Summary
Development of an effective AIDS vaccine is a global priority. However, the extreme diversity of human immunodeficiency virus type 1 (HIV-1), which is a consequence of its propensity to mutate to escape immune responses, along with host factors that prevent the elicitation of protective immune responses, continue to hinder vaccine development. Breakthroughs in understanding of the biology of the transmitted virus, the structure and nature of its envelope trimer, vaccine-induced CD8 T cell control in primates, and host control of broadly neutralizing antibody elicitation have given rise to new vaccine strategies. Despite this promise, emerging data from preclinical trials reinforce the need for gaining additional insight into virus – host biology in order to facilitate the development of a successful vaccine.
Introduction
After over 30 years of intense research, an effective human immunodeficiency virus type 1 (HIV-1) vaccine remains elusive. Vaccines for non-integrating viruses such as influenza, smallpox and measles elicit robust B cell neutralizing antibody and T cell responses that provide protection from clinical disease (Plotkin, 2010). For HIV-1, passive transfer of potent broadly neutralizing antibodies (bnAbs) to rhesus macaques confers sterilizing protection from simian-immunodeficiency virus (SHIV) challenge (Barouch et al., 2015; Barouch et al., 2013). Similarly, adenovirus vectored immunogens confer partial protection from simian immunodeficiency virus (SIV) infection (Moldt et al., 2012), while attenuated rhesus cytomegalovirus (RhCMV) vectored immunogens induce CD8+ T cell responses that clear SIV infection in over half of vaccinated animals (Hansen et al., 2011; Hansen et al., 2013a). Despite these early promising findings, only one of five HIV-1 vaccine efficacy trials in humans, the RV144 vaccine trial in Thailand testing the ALVAC/gp120 B/E vaccine, has shown any protection from transmission, with an estimated vaccine efficacy of 31.2% (Rerks-Ngarm et al., 2009). An RV144 immune correlates analysis raised the hypothesis that reduced transmission risk in this trial was mediated by Env second variable loop (V2)-directed ADCC antibodies (Haynes et al., 2012a). The marginal success of these preclinical and clinical vaccine trials highlights the complexity of host immune responses to HIV-1 and the challenges associated with developing a vaccine that can elicit protective B or T cell responses. This review highlights some of these challenges and potential avenues to overcome them.
The Transmitted Founder Virus
The majority (~70%) of HIV-1 infections worldwide result from heterosexual contact, which in the absence of confounding risk factors (e.g., genital ulceration), is generally an inefficient process. This is reflected in a mucosal bottleneck that reduces the genetic diversity of the HIV-1 quasispecies in the transmitting donor to only one or very few variants that seed the recipient (Joseph et al., 2015; Shaw and Hunter, 2012). This virus population bottleneck is likely due to both stochastic and selective forces in the mucosal tissues that act during the transmission process where virus is most vulnerable to elimination.
Understanding the viral and host factors that contribute to the mucosal bottleneck may inform vaccine design. One approach to dissect transmission barriers is to study the genotype and phenotype of viruses that establish new infections. Humans cannot be sampled at the moment of transmission, but by analyzing plasma viral sequences from acutely infected individuals, it is possible to infer the genomes of the viruses that had initiated productive infection weeks earlier (Keele et al., 2008). In the absence of adaptive immune responses, HIV-1 diversifies in an essentially random fashion. As a consequence, viral sequences that evolve from individual transmitted founder (TF) viruses exhibit a Poisson distribution of mutations and a star-like phylogeny that coalesces to an inferred consensus sequence at or near the time of transmission (Keele et al., 2008). Using single template amplification, which generates HIV-1 sequences devoid of PCR artifacts, it was shown that in ~80% of heterosexual transmission cases, a single virus was responsible for establishing the new infection (Keele et al., 2008). The same approach revealed that ~40% of men who have sex with men (MSM) and ~60% of intravenous drug users (IVDU) acquired two or more variants that led to productive infection. The higher multiplicity of HIV-1 infection observed in MSM and IVDU is consistent with a higher epidemiological risk of virus acquisition and suggests a greater challenge for HIV-1 vaccines.
Many factors influence whether virus exposure at mucosal surfaces leads to productive infection, including the virus load in the infecting partner, the integrity of the mucosa, target cell availability in mucosal and submucosal tissues, immune activation, genital inflammation, and altered mucosal microbiota (Joseph et al., 2015). In addition, there is increasing evidence that the transmission process selects for viruses with enhanced transmission fitness (Figure 1). Comparing Gag, Pol and Nef protein sequences from 137 heterosexual transmission pairs, Carlson and colleagues found that viruses with amino acid residues matching the consensus sequence of the study population were preferentially transmitted (Carlson et al., 2014). This finding is consistent with previous observations that most within-host diversification reduces transmission fitness and represents an evolutionary dead-end at a population level (Fraser et al., 2014; Pybus and Rambaut, 2009). The observed selection was more stringent in female-to-male than male-to-female transmissions, and was mitigated by factors that elevate transmission risk such as higher donor viral loads, genital ulcer disease and genital tract inflammation (Carlson et al., 2014).
Figure 1. Model of the HIV-1 transmission bottleneck.
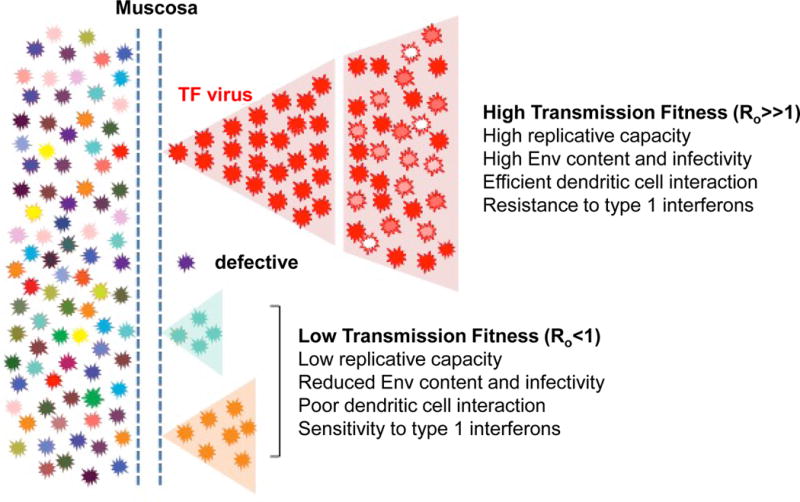
Mucosal transmission reduces the genetic and phenotypic diversity of the donor HIV-1 quasi-species to only one or very few variants that seed infection in the recipient. Viruses that traverse the mucosa, but are defective or fail to initiate a productive infection (i.e., have a basic reproductive ratio Ro of lower than 1), will be extinguished. In contrast, the mucosal bottleneck selects for viruses with a high transmission fitness. Although the biological properties that comprise this phenotype remain to be fully elucidated, a high replicative capacity, increased infectivity, enhanced dendritic cell interaction, and greater resistance to the antiviral effects of type 1 interferons (IFNs) are likely to contribute (Parrish et al., 2013).
Studies in experimentally infected macaques and human mucosal explant cultures have shown that HIV-1 is first intercepted by intraepithelial Langerhans or mucosal dendritic cells, which pass the transmitted viruses on to resident CD4+ T cells without themselves becoming productively infected (Joseph et al., 2015; Shaw and Hunter, 2012). In the macaque model, the resident CD4+ T cells exhibit a “resting” phenotype, characterized by the lack of classical activation markers and low-level CCR5 expression. Macrophages, which are also present, do not play a significant role. Consistent with these findings, TF viruses almost exclusively use CD4 and CCR5 as their receptor and co-receptor, and replicate efficiently in activated CD4+ T cells, but not macrophages. Moreover, analysis of a large panel of full-length infectious molecular clones demonstrated that TF viruses are twice as infectious and contain twice as much envelope glycoprotein (Env) compared to viruses that predominate during chronic infection (Parrish et al., 2013). TF viruses are also captured twice as efficiently by monocyte-derived dendritic cells and are more readily transferred to CD4+ T cells than chronic viruses. Thus, higher Env content, increased cell-free infectivity and improved dendritic cell interaction likely contribute to the ability of the TF virus to gain a foothold in the new host (Figure 1).
