Abstract
Background
The German Diabetes Study (GDS) is a prospective longitudinal cohort study describing the impact of subphenotypes on the course of the disease. GDS aims at identifying prognostic factors and mechanisms underlying the development of related comorbidities.
Study design and methods
The study comprises intensive phenotyping within 12 months after clinical diagnosis, at 5-year intervals for 20 years and annual telephone interviews in between. Dynamic tests, including glucagon, mixed meal, intravenous glucose tolerance and hyperinsulinemic clamp tests, serve to assess beta-cell function and tissue-specific insulin sensitivity. Magnetic resonance imaging and multinuclei spectroscopy allow quantifying whole-body fat distribution, tissue-specific lipid deposition and energy metabolism. Comprehensive analyses of microvascular (nerve, eye, kidney) and macrovascular (endothelial, cardiorespiratory) morphology and function enable identification and monitoring of comorbidities. The GDS biobank stores specimens from blood, stool, skeletal muscle, subcutaneous adipose tissue and skin for future analyses including multiomics, expression profiles and histology. Repeated questionnaires on socioeconomic conditions, patient-reported outcomes as quality of life, health-related behavior as physical activity and nutritional habits are a specific asset of GDS. This study will recruit 3000 patients and a group of humans without familiy history of diabetes. 237 type 1 and 456 type 2 diabetes patients have been already included.
Electronic supplementary material
The online version of this article (doi:10.1186/s12933-016-0374-9) contains supplementary material, which is available to authorized users.
Keywords: Insulin resistance, Magnetic resonance spectroscopy, Beta cell function, Metabolic phenotyping, Diabetes comorbidities
Background
Why was the cohort set up?
Diabetes mellitus (DM), particularly type 2 DM, is a global health issue affecting about 387 million people [1]. Also type 1 DM, characterized by insulin deficiency due to autoimmune-mediated beta-cell destruction, is rising [2]. All DM types tightly associate with microvascular (retinopathy, nephropathy, neuropathy) and macrovascular comorbidities (ischemic heart disease, peripheral vascular disease, cerebrovascular disease) and several malignancies. The resulting organ dysfunctions affect at least one-third of individuals with DM accounting for approximately 8 % of global all-cause mortality in humans aged between 20 and 79 years [3].
Subphenotypes of DM
Type 2 DM is characterized by a long “prediabetic” state with impaired insulin sensitivity, which promotes hyperinsulinemia. With failing insulin secretion, glycemia increases until diagnostic thresholds for overt diabetes are exceeded [4]. There is evidence for the existence of subphenotypes even in the prediabetic state, possibly resulting from differences in the pathogenesis, and also with regard to the course of disease and related complications. In most forms of DM, beta cell dysfunction is a major driving force for disease development and progression [5]. Despite extensive research on potential underlying mechanisms, such as glucose- or lipid-mediated toxicity [6, 7], mitochondrial dysfunction [8] or inflammation [9] the processes involved in beta cell failure are not fully understood [10]. Defects in pancreatic beta-cell function are often preceded by insulin resistance [11–13] which is not only a feature of type 2 DM, but is also found in patients with type 1 DM [14, 15]. Moreover, obesity associates with increased incidence of type 1 DM [16, 17], which has led to the term ‘double diabetes’. The mechanisms linking type 1 DM and insulin resistance, and thereby type 2 DM, are yet unknown.
Gene variants identified by genome-wide association studies seem to affect DM susceptibility predominantly through beta-cell dysfunction but also with insulin resistance [18]. The risk alleles are common in the population, but their effect size is small and not suitable for general genetic screening [19]. Identification of genetic determinants for insulin resistance relied on its surrogate markers such as fasting insulin [20, 21]. If such genetic variants are studied in comprehensively phenotyped cohorts [22], novel therapy targets might be identified [23]. The distribution of subphenotypes, the predictive value for the development of comorbidities and the effectiveness of stratified or personalized treatment strategies are yet unclear.
Complications and diagnosis of early manifestations
In individuals at low risk of cardiovascular diseases, i.e. younger non-smoking normotensive women, the relative cardiovascular risk is multiplied by the occurrence of type 2 DM [24]. Individuals with newly diagnosed type 2 DM already present comorbidities presumably due to longer-term undetected hyperglycemia [25], while some patients with type 1 DM show a rapid progress of diabetes-associated diseases [26] despite good metabolic control [27]. Regarding macrovascular diseases, the prognostic importance of the metabolic syndrome compared to the sum of its individual components has been challenged [28]. Even the obvious link between hyperglycemia and comorbidities appears complex [29]. The predictive value of subphenotypes for the development of comorbidities and the effectiveness of individualized treatment strategies is far from being understood. GDS focusses on the presumably formative early period after diagnosis of the disease and the thorough prospective assessment of (pre) clinical manifestations of comorbidities over 10 years.
Individualized intervention strategies
There is evidence for sustained protection by intensive glycemic control early after onset of DM, with reduction of mortality and micro- and macrovascular comorbidities decades thereafter, referred to as legacy effect [24, 29]. Current glucose-lowering drugs have modest efficacy on diabetes endpoints, so novel therapy strategies need to prove additional positive effects on the development of comorbidities.
Nutritional behavior is an important lifestyle factor influencing the risk of developing type 2 DM [30] and to some extent of type 1 DM [31, 32]. Dietary intervention in type 2 DM can improve insulin sensitivity and beta-cell function [33]. Accordingly, excess availability of certain metabolites such as free fatty acids and branched-chain amino acids induces whole-body insulin resistance [34–36]. Together with gene variants related to response to lifestyle intervention [37], metabolome profiling will have impact for the development of treatment and prevention strategies. The GDS has the potential to imply targeted interventions in selected subgroups covering a broad range of potential mechanisms as well as diabetic comorbidities.
Classical and novel risk factors
Despite the vast amount of data on classical risk factors, the impact of novel biomarkers, e.g. identified by multiomics technologies or innovative imaging tools is incompletely understood [38, 39]. The majority of epidemiological studies have estimated insulin resistance from surrogate parameters, yielding incorrect results in patients with impaired beta-cell function [40–42]. Therefore, we aimed at standardizing methods for comprehensive metabolic phenotyping and implementation of novel tools in the initial period between 2005 and 2009. The updated validated study program started in 2009 with a focus on cellular mechanisms of insulin resistance. High lipid availability and ectopic lipid deposition in skeletal muscle and the liver play a central role in the development of insulin resistance [43] but the cellular mechanisms remain unknown [44, 45]. Regulators of subcellular lipid partitioning and mitochondrial oxidation strongly determine insulin sensitivity but are only partially understood [46].
Both DM and its comorbidities have in common that inflammation-related processes are involved in their development [47–49]. The current knowledge on the relevance of biomarkers of subclinical inflammation is mainly restricted to studies on hard cardiovascular endpoints in patients with type 2 DM [47–50]. Prospective data are limited on associations of inflammation and quantitative phenotypes in earlier stages of cardiovascular disease and all stages of microvascular comorbidities or cognitive decline and are mainly derived from cohorts of patients with type 1 DM. We hypothesize that circulating biomarkers of subclinical inflammation predict the deterioration of insulin sensitivity and beta-cell function and the progression of micro- and macrovascular comorbidities in patients with newly diagnosed DM.
For the identification of novel predictors, most previous studies included patients with longer DM duration [51–53]. Therefore, we acquire circulating biomarkers of subclinical inflammation, non-invasive data on energy and lipid metabolism in liver and skeletal muscle and store biopsy samples of skeletal muscle and subcutaneous adipose tissue for future analyses.
Sociodemographic and psychosocial determinants have been discussed as modifiable regulators of the course of DM. These include e.g. socioeconomic position, health-related quality of life and mental disorders [54–57]. Knowledge about the changes of innovative patient reported measures such as patients’ preferences, information needs and time needed for health-related activities [58, 59] during the progression of the disease is lacking so far.
The aims for setting up the GDS were identification of (i) sub-phenotypes of DM with respect to insulin sensitivity, insulin secretion and (ii) predictors of early diabetes-related comorbidities, (iii) to develop individualized intervention strategies for the treatment and prevention of diabetes and related comorbidities (iv) to analyze the impact of known and novel risk factors (i.e. nutrition, subclinical inflammation, energy metabolism, body fat distribution, metabolites and other biomarkers, socioeconomic and psychosocial conditions) on disease progression and development of diabetes-related comorbidities, in recently diagnosed patients with type 1 and type 2 DM. GDS will test the hypothesis that the course of DM and related comorbidities is determined by processes that depend on diabetes type, affecting immunological factors, energy homeostasis, body fat composition and distribution and patient reported measures.
