Abstract
The IL-12 family of heterodimeric cytokines, consisting of IL-12, IL-23, IL-27, and IL-35, has important roles in regulating the immune response. IL-12 family members are comprised of a heterodimer consisting of α and β chains: IL-12 (p40 and p35), IL-23 (p40 and p19), IL-27 (Ebi3 and p28), and IL-35 (Ebi3 and p35). Given the combinatorial nature of the IL-12 family, we generated adenoviral vectors expressing two putative IL-12 family members not yet found naturally, termed IL-X (Ebi3 and p19) and IL-Y (p40 and p28), as single-chain molecules. scIL-Y, but not scIL-X, was able to stimulate significantly a unique cytokine/chemokine expression profile as well as activate STAT3 in mice, in part, through a pathway involving IL-27Rα in splenocytes. Adenoviral-mediated, intra-tumoral delivery of scIL-Y increased tumor growth in contrast to the anti-tumor effects of scIL-12 and scIL-23. Similarly, treatment of pre-diabetic non-obese diabetic (NOD) mice by intravenous injection of Ad.scIL-Y prevented the onset of hyperglycemia. Analysis of cells from Ad.scIL-Y-treated NOD mice demonstrated that scIL-Y reduced expression of inflammatory mediators such as IFN-γ. Our data demonstrates that a novel, synthetic member of the IL-12 family, termed IL-Y, confers unique immunosuppressive effects in two different disease models and thus could have therapeutic applications.
Keywords: type 1 diabetes, regulatory T cells, interleukin-12, NOD mice, adenovirus
Introduction
The IL-12 family of heterodimeric cytokines currently has four members, termed IL-12, IL-23, IL-27 and IL-35, consisting of one of two α (p40, Ebi3) and one of three β chains (p19, p28, p35) [1]. IL-12 is comprised of a p40 and p35 heterodimer, whereas IL-23, IL-27 and IL-35 are comprised of heterodimers p40 and p19, Ebi3 and p28, Ebi3 and p35, respectively. The α chains are structurally homologous to the type I cytokine IL-6, whereas the β chains are homologous to the soluble form of the IL-6 receptor. There are notable differences in the expression of the subunits for these cytokines with IL-12, IL-23, and IL-27 being expressed and secreted by antigen presenting cells such as dendritic cells (DCs) and macrophages (MΦ). Activation of innate receptors, like toll-like receptors, induces the expression of these cytokines. However, T-cell help in the form of IFN-γ or CD40L also can enhance their expression [2-4]. IL-35 is expressed primarily by FoxP3+ regulatory T-cells (Tregs), but its subunits also are expressed by γδ T-cells, CD8+ T-cells, and placental trophoblasts [5].
Members of the IL-12 family of cytokines also exhibit different functions. Both IL-12 and IL-23 are pro-inflammatory cytokines with the former being able to induce Th1 cells while the latter plays a major role in the induction of Th17 cells. IL-27 exhibits a pleotropic functional phenotype capable of augmenting both pro and anti-inflammatory responses [6, 7]. For example, IL-27 suppresses expression of certain anti-inflammatory cytokines, which skews the adaptive immune response towards pro-inflammatory [8, 9]. In contrast, IL-27 also up-regulates the expression of IL-10, leading to the induction of type I regulatory T (Tr-1) cells [10-12]. IL-35 is a suppressive cytokine inhibiting effector T-cells responses [1, 5] and suppressing the development of Th17 cells as well the proliferation of effector T-cells [13]. The suppression of T-cells mediated by IL-35 is IL-10 dependent [14, 15].
Previously, we and others generated adenoviral-based vectors expressing either IL-12 or IL-23 and demonstrated their ability to induce anti-tumor immunity following intra-tumor injection [16-18]. Both IL-12 and IL-23 can induce potent anti-tumor immune responses, but at different kinetics. IL-12 is effective in the early stages of tumor formation whereas IL-23 is more effective during the later stages of tumor growth. IL-23 also is more effective in inducing anti-tumor immunity. The anti-tumor activities of IL-12 and IL-23 also were significantly enhanced when their two subunits were expressed as a single chain using a 15 amino acid linker [17]. The enhanced function of the linked cytokines is reminiscent of previous studies with IL-12 (p35 and p40), G-CSF (GM-CSF fusion), and GM-CSF (IL-3 fusion) [19-21].
Given the promiscuous use of receptor and ligand subunits between IL-12, IL-23, IL-27, and IL-35, additional pairings between these components or interactions with other partners are possible. Thus, we generated adenoviral vectors expressing two additional hypothetical IL-12 family heterodimers not yet found naturally, termed IL-Y (p40 and p28) and IL-X (Ebi3 and p19), as single chain molecules. Here we demonstrate that scIL-Y in particular is able to induce a specific subset of chemokines/cytokines from primary splenocytes, in part, through an IL-27R pathway. Moreover, we demonstrate that scIL-Y is immunosuppressive in vivo, blocking anti-tumor responses and preventing onset of hyperglycemia in the NOD mice, a model for Type 1 Diabetes (T1D). Although scIL-Y induced IFN-γ expression in cultured naïve splencoytes, treatment of NOD mice with Ad.scIL-Y decreased expression of IFN-γ in T-cells. Interestingly, scIL-Y decreased the frequency of FoxP3+Helios+ Treg cells while having little effect on FoxP3 single positive Treg cells. Overall, the data suggest that a novel heterodimeric IL-12 family member, scIL-Y, comprised of p40 and p28, is able to antagonize Th1-driven immune responses. Thus scIL-Y could have therapeutic applications for treating autoimmune diseases.
Results
The novel IL-12 family heterodimer, scIL-Y, exhibits biological activity
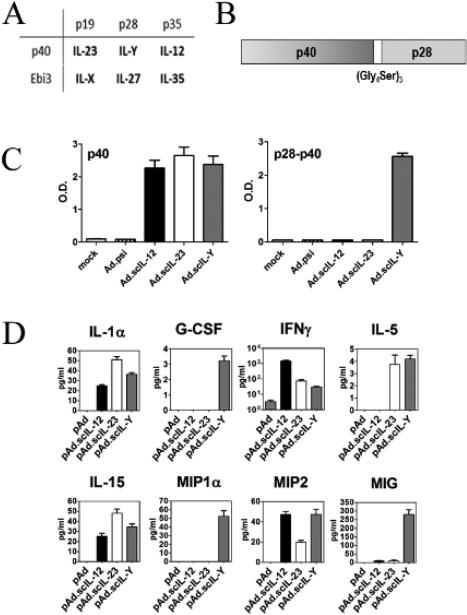
We generated adenoviral vectors expressing the two hypothetical IL-12 family members, termed IL-Y and IL-X, comprised of p40 and p28 and Ebi3 and p19, respectively (Fig. 1A and B). These novel IL-12 family members were generated as single chain molecules using a 15 amino acid (Gly4Ser)3 spacer as described for scIL-23 [17]. We verified that scIL-Y and scIL-X were secreted following infection of A549 cells by ELISA as described in the Materials and Method section. We initially tested the biological activity of Ad.scIL-Y and Ad.scIL-X by treating naïve splenocytes with conditioned media (CM) from Ad.scIL-Y and Ad.scIL-X infected MCA205 cells. As shown in Figure 1D, we analyzed these supernatants using a limited multiplex Luminex© assay for the expression of cytokines/chemokines associated with inflammatory (IFN-γ, MIP1α, CCL9) and anti-inflammatory (IL-5, IL-15) responses. We chose a subset of cytokine/chemokines representative of these diverse immune responses. In contrast, Ad.scIL-X had a minimal effect on cytokine/chemokine expression (data not shown).