Given that Envs from TF viruses frequently encode shorter variable loops and contain fewer glycosylation sites than Envs from chronically infected individuals, the question arose whether TF viruses might be more neutralization sensitive (Derdeyn et al., 2004; Ping et al., 2013). One early study of heterosexual transmission reported that Envs from acutely infected recipients were uniquely sensitive to neutralization by plasma from their respective donors, although this was not observed for plasma from other infected individuals (Derdeyn et al., 2004). However, subsequent studies of large numbers of TF Envs showed that they were invariantly resistant to heterologous neutralization (Keele et al., 2008; Ping et al., 2013). Dissection of the antibody response in chronically infected individuals provided a plausible explanation (Moody et al., 2015): Common CD4 binding site (CDbs) and third variable (V3) loop directed monoclonal antibodies, which were unable to neutralize heterologous tier 2 (difficult-to-neutralize) viruses, were nonetheless capable of neutralizing autologous tier 2 viruses and selected for viral escape mutations (Moody et al., 2015). These results indicated that the autologous neutralization potently constrains the native Env trimer to a conformationally closed state, thus explaining the neutralization-resistant phenotype of TF viruses.
Although innate immune activation can promote viral replication, there is increasing evidence that innate responses activated early on mediate potent antiviral activity that contributes to the HIV-1 transmission bottleneck. Studies in macaques have shown that plasmacytoid dendritic cells recruited to the site of virus entry (Li et al., 2009) secrete cytokines and chemokines, including type I interferons (IFNs). This induces a rapid innate immune response through the upregulation of interferon stimulated genes (ISGs), many of which have potent anti-HIV-1 activity (Doyle et al., 2015). Furthermore, treatment of rhesus macaques with IFNα2 increased the number of intrarectal challenges required for systemic SIVmac infection and decreased the number of TF viruses (Sandler et al., 2014), indicating that increased levels of type 1 IFNs in the mucosa at the initial sites of viral replication has a substantial protective effect. Consistent with this, HIV-1 TF viruses are commonly less sensitive to inhibition by type 1 IFNs than viruses that predominate during chronic infection (Fenton-May et al., 2013; Parrish et al., 2013), but IFN resistance decreases rapidly over time in part as a result of immune escape mutations (Fenton-May et al., 2013). These results suggest that type 1 IFNs exert considerable selective pressure on the transmitted virus pool, (Figure 1). A careful dissection of the innate immune mechanisms that both combat and aid HIV-1 replication at the site of entry may help guide rational vaccine design.
Virus-Neutralizing Antibody Co-evolution
Single genome sequencing has been used to fine-map the evolution of TF viruses in prospectively followed individuals by sequencing their plasma virus over the course of infection. Within a few months of infection (Keele et al., 2008), TF viruses are almost completely replaced by viruses differing at several highly selected genomic loci, initially predominantly mediated by CTL-driven immune escape (Goonetelike et al. 2009b). Extending this approach into longitudinal studies spanning years, we and others have mapped HIV-1 diversification in several individuals who developed broad and potent neutralizing antibodies, revealing that a virus-antibody “arms race” ensues in which the HIV-1 TF Env induces autologous neutralizing antibodies that can neutralize the TF virus and drive selection of virus escape mutants (Figure 2) (Doria-Rose et al., 2014; Liao et al., 2013). This process is repeated throughout virus evolution, leading to the induction of neutralizing antibodies with varying degrees of cross-reactive breadth, such that sera from 50% of HIV-1-infected individuals can neutralize ~50% of primary viruses, and ~15–20% of HIV-1 infected individuals have potent bnAbs with extensive breadth (Hraber et al., 2014). While some degree of breadth in bnAb development is common, bnAbs with a high degree of potency and breadth require prolonged development, taking ~2–4 years (Burton and Mascola, 2015). Study of HIV-1-infected individuals have provided a rich source for isolation of a large number of bnAbs (Burton and Mascola, 2015; Mascola and Haynes, 2013), many of which are now being developed for passive administration to prevent and treat HIV-1 infection (Burton and Mascola, 2015). Identification of recurrent antibody targets suggest there are five main targets for bnAbs on the HIV-1 envelope trimer: the CD4 binding site (bs), the V3- and V1V2-glycan sites, gp120-gp41 bridging bnAbs, and the gp41 membrane proximal external region (MPER) (Figure 3) (Burton and Mascola, 2015; Klein et al., 2013; West et al., 2014). Phase I clinical trials have been completed with CD4 binding site bnAbs 3BNC117 (Caskey et al., 2015) and VRC01 (Ledgerwood et al., 2015) with the goal of developing them for both preventative and therapeutic uses.
Figure 2.
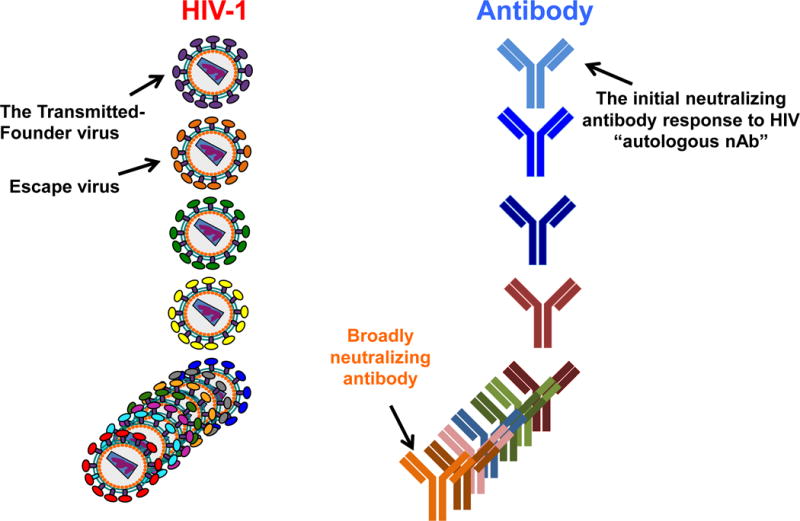
Co-evolution of HIV transmitted-founder virus and evolving neutralizing antibodies. The initial transmission event of sexually transmitted HIV-1 is mediated by one transmitted founder (TF) virus. The TF virus induces an initial antibody response, called the autologous neutralizing antibody, that is specific for the TF virus. The autologous neutralizing antibody neutralizes the TF but rapidly selects virus escape mutants, which in turn induces new antibody specifities. This process is repeated throughout virus evolution such that after years of infection, a spectrum of cross-reactive neutralizing antibodies are induced, with ~20% of chronically infected individuals making high levels of very broadly reactive neutralizing antibodies.
Figure 3.
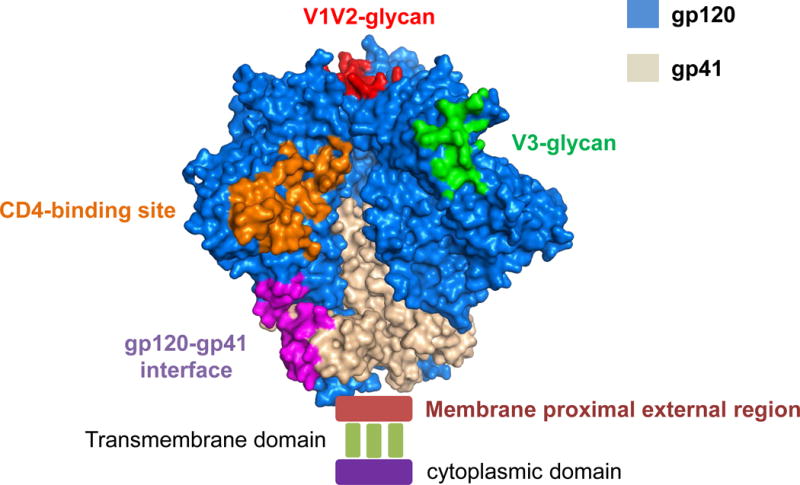
HIV-1 trimer and Broadly Neutralizing Antibody Binding Sites. Co-crystal structure of the HIV-1 trimer (Pancera et al. 2014) with gp120 in blue and gp41 in grey. The five areas targeted by broadly neutralizing antibodies are the CD4 binding site (orange), V1V2 glycans (red), V3 glycans (green), gp120-gp41 bridging site (purple) and the membrane proximal external region (dark red). The area of insertion of the envelope trimer into the membrane is noted by the transmembrane domain and the gp160 cytoplasmic domain is noted.