Study design and methods
The GDS is an ongoing prospective observational study comprising intensive phenotyping within 12 months after clinical diagnosis, at 5-year intervals for at least 20 years and annual telephone interviews in between. The study is performed according to the Declaration of Helsinki, approved by the ethics committee of the University of Düsseldorf (previous reference number 2478, current reference number 4508) and was registered at Clinicaltrials.gov (Identifier number: NCT01055093).
Who is in the cohort?
The primary inclusion criterion is diagnosis of DM according to current ADA recommendations [60] within the last 12 months in individuals aged between 18 and 69 years. These include maturity onset diabetes of the young (MODY) and latent autoimmune diabetes of the adult (LADA), while individuals suffering from type 3 (e.g. pancreoprive DM) or type 4 (gestational) DM are not included. The main inclusion criteria are provided in detail in Table 1. The recruitment was performed via advertisements in local newspapers and the institutional homepage and via practitioners we supplied with information and flyers. After receiving the contact details, the potential participants were contacted and prescreened in a detailed telephone interview to check the inclusion and exclusion criteria. Appropriate applicants were then invited to the first study day including physical examination and anamnesis, thereafter participation in the more advanced examination was decided upon. From 2015 on a glucose tolerant subgroup matched for sex, BMI and age will be included to the data base. Participants give written informed consent to the study protocol.
Table 1.
Key exclusion criteria
| Key inclusion criteria | Key exclusion criteria | Exclusion criteria for specific examinations |
|---|---|---|
| Diagnosis of type 1 DM and type 2 DM including maturity onset diabetes of the young (MODY) and latent autoimmune diabetes of the adult (LADA) based on current ADA recommendationsa
Onset of DM within the last 12 months Diagnosis of type 1 DM based on diabetes manifestation with ketoacidosis or immediate insulin requirement along with the presence of at least one islet cell directed autoantibody or C-peptide levels below detection limita Age of 18–69 years |
Secondary DM according to ADA criteria (Type 3 B-H, e.g. pancreoprive DM) Type 4 (gestational) DM, pregnancy Poor glycemic control (HbA1c > 9.0 %) Hyperlipidemia (triglycerides and low-density lipoproteins ≥double upper reference limit) Heart, renal, liver failure (NYHA ≥II, serum creatinine ≥1.6 mg/dl, Aspartate-Aminotransferase/Alanine-Aminotransferase/Gamma-Glutamyltransferase Peripheral artery occlusive disease IV Venous thromboembolic events Anemia, blood donation or participation in a clinical study within the past 3 months Acute infection, leukocytosis, immunosuppressive therapy, autoimmune diseases, infection with human immunodeficiency virus, other severe diseases (e.g. active cancer disease) Psychiatric disorders, limited cooperation ability |
Neurologic examination: corneal disorders, and neuropathy from causes other than diabetes Spiroergometry: electrocardiogram abnormalities (alterations of the ST segment, higher-grade arrhythmia), unstable angina pectoris, uncontrolled hypertonia Magnetic resonance spectroscopy/imaging: metallic implants (cardiac pacemaker or defibrillator, cochlear implants, implanted catheters, clips, prosthetic valves), metallic fragments (metal removed from eye, ever worked as metal worker), larger tattoos, waist circumference > 135 cm, claustrophobia Tissue biopsies: effective anticoagulation therapy, platelet aggregation inhibitors >100 mg acetylsalicylate |
a American Diabetes Association [60]
By 01/2015 240 type 1 and 458 type 2 DM patients and three patients with MODY were included. GDS (in Germany named Deutsche Diabetes-Studie, DDS) was initiated at the German Diabetes Center (Deutsches Diabetes Zentrum, DDZ), Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany and developed into a national multicenter study with ongoing inclusion of patients from different regions of Germany being a core research project within the German Center of Diabetes Research (DZD e.V.) since 2014. The other partners and associates of DZD e.V. now contributing to GDS with increasing numbers include alphabetically listed the Department of Endocrinology/University Hospital Schleswig–Holstein; Department of Medicine I and Clinical Chemistry, University of Heidelberg; Faculty of Medicine/University Hospital Carl Gustav Carus, Dresden; Faculty of Medicine/Ludwig Maximilians University, Munich; German Institute of Human Nutrition Potsdam Rehbruecke; and Institute for Diabetes Research and Metabolic Diseases at Eberhard Karls University, Tübingen.
How often will they be followed up?
All patients undergo the full test program at baseline, at 5 and 10 years after diagnosis, and shall be continued thereafter. In between, a standardized telephone interview is conducted in annual intervals. During the initial phase of the study, the full test program was also repeated after 2 years in 137 patients. The annual telephone interviews reach a response rate of around 83 %. The 5-year follow-up started in 2014 and shows a response rate of 61 %. Another 33 % of patients are potential responders as they are still within the allowed time frame, while 6 % are lost to follow-up. Of those lost to follow up, 48 % refrained from further participation, 33 % were not accessible, 11 % died, 4 % refused for lack of time and 4 % showed non-compliance to the protocol.
What is measured?
In addition to demographic data (Table 2), baseline and follow up assessments include clinical and metabolic variables at baseline and follow up, summarized in Table 3. To address the issue of beta-cell dysfunction, the phenotyping includes assessment of beta-cell function using the glucagon stimulation test and mixed meal tolerance test (MMTT) [61]. In addition, the Botnia clamp consists of an intravenous glucose tolerance test (IVGTT [62]) for further testing of beta-cell function followed by a hyperinsulinemic-euglycemic clamp test providing precise dynamic measures of insulin secretion and sensitivity [63, 64]. Hepatic insulin sensitivity is measured by co-infusion of [6,6-2H2]glucose [65]. Cycling ergospirometry is performed to measure cardiorespiratory performance [37], indirect calorimetry to assess energy expenditure and substrate oxidation during fasting and hyperinsulinemia [66]. The experimental protocols are described in the Additional file 1: Annexure 1.
Table 2.
