Abstract
MGd1, a monoclonal antibody raised against gastric cancer cells, possesses a high degree of specificity for gastric cancer (GC). Here we identified that the antigen of MGd1 is CEACAM5, and used MGd1 to investigate the expression of CEACAM5 in non-GC and GC tissues (N=643), as a biomarker for prewarning and prognosis. The expression of CEACAM5 was detected by immunohistochemistry in numerous tissues; its clinicopathological correlation was statistically analyzed. CEACAM5 expression was increased progressively from normal gastric mucosa to chronic atrophic gastritis, intestinal metaplasia, dysplasia and finally to GC (p<0.05). In gastric precancerous lesions (intestinal metaplasia and dysplasia), CEACAM5-positive patients had a higher risk of developing GC as compared with CEACAM5-negative patients (OR = 12.68, p<0.001). Besides, CEACAM5 was found positively correlated with invasion depth of gastric adenocarcinoma (p<0.001). In survival analysis, CEACAM5 was demonstrated to be an independent prognostic predictor for patients with GC of clinical stage IIIA/IV (p=0.033). Our results demonstrate that CEACAM5 is a promising biomarker for GC prewarning and prognostic evaluation.
Keywords: gastric cancer, CEACAM5, biomarker, prognosis, immunohistochemistry
Introduction
Gastric cancer represents one of the most prevalent malignancies in China and the second most common cause of cancer-related death around the world (Wu et al. 2009). Biomarkers for early detection are urgently needed to improve patient prognoses. Molecule-directed immunotherapy is a promising avenue for curing this disease. Our laboratory has produced a batch of gastric cancer-associated monoclonal antibodies (Abs)—MG7, MGd1, MG5, among others—through hybridoma techniques after immunizing BALB/C mice directly with gastric cancer (GC) MKN-46-9 cells or cells from GC tissues. MG7 has been acknowledged as a specific antibody for the detection (Chen et al. 2010), prewarning (Hong et al. 2010), in vivo imaging (Li et al. 2013; Xu et al. 2015), and targeted therapy (Lu et al. 2013) of GC. The utility of the other antibodies is largely unknown, with the exception of MGd1-Ag (a target of MGd1), which possesses high specificity for GC and may have the potential to be used diagnostically (Chao et al. 1989).
Tissue microarray (TMA) techniques can simultaneously produce comprehensive protein expression profiles for numerous tumors (Kallioniemi et al. 2001). This technology has been used extensively and is the subject of multiple reviews (Williamson et al. 2001; Shergill et al. 2004; van de Rijn and Gilks 2004). TMA allows for the simultaneous analysis of large numbers of specimens, resulting in high-throughput data acquisition. Because all tissue specimens analyzed are arrayed on one identical TMA, antigen retrieval, reagent concentrations, incubation times with primary antibodies, temperatures and wash conditions are identical for each core, resulting in an unprecedented level of standardization over and above that available with standard histopathological techniques.
In the current study, we have identified the antigen recognized by MGd1 as CEACAM5. CEACAM5 belongs to the CEACAM family. It serves as a cell adhesion protein. CEACAM5 is overexpressed in about 90% of gastrointestinal, colorectal and pancreatic cancers, 70% of non-small cell lung cancers, and 50% of breast cancers (Thompson et al. 1991). CEACAM5 surveillance has been applied in the clinical detection of liver metastasis from colorectal cancers and during post-surgical surveillance of colon cancer relapse (Duffy 2001). However, the utility of CEACAM5 for prewarning and prognosis of GC has been less investigated. Thus, we adopted a TMA-based IHC assay as a simple and practical method to investigate CEACAM5/MGd1-Ag expression and its clinicopathological values, including its dynamic expression in non-cancerous lesions, its prewarning and prognostic utilities as indicators of the disease, and its expression profile in multiple normal and malignant tissues.
