Abstract
Background—
This randomized controlled trial evaluated clinical durability of Zilver PTX, a paclitaxel-coated drug-eluting stent (DES), for femoropopliteal artery lesions. Outcomes compare primary DES versus percutaneous transluminal angioplasty (PTA), overall DES (primary and provisional) versus standard care (PTA and provisional Zilver bare metal stent [BMS]), and provisional DES versus provisional BMS.
Methods and Results—
Patients with symptomatic femoropopliteal artery disease were randomly assigned to DES (n=236) or PTA (n=238). Approximately 91% had claudication; 9% had critical limb ischemia. Patients experiencing acute PTA failure underwent secondary randomization to provisional BMS (n=59) or DES (n=61). The 1-year primary end points of event-free survival and patency showed superiority of primary DES in comparison with PTA; these results were sustained through 5 years. Clinical benefit (freedom from persistent or worsening symptoms of ischemia; 79.8% versus 59.3%, P<0.01), patency (66.4% versus 43.4%, P<0.01), and freedom from reintervention (target lesion revascularization, 83.1% versus 67.6%, P<0.01) for the overall DES group were superior to standard care in nonrandomized comparisons. Similarly, clinical benefit (81.8% versus 63.8%, P=0.02), patency (72.4% versus 53.0%, P=0.03), and freedom from target lesion revascularization (84.9% versus 71.6%, P=0.06) with provisional DES were improved over provisional BMS. These results represent >40% relative risk reduction for restenosis and target lesion revascularization through 5 years for the overall DES in comparison with standard care and for provisional DES in comparison with provisional BMS.
Conclusions—
The 5-year results from this large study provide long-term information previously unavailable regarding endovascular treatment of femoropopliteal artery disease. The Zilver PTX DES provided sustained safety and clinical durability in comparison with standard endovascular treatments.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00120406.
Keywords: angioplasty, drug-eluting stents, paclitaxel, peripheral artery disease, stents
Endovascular management of symptomatic peripheral artery disease (PAD) remains challenging despite its adoption by many as the initial preferred revascularization strategy when anatomically feasible. A wide range of percutaneous therapies using a variety of endovascular devices, including percutaneous transluminal angioplasty (PTA), atherectomy, and stent placement, have been used; however, 5-year results are confined to isolated reports.1–5
Editorial, see p 1435
Clinical Perspective on p 1483
In an effort to reduce restenosis rates, the most frequent cause of failure following any endovascular intervention, drug-eluting stents (DES) were developed. Their success in coronary artery intervention led to the investigation of DES in the superficial femoral artery (SFA) in hopes of providing patients who have claudication or critical limb ischemia a minimally invasive treatment option that is both safe and durable. As previously reported, the Evaluation of the Zilver PTX Drug-Eluting Stent in the Above-the-Knee Femoropopliteal Artery (Zilver PTX Randomized Clinical Trial [RCT]) assessed a self-expanding nitinol DES with polymer-free paclitaxel coating on its outer surfaces. The 1-year primary safety and effectiveness end points were met, and the results demonstrated the benefits at 1 and 2 years of this DES relative to both PTA and provisional bare metal stent (BMS) placement in patients with de novo or restenotic lesions of the above-the-knee femoropopliteal artery.6,7 In addition to DES, other interventional therapies that provide local drug delivery to the SFA with the intent of limiting neointimal hyperplasia have included paclitaxel-coated balloons, which have shown improved initial results in comparison with PTA alone.8–10
PAD is associated with functional decline, reduced quality of life, increased anxiety and depression, and increased cardiovascular morbidity and mortality.11,12 Repeat treatment for PAD is also costly and inconvenient. To evaluate the safety and durability of treatment with the paclitaxel-coated stent, the Zilver PTX RCT included long-term follow-up of this DES through 5 years. This is the largest randomized SFA endovascular device trial reported, and the 5-year outcomes detailed herein provide the longest known comparative outcomes of DES to standard endovascular treatments for femoropopliteal disease.
Methods
A detailed description of the study design, inclusion and exclusion criteria, methods, primary end points at 1 year, and follow-up through 2 years for the Zilver PTX RCT have been previously reported.6,7 In brief, this study included 55 sites in the United States, Japan, and Germany with planned follow-up through 5 years. The inclusion criteria included Rutherford category ≥2, ≥50% diameter stenosis, reference vessel diameter 4 to 9 mm, lesion length up to 14 cm, and at least 1 patent runoff vessel with <50% stenosis throughout its course. Exclusion criteria included untreated >50% stenosis of the inflow tract and previous target vessel stenting. Approval was obtained from each site’s institutional review board or ethics committee, and patients provided written informed consent. This study was overseen by an independent data safety–monitoring board and monitored in accordance with International Conference on Harmonisation – Good Clinical Practices. To determine their relationship to the study procedure or device, major adverse events were adjudicated by an independent clinical events committee. Core laboratories provided independent analyses for radiographic imaging (Brigham and Women’s Hospital Angiographic Core Laboratory, Boston, MA) and duplex ultrasound imaging (VasCore, Massachusetts General Hospital, Boston, MA).
Patients with symptomatic disease of the above-the-knee femoropopliteal arteries were enrolled in this study, with 238 patients in the PTA group and 241 patients in the primary DES group (Zilver PTX, Cook Medical, Bloomington, IN). One hundred twenty patients with acute PTA failure were subsequently randomly assigned to provisional DES (n=61 patients) or provisional BMS placement (n=59 patients; Zilver, Cook Medical, Bloomington, IN). The study design included prespecified comparisons of the primary DES group with the PTA group and the provisional DES group with the provisional BMS group through 5 years. In addition, the overall DES group (including both primary and provisional DES) was compared with a standard care group comprising patients with provisional BMS placement plus patients with optimal PTA. Although not prespecified, through 5-year long-term follow-up, this nonrandomized comparison of the overall DES group with the standard care group is considered more relevant to current clinical practice.
Interventions
It was recommended that stents be oversized by 1 to 2 mm with respect to the reference vessel and placed at least 1 cm below the SFA origin and above the medial femoral epicondyle to fully cover the target lesion(s). Pre- and postdilation were at the physician’s discretion, with residual stenosis <30% required for procedural success. For patients randomly assigned to the PTA group, PTA was performed according to institutional standard practice. Acute PTA failure was defined as ≥30% residual stenosis or a ≥5 mm Hg mean transstenotic pressure gradient, and the protocol required 1 repeat, 2- to 3-minute balloon inflation before PTA was considered a failure. Patients experiencing acute PTA failure underwent secondary randomization to provisional BMS or provisional DES placement.
Medical Therapy
The same antiplatelet regimen was recommended for all patients: clopidogrel (ticlopidine in Japan) starting at least 24 hours before the intervention or a procedural loading dose of 300 mg; continued clopidogrel/ticlopidine therapy for at least 60 days postprocedure; and lifelong aspirin therapy.