Figure 1. Mouse IL-Y exhibits biological activity.
(A) The IL-12 family heterodimers. (B) A schematic illustration of scIL-Y. (C) ELISA probing the supernatant of A549 infected cell for secreted proteins containing either IL-12p40 subunit only (left) or the IL-12p40/IL-27p28 combination (right). Bar graph show mean + SEM from 3 replicate representing 3 independent experiments. (D) The supernatants of splenocytes treated with CM were assessed using a Luminex assay which screened for a small panel of cytokines/chemokines. These finding were confirmed by a second Luminex assay. Bar graph show mean + SEM from 3 replicate representing 2 independent experiments.
scIL-Y induces phosphorylation of STAT3
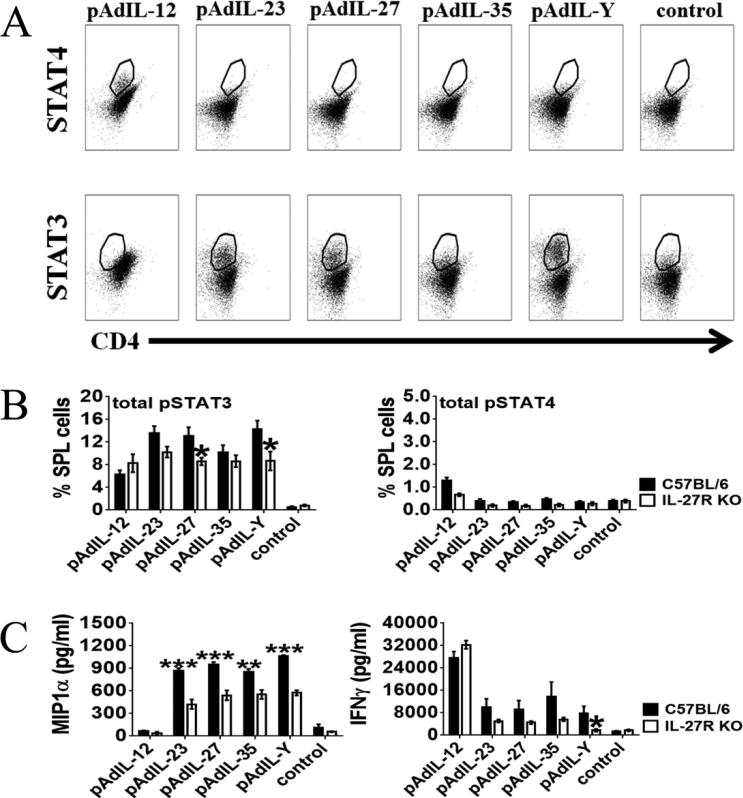
Members of the IL-12 cytokine family signal through cell surface receptors consisting of heterodimers [1]. Each monomer binds individually to its corresponding receptor subunit. For example, IL-12p35 binds to the IL-12Rβ2 subunit and IL-12p40 to IL-12Rß1 subunit of the IL-12R. The potential receptor for scIL-Y would consist of IL-27Rα, binding p28, and IL-12Rβ1, binding p40. Immediately following activation, the IL-12 family member receptors activate the JAK/STAT pathway. To determine if scIL-Y stimulation leads to phosphorylation of either STAT3 or STAT4, which would be activated by the potential scIL-Y receptor, we stimulated C57BL/6 splenocytes with CM for 48 hours and cells prepared for intracellular staining to detect phosphorylated STATs (pSTAT). As shown in Figure 2A, scIL-12 CM induced pSTAT4, consistent with previous observations [22], whereas none of the other CMs tested (scIL-23, scIL-27, scIL-35, scIL-Y) induced pSTAT4 indicating the specificity of the CMs. In contrast, phosphorylation of STAT3 was induced by scIL-23, scIL-27 and scIL-Y in wild type splenocytes. Quantitative analysis demonstrated that pSTAT3 was significantly induced by scIL-Y in comparison to control splenocytes (Fig. 2B). We verified activation of STAT3 by Western Blot analysis in which phosphorylation of STAT3 was induced 3 hours following stimulation with scIL-Y CM (Supporting Information Fig. 1). Interestingly, the analysis revealed that activation of STAT3 was slower than with the other IL-12 family members as very little phosphorylation of STAT3 was detected at 10 minutes or 1 hour following stimulation with CM (Supporting Information Fig. 1). This could be due to a reduced affinity of scIL-Y for its receptor compared to scIL-23 and scIL-27. Importantly, pSTAT3 was significantly reduced in IL-27Rα knockout (KO) splenocytes treated with either scIL-Y or scIL-27 CM, suggesting that IL-27Rα is part of the scIL-Y receptor. There also was a reduction of pSTAT3 in scIL-23 and scIL-35 CM-treated splenocytes, but not to the extent as seen with scIL-Y or scIL-27. No significant differences were observed with pSTAT4 in IL-27Rα KO splenocytes.
Figure 2. Plasmid Ad.scIL-Y conditioned media suppresses cytokine production of agonist T-cell receptor-stimulated splenocytes.
Splenocytes from C57BL/6 and IL-27Rα KO mice were cultured (2 × 106 cells per well) in 96-well plate and incubated with CM (1:2 ratio) or with culture media only (control) for two-days. STAT4 and STAT3 phosphorylation was analyzed on CD4+ cells by flow cytometry. (A) Dot-plots showing the phosphorylated STAT4 and STAT3 in C57BL/6 splenocytes. One representative plot out of 2 independent experiments is shown. (B) Bar graphs depict the percent of wild type or IL-27Rα KO splenocytes that were either STAT3+CD4+ or STAT4+CD4+ following incubation with CM (n=3 ± SEM) or with culture media only (control). Bars show mean ± SEM from 2 independent experiments with 3 samples each. (C) Supernatants from these cultures were collected and analyzed by ELISA for the presence of MIP1α and IFN-γ. The data represents the results of three mice (three replicates per mouse, from one culture per mouse) ± SEM. The data was analyzed by two-way ANOVA (*p<0.05, **p<0.01, ***p<0.001).