Materials & Methods
Cells and Specimens
Gastric cancer cell lines (AGS, MKN45, KATO3, and SGC7901), immortalized gastric epithelial cell (GES), and HEK293 cells were employed in this study. In addition, we obtained 643 stomach specimens from patients who had undergone endoscopic biopsy at the Xijing Hospital (Xi’an, China) in 2010 and 2011. This included 266 cases of chronic atrophic gastritis (CAG), 80 cases of intestinal metaplasia (IM), 104 cases of dysplasia, and 50 normal gastric tissues samples. In all cases, informed consent was obtained for specimen use. For survival analysis, we made two different TMAs containing 143 cases of GC for which detailed clinicopathological data, such as age, sex, depth of invasion, pathological grade, and clinical stage (ACJJ 7th), were available, with all of the tissues obtained from resectable, non-metastatic patients who had undergone subtotal gastrectomy with D2 lymph node dissection from 2006–2011. These cases included 97 men and 46 women, and comprised 51 clinical stage I-II and 92 clinical stage III-IV. All patients received a fluorouracil-based regimen postoperatively. No patient had received preoperative chemotherapy or radiotherapy. Patients were followed up from the date of surgery to either the date of death or to September 29, 2011, resulting in follow-up periods ranging from 1 to 61 months. Those cases lost to follow-up or those who died of any cause other than cancer were regarded as censored data. Informed consent was obtained for the use of all the resected tumor specimens.
Tissue Array Methods
Tissue microarrays were prepared by Tissue Microarrayer (Beecher Instruments; Sun Prairie, WI). Briefly, core tissue biopsies (2 mm in diameter) were taken from individual paraffin-embedded gastric tumors (donor blocks) and arranged in a new recipient paraffin block (tissue array block) using a trephine apparatus. Staining results for the different intratumoral areas of gastric carcinomas in these tissue array blocks were in excellent agreement. We defined an adequate case as a tumor that occupied at least 10% of the core area. Sections (4-μm thick) were cut from each tissue array block, deparaffinized and rehydrated.
Primary Antibodies
The mouse monoclonal antibody MGD1 was developed in our laboratory and was purified from the induced ascites by DEAE-52 cellulose affinity chromatography. Anti-CEACAM5 antibody (ab4451) was purchased from Abcam (Cambridge, MA).
Immunoprecipitation (IP)
IP was performed with Pierce Classic IP Kit (Pierce Biotechnology; Rockford, IL). Cells were lysed with IP Lysis/Wash Buffer on ice for 5 min, and the lysate centrifuged at 13000 ×g for 10 min. The supernatant was pre-cleared using Control Agarose Resin for 1 hr, and then 600 µl pre-cleared lysate was incubated with 10 µg primary antibodies and 40 µl Protein A/G Agarose Resin overnight at 4°C. Normal IgG (10 µg) was used as a control antibody. The resin was then washed twice with 200 µl Lysis/Wash Buffer, and once with 100 µl of 1× conditioning Buffer. The resin was suspended with 50 µl 2 × sample buffer containing 20 mM DDT. Samples were boiled at 100°C for 10 min and centrifuged at 1000 ×g for 3 min to collect the elute.
Cell Culture and Lentivirus Infection
KATO3 cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum in a humidified atmosphere containing 5% CO2 at 37°C. Lentivirus-targeted shRNA for CEACAM5 and control shRNA were purchased from GeneChem Company (Shanghai, China). Cells were infected with lentivirus according to the manufacturer’s procedure.
General Methods
Immunohistochemistry (IHC), confocal immunofluorescence (IF) and western blotting were performed as described previously (Zhou et al. 2011), using different primary antibodies and dilutions (MGd1, 2 µg/ml for IHC; 2 µg/ml MGd1 and 2 µg/ml anti-CEACAM5 for IF; 1 µg/ml MGd1, 1 µg/ml anti-CEACAM5 for western blotting). The expression was scored as negative (-) or positive (+) according to the intensity of staining of the cancer cells by optical evaluation.
Statistical Analysis
All statistical analyses were performed using IBM SPSS 19.0 software (Armonk, NY). Measurement data were analyzed using Student’s t or one-way ANOVA tests, whereas categorical data were studied using the χ2 or nonparametric tests. Survival curves were estimated using the Kaplan–Meier method, and the log-rank test was used to calculate differences between the curves. A multivariate analysis using the Cox proportional hazards regression model was performed to assess the prognostic values of protein expression. Statistical significance was set at p<0.05.