Follow-Up
Follow-up included telephone contact at 1, 3, 9, and 18 months to assess overall patient condition. At 6 months, 1 year, and annually thereafter through 5 years, patients underwent an in-clinic assessment, which included Rutherford classification, ankle brachial index, and Walking Impairment Questionnaire.13 Duplex ultrasound evaluation of patency was performed for all patients at 6 months and 1 year. In years 2 through 5, duplex ultrasound evaluation of patency was performed for all stent patients and a subgroup of PTA patients (long-term PTA subgroup). As previously described, patients were assigned to the long-term PTA subgroup using a process of adaptive random selection.7 PTA patients not assigned to the subgroup were censored at 13.5 months. The resulting long-term PTA subgroup represented 58% of the PTA patients who maintained patency through 1 year. High-resolution stent radiographs in 2 planes (1 with the leg straight and 1 with the leg bent 90° at the knee) were obtained at 1, 3, and 5 years to assess stent integrity.
Definitions
Event-free survival (EFS) was defined as freedom from adjudicated major adverse events and freedom from worsening of the Rutherford classification by 2 classes or to class 5 or 6. Major adverse events included death, amputation, clinically driven target lesion revascularization (TLR), target limb ischemia requiring surgical intervention, or surgical vessel repair. Clinically driven TLR was defined as reintervention performed for ≥50% diameter stenosis within ±5 mm of the target lesion after documentation of recurrent clinical symptoms. Thrombosis was defined as site-reported total occlusion of suspected thrombotic origin. Primary patency was defined as <50% stenosis from duplex ultrasonography (peak systolic velocity ratio <2.0) or from arteriography when available.1 Ultrasound examinations were independently analyzed by a core laboratory (VasCore, the Vascular Ultrasound Core Laboratory, Massachusetts General Hospital, Boston, MA). Clinical benefit was defined as freedom from persistent or worsening symptoms of ischemia (ie, claudication, rest pain, ulcer, or tissue loss) after the initial study treatment.
Statistical Analysis
Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). The number of observations represents patients, treated lesions, or implanted stents as specified. According to the analysis plan, primary patency was analyzed on an intent-to-treat basis, defined as the originally randomly assigned treatment group regardless of the actual treatment received. EFS and TLR were analyzed on a per protocol basis, with the per protocol cohort defined as the subset of the intent-to-treat group where patients received their intended original randomly assigned treatment; any patient not treated with the original randomized treatment assignment was excluded from the per protocol group. All analyses of clinical benefit and all comparisons of the overall DES group with the standard care group were performed by using the as-treated population, defined by the treatment actually received regardless of the original randomization assignment, including cases enrolled in continuing medical education conferences. Primary patency was analyzed on an intent-to-treat basis, defined as the originally randomized treatment group regardless of the actual treatment received. All analyses of clinical benefit and all comparisons of the overall DES group with the standard care group were performed by using the as-treated populations, defined as the treatment actually received regardless of the original randomization assignment. Continuous variables were summarized with means and standard deviations or medians and interquartile ranges, with P values calculated by using the standard t test or rank sum (Wilcoxon) test, where appropriate. Dichotomous and polytomous variables were reported as counts and percentages, with P values calculated by using the Fisher exact test. Analysis of patency was also performed by using a multivariable Cox proportional hazards model that accounted for multiple lesions14 and included relevant covariates previously described (ie, lesion length, lesion location, total occlusion, percent diameter stenosis, diabetes mellitus, Rutherford status, calcification, reference vessel diameter, smoking status, dual-antiplatelet therapy status, number of patent runoff vessels, acute PTA failure),7 and their interactions with the treatment arm, as well. Additional subgroup analyses were also performed using univariable hazard rates from a Cox model and presented with forest plots. For the primary safety and effectiveness end points, Kaplan-Meier analyses were performed to assess EFS and primary patency for the primary DES group in comparison with the PTA group; freedom from thrombosis/occlusion and stent integrity for the overall DES group; and freedom from TLR, primary patency, and clinical benefit for the overall DES group in comparison with the standard care group and for the provisional DES group in comparison with the provisional BMS group. P values for the Kaplan-Meier analyses were calculated using the log-rank test. P values for freedom from TLR and patency were adjusted for multiplicity by using the Holm method, as called for in the analysis of these end points at 1 year. Patients completing 5-year follow-up were censored at the end of the 5-year follow-up window.
Results
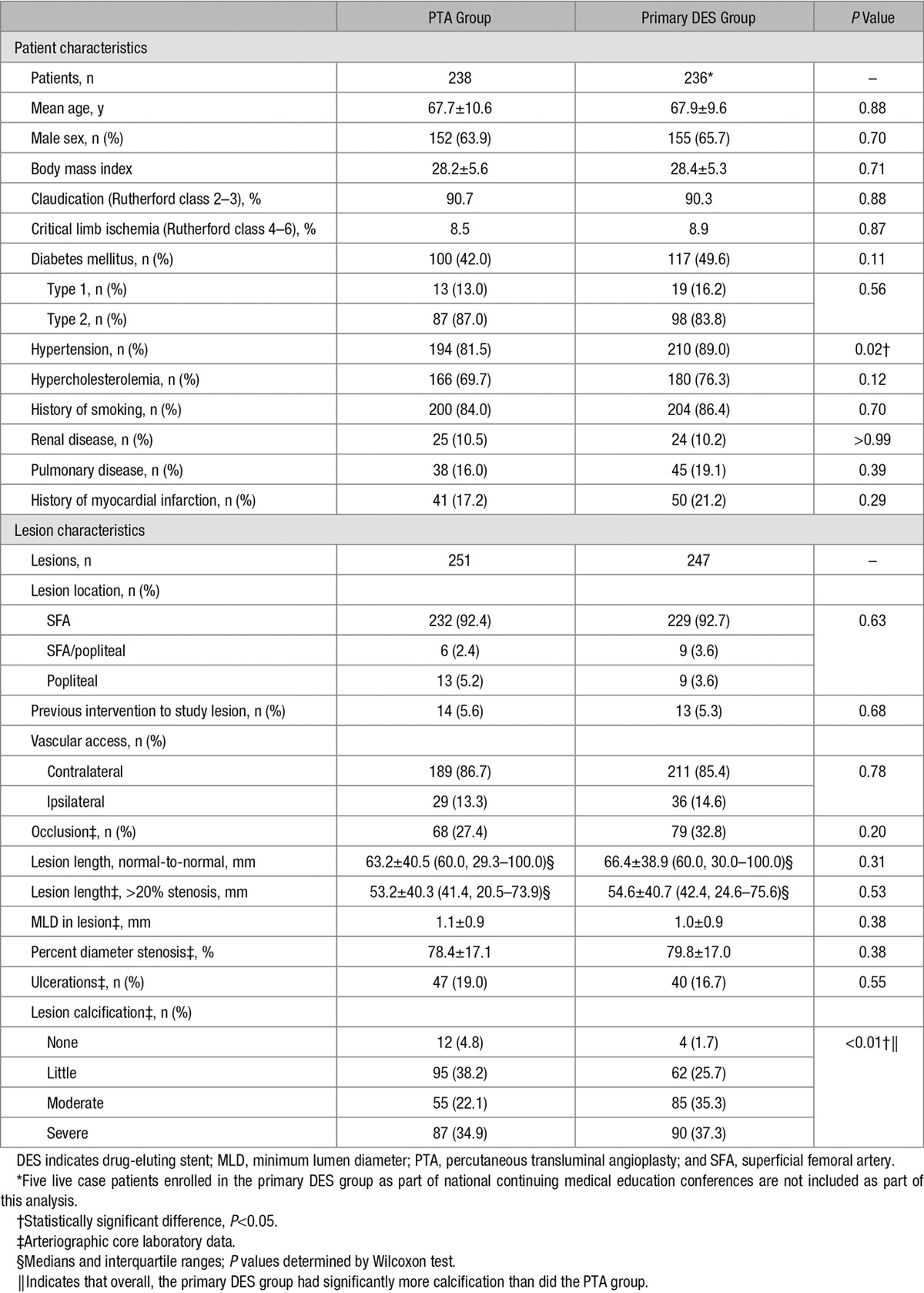
Patient flow diagram is detailed in Figure 1. Through 5 years, the combined withdrawal plus loss to follow-up were ≈6.4% per year. Demographics, comorbidities, and lesion characteristics are shown in the Table. The average lesion length was ≈65±40 mm.