We next examined the effect of scIL-Y on cytokine/chemokine production by activated splenocytes. Analysis of the splenocyte supernatants by ELISA revealed that the loss of IL-27Rα reduced the level MIP1α stimulated by scIL-23, scIL-27 and scIL-Y CM, but not scIL-12 (Fig. 2C). A similar trend was observed for IFN-γ. However, the reduction of IFN-γ was only significant for scIL-Y stimulated IL-27Rα KO splenocytes.
Activation of antigen-presenting cells by scIL-Y
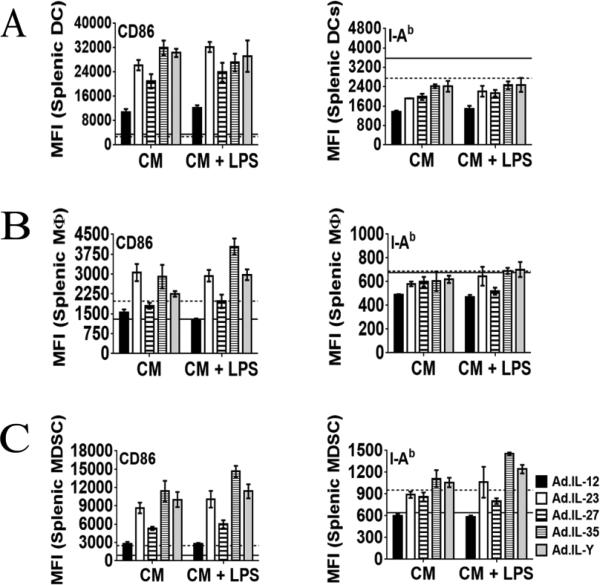
To determine the effect of scIL-Y on antigen presenting cells (APCs), splenocytes were cultured in the presence of CM ± LPS. After 48 hours, the cells were collected and assessed for activation by measuring the up-regulation of CD86 and MHC II. No difference in the activation of DCs (CD11b+CD11c+) was observed with any CM tested (Fig. 3A). A similar pattern for MΦ (CD11b+F4/80+) was observed, with the exception of a greater up-regulation of CD86 by scIL-35 CM + LPS (Fig. 3B). In addition, both CD86 and MHC II were significantly up-regulated by scIL-35 and scIL-Y CM (± LPS) on myeloid derived-suppressor cells (MDSCs; Gr-1+CD11b+) (Fig. 3C).
Figure 3. scILY enhanced the activation of MDSCs.
Splenocytes from wild type C57BL/6 mice (2 × 106 per well) were cultured in the presence of CM ± LPS and stimulated with the indicated cytokines for 48 hours. The cells were collected and prepared for flow cytometry. Antigen presenting cell subsets were gated as follows: for DCs CD11c+CD11b+; for MΦ CD11b+F4/80+; for MDSCs Gr-1+CD11b+. The expression of CD86 and MHC II by DCs (A), MΦ (B) and MDSCs (C) was measured by flow cytometry [dash line positive control (LPS only); solid line negative control (no stimulation)]. The data represents the results of two mice (three replicates per mouse) ± SEM. Two-way ANOVA; *p<0.05, **p<0.01, ***p<0.001].
scIL-Y suppresses the anti-tumor immune response
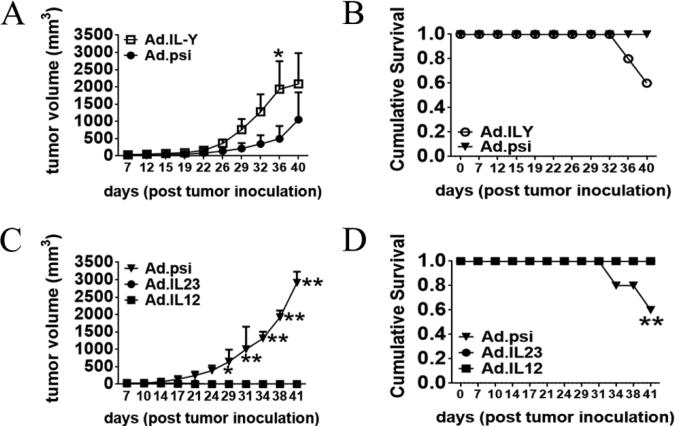
To determine if scIL-Y had any immune activity in vivo, the effect of Ad.scIL-Y on murine tumor growth was evaluated, given that we previously demonstrated significant anti-tumor effects following intra-tumor injection of Ad.IL-12 and Ad.IL-23. MCA205 fibrosarcoma cells were implanted subcutaneously in the flank of C57BL/6 mice and either Ad.scIL-Y or Ad.psi5 injected intra-tumorally on days 7, 9, and 11. Interestingly, mice treated with Ad.scIL-Y exhibited enhanced tumor growth, compared to control mice (Fig. 4A and B). In contrast, tumor-bearing mice treated with either Ad.scIL-12 or Ad.scIL-23 (Fig. 4C and D) quickly rejected the tumor, consistent with previous results [17, 18]. These data suggest that intra-tumor expression of scIL-Y suppresses anti-tumor immunity in vivo.
Figure 4. Ad.scIL-Y is less effective in resisting the tumor growth in vivo.
The anti-tumor effect of Ad.scIL-Y (A and B), Ad.IL-12 (C and D), and Ad.IL-23 (C and D) was evaluated in mice implanted with tumors. Virus was injected intratumorally on days 7, 9 and 11 post-tumor inoculations with 5 × 1010 viral particles. In all experiments, tumor volume was monitored using a metric caliper every 3 or 4 days or until mice were sacrificed due to excessive tumor size or tumor ulceration. Each data point was statistically tested using two-way ANOVA (*p<0.05, **p<0.01, ***p<0.001). The data shown represents the results of five mice + SEM for each group.
scIL-Y suppresses the development of diabetes in NOD mice
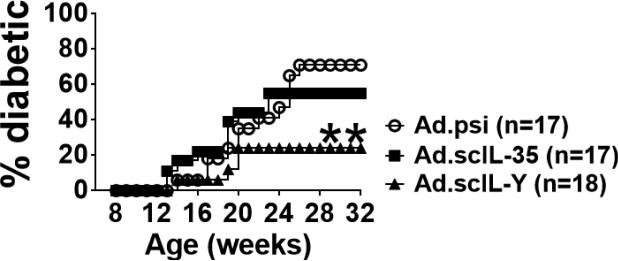
To examine the possible immune suppressive properties of scIL-Y, we utilized the NOD mouse model of T1D [23]. T1D is a T-cell-driven autoimmune disease which results in the destruction of insulin-producing β cells in the pancreatic islets of Langerhans [24]. Eight week old female, pre-diabetic NOD mice were injected intravenously with 5 × 108 infectious units (IU) of Ad.scIL-Y, Ad.scIL-35, or control Ad.psi5 virus and monitored for the onset of hyperglycemia over 6 months. As shown in Figure 5, nearly 80% of the mice infected with Ad.scIL-Y (n=17) were protected from developing diabetes. In contrast, treatment with Ad.scIL-35 (n=18) was less effective in preventing diabetes with approximately 50% of the mice remaining diabetes free. The reduction in frequency as well as the delay in the time of onset of hyperglycemia in NOD mice following Ad.scIL-Y treatment further supports an immunosuppressive function for scIL-Y.