The first individual studied from the time of transmission to bnAb development (CH505) produced the heavy chain third complementarity determining region (HCDR3)-type of CD4 bs bnAbs and had one TF virus that initiated their infection (Liao et al., 2013). Binding of the TF Env to the bnAb unmutated common ancestor (UCA) B cell antigen receptor (BCR) induced the bnAb lineage, followed by intense selection of virus escape-mutations and envelope (Env) epitope diversification, that preceded acquisition of plasma bnAb activity. These observations are consistent with the hypothesis that exposure to autologous variants that confer some degree of resistance to antibodies may foster selection of antibodies during affinity maturation with extended capacity to recognize virus variants (Liao et al., 2013). Doria-Rose et al. (Doria-Rose et al., 2014) recently made similar observations about the development of V1V2-glycan bnAb targeted bnAbs.
The study of antibody-virus co-evolution in the CH505 individual revealed that two distinct B cell lineages can act “cooperatively” in driving bnAb development: one cooperating B cell lineage selected for TF virus escape mutants that were resistant to that lineage, but that enhanced sensitivity to neutralization by a second bnAb lineage (Figure 4) (Gao et al., 2014). In a case of V3 glycan bnAb B cell lineage induction, plasma autologous neutralizing antibodies selected an escape mutant creating a new V3-glycan epitope essential for bnAb Env recognition (Moore et al., 2012). Similarly, Bonsignori et al. have found multiple cooperating B cell lineages for V3-glycan bnAbs (Bonsignori et al., 2015). In addition to the importance of cooperating lineages for understanding how bnAbs develop, the elucidation of such lineages allows emergence of patterns of Env sensitivity and resistance to these lineages to be defined through time, enabling the definition of key mutations that impact the evolution of antibody breadth; such information can be directly employed to inform vaccine antigen design.
Figure 4.
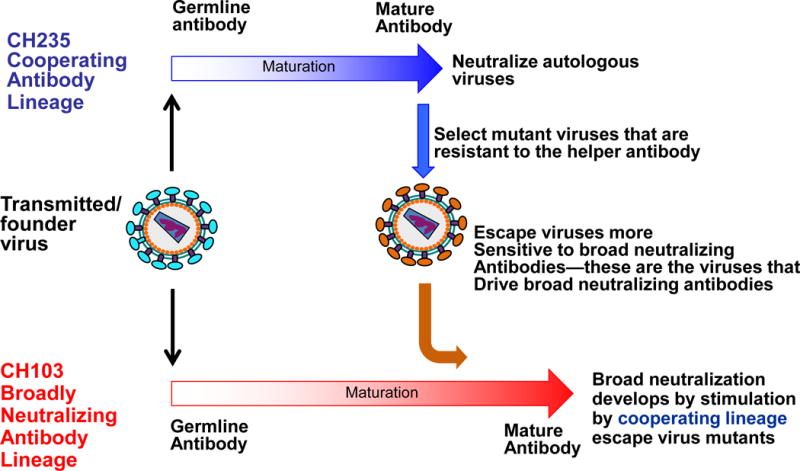
Cooperation of` B cell lineages in induction of HIV-1 broadly neutralizing antibodies. The transmitted founder (TF) virus induces both a broadly neutralizing antibody (bnAb) lineage (CH103 lineage in red) as well as a second lineage (the CH235 cooperating lineage in blue). The TF directly drives the bnAb lineage while the cooperating antibody lineage selects virus escape mutants that bind to and are neutralized by the bnAb lineage. Thus, in this case, the bnAb lineage is driven both by escape mutants from other cooperating lineages.
Host controls of broadly neutralizing antibody induction
All bnAbs have one or more unusual antibody traits: long hydrophobic HCDR3s, autoreactivity, and high frequencies of somatic mutations, all traits associated with triggering of B cell deletion or anergy by immune tolerance (Burton and Mascola, 2015; Haynes and Verkoczy, 2014; Mascola and Haynes, 2013). Thus, immune tolerance controls may constrain vaccinees’ ability to make bnAbs (Haynes et al., 2005; Haynes and Verkoczy, 2014; Yang et al., 2013). Moreover, HIV-1 Env is highly glycosylated and glycans shield critical neutralizing antibody target sites (Burton and Mascola, 2015). Env carbohydrates are synthesized by the host glycosylation machinery and have been proposed to be poorly immunogenic by mimicking self glycans (Scanlan et al., 2007). Despite this, carbohydrates are often critical components of bnAb epitopes, for example the V1V2 and V3 glycan bnAbs.
The higher frequencies of autoreactivity and/or polyreactivity among HIV-1 bnAbs (Liu et al., 2015) suggest that conserved, neutralizing epitopes present on the HIV-1 Env may have been selected to resemble host determinants (Haynes et al., 2005; Haynes et al., 2012b; Yang et al., 2013). Studies of knock-in mice that express the V(D)J rearrangements of human bnAbs or their germline ancestors (Chen et al., 2013; Doyle-Cooper et al., 2013; Verkoczy et al., 2011; Verkoczy et al., 2010), and the identification of host antigens that structurally resemble or are identical to HIV-1 determinants, both support this hypothesis (Liu et al., 2015; Yang et al., 2013). Thus, a key hypothesis pursued by our group is that HIV-1 infection induces immune perturbations that relax immune tolerance controls and eventually promote the expression of bnAbs with traits of antibodies normally disfavored by immune tolerance mechanisms (Haynes and Verkoczy, 2014).
Immunogen concepts for inducing bnAbs
The first antibodies to arise after HIV-1 transmission are to Env gp41, at ~13 days post transmission (Tomaras et al., 2008). The predominant specificity of plasma cells in acute HIV-1 infection (AHI) in both blood and terminal ileum is directed to gp41 (Liao et al., 2011; Trama et al., 2014). Many of these gp41 antibodies were class-switched to IgG, mutated, and polyreactive to host and environmental antigens, including the microbiome. Thus we hypothesized that the microbiome primed and expanded the pre-infection B cell repertoire to be cross-reactive with gp41, enabling stimulation by HIV-1 gp41 at the time of AHI (Liao et al., 2011; Trama et al., 2014). Recently, a DNA prime, recombinant adenovirus (rAd5) boost vaccine containing both Env gp120 and gp41 was tested in an efficacy trial (Williams et al., 2015). Vaccinees had a dominant gp41 antibody response both in serum and in the memory B cell repertoire. These gp41 antibodies were non-neutralizing and polyreactive for host and microbiome antigens. A study of the pre-vaccination B cell repertoire proved the presence of a pre-existing pool of microbiome-gp41 cross-reactive B cells that was stimulated by the vaccine (Williams et al., 2015).
The pre-existing CD4 T cell repertoire in HIV-1 uninfected individuals also contains T cells that cross-react with both HIV-1 and microbiome peptides (Campion et al., 2014; Su et al., 2013). This implies that previous exposure to immunogens from many sources may influence the primary immune response to an agent such as HIV-1, probably influencing the immunodominance of the responding T cells. Thus, both the CD4 T cell repertoire and the B cell repertoire are primed to respond to HIV-1 antigens due to the presence of microbiome-HIV cross-reactive antigens. These findings have provided a rationale for either deleting or modifying cross-reactive Env epitopes to minimize non-neutralizing dominant antibody responses induced by HIV-1 immunogens.
Minimal immunogens
Minimal Env immunogens range from small glycopeptides to monomer Env subunits, and have the potential to focus the immune response on a vulnerable epitope. For the V1V2- and V3-glycan bnAb epitopes, synthetic glycopeptides have been produced (Alam et al., 2013; Fernandez-Tejada et al., 2015). The best-studied minimal immunogens have been developed for the CD4 bs (Jardine et al., 2015) and for the MPER (Kim et al., 2013; Ofek et al., 2010; Verkoczy et al., 2013).
