Abstract
Zebrafish (Danio rerio) and their transparent embryos are becoming an increasingly popular tool for studying processes involved in tumor progression and in the search for novel tumor treatment approaches. The xenotransplantation of fluorescently labeled mammalian cancer cells into zebrafish embryos is an approach enabling relatively high-throughput in vivo analyses. The small size of the embryos as well as the relative simplicity of their manipulation and maintenance allow for large numbers of embryos to be processed efficiently in a short time and at low cost. Furthermore, the possibility of fluorescence microscopic imaging of tumor progression within zebrafish embryos and larvae holds unprecedented potential for the real-time visualization of these processes in vivo. This review presents the methodologies of xenotransplantation studies on zebrafish involving research on tumor invasion, proliferation, tumor-induced angiogenesis and screening for antitumor therapeutics. We further focus on the application of these zebrafish to the study of glioma; in particular, its most common and malignant form, glioblastoma.
Keywords: cancer models, glioma, intravital microscopy, drug screening
Introduction
It is recognized that cancer results from complex interactions of cancer cells with their microenvironment and the whole organism. Thus, established in vitro cell and tissue models of cancer must be complemented by in vivo models, the former aiming at deciphering molecular mechanisms of tumor progression, and the latter, elucidating multicellular interactions during tumor progression. Commonly used mammalian models have several drawbacks in that they are expensive, time consuming, and ethically questionable; yet are not necessarily a good approximation of physiological processes taking place in the human body. In recent years, steps have been taken towards bridging the gap between high-throughput in vitro studies on the one hand and animal cancer models on the other by introducing models with higher throughput that are less ethically problematic. Recently, the zebrafish (Danio rerio) and its embryos have become a popular in vivo experimental model that enables rapid, medium-throughput studies at low cost and offers the possibility to image tumor progression directly at single-cell resolution in real time (Armatruda et al. 2002; Konantz et al. 2005; Mione and Trede 2010; White at al. 2013; Zon and Peterson 2005).
This review aims to provide a brief overview of the methodology involved in studying human tumors by xenotransplantation into zebrafish embryos and the application of this model to the study of gliomas, focusing on glioblastoma multiforme (GBM), the most common and aggressive form of glioma (Behin et al. 2003; Ohgaki and Kleihues 2013). Several properties of GBM make these tumors difficult to treat. Their diffuse growth and invasion into surrounding areas of the brain prevent their complete surgical removal, whereas the presence of the blood-brain permeability barrier (BBB) limits drug delivery (Claes et al. 2007; Persano et al. 2013). The utilization of a complex in vivo model that enables real-time imaging of cellular interactions during GBM progression and its interactions with the environment at single-cell resolution can contribute greatly to the development of methods to improve the treatment of this devastating disease. We discuss the potential use of zebrafish xenotransplantation for the discovery of novel pharmaceuticals and molecular markers with potential diagnostic, prognostic and therapeutic value.
The Zebrafish Cancer Model
Zebrafish and Their Early Life Stages in Experimental Research
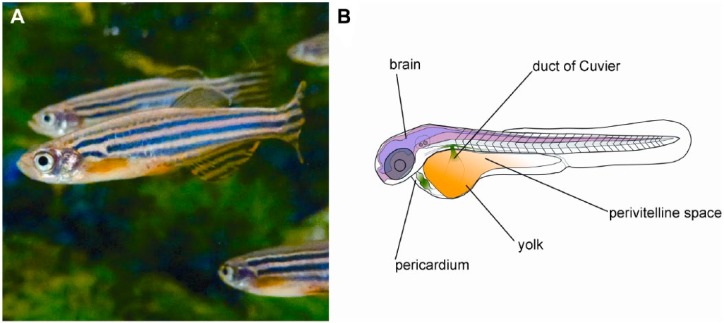
The zebrafish (Fig. 1) is a freshwater teleost that has been extensively studied from developmental and genetic points of view (Grunwald and Eisen 2002). It is an established developmental model organism due to the ease of obtaining and studying its embryos. The fecundity of zebrafish allows even a small breeding facility to achieve a daily production of embryos in their hundreds (Kari et al. 2007). Zebrafish embryos are convenient, as they develop outside of their parents’ bodies and can thus be monitored easily throughout their development. The development of zebrafish embryos is rapid: at 48 hours, an embryo already possesses a well-developed nervous system and displays a functional circulation as well as motility (Kimmel et al. 1995). In addition, zebrafish embryos are small and are suitable for maintenance on multi-well plates, making them an in vivo experimental system with relatively high throughput at a reasonable cost (Geiger et al. 2008; Kari et al. 2007; Zon and Peterson 2005). Reverse genetic approaches are well developed in this species (Hwang et al. 2013a, 2013b; Lawson and Wolfe 2011; Zu et al. 2013), with a perspective to produce knockout mutants for various genes in its genome (Kettleborough et al. 2013).
Figure 1.
The zebrafish (Danio rerio) and its embryo. (A) Adult zebrafish. (B) The anatomy of a zebrafish embryo at 2 days after fertilization. The areas of cancer cell implantation discussed in text are marked.
Besides these benefits, the major advantage of zebrafish embryos and larvae is their potential for in vivo visualization of cellular processes at high resolution. This is due to their small size and optical transparency, making in vivo observations of developmental processes easy to accomplish at single cell resolution (Hendricks and Jesuthasan 2007; Keller et al. 2008). Intravital fluorescence microscopy of zebrafish embryos has been further enhanced by the application of light sheet microscopy (Jung et al. 2012; Kobitski et al. 2015). Although in vivo fluorescence imaging of engrafted tumors has been performed on mammals (Yang et al. 2001), they do not offer the high-resolution imaging that can be performed in zebrafish embryos. Furthermore, transgenic zebrafish strains with tissue-specific expression of fluorescent proteins are being produced and are readily available for use (Distel et al. 2009; Lawson and Weinstein 2002), enabling fluorescence imaging of microanatomical structures and gene expression patterns; this adds greatly to the value of this model.
Zebrafish in Cancer Research and the Tumor Xenotransplantation Model
The zebrafish has been successfully utilized in cancer research over the past decade (Feitsma and Cuppen 2008; Konantz et al. 2012; Mimeault and Batra 2012; Veinotte et al. 2014). Cancer in zebrafish has been induced by genetic manipulation (Blackburn and Langenau 2014; Ignatius et al. 2012; Langenau et al. 2003; Sabaawy et al. 2006) or by xenotransplantation of cultured mammalian cancer cells (Geiger et al. 2008; Haldi et al. 2006; Lee et al. 2005; Konantz et al. 2012). Gene expression patterns of cancer cells were shown to be highly similar in zebrafish and humans (Lam et al. 2006; Zheng et al. 2014). Similar molecular pathways may lead to tumor development in the two species (Jung et al. 2013; Mione and Trede 2010) and tumors in zebrafish were found to be histologically similar to their mammalian counterparts (Armatruda et al. 2002; Eden et al. 2014; Stern and Zon 2003). Furthermore, zebrafish cells can respond to human signaling molecules (Drabsch et al. 2013).
Because of the small size of zebrafish embryos, only a few hundreds of cancer cells per embryo are generally implanted (Konantz et al. 2012), and single-cell imaging enables monitoring of the xenograft’s behavior in the embryo. It has been proposed that implantation of a low number of cells mirrors the early stages of tumor development (Lal et al. 2012; Nicoli and Presta 2007). This also makes the zebrafish xenotransplantation model particularly suitable for the study of cells that are difficult to obtain, such as cancer stem cells (Yang et al. 2013a).