Figure 1.
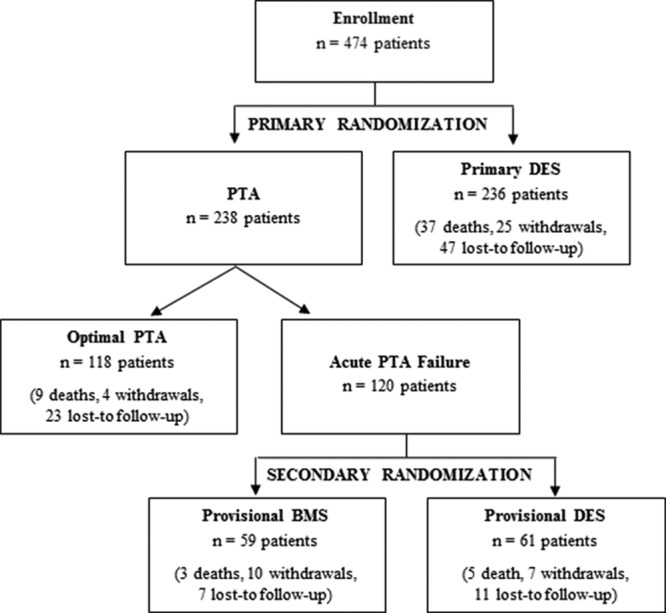
Patient flow diagram. Enrollment by original random assignment, death (all-cause), withdrawal, and loss to follow-up through 5 years are shown. BMS indicates bare metal stent; DES, drug-eluting stent; and PTA, percutaneous transluminal angioplasty.
Table.
Baseline Patient and Lesion Characteristics6,7
Primary End Points
As previously reported, the 1-year primary end points of EFS and primary patency showed superiority of primary DES in comparison with PTA,6 and these results were sustained through 5 years. The EFS rate through 5 years for the primary DES group was significantly greater than that for PTA (Kaplan-Meier estimates 81.4% versus 70.1%, P<0.01, log-rank). The most common end to EFS through 5 years was TLR, which occurred at rates of 16.1% for primary DES and 28.0% for PTA (P<0.01). In the per protocol analyses of EFS and TLR, the PTA group included patients with optimal PTA (n=118), patients receiving provisional BMS (n=59), and patients receiving provisional DES (n=61). The primary patency rate through 5 years for the primary DES group was also significantly greater than that for the PTA group (Kaplan-Meier estimates 64.9% versus 19.0%, P<0.01, log-rank).
Safety
The most common major adverse event through 5 years was clinically driven TLR. The 5-year all-cause mortality rate was 13.6% (10.2% for the primary DES group and 16.9% for the PTA group, P=0.03), and no deaths were adjudicated as procedure or device related.
The thrombosis/occlusion rate was low in both the DES and BMS groups. There was no difference in the rate of freedom from thrombosis/occlusion through 5 years in these 2 groups, with Kaplan-Meier estimates of 97.3% in the overall DES group versus 96.3% in the BMS group (P=0.68, log-rank).
At 1 year, there were 4 stent fractures representing a 0.9% fracture rate.6 At 3 years, 3 additional fractures were identified in the 286 BMS and DES evaluated by x-ray, representing a 1.9% cumulative fracture rate based on Kaplan-Meier estimates. No additional fractures were identified in the 177 BMS and DES with x-ray evaluation at 5 years, representing a 1.9% cumulative rate through 5 years.
Durability of Effectiveness
The 5-year freedom from clinically driven TLR rate was significantly higher for the overall DES group than for the standard care group (83.1% versus 67.6%, P<0.01, log-rank; Figure 2). Similarly, the 5-year primary patency rate for the overall DES group was superior to the standard care group (66.4% versus 43.4%, P<0.01, log-rank; Figure 3). A multivariable Cox proportional hazards analysis of primary patency indicated that there were no significant interactions (all P values >0.05) between the treatment arm (ie, overall DES or standard care) and any of the covariates in the model. In a univariable model, the overall DES group compared favorably with the standard care group for each of several clinically important patient and lesion subgroups analyzed at 5 years (Figure 4). In the optimal PTA group, the Kaplan-Meier estimates for 5-year freedom from TLR and patency rates were 66.2% and 38.3%, respectively.
Figure 2.
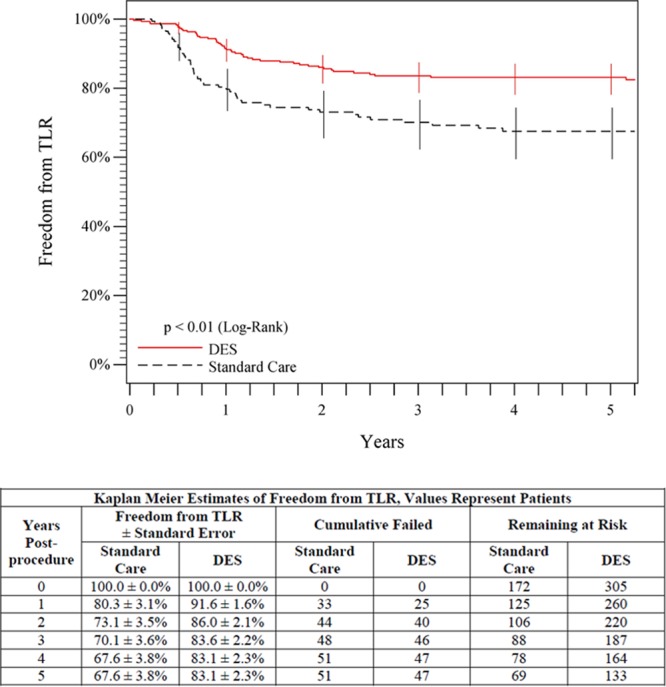
Five-year freedom from TLR outcomes comparing overall DES (primary DES + provisional DES) with standard care (provisional BMS placement + optimal PTA). The black curve shows 67.6% freedom from TLR for the standard care group, and the red curve shows the significantly higher (P<0.01) 83.1% freedom from TLR rate for the DES group. The life table is included. BMS indicates bare metal stent; DES, drug-eluting stent; PTA, percutaneous transluminal angioplasty; and TLR, target lesion revascularization.
