diploid than in the polyploid between low and high soil nutrient environments (nutrient × cytotype interaction, F1,2 = 22.41, P = 0.0418; Fig. 4B). Leaf Chl a and Chl b were highly variable within the diploid cytotype among all treatments, but were lower for the polyploid in high light vs. low light environments (Chl a: light × nutrient × cytotype interaction, F1,2 = 105.22, P = 0.0094; Chl b: F1,2 = 54.49, P = 0.0179; Fig. 4C and D).
Figure 4.
Means (±1 SE) for leaf chemistry traits in two cytotypes, L. peploides subsp. montevidensis (diploid) and L. hexapetala (polyploid), in response to light (shade and sun) and soil nutrient (low and high) treatments. Letters above bars indicate significance in Tukey–Kramer multiple comparisons tests (α level is P = 0.00625).
Flowering phenology
By the end of the 6-week growing period, the two cytotypes vastly differed in reproductive phenology (Fig. 5). Mean time to anthesis in the diploid cytotype was 27 days, and all diploid plants flowered during the 42-day study period (Fig. 5). The polyploid was slower to transition to anthesis. Only three polyploid individuals produced flowers during the study period.
Figure 5.
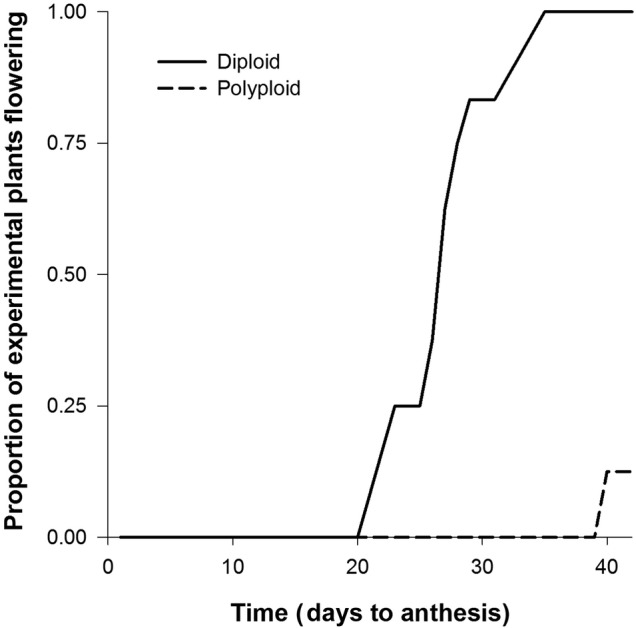
Cumulative proportion of experimental plants (n = 24) flowering over the 42-day study period.
Discussion
Our experiment focussed on the ability of stolon fragments of Ludwigia spp. to colonize across light and soil nutrient gradients in shallow water. Overall, growth and biomass allocation responses in early life stages were dominantly related to the interactions between soil nutrient availability and cytotype identity. It often is assumed that polyploid invasive plants will have higher growth rates than diploid congeners (Pandit et al. 2011). In this establishment stage of growth, our expectation that the decaploid species would produce more biomass than the diploid congener was not supported. In fact, for most trait responses, the diploid congener outperformed the decaploid L. hexapetala in this early stage of growth. One of the most striking differences between the congeners was the far superior growth rate and biomass accumulation of the diploid cytotype with nutrient enrichment, irrespective of light environment, despite similar biomass accumulation when nutrient availability was low. These data suggest that the diploid is capable of greater plasticity in response to nutrients than the polyploid Ludwigia species during early growth. Our results support the findings of Černá and Münzbergová (2013), who demonstrated the need to account for contrasting habitat conditions when comparing single life-history traits or demographic performances of diploid and polyploid congeners.
The architecture of individual plants was dependent on resource environment and cytotype identity. Resources strongly influenced the shape of the plant, particularly emergent shoot and floating shoot internode lengths. For emergent shoots, the driving factor was light availability, while floating shoots responded to nutrient availability. It is expected that shade avoiding plants will increase stem elongation and increase apical dominance through reduced branching in an attempt to access more light (Lambers et al. 2008; Dlugos et al. 2015). For floating shoots, our results suggest that cytotype identity strongly influences architectural traits ultimately related to dispersal ability, such as shoot length, particularly when soil nutrient availability is high. The early investment and superior ability of L. hexapetala to grow longer shoots and produce more rooting nodes along stems may support foraging opportunities for limited heterogeneous resources. These measured trait responses resulted in greater extension of buoyant L. hexapetala shoots across the water surface than was observed for L. peploides. In a natural river setting, these long shoots, growing perpendicular to the river bank, can facilitate the hydrochorous dispersal of rooted shoot fragments with water currents. The asexual propagules can root and establish elsewhere, supporting colonization of new population patches downstream.