Nevertheless, there are drawbacks or limitations to the use of zebrafish in xenotransplantation experiments. Because of the phylogenetic distance between teleost fish and mammals, zebrafish might provide the implanted cells with a different microenvironment than the human body, especially when orthotopic implantation is impossible due to the lack of corresponding organs in zebrafish (Konantz et al. 2012). Furthermore, the use of embryos can be problematic due to the immaturity of their developing tissues. For example, myelinated axonal sheaths do not develop in the zebrafish central nervous system (CNS) until 4–7 days post-fertilization (dpf) (Brösamle and Halpern 2002), which may affect the invasion of implanted glioma cells (Lal et al. 2012). Furthermore, the BBB does not develop in zebrafish embryos until 3 dpf (Xie et al. 2010) and is not mature for another 7 days (Fleming et al. 2013), an issue important for glioma drug screening.
Growing Human Tumors Underwater: Approaches to Zebrafish Xenotransplantation
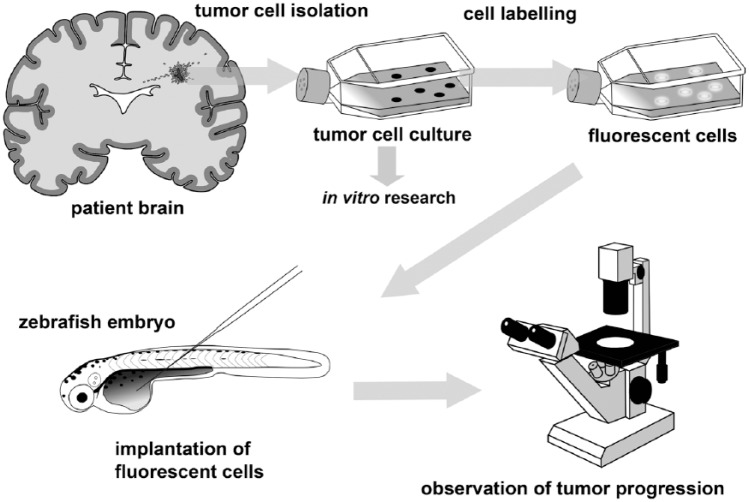
For cancer cell xenotransplantation, cells isolated from tumors are grown in culture, labeled in order to distinguish them from the recipient tissues, and implanted into the zebrafish, after which the effect of experimental manipulations on tumor progression are monitored (Fig. 2). Since the zebrafish adaptive immune system matures at 3–4 weeks after fertilization (Lam et al. 2004; Willett et al. 1999), xenotransplantation in early life-stages does not necessitate immunosuppression, making embryos particularly suitable for xenotransplantation. An additional benefit is that embryos do not need to be fed, relying on the yolk for nutrition.
Figure 2.
Overview of the zebrafish glioma xenotransplantation model workflow. Cells isolated from a patient’s tumor are grown in culture, fluorescently labeled, and implanted into the zebrafish embryos. After implantation, the embryos are incubated for several days, during which time the behavior of the implanted cells can be monitored using fluorescence microscopy.
The zebrafish model is relatively novel and therefore most methodology involved is not yet standardized. A variety of incubation conditions such as temperature and composition of the medium vary between laboratories, with no agreement as yet on best practice.
A major issue in the use of zebrafish as recipients of implanted mammalian cells is that the optimal temperature for the development of zebrafish embryos is 28°C (Kimmel et al. 1995), whereas human cells grow optimally at 37°C. In some studies, some cancer cell lines are able to tolerate 28°C (Nicoli and Presta 2007; Zhao et al. 2009, 2011a). For the GBM cell line, U251, it has been demonstrated that varying the incubation temperature between 28°C and 35°C has no effect on the survival of cells after engraftment (Geiger et al., 2008). In other cases, the temperature has been raised to 30°C (Geiger et al. 2008; Haldi et al. 2006; Lally et al. 2007) or even as high as 35°C (Marques et al. 2009; Yang et al. 2013a, 2013b). Embryos can tolerate up to 35°C, although their survival is reportedly best at temperatures below 33°C (Kimmel et al. 1995).
Implanted cells are generally rendered fluorescent to visualize them within the body of the recipient embryo. This can be achieved by staining cells prior to implantation with stable fluorescent dyes, such as DiI, which are passed onto the cell progeny (Jung et al. 2012; Lal et al. 2012; Lee et al. 2009; Rouhi et al. 2010; Teng et al. 2013). The staining of cells prior to implantation is relatively fast and can also be performed in primary cultures. In this way, the invasive potential of cancer cells obtained from biopsies has been assessed in vivo using the zebrafish model (Marques et al. 2009), but studies on established cell lines have also been performed with the use of dyes (Jung et al. 2012; Lal et al. 2012). The disadvantage of using fluorescent dyes is that their fluorescence can only decrease during the experiment, making the quantification of cell proliferation by means of fluorescence impossible. Furthermore, fluorescent dyes may be transferred to other cells.
An alternative to the fluorescence-labeling approach is the establishment of cancer cell lines that stably express fluorescent proteins (Drabsch et al. 2012; Eden et al. 2014; Geiger et al. 2008; He et al. 2012; Yang et al. 2013a). The fluorescence of endogenous fluorescent proteins is more stable and correlates with cell number, enabling the quantification of proliferation by measuring fluorescence intensity (Geiger et al. 2008; Vittori et al. 2014; Yang et al. 2014).
The crossing of different mutant zebrafish strains led to the development of the Casper strain, which does not develop skin pigmentation (White et al. 2008). The absence of pigmentation aids microscopic observations because pigments in the embryo may conceal the fluorescence emitted by fluorescently labeled cells and only non-pigmented fish enable quantitative intravital fluorescence imaging of implanted cells. Despite its benefits, the Casper strain has rarely been used in xenotransplantation studies (Corkery et al. 2011; Eden et al. 2014). Instead, low concentrations of phenylthiourea (PTU) are often used to inhibit melanin synthesis (Lee et al. 2009; Yang et al. 2013a, 2013b, 2014). Numerous studies have also used wild type embryos with normally developed pigmentation for xenotransplantation (Kitambi et al. 2014; Lally et al. 2007; Rampazzo et al. 2013).
Although human cells have been successfully engrafted into zebrafish embryos during the late blastula stage (Geiger et al. 2008; Lee et al. 2005; Zhao et al. 2009), cells are most often implanted at 2 dpf, when the embryos develop all of their major organ systems. Whereas some observations, especially on angiogenesis and cell invasion, are concluded at 5 dpf (Nicoli and Presta 2007; Yang et al. 2013a, 2013b), longer studies, extending into the period of larval development, have also been performed (Kitambi et al. 2014; Pruvot et al. 2011). It is worth noting that the yolk is generally degraded at 5 dpf (Kimmel et al. 1995). When the yolk mass is the site of implantation, this should be taken into account, as the microenvironment of the implanted cells will change with yolk resorption.
Applications of the Zebrafish Model
Studies on Cell Invasion and Metastasis
In the studies of cell invasion, the yolk sac is the most common area of implantation (Eguiara et al. 2010; Jung et al. 2012; Marques et al. 2009; Yang et al. 2013a). In this case, cells are injected into the center of the yolk mass, a syncytium containing nutrients required for embryonic development. The movement of engrafted cells from the yolk to other parts of the embryo can then be observed and quantified over a period of several days. The number of migrated cells or the number of embryos in which invasion occurs are determined (Eguiara et al. 2010; Marques et al. 2009; Yang et al. 2013a).
It has been argued that quantification of the invasion of cells from the yolk sac does not reflect their invasive potential, as cells may be passively transported to other parts of the body via blood vessels (Drabsch et al. 2013). However, differences in the capacity of cells to leave the yolk sac have been demonstrated among cells grown in different culture conditions (Eguiara et al. 2011; Yang et al. 2013a) and among different cancer cell lines (Lee et al. 2009; Marques et al. 2009), indicating that this is a valid model. The correlation between the in vitro invasive potential of cell lines and their ability to invade the body of the embryos has also been established for cells implanted into the perivitelline space, the cavity between the periderm forming the body wall and yolk (Fig. 1B; Teng et al. 2013).