Jardine and colleagues have designed a series of immunogens that mimic the CD4 bs and bind well to the UCA of the CD4 bs bnAb, VRC01, and activate the VRC01 UCA B cell receptor in the context of VRC01 UCA VH knock-in mice (Jardine et al., 2015). Similarly, Dosenovic et al. have driven a VRC01 antibody lacking key mutations to further mature and make plasma bnAb activity in a bnAb VH knock in mouse system (Dosenovic et al., 2015). Prototype gp41 bnAbs are 2F5, that binds at the MPER N-terminus, and 4E10 and 10E8, that bind at the C-terminus (Burton and Mascola, 2015). 2F5, 4E10 and 10E8 are autoreactive (Liu et al., 2015; Yang et al., 2013), and in knock-in mice that express the 2F5 or 4E10 BCR, B cell development is blocked by clonal deletion in the bone marrow (Chen et al., 2013; Verkoczy et al., 2011; Verkoczy et al., 2010). All three have a long hydrophobic HCDR3 that interacts with lipid membranes (Alam et al., 2013; Chen et al., 2014; Huang et al., 2012) — a trait necessary for binding to and neutralization of HIV-1 (Alam et al., 2013). Because of the involvement of the virion membrane in the epitopes, minimal MPER peptide-liposome immunogens have been designed that project the MPER bnAb epitopes in a manner similar to that seen in the virion (Alam et al., 2013; Verkoczy et al., 2013). In mature 2F5 bnAb VH and VL knock-in mice, an MPER peptide-liposome induced the few anergic peripheral B cells that escaped deletion to produce high levels of plasma bnAbs (Verkoczy et al., 2013). Immunization of 2F5 UA VH and VL mice with an MPER peptide-liposome has initiated the UA 2F5 lineage (L.Verkoczy and B.F. Haynes, personal communication).
Rationale for native-like trimer immunogens
Recently, several structures of HIV-1 Env trimers have been reported, with the highest resolution that of the soluble BG505 SOSIP Env trimer (Julien et al., 2013; Pancera et al., 2014) (Figure 3). The main rationales for use of an Env trimer immunogen are: 1) native trimers do not express non-neutralizing epitopes to divert protective immune responses, 2) they display bnAb epitopes in correct orientations to facilitate the activation of bnAb UCAs, and 3) multiple epitopes will be present, potentially giving rise to polyclonal antibody responses that target different epitopes, thus limit the potential for resistant variants to evade a vaccine response. Currently, the best-defined “native-like” Env vaccine is the soluble BG505 SOSIP, however, this immunogen only elicited antibodies that mediate neutralization of the autologous BG505 Env, with no heterologous HIV-1 neutralization reported (Sanders et al., 2015). Recently membrane-bound trimers on virus-like particles (VLPs) have been produced, and were found to induce occasional tier 2 neutralizing antibodies for viruses with deleted glycans (Crooks et al., 2015). Immunization with fungal high mannose glycans have induced antibodies that neutralize HIV-1 produced to express only high mannose glycans (Zhang et al., 2015).
Sequential Env immunogens
In early studies of bnAbs, it was noted that only a few of the bnAb UCAs reacted with heterologous HIV-1 envelope proteins. However, studies of HIV-1 infected individuals from the time of transmission until development of bnAbs have shown that many TF Envs bind antigen-receptors of bnAb UCAs, and are therefore key to driving bnAb development. The HIV-bnAb co-evolution studies suggested selecting sequential Env immunogens that target germline UCA antibodies and the receptors of subsequent lineage intermediates (Haynes et al., 2012b; Liao et al., 2013). Using immunogens optimized for binding to specific BCRs (e.g., UCA, subsequent intermediates, and finally bnAb precursors), we propose to provide a selective growth and development advantage to the B-cell lineages with the greatest potentials for producing bnAbs with high potency and breadth (Haynes et al., 2012b). BnAb B cell lineages of CD4bs bnAbs (Zhou et al., 2015) and V1V2-glycan antibodies (Andrabi et al., 2015; Gorman Jason, 2015) are similar in VH usage, bnAb structures, and recognition of specific Envs by bnAb germline antibodies, regardless of the individuals from whom they were isolated. Thus, immunogens designed from one individual may successfully drive bnAb lineages in others.
From the antibody-virus evolution work discussed above, sequential gp120 Env monomer immunogens have been designed to study the ability of sequential TF mutant Env immunizations to recreate the Env events that drive bnAb development (Liao et al., 2013). Similarly, Moore et al. have identified the mutations that were key for V1V2 bnAb development in an HIV-1-infected African individual and have proposed a sequential Env immunization regimen for inducing V1V2 bnAbs (Bhiman et al., 2015).
Rationale for combining HIV-1 Env immunization with immune modulation
The unique features of bnAbs, both the extensive somatic hypermutation and the host tolerance controls that constrain their development, suggest a need for an appropriate immunologic environment for their induction. This includes adequate CD4+ T cell help and relaxation of host tolerance mechanisms at the time of vaccination.
CD4 T follicular helper cells (TFH) were first recognized by their association with normal and cancerous germinal centers (GC) (Stein et al., 1982; Velardi et al., 1986), and subsequently shown to be antigen-specific, CD4+ immigrants into the B cell follicle necessary for both GC B cell survival and for the somatic evolution of B cell populations towards higher affinity (Crotty, 2014). Immune checkpoints also control the function of TFH and prevent systemic autoimmune disease mediated by autoantibodies (Yu and Vinuesa, 2010). TFH enforce peripheral tolerance on GC B cells via mechanisms that are not yet elucidated (Han et al., 1995; Pulendran et al., 1995; Shokat and Goodnow, 1995). CD4 T follicular helper cells (TFH) are more frequent in the blood of HIV-infected individuals with comparatively high plasma bnAb activity (Locci et al., 2013). Immunization regimens that selectively induce increased TFH for Env antibody induction are under development.
BnAb vaccines may further require limiting host tolerance controls on disfavored bnAb lineages, a strategy we term Vaccine Transient Immune Modulation (VTIM). VTIM is modeled after cancer immunotherapy immune checkpoint blockades (Mueller, 2015), based on the recognition that tumors may commandeer peripheral tolerance to persist. The most successful of these interfere with the engagement of T-cell checkpoint receptors and their ligands (Armand, 2015). A similar conceptual approach could be employed to enable expansion of the primary B cell repertoire to include specificities normally limited by central tolerance.
CD8 T cell anti-HIV-1 responses
To complement antibody-mediated protection, there is also a need for vaccines to induce cellular immune responses capable of eradicating or effectively containing virus replication following the establishment of HIV-1 infection. Although strong virus-specific CD8+ T cell responses are activated during acute HIV-1 infection they fail to eliminate the virus (Borrow et al., 1997; Goonetilleke et al., 2009; Jones et al., 2004). Analysis of HIV’s strategies for evasion of T cell control and features of virus-specific CD8+ T cell responses that are associated with superior virus control helps inform the rational design of T cell-based vaccine strategies.
HIV-1 evades CD8+ T cell-mediated control during acute infection by rapid evolution of escape mutations in and around the epitopes targeted by CD8+ T cells and by down-regulation of MHC class I levels on the surface of infected cells to reduce CD8+ T cell recognition. HIV-1 elimination is further hampered by infection of CD4+ cells in sites such as lymph node GCs, which are poorly accessed by CD8+ T cells, and by the rapid establishment of a reservoir of latently-infected cells. Continued high-level antigenic stimulation by persisting virus, exacerbated by defects in CD4+ T cell function, leads to exhaustion of CD8+ T cell antiviral activity that further impairs control of viral replication from early infection onwards.