Results
Identification of MGd1 as a Specific Antibody against CEACAM5
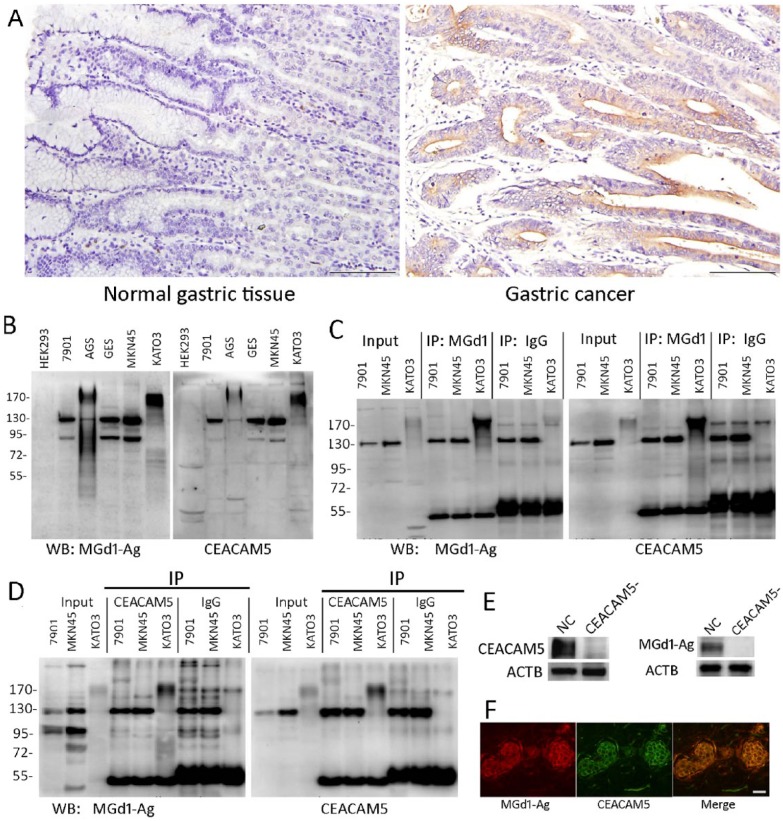
We examined MGd1-Ag expression in 50 normal tissues and 143 cases of GC by IHC, and found that it was not expressed in normal tissues but highly expressed in cancer tissues (Fig. 1A), with 57.3% positivity in GC, suggesting that MGd1 is high specific to GC tissues. We next decided to identify the target of MGd1. We immunoprecipitated MGd1-Ag (the antigen of MGd1) from the KATO3 GC cell line and found an enrichment at 170 Kd. Mass spectrum analysis revealed that the 170-Kd protein was possibly CEACAM5 (data not shown). Western blotting in a panel of cell lines showed that the expression of MGd1-Ag was identical to that of CEACAM5 (Fig. 1B). Reciprocal IP experiments in KATO3 cell line revealed that anti-CEACAM5 recognized the immunoprecipitated MGd1-Ag at 170 Kd (Fig. 1C) and Mgd1-Ag recognized the immunoprecipitated CEACAM5 at the same level (Fig. 1D). Silencing CEACAM5 in KATO3 cells by lentivirus-targeted shRNA led to a consistent reduction in MGd1-Ag expression (Fig. 1E) and co-immunofluorescence assay showed that MGd1-Ag was completely co-localized with CEACAM5 in GC tissues. All of these data demonstrate that MGd1 is a specific antibody against CEACAM5. Thus, in the following study, we used this antibody for CEACAM5 detection.
Figure 1.
(A) IHC staining of CEACM5 in normal gastric tissues and gastric cancer. (B) Western blot (WB) of MGd1-Ag and CEACMA5 in different cell lines (the entire blot is shown). (C) MGd-1 was immunoprecipitated from KATO3 cells and WB performed using MGd1 and anti-CEACAM5; SCG7901 (7901) and MKN45 were used as negative control cell lines. (D) CEACAM5 was immunoprecipitated from KATO3 cells and the blots stained using MGd1 and anti-CEACAM5; SCG7901 (7901) and MKN45 were again used as negative control cell lines. (E) CEACAM5 was silenced by lentivirus targeted shRNA and control shRNA (NC), and lysates subjected to western blotting using anti-CEACAM5 and MGd1. ACTB (Actin, Beta) was used as an internal loading control . (F) Immunofluorescence of MDd1-Ag and CEACM5 in a GC tissue slide. Scale (A, F) 200 μm.