Flowering time greatly influences ecological and evolutionary processes ranging from gene flow to species interactions (Elzinga et al. 2007), with effects that flow from individual to population, community and ecosystem levels (Franks 2015). Our results revealed extreme differences in time to anthesis between the cytotypes. The earlier flowering and higher RGR of the diploid congener represents more rapid development and a much earlier shift in phenology to reproduction compared with the polyploid. We would assume a trade-off between growth and/or defence due to this early shift to a reproductive life stage in the diploid. However, in our experiment, overall growth of the diploid surpassed that of the polyploid at this initial life stage; other work suggests slower growth rates in polyploids can be linked to delayed onset of anthesis (te Beest et al. 2012). Additionally, polyploids tend to allocate resources toward growth for vegetative reproduction/clonality, which in some examples has contrasted with greater tendency toward sexual reproduction in diploids (Hroudová and Zákravský 1993; Henery et al. 2010; Černá and Münzbergová 2013). Although our focus on the early colonizing life stage does not allow for a comparison of final sexual reproductive output between species, the biomass allocation results suggest a greater investment in sexual reproduction by the diploid. The advantages of the shift to early flowering of plants in response to climate change or other ecological reasons are not well understood (Franks 2015). However, an accelerated life cycle can ultimately affect overall production of biomass and rates of nutrient cycling.
Conclusions
Field observations suggest that polyploid L. hexapetala is a more aggressive invader that reaches higher levels of abundance and colonizes a much wider niche breath than the diploid L. peploides subsp. montevidensis, suggesting greater plasticity through the life cycle of the polyploid. Our experiment revealed, however, that in the early, colonizing phase of growth, the diploid taxa has a superior ability to maximize resource uptake, use and allocation across contrasting resource gradients. The growth and abundance of L. p. subsp. montevidensis was also much more responsive to nutrient loading than the polyploid species, suggesting that reductions in nutrient loads to aquatic environments may be more effective toward controlling the diploid congener than the decaploid.
The rapid, early season growth of the diploid congener is supported by field observations in which the taxon has an accelerated life cycle, produces copious seed before seasonal hydrologic drawdown and completes its seasonal life cycle much earlier than L. hexapetala. This temporal growth pattern suggests that managers should target L. p. subsp. montevidensis for control early in the growing season. In contrast, the measured trait responses of L. hexapetala suggest that its overall success as an aggressive invader at source population sites is supported by a more long-term investment in growth that may explain higher biomass production in established perennial stands by season's end (B. Grewell, unpubl. data).
The unexpected higher performance of the diploid relative to the polyploid congener at the early stage of growth suggests that functional trait responses underlying invader success may change with ontogeny. Appropriate management strategies for aquatic Ludwigia invaders must, therefore, be tailored for specific cytotypes and the unique characteristics of their life stages. Additional studies are needed to illuminate trait responses and impacts of invasive Ludwigia cytotypes for a comprehensive understanding of potential strengths and weaknesses throughout their life cycles.
Sources of Funding
Our work was funded by the US Army Corps of Engineers, Engineer Research and Development Center, Aquatic Plant Control Research Program, Vicksburg, Mississippi (USA).
Contributions by the Authors
B.J.G., R.E.D. and M.I. conceived the study and designed the experiment; all authors contributed to execution of the experiment; M.J.S.T., R.E.D., C.J.F. and B.J.G. analysed the data; and B.J.G., R.E.D. and M.J.S.T. wrote and revised the manuscript.
Conflict of Interest Statement
None declared.
Supporting Information
The following additional information is available in the online version of this article —
Figure S1. Ludwigia peploides subsp. montevidensis at reproductive life stage, Sage Creek upstream of Lake Hennessey, Napa River Watershed, California.
Figure S2. Ludwigia hexapetala at reproductive life stage, Russian River upstream of Duncan's Mills, Russian River Watershed California.
Figure S3. Early season to mid-summer drawdown conditions in Ludwigia peploides subsp. montevidensis habitat along the banks of Sage Creek, California.
Figure S4. High density seed capsule production of (A) Ludwigia peploides subsp. montevidensis at Sage Creek confluence with Lake Hennessey, Napa River Watershed, July 20, 2006. Ludwigia hexapetala growth characteristics during (B) pre-reproductive growth, and (C) flowering life stages along the Russian River, California.
Figure S5. Buoyant shoots of Ludwigia hexapetala (A) with floating roots at stem nodes, (B) early season floating shoot elongation and (C) late summer shoot elongation along the Russian River, California.
Table S1. Results from MANOVA (df = 7.30) and univariate models (df = 1.2) for biomass production and allocation traits of the two cytotypes, Ludwigia peploides (diploid) and L. hexapetala (polyploid), in response to light (high and low) and nutrient (high and low) treatments.