Figure 3.
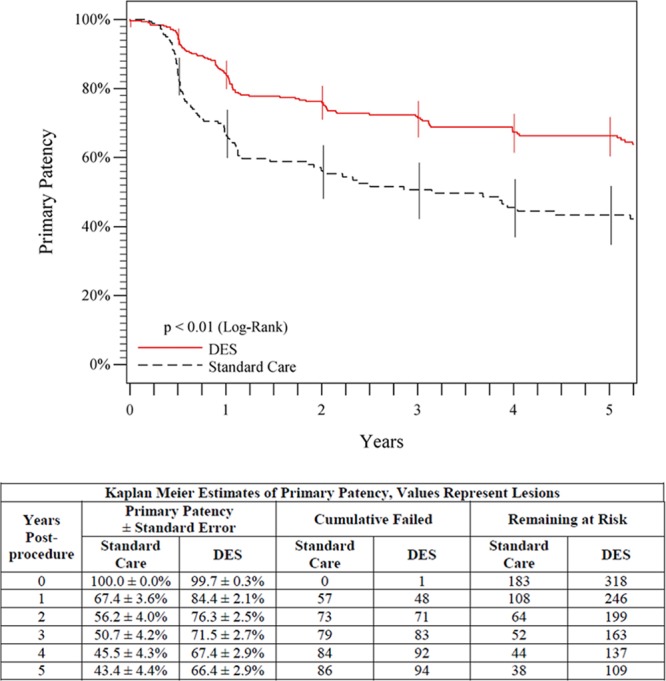
Five-year primary patency outcomes comparing overall DES (primary DES + provisional DES) to standard care (provisional BMS placement + optimal PTA). The black curve shows 43.4% primary patency for the standard care group, and the red curve shows the significantly higher (P<0.01) 66.4% primary patency rate for the DES group. The life table is included. BMS indicates bare metal stent; DES, drug-eluting stent; and PTA, percutaneous transluminal angioplasty.
Figure 4.
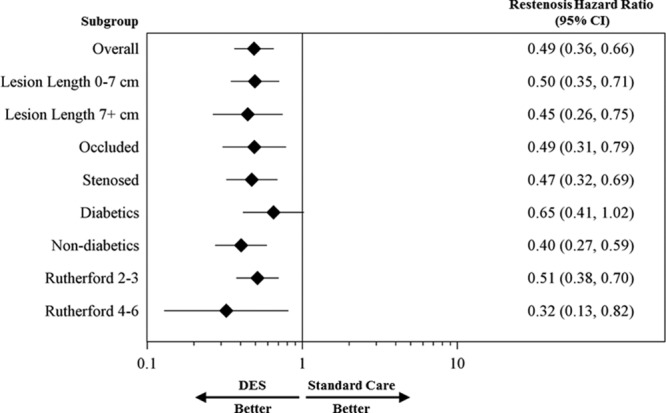
Restenosis hazard ratio for overall DES in comparison with standard care among subgroups of interest through 5 years. The diamonds indicate the hazard ratios, and the lines indicate the 95% confidence intervals (CIs). DES indicates drug-eluting stent.
Evaluation of the provisional stent groups, which provides a direct assessment of the paclitaxel effect, showed 5-year freedom from TLR rates of 84.9% for provisional DES in comparison with 71.6% for provisional BMS (P=0.06, log-rank; Figure 5), and a superior 5-year primary patency rate of 72.4% for provisional DES in comparison with 53.0% for provisional BMS (P=0.03, log-rank; Figure 6). In addition, the primary patency rates for the provisional and primary DES groups were not significantly different through 5 years (72.4% versus 64.9%, P=0.17, log-rank).
Figure 5.
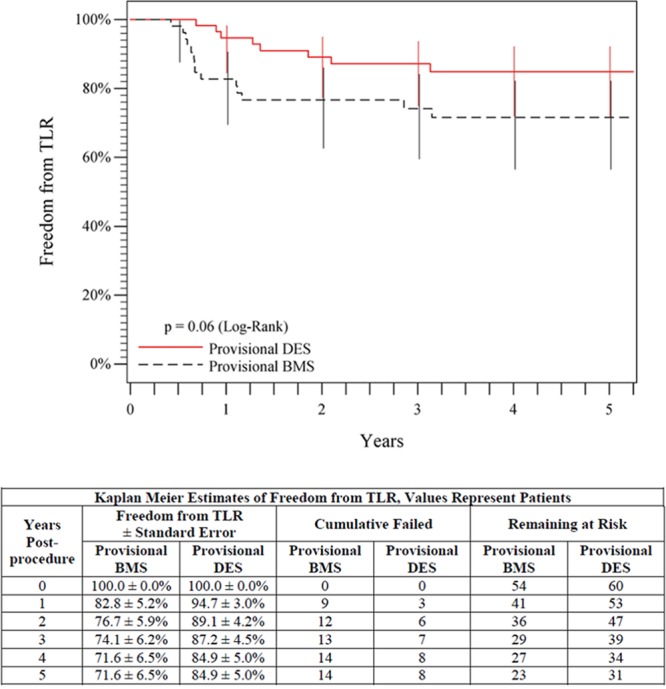
Five-year freedom from TLR outcomes comparing provisional DES with provisional BMS. The black curve shows 71.6% freedom from TLR for the provisional BMS group, and the red curve shows the 84.9% freedom from TLR rate for the provisional DES group. The life table is included. BMS indicates bare metal stent; DES, drug-eluting stent; and TLR, target lesion revascularization.
Figure 6.
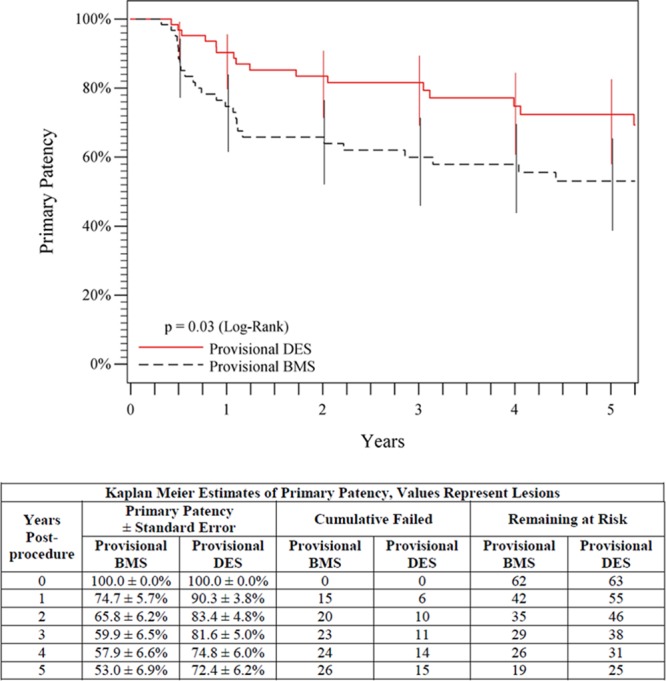
Five-year primary patency outcomes comparing provisional DES with provisional BMS. The black curve shows 53.0% primary patency for the provisional BMS group, and the red curve shows the significantly higher (P=0.03) 72.4% primary patency rate for the provisional DES group. The life table is included. BMS indicates bare metal stent; and DES, drug-eluting stent.