CEACAM5 Expression as a Prewarning Biomarker for Non-cancerous Tissue Lesions of the Stomach
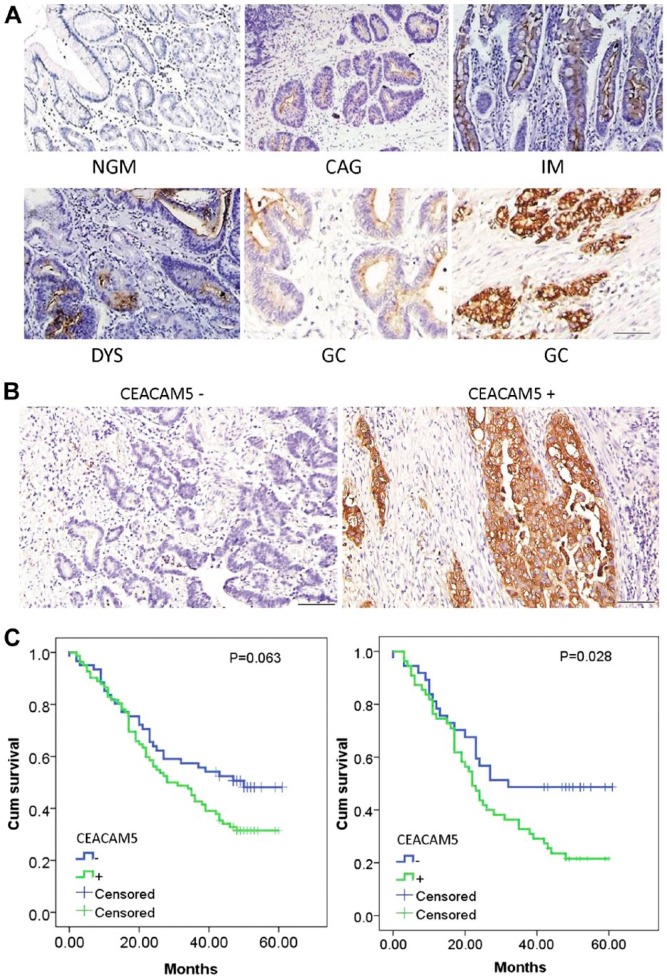
To clarify the expression patterns of CEACAM5 at different stages of gastric carcinogenesis, 643 stomach specimens were examined by IHC. CEACAM5 was absent in normal gastric mucosa (NGM), and positively expressed on the luminal surface of samples taken from patients with chronic atrophic gastritis (CAG), dysplasia (DYS), and GC. CEACAM5 could also be detected in the cytoplasm of cancerous cells in 64.2% of GC tissues (Fig. 2A). The positivity of CEACAM5 in NGM, CAG, intestinal metaplasia (IM), DYS and GC were 0/50 (0%), 21/266 (7.8%), 22/80 (27.5%), 44/104 (42.3%) and 82/143 (57.3%), respectively. Thus, positive rates of CEACAM5 gradually increased along the NGM-CAG-IM-DYS-GC axis (Table 1; p<0.05). All patients with these non-cancerous lesions were followed up for at least 4 years (4–7 years), and the correlation between CEACAM5 and GC development was analyzed by unconditional logistic regression. For patients with CAG, 4 out of 139 (3%) patients developed GC in the CEACAM5-negative (CEACAM5-) group, 5 out of 127 (4%) patients developed GC in the CEACAM5-positive (CEACAM5+) group, with no significant difference in GC risk (Table 2; p=0.675). For patients with IM and DYS, 2 out of 36 (5.5%) patients developed GC in the CEACAM5- group, 57 out of 148 (38.5%) patients developed GC in the CEACAM5+ group, with the GC risk for CEACAM5+ patients 12.68-times that of the CEACAM5- group (Table 2; p<0.001); this suggests that CEACAM5 is a promising classifier for IM/DYS patients with a high risk of GC development.
Figure 2.
(A) Representative IHC staining of CEACAM5 in different gastric lesions. NGM, normal gastric mucosa; CAG, chronic atrophic gastritis; IM, intestinal metaplasia; DYS, dysplasia; GC, gastric cancer. (B) Negative (left) and positive (right) expression of CEACAM5 in representative GC tissues. (C) Kaplan-Meier curves for postoperative survival of gastric cancer patients with regard to CEACAM5 expression. Left, Kaplan-Meier curves for all the patients (p=0.063); right, Kaplan-Meier curves for gastric cancer patients at advanced clinic stage (IIIA-IV) (p=0.028). Scale (A, B) 200 μm.
Table 1.