T cell escape mutations are selected if the replicative advantage conferred by the mutation is outweighed by any costs of the mutation to intrinsic viral fitness. The most rapidly-escaped responses are selected by immunodominant T cells targeting epitopes in variable viral sequences where mutations are readily accommodated (Ferrari et al., 2011; Liu et al., 2013). Hence broadly-directed, co-dominant responses to conserved epitopes where escape incurs high fitness costs, in the absence of mutations, are associated with good HIV-1 control (Migueles and Connors, 2015; Yue et al., 2015). The associations between CD8+ T cell responses to particular viral proteins and/or epitopes and the efficiency of HIV-1 control (Kiepiela et al., 2007; Mothe et al., 2011) are due in part to their relative sequence conservation. Likewise associations between certain HLA class I alleles and better virus control are partly attributable to epitope conservation and the fitness costs of mutational escape (Goulder and Walker, 2012). Thus, it should be beneficial for T cell-inducing HIV-1 vaccines to focus on conserved viral epitopes.
Associations between HLA alleles and/or responses of particular protein/epitope specificity and HIV-1 control also give insight into the relative antiviral efficacy of different CD8+ T cell responses. The association of responses to Gag epitopes and HLA alleles that restrict immunodominant Gag responses is attributable in part to the rapid presentation of Gag derived from infecting virions on HIV-infected cells, which enables them to be targeted by CD8+ T cells prior to MHC class I down-regulation (Sacha et al., 2007).
The points discussed above are also relevant to the design of T cell based HIV therapeutic strategies. Here, induction of a breadth of co-dominant responses is important not only to prevent de novo escape, but also to enable targeting of responses to additional epitopes that latent virus has not already escaped (Deng et al., 2015). Targeting of epitopes that are rapidly and continuously displayed on cells following reactivation of latent virus is also a priority. In addition, therapeutic strategies need to overcome the defects in immune function, including the decline in CD8+ T cell antiviral activity induced by prior high-level virus replication.
T cell responses elicited by select vaccine vectors
There has been a sustained effort to use vaccines to stimulate directly protective CD8 and CD4 T cell responses. Initially it was thought that such approaches might lead to partial but beneficial control of chronic HIV infection, rather than sterile prevention or virus elimination. SIV monkey infection models showed that SIV set point could be reduced ten-fold or more, depending on the challenge virus, the strength of the T cell response (Casimiro et al., 2005), and the vaccine approach used. Because the induction of CD8 T cells requires intracellular antigen processing, most approaches have used transfection of DNA or viral vectors. The combinations that have generated the strongest T cell responses in humans so far have been electroporated DNA (Kalams et al., 2013) recombinant adenoviruses (Liu et al., 2009; Shiver et al., 2002) (Borthwick et al., 2014) and poxviruses (Goepfert et al., 2014) often in heterologous prime-boost combinations. However, when these approaches to stimulate anti-HIV-1 specific CD8 T cell responses were applied to humans in vaccine efficacy trials, no protection was seen in the STEP, Phambili or HVTN505 trials (Gray et al., 2010; Huang et al., 2015; McElrath et al., 2008).
These negative results led to the notion that vaccine-induced CD8 T cells offer little protection but this is challenged by the recent findings of Picker and colleagues who have tested a cytomegalovirus (CMV) vaccine vector (Hansen et al., 2011; Hansen et al., 2013a). They intended to stimulate a high level of SIV specific effector-memory CD8 T cells that would be continually stimulated by the persisting virus vector, a laboratory-adapted Rhesus CMV vector including all of SIV except vif. The results were surprising. When challenged with SIVmac239, all animals were infected, but over the following weeks 55% of animals completely cleared the SIV infection (Hansen et al., 2011; Hansen et al., 2013a). This result has been seen in more than 100 SIVmac239 challenged animals, and is the only clearly-documented example of immune clearance of a persisting lentivirus. The effect is mediated by CD8 T cells, which make exceptionally broad responses to ~100 epitopes (Hansen et al., 2013b). In contrast, the vaccinated animals make virtually no anti-SIV antibodies. Subsequent detailed analysis has shown that the responsible CD8 T cells are unusual, two thirds restricted by MHC class II (Hansen et al., 2013b) and one third by the non-classical MHC-E molecule (Hansen et al., 2016).
It is likely, though not yet certain, that the atypical CD8 T cell responses are essential for the protection. These T cell responses are dependent on the RhCMV gene US11 which down regulates classical MHC1a expression, and on the absence of the UL128/130 genes which determine virus tropism for epithelial and endothelial cells (Hansen et al., 2013b). The CMV must also impair the cross-priming process that is normally important for generation of classical T cell responses. There is a similarity to primary T cell responses to mycobacterial antigens in humans, which are primarily class II restricted CD4 T cells and HLA-E restricted CD8 T cells (Joosten et al., 2010; Lewinsohn et al., 1998).
It is clear that, at least in the protected monkeys, SIV infected cells must express some of the same peptides in MHC-E and MHC-II so that they are recognized by the vaccine stimulated T cells. They may be expressed in low numbers, because CD8 T cells can recognize cells expressing <20 epitope peptides per cell (Irvine et al., 2002). The level is probably insufficient to prime T cell responses.
The simplest explanation for the protection offered by the RhCMV68.1 vaccine would be the exceptional breadth of the T cell responses, making it impossible for SIV to escape. Figure 5 compares SIV and HIV vaccines aimed at generating T cell responses, comparing the strength of the responses they elicit and their breadth, corrected for the degree or matching with the challenge or infecting viruses. HIV can simultaneously escape at least three T cell responses (Goonetilleke et al., 2009), such escape being made easier by the uneven immunodominance pattern of classical CD8 T cell responses (Liu et al., 2013). Thus, extreme breadth of T cell responses of even strength could contribute to the unique protection offered by the CMV vaccine. The functional phenotype of the effector T cells and the distribution of SIV infected MHC-II and MHC-E positive cells could also be critical factors.
Figure 5.
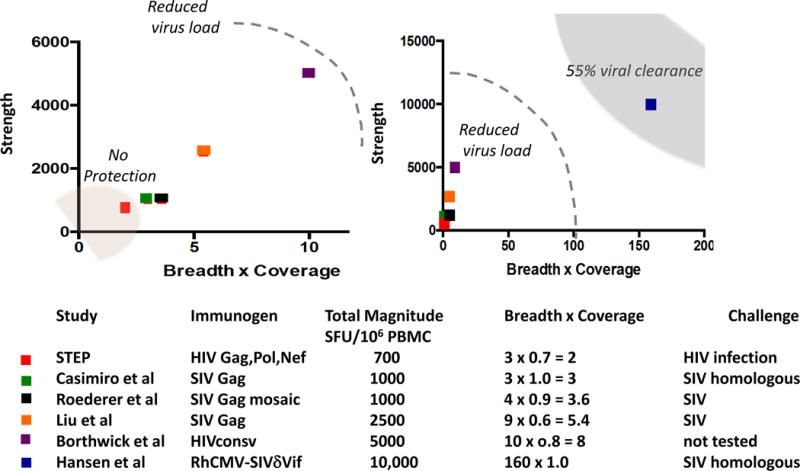
Degrees of HIV-1 control by vaccines that elicit CD8 T cell responses: Each vaccine was designed to elicit only or primarily, T cell responses. These were the STEP Adenovirus-5-Gag-Pol-Nef vaccine (McElrath et al., 2008), DNA-Adenovirus-5 SIV Gag (Casimiro et al., 2005), DNA-Adenovirus-5-mosaic Gag (Roederer et al., 2014), Adenovirus-26 gag + Adenovirus 5 gag (Liu et al., 2009), RhCMV68-1 – SIV (Hansen et al., 2011), and ChAd63-HIVconsv – MVA-HIVconsv (Borthwick et al., 2014) vaccines. The peak responses after vaccination are shown. On the y axis is the magnitude as virus specific T cells per million PBMC (either directly from Elispot values or converted from % CD8 T cells in flow cytometry assays). On the x axis is shown the number of epitopes reported (breadth) corrected for the degree of matching between vaccine and challenge/infecting virus. For perfect matches between the vaccine and challenge the correction value is 1.0, for the STEP vaccine it is estimated to be 0.7. The left hand plot shows values for the STEP trial compared to similar vaccines in the SIV model. In the right panel these are compared to the values for the RhCMV-68.1-SIV vaccine. Also shown here are the values for the HIVconsv conserved region vaccine in a phase I trial in humans where there was no HIV-1 exposure. Where known, the outcomes of SIV challenge of HIV-1 exposure are shown.