These effectiveness outcomes each represent a >40% relative risk reduction for reintervention or restenosis through 5 years for patients treated with the overall DES group in comparison with the standard care group and with provisional DES in comparison with provisional BMS.
Clinical Benefit
The Rutherford classification, ankle brachial index, and Walking Impairment Questionnaire score each significantly improved (P<0.05) from preprocedure to 5 years both in the overall DES group and in the standard care group. Furthermore, durability of clinical benefit, defined as freedom from persistent or worsening symptoms of ischemia (ie, claudication, rest pain, ulcer, or tissue loss), in the overall DES group was superior to the standard care group (79.8% versus 59.3%, P<0.01, log-rank; Figure 7) at 5 years. Evaluation of the provisional stent groups also showed a significantly higher 5-year clinical benefit for provisional DES than for provisional BMS (81.8% versus 63.8%, P=0.02, log-rank; Figure 8).
Figure 7.
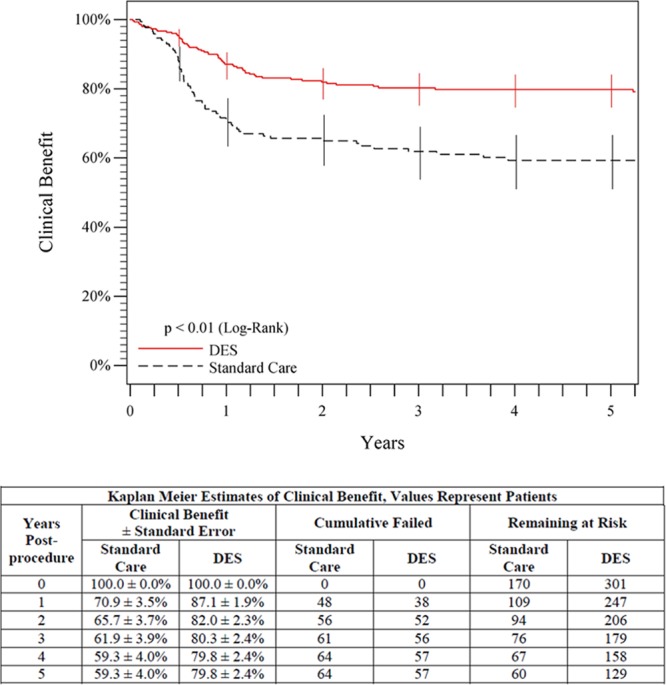
Five-year posttreatment clinical benefit outcomes comparing overall DES (primary DES + provisional DES) with standard care (provisional BMS placement + optimal PTA). Clinical benefit was defined as freedom from persistent or worsening claudication, rest pain, ulcer, or tissue loss after the initial study treatment. The black curve shows that 59.3% of patients in the standard care group maintained clinical benefit, and the red curve shows that 79.8% of patients in the DES group maintained clinical benefit (P<0.01). The life table is included. BMS indicates bare metal stent; DES, drug-eluting stent; and PTA, percutaneous transluminal angioplasty.
Figure 8.
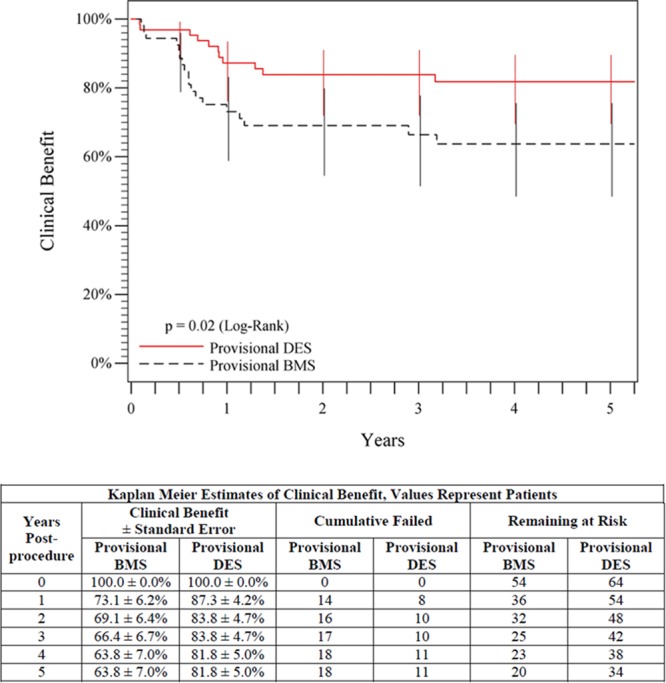
Five-year posttreatment clinical benefit outcomes comparing provisional DES with provisional BMS. Clinical benefit was defined as freedom from persistent or worsening claudication, rest pain, ulcer, or tissue loss after the initial study treatment. The black curve shows that 63.8% of patients in the provisional BMS group maintained clinical benefit, and the red curve shows that 81.8% of patients in the provisional DES group maintained clinical benefit (P=0.02). The life table is included. BMS indicates bare metal stent; and DES, drug-eluting stent.
Discussion
As one of the largest randomized controlled trials of an endovascular device to treat patients with femoropopliteal artery disease, and the first to provide 5-year follow-up, the Zilver PTX RCT provides long-term data and insights regarding the durability of endovascular therapies of the femoropopliteal arteries for patients experiencing claudication or critical limb ischemia. It has been shown that endovascular treatment of PAD improves quality of life, and that subsequent restenosis and reintervention are associated with increased patient risk, increased cost, and reduced quality of life.15–17 Accordingly, a durable treatment option could help limit the functional decline, reduced quality of life, anxiety and depression, and cardiovascular morbidity and mortality that are associated with PAD.11,12
Previous reports at 1 and 2 years6,7 detailed measures of device safety, including EFS, and low frequencies of thrombotic occlusion and stent fracture after DES placement, comparable to the published rates of these adverse events with the use of nitinol BMS.18–22 Through 5 years, the safety and low frequency of device fracture (1.9%) are sustained. There was no procedure- or device-related mortality in this study. The all-cause mortality rate was ≈2.6% per year, illustrating the risk associated with PAD. This rate is also consistent with the ≈3% per year observed in other similar endovascular device trials reporting long-term follow-up.3,23
Measures of effectiveness through 5 years significantly favor the primary DES group versus the PTA group. Furthermore, because it is currently a more clinically relevant comparison, the overall DES group (including primary and provisional DES) was compared with the standard care group (including optimal PTA and provisional BMS). This nonrandomized comparison has the potential for bias; however, the multivariable Cox proportional hazards analysis showed no significant interactions between the treatment arm and any of the covariates in the model, supporting the validity of this comparison. Notably, there is a statistically significant difference through 5 years between the outcomes for the overall DES group in comparison with the standard care group in terms of clinical benefit, TLR, and patency, with the reintervention and restenosis rates both reduced by >40%. In addition, the patency results (Figure 2C) continue to diverge over time, with the difference between the overall DES and standard care groups increasing by a relative 35.3% from 1 to 5 years.