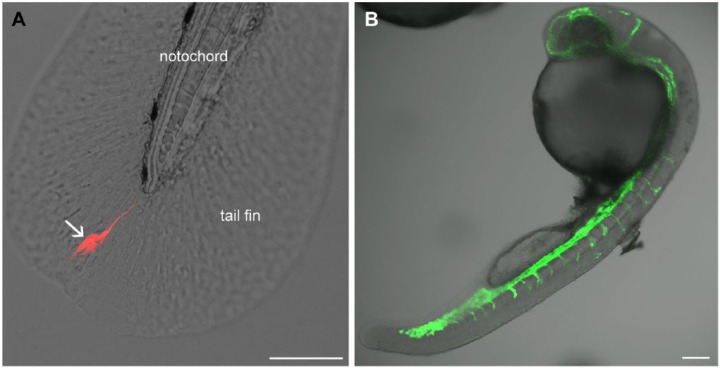
Alternatively, cells have been injected into to duct of Cuvier (the cardinal vein of zebrafish embryos; Fig. 1B) and allowed to spread throughout the body via the blood circulation (Drabsch et al. 2013; He et al. 2012). Later, their invasive potential can be assessed by counting cells located within the tail fin, a structure that possesses no blood vessels, thus ensuring that the cells had actively invaded the tissue (Fig. 3A). This approach has been successfully employed to study the involvement of neutrophils, which may help to process the collagen matrix to facilitate cancer cell invasion (He et al. 2012).
Figure 3.
Fluorescence observation of processes in zebrafish embryos in vivo. (A) A U87 DsRed cell (arrow) invading the tail fin of a zebrafish embryo 2 days after implantation from the spinal cord. (B) Visualization of the vasculature in a living transgenic embryo at 1 day after fertilization (courtesy of Marchien Dallinga). The embryo expresses GFP in the vascular endothelium. Scale, 100 µm.
Angiogenesis may be involved in cancer cell dissemination from the injection site. For example, vascular endothelial growth factor receptor (VEGFR) blockage was shown to inhibit cell invasion from the perivitelline space (Lee et al. 2009), whereas hypoxia promoted it (Lee et al. 2009; Rouhi et al. 2010). For the study of hypoxia-induced effects, zebrafish embryos may be maintained in a hypoxic chamber (Rouhi et al. 2010), although hypoxia may lead to developmental abnormalities (Lee at al. 2009; Padilla and Roth 2001).
Zebrafish have also been used to study metastatic processes. The transparency of embryos and the availability of transgenic zebrafish embryos (fli1:GFP) expressing GFP in their vascular endothelium (Lawson and Weinstein 2002) has enabled the high-resolution imaging of processes involved in metastasis (Stoletov et al. 2007). The metastatic potential of cancer cells was associated with the upregulation of specific molecular markers that are involved in metastasis (Drabsch et al. 2013; He et al. 2012; Stoletov et al. 2010). With cells implanted in the pericardium (Fig. 1B), cell invasion along the abluminal side of blood vessels was also observed (Zhao et al. 2011). The results of these studies show that zebrafish embryos can be used to evaluate the invasive potential of cancer cells as well as the mechanisms of metastasis.
Quantification of Cell Survival and Cell Proliferation
The assessment of the effects of different treatments on the proliferation of cancer cells through fluorescence quantification has been successfully implemented (Eden et al. 2014; Geiger et al. 2008; Lally et al. 2007; Yang et al. 2014). An alternative approach is the use of bioluminescence imaging of implanted cells genetically modified to express luciferase (Zhao et al. 2009). The advantage of bioluminescence is the low background signal as compared to fluorescence, but it is less suitable for single-cell visualization (Klerk et al. 2007). Alternative approaches for the quantification of cancer cell proliferation include measuring the projected tumor area on fluorescence micrographs (Lal et al. 2012) and estimating the volume occupied by cancer cells (Zhao et al. 2011a); however, these approaches may not be indicative for proliferation, as an increase in tumor volume can also result from cell dispersion or the accumulation of host cells in the tumor (Lal et al. 2012).
Modeling Tumor-Induced Angiogenesis
Zebrafish embryos are particularly suitable for the study of tumor-induced angiogenesis owing to the simplicity of their cardiovascular system and its predictable patterning (Seabra and Bhogal 2010; Stoletov et al. 2010; Tobia et al. 2011; 2013). The possibility to directly visualize the developing vasculature is facilitated by the transparency of the embryos and the availability of transgenic strains with fluorescent blood vessels (Fig. 3B). To study tumor-induced angiogenesis, cells are usually implanted into the perivitelline space, where the effect of implanted cells on the development of the subintestinal vasculature is then monitored (Nicoli and Presta 2007; Nicoli et al. 2007; Vitale et al. 2014; Zhao et al. 2011a). Assessment of this effect can be rapid, as changes can be observed within two days after tumor xenografting (Vitale et al. 2014; Yang et al. 2013b, 2014). The quantification of the xenograft’s angiogenic effect involves the determination of the percentage of embryos in which abnormal angiogenesis is observed (Nicoli and Presta 2007; Yang et al. 2013b) or measurement of parameters such as vessel length, diameter, or the number of branching points (Yang et al. 2013; Zhao et al. 2011). Alternatively, ex vivo quantification of the frequency of tumor-induced angiogenesis and the number of generated blood vessels has been performed using whole-mount alkaline phosphatase activity staining of the vasculature (Nicoli and Presta 2007; Nicoli et al. 2007; Tobia et al. 2011).
The Zebrafish Xenotransplantation Model in the Study of Glioma
Introducing Glioma to Zebrafish
Human glioma cells have successfully been transplanted into zebrafish, and most of the methodological approaches outlined above have been applied to study glioma. Unlike many other mammalian tumors, glioma may be particularly suitable for orthotopic xenotransplantation experiments in zebrafish.
The yolk was the initial area of GBM cell implantation, at first in blastula stage embryos and later in 2 dpf embryos (Geiger et al. 2008; Lally et al. 2007; Yang et al. 2013a, 2013b). Apart from survival and proliferation, the assessment of the invasion potential of glioma cells may be particularly important for the development of new treatment approaches for this highly invasive type of cancer. Due to the possible invasion of glioma cells into other parts of the CNS, successful treatment of glioma by surgical removal and directed radiotherapy is impossible (Claes et al. 2007). Glioma cell lines that have so far been studied in zebrafish do not demonstrate a great tendency to invade if implanted into the yolk sac. Differentiated U87 GBM cells are not invasive when implanted in this area (Lal et al. 2012; Yang et al. 2013a, 2014), and invasion of U251 GBM cells has not been reported (Geiger et al. 2008; Lally et al. 2007). The inability of implanted cells to leave the yolk sac likely reflects their low metastatic potential.
On the other hand, implantation into the yolk sac has provided new insights into the role of glioma stem-like cells (GSLCs) in tumor progression. One of the hallmarks of GSLCs is the expression of the cell-surface marker CD133, which is also used for isolating these cells. GSLCs have been proposed to play important roles in glioma progression and radiotherapy resistance, which is attributed to their more efficient DNA repair mechanisms (Bao et al. 2006; Campos and Herold-Mende 2011; Hira et al. 2015; Molina et al. 2014; Persano et al. 2013; Tabatabai and Weller 2011; Wang et al. 2009). As a result, GSLCs are the focus for research into targeted therapy, including studies using the zebrafish model. To this end, CD133+ U87 cells were obtained by single-cell cloning of U87 cells and growing them under specific culture conditions (Yang et al. 2013a) or by obtaining CD133+ cells by flow cytometry-based cell sorting (Yang et al. 2013b, 2014). The CD133+ subpopulation of U87 cells implanted into the yolk sac of 2 dpf zebrafish embryos invaded the body more frequently than CD133- cells (Yang et al. 2013a). These findings suggest that GBM cells with stem cell properties are more invasive than their differentiated counterparts.