Baseline characteristics of type 1 and type 2 diabetes patients
| Type 1 DM | Type 2 DM | |
|---|---|---|
| Age | (%) | (%) |
| 15–19 | 5.4 | 0.2 |
| 20–24 | 15.0 | 1.1 |
| 25–29 | 16.7 | 1.7 |
| 30–34 | 18.0 | 3.1 |
| 35–39 | 10.0 | 4.6 |
| 40–44 | 9.6 | 8.5 |
| 45–49 | 8.7 | 15.3 |
| 50–54 | 10.4 | 16.2 |
| 55–59 | 2.5 | 17.2 |
| 60–64 | 3.0 | 18.8 |
| 65–69 | 0.8 | 13.1 |
| 70–74 | 0 | 0.2 |
| Sex | ||
| Male | 62.5 | 67.0 |
| Female | 37.5 | 33.0 |
| Marital status | ||
| Single | 55.0 | 21.8 |
| Co-habiting/married | 40.8 | 61.6 |
| Separated/divorced | 3.7 | 12.4 |
| Widowed | 0.4 | 4.1 |
| Higher education | ||
| Up to class 8 | 9.6 | 27.1 |
| Junior high school | 20.0 | 22.5 |
| Up to class 10 | 0.0 | 5.0 |
| Advanced technical college | 13.3 | 12.2 |
| Secondary school examination | 55.0 | 30.6 |
| No education | 0.4 | 0.7 |
| Other types of education | 1.2 | 1.7 |
| No response* | 0.4 | 0.2 |
| Employment | ||
| Laborer | 9.2 | 16.2 |
| Employee | 54.6 | 61.3 |
| Official | 5.4 | 5.0 |
| Business man | 7.2 | 10.0 |
| Farmer | 0 | 0.2 |
| Self-employed worker | 2.1 | 3.5 |
| Non-employed | 0.8 | 0 |
| Other professions | 5.4 | 1.5 |
| No response/advanced educationa | 1.2 | 0.2 |
| Medical insurance | ||
| Private | 15.8 | 12.4 |
| Government/public | 77.9 | 81.9 |
| Other insurance | 5.8 | 4.6 |
| No insurance | 0 | 0 |
| No response | 0.5 | 1.1 |
| Regular medical checks | ||
| Yes | 35.8 | 56.1 |
| No | 63.7 | 42.8 |
| No responsea | 0.4 | 0.9 |
| Family history of diabetes | ||
| Mother | 16.1 | 32.7 |
| Father | 17.3 | 26.3 |
| Children | 0.8 | 1.7 |
| Brothers and sisters | 7.6 | 18.4 |
| Grandparents | 37.3 | 35.2 |
| Uncles and aunts | 15.8 | 20.0 |
| Other diseases/risk factors/comorbidities | ||
| Hypertension | 18.5 | 63.3 |
| History of myocardial infarction | 0.4 | 2.8 |
| Retinopathy | 0.8 | 1.3 |
| History of or current smoking | 73.3 | 88.9 |
| Subclinical DSPN | 9.8 | 6.9 |
| Confirmed asymptomatic DSPN | 0.4 | 2.6 |
| Confirmed symptomatic DSPN | 2.2 | 4.0 |
| Possible DSPN | 7.2 | 23.2 |
| Probable DSPN | 0.5 | 5.3 |
| Subclinical/borderline CAN | 0.9 | 2.1 |
| Definite CAN | 1.4 | 2.4 |
| Medication | ||
| Glucose lowering therapy | ||
| Insulin, short acting | 87.9 | 5.9 |
| Insulin, long acting | 54.2 | 4.8 |
| Metformin | 14.6 | 56.1 |
| Sulfonylurea | 1.3 | 3.5 |
| Dipeptidyl-peptidase-4 inhibitors | 1.3 | 6.6 |
| GLP-1-Agonists | 0.4 | 2.0 |
| Other therapies | ||
| Acetylsalicylic acid | 1.7 | 11.1 |
| Statins | 2.5 | 17.5 |
| Fibrates | 0.0 | 0.7 |
| Any antihypertensive therapy | ||
| Blockers of the renin-angiotensin system | 2.1 | 15.9 |
| Beta blockers | 2.9 | 25.3 |
| Calcium channel blockers | 0.8 | 13.8 |
| Diuretics | 0.4 | 8.3 |
Demographic characteristics of the type 1 and type 2 diabetes mellitus (DM) patients included until 01/2015 at baseline in % of whole study group. DSPN: diabetic sensorimotor polyneuropathy, CAN: cardiovascular autonomic neuropathy. DSPN and CAN were defined as previously reported (68, 98)
aNo response refers to the number of participants, who prefer not to answer to a specific question during the interview
Table 3.
Clinical parameters at baseline in (a) type 1 and (b) 2 diabetes patients
| N | M ± SD | LQ/UQ | Med | |
|---|---|---|---|---|
| Panel a | ||||
| Age (years) | 240 | 36.0 ± 11.8 | 26.3/45.4 | 34.0 |
| Body mass index (kg/m2) | 240 | 24.8 ± 4.1 | 22.0/26.6 | 24.0 |
| Waist circumference (cm) | 239 | 86.1 ± 12.6 | 76.2/94.0 | 85.0 |
| Hemoglobin A1c (%) | 237 | 6.5 ± 1.2 | 5.8/6.9 | 6.3 |
| Glucose (mg/dl) | 232 | 133.4 ± 48.0 | 106.0/150.5 | 121.0 |
| Total cholesterol (mg/dl) | 238 | 184.9 ± 38.6 | 160.5/207.5 | 180.5 |
| HDL cholesterol (mg/dl) | 236 | 60.5 ± 17.3 | 48.5/70.5 | 59 |
| LDL cholesterol (mg/dl) | 236 | 108.9 ± 33.5 | 87.0/126.5 | 105.0 |
| Triglycerides (mg/dl) | 238 | 89.6 ± 58.2 | 56.0/102.0 | 74.0 |
| ASAT (U/l) | 238 | 22.4 ± 8.5 | 17.0/25.0 | 20.2 |
| ALAT (U/l) | 238 | 25.2 ± 18.5 | 15.9/28.0 | 20.9 |
| GGT (U/l) | 238 | 22.1 ± 21.7 | 11.1/26.0 | 16.0 |
| hsCRP (mg/dl) | 237 | 0.2 ± 0.3 | 0.1/0.2 | 0.1 |
| C-peptide (ng/ml) | 236 | 1.2 ± 0.9± | 0.5/1.4/ | 1.0 |
| SBP (mmHg) | 237 | 129.5 ± 14.9 | 120.5/138.0 | 129.0 |
| DBP (mmHg) | 237 | 78.0 ± 9.8 | 71.0/84.0 | 77.0 |
| VO2max (ml min.−1 kg−1) | 203 | 27.0 ± 7.8 | 22.1/31.3 | 25.7 |
| FMD (%) | 201 | 6.8 ± 6.6 | 2.2/10.6 | 5.6 |
| NMD (%) | 196 | 16.4 ± 9.5 | 9.3/22.1 | 15.8 |
| Panel b | ||||
| Age (years) | 458 | 53.5 ± 10.4 | 47.1/62.4 | 54.8 |
| Body mass index (kg/m2) | 456 | 31.7 ± 6.0 | 27.1/35.4 | 31.1 |
| Waist circumference (cm) | 455 | 105.9 ± 14.7 | 95.0/115.5 | 105.5 |
| Hemoglobin A1c (%) | 452 | 6.4 ± 0.8 | 5.8/6.8 | 6.2 |
| Fasting glucose (mg/dl) | 438 | 125.0 ± 28.6 | 107.0/137.0 | 122.0 |
| Total cholesterol (mg/dl) | 451 | 206.2 ± 42.0 | 180.0/234.0 | 203.0 |
| HDL cholesterol (mg/dl) | 448 | 46.4 ± 12.8 | 37.0/53.0 | 45.0 |
| LDL cholesterol (mg/dl) | 448 | 130.5 ± 36.1 | 105.2/153.5 | 129.0 |
| Triglycerides (mg/dl) | 451 | 176.0 ± 165.2 | 98.0/203.8 | 137.0 |
| ASAT (U/l) | 451 | 25.4 ± 11.0 | 19.0/29.0 | 23.0 |
| ALAT (U/l) | 451 | 34.5 ± 19.5 | 21.9/41.8 | 29.0 |
| GGT (U/l) | 451 | 43.0 ± 51.0 | 21.4/48.0 | 31.6 |
| hsCRP (mg/dl) | 446 | 0.4 ± 0.7 | 0.1/0.5 | 0.3 |
| C-peptide (ng/ml) | 445 | 3.3 ± 1.6 | 2.2/4.2 | 3.0 |
| SBP (mmHg) | 447 | 141.6 ± 17.1 | 129.5/152.0 | 141.5 |
| DBP (mmHg) | 447 | 85.1 ± 10.5 | 78.0/91.5 | 84.5 |
| VO2max (ml min.−1 kg−1) | 317 | 19.1 ± 4.9 | 15.6/21.8 | 18.7 |
| FMD (%) | 332 | 5.6 ± 5.3 | 1.9/8.25 | 4.5 |
| NMD (%) | 338 | 12.2 ± 7.3 | 6.6/16.2 | 11.5 |
Number (N) of type 1 (DM) participants or samples, M mean, SD standard deviation, Med median, LQ lower quartile, UQ upper quartile, HDL high-density lipoprotein, LDL low-density lipoprotein, ASAT Aspartate-Aminotransferase, ALAT Alanine-Aminotransferase, GGT Gamma-Glutamyltransferase, hsCRP high sensitive C-reactive protein, SBP systolic blood pressure, DBP diastolic blood pressure. VO 2 max maximal oxygen uptake, FMD flow-mediated (endothelium-dependent) vasodilatation, NMD nitrogen-mediated (endothelium independent) vasodilatation
RNA and DNA samples are purified from whole-blood and peripheral blood mononuclear cells (PBMCs), serum and plasma samples (citrate, EDTA) are stored at −80 °C for analysis of biomarkers [67, 68]. Neurological and cutaneous microvascular assessments include nerve conduction studies, quantitative sensory testing, neuropathic symptoms and deficits, heart rate variability, baroreflex sensitivity, pupillography, sudomotor function, sexual function, intraepidermal nerve fiber density, corneal confocal microscopy, laser Doppler flowmetry, skin autofluorescence [69–72], opthalmological examinations include funduscopy, corneal esthesiometry [73] and optical coherence tomography (OCT) [74].