To further evaluate the effect of the drug, a randomized comparison of provisional BMS (non–drug-coated Zilver stent) with provisional DES was performed in 120 patients who had acute PTA failure. The previously published results detailed a statistically significant difference in patency in favor of the drug-coated stent through 2 years.7 After 5 years, this difference is sustained with a patency rate of 72.4% for the provisional DES group versus 53.0% for the provisional BMS patients. Similar to the comparison of the overall DES and standard care groups, this direct comparison of the provisional DES and BMS groups shows a >40% reduction in restenosis at 5 years.
Other studies have also shown benefits of local paclitaxel delivery in the SFA. These include 1-year results from clinical trials of paclitaxel drug-coated balloons randomly assigned against PTA.8–10 However, the long-term results with drug-coated balloons for the SFA are largely unknown.4,24 In this regard, the 5-year outcomes of the Zilver PTX RCT raise some interesting questions regarding the potential long-term effectiveness of balloon versus stent devices. In the Zilver PTX RCT, the patency rate for optimal PTA decreases more than the patency rate for BMS from 1 through 5 years. As a result, the patency rates for BMS and optimal PTA continue to diverge between 1 and 5 years with a relative increase in separation of 44.1%. This new observation reinforces the value of long-term results. Therefore, long-term results will be important to determine if drug-coated balloons follow a PTA-like trajectory, or if the initial drug application will confer a more durable benefit that resists the progressive remodeling and late restenosis of PTA.
Although coronary DES have demonstrated 5-year benefits in comparison with BMS,25,26 published long-term results for other endovascular therapies for the SFA are limited. For example, randomized study results have only been reported to 3 years,3,23 and results through 5 years are limited to a small single-arm study.27 Without comparable 5-year data available for other endovascular SFA devices, published 5-year outcomes for surgical bypass may be the only currently available data for comparison with the DES results. Primary patency rates from RCTs range from 74% to 76% for saphenous vein grafts and from 39% to 68% for polytetrafluoroethylene grafts at 5 years, where loss of patency is determined as absence of flow.28–30 The 5-year patency rate of 66.4% for the overall DES group, where patency is more conservatively determined as <50% diameter stenosis, compares favorably with the patency results for surgical bypass grafting.
A limitation of this RCT is the lack of a primary BMS group for direct comparison with the primary DES group. However, the randomized comparison of provisional DES and provisional BMS placement allowed a direct assessment of the drug effect and showed significant benefit sustained through 5 years. The combined 6.4% per year withdrawal plus loss-to-follow-up rate in this 5-year study was also considered. This is similar to the 5% to 8% per year withdrawal plus loss-to-follow-up rate for other recently published randomized studies of endovascular therapies for the SFA, which are also limited to results through 3 years.3,23 Another limitation of this RCT was the use of a conservative peak systolic velocity ratio <2.0 for evaluating patency by duplex ultrasonography. More recent studies have used a ratio of <2.4 or 2.5, which may result in higher patency rates than the ratio of <2.0 used in this study and make comparison more difficult.9,31,32 The results of the RCT also include only patients with femoropopliteal artery disease of moderate lesion lengths (mean ≈6.5 cm). These lesion lengths were limited to <14 cm by regulatory agency requirements; however, these moderate lesion lengths are common in the large claudicant population.33,34 Furthermore, because PTA and BMS perform better in shorter versus longer lesions, this could make it more challenging to show differences with the DES. Nevertheless, the DES resulted in significant benefit in comparison with PTA and BMS in this study. In addition, 1- and 2-year outcomes from additional Zilver PTX studies support the benefit of the DES in longer and more complex lesions.6,7,34
Conclusion
The Zilver PTX RCT was a large randomized trial that now provides long-term, 5-year results for endovascular treatment of patients with femoropopliteal disease. The outcomes support the sustained safety and more durable clinical benefit of the overall DES group in comparison with the standard care group. The patency benefit is not only sustained, but continues to increase through 5 years. The data also demonstrate the long-term benefit of the drug coating over the corresponding BMS. Together, these results with the DES may help to set a new benchmark for long-term outcomes in the endovascular treatment of femoropopliteal disease.
Acknowledgments
We thank Dr Jeffrey Popma and the team at Brigham and Women’s Hospital Angiographic Core Laboratory, Boston, MA, for radiographic imaging analysis, and Dr Michael Jaff and the team at VasCore, Massachusetts General Hospital, Boston, MA, for duplex ultrasonography imaging analysis. We also thank Aaron E. Lottes, PhD, Alan T. Saunders, MS, and Bret Teany of Cook Research Incorporated (a contract research organization and Cook Group Company) and Theodore Heise, PhD, of Cook Incorporated for assistance with data analysis, figure preparation, and critical review of the manuscript.
Sources of Funding
This study was sponsored by Cook Medical.
Disclosures
Dr Dake is a member of the scientific advisory board for Abbott Vascular, 480 Medical, Inc., and W.L. Gore and Associates; and has received consulting fees from Medtronic, Inc. and Cook Medical. Dr Ansel serves on the medical advisory boards for Boston Scientific Vascular, Cordis Corporation, Abbott Vascular, Medtronic, CR Bard, and W.L. Gore and Associates; has received royalties for vascular products from Cook Medical; and serves as a board member for VIVA Physicians, a 501(c)(3) not-for-profit education and research organization. Dr Jaff is the medical director of VasCore; VasCore is part of the Massachusetts General Physicians Organization, a not-for-profit organization. Dr Jaff receives no compensation from Cook or any sponsor for his work at VasCore. Dr Jaff also serves as a noncompensated advisor for Boston Scientific, Cordis Corporation, Covidien Vascular, and Medtronic Vascular; is an equity shareholder with PQ Bypass; and is a board member for VIVA Physicians, a 501(c)(3) not-for-profit education and research organization. Dr Ohki is a paid consultant for Terumo, Gore, and Cordis. Dr Saxon has received honoraria from Penumbra, consulting fees from Abbott, Control Medical, CR Bard, Penumbra, W.L. Gore and Associates, and has received research, clinical trial, or drug study funds from CR Bard (Lutonix), Penumbra, W.L. Gore and Associates. Dr Smouse has received consulting fees from Novate, Veryan, BSC, and Endotronix; has stock in Novate, Veryan, and Endotronix; has received research or clinical trial funds from Cook Medical; and serves on the scientific advisory board for BSC. Dr Machan serves as a noncompensated advisor for Boston Scientific and is an equity shareholder with Ikomed Inc., and NDC Inc. Drs Snyder, O’Leary, and Ragheb are paid employees of Cook Research Incorporated, a contract research organization and Cook Group Company. Dr Zeller has received honoraria from Abbott Vascular, Angioslide, CR Bard Peripheral Vascular, Veryan, Biotronik, Boston Scientific Corporation, Cook Medical, Cordis Corporation, Covidien, W.L. Gore and Associates, Medtronic, Spectranetics, Straub Medical, TriReme, and VIVA Physicians; has received consulting fees for Abbott Vascular, CR Bard Peripheral Vascular, Boston Scientific Corporation, Cook Medical, W.L. Gore and Associates, Medtronic, Spectranetics, and ReCor; and has received research, clinical trial, or drug study funds from 480 Biomedical, CR Bard Peripheral Vascular, Veryan, Biotronik, Cook Medical, Cordis Corporation, Covidien, W.L. Gore and Associates, Abbott Vascular - IDEV Technologies, Inc, Medtronic, Spectranetics, Terumo, TriReme, and Volcano.