HLA-E restricted HIV-reactive CD8 T cells have not yet been shown, but humans do make broad CD8 T cell responses to mycobacterial antigens (Joosten et al., 2010). It is likely that similar HIV specific T cells could be stimulated by a vaccine. Similarly for HLA-II restricted CD8 T cells, which are normally rare, for both to be considered as targets for vaccine design it will be necessary to show that HIV-infected cells express these antigens on their surface.
If direct translation of a CMV vectored vaccine to humans is not yet practical, responses that are much broader than generated by current human vaccine candidates (Figure 5) might still be effective, especially if there was good match of vaccine to virus. There are two promising approaches to this problem, focusing on conserved regions, and using mosaic strategies to design antigens.
Mosaic vaccine antigens are designed using a computational machine learning strategy to include the most common forms of all potential epitopes across a protein in a sample population. This minimizes the inclusion of rare epitopes commonly found in natural proteins, and maximizes the cross-reactive potential of the vaccine response (Fischer et al., 2007). Mosaic vaccines are polyvalent sets of 2 or 3 antigens that resemble natural proteins, and are complementary in terms of carrying distinct (but common) epitopes. They have proven to be highly immunogenic, eliciting T-cell responses with greater breadth and potency than do natural proteins (Barouch et al., 2010; Santra et al., 2010). Env mosaic vaccines provided significant protection from infection in a SHIV challenge model, likely due to antibody responses, and also enabled improved viral control in infected animals (Barouch et al., 2013). The inclusion of multiple mosaics in a polyvalent vaccine elicits T-cell responses that are better able to recognize natural epitope variants (Abdul-Jawad et al., 2016).
Conserved region vaccines include only conserved regions as they are most likely to elicit cross-reactive responses, and most likely to be constrained in escape by fitness costs (Letourneau et al., 2007; Rolland et al., 2007). Given that vaccines of concatenated epitopes have not been immunogenic in human trials (Gorse et al., 2008; Jaoko et al., 2008), the use of multiple short but highly conserved peptides (Rolland et al., 2007) may not be feasible. Thus the use of longer, more modestly conserved stretches has been explored (Letourneau et al., 2007). These longer conserved stretches provide local context for processing pathways, and are highly immunogenic in both NHPs and humans. They encompass many known and predicted overlapping epitopes that could be targeted by people with diverse HLA haplotypes. It remains to be resolved which vaccine approach will be most beneficial: one that only includes conserved epitopes (Borthwick et al., 2014); one that uses full length proteins, so elicits more responses overall, but includes less cross-reactive responses that are more vulnerable to escape (Barouch et al., 2013; Hulot et al., 2015); or one that specifically targets epitopes that have been found to be associated with reduced viral loads and better outcome in natural infection (Mothe et al., 2015). Combinations of these approaches are also possible.
Conclusions
During the first 20 years of the HIV-1 epidemic, vaccine efforts were targeted at making an HIV-1 vaccine using strategies that were previously successful for currently licensed vaccines, such as live attenuated, Env subunit, killed virus HIV-1 experimental vaccines. Over the last 10 years the HIV-1 vaccine development field has realized that for bnAb induction, both understanding the structures of the Env immunogens, the implications of antigenic diversification on the acquisition of breadth, and an in-depth understanding of the host controls of bnAb induction are critical for vaccine success. That bnAbs can develop in HIV-1-infected individuals and can be mapped from the time of infection to bnAb development has provided hope that the immunogenic events that transpire in infection, once fully deciphered, can be recreated with a vaccine. The need for generation of extensive and unprecedented levels of somatic hypermutation as well as circumventing roadblocks at various levels of host immune tolerance control will require understanding at a deep level of the biology of regulation of B cell maturation in germinal center. Furthermore, CD8 T cell control of HIV-1, robust, co-dominant, broadly- directed virus-specific CD8 T cell responses will need to be induced for an optimum vaccination strategy. Future work is underway to resolve if CD8 T cells may need to be induced in a manner that specifically promotes target cell recognition via MHC-E or class II or both.
No other infectious agent vaccine has required such detailed understanding of host-pathogen interactions as has HIV-1. In gaining this understanding, research progress in HIV-1 vaccine development has catapulted forward the fields of non-human primate and human immunology, retroviral biology, structural biology, genetics of the immune response, viral drug development and vector development of gene delivery—all accomplishments that are benefitting many non-HIV-1 areas of research. Moving the host-virus insights and new HIV-1 vaccine candidates into experimental medicine trials in man will speed progress in HIV-1 vaccine development and allow for iterative improvements in immunogens until sufficient protection can be achieved. The goal is that when the vaccine is implemented, it will join with other preventive modalities such as pre-exposure administration of anti-retroviral drugs, to eventually end the HIV-1 epidemic.
Acknowledgments
This work was supported by NIH, NIAID grant UM1-AI10064 for the Duke Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery and Collaboration for AIDS Vaccine Discovery grants from the Bill and Melinda Gates Foundation to Barton F. Haynes. We thank Todd Bradley and Kevin Wiehe for Figure 2. The authors also thank Kelly Soderberg, Thomas Denny and Cherie Lahti for administrative leadership, and all of our collaborators in the Duke CHAVI-ID for their invaluable work over the past 12 years. We apologize that due to space limitations we could not fully reference all the fine work of our colleagues in the field. We thank Stephanie Risbon for expert secretarial assistance.
Footnotes
Author contributions
All authors wrote and edited the paper.