Relation between CEACAM5 Expression and Different Gastric Tissues.
| CEACAM5 Expression |
|||||
|---|---|---|---|---|---|
| Tissues | N | Expression Site | Negative | Positive | P value* |
| NGM | 50 | Luminal surface | 50 | 0 (0%) | |
| CAG | 266 | Luminal surface | 249 | 21(7.8%) | 0.041 |
| IM | 80 | Luminal surface | 58 | 22 (27.5%) | <0.001 |
| DYS | 104 | Luminal surface | 60 | 44 (42.3%) | 0.038 |
| GC | 143 | Surface/cytoplasm | 61 | 82 (57.3%) | 0.020 |
chi-square test, significance (p<0.10). NGM, normal gastric mucosa; CAG, chronic atrophic gastritis; IM, intestinal metaplasia; DYS, dysplasia; GC, gastric cancer.
Table 2.
Correlation between CEACAM5 and Gastric Development in Gastric Precancerosis.
| Tissues | CEACAM5 | Non-GC | GC | OR (95%CI) | P value |
|---|---|---|---|---|---|
| CAG | - | 135 | 4 | 1 | |
| + | 122 | 5 | 1.43 (0.23–3.56) | 0.675 | |
| IM/DYS | - | 34 | 2 | 1 | |
| + | 91 | 57 | 12.68 (3.72–66.26) | <0.001 |
Unconditional logistic regression, adjusting age, gender and H. pylori infection. CAG, chronic atrophic gastritis; IM, intestinal metaplasia; DYS, dysplasia; GC, gastric cancer.
CEACAM5 is an Independent Prognostic Indicator for Late Stage Gastric Adenocarcinoma
We investigated 143 cases of GC to evaluate the predictive value of CEACAM5 for patient survival. The relation between CEACAM5 expression and various clinicopathological parameters is summarized in Table 3. We found CEACAM5 expression to be positively correlated with invasion depth of GC (p<0.001); but found no other significant correlation. Representative IHC images are shown in Fig. 2B. Patients were divided into negative and positive groups according to CEACAM5 expression analyses. The median survival time of CEACAM5+ and CEACAM5- patients was 28 ± 4.9 months and 50 ± 6.3 months, respectively, with no significant difference between these two groups (Fig. 2 left, log-rank test: p=0.063). As clinical stage is considered to an important factor that could influence the survival time of cancer patients, we stratified patients into Stage IA-IIB group and Stage IIIA-IV group, and re-analyzed for survival. For patients at Stage IA-IIB, CEACAM5 expression could not be used separate the survival curves between the two groups (log-rank test: P=0.922). Notably, by Stage IIIA-IV, the median survival time for CEACAM5+ patients was significantly shortened as compared with that of the CEACAM5- group (22 ± 2.1 vs 32 ± 3.3 months, Fig. 2 right, p=0.028). In the multivariate analysis using a Cox proportional hazards model (all parameters in Table 3 were enrolled), CEACAM5 was selected as an independent prognostic indicator for patients with stage IIIA-IV cancer (p=0.033).
Table 3.
Statistical Results of Immunohistochemical Assay (N=143).
| N | CEACAM5 Expression | P* | ||
|---|---|---|---|---|
| - | + | |||
| Gender | 0.822 | |||
| Men | 97 | 42 | 55 | |
| Women | 46 | 19 | 27 | |
| Age (years) | 0.852 | |||
| <60 | 55 | 24 | 31 | |
| >60 | 88 | 37 | 51 | |
| Clinic stage (ACJJ 7th) | 0.428 | |||
| Early (IA-IIB) | 51 | 24 | 27 | |
| Advanced (IIIA-IV) | 92 | 37 | 55 | |
| Pathological Grade | 0.398 | |||
| I-II | 38 | 14 | 24 | |
| III-IV | 105 | 47 | 58 | |
| Invasive Depth | <0.001 | |||
| T1-T2 | 18 | 14 | 4 | |
| T3-T4 | 125 | 30 | 95 | |
| Lymph Node Status | 0.750 | |||
| N0 | 31 | 14 | 17 | |
| N1-N3 | 112 | 47 | 65 | |
| Distant Metastasis | 0.992 | |||
| M0 | 131 | 56 | 75 | |
| M1 | 12 | 5 | 7 | |
Chi-square test, significance (P<0.10).