Studies on the proliferation of GBM cells in the yolk sac have provided somewhat conflicting results, as U87 cells generally do not proliferate in this area (Lal et al. 2012; Yang et al. 2014; Vittori et al. 2014) unless injected together with Matrigel (Yang et al. 2014). In contrast, a study assessing proliferation through bioluminescence showed the proliferation of U87 cells within the yolk sac (Zhao et al. 2009). Experiments on the cell line U251 also demonstrated measurable proliferation in this area (Lally et al. 2007; Geiger et al. 2008).
To study GBM-induced angiogenesis in zebrafish embryos, implantation into the perivitelline space—as has been suggested for other tumor types (Nicoli and Presta 2007)—may not be necessary, as remodeling of the vasculature has been observed in embryos in which U87 cells were implanted in the center of the yolk mass (Yang et al. 2013b). The implantation of U87 cells in the yolk resulted in better survival as compared with their implantation into the perivitelline space. Angiogenic activity of xenotransplanted U251 cells in the yolk mass has also been demonstrated (Geiger et al. 2008). This approach has been applied to study transforming growth factor-β (TGF-β) involvement in angiogenesis by pretreating U87 cells with TGF-β and implanting them in the yolk sac of 2 dpf embryos. Pretreatment resulted in enhanced xenograft-induced angiogenesis in the area of U87 cell implantation (Yang et al. 2013b).
Location Matters: Orthotopic Studies on Glioma
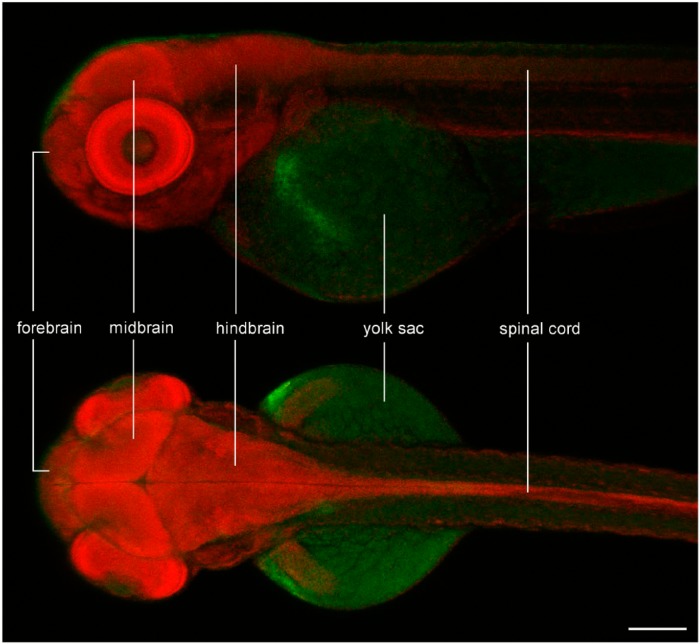
In zebrafish, the CNS begins to form as early as 9 hr after fertilization and, by 2 dpf, the brain, spinal cord, eyes and the inner ear are well formed. The CNS is a relatively large structure within the embryo’s body (Fig. 4), which speaks in favor of orthotopic xenotransplantation of GBM cells. The advantages and disadvantages of implanting cells in the embryonic brain are summarized in Table 1.
Figure 4.
Confocal image of methyl green staining in a zebrafish embryo at 3 days after fertilization, showing the relative size and basic anatomy of its central nervous system. The brain is one of the largest structures within the body of the embryo, facilitating orthotopic implantation of glioma cells. Scale, 200 µm.
Table 1.
Advantages and Disadvantages of the Yolk Sac or Brain for Glioma Cell Implantation.
| Yolk Sac |
Brain |
||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Studying angiogenic effects is straightforward | Yolk sac is degraded after a few days | Is a permanent structure | Studying angiogenic effects may be difficult |
| Survival and proliferation of glioma cells are limited | Glioma cells survive and proliferate better | ||
| Limited suitability for studying cell invasion | Suitable for studying cell invasion | ||
| Orthotopic implantation may give more relevant results | |||
Orthotopic implantation of glioma cells is advantageous over yolk sac implantation, as the CNS represents an environment that is similar to that of humans. In addition, orthotopic implantation can improve the survival and proliferation of glioma cells as compared to implantation into the yolk sac (Figs. 5, 6; Vittori et al. 2014). Human GBM cells have been successfully implanted into the embryonic brain at two days after fertilization (Kitambi et al. 2014; Vittori et al. 2014). However, most orthotopic studies have used older developmental stages, such as larvae and juveniles (Eden et al. 2014; Lal et al. 2012; Rampazzo et al. 2013). Lal et al. (2012) assessed the invasiveness of GBM cells implanted into the brain of either 4-day-old embryos or 10-day-old larval zebrafish. Implanted DiI-labeled U87 cells were shown to disperse in the larval brain, moving predominantly along the brain vasculature, which is also a major track for invading GBM cells in the human brain.
Figure 5.
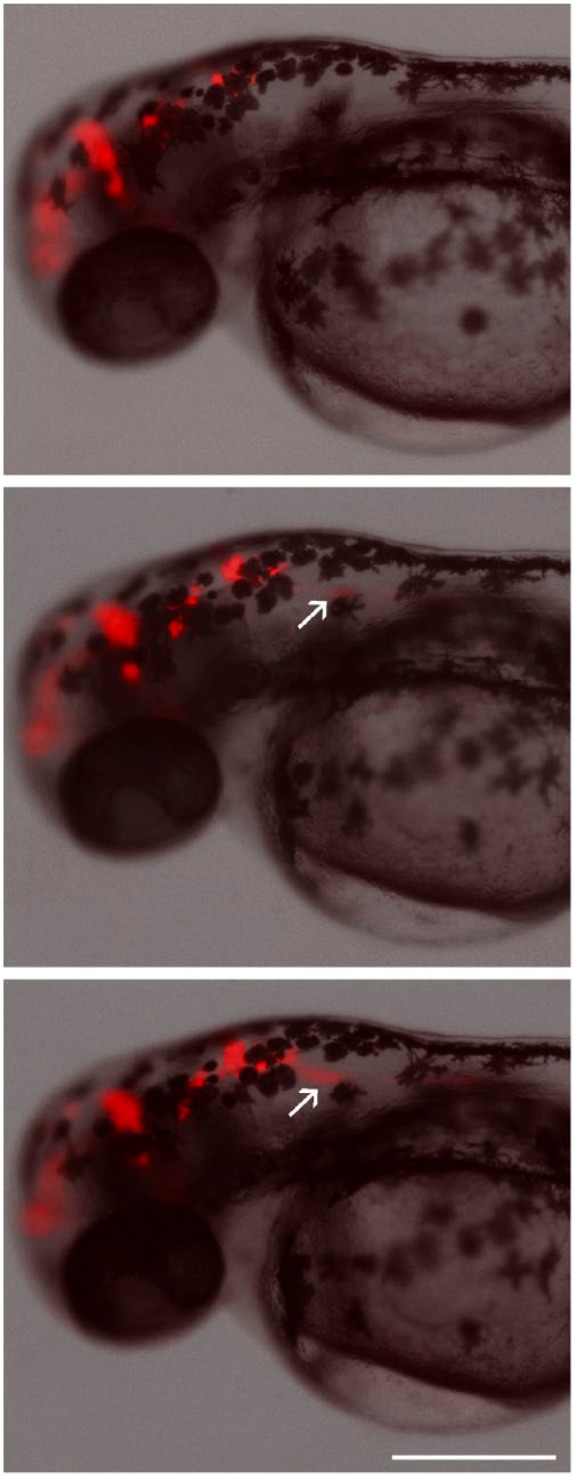
Time-lapse imaging of U87 DsRed glioblastoma cells implanted in the brain of a zebrafish embryo at 2 days after fertilization. Images were obtained at 4-hr intervals. Cells can be seen invading the spinal cord in the posterior direction (arrows). Scale, 300 µm.
Figure 6.