References
- Abdul-Jawad S, Ondondo B, van Hateren A, Gardner A, Elliott T, Korber B, Hanke T. Increased valency of conserved-mosaic vaccines enhances the breadth and depth of epitope recognition. Molecular therapy: the journal of the American Society of Gene Therapy. 2016 doi: 10.1038/mt.2015.210. epub ahead of print Jan 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, Dennison SM, Aussedat B, Vohra Y, Park PK, Fernandez-Tejada A, Stewart S, Jaeger FH, Anasti K, Blinn JH, et al. Recognition of synthetic glycopeptides by HIV-1 broadly neutralizing antibodies and their unmutated ancestors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18214–18219. doi: 10.1073/pnas.1317855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi R, Voss JE, Liang CH, Briney B, McCoy LE, Wu CY, Wong CH, Poignard P, Burton DR. Identification of Common Features in Prototype Broadly Neutralizing Antibodies to HIV Envelope V2 Apex to Facilitate Vaccine Design. Immunity. 2015;43:959–973. doi: 10.1016/j.immuni.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015;125:3393–3400. doi: 10.1182/blood-2015-02-567453. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349:320–324. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nature medicine. 2010;16:319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155:531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhiman JN, Anthony C, Doria-Rose NA, Karimanzira O, Schramm CA, Khoza T, Kitchin D, Botha G, Gorman J, Garrett NJ, et al. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nature medicine. 2015;21:1332–1336. doi: 10.1038/nm.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Kreider T, Fera D, Meryhoff R, Hwang KK, Lu XZ, Mangiapani M, Iyengar SM, Arora HG, Gladden M, Parks R, Korber BT, Hraber P, Kepler TB, Shaw G, Harrison SC, Hahn B, Moody MA, Gao F, Liao HX, Haynes BF, et al. Co-Evolution of a N332-sensitive V3-glacan Bradly Neutralizing Antibody Lineage and founder virus from Acute through Chronic In Keystone Symposia (Banff, Alberta, Canada) 2015 [Google Scholar]
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nature medicine. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- Borthwick N, Ahmed T, Ondondo B, Hayes P, Rose A, Ebrahimsa U, Hayton EJ, Black A, Bridgeman A, Rosario M, et al. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22:464–475. doi: 10.1038/mt.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nature immunology. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion SL, Brodie TM, Fischer W, Korber BT, Rossetti A, Goonetilleke N, McMichael AJ, Sallusto F. Proteome-wide analysis of HIV-specific naive and memory CD4(+) T cells in unexposed blood donors. The Journal of experimental medicine. 2014;211:1273–1280. doi: 10.1084/jem.20130555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Schaefer M, Monaco DC, Batorsky R, Claiborne DT, Prince J, Deymier MJ, Ende ZS, Klatt NR, DeZiel CE, et al. HIV transmission. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science. 2014;345:1254031. doi: 10.1126/science.1254031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, Davies ME, McDermott AB, O’Connor DH, Fridman A, et al. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. Journal of virology. 2005;79:15547–15555. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Frey G, Peng H, Rits-Volloch S, Garrity J, Seaman MS, Chen B. Mechanism of HIV-1 neutralization by antibodies targeting a membrane-proximal region of gp41. Journal of virology. 2014;88:1249–1258. doi: 10.1128/JVI.02664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang J, Hwang KK, Bouton-Verville H, Xia SM, Newman A, Ouyang YB, Haynes BF, Verkoczy L. Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10. Journal of immunology. 2013;191:1260–1275. doi: 10.4049/jimmunol.1300770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks ET, Tong T, Chakrabarti B, Narayan K, Georgiev IS, Menis S, Huang X, Kulp D, Osawa K, Muranaka J, et al. Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site. PLoS Pathog. 2015;11:e1004932. doi: 10.1371/journal.ppat.1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenovic P, von Boehmer L, Escolano A, Jardine J, Freund NT, Gitlin AD, McGuire AT, Kulp DW, Oliveira T, Scharf L, et al. Immunization for HIV-1 Broadly Neutralizing Antibodies in Human Ig Knockin Mice. Cell. 2015;161:1505–1515. doi: 10.1016/j.cell.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle-Cooper C, Hudson KE, Cooper AB, Ota T, Skog P, Dawson PE, Zwick MB, Schief WR, Burton DR, Nemazee D. Immune tolerance negatively regulates B cells in knock-in mice expressing broadly neutralizing HIV antibody 4E10. Journal of immunology. 2013;191:3186–3191. doi: 10.4049/jimmunol.1301285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T, Goujon C, Malim MH. HIV-1 and interferons: who’s interfering with whom? Nature reviews Microbiology. 2015;13:403–413. doi: 10.1038/nrmicro3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton-May AE, Dibben O, Emmerich T, Ding H, Pfafferott K, Aasa-Chapman MM, Pellegrino P, Williams I, Cohen MS, Gao F, et al. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology. 2013;10:146. doi: 10.1186/1742-4690-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Tejada A, Haynes BF, Danishefsky SJ. Designing synthetic vaccines for HIV. Expert review of vaccines. 2015;14:815–831. doi: 10.1586/14760584.2015.1027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Korber B, Goonetilleke N, Liu MK, Turnbull EL, Salazar-Gonzalez JF, Hawkins N, Self S, Watson S, Betts MR, et al. Relationship between functional profile of HIV-1 specific CD8 T cells and epitope variability with the selection of escape mutants in acute HIV-1 infection. PLoS Pathog. 2011;7:e1001273. doi: 10.1371/journal.ppat.1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R, Kuiken C, Haynes B, Letvin NL, Walker BD, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nature medicine. 2007;13:100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- Fraser C, Lythgoe K, Leventhal GE, Shirreff G, Hollingsworth TD, Alizon S, Bonhoeffer S. Virulence and pathogenesis of HIV-1 infection: an evolutionary perspective. Science. 2014;343:1243727. doi: 10.1126/science.1243727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Bonsignori M, Liao HX, Kumar A, Xia SM, Lu X, Cai F, Hwang KK, Song H, Zhou T, et al. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell. 2014;158:481–491. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert PA, Elizaga ML, Seaton K, Tomaras GD, Montefiori DC, Sato A, Hural J, DeRosa SC, Kalams SA, McElrath MJ, et al. Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. The Journal of infectious diseases. 2014;210:99–110. doi: 10.1093/infdis/jiu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. The Journal of experimental medicine. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman Jason SC, Yan Max, Deavenport Thaddeus, Guttman Miklos, Bailer Robert, Chambers Michael, Chuang Gwo-yu, DeKosky Brandon, Doria-Rose Nicole, Druz Aliaksandr, Ernandes Michael, Georgiev Ivelin, Jarosinksi Marissa, Gordon Joyce M, Lemmin Thomas, et al. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nature Structural and Molecular Biology. 2015 doi: 10.1038/nsmb.3144. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse GJ, Baden LR, Wecker M, Newman MJ, Ferrari G, Weinhold KJ, Livingston BD, Villafana TL, Li H, Noonan E, et al. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008;26:215–223. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Goulder PJ, Walker BD. HIV and HLA class I: an evolving relationship. Immunity. 2012;37:426–440. doi: 10.1016/j.immuni.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Current opinion in HIV and AIDS. 2010;5:357–361. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3- nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self- tolerance. J Exp Med. 1995;182:1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013a;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013b;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science. 2016 doi: 10.1126/science.aac9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012a;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nature biotechnology. 2012b;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Verkoczy L. AIDS/HIV. Host controls of HIV neutralizing antibodies. Science. 2014;344:588–589. doi: 10.1126/science.1254990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. Aids. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Follmann D, Nason M, Zhang L, Huang Y, Mehrotra DV, Moodie Z, Metch B, Janes H, Keefer MC, et al. Effect of rAd5-Vector HIV-1 Preventive Vaccines on HIV-1 Acquisition: A Participant-Level Meta-Analysis of Randomized Trials. PloS one. 2015;10:e0136626. doi: 10.1371/journal.pone.0136626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulot SL, Korber B, Giorgi EE, Vandergrift N, Saunders KO, Balachandran H, Mach LV, Lifton MA, Pantaleo G, Tartaglia J, et al. Comparison of Immunogenicity in Rhesus Macaques of Transmitted-Founder, HIV-1 Group M Consensus, and Trivalent Mosaic Envelope Vaccines Formulated as a DNA Prime, NYVAC, and Envelope Protein Boost. Journal of virology. 2015;89:6462–6480. doi: 10.1128/JVI.00383-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, Nanvubya A, Bashir F, Bhatt K, Ogutu H, Wakasiaka S, et al. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine. 2008;26:2788–2795. doi: 10.1016/j.vaccine.2008.02.071. [DOI] [PubMed] [Google Scholar]
- Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, Kalyuzhniy O, Skog PD, Thinnes TC, Bhullar D, Briney B, et al. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science. 2015;349:156–161. doi: 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NA, Wei X, Flower DR, Wong M, Michor F, Saag MS, Hahn BH, Nowak MA, Shaw GM, Borrow P. Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. The Journal of experimental medicine. 2004;200:1243–1256. doi: 10.1084/jem.20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten SA, van Meijgaarden KE, van Weeren PC, Kazi F, Geluk A, Savage ND, Drijfhout JW, Flower DR, Hanekom WA, Klein MR, et al. Mycobacterium tuberculosis peptides presented by HLA-E molecules are targets for human CD8 T-cells with cytotoxic as well as regulatory activity. PLoS Pathog. 2010;6:e1000782. doi: 10.1371/journal.ppat.1000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Swanstrom R, Kashuba AD, Cohen MS. Bottlenecks in HIV-1 transmission: insights from the study of founder viruses. Nature reviews Microbiology. 2015;13:414–425. doi: 10.1038/nrmicro3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, De Rosa S, Carter DK, Rybczyk K, Frank I, et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. The Journal of infectious diseases. 2013;208:818–829. doi: 10.1093/infdis/jit236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nature medicine. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Kim M, Song L, Moon J, Sun ZY, Bershteyn A, Hanson M, Cain D, Goka S, Kelsoe G, Wagner G, et al. Immunogenicity of membrane-bound HIV-1 gp41 membrane-proximal external region (MPER) segments is dominated by residue accessibility and modulated by stereochemistry. The Journal of biological chemistry. 2013;288:31888–31901. doi: 10.1074/jbc.M113.494609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood JE, Coates EE, Yamshchikov G, Saunders JG, Holman L, Enama ME, DeZure A, Lynch RM, Gordon I, Plummer S, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clinical and experimental immunology. 2015;182:289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, Dorrell L, Dong T, Korber B, McMichael AJ, et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PloS one. 2007;2:e984. doi: 10.1371/journal.pone.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn DM, Alderson MR, Briden AL, Riddell SR, Reed SG, Grabstein KH. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. The Journal of experimental medicine. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, Whitesides JF, Lu X, Yu JS, Hwang KK, et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. The Journal of experimental medicine. 2011;208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Yang G, Wiehe K, Nicely NI, Vandergrift NA, Rountree W, Bonsignori M, Alam SM, Gao J, Haynes BF, et al. Polyreactivity and autoreactivity among HIV-1 antibodies. Journal of virology. 2015;89:784–798. doi: 10.1128/JVI.02378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MK, Hawkins N, Ritchie AJ, Ganusov VV, Whale V, Brackenridge S, Li H, Pavlicek JW, Cai F, Rose-Abrahams M, et al. Vertical T cell immunodominance and epitope entropy determine HIV-1 escape. The Journal of clinical investigation. 2013;123:380–393. doi: 10.1172/JCI65330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al. Human circulating PD-1+CXCR3-CXCR5+memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunological reviews. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migueles SA, Connors M. Success and failure of the cellular immune response against HIV-1. Nature immunology. 2015;16:563–570. doi: 10.1038/ni.3161. [DOI] [PubMed] [Google Scholar]
- Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody MA, Gao F, Gurley TC, Amos JD, Kumar A, Hora B, Marshall DJ, Whitesides JF, Xia SM, Parks R, et al. Strain-Specific V3 and CD4 Binding Site Autologous HIV-1 Neutralizing Antibodies Select Neutralization-Resistant Viruses. Cell host & microbe. 2015;18:354–362. doi: 10.1016/j.chom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, Hermanus T, Bajimaya S, Tumba NL, Abrahams MR, et al. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nature medicine. 2012;18:1688–1692. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe B, Hu X, Llano A, Rosati M, Olvera A, Kulkarni V, Valentin A, Alicea C, Pilkington GR, Sardesai NY, et al. A human immune data-informed vaccine concept elicits strong and broad T-cell specificities associated with HIV-1 control in mice and macaques. J Transl Med. 2015;13:60. doi: 10.1186/s12967-015-0392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe B, Llano A, Ibarrondo J, Daniels M, Miranda C, Zamarreno J, Bach V, Zuniga R, Perez-Alvarez S, Berger CT, et al. Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med. 2011;9:208. doi: 10.1186/1479-5876-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL. Cancer immunology and immunotherapy. Realizing the promise. Introduction. Science. 2015;348:54–55. doi: 10.1126/science.348.6230.54. [DOI] [PubMed] [Google Scholar]
- Ofek G, Guenaga FJ, Schief WR, Skinner J, Baker D, Wyatt R, Kwong PD. Elicitation of structure-specific antibodies by epitope scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17880–17887. doi: 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, et al. Phenotypic properties of transmitted founder HIV-1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping LH, Joseph SB, Anderson JA, Abrahams MR, Salazar-Gonzalez JF, Kincer LP, Treurnicht FK, Arney L, Ojeda S, Zhang M, et al. Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. Journal of virology. 2013;87:7218–7233. doi: 10.1128/JVI.03577-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA. Correlates of protection induced by vaccination. Clinical and vaccine immunology: CVI. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Kannourakis G, Nouri S, Smith KG, Nossal GJ. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995;375:331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Pybus OG, Rambaut A. Evolutionary analysis of the dynamics of viral infectious disease. Nature reviews Genetics. 2009;10:540–550. doi: 10.1038/nrg2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, et al. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014;505:502–508. doi: 10.1038/nature12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M, Nickle DC, Mullins JI. HIV-1 group M conserved elements vaccine. PLoS Pathog. 2007;3:e157. doi: 10.1371/journal.ppat.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacha JB, Chung C, Rakasz EG, Spencer SP, Jonas AK, Bean AT, Lee W, Burwitz BJ, Stephany JJ, Loffredo JT, et al. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. Journal of immunology. 2007;178:2746–2754. doi: 10.4049/jimmunol.178.5.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, et al. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science. 2015;349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S, Liao HX, Zhang R, Muldoon M, Watson S, Fischer W, Theiler J, Szinger J, Balachandran H, Buzby A, et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nature medicine. 2010;16:324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan CN, Ritchie GE, Baruah K, Crispin M, Harvey DJ, Singer BB, Lucka L, Wormald MR, Wentworth P, Jr, Zitzmann N, et al. Inhibition of mammalian glycan biosynthesis produces non-self antigens for a broadly neutralising, HIV-1 specific antibody. Journal of molecular biology. 2007;372:16–22. doi: 10.1016/j.jmb.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Hunter E. HIV transmission. Cold Spring Harbor. 2012;2 doi: 10.1101/cshperspect.a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Stein H, Gerdes J, Mason DY. The normal and malignant germinal centre. Clinics in haematology. 1982;11:531–559. [PubMed] [Google Scholar]
- Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. Journal of virology. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trama AM, Moody MA, Alam SM, Jaeger FH, Lockwood B, Parks R, Lloyd KE, Stolarchuk C, Scearce R, Foulger A, et al. HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria. Cell host & microbe. 2014;16:215–226. doi: 10.1016/j.chom.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velardi A, Mingari MC, Moretta L, Grossi CE. Functional analysis of cloned germinal center CD4+ cells with natural killer cell-related features. Divergence from typical T helper cells. Journal of immunology. 1986;137:2808–2813. [PubMed] [Google Scholar]
- Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, Ouyang YB, Alam SM, Holl TM, Hwang KK, et al. Rescue of HIV-1 broad neutralizing antibody-expressing B cells in 2F5 VH × VL knockin mice reveals multiple tolerance controls. Journal of immunology. 2011;187:3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Chen Y, Zhang J, Bouton-Verville H, Newman A, Lockwood B, Scearce RM, Montefiori DC, Dennison SM, Xia SM, et al. Induction of HIV-1 broad neutralizing antibodies in 2F5 knock-in mice: selection against membrane proximal external region-associated autoreactivity limits T-dependent responses. Journal of immunology. 2013;191:2538–2550. doi: 10.4049/jimmunol.1300971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Jr, Scharf L, Scheid JF, Klein F, Bjorkman PJ, Nussenzweig MC. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WB, Liao HX, Moody MA, Kepler TB, Alam SM, Gao F, Wiehe K, Trama AM, Jones K, Zhang R, et al. HIV-1 VACCINES. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science. 2015;349:aab1253. doi: 10.1126/science.aab1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Holl TM, Liu Y, Li Y, Lu X, Nicely NI, Kepler TB, Alam SM, Liao HX, Cain DW, et al. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. The Journal of experimental medicine. 2013;210:241–256. doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Vinuesa CG. Multiple checkpoints keep follicular helper T cells under control to prevent autoimmunity. Cellular & molecular immunology. 2010;7:198–203. doi: 10.1038/cmi.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Pfafferott KJ, Baalwa J, Conrod K, Dong CC, Chui C, Rong R, Claiborne DT, Prince JL, Tang J, et al. Transmitted virus fitness and host T cell responses collectively define divergent infection outcomes in two HIV-1 recipients. PLoS Pathog. 2015;11:e1004565. doi: 10.1371/journal.ppat.1004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Fu H, Luallen RJ, Liu B, Lee FH, Doms RW, Geng Y. Antibodies elicited by yeast glycoproteins recognize HIV-1 virions and potently neutralize virions with high mannose N-glycans. Vaccine. 2015;33:5140–5147. doi: 10.1016/j.vaccine.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Lynch RM, Chen L, Acharya P, Wu X, Doria-Rose NA, Joyce MG, Lingwood D, Soto C, Bailer RT, et al. Structural Repertoire of HIV-1-Neutralizing Antibodies Targeting the CD4 Supersite in 14 Donors. Cell. 2015;161:1280–1292. doi: 10.1016/j.cell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