In mid-2012 acquisition of skeletal muscle and subcutaneous abdominal adipose tissue samples and magnetic resonance imaging and spectroscopy (MRI/S) examinations were implemented. Absolute quantification of phosphorus metabolites in the liver and recovery of phosphocreatine after depletion through exercise in skeletal muscle have been applied for the estimation of tissue specific energy metabolism [75]. Assessment of whole body fat distribution and ectopic lipid storage is assessed from proton spectroscopy and MRI.
Patient-reported outcomes are assessed via questionnaires at baseline and follow-up investigations and during annual telephone interviews (Table 3), reflecting quantitative analysis of lifestyle, course of the disease, compliance and socio-economic factors associated with the disease, and dietary habits. Participation preferences, information needs and time for health related activities are assessed by the Control Preferences Scale, the Autonomy Preference Index and questionnaires developed and validated in the DDZ, and diabetes self-management instruments which are well established in cohort studies [76–80]. Quality of life and depression are assessed by common questionnaires (Table 4).
Table 4.
Questionnaires at baseline and follow-up
| Baseline | 5-year follow-up | 10-year follow-up | Telephone interview | |
|---|---|---|---|---|
| Demographics | ||||
| Age | Y | N | N | N |
| Sex | Y | N | N | N |
| Marital status | Y | Y | Y | Y |
| Retardation/physical disabilities | Y | Y | Y | Y |
| Diabetes | ||||
| Time of diagnosis | Y | N | N | N |
| Symptoms at time of diagnosis | Y | N | N | N |
| Diabetes treatment regime | Y | Y | Y | Y |
| Diet plan and advice | Y | Y | Y | Y |
| Diabetes education for the patients | Y | Y | Y | Y |
| Ophthalmological complications | Y | Y | Y | Y |
| Kidney complications | Y | Y | Y | Y |
| Cardiovascular complications | Y | Y | Y | Y |
| Neurological complications | Y | Y | Y | Y |
| Cerebrovascular complications | Y | Y | Y | Y |
| Radiation exposure in last 10 years | Y | Y | Y | Y |
| Family history of diabetes and other diseases | Y | Y | Y | Y |
| Socio-economic status | ||||
| Household composition | Y | Y | Y | Y |
| Education | Y | Y | Y | Y |
| Health insurance | Y | Y | Y | Y |
| Employment | Y | Y | Y | Y |
| Net household income | Y | Y | Y | Y |
| Personal health behavior, life style | ||||
| Smoking | Y | Y | Y | Y |
| Alcohol | Y | Y | Y | Y |
| Physical activity | Y | Y | Y | Y |
| Food frequency questionnaire | Y | Y | Y | Y |
| Regular medical checks | Y | Y | Y | Y |
| Personal health history | ||||
| Other diseases | Y | Y | Y | Y |
| Food supplement intake | Y | Y | Y | Y |
| Other medication | Y | Y | Y | Y |
| Self reported weight and weight change | Y | Y | Y | Y |
| Mental health | Y | Y | Y | Y |
| Reproductive history | Y | Y | Y | Y |
| Health-related quality of life | ||||
| WHO-5, SF-36 | Y | Y | Y | Y |
| WHOQUOL-Bref, SCL-14 | Y | Y | Y | N |
| Depression | ||||
| PHQ, PAID, ADS-L | Y | Y | Y | Y |
| Information needs, patient time | ||||
| CPS, API |
Questions and questionnaires at baseline, 5-year follow-up, 10-year follow-up, and annual telephone interview. Y stands for ‘yes’ (question asked) and N stands for ‘no’ (question not asked)
Short form 36 health survey questionnaire (SF36), World Health Organization 5 questions health survey (WHO5), World Health Organisation quality of life assessment (WHOQOL-Bref), symptom checklist 14 (SCL-14), patient health questionnaire (PHQ), problem areas in diabetes (PAID), Allgemeine Depressionsskala (ADS-L), Control Preferences Scale (CPS), Autonomy Preference Index (API)
Discussion
What has it found? key findings and publications
Baseline characteristics
The basal characteristics of the cohort recruited between 01/2009 and 1/2015 are summarized in Table 2. In our cohort, the percentage of patients diagnosed with type 2 DM at an age of less than 45 years, commonly referred to as “early manifestation of type 2 DM” is doubled compared to US registry data (Table 2) [81–83]. This might result from the higher willingness of younger persons to participate in time-consuming examinations. Such higher motivation could also reflect their higher educational levels compared to other cohorts of newly diagnosed type 2 DM. Accordingly, mean age at diagnosis was comparable to cohort studies that excluded elderly patients [84, 85] (Table 2b). The percentage of male type 2 DM participants is higher compared to other cohorts, which might result from more frequent manifestation in males <65 years [86–88]. Our observational study is not designed as a population-based study and therefore does not claim to represent the German diabetes population, but intends to reveal predictors of later outcome in specific subgroups and to unravel underlying mechanisms. We now post this statement and compare our baseline description of the first included patients to one report of Hartwig et al., who reviewed four large representative regional cohorts of incidental type 2 diabetes and the data from the national German Health Interview and Examination Survey for Adults (DEGS), which combines a nationally representative health survey and a longitudinal follow-up of participants from the previous German National Health Interview and Examination Survey in 1997–1999 [89]. The participants of our cohort include younger patients and more males than represented in this reference study, while the body mass index is comparable. The mean body mass index at baseline is also comparable to other European cohorts [84, 86–88].
The mean body mass index at baseline is comparable to other European cohorts [84, 86–88]. A high proportion of type 2 DM patients demonstrated evidence of modifiable cardiovascular risk factors at diagnosis. Participants with newly diagnosed type 1 DM were leaner and younger compared to type 2 participants (Table 2a). In line with type 1 DM intervention studies, a remarkable number still had residual beta-cell function (Fig. 1a) [90–93]. We reported the possible effects of low-grad inflammation and dietary habits on maintenance of residual beta-cell function in follow-up examinations 2 years after diagnosis [94]. Future follow-up examinations will unravel the role of other factors, that are currently under discussion, body fat distribution i.e. liver fat content. On the other hand, we found that a substantial number of type 1 DM patients showed decreased insulin sensitivity, which is in line with previous reports [14, 95] and might relate to poor glycemic control [96] or other mechanisms also attributable to type 2 DM [97] (Fig. 1b). In addition to insulin treatment 15 % of type 1 DM patients were treated with metformin, a few had oral glucose lowering agents and 8 % were not treated with insulin yet (Table 2a). These data show that type 1 DM patients may have oral glucose lowering at diagnosis of the disease before establishing the correct diagnosis. Cardiorespiratory fitness as assessed from oxygen uptake at maximal workload during spiroergometry (VO2max) is often impaired in patients with type 2 DM [98]. Accordingly, VO2max was lower in patients with type 2 DM compared to patients with type 1 DM and corresponded to values reported previously (Table 3a, b). Reduced VO2max might also be due to reduced mitochondrial oxidative capacity, sedentary life style and possibly due to higher hepatocellular lipids and might predispose to the development of insulin resistance [99, 100]. Inverse correlation of VO2max with IL-6 and hsCRP in healthy men [101] and with hsCRP, white blood cell count and fibrinogen in men with T2D [102] suggest that lower VO2max might predispose to the development of cardiovascular disease in diabetes [103].
Fig. 1.