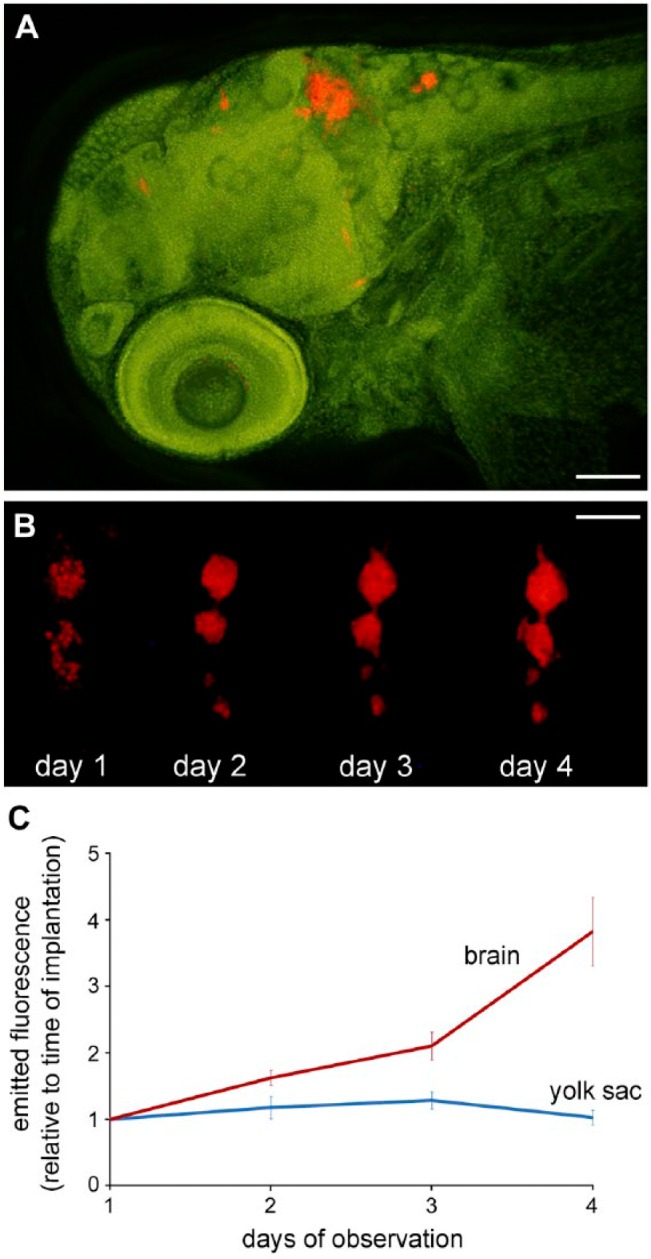
Orthotopic xenotransplantation of fluorescent glioma cells. (A) Fluorescent human glioblastoma cells (red) within the brain of a zebrafish embryo at 3 days after implantation. (B) Proliferation of implanted U-87 glioblastoma cells expressing the fluorescent protein DsRed within the brain of a zebrafish embryo over the course of several days. Day 1 marks the time point of cell implantation at 2 days after fertilization. (C) Comparison of the proliferation of implanted U-87 glioblastoma cells within the brain and within the yolk sac of zebrafish embryos. Approximately 100 DsRed-expressing cells were implanted into the brain or the yolk sac of zebrafish embryos at 2 days after fertilization, and the embryos were incubated at 31°C for 3 days. The fluorescence emitted by the cells was measured daily. Scale (A) 100 µm; (B) 250 µm.
Rampazzo et al. (2013) implanted patient-derived CD133+ glioma cells, obtained by cell sorting and transfected with the GFP gene, into zebrafish larvae at 7 dpf. In this study, the implanted CD133+ cells underwent cell cycle arrest, decreased the expression of stem cell markers and increased the expression of neuronal markers. It was suggested that the apparent loss of stemness is linked to Wnt-signaling. The activity of Wnt signaling was visualized in vivo by using transgenic zebrafish larvae expressing a fluorescent protein upon activation of a Wnt-associated transcription regulator. As demonstrated by Yang et al. (2013a), the percentage of CD133+ U87 cells was greatly reduced in the body of embryos at two days after implantation of a CD133+-enriched population of this cell line into the yolk sac. The mechanism of this reduction is not clear. Taken together, these results suggest that the zebrafish embryonal environment may promote differentiation of GSLCs, which would have great impact on the type of research that can be applied to GSLCs.
Bigger Fish to Fry: Studying Glioma in Juvenile Zebrafish
The xenotransplantation approach has also been applied to older zebrafish that are approximately 30 days old (Eden et al. 2014; Stoletov and Klemke 2008; Stoletov et al. 2007). The possible advantages and disadvantages of this approach are summarized in Table 2. It has been argued that the use of juveniles may provide more similarity to the native tumor environment. However, the application of xenotransplantation to older life stages comes at a cost. The brain is not as easily imaged by optical microscopy in juvenile and adult fish as in embryos and larvae, even in the Casper strain (White at al. 2008), making older life stages less suitable for in vivo imaging. Xenotransplantation of cancer cells into juvenile fish requires immunosuppression of the recipient fish through the delivery of dexamethasone (Eden et al. 2014; Stoletov and Klemke 2008; Tobia et al. 2013) or radiation (Taylor and Zon 2009; Zhang et al. 2014). However, this is not necessarily a disadvantage, as GBM patients are often exposed to these treatments as well.
Table 2.
Advantages and Disadvantages of Zebrafish Embryos or Juveniles in Xenotransplantation Studies of Brain Tumors.
| Embryos |
Juvenile Fish |
||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Transparency enables high-resolution microscopic observations | Experiments last only a few days | Longer-term experiments are possible | Reduced transparency limits the resolution of microscopic observations |
| No need for immunosuppression | Can accommodate only a few hundred mammalian cells | Can accommodate more mammalian cells | Xenotransplantation requires immunosuppression |
| No need for feeding | Organ systems are still developing | Most organ systems are mature | Require feeding |
| Blood-brain barrier is developing | Blood-brain barrier is functional | ||
In the study of Eden et al. (2014), xenografting of cells derived from mouse brain tumors (including GBM) was performed in 30-day-old zebrafish. The formation of secondary tumor nodules elsewhere in the brain was observed in these experiments, and tumor progression in zebrafish was found to recapitulate that of the parent tumor. In addition, the authors demonstrated similarities between the transcriptomes of zebrafish tumors and their corresponding mammalian tumors, which is an encouraging validation of the orthotopic xenografting approach in zebrafish.
Application of Zebrafish Studies to Drug Screening
Zebrafish embryos are an attractive model to study the effects of novel pharmaceuticals, since small molecules are taken up directly from the aqueous environment, making chemical delivery simple and non-invasive (Drabsch et al. 2013; Taylor and Zon 2009; Yang et al. 2014). The simultaneous treatment of numerous embryos with small amounts of chemicals enables a relatively high throughput of such studies at low cost. Zebrafish embryos and larvae can thus potentially bridge the gap between high-throughput in vitro screening and mammalian models, as promising chemical hits can first be validated in zebrafish and later studied in greater detail in mammals (Jung et al. 2012; Stern and Weinstein 2003).
The earliest application of zebrafish embryos to validate glioma-related cytotoxic drug screening served to identify potential small-molecule radiosensitizers. In this approach, drug candidates were identified by in vitro screening and the zebrafish embryo model was used to validate the in vitro findings in an in vivo model. The novel radiosensitizer NS-123, which increases the effects of radiation treatment on GBM cells in vitro, was validated in vivo by implanting U251 GBM cells in the yolk of blastula stage embryos (Lally et al. 2007). This test enabled the simultaneous evaluation of the potential toxic and teratogenic effects of the chemical. The study demonstrated that the compound effectively decreased the survival of GBM cells in the yolk sac following radiation treatment without toxic effects to the zebrafish embryos. Another validation of a therapeutic candidate in zebrafish was performed with the macropinocytosis-targeting compound Vacquinol-1, which is reported to selectively induce vacuolization and death of GBM cells (Kitambi et al. 2014). Zebrafish embryos at 2 dpf were orthotopically implanted with fluorescently labeled U3013 GBM cells and the tumor area was monitored for 10 days. The use of Islet1:GFP transgenic zebrafish (Higashijima et al. 2000) with a fluorescent CNS facilitated the visualization of implanted cancer cells in the CNS. In the study, several drug candidates were first tested for toxicity in zebrafish embryos, which helped to narrow down the candidates to a few compounds. Vacquinol-1 was then demonstrated to most effectively decrease the survival of GBM cells in the brain of zebrafish larvae without causing toxic effects.