Table S2. Results from MANOVA (df = 7.31) and univariate models (df = 1.2) for plant architecture traits of the two cytotypes, Ludwigia peploides (diploid) and L. hexapetala (polyploid), in response to light (high and low) and nutrient (high and low) treatments.
Table S3. Results from MANOVA (df = 4.33) and univariate models (df = 1.2) for leaf growth traits of the two cytotypes, Ludwigia peploides (diploid) and L. hexapetala (polyploid), in response to light (high and low) and nutrient (high and low) treatments.
Table S4. Results from MANOVA (df = 4.33) and univariate models (df = 1.2) for leaf chemical traits of the two cytotypes, Ludwigia peploides (diploid) and L. hexapetala (polyploid), in response to light (high and low) and nutrient (high and low) treatments.
Acknowledgements
We thank the journal editors, Michael D. Netherland and anonymous reviewers for their valuable comments on the manuscript, Michael D. Netherland for helpful discussions and support and Zac Miller-Smith and Ashley McBride for their help with the experiment and post-harvest measurements.
Literature Cited
- Buggs RJA, Pannell JR. 2007. Ecological differentiation and diploid superiority across a moving ploidy contact zone. Evolution 61:125–140. 10.1111/j.1558-5646.2007.00010.x [DOI] [PubMed] [Google Scholar]
- Černá L, Münzbergová Z. 2013. Comparative population dynamics of two closely related species differing in ploidy level. PLoS ONE 8:e75563. 10.1371/journal.pone.0075563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos DM, Collins H, Bartelme EM, Drenovsky RE. 2015. The non-native plant Rosa multiflora expresses shade avoidance traits under low light availability. American Journal of Botany 102:1323–1331. 10.3732/ajb.1500115 [DOI] [PubMed] [Google Scholar]
- Drenovsky RE, Grewell BJ, D'antonio CM, Funk JL, James JJ, Molinari N, Parker IM, Richards CL. 2012. A functional trait perspective on plant invasion. Annals of Botany 110:141–153. 10.1093/aob/mcs100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga JA, Atlan A, Arjen B, Gigord L, Weis AE, Bernasconi G. 2007. Time after time: flowering phenology and biotic interactions. Trends in Ecology and Evolution 22:432–439. 10.1016/j.tree.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Franks SJ. 2015. The unique and multifaceted importance of the timing of flowering. American Journal of Botany 102:1401–1402. 10.3732/ajb.1500234 [DOI] [PubMed] [Google Scholar]
- García-Álvarez A, Van Leeuwen CHA, Luque CJ, Hussner A, Vélez-Martín A, Pérez-Vázquez A, Green AJ, Castellanos EM. 2015. Internal transport of alien and native plants by geese and ducks: an experimental study. Freshwater Biology 60:1316–1329. 10.1111/fwb.12567 [DOI] [Google Scholar]
- Godsoe W, Larson MA, Glennon KL, Segraves KA. 2013. Polyploidization in Heuchera cylindrical (Saxifragaceae) did not result in a shift in climatic requirements. American Journal of Botany 100:496–508. 10.3732/ajb.1200275 [DOI] [PubMed] [Google Scholar]
- Hahn MA, Van Kluenen M, Müller-Schärer H. 2012. Increased phenotypic plasticity to climate may have boosted the invasion success of polyploid Centaurea stoebe. PLoS ONE 7:e50284 10.1371/journal.pone.0050284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henery ML, Bowman G, Mráz P, Treier UA, Gex-Fabry E, Schaffner U, Müller-Schärer H. 2010. Evidence for a combination of pre-adapted traits and rapid adaptive change in the invasive plant Centaurea stoebe. Journal of Ecology 98:800–813. 10.1111/j.1365-2745.2010.01672.x [DOI] [Google Scholar]
- Hoch PC, Grewell BJ. 2012. Ludwigia. In: Baldwin BF, Goldman HD, Keil DJ, Patterson R, Rosatti TJ, Wilkin DH, eds. The Jepson manual: vascular plants of California, 2nd edn Berkeley: University of California Press, 948–951. [Google Scholar]
- Hoch PC, Wagner WL, Raven PH. 2015. The correct name for a section of Ludwigia L. (Onagraceae). PhytoKeys 50:31–34. 10.3897/phytokeys.50.4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hroudová Z, Zákravský P. 1993. Ecology of two cytotypes of Butomus umbellatus II. Reproduction, growth and biomass production. Folia Geobotanica et Phytotaxonomica 28:413–424. 10.1007/BF02853307 [DOI] [Google Scholar]