Expression Profiles of CEACAM5 in Various Tumors and Normal Tissues
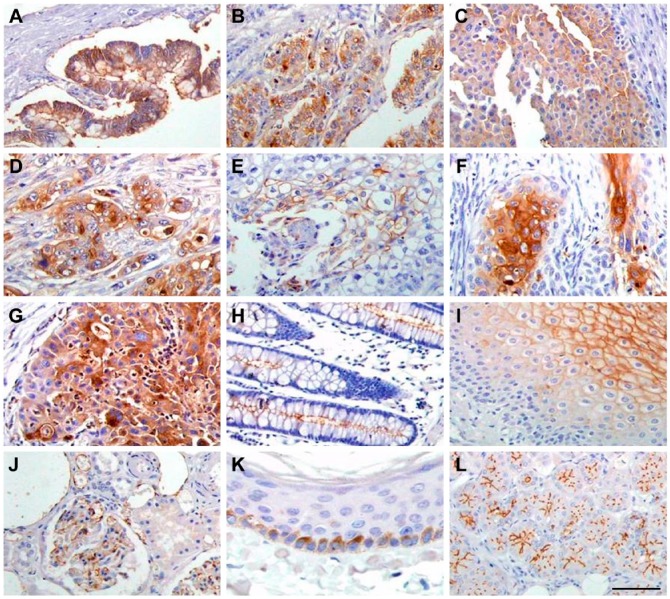
CEACAM5 expression was detected in tissues of gastric carcinoma, colon adenocarcinoma, rectum adenocarcinoma, squamous cell carcinoma of the lung, epithelial cancer of the bladder, duct carcinoma of the breast, ovary endometriosis carcinoma, transitional cell carcinoma of the prostate, pancreatic adenocarcinoma, and squamous cell carcinoma of the cervix. CEACAM5 was found to be distributed throughout the cellular surface and in the cytoplasm of cancerous cells (Fig. 3A–3G). In contrast, CEACAM5 expression was not detected in hepatocellular liver cancer and many other cancers (data not shown). In normal tissues, CEACAM5 expression could be detected in kidney, bladder, larynx, epiglottis, skin, submaxillary gland, colon, esophagus, duodenum, parotid, and sublingual gland tissues (Fig. 3H–3L). In addition, the distribution of CEACAM5 was observed to be tissue-dependent. For example, CEACAM5 localized to the apical and/or lateral membranes of gland cells of the duodenum, parotid gland, submaxillary gland, colon, and sublingual gland, and was distributed throughout the membranes of the stratified squamous epithelium of the esophagus, larynx, epiglottis, and bladder. CEACAM5 was also expressed on the membranes of basal cells of the skin, and in endothelial cells of the kidney glomerulus. Conversely, CEACAM5 expression was not detected in tissues of the stomach, pancreas and many other normal tissues (data not shown).
Figure 3.
CEACAM5 immunostaining in different tumor tissues and normal tissues. (A) gastric carcinoma; (B) adenocarcinoma of colon; (C) epithelial cancer of bladder; (D) adenocarcinoma of rectum; (E) squamous cell carcinoma of lung; (F) squamous cell carcinoma of cervix; (G) pancreatic adenocarcinoma; (H) colon; (I) esophagus; (J) kidney; (K) skin; (L) sublingual gland. Scale, 200 μm.
Discussion
Although the gastric cancer-associated monoclonal antibody MGd1 was produced many years ago (Chao et al. 1989), the antigen it recognized remained a mystery until now, and this hindered the exploitation and application of this antibody. In the current study, we successfully identified CEACAM5 as the antigen of MGd1 through a series of assays, and then used MGd1 as a specific antibody to investigate CEACAM5. CEACAM5 belongs to the CEACAM family and serves as a cell adhesion protein. It is overexpressed in many different cancers (Thompson et al. 1991), and has been used as a biomarker in the clinic to detect liver metastasis from colorectal cancers and to predict colon cancer relapse (Duffy 2001). However, the potential utility of CEACAM5 in prewarning and prognosis of GC are largely unknown.