The validity of zebrafish xenotransplantation in the search of novel therapeutics for the treatment of glioma has been shown in various studies. The antiangiogenic effects of Axitinib, Suntinib and Vatalanib were tested in zebrafish with U87 GBM cells implanted in the yolk sac. These chemicals all inhibited tumor-induced vessel formation (Yang et al. 2014). This study also demonstrated that the novel anti-tumor compound Nordy inhibited angiogenesis and increased the antiangiogenic effects of Vatalanib. The radiosensitizing effects of temozolomide on U251 GBM cells implanted in the yolk sac of blastula stage embryos were also confirmed. In addition, temozolomide in concentrations that increased the toxicity of radiation to GBM cells did not have a negative effect on embryo development (Geiger et al. 2008). In a study on juvenile zebrafish at 30 dpf, the effects of two chemotherapeutics on the survival and proliferation of implanted cancer cells were assessed: tyrosine kinase inhibitor erlotinib and the pyrimidine analog 5-fluorouracil (5-FU). In juveniles, 5-FU was administered by dissolving it in the fish maintenance water, whereas erlotinib was administered orally because of its low solubility (Eden et al. 2014). Quantification of cancer cell fluorescence demonstrated that 5-FU and erlotinib inhibited GBM cell proliferation in vivo. Taken together, these studies demonstrate that the zebrafish embryo model is a good model for drug screening and preclinical validation.
Issues, Prospects and Conclusions
The zebrafish xenotransplantation cancer model is emerging from its infancy and holds great promise for the visualization of tumor progression and high-throughput therapeutic compound validation. In this regard, the model fits between in vitro cell studies and in vivo animal studies (Eden et al. 2014). It offers intermediate throughput, being more time and labor consuming than in vitro experiments, yet faster and more ethical than animal experimentation.
The strength of the model lies in its potential for single-cell visualization and the ease of genetic manipulation; the transgenic arsenal available for zebrafish has likely not been fully exploited yet. The use of zebrafish with a fluorescent vasculature is becoming routine in xenotransplantation experiments (Geiger et al. 2008; Lee et al. 2009; Stoletov et al. 2007; Yang et al. 2014), whereas other structures, such as the CNS (Higashijima et al. 2000; Zhang and Gong 2013) can also express fluorescent proteins, enabling high-resolution imaging of implanted cells and their microenvironment in vivo. In the future, several genetically coded fluorescent labels may be used simultaneously to highlight different structures interacting with the engrafted cells.
Zebrafish embryos are ideal for the use of fluorescent reporter genes. For example, the generation of transgenic zebrafish expressing three different molecular markers that are characteristic for different levels of muscle cell differentiation linked to three different fluorescent proteins provided unprecedented insights into the progression of rhabdomyosarcoma induced by KRAS overexpression (Ignatius et al. 2012). This methodology can also be applied to xenotransplants, enabling visualization of gene expression patterns during cancer progression at single-cell resolution. Indeed, the combination of the visualization of implanted cells with the expression of fluorescent reporter genes has been successfully applied to study glioma xenografts (Rampazzo et al. 2013), showing that this approach has great potential.
Another possibility for future developments of the zebrafish model is to study cancer cell heterogeneity (Blackburn and Langenau 2014). For example, two cancer cell lines expressing different fluorescent proteins have been implanted simultaneously in a single embryo in order to demonstrate the difference in their invasive potential (Marques et al. 2009). In another study, wild type cells and cells in which the expression of certain genes was upregulated were labeled with different fluorescent proteins and simultaneously implanted into zebrafish (Stoletov et al. 2010). This approach not only enables the simultaneous visualization of the behavior of several cell types, but also holds potential for the analysis of cell–cell interactions in tumor progression.
Steps have been taken to increase the throughput of zebrafish xenotransplantation experiments (Snaar-Jagalska 2009), leading to a proposal of automated approaches to cell implantation and imaging that focus predominantly on the quantification of cell invasion from the yolk sac (Ghotra et al. 2012). The study of this process can be primarily applied to the screening and validation of anti-angiogenic and anti-metastatic drugs. Nevertheless, ethical considerations with respect to the use of embryos in large-scale screens should not be ignored entirely.
Acknowledgments
The authors wish to thank Matjaž Novak, Dr. David Dobnik (National Institute of Biology, Ljubljana) and Marchien Dallinga (Academic Medical Center, Amsterdam) for their assistance.
Footnotes
Declaration of Competing Interests: The authors declared no potential competing interests with respect to the research, authorship, and/or publication of this article.
Author Contributions: MV, HM and TLT all contributed to literature overview and writing of the manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Slovenian Research Agency Program P1-0245; INTERREG EC Project 2011, Ref. No. 42: Identification of novel biomarkers to brain tumors - glioma, for diagnosis and as new therapeutic targets, Acronym: GLIOMA.
References
- Armatruda JF, Shepard JL, Stern HM, Zon LI. (2002). Zebrafish as a cancer model system. Cancer Cell 1: 229-231. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon R, Hao Y, Shi Q, Hjelmeland A, Dewhirst M, Bignern D, Rich J. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756-760. [DOI] [PubMed] [Google Scholar]
- Behin A, Hoang-Xuan K, Carpentier A, Delattre J-Y. (2003). Primary brain tumours in adults. Lancet 361:323-331. [DOI] [PubMed] [Google Scholar]
- Blackburn J, Langenau D. (2014). Zebrafish as a model to assess cancer heterogeneity, progression and relapse. Dis Model Mech 7:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brösamle C, Halpern M. (2002). Characterization of myelination in the developing zebrafish. Glia 39:47-57. [DOI] [PubMed] [Google Scholar]
- Campos B, Herold-Mende CC. (2011). Insight into the complex regulation of CD133 in glioma. Int J Cancer 128:501-510. [DOI] [PubMed] [Google Scholar]
- Claes A, Idema A, Wesseling P. (2007). Diffuse glioma growth: a guerilla war. Acta Neuropathol 114:443-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkery D, Dellaire G, Berman J. (2011). Leukaemia xenotransplantation in zebrafish – chemotherapy response assay in vivo. Br J Haematol 153:786-789. [DOI] [PubMed] [Google Scholar]
- Distel M, Wullimann M, Köster R. (2009). Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc Natl Acad Sci USA 106:13365-13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabsch Y, He S, Zhang L, Snaar-Jagalska B, Dijke P. (2013). Transforming growth factor beta signalling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res 15:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden C, Ju B, Murugesan M, Phoenix T, Nimmervoll B, Tong Y, Ellison D, Finkelstein D, Wright K, Boulos N, Dapper J, Thiruvenkatam R, Lessman C, Taylor M, Gilbertson R. (2014). Orthotopic models of pediatric brain tumors in zebrafish. Oncogene 34:1736-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguiara A, Holgado O, Beloqui I, Abalde L, Sanchez Y, Callol C, Martin A. (2011). Xenografts in zebrafish embryos as a rapid functional assay for breast cancer stem-like cell identification. Cell Cycle 10:3751-3757. [DOI] [PubMed] [Google Scholar]