Figure 5. Ad.scIL-Y suppresses the development of diabetes in pre-diabetic NOD mice.
Eight week old NOD mice were infected with 5 × 108 IFU of each of the indicated adeno-virus through i.v. injection. The mice were monitored for the development of diabetes by screening blood glucose levels weekly. Mice were considered diabetic following two consecutive readings of ≥ 250 mg/dl. The graph representative 2 independent experiments with the indicated numbers of mice is shown (One-way ANOVA; *p<0.05, **p<0.01, ***p<0.001).
Immunosuppression associated with scIL-Y is not mediated through regulatory T-cells
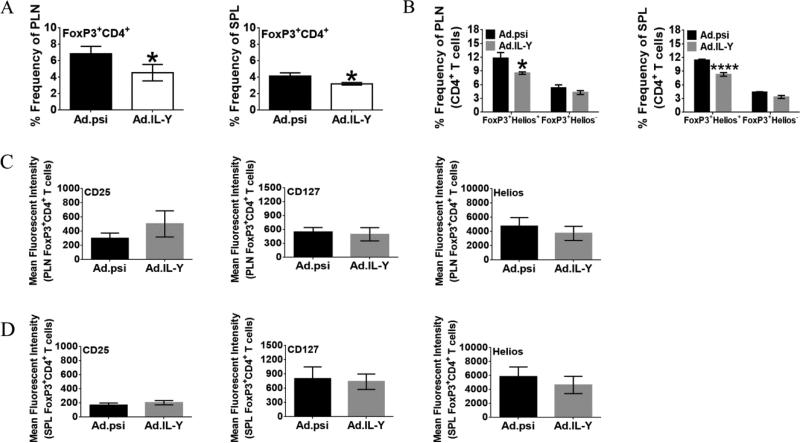
To determine the mechanism of suppression mediated by scIL-Y, we initially examined the possible role of CD4+ Treg cells in conferring protection from T1D. In T1D, studies have shown deficiencies in Treg cells in both their numbers and function [25-27] whereas replacement therapy or reagents that activate Treg cells protect against the development of T1D [28, 29]. Eight week old female NOD mice were infected with Ad.scIL-Y or control Ad.psi5 virus. After four weeks, SPL and PLN cells were harvested and the frequency of Treg cells determined. A significant decrease in the percentage of Treg cells from Ad.scIL-Y treated mice in both the SPL and PLN (Fig. 6A) was observed when gating on the CD4+FoxP3+ population. The reduction of Treg cells was seen predominantly with native Treg cells (FoxP3+Helios+), but not the inducible subset (FoxP3+Helios−), as shown in Figure 6B for both PLN and SPL. Within the CD4+FoxP3+ Treg cell population, analysis of the level of CD25, CD127 and Helios expression showed no significant differences in PLN or SPL Treg cells (Fig. 6C and D). Analysis of mice at 2-weeks post-infection showed no differences between treatment groups (data not shown). Thus, treatment of NOD mice with Ad.scIL-Y induces a protective mechanism that prevents the onset of diabetes. However, this mechanism does not appear to enhance the frequency or activation of CD4+FoxP3+ Treg cells.
Figure 6. Ad.scIL-Y treatment reduces frequency of FoxP3+CD4+ regulatory T cells in wild type NOD mice.
NOD mice were infected with Ad.psi or Ad.scIL-Y at 8 weeks of age. Four weeks post infection both the PLN and SPL were removed and prepared for flow cytometry. (A) Shows the percentage of CD4+FoxP3+ Tregs in the PLN and SPL (Ad.psi n=7, Ad.scIL-Y n=8 ± SEM). (B) Shows the percentage of PLN and SPL Treg cell subsets which were defined on the basis of FoxP3 and Helios expression (nTregs FoxP3+Helios+; iTregs FoxP3+Helios−; Ad.psi and Ad.scIL-Y n=5 ± SEM). (C) Shows the MFI of CD25, Helios, and CD127 expressed by CD4+FoxP3+ Treg cells in the PLN while (D) shows the MFI of these surface markers by Treg cells in the SPL. (A-D) The graph shown represents the cumulative of 2 independent experiments. (Mann-Whitney test; *p<0.05, **p<0.01, ***p<0.001; Ad.psi treated mice n=7. Ad.scIL-Y treated mice n=8 ± SEM).
Re-stimulation of T cells reveals suppression following Ad.scIL-Y treatment
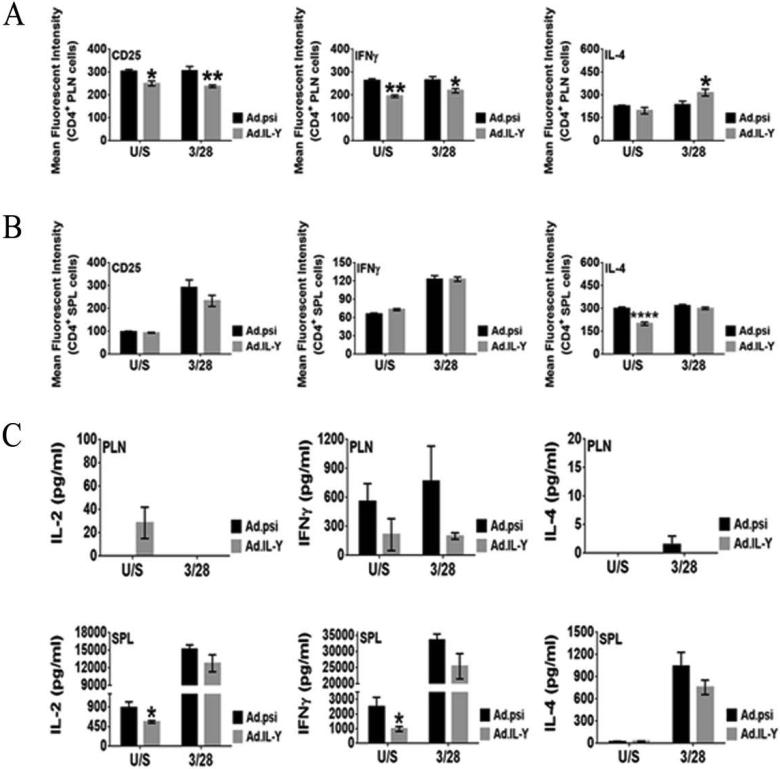
To evaluate further the effect of scIL-Y on effector T-cells, we performed in vitro re-stimulation assay on whole SPL and PLN cells from Ad.scIL-Y treated NOD mice, which were activated through the T-cell receptor (TCR). Four weeks following infection, cells were prepared, transferred into wells previously coated with anti-CD3 mAb and cultured with anti-CD28 mAb for 2-days. Cells were probed for the expression of activation markers and for the production of cytokines such as IFN-γ and IL-4. PLN CD4+ T-cells showed a significant reduction in the expression of both CD25 and IFN-γ in mice infected with Ad.scIL-Y if cells were either untreated or activated through the TCR (Fig. 7A). IL-4 expressing T-cells also were elevated in the PLN of Ad.scIL-Y treated mice only following activation through the TCR. Splenic CD4+ T-cells did not exhibit these changes; however, unstimulated IL-4 expressing cells were significantly reduced (Fig. 7B). We also probed T-cells for the production of IL-10 (Tr1) and IL-17 (Th17) and were unable to detect these cytokines (data not shown). The data from this experiment suggest that the treatment of NOD mice with Ad.scIL-Y antagonized the pro-inflammatory response (IFN-γ) while simultaneously inducing an anti-inflammatory (IL-4) response at least in the PLN.