Intestinal-type gastric carcinoma was thought to be preceded by a precancerous stage characterized by sequential progress through atrophic gastritis, intestinal metaplasia, dysplasia, and intramucosal carcinoma (Correa 1995). In the current study, we found a gradual increase in CEACAM5 expression during this carcinogenesis process (Correa pathway), suggesting that CEACAM5 could serve as a biomarker to warn patients who may be at risk of developing gastric cancer. Thus, we next examined the use of CEACAM5 as a prewarning biomarker in numerous cases of gastric non-cancer lesions. We found that precancerous lesions (IM/DYS) with positive expression of CEACAM5 were more likely to evolve into GC than negative cases. Although CAG, IM and DYS are considered as precancerous lesions of GC, there are no unified clinical guidelines for the treatment of these lesions (Haruma et al. 2013; Park and Kim 2015). We propose that IM and DYS lesions should be surgically excised if the patient is positive for CEACAM5. Besides, as cytoplasmic CEACAM5 was detected in 64.2% of GC tissues, this marker should be of great importance in the differential diagnosis between gastric cancer and high-grade dysplasia.
Patients with advanced cancer often want to know how long they have left to live to prepare for their future but clinicians are not confident at estimating prognosis. Biomarkers for prognosis would thus offer a potentially useful tool to help clinicians in this prediction. Here, we found that CEACAM5 could predict the prognosis of patients with clinically advanced gastric cancer (Stage IIIA-IV), without considering age, sex, differentiation, pathological TNM stage or lymph node metastasis of patients. Aside from its function in cell adhesion and migration, CEACAM5 also inhibits anoikis (Ordonez et al. 2000). Because resistance to anoikis is a feature of cancer cells, this implicates a role for CEACAM5 in facilitating tumorigenesis and metastasis. Indeed, the tumorigenic functions of CEACAM5 have been shown in 3D cultures of colon carcinoma cell lines (Ilantzis et al. 2002) and also in transgenic mice (Chan et al. 2006; Chan et al. 2007); some studies have also validated the contribution of CEACAM5 in cancer invasion and metastasis (Hostetter et al. 1990; Hashino et al. 1994). These data may partly explain CEACAM5’s correlation with poor prognosis in GC patients.
Antibody-directed, CEACAM5-targeted immunotherapy is a promising treatment for colonic and pancreatic cancers (Govindan et al. 2009; Zheng et al. 2011) and probably other CEACAM5-expressing cancers. However, it is first important to determine the expression pattern of CEACAM5 in various normal tissues and organs. In the present study, the expression of CEACAM5 was detected in a variety of normal tissues, particularly in the apical membranes of gland cells of the duodenum, parotid gland, submaxillary gland, colon, and stomach. Thus, the effectiveness and side-effects associated with CEACAM5-targeted therapy need to be evaluated by animal experimentation and other approaches.
In summary, this is the first TMA-based IHC study to have comprehensively evaluated the implications of CEACAM5 as a biomarker for prewarning and prognosis in GC. CEACAM5 expression is gradually increased along the Correa pathway, supporting the notion that it plays an important role in tumorigenesis. We show that CEACAM5 is a prewarning biomarker that could be used to classify IM and DYS patients who may be at high risk of GC occurrence. It is also an independent prognostic indicator for clinically advanced GC, and thus may serve as a prognostic classifier for advanced GC in practice. CEACAM5 is widely expressed in diverse tumors and normal tissues, and therefore CEACAM5-targeted therapy needs to be evaluated in greater detail.
Footnotes
Author Contributions: JZ and XF performed the immunohistochemistry and statistical analysis. JZ, XF, FZ and JD collected the tissues and prepared the TMA blocks. NC performed the western blot and IP assays. YN and JZ designed the study and drafted the manuscript. DF revised the manuscript. All authors have read and approved the final manuscript.