- Hufbauer RA, Torchin ME. 2007. Integrating ecological and evolutionary theory of biological invasions. In: Nentwig W, ed. Biological invasions. Berlin: Springer, 79–96. [Google Scholar]
- Lambers H, Chapin FS III, Pons TL. 2008. Plant physiological ecology, 2nd edn New York, NY, USA: Springer Science+Business Media LLC. [Google Scholar]
- Levin D. 2002. The role of chromosomal change in plant evolution. Oxford: Oxford University Press. [Google Scholar]
- Lichtenthaler HK. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 148:350–382. 10.1016/0076-6879(87)48036-1 [DOI] [Google Scholar]
- Manzaneda AJ, Rey PJ, Bastida JM, Weiss-Lehman C, Raskin E, Mitchell-Olds T. 2012. Environmental aridity is associated with cytotype segregation and polyploidy occurrence in Brachypodium distachyon (Poaceae). New Phytologist 193:797–805. 10.1111/j.1469-8137.2011.03988.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzbergová Z. 2007a. No effect of ploidy level in plant response to competition in a common garden experiment. Biological Journal of the Linnean Society 92:211–219. 10.1111/j.1095-8312.2007.00820.x [DOI] [Google Scholar]
- Münzbergová Z. 2007b. Population dynamics of diploid and hexaploid populations of a perennial herb. Annals of Botany 100:1259–1270. 10.1093/aob/mcm204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27:31–36. 10.1016/S0003-2670(00)88444-5 [DOI] [Google Scholar]
- Okada M, Grewell BJ, Jasieniuk M. 2009. Clonal spread of invasive Ludwigia hexapetala and L. grandiflora in freshwater wetlands of California. Aquatic Botany 91:123–129. 10.1016/j.aquabot.2009.03.006 [DOI] [Google Scholar]
- Pandit MK, Pocock MJO, Kunin WE. 2011. Ploidy influences rarity and invasiveness in plants. Journal of Ecology 99:1108–1115. 10.1111/j.1365-2745.2011.01838.x [DOI] [Google Scholar]
- Richardson DM, Pyšek P. 2006. Plant invasions: merging the concepts of species invasiveness and community invasibility. Progress in Physical Geography 30:409–431. 10.1191/0309133306pp490pr [DOI] [Google Scholar]
- Ruaux B, Greulich S, Haury J, Berton J-P. 2009. Sexual reproduction of two alien invasive Ludwigia (Onagraceae) on the middle Loire River, France. Aquatic Botany 90:143–148. 10.1016/j.aquabot.2008.08.003 [DOI] [Google Scholar]
- Schiener SM. 2001. MANOVA: multiple response variables and multispecies interactions. In: Schiener SM, Gurevitch J, eds. Design and analysis of ecological experiments. New York: Chapman & Hall, 94–112. [Google Scholar]
- Soltis DE, Soltis PS, Tate JA. 2004. Advances in the study of polyploidy since Plant speciation. New Phytologist 161:173–191. 10.1046/j.1469-8137.2003.00948.x [DOI] [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Patison AH, Zheng C, Sankoff D, Depamphilis CW, Wall PK, Soltis PS. 2009. Polyploidy and angiosperm diversification. American Journal of Botany 96:336–348. 10.3732/ajb.0800079 [DOI] [PubMed] [Google Scholar]
- Soltis DE, Buggs RJA, Doyle JJ, Soltis PS. 2010. What we still don't know about polyploidy. Taxon 59:1387–1403. [Google Scholar]
- Soltis PS, Soltis DE. 2000. The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences of the USA 97:7051–7057. 10.1073/pnas.97.13.7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Beest M, Le Roux JJ, Richardson DM, Brysting AK, Suda J, Kubešová M, Pyšek P. 2012. The more the better? The role of polyploidy in facilitating plant invasions. Annals of Botany 1:19–45. 10.1093/aob/mcr277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thébault A, Gillet F, Müller-Schärer H, Buttler A. 2011. Polyploidy and invasion success: trait trade-offs in native and introduced cytotypes of two Asteraceae species. Plant Ecology 212:315–325. 10.1007/s11258-010-9824-8 [DOI] [Google Scholar]
- Thouvenot L, Haury J, Thiebaut G. 2013. A success story: water primroses, aquatic plant pests. Aquatic Conservation-Marine and Freshwater Ecosystems 23:790–803. [Google Scholar]
- Wagner WL, Hoch PC, Raven PH. 2007. Revised classification of the Onagraceae. Systematic Botany Monographs 83:1–240. [Google Scholar]
- Zardini EM, Peng C-I, Hoch PC. 1991. Chromosome numbers in Ludwigia sect. Oligospermum and sect. Oocarpon (Onagraceae). Taxon 40:221–230. 10.2307/1222976 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.