Figure 7. In vitro re-stimulation of splenic and pancreatic lymph node cells reveals suppression of effector T cells following Ad.scIL-Y treatment.
Cells were transferred into a 96-well plate at 2 × 106 cell per well. The cells were stimulated with plate bound anti-CD3 mAb and soluble anti-CD28 mAb for 2-days. The spleens from five mouse were tested individually (n=5 ± SEM) while the PLN these same mice were pooled toghether and four replicates per condition (±SEM) were tested. Gating on CD4+ cells the expressions of CD25, IFN-γ from the PLN (A) and SPL (B) was determined by flow cytometry. (C) The secretion of IL-2, IFN-γ, and IL-4 was assayed by ELISA (Two-way ANOVA; *p<0.05, **p<0.01, ***p<0.001).
Activation of APCs following treatment of NOD mice with Ad.scIL-Y
To test the effect of scIL-Y on APCs, NOD mice were infected with Ad.scIL-Y or Ad.psi5 and after 4 weeks, SPL and PLN were harvested and prepared for FACS analysis. The frequency of APC subsets (DCs, MΦ, and MDSCs) in the SPL did not differ between the treatment groups (Supporting Information Fig. 2A). However, the expression of CD86, a marker of activation, was elevated in splenic DCs (CD11c+CD11b+) following Ad.scIL-Y treatment. In addition, the frequencies of DCs and MΦ from Ad.scIL-Y infected mice were reduced while MDSCs remained at similar levels between each group (Supporting Information Fig. 2B). The activation of these APC subsets did not differ between each group of mice.
Discussion
The IL-12 family of heterodimeric cytokines, consisting of IL-12, IL-23, IL-27, and IL-35, has important roles in regulating the immune response. Given the promiscuous interactions between the IL-12 family subunits and with their heterodimeric receptors, it is possible that there are additional functional interactions between the IL-12 family of subunits. Thus we generated adenoviral vectors expressing two additional hypothetical IL-12 family heterodimers not yet found naturally, termed IL-Y (p40 and p28) and IL-X (Ebi3 and p19), as single chain molecules. Other studies have shown that single chain fusions of G-CSF and GM-CSF and G-CSF and IL-3 are biologically active and that single chain IL-12 and Il-23 are as or more active than the normal dimeric cytokine [19-21]. Our analysis suggests that IL-Y in particular is able to stimulate a specific subset of cytokines/chemokines, distinct from the IL-12 family members. For example, scIL-Y stimulated expression of MIP1α (CCL3), a chemokine involved in the recruitment of mononuclear cells, most notably neutrophils to the site of inflammation or injury [30, 31] and CCR5+ Treg cells [32]. scIL-Y also specifically stimulated expression of the chemokine MIG (CXCL9), a T-cell chemoattractant, and the cytokines G-CSF, able to stimulate granulocytes and certain stem cell populations, IL-5 and IL-15. However, despite the ability of scIL-Y to stimulate expression of a specific subset of chemokines/cytokines, we have been unable to detect the secretion of endogenous IL-Y from stimulated mouse splenocytes.
The fact that IL12p40 binds to the IL-12Rβ1 whereas IL-27p28 binds primarily to IL-27Rα subunit suggests that the IL-Y receptor could consist of a heterodimer between IL-12Rβ1 and IL-27Rα. IL-27Rα is expressed on CD4+ T-cells, NK cells, NKT cells, and macrophages, but also is expressed on activated B cells, mast cells, monocytes, dendritic cells and polarized Th cells [33-35]. Activation of IL-27Rα drives the phosphorylation of JAK1 and to a lesser extent JAK2 and TyK2, resulting in induction of STAT3 and STAT4 [1, 33, 35]. Our analysis in splenocytes demonstrated that scIL-Y stimulated phosphorylation of STAT3 and that the level of pSTAT3 induction by scIL-Y was reduced, but not completely eliminated, by KO of IL-27Rα. These observations suggest that scIL-Y signals through binding to the IL-27R, but that other interactions, such as with IL-12Rβ1, also could be important.
Given the genes stimulated by scIL-Y in splenocytes including G-CSF, IL-5, IL-15, MIP1α and MIG, we initially hypothesized that localize, intra-tumoral expression would lead to induction of an anti-tumor immune response, similar to those observed with intra-tumoral injection of IL-12 and IL-23. However, tumor-bearing mice treated intra-tumorally with Ad.scIL-Y had enhanced tumor growth, compared to the control, suggesting that localized scIL-Y expression inhibits the anti-tumor response in vivo, similar to IL-35. Thus we explored the effects of transient expression of scIL-Y in the well-characterized NOD mouse model of autoimmune diabetes.
Members of the IL-12 cytokine family have been linked to autoimmune diseases [33]. IL-12 is noted for its ability to skew the development of naïve CD4+ T-cells towards a Th1 phenotype. Also, NOD mice injected with recombinant IL-12 during the early pre-diabetic stage developed diabetes at an accelerated rate [36-38] whereas injection of IL-12 late during the pre-diabetic stage had no effect. In addition, ectopic expression of IL-35 under the control of the rat insulin promoter in NOD mice prevents the development of diabetes [39]. Interestingly, our results demonstrate that following intravenous injection of the adenoviral vectors expressing scIL-Y and scIL-35, transient expression of scIL-Y was more effective than scIL-35 in suppressing the onset of hyperglycemia in NOD mice. scIL-Y expression reduced the percentage of Tregs cells in both the PLN and SPL while maintaining a consistent level of expression for Treg cell associated markers (CD25, Helios, and CD127). Furthermore, the analysis of splenocytes from Ad.scIL-Y treated mice showed a reduction of IL-2 production following TCR activation, similar to what has been shown for IL-27. Since the prevention of diabetes conferred by scIL-Y appears not to be mediated through Treg cells, we analyzed the functional profile of effector T-cells. Here TCR stimulation showed a significant reduction in the expression of CD25 and IFN-γ by CD4+ T-cells in the PLN from scIL-Y treated mice. In addition, IL-4 expression was increased in PLN CD4+ T-cells. Furthermore, ex vivo analysis of splenic and PLN T-cells showed no effect of Ad.scIL-Y on IFN-γ or IL-4 production by effector T-cells (data not shown). Additionally, we were unable to detect IL-10 (Tr1 cells) or IL-17 (Th17 cells) positive effector T-cells (data not known). These data suggest that scIL-Y prevents the onset of hyperglycemia in NOD, in part, through the suppression of effector T-cells function, either directly or indirectly.