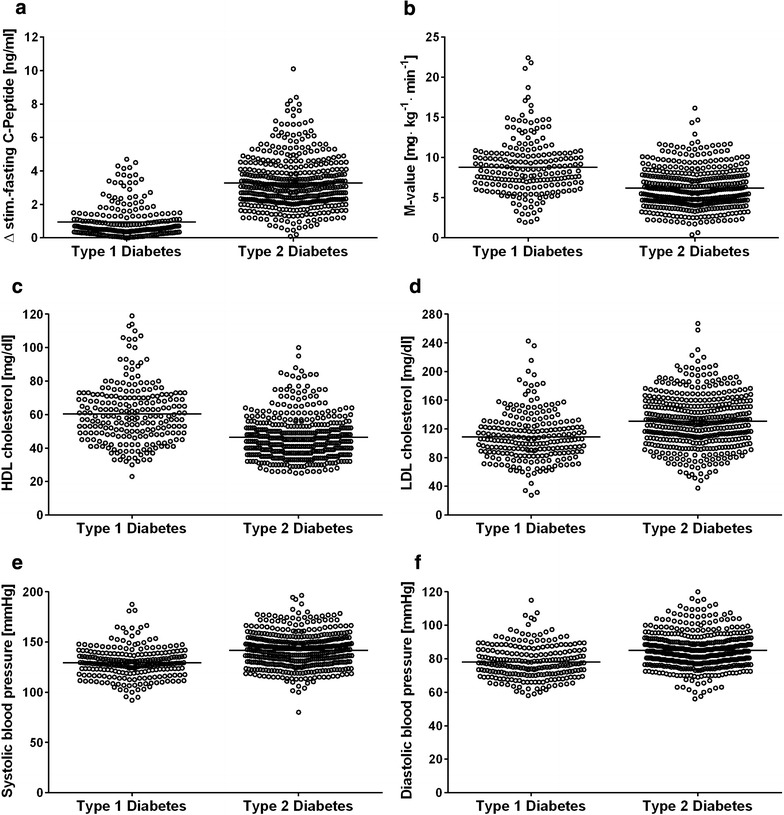
Beta-cell function as assessed from increase of C-peptide during the glucagon stimulation test (C-peptide concentration at 6 min after injection minus C-peptide concentration at baseline) (a, n = 216 patients with type 1 DM, n = 374 patients with type 2 DM), insulin sensitivity (M value) as assessed from euglycemic-hyperinsulinemic clamp tests in patients with type 1 DM or type 2 DM (b, n = 204 patients with type 1 DM, n = 351 patients with type 2 DM), high-density lipoprotein levels (HDL, c, n = 236 patients with type 1 DM, n = 448 patients with type 2 DM), low-density lipoprotein levels (LDL, d, n = 236 patients with type 1 DM, n = 448 patients with type 2 DM), systolic blood pressure (e, n = 237 patients with type 1 DM, n = 447 patients with type 2 DM), and diastolic blood pressure (f, n = 237 patients with type 1 DM, n = 447 patients with type 2 DM)
Endothelial-mediated (flow-mediated) and endothelial-independent (nitrogen-induced) vasodilatation of patients with type 2 DM (Table 3b) were in the range of previous studies in patients with type 2 DM without previous cardiovascular events [104] and higher compared to baseline parameters in a number of statin intervention studies [105] which might be due to the shorter period of diabetes, the lower cholesterol levels, better glycemic control and the inclusion of patients undergoing statin treatment, factors that are known to positively correlate with vasodilatation. Endothelial function of type 1 DM patients corresponded to values in healthy control of similar body mass and age [104].
Overall in case of type 2 DM patients, 41.9 % had plasma triglycerides >150 mg/dl, 23.3 % had plasma low-density lipoproteins <160 > 150 mg/dl, 73.6 % had blood pressure >130/85 mmHg and 62.5 % were overweight or obese (Fig. 1c–f). At baseline, 18 % of type 2 DM patients were receiving a statin, 9 % were receiving a fibrate, 11 % were being treated with an acetylsalicylic acid agent, and 55 % were receiving any drug for blood pressure reduction (Table 2). The percentage of statin use is low, particularly in type 2 diabetes patients and cardiovascular risk factors are not perfectly controlled at the onset of diabetes (Fig. 1; Table 2).
Summary of the results obtained so far
By beginning of 2015, GDS yielded 13 original articles published in peer-reviewed journals [35, 64, 66, 69–71, 75, 106–111].
Standardization of experimental protocols
The measures of insulin sensitivity derived from the Botnia clamp were validated against the standard hyperinsulinemic-euglycemic clamps in patients with type 2 DM [64].
Post-calorimetric individual calibration procedures have been developed to increase the accuracy and comparability of indirect calorimetry assessed in different centers [66].
Blood glucose measuring instruments were validated against gold-standard method and the method with the highest accuracy was selected [107].
Establishment of novel methods for metabolic imaging
At DDZ, noninvasive phosphorous (31P) MRS of liver was established and optimized with short examination time on a 3-T clinical magnet [75]. With this method, GDS started to employ quantifying absolute concentrations of hepatic adenosine triphosphate (ATP) and inorganic phosphate (Pi) as measures of liver energy metabolism [112].
Rapid methods for the quantification of hepatic and pancreatic fat were developed applicable to larger cohorts, showing that no relationship exists between pancreatic adipose tissue infiltration and beta cell function, regardless of glucose tolerance status [109, 110].
Development of comorbidities
Immune cell phenotyping showed distinct occurrence of certain white blood cell subtypes and associations with insulin sensitivity, glycemia and lipidemia in patients with type 1 and type 2 DM [106].
The sensitivity of an indicator test for sudomotor dysfunction on the foot (Neuropad) for detecting small fiber dysfunction was relatively high in recently diagnosed type 1 DM (80 %) and somewhat lower in type 2 DM (68 %) (64). Thus, early sudomotor dysfunction may be demonstrated by screening in recent-onset diabetes.
Using novel methods to quantify small nerve fiber density (NFD) including corneal confocal microscopy and skin biopsy early nerve pathology was detected in up to 20 % of subgroups with type 2 DM participating in the GDS [69–71]. However, the vast majority of patients with abnormal corneal NFD showed concomitantly normal intraepidermal NFD and vice versa. Thus, both techniques detect early nerve fiber loss in recently diagnosed type 2 DM, but largely in different patients, suggesting a patchy manifestation pattern of small fiber neuropathy. Recently diagnosed type 2 DM patients also demonstrate a marked reduction of cutaneous Langerhans cell density, which relates to insulin resistance in women [69]. Prospective data will establish whether the initial Langerhans cell decline could promote a cutaneous immunogenic imbalance toward inflammation predisposing to polyneuropathy and foot ulcers. Moreover, dermal expression of mitochondrial superoxide dismutase (SOD2) expression in the lower limbs was augmented by ≈60 % and correlated with increasing diabetes duration, cardiac sympathetic predominance, and diminished vagal activity, while subepidermal endothelial cell area was not altered. The SOD2 overexpression points to an early enhanced, presumably compensatory, cutaneous anti-oxidative defence in type 2 DM [111]. Whether cutaneous SOD2 levels can predict the development of diabetic neuropathy will be determined during the prospective GDS follow-up.
Assessing various single nucleotide polymorphisms (SNPs) in the transketolase gene, we observed associations of genetic variability in transketolase enzyme with neuropathic symptoms and reduced thermal sensation in the GDS baseline cohort, suggesting a role of pathways metabolizing glycolytic intermediates in early diabetic neuropathy.
Using the diagnostic criteria for diabetic sensorimotor polyneuropathy (DSPN) based on the Toronto Consensus (85), the prevalence of DSPN was relatively high, achieving 20 % in individuals with type 1 DM and 42 % in those with type 2 DM (Table 3). DSPN was subclinical in 10 % of the type 1 DM subjects and possible in 23 % of those with type 2 DM. The prevalence of confirmed DSPN was relatively low, with 3 % in individuals with type 1 DM and 7 % in those with type 2 DM, similar to the prevalence of cardiovascular autonomic neuropathy (CAN) at 2 % in type 1 DM and 5 % in type 2 DM patients. The rate of DSPN strongly depends on the definition of DSPN and is considerably lower, if both clinical and electrodiagnostic criteria are combined. The prevalence of definite CAN in GDS participants with type 2 DM (2.4 %) is similar to the rate of 1.8 % observed by the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS) [113]. Likewise, the prevalence of DSPN found in the present study is compatible with the percentages of 4–39 % depending on the different definition criteria for DSPN used in cohorts of newly diagnosed DM patients [114].
Biomarkers of subclinical inflammation are associated with DSPN and both motor and sensory nerve conduction velocity (NCV). High serum IL-6 was associated with the presence of DSPN and reduced motor NCV in type 2 DM. In addition, higher levels of high-molecular weight (HMW) and total adiponectin were consistently associated with DSPN and both reduced motor and sensory NCV in individuals with type 2 DM. In participants with type 1 DM however, associations between high adiponectin and higher motor NCV were found. Thus, our data support the hypothesis that the pathomechanisms leading to DSPN may only partially overlap between type 1 and type 2 DM [115].