CLINICAL PERSPECTIVE
Lower extremity peripheral artery disease is a manifestation of atherosclerosis that is associated with functional decline, and the reduced quality of life is related to increased cardiovascular morbidity and mortality. Currently, several treatment options for peripheral artery disease exist; specifically, standard treatment options have included percutaneous transluminal angioplasty, atherectomy, and bare metal stent placement. Despite the treatments available, endovascular management of symptomatic peripheral artery disease remains challenging and is often burdened by restenosis that results in treatment failure. Resulting repeat treatments after primary failure are associated with increased cost and inconvenience to the patient. New treatment strategies were developed in an effort to reduce restenosis rates; namely, drug-eluting stents (DES), which had clinical success in the treatment of coronary artery disease, were expanded to include treatment of the superficial femoral artery. This multicenter study, with sites in the United States, Japan, and Germany, was initiated to evaluate the safety and durability of treatment with the paclitaxel-coated DES, the Zilver PTX. Patients with symptomatic disease (ie, Rutherford category ≥2 and ≥50% diameter stenosis) of above-the-knee femoropopliteal arteries were randomly assigned to either receive percutaneous transluminal angioplasty or primary DES. In total, 238 patients were randomly assigned to the percutaneous transluminal angioplasty group and 241 patients were randomly assigned to the primary DES group. Subsequently, 120 patients with acute percutaneous transluminal angioplasty failure were further randomly assigned to provisional DES (n=61) or provisional bare metal stent placement (n=59). Comparisons and outcomes were followed through 5 years. The outcomes support the sustained safety and more durable clinical benefit of the DES treatments in comparison with standard care treatment options; namely, the patency benefit is not only sustained, but continues to increase through 5 years. The data also demonstrate the long-term benefit of DES use over bare metal stent placement. Therefore, as one of the largest randomized controlled trials of an endovascular device to treat patients with femoropopliteal artery disease, and the first to provide 5-year follow-up, the Evaluation of the Zilver PTX Drug-Eluting Stent in the Above-the-Knee Femoropopliteal Artery (Zilver PTX Randomized Clinical Trial) provides long-term data and insights regarding the durability of endovascular therapies of the femoropopliteal arteries for patients who have claudication or critical limb ischemia.
References
- 1.Rocha-Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G VIVA Physicians, Inc. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69:910–919. doi: 10.1002/ccd.21104. doi: 10.1002/ccd.21104. [DOI] [PubMed] [Google Scholar]
- 2.Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, Loewe C, Cejna M, Lammer J, Minar E. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–2749. doi: 10.1161/CIRCULATIONAHA.107.688341. doi: 10.1161/CIRCULATIONAHA.107.688341. [DOI] [PubMed] [Google Scholar]
- 3.Geraghty PJ, Mewissen MW, Jaff MR, Ansel GM VIBRANT Investigators. Three-year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. J Vasc Surg. 2013;58:386–95.e4. doi: 10.1016/j.jvs.2013.01.050. doi: 10.1016/j.jvs.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 4.Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, Speck U, Zeller T. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. 2015;8(1 pt A):102–108. doi: 10.1016/j.jcin.2014.07.023. doi: 10.1016/j.jcin.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Marmagkiolis K, Hakeem A, Choksi N, Al-Hawwas M, Edupuganti MM, Leesar MA, Cilingiroglu M. 12-month primary patency rates of contemporary endovascular device therapy for femoro-popliteal occlusive disease in 6,024 patients: beyond balloon angioplasty. Catheter Cardiovasc Interv. 2014;84:555–564. doi: 10.1002/ccd.25510. doi: 10.1002/ccd.25510. [DOI] [PubMed] [Google Scholar]
- 6.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS Zilver PTX Investigators. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 7.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Snyder SA, O’Leary EE, Tepe G, Scheinert D, Zeller T Zilver PTX Investigators. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J Am Coll Cardiol. 2013;61:2417–2427. doi: 10.1016/j.jacc.2013.03.034. doi: 10.1016/j.jacc.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Werk M, Albrecht T, Meyer DR, Ahmed MN, Behne A, Dietz U, Eschenbach G, Hartmann H, Lange C, Schnorr B, Stiepani H, Zoccai GB, Hänninen EL. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5:831–840. doi: 10.1161/CIRCINTERVENTIONS.112.971630. doi: 10.1161/CIRCINTERVENTIONS.112.971630. [DOI] [PubMed] [Google Scholar]
- 9.Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Müller-Hülsbeck S, Nehler MR, Benenati JF, Scheinert D LEVANT 2 Investigators. Trial of a Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease. N Engl J Med. 2015;373:145–153. doi: 10.1056/NEJMoa1406235. doi: 10.1056/NEJMoa1406235. [DOI] [PubMed] [Google Scholar]
- 11.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011;123:87–97. doi: 10.1161/CIRCULATIONAHA.109.881888. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger JS, Hiatt WR. Medical therapy in peripheral artery disease. Circulation. 2012;126:491–500. doi: 10.1161/CIRCULATIONAHA.111.033886. doi: 10.1161/CIRCULATIONAHA.111.033886. [DOI] [PubMed] [Google Scholar]
- 13.Hiatt WR, Hirsch AT, Regensteiner JG, Brass EP. Clinical trials for claudication. Assessment of exercise performance, functional status, and clinical end points. Vascular Clinical Trialists. Circulation. 1995;92:614–621. doi: 10.1161/01.cir.92.3.614. [DOI] [PubMed] [Google Scholar]
- 14.Lee EW, Wei LJ, Amato DA. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, editors. Survival Analysis: State of the Art. Dordrecht, Netherlands: Kluwer Academic Publishers; 1992. pp. 237–247. [Google Scholar]