IL-Y is structurally similar to IL-27, sharing the IL-27p28 subunit, and thus potentially may share similar functions. IL-27 is a pleotropic cytokine, which links it to various conditions ranging from cancer to autoimmunity [1, 6, 35]. IL-27 was initially considered a pro-inflammatory cytokine since it induces the activation of STAT1 and T-bet and expression of IFN-γ [8, 40-42]. However, subsequent studies showed that the pro-inflammatory phenotype was the result of effective suppression of the anti-inflammatory arm of the immune response (Th2 and Treg cells) [9, 43, 44]. Similar to IL-27, Ad.scIL-Y treatment of NOD mice reduced the percentage of Treg cells and the number of IL-4 expressing CD4+ T-cells in the spleen. However, unlike IL-27, scIL-Y reduces the level of IFN-γ, possibly making it a more immunosuppressive.
Our results demonstrate that not only does scIL-Y exhibit biological activity, but scIL-Y functions as a suppressive cytokine to limit autoimmunity. Our data is similar to those obtained in a recent study examining the biologically effects of a recombinant IL-12p40/IL-27p28 heterodimeric protein [45]. In this study, the recombinant protein was injected into an animal model of uveitis where it prevented the destructive autoimmune response. However, unlike the reduction of Treg cells we observed in our diabetes model, they observed an increase in Treg cells along with a significant suppression of Th17, both of which are active participants in the pathogenesis of uveitis. We saw no effect of scIL-Y on the generation of Th17 cells both in ex vivo (data not shown) and in vitro (data not shown) assays. However, in both studies, the expression of IFN-γ was significantly reduced. Taken together, these results suggest that scIL-Y may serve as a useful therapeutic for reducing inflammation and ameliorating autoimmune diseases.
Materials and Methods
Mouse models
All female C57BL/6, NOD ShijL, and IL-27Rα (C57BL/6 background) knockout mice were obtained from The Jackson Laboratory (Bar Harbor, ME) [46]. For the tumor experiments, six to seven week old C57BL/6 mice were used, whereas for the Type 1 Diabetes analysis, the NOD mouse model was utilized. Animals were maintained under pathogen-free conditions at the animal facility at the University of Pittsburgh and Scripps-Florida. All procedures performed were approved by the University of Pittsburgh and Scripps-Florida Institutional Animal Care and Use Committee.
Cell Lines
MCA205 fibrosarcoma cells were maintained in RPMI supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), and 1% penicillin/streptomycin (Gibco, Carlsbad, CA) and L-glutamine (Gibco, Carlsbad, CA). Cells were kept in a humidified chamber at 37°C/5% CO2 and passaged every 2–3 days.
Adenovirus
Adenoviruses expressing single-chain version of IL-12 and IL-23 have been described previously [17]. For the construction of Ad.scIL-27, the full length clone of the murine EBI3 gene (GenBank NP056581, amino acids 1 to 228) was linked to a truncated version of the IL-27p28 gene (GenBank NP663611, amino acids 29 to 234) by a 15 amino acid (Gly4Ser)3 spacer containing the Bam HI restriction enzyme site (GGATCC, GlySer). The scIL-27 clone was codon-optimized for expression in mammalian cells using the UpGene codon optimization algorithm [47] and synthesized by GenScript, Inc (Piscataway, New Jersey). The scIL-27 open reading frame was inserted into the shuttle vector, pAd.Lox (GenBank U62024), at Sal I/Not I sites engineered in pAd.scIL-27. For the construction of pAd.scIL-35, the p35 gene was amplified with p35-S (5'-GGTGGATCCCGGGTGATTCCGGTGTCCGGT-3') and Ad-R (5'-GTAACCATTATAAGCTGC-3') primers off pAd.scIL-12, digested with BamH I and Not I restriction enzymes and then inserted into the pAd.scIL-27 thus replacing p28. For the construction of pAd.scIL-X, the p19 gene was amplified with p19-S (5'-GGTAGATCTGTACCTAGATCCTCCAGCCCC-3') and Ad-R primers off pAd.scIL-23, digested with Bgl II and Not I restriction enzymes and then inserted into Bam H I and Not I site of pAd.scIL-27 thus replacing p28. For the construction of pAd.scIL-Y, the p40 gene was amplified with Ad-S (5'-CAAGTCTCCACCCCATTG-3') and p40-AS (5'-TACGGATCCACCCCCGCCCGAGCCTCCTCCACCGGAGCC-3') primers off the pAd.scIL-12, digested with Sal I and Bam H I restriction enzymes and then inserted into the Sal 1/BamH I site of pAd.scIL-27 thus replacing the EBI3 gene. All constructions were confirmed by sequencing. Viruses were propagated on HEK-293 cells and purified by CsCl banding, followed by dialysis in 3% sucrose solution. Particle titer of purified viruses was determined by spectroscopy using the equation (OD260) (dilution factor)/9.09×10−13, with the virus being diluted 1:50 prior to measuring OD. The particle titer was used to calculate MOI in all experiments. Infectious titers were determined using quantitative real-time PCR as previously described and were approximately 100-fold less than particle titers [48]. Viruses were aliquoted and stored at −80°C until use. The expression and secretion of scIL-Y by recombinant adenoviruses was verified by ELISA. Briefly, 96-well plates were coated overnight with (1 μg/well) of anti-p40 monoclonal antibodies (Pharmingen) in carbonate coating buffer (pH 9.5) and blocked with PBS containing 2% BSA and 0,05% Tween 20 for 1 hour. The supernatant from A549 cells infected with 10 MOI of Ad.scIL-Y was added and incubated for 2 hours. After the plates were washed, biotin-conjugated anti-p28 (100 ng/well; R&D systems) was added to each well and incubated for one hour. For detection of p40, 96-well plates were coated overnight with 200 μl of the supernatant from A549 cells infected with 10 MOI of Ad.scIL-Y in carbonate coating buffer (pH 9.5) (1:3) followed by biotin-conjugated anti-p40 50 ng/well (R&D systems) and avidin-horse radish peroxidase (HRP) (1:500, Pharmingen) were added to each well and incubated for one hour. The plates were washed three times and developed with 3,3’5,5’-tetramethylbenzidine, and the reaction was stopped with 1M H2SO4 and absorbance at 450nm was determined using an ELISA reader (BIO-TEK instruments).