Cellular mechanisms of insulin resistance
In a subgroup of type 2 DM patients, we assessed cellular mechanisms of insulin resistance in skeletal muscle [35]. These data provided evidence that specific diacylglycerol species underlie activation of protein kinase C, which impairs insulin signaling.
In a subgroup of type 2 DM participants, we analysed effect of low-caloric interventions on insulin sensitivity and found that energy restriction per se seems to be key for improving insulin action in phases of active weight loss in obese type 2 DM, with a potential improvement of subclinical inflammation with a diet free of red meat [108].
Higher levels of biomarkers of subclinical and vascular inflammation were found associated with the deterioration of glycemic control and decreases in beta-cell function in study participants with recently diagnosed type 1 and type 2 DM [94].
What are the main strengths and weaknesses?
The main strength is the broad spectrum of comprehensive metabolic phenotyping combining gold standard methods with novel techniques in humans shortly after diagnosis of DM at regular intervals for 20 years. The examinations combine highly specialized tests, such as non-invasive cutting-edge metabolic imaging, micro methods applied to tissue biobanks and blood samples, which have not been used in previous long-term studies. The array of morphological and functional measures allows for recording preclinical occurrence of comorbidities and will allow for large scale interventional studies.
Detailed test results are sent to both participants and their physicians, and diabetes information days are organized at DDZ to keep patients informed and to increase compliance of the participants.
One weakness is that GDS includes only people living in Germany and cannot represent all ethnicities (Additional file 2: Annexure 2).
Another challenge is present by potential sample size limitations. Since the GDS study is not a randomized trial with a single pre-specified hypothesis, but a very complex cohort study with many observed and calculated variables, all exemplarily performed sample size calculations were based on a logistic regression model for a binary response and a single binary or continuous covariate. Standard regression analyses in cohort studies in general involve additional covariates for confounder adjustment. Therefore, all initially calculated sample sizes can easily be inflated by a variance inflation factor (VIF) and a factor for the expected dropout rate in the study [116]. As an example, considering as a response the incidence of peripheral diabetic neuropathy (DPN) within 10 years after baseline and a properly/poorly regulated HbA1c value (defined as ≤/> 6.5 %) as covariate, further, assuming an annual incidence of DPN of approx. 2 %, a poorly regulated HbA1c in the GDS cohort of 42.5 %, and an odds ratio of 1.95 to be clinically relevant, a test at level α = 0.05 with power of 80 % to detect this odds ratio if 480 probands are included in the cohort. when additionally allowing for confounder adjustment by a VIF of 20 % and a dropout rate of 40 %, then at least 999 participants are required.
Potential selection bias includes higher social standards, the acquisition of scientific knowledge and gathering more information on the disease being the most important motivation for the participants since the study protocol is time consuming and demanding and includes invasive examinations at low expense allowance. Deep phenotyping results in detailed information that is provided to the practitioner. This may induce a change of the treatment strategy. Baseline assessment, yearly phone interviews and reassessments every 5 years might increase motivation to adhere to the therapy recommendations and thereby might refer to as a kind of intervention compared to other patients that were not included. Thus, in addition to the mentioned selection bias, these factors might confound the outcome measures. The participants received the results on the clinical examinations such as beta cell function, insulin sensitivity, cardiorespiratory fitness, liver fat content, (pre)clinical onset of retinopathy or neuropathy, assessment of dietary habits and laboratory measures and had the opportunity to discuss these findings with a medical doctor and a nutritionist. Assessment of preclinical manifestation of comorbidities may remain without distinct immediate clinical consequence. Incidental findings, e.g. abnormalities found during magnetic resonance imaging are communicated to the participant and the practitioner for further diagnostic steps to be taken. At the same time the investigator is not obligated to assess clinical findings beneath the scope of the research question. This approach is approved by the ethical committee and explained to the participants prior to inclusion.
Can I get hold of the data? Where can I find out more?
A request and transfer process has been established so that researches may apply for data by contacting the study coordinators via email (GDS@ddz.uni-duesseldorf.de). Once approved by the steering committee, the requesting researcher and the principal investigator of GDS sign a contract on the terms and conditions of data transfer and transmission of results back to the DDZ.
Authors' contributions
JS, AS, KSW, KS, CH, BN, AI, DZ wrote the manuscript and researched data; KS performed the statistical analysis; VB, OK, DZ, HAH, KM and MR contributed to discussion and reviewed/edited the manuscript; MR designed the study, contributed to discussion and reviewed/edited the manuscript. All authors critically reviewed the manuscript. MR is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Acknowledgements
We thank all GDS participants, all physicians for their continued encouragement of their patients to participate in GDS, all former and present researchers, study nurses, administrators and interviewers involved at DDZ and all other study centers. We thank Markku Laakso for determination of genome-wide associations in the first hundreds of the cohort.
The Members of the GDS group contributed to the design and conduct of the GDS:
The GDS Group consists of M. Roden (speaker), H. Al-Hasani, S. Bornstein, A. E. Buyken, J. Eckel, G. Geerling, H.-U. Häring, C. Herder, A. Icks, J. Kotzka, O. Kuß, E. Lammert, H. Lehnert, J. Lundbom, K. Müssig, P. Nawroth, P. Nowotny, A. F. H. Pfeiffer, W. Rathmann, J. Seißler, M. Stumvoll, J. Szendroedi, D. Ziegler and their co-workers who contributed to the design and conduct of the GDS.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was initially exclusively supported by DDZ, which is funded by the German Federal Ministry of Health (Berlin, Germany) and the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany) and as a multicenter study now receives additional funding by the German Federal Ministry of Education and Research (BMBF) through the German Center for Diabetes Research (DZD e.V.). Third-party funding supports sub-projects within GDS. There is no conflict of interest.
Additional files
10.1186/s12933-016-0374-9 Risk analysis and risk mitigation measures.
Contributor Information
Julia Szendroedi, Email: Julia.Szendroedi@ddz.uni-duesseldorf.de.
Aaruni Saxena, Email: Aaruni.Saxena@ddz.uni-duesseldorf.de.
Katharina S. Weber, Email: Katharina.Weber@ddz.uni-duesseldorf.de
Klaus Strassburger, Email: Klaus.Strassburger@ddz.uni-duesseldorf.de.
Christian Herder, Email: Christian.Herder@ddz.uni-duesseldorf.de.
Volker Burkart, Email: Volker.Burkart@ddz.uni-duesseldorf.de.
Bettina Nowotny, Email: Bettina.Nowotny@ddz.uni-duesseldorf.de.
Andrea Icks, Email: Andrea.Icks@ddz.uni-duesseldorf.de.
Oliver Kuss, Email: Oliver.Kuss@ddz.uni-duesseldorf.de.
Dan Ziegler, Email: Dan.Ziegler@ddz.uni-duesseldorf.de.
Hadi Al-Hasani, Email: Hadi.Al-Hasani@ddz.uni-duesseldorf.de.
Karsten Müssig, Email: Karsten.Muessig@ddz.uni-duesseldorf.de.
Michael Roden, Phone: + 49 211 3382 201, Email: Michael.Roden@ddz.uni-duesseldorf.de.
References
- 1.Federation ID, Diabetes Atlas. Sixth Edition (Brussels); 2013.