- Feitsma H, Cuppen E. (2008). Zebrafish as a cancer model. Mol Cancer Res 6:685-694. [DOI] [PubMed] [Google Scholar]
- Fleming A, Diekmann H, Goldsmith PF. (2013). Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS One 8:e77548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger G, Fu W, Kao G. (2008). Temozolomide-mediated radiosensitization of human glioma cells in a zebrafish embryonic system. Cancer Res 68:3396-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghotra V, He S, Bont H, Ent W, Spaink H, Water B, Snaar-Jagalska B, Danen E. (2012). Automated whole animal bio-imaging assay for human cancer dissemination. PloS One 7:e31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. (2002). Headwaters of the zebrafish – emergence of a new model vertebrate. Nat Rev Genet 3:717-724. [DOI] [PubMed] [Google Scholar]
- Haldi M, Ton C, Seng W, McGrath P. (2006). Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 9:139-151. [DOI] [PubMed] [Google Scholar]
- He S, Lamers G, Beenakker J, Cui C, Ghotra V, Danen E, Meijer A, Spaink H, Snaar-Jagalska B. (2012). Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J Pathol 227:431-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M, Jesuthasan S. (2007). Electroporation-based methods for in vivo, whole mount and primary culture analysis of zebrafish brain development. Neural Dev 2:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. (2000). Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the Islet-1 promoter/enhancer. J Neurosci 20:206-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hira VVV, Ploegmakers KJ, Grevers F, Verbovšek U, Silvestre-Roig C, Aronica E, Tigchelaar W, Turnsek TL, Molenaar RJ, Van Noorden CJF. (2015). CD133+ and nestin+ glioma stem-like cells reside around CD31+ arterioles in niches that express SDF-1α, CXCR4, osteopontin and cathepsin K. J Histochem Cytochem 63:481-493. [DOI] [PubMed] [Google Scholar]
- Hwang W, Fu Y, Reyon D, Maeder M, Tsai S, Sander J, Peterson R, Yeh J-R, Joung J. (2013a). Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31:227-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W, Fu Y, Reyon D, Maeder M, Kaini P, Sander J, Joung J, Peterson R, Yeh JR. (2013b). Heritable and precise zebrafish genome editing using a CRISPR-Cas system. PLoS One 8:e68708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius M, Chen E, Elpek N, Fuller A, Tenente I, Clagg R, Liu S, Blackburn J, Linardic C, Rosenberg A, Nielsen P, Mempel T, Langenau D. (2012). In vivo imaging of tumor-propagating cells, regional tumor heterogeneity, and dynamic cell movements in embryonal rhabdomyosarcoma. Cancer Cell 21:680-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung DW, Oh ES, Park SH, Chang YT, Kim CH, Choi SY, Williams D. (2012). A novel zebrafish human tumor xenograft model validated for anti-cancer drug screening. Mol Biosyst 8:1930-1939. [DOI] [PubMed] [Google Scholar]
- Jung I, Leem G, Jung D, Kim M, Kim E, Kim S, Park HC, Park S. (2013). Glioma is formed by active Akt1 alone and promoted by active Rac1 in transgenic zebrafish. Neuro Oncol 15:290-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari G, Rodeck U, Dicker A. (2007). Zebrafish: An emerging model system for human disease and drug discovery. Clin Pharmacol Ther 82:70-80. [DOI] [PubMed] [Google Scholar]
- Keller P, Schmidt A, Wittbrodt J, Stelzer E. (2008). Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322:1065-1069. [DOI] [PubMed] [Google Scholar]
- Kettleborough R, Busch-Nentwich E, Harvey S, Dooley C, Bruijn E, Eeden F, Sealy I, White R, Herd C, Nijman I, Fényes F, Mehroke S, Scahill C, Gibbons R, Wali N, Carruthers S, Hall A, Yen J, Cuppen E, Stemple D. (2013). A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496:494-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. (1995). Stages of embryonic development of the zebrafish. Dev Dyn 203:253-310. [DOI] [PubMed] [Google Scholar]
- Kitambi S, Toledo E, Usoskin D, Wee S, Harisankar A, Svensson R, Sigmundsson K, Kalderén C, Niklasson M, Kundu S, Aranda S, Westermark B, Uhrbom L, Andäng M, Damberg P, Nelander S, Arenas E, Artursson P, Walfridsson J, Nilsson K, Hammarström L, Ernfors P. (2014). Vulnerability of glioblastoma cells to catastrophic vacuolization and death induced by a small molecule. Cell 157:313-328. [DOI] [PubMed] [Google Scholar]
- Klerk CPW, Overmeer RM, Niers TMH, Versteeg HH, Richel DJ, Buckle T, Van Noorden CJF, van Tellingen O. (2007). Validity of bioluminescence measurements for noninvasive in vivo imaging of tumor load in small animals. BioTechniques 43:S7-S13, S30. [DOI] [PubMed] [Google Scholar]
- Kobitski A, Otte J, Takamiya M, Schäfer B, Mertes J, Stegmaier J, Rastegar S, Rindone F, Hartmann V, Stotzka R, García A, Wezel J, Mikut R, Strähle U, Nienhaus G. (2015). An ensemble-averaged, cell density-based digital model of zebrafish embryo development derived from light-sheet microscopy data with single-cell resolution. Sci Rep 5:8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konantz M, Balci T, Hartwig U, Dellaire G, André M, Berman J, Lengerke C. (2012). Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann N Y Acad Sci 1266:124-137. [DOI] [PubMed] [Google Scholar]
- Lal S, Du J, Tanguay R, Greenwood J. (2012). Calpain 2 is required for the invasion of glioblastoma cells in the zebrafish brain microenvironment. J Neurosci Res 90:769-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally B, Geiger G, Kridel S, Arcury-Quandt A, Robbins M, Kock N, Wheeler K, Peddi P, Georgakilas A, Kao G, Koumenis C. (2007). Identification and biological evaluation of a novel and potent small molecule radiation sensitizer via an unbiased screen of a chemical library. Cancer Res 67:8791-8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S., Chua H., Gong Z, Lam T., Sin Y. (2004). Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol 28:9-28. [DOI] [PubMed] [Google Scholar]
- Lam S, Wu Y, Vega V, Miller L, Spitsbergen J, Tong Y, Zhan H, Govindarajan K, Lee S, Mathavan S, Murthy K, Buhler D, Liu E, Gong Z. (2006). Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol 24:73-75. [DOI] [PubMed] [Google Scholar]
- Langenau D, Traver D, Ferrando A, Kutok J, Aster J, Kanki J, Lin S, Prochownik E, Trede N, Zon L, Look A. (2003). Myc-induced T cell leukemia in transgenic zebrafish. Science 299:887-890. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. (2002). In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248:307-318. [DOI] [PubMed] [Google Scholar]
- Lawson N, Wolfe S. (2011). Forward and Reverse Genetic Approaches for the Analysis of Vertebrate Development in the Zebrafish. Dev Cell 21:48-64. [DOI] [PubMed] [Google Scholar]
- Lee LMJ, Seftor EA, Bonde G, Cornell RA, Hendrix MJC. (2005). The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn 233:1560-1570. [DOI] [PubMed] [Google Scholar]
- Lee S, Rouhi P, Jensen L, Zhang D, Ji H, Hauptmann G, Ingham P, Cao Y. (2009). Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci U S A 106:19485-19490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Gen 2007:8:353-367. [DOI] [PubMed] [Google Scholar]
- Marques I, Weiss F, Vlecken D, Nitsche C, Bakkers J, Lagendijk A, Partecke L, Heidecke C-D, Lerch M, Bagowski C. (2009). Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra S. (2013). Emergence of zebrafish models in oncology for validating novel anticancer drug targets and nanomaterials. Drug Discov Today 18:128-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mione M, Trede N. (2010). The zebrafish as a model for cancer. Dis Model Mech 3:517-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina ES, Pillat MM, Moura-Neto V, Lah TT, Ulrich H. (2014). Glioblastoma stem-like cells: approaches for isolation and characterization. J Cancer Stem Cell Res 2:e1007. [Google Scholar]