Adenovirus functional assay
Relative cytokine expression of each adenoviral preparation was analyzed by infecting 4 × 104 MCA205 cells with a 500 MOI for 1 hour at 37°C in serum free-media. Complete media was added and cells were incubated for 72 hours, after which supernatants were harvested. IL-12 and IL-23 content was analyzed using the mouse IL-12 p70 and mouse IL-23 p19/p40 IL-23 ELISA kits (eBioscience, San Diego, CA), respectively, following the manufacturers’ instructions. Biological activity of Ad.scIL-23 was assayed as follows: 4 × 104 MCA205 cells were infected with a MOI 1000 of Ad.scIL-23 or Ad.psi5 and supernatants were harvested 72 hours post-infection. Splenocytes were then harvested from C57BL/6 mice, mechanically dissociated and treated with red blood cell (RBC) lysis buffer (150 mM ammonia chloride, 1 mM sodium bicarbonate, and 0.1 mM EDTA at pH 7.7). Splenocytes were plated at a concentration of 2 × 106 cells per well in a 24-well plate and 24 hours later treated with supernatants from adenovirus-infected MCA205s. Forty-eight hours after treatment, splenocyte supernatants were collected and analyzed for induction of cytokines. The supernatants were assessed with the Luminex® Multiplex mouse 32 cytokines/chemokines panel (Millipore, Billerica, MA).
In vivo tumor assay
Mice were inoculated with 1 × 105 MCA tumor cells via subcutaneous injection into the abdomen. Ad.scIL-12, Ad.scIL-23, Ad.scIL-Y, and Ad.psi5 (5 × 108 I.U.) were injected intratumorally on days 7, 9 and 11 post-inoculations. In all experiments, tumor volume was monitored using a metric caliper every 3 to 4 days or until mice were sacrificed due to excessive tumor size or tumor ulceration.
Diabetes study
At eight weeks of age, NOD mice were treated with 5 × 1010 v.p. per mouse. Viruses were administered through intra-venous (i.v.) injection in a total volume of 100 μl sterile saline solution. Blood glucose monitoring was carried out once per week on restrained, un-anesthetized mice through tail vein bleeds using a FreeStyle Lite glucometer and test strips (Abbott Laboratory, Abbott Park, IL USA).
Flow cytometric analysis of pancreatic lymph nodes and spleen
The SPL and PLN were converted into single cell suspension and washed with sterile 1× PBS. For these tissues, RBCs were depleted, extensively washed with 1× PBS and then passed through a cell strainer. Subsequently, the cells were re-suspended in FACS buffer (2% FBS, 1× PBS, 2mM EDTA, and 0.04% sodium azide) at a concentration of 3.75 × 106 cells per ml. A 200 μl aliquot of each sample was transferred into 96-well polypropylene round-bottom plates (BD Bioscience San Jose, CA). Fc receptors were blocked using anti-CD16/CD32 mAb (purchased from BD Pharmagin) at a 1:600 dilution for 20 minutes at 10°C. Subsequently, the cells were stained with fluorochrome conjugated mAb (purchased from either BD Pharmagin or eBioscience) at the appropriate titer for 45 minutes at 10°C. The cells were washed with FACS buffer twice. For intracellular cytokine staining, the cells were processed using a mouse FoxP3 staining buffer kit purchased from BD Pharmingen (San Diego, CA USA) and used according to the manufacturer's instructions. All flow cytometry samples were processed through a BD LSR II flow cytometer (BD Bioscience San Jose, CA) and analyzed using Flowjo software (Tristar, Inc. Ashland, OR).
In vitro stimulation of wild type and IL-27RαKO splenocytes with conditioned media
All T-cell assays are in compliance with MIATA guidelines. CM was generated using HEK-293 cells. HEK-293 cells (1 × 106) were transfected with plasmid encoding scIL12, scIL23, scIL-27, scIL-35, and scIL-Y for 48 hours. The supernatants were aliquoted and frozen at −20°C until further use. For the in vitro stimulation assay, splenocytes were converted into a single cell suspension and depleted of RBCs, washed extensively and then re-suspended at 2 × 106 per well prior to transfer into a 96-well plate. CM was added at a 1:2 dilution. The cells were cultured for 48 hours at which point supernatants and cells were harvested. The supernatants were assayed specifically for the chemokine MIP1α (CCL3) using an ELISA kit purchased from Sigma-Aldrich (St. Louis, MO) or IL-2, IFN-γ (Abs purchased from BD Pharmingen). The splenocytes were directly assayed for the production of cytokine by intracellular staining by flow cytometry as previously described. Splenocytes from wild type and IL-27Rα KO mice were also analyzed for the phosphorylation of either STAT3 or STAT4 following stimulation by CM. For phospho-STAT (pSTAT) analysis, single cell suspended splenocytes were washed with 1× PBS and then fixed in 2% paraformaldehyde for 30 minutes. Afterwards, the cells were washed twice with 1× PBS and permeabilized with 100% ice cold methanol for 45 minutes. The cells were washed with FACS buffer twice and stained with anti-mouse pSTAT3 (pY705) and anti-mouse pSTAT4 (pY693) (BD Biosciences, San Jose, CA) and T-cell marker CD4 for one hour at 10°C. The cells were washed with FACS buffer twice prior to analysis by flow cytometry. For western blot analysis, splenocytes from wild type mice (2 × 106 cells/well) were stimulated with CM for 10 minutes, 1 hour, and 3 hours in 96 well round bottom plates. Afterwards, the cells were washed twice with 1× PBS and then re-suspended in lysis buffer (1× protease inhibitor (Sigma-Aldrich Inc.), 1× phosphatase inhibitor (Thermo Scientific), 1× cells lysis buffer (Cell Signaling)) and incubated on ice for 30 minutes. The extracts were centrifuged at 13,000g for 20 minutes at 4°C and the supernatants saved. The protein content for each extract was quantified and then prepared for western blot analysis. For the western blot, pSTAT3 was probed for using anti-phosphoSTAT3 (Tyr 705) purchased from Cell Signaling while total STAT3 was assayed using anti-STAT3 purchased from Santa Cruz, Inc. As a loading control, β-actin was probed with anti-β-actin purchased from Cell Signaling.
In vitro re-stimulation assay
The effect of Ad.scIL-Y on effector T-cells was assayed using splenocytes from NOD mice one month post-infection. SPLs and PLNs were recovered from these treated mice. Splenocytes were transferred into a 96-well plate at 2 × 106 cells per well. For this experiment, the SPLs were assayed on an individual bases with multiple replicates. PLNs for each treatment group were pooled together and multiple replicates were assayed. The cells were stimulated through the T-cell receptor using plate bound anti-CD3 mAb (1 μg/ml) and soluble anti-CD28 mAb (0.5 μg/ml) for two days. The supernatants were saved and analyzed by ELISA (IL-2, IL-4, and IFN-γ) while the cells were processed for intracellular cytokine FACS analysis (IL-4 and IFN-γ).