- 2.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, Group ES Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 3.Group IDFDA Update of mortality attributable to diabetes for the IDF Diabetes Atlas: estimates for the year 2011. Diabet Res clin Prac. 2013;100(2):277–279. doi: 10.1016/j.diabres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340(8825):925–929. doi: 10.1016/0140-6736(92)92814-V. [DOI] [PubMed] [Google Scholar]
- 5.Rhee SY, Chon S, Oh S, Kim SW, Kim JW, Kim YS, et al. Insulin secretion and insulin resistance in newly diagnosed, drug naive prediabetes and type 2 diabetes patients with/without metabolic syndrome. Diabet Res clin Prac. 2007;76(3):397–403. doi: 10.1016/j.diabres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Kaneto H. Pancreatic beta-cell glucose toxicity in type 2 diabetes mellitus. Curr Diabet Rev. 2015;11(1):2–6. doi: 10.2174/1573399811666141216160217. [DOI] [PubMed] [Google Scholar]
- 7.Janikiewicz J, Hanzelka K, Kozinski K, Kolczynska K, Dobrzyn A. Islet beta-cell failure in type 2 diabetes—Within the network of toxic lipids. Biochem Biophys Res Commun. 2015;460(3):491–496. doi: 10.1016/j.bbrc.2015.03.153. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman BA, Li C, Soleimanpour SA. Mitochondrial regulation of beta-cell function: maintaining the momentum for insulin release. Mol Asp Med. 2015;42:91–104. doi: 10.1016/j.mam.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and beta-cell dysfunction. Trans Res: J Lab Clin Med. 2016;167(1):228–256. doi: 10.1016/j.trsl.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, et al. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37(6):1751–1758. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–1139. doi: 10.2337/dc05-2179. [DOI] [PubMed] [Google Scholar]
- 12.Faerch K, Johansen NB, Witte DR, Lauritzen T, Jorgensen ME, Vistisen D. Relationship between insulin resistance and beta-cell dysfunction in subphenotypes of prediabetes and type 2 Diabetes. J Clin Endocrinol Metab. 2015;100(2):707–716. doi: 10.1210/jc.2014-2853. [DOI] [PubMed] [Google Scholar]
- 13.Vangipurapu J, Stancakova A, Kuulasmaa T, Kuusisto J, Laakso M. Both fasting and glucose-stimulated proinsulin levels predict hyperglycemia and incident type 2 diabetes: a population-based study of 9,396 finnish men. PLoS ONE. 2015;10(4):e0124028. doi: 10.1371/journal.pone.0124028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kacerovsky M, Brehm A, Chmelik M, Schmid AI, Szendroedi J, Kacerovsky-Bielesz G, et al. Impaired insulin stimulation of muscular ATP production in patients with type 1 diabetes. J Int Med. 2011;269(2):189–199. doi: 10.1111/j.1365-2796.2010.02298.x. [DOI] [PubMed] [Google Scholar]
- 15.Perseghin G, Lattuada G, Danna M, Sereni LP, Maffi P, De Cobelli F, et al. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2003;285(6):E1174–E1181. doi: 10.1152/ajpendo.00279.2003. [DOI] [PubMed] [Google Scholar]
- 16.Kibirige M, Metcalf B, Renuka R, Wilkin TJ. Testing the accelerator hypothesis: the relationship between body mass and age at diagnosis of type 1 diabetes. Diabetes Care. 2003;26(10):2865–2870. doi: 10.2337/diacare.26.10.2865. [DOI] [PubMed] [Google Scholar]
- 17.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003;26(10):2871–2875. doi: 10.2337/diacare.26.10.2871. [DOI] [PubMed] [Google Scholar]
- 18.Rung J, Cauchi S, Albrechtsen A, Shen L, Rocheleau G, Cavalcanti-Proenca C, et al. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet. 2009;41(10):1110–1115. doi: 10.1038/ng.443. [DOI] [PubMed] [Google Scholar]
- 19.Replication DIG. Meta-analysis C. Asian Genetic Epidemiology Network Type 2 Diabetes C. South Asian Type 2 Diabetes C. Mexican American Type 2 Diabetes C. Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in muylti-Ethnic Samples C et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46(3):234–244. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44(9):991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659–669. doi: 10.1038/ng.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva Xavier G, Loder MK, McDonald A, Tarasov AI, Carzaniga R, Kronenberger K, et al. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes. 2009;58(4):894–905. doi: 10.2337/db08-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pehmoller C, Brandt N, Birk JB, Hoeg LD, Sjoberg KA, Goodyear LJ, et al. Exercise alleviates lipid-induced insulin resistance in human skeletal muscle-signaling interaction at the level of TBC1 domain family member 4. Diabetes. 2012;61(11):2743–2752. doi: 10.2337/db11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373(9677):1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 25.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, Group KS Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3):464–469. doi: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]
- 26.Soedamah-Muthu SS, Vergouwe Y, Costacou T, Miller RG, Zgibor J, Chaturvedi N, et al. Predicting major outcomes in type 1 diabetes: a model development and validation study. Diabetologia. 2014;57(11):2304–2314. doi: 10.1007/s00125-014-3358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordwall M, Hyllienmark L, Ludvigsson J. Early diabetic complications in a population of young patients with type 1 diabetes mellitus despite intensive treatment. JPEM. 2006;19(1):45–54. doi: 10.1515/JPEM.2006.19.1.45. [DOI] [PubMed] [Google Scholar]
- 28.Kahn R, Buse J, Ferrannini E, Stern M, American Diabetes A. European Association for the Study of D The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 29.Diabetes Control and Complications Trial Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968–983. doi: 10.2337/diab.44.8.968. [DOI] [PubMed] [Google Scholar]
- 30.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Eng J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 31.Virtanen SM, Knip M. Nutritional risk predictors of beta cell autoimmunity and type 1 diabetes at a young age. Am J Clin Nutr. 2003;78(6):1053–1067. doi: 10.1093/ajcn/78.6.1053. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen DS, Krych L, Buschard K, Hansen CH, Hansen AK. Beyond genetics. Influence of dietary factors and gut microbiota on type 1 diabetes. FEBS Lett. 2014;588(22):4234–4243. doi: 10.1016/j.febslet.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Delahanty LM. The look AHEAD study: implications for clinical practice go beyond the headlines. J Acad Nutr Diet. 2014;114(4):537–542. doi: 10.1016/j.jand.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Investig. 1996;97(12):2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, et al. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci USA. 2014;111(26):9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, et al. Over activation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54(9):2674–2684. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 37.Kacerovsky-Bielesz G, Chmelik M, Ling C, Pokan R, Szendroedi J, Farukuoye M, et al. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes. 2009;58(6):1333–1341. doi: 10.2337/db08-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tousoulis D, Papageorgiou N, Androulakis E, Siasos G, Latsios G, Tentolouris K, et al. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol. 2013;62(8):667–676. doi: 10.1016/j.jacc.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 39.Keshari KR, Wilson DM, Sai V, Bok R, Jen KY, Larson P, et al. Noninvasive in vivo imaging of diabetes-induced renal oxidative stress and response to therapy using hyperpolarized 13C dehydroascorbate magnetic resonance. Diabetes. 2015;64(2):344–352. doi: 10.2337/db13-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 41.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222–2231. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wlazlo N, van Greevenbroek MM, Ferreira I, Jansen EJ, Feskens EJ, van Kallen C. Low-grade inflammation and insulin resistance independently explain substantial parts of the association between body fat and serum C3: the CODAM study. Metab, Clin Exp. 2012;61(12):1787–1796. doi: 10.1016/j.metabol.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Roden M. Mechanisms of disease: hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2(6):335–348. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- 44.Roden M. How free fatty acids inhibit glucose utilization in human skeletal muscle. Physiology. 2004;19:92–96. doi: 10.1152/nips.01459.2003. [DOI] [PubMed] [Google Scholar]
- 45.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szendroedi J, Kaul K, Kloock L, Strassburger K, Schmid AI, Chmelik M, et al. Lower fasting muscle mitochondrial activity relates to hepatic steatosis in humans. Diabetes Care. 2014;37(2):468–474. doi: 10.2337/dc13-1359. [DOI] [PubMed] [Google Scholar]