Competing Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Chan CH, Camacho-Leal P, Stanners CP. (2007). Colorectal hyperplasia and dysplasia due to human carcinoembryonic antigen (CEA) family member expression in transgenic mice. PLoS One 2:e1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Cook D, Stanners CP. (2006). Increased colon tumor susceptibility in azoxymethane treated CEABAC transgenic mice. Carcinogenesis 27:1909-1916. [DOI] [PubMed] [Google Scholar]
- Chao CY, Lie J, Fan DM. (1989). Immunohistochemical study of monoclonal antibody MGD-1 in gastric carcinoma. Histopathology 15:523-529. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hong L, Liu L, Peng D, Li Q, Jin B, Qiao T, Wu K, Fan D. (2010). Monoclonal antibody MG7 as a screening tool for gastric cancer. Hybridoma (Larchmt) 29:27-30. [DOI] [PubMed] [Google Scholar]
- Correa P. (1995). Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol 19 Suppl 1:S37-43. [PubMed] [Google Scholar]
- Duffy MJ. (2001). Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem 47:624-630. [PubMed] [Google Scholar]
- Govindan SV, Cardillo TM, Moon SJ, Hansen HJ, Goldenberg DM. (2009). CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates. Clin Cancer Res 15:6052-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruma K, Kamada T, Murao T, Yamanaka Y, Ohsawa M, Shiotani A, Inoue K. (2013). [European guideline for the management of precancerous conditions in the stomach and it’s application to Japan]. Nihon Rinsho 71:1479-1484. [PubMed] [Google Scholar]
- Hashino J, Fukuda Y, Oikawa S, Nakazato H, Nakanishi T. (1994). Metastatic potential of human colorectal carcinoma SW1222 cells transfected with cDNA encoding carcinoembryonic antigen. Clin Exp Metastasis 12:324-328. [DOI] [PubMed] [Google Scholar]
- Hong L, Li S, Liu L, Shi Y, Wu K, Fan D. (2010). The value of MG7-Ag and COX-2 for predicting malignancy in gastric precancerous lesions. Cell Biol Int 34:873-876. [DOI] [PubMed] [Google Scholar]
- Hostetter RB, Campbell DE, Chi KF, Kerckhoff S, Cleary KR, Ullrich S, Thomas P, Jessup JM. (1990). Carcinoembryonic antigen enhances metastatic potential of human colorectal carcinoma. Arch Surg 125:300-304. [DOI] [PubMed] [Google Scholar]
- Ilantzis C, DeMarte L, Screaton RA, Stanners CP. (2002). Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia 4:151-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi OP, Wagner U, Kononen J, Sauter G. (2001). Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet 10:657-662. [DOI] [PubMed] [Google Scholar]
- Li Z, Zuo XL, Li CQ, Zhou CJ, Liu J, Goetz M, Kiesslich R, Wu KC, Fan DM, Li YQ. (2013). In vivo molecular imaging of gastric cancer by targeting MG7 antigen with confocal laser endomicroscopy. Endoscopy 45:79-85. [DOI] [PubMed] [Google Scholar]
- Lu X, Cheng C, Wang G, Shu X, Ma J, Tong Q. (2013). Synergistic enhancement of cancer therapy using a combination of fusion protein MG7-scFv/SEB and tumor necrosis factor alpha. Protein Pept Lett 20:467-472. [PubMed] [Google Scholar]
- Ordonez C, Screaton RA, Ilantzis C, Stanners CP. (2000). Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res 60:3419-3424. [PubMed] [Google Scholar]
- Park YH, Kim N. (2015). Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev 20:25-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill IS, Shergill NK, Arya M, Patel HR. (2004). Tissue microarrays: a current medical research tool. Curr Med Res Opin 20:707-712. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Grunert F, Zimmermann W. (1991). Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal 5:344-366. [DOI] [PubMed] [Google Scholar]
- van de Rijn M, Gilks CB. (2004). Applications of microarrays to histopathology. Histopathology 44:97-108. [DOI] [PubMed] [Google Scholar]
- Williamson M, Naaby-Hansen S, Masters JR. (2001). 21st century molecular biology in urology. BJU Int 88:451-457. [DOI] [PubMed] [Google Scholar]
- Wu K, Nie Y, Guo C, Chen Y, Ding J, Fan D. (2009). Molecular basis of therapeutic approaches to gastric cancer. J Gastroenterol Hepatol 24:37-41. [DOI] [PubMed] [Google Scholar]
- Xu B, Li X, Yin J, Liang C, Liu L, Qiu Z, Yao L, Nie Y, Wang J, Wu K. (2015). Evaluation of 68Ga-labeled MG7 antibody: a targeted probe for PET/CT imaging of gastric cancer. Sci Rep 5:8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Feng J, Lu D, Wang P, Xing S, Coll JL, Yang D, Yan X. (2011). A novel anti-CEACAM5 monoclonal antibody, CC4, suppresses colorectal tumor growth and enhances NK cells-mediated tumor immunity. PLoS One 6:e21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li K, Gu Y, Feng B, Ren G, Zhang L, Wang Y, Nie Y, Fan D. (2011). Transcriptional up-regulation of RhoE by hypoxia-inducible factor (HIF)-1 promotes epithelial to mesenchymal transition of gastric cancer cells during hypoxia. Biochem Biophys Res Commun 415:348-354. [DOI] [PubMed] [Google Scholar]