- Nicoli S, Presta M. (2007). The zebrafish/tumor xenograft angiogenesis assay. Nat Protoc 2:2918-2923. [DOI] [PubMed] [Google Scholar]
- Nicoli S, Ribatti D, Cotelli F, Presta M. (2007). Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res 67:2927-2931. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. (2013). The definition of primary and secondary glioblastoma. Clin Cancer Res 19:764-772. [DOI] [PubMed] [Google Scholar]
- Padilla PA, Roth MB. (2001). Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc Natl Acad Sci USA 98:7331-7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persano L, Rampazzo E, Basso G, Viola G. (2013). Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem Pharmacol 85:612-622. [DOI] [PubMed] [Google Scholar]
- Pruvot B, Jacquel A, Droin N, Auberger P, Bouscary D, Tamburini J, Muller M, Fontenay M, Chluba J, Solary E. (2011). Leukemic cell xenograft in zebrafish embryo for investigating drug efficacy. Haematologica 96:612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampazzo E, Persano L, Pistollato F, Moro E, Frasson C, Porazzi P, Puppa A, Bresolin S, Battilana G, Indraccolo S, Kronnie G, Argenton F, Tiso N, Basso G. (2013). Wnt activation promotes neuronal differentiation of glioblastoma. Cell Death Dis 4:e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhi P, Jensen L, Cao Z, Hosaka K, Länne T, Wahlberg E, Steffensen J, Cao Y. (2010). Hypoxia-induced metastasis model in embryonic zebrafish. Nat Protoc 5:1911-1918. [DOI] [PubMed] [Google Scholar]
- Sabaawy H, Azuma M, Embree L, Tsai H-J, Starost M, Hickstein D. (2006). TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 103:15166-15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra R, Bhogal N. (2010). In vivo research using early life stage models. In Vivo 24:457-462. [PubMed] [Google Scholar]
- Snaar-Jagalska BE. (2009). ZF-CANCER: developing high-throughput bioassays for human cancers in zebrafish. Zebrafish 6:441-443. [DOI] [PubMed] [Google Scholar]
- Stern HM, Zon LI. (2003). Cancer genetics and drug discovery in the zebrafish. Nat Rev Cancer 3:1-7. [DOI] [PubMed] [Google Scholar]
- Stoletov K, Montel V, Lester R, Gonias S, Klemke R. (2007). High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proc Natl Acad Sci U S A 104:17406-17411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoletov K, Klemke R. (2008). Catch of the day: zebrafish as a human cancer model. Oncogene 27:4509-4520. [DOI] [PubMed] [Google Scholar]
- Stoletov K, Kato H, Zardouzian E, Kelber J, Yang J, Shattil S, Klemke R. (2010). Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci 123:2332-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai G, Weller M. (2011). Glioblastoma stem cells. Cell Tissue Res 343:459-465. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Zon LI. (2009). Zebrafish tumor assays: the state of transplantation. Zebrafish 6:339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Xie X, Wlaker S, White DT, Mumm JS, Cowel JK. (2013). Evaluating human cancer cell metastasis in zebrafish. BMC Cancer 13:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobia C, Gariano G, Sena G, Presta M. (2013). Zebrafish embryo as a tool to study tumor/endothelial cell cross-talk. Biochim Biophys Acta 1832:1371-1377. [DOI] [PubMed] [Google Scholar]
- Tobia C, Sena G, Presta M. (2011). Zebrafish embryo, a tool to study tumor angiogenesis. Int J Dev Biol 55:505-509. [DOI] [PubMed] [Google Scholar]
- Veinotte C, Dellaire G, Berman J. (2014). Hooking the big one: the potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Dis Model Mech 7:745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Gaudenzi G, Dicitore A, Cotelli F, Ferone D, Persani L. (2014). Zebrafish as an innovative model for neuroendocrine tumors. Endocr Relat Cancer 21:R67-R83. [DOI] [PubMed] [Google Scholar]
- Vittori M, Podergajs N, Motaln H, Lah TT. (2014). Orthotopic xenotransplantation of human cancer cells into zebrafish embryos for the in vivo study of glioblastoma. In: Heart of Europe Zebrafish Meeting, September 17-19, 2014 Warsaw: International Institute of Molecular and Cell Biology: p. 65. [Google Scholar]
- Wang J, Wakeman T, Lathia J, Hjelmeland A, Wang X-F, White R, Rich J, Sullenger B. (2010). Notch promotes radioresistance of glioma stem cells. Stem Cells 28:17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wakeman T, Lathia J, Hjelmeland A, Wang X-F, White R, Rich J, Sullenger B. (2008). Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2:183-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Rose K, Zon L. (2013). Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer 13:624-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett C, Cortes A, Zuasti A, Zapata A. (1999). Early hematopoiesis and developing lymphoid organs in the zebrafish. Dev Dyn 214:323-336. [DOI] [PubMed] [Google Scholar]
- Yang M, Baranov E, Li XM, Wang JW, Jiang P, Li L, Moossa AR, Penman S, Hoffman M. (2001). Whole-body and intravital optical imaging of angiogenesis in orthotopically implanted tumors. Proc Natl Acad Sci U S A 98:2616-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen G, Yu S, Xu C, Xin Y, Li T, Shi Y, Gu A, Duan J, Qian C, Cui Y, Zhang X, Bian X. (2013b). TGF-β1 enhances tumor-induced angiogenesis via JNK pathway and macrophage infiltration in an improved zebrafish embryo/xenograft glioma model. Int Immunopharmacol 15:191-198. [DOI] [PubMed] [Google Scholar]
- Yang X, Cui W, Gu A, Xu C, Yu S, Li T, Cui Y, Zhang X, Bian X. (2013a). A novel zebrafish xenotransplantation model for study of glioma stem cell invasion. PLoS One 8:e61801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Cui W, Yu S, Xu C, Chen G, Gu A, Li T, Cui Y, Zhang X, Bian X. (2014). A synthetic dl-nordihydroguaiaretic acid (Nordy), inhibits angiogenesis, invasion and proliferation of glioma stem cells within a zebrafish xenotransplantation model. PLoS One 9:e85759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Shimada Y, Kuroyanagi J, Nishimura Y, Umemoto N, Nomoto T, Shintou T, Miyazaki T, Tanaka T. (2014). Zebrafish xenotransplantation model for cancer stem-like cell study and high-throughput screening of inhibitors. Tumor Biol 35:11861-11869. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gong Z. (2013). Fluorescent transgenic zebrafish Tg(nkx2.2a:mEGFP) provides a highly sensitive monitoring tool for neurotoxins. PLoS One 8:e55474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Wang X, Zhao Y, Li Z, Lin S, Wei Y, Yang H. (2011a). A novel xenograft model in zebrafish for high-resolution investigating dynamics of neovascularization in tumors. PloS One 6:e21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Yang H, Shi H, Wang X, Chen X, Yuan Y, Lin S, Wei Y. (2011b). Distinct contributions of angiogenesis and vascular co-option during the initiation of primary microtumors and micrometastases. Carcinogenesis 32:1143-1150. [DOI] [PubMed] [Google Scholar]
- Zhao H, Tang C, Cui K, Ang B-T, Wong S. (2009). A screening platform for glioma growth and invasion using bioluminescence imaging. J Neurosurg 111:238-246. [DOI] [PubMed] [Google Scholar]
- Zheng W, Li Z, Nguyen A, Li C, Emelyanov A, Gong Z. (2014). Xmrk, Kras and Myc transgenic zebrafish liver cancer models share molecular signatures with subsets of human hepatocellular carcinoma. PLoS One 9:e91179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon LI, Peterson RT. (2005). In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 4:35-44. [DOI] [PubMed] [Google Scholar]
- Zu Y, Tong X, Wang Z, Liu D, Pan R, Li Z, Hu Y, Luo Z, Huang P, Wu Q, Zhu Z, Zhang B, Lin S. (2013). TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat Methods 10:329-331. [DOI] [PubMed] [Google Scholar]