Supplementary Material
Acknowledgements
The authors would like to thank Dewayne Faulkner (University of Pittsburgh) for his assistance with flow cytometry, Joan Nash (University of Pittsburgh) for her administrative support, and Sara McGowan for providing animal husbandry services (The Scripps Research Institute-Florida). This work was supported by grants AG024827 and AR051456 from the National Institutes of Health and a program grant from the Juvenile Diabetes Research Foundation (JDRF) to PDR. RRF was supported by a T32 grant from NIH on Autoimmunity and Immunopathology. This project used the University of Pittsburgh Cancer Institute (UPCI) Vector Facilities supported by the University of Pittsburgh's National Institutes of Health (NIH) Cancer Center Support Grant (CCSG) P30 CA047904.
Abbreviations
- SPL
spleen
- PLN
pancreatic lymph node
- sc
single chain
- Tregs
regulatory T cells
- MOI
multiplicity of infection
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat. Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis KL, Reizis B. Dendritic cells: arbiters of immunity and immunological tolerance. Cold Spring Harb. Persp. Biol. 2012;4:a007401. doi: 10.1101/cshperspect.a007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol. Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall AO, Silver JS, Hunter CA. The immunobiology of IL-27. Adv. in Immunol. 2012;115:1–44. doi: 10.1016/B978-0-12-394299-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 7.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J. Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 10.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 12.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 13.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 14.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J. Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J. Immunol. 2010;184:7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gambotto A, Tuting T, McVey DL, Kovesdi I, Tahara H, Lotze MT, Robbins PD. Induction of antitumor immunity by direct intratumoral injection of a recombinant adenovirus vector expressing interleukin-12. Can. Gene Ther. 1999;6:45–53. doi: 10.1038/sj.cgt.7700013. [DOI] [PubMed] [Google Scholar]
- 17.Reay J, Gambotto A, Robbins PD. The antitumor effects of adenoviral-mediated, intratumoral delivery of interleukin 23 require endogenous IL-12. Can. Gene Ther. 2012;19:135–143. doi: 10.1038/cgt.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reay J, Kim SH, Lockhart E, Kolls J, Robbins PD. Adenoviral-mediated, intratumor gene transfer of interleukin 23 induces a therapeutic antitumor response. Can. Gene Ther. 2009;16:776–785. doi: 10.1038/cgt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhalla K, Tang C, Ibrado AM, Grant S, Tourkina E, Holladay C, Hughes M, et al. Granulocyte-macrophage colony-stimulating factor/interleukin-3 fusion protein (pIXY 321) enhances high-dose Ara-C-induced programmed cell death or apoptosis in human myeloid leukemia cells. Blood. 1992;80:2883–2890. [PubMed] [Google Scholar]
- 20.Lee AY, Chung HK, Bae EK, Hwang JS, Sung BW, Cho CW, Kim JK, et al. A recombinant human G-CSF/GM-CSF fusion protein from E. coli showing colony stimulating activity on human bone marrow cells. Biotech. Lett. 2003;25:205–211. doi: 10.1023/a:1022346800375. [DOI] [PubMed] [Google Scholar]
- 21.Lieschke GJ, Rao PK, Gately MK, Mulligan RC. Bioactive murine and human interleukin-12 fusion proteins which retain antitumor activity in vivo. Nat. Biotech. 1997;15:35–40. doi: 10.1038/nbt0197-35. [DOI] [PubMed] [Google Scholar]
- 22.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson MS, Bluestone JA. The NOD Mouse: A Model of Immune Dysregulation. Annu. Rev. Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson MA. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harb. Persp. Med. 2012;2:a007641. doi: 10.1101/cshperspect.a007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goudy KS, Johnson MC, Garland A, Li C, Samulski RJ, Wang B, Tisch R. Reduced IL-2 expression in NOD mice leads to a temporal increase in CD62Llo FoxP3+ CD4+ T cells with limited suppressor activity. Eur. J. Immunol. 2011;41:1480–1490. doi: 10.1002/eji.201040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J. Exp. Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michels AW, Eisenbarth GS. Immune intervention in type 1 diabetes. Sem. in Immunol. 2011;23:214–219. doi: 10.1016/j.smim.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Maurer M, von Stebut E. Macrophage inflammatory protein-1. The Inter. J. Biochem. and Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, Cunha FQ. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J. Leuk. Biol. 2005;78:167–177. doi: 10.1189/jlb.0404237. [DOI] [PubMed] [Google Scholar]
- 32.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol. 2012;189:5602–5611. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 33.Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. and Aller. Drug Targ. 2009;8:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- 34.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H, Miyazaki Y. Regulation of immune responses by interleukin-27. Immunol. Rev. 2008;226:234–247. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 36.Trembleau S, Penna G, Bosi E, Mortara A, Gately MK, Adorini L. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J. Exp. Med. 1995;181:817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trembleau S, Penna G, Gregori S, Giarratana N, Adorini L. IL-12 administration accelerates autoimmune diabetes in both wild-type and IFN-gamma-deficient nonobese diabetic mice, revealing pathogenic and protective effects of IL-12-induced IFN-gamma. J. Immunol. 2003;170:5491–5501. doi: 10.4049/jimmunol.170.11.5491. [DOI] [PubMed] [Google Scholar]
- 38.Trembleau S, Penna G, Gregori S, Magistrelli G, Isacchi A, Adorini L. Early Th1 response in unprimed nonobese diabetic mice to the tyrosine phosphatase-like insulinoma-associated protein 2, an autoantigen in type 1 diabetes. J. Immunol. 2000;165:6748–6755. doi: 10.4049/jimmunol.165.12.6748. [DOI] [PubMed] [Google Scholar]
- 39.Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35. Diabetes. 2012;61:1519–1526. doi: 10.2337/db11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J. Immunol. 2008;180:922–930. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 41.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J. Interf. and Cyto. Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 42.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J. Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 43.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 44.Wojno ED, Hosken N, Stumhofer JS, O'Hara AC, Mauldin E, Fang Q, Turka LA, et al. A role for IL-27 in limiting T regulatory cell populations. J. Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang RX, Yu CR, Mahdi RM, Egwuagu CE. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J. Biol. Chem. 2012;287:36012–36021. doi: 10.1074/jbc.M112.390625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 47.Gao W, Rzewski A, Sun H, Robbins PD, Gambotto A. UpGene: Application of a web-based DNA codon optimization algorithm. Biotech. Prog. 2004;20:443–448. doi: 10.1021/bp0300467. [DOI] [PubMed] [Google Scholar]
- 48.Bilbao R, Reay DP, Hughes T, Biermann V, Volpers C, Goldberg L, Bergelson J, et al. Fetal muscle gene transfer is not enhanced by an RGD capsid modification to high-capacity adenoviral vectors. Gene Ther. 2003;10:1821–1829. doi: 10.1038/sj.gt.3302084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.