- 15.Sabeti S, Czerwenka-Wenkstetten A, Dick P, Schlager O, Amighi J, Mlekusch I, Mlekusch W, Loewe C, Cejna M, Lammer J, Minar E, Schillinger M. Quality of life after balloon angioplasty versus stent implantation in the superficial femoral artery: findings from a randomized controlled trial. J Endovasc Ther. 2007;14:431–437. doi: 10.1177/152660280701400401. doi: 10.1583/1545-1550(2007)14[431:QOLABA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Chetter IC, Spark JI, Kent PJ, Berridge DC, Scott DJ, Kester RC. Percutaneous transluminal angioplasty for intermittent claudication: evidence on which to base the medicine. Eur J Vasc Endovasc Surg. 1998;16:477–484. doi: 10.1016/s1078-5884(98)80237-2. [DOI] [PubMed] [Google Scholar]
- 17.Deutschmann HA, Schoellnast H, Temmel W, Deutschmann M, Schwantzer G, Fritz GA, Brodmann M, Hausegger KA. Endoluminal therapy in patients with peripheral arterial disease: prospective assessment of quality of life in 190 patients. AJR Am J Roentgenol. 2007;188:169–175. doi: 10.2214/AJR.05.1408. doi: 10.2214/AJR.05.1408. [DOI] [PubMed] [Google Scholar]
- 18.Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J, Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 19.Cheng SW, Ting AC, Wong J. Endovascular stenting of superficial femoral artery stenosis and occlusions: results and risk factor analysis. Cardiovasc Surg. 2001;9:133–140. doi: 10.1016/s0967-2109(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 20.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Tielbeek A, Anderson J, Wiesinger B, Tepe G, Lansky A, Mudde C, Tielemans H, Bérégi JP. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J Vasc Interv Radiol. 2005;16:331–338. doi: 10.1097/01.RVI.0000151260.74519.CA. doi: 10.1097/01.RVI.0000151260.74519.CA. [DOI] [PubMed] [Google Scholar]
- 21.Cejna M, Thurnher S, Illiasch H, Horvath W, Waldenberger P, Hornik K, Lammer J. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol. 2001;12:23–31. doi: 10.1016/s1051-0443(07)61397-9. [DOI] [PubMed] [Google Scholar]
- 22.Bosiers M, Deloose K, Callaert J, Moreels N, Keirse K, Verbist J, Peeters P. Results of the Protégé EverFlex 200-mm-long nitinol stent (ev3) in TASC C and D femoropopliteal lesions. J Vasc Surg. 2011;54:1042–1050. doi: 10.1016/j.jvs.2011.03.272. doi: 10.1016/j.jvs.2011.03.272. [DOI] [PubMed] [Google Scholar]
- 23.Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ, Goldstein J, Cao AY, Jaff MR RESILIENT Investigators. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther. 2012;19:1–9. doi: 10.1583/11-3627.1. doi: 10.1583/11-3627.1. [DOI] [PubMed] [Google Scholar]
- 24.Micari A, Cioppa A, Vadalà G, Castriota F, Liso A, Marchese A, Grattoni C, Pantaleo P, Cremonesi A, Rubino P, Biamino G. 2-year results of paclitaxel-eluting balloons for femoropopliteal artery disease: evidence from a multicenter registry. JACC Cardiovasc Interv. 2013;6:282–289. doi: 10.1016/j.jcin.2013.01.128. doi: 10.1016/j.jcin.2013.01.128. [DOI] [PubMed] [Google Scholar]
- 25.Ellis SG, Stone GW, Cox DA, Hermiller J, O’Shaughnessy C, Mann T, Turco M, Caputo R, Bergin PJ, Bowman TS, Baim DS TAXUS IV Investigators. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV-SR: Treatment of De Novo Coronary Disease Using a Single Paclitaxel-Eluting Stent). JACC Cardiovasc Interv. 2009;2:1248–1259. doi: 10.1016/j.jcin.2009.10.003. doi: 10.1016/j.jcin.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Weisz G, Leon MB, Holmes DR, Jr, Kereiakes DJ, Popma JJ, Teirstein PS, Cohen SA, Wang H, Cutlip DE, Moses JW. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) Trial. J Am Coll Cardiol. 2009;53:1488–1497. doi: 10.1016/j.jacc.2009.01.050. doi: 10.1016/j.jacc.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 27.Fischer M, Schwabe C, Schulte KL. Value of the hemobahn/viabahn endoprosthesis in the treatment of long chronic lesions of the superficial femoral artery: 6 years of experience. J Endovasc Ther. 2006;13:281–290. doi: 10.1583/05-1799.1. doi: 10.1583/05-1799.1. [DOI] [PubMed] [Google Scholar]
- 28.Prendiville EJ, Yeager A, O’Donnell TF, Jr, Coleman JC, Jaworek A, Callow AD, Mackey WC, Deterling RA. Long-term results with the above-knee popliteal expanded polytetrafluoroethylene graft. J Vasc Surg. 1990;11:517–524. [PubMed] [Google Scholar]
- 29.Green RM, Abbott WM, Matsumoto T, Wheeler JR, Miller N, Veith FJ, Money S, Garrett HE. Prosthetic above-knee femoropopliteal bypass grafting: five-year results of a randomized trial. J Vasc Surg. 2000;31:417–425. [PubMed] [Google Scholar]
- 30.Ballotta E, Renon L, Toffano M, Da Giau G. Prospective randomized study on bilateral above-knee femoropopliteal revascularization: polytetrafluoroethylene graft versus reversed saphenous vein. J Vasc Surg. 2003;38:1051–1055. doi: 10.1016/s0741-5214(03)00608-6. doi: 10.1016/S0741. [DOI] [PubMed] [Google Scholar]
- 31.Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ, Goldstein J, Jaff MR RESILIENT Investigators. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3:267–276. doi: 10.1161/CIRCINTERVENTIONS.109.903468. doi: 10.1161/CIRCINTERVENTIONS.109.903468. [DOI] [PubMed] [Google Scholar]
- 32.Bosiers M, Torsello G, Gissler HM, Ruef J, Müller-Hülsbeck S, Jahnke T, Peeters P, Daenens K, Lammer J, Schroë H, Mathias K, Koppensteiner R, Vermassen F, Scheinert D. Nitinol stent implantation in long superficial femoral artery lesions: 12-month results of the DURABILITY I study. J Endovasc Ther. 2009;16:261–269. doi: 10.1583/08-2676.1. doi: 10.1583/08-2676.1. [DOI] [PubMed] [Google Scholar]
- 33.Dake MD, Scheinert D, Tepe G, Tessarek J, Fanelli F, Bosiers M, Ruhlmann C, Kavteladze Z, Lottes AE, Ragheb AO, Zeller T Zilver PTX Single-Arm Study Investigators. Nitinol stents with polymer-free paclitaxel coating for lesions in the superficial femoral and popliteal arteries above the knee: twelve-month safety and effectiveness results from the Zilver PTX single-arm clinical study. J Endovasc Ther. 2011;18:613–623. doi: 10.1583/11-3560.1. doi: 10.1583/11-3560.1. [DOI] [PubMed] [Google Scholar]
- 34.Yokoi H, Ohki T, Kichikawa K, Nakamura M, Komori K, Nanto S, O’Leary EE, Lottes AE, Snyder SA, Dake MD. Zilver PTX Post-market surveillance study of paclitaxel-eluting stents for treating femoropopliteal artery disease in Japan: 12-month results. JACC. Cardiovasc Interv. 2016;9:271–277. doi: 10.1016/j.jcin.2015.09.035. doi: 10.1016/j.jcin.2015.09.035. [DOI] [PubMed] [Google Scholar]