- 47.Herder C, Karakas M, Koenig W. Biomarkers for the prediction of type 2 diabetes and cardiovascular disease. Clin Pharmacol Ther. 2011;90(1):52–66. doi: 10.1038/clpt.2011.93. [DOI] [PubMed] [Google Scholar]
- 48.Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four UK prospective cohort studies. Diabetes Care. 2012;35(2):396–403. doi: 10.2337/dc11-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walraven I, van Riet E, Kamp O, Schalkwijk CG, Stehouwer CD, van den Hurk K, et al. Low-grade inflammation and endothelial dysfunction explain the association between retinopathy and left ventricular ejection fraction in men: an 8-year follow-up of the Hoorn Study. J Diabet Complicat. 2014;28(6):819–823. doi: 10.1016/j.jdiacomp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Schottker B, Herder C, Rothenbacher D, Roden M, Kolb H, Muller H, et al. Proinflammatory cytokines, adiponectin, and increased risk of primary cardiovascular events in diabetic patients with or without renal dysfunction: results from the ESTHER study. Diabetes Care. 2013;36(6):1703–1711. doi: 10.2337/dc12-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pop-Busui R, Lu J, Brooks MM, Albert S, Althouse AD, Escobedo J, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. 2013;36(10):3208–3215. doi: 10.2337/dc13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanai K, Babazono T, Yoshida N, Nyumura I, Toya K, Hayashi T, et al. Gender differences in the association between HDL cholesterol and the progression of diabetic kidney disease in type 2 diabetic patients. Nephrol Dial Transplant. 2012;27(3):1070–1075. doi: 10.1093/ndt/gfr417. [DOI] [PubMed] [Google Scholar]
- 53.Younes N, Cleary PA, Steffes MW, de Boer IH, Molitch ME, Rutledge BN, et al. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. CJASN. 2010;5(7):1235–1242. doi: 10.2215/CJN.07901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manuel DG, Schultz SE. Health-related quality of life and health-adjusted life expectancy of people with diabetes in Ontario, Canada, 1996–1997. Diabetes Care. 2004;27(2):407–414. doi: 10.2337/diacare.27.2.407. [DOI] [PubMed] [Google Scholar]
- 55.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 56.Icks A, Kruse J, Dragano N, Broecker-Preuss M, Slomiany U, Mann K, et al. Are symptoms of depression more common in diabetes? Results from the Heinz Nixdorf Recall study. Diabet Med. 2008;25(11):1330–1336. doi: 10.1111/j.1464-5491.2008.02585.x. [DOI] [PubMed] [Google Scholar]
- 57.Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JF. Patient preferences for the treatment of type 2 diabetes: a scoping review. Pharmacoeconomics. 2013;31(10):877–892. doi: 10.1007/s40273-013-0089-7. [DOI] [PubMed] [Google Scholar]
- 58.Russell LB. Completing costs: patients’ time. Med Care. 2009;47(7 Suppl 1):S89–S93. doi: 10.1097/MLR.0b013e31819bc077. [DOI] [PubMed] [Google Scholar]
- 59.Ettner SL, Cadwell BL, Russell LB, Brown A, Karter AJ, Safford M, et al. Investing time in health: do socioeconomically disadvantaged patients spend more or less extra time on diabetes self-care? Health Econ. 2009;18(6):645–663. doi: 10.1002/hec.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, Battelino T, Haastert B, Ludvigsson J, et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31(10):1966–1971. doi: 10.2337/dc07-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pacini G, Tonolo G, Sambataro M, Maioli M, Ciccarese M, Brocco E, et al. Insulin sensitivity and glucose effectiveness: minimal model analysis of regular and insulin-modified FSIGT. Am J Physiol. 1998;274(4 Pt 1):E592–E599. doi: 10.1152/ajpendo.1998.274.4.E592. [DOI] [PubMed] [Google Scholar]
- 63.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 64.Kahl S, Nowotny B, Piepel S, Nowotny PJ, Strassburger K, Herder C, et al. Estimates of insulin sensitivity from the intravenous-glucose-modified-clamp test depend on suppression of lipolysis in type 2 diabetes: a randomised controlled trial. Diabetologia. 2014;57(10):2094–2102. doi: 10.1007/s00125-014-3328-3. [DOI] [PubMed] [Google Scholar]
- 65.Bischof MG, Bernroider E, Krssak M, Krebs M, Stingl H, Nowotny P, et al. Hepatic glycogen metabolism in type 1 diabetes after long-term near normoglycemia. Diabetes. 2002;51(1):49–54. doi: 10.2337/diabetes.51.1.49. [DOI] [PubMed] [Google Scholar]
- 66.Schadewaldt P, Nowotny B, Strassburger K, Kotzka J, Roden M. Indirect calorimetry in humans: a postcalorimetric evaluation procedure for correction of metabolic monitor variability. Am J Clin Nutr. 2013;97(4):763–773. doi: 10.3945/ajcn.112.035014. [DOI] [PubMed] [Google Scholar]
- 67.Herder C, Baumert J, Zierer A, Roden M, Meisinger C, Karakas M, et al. Immunological and cardiometabolic risk factors in the prediction of type 2 diabetes and coronary events: MONICA/KORA Augsburg case-cohort study. PLoS ONE. 2011;6(6):e19852. doi: 10.1371/journal.pone.0019852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herder C, Bongaerts BW, Rathmann W, Heier M, Kowall B, Koenig W, et al. Association of subclinical inflammation with polyneuropathy in the older population: KORA F4 study. Diabetes Care. 2013;36(11):3663–3670. doi: 10.2337/dc13-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strom A, Bruggemann J, Ziegler I, Jeruschke K, Weiss J, Al-Hasani H, et al. Pronounced reduction of cutaneous Langerhans cell density in recently diagnosed type 2 diabetes. Diabetes. 2014;63(3):1148–1153. doi: 10.2337/db13-1444. [DOI] [PubMed] [Google Scholar]
- 70.Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63(7):2454–2463. doi: 10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- 71.Ziegler D, Papanas N, Roden M, Group GDCS Neuropad: evaluation of three cut-off points of sudomotor dysfunction for early detection of polyneuropathy in recently diagnosed diabetes. Diabet Med. 2011;28(11):1412–1415. doi: 10.1111/j.1464-5491.2011.03345.x. [DOI] [PubMed] [Google Scholar]
- 72.Ziegler D, Laux G, Dannehl K, Spuler M, Muhlen H, Mayer P, et al. Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med. 1992;9(2):166–175. doi: 10.1111/j.1464-5491.1992.tb01754.x. [DOI] [PubMed] [Google Scholar]
- 73.Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;108(3):586–592. doi: 10.1016/S0161-6420(00)00599-6. [DOI] [PubMed] [Google Scholar]
- 74.Sng CC, Cheung CY, Man RE, Wong W, Lavanya R, Mitchell P, et al. Influence of diabetes on macular thickness measured using optical coherence tomography: the Singapore Indian Eye Study. Eye. 2012;26(5):690–698. doi: 10.1038/eye.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laufs A, Livingstone R, Nowotny B, Nowotny P, Wickrath F, Giani G, et al. Quantitative liver 31P magnetic resonance spectroscopy at 3T on a clinical scanner. Mag Resonan Med. 2014;71(5):1670–1675. doi: 10.1002/mrm.24835. [DOI] [PubMed] [Google Scholar]
- 76.Ende J, Kazis L, Ash A, Moskowitz MA. Measuring patients’ desire for autonomy: decision making and information-seeking preferences among medical patients. J Gen Intern Med. 1989;4(1):23–30. doi: 10.1007/BF02596485. [DOI] [PubMed] [Google Scholar]
- 77.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. The Canadian journal of nursing research = Revue canadienne de recherche en sciences infirmieres. 1997;29(3):21–43. [PubMed]
- 78.Giersdorf N, Loh A, Bieber C, Caspari C, Deinzer A, Doering T, et al. Development and validation of assessment instruments for shared decision making. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2004;47(10):969–976. doi: 10.1007/s00103-004-0905-5. [DOI] [PubMed] [Google Scholar]
- 79.Schunk M, Schweikert B, Gapp O, Reitmeir P, Meisinger C, Mielck A, et al. Time trends in type 2 diabetes patients’ disease management and outcomes: evidence from two KORA surveys in Germany. Exp Clin Endocrinol Diabet. 2009;117(2):88–94. doi: 10.1055/s-2008-1078734. [DOI] [PubMed] [Google Scholar]
- 80.Chernyak N, Stephan A, Bachle C, Genz J, Julich F, Icks A. Assessment of information needs in diabetes: development and evaluation of a questionnaire. Primary care diabetes. 2016. [DOI] [PubMed]
- 81.Kenny SJ, Aubert R, Geiss LS. Washington, DC, US Govt. Printing Office, Prevalence and incidence of non-insulin-dependent diabetes. Diabetes in America. 2nd ed. National Diabetes Data Group (NIH publ. no. 95–1468). 1995; 47– 67.
- 82.Cowie CC, Eberhardt EM. Washington, DC, US Govt. Printing Office, Sociodemographic characteristics of persons with diabetes. Diabetes in America. 2nd ed. National Diabetes Data Group (NIH publ. no. 95–1468). 1995: 85–116.
- 83.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001;24(9):1522–1527. doi: 10.2337/diacare.24.9.1522. [DOI] [PubMed] [Google Scholar]
- 84.Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. BMJ. 1998;317(7167):1202–1208. doi: 10.1136/bmj.317.7167.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.United Kingdom Prospective Diabetes Study. III Prevalence of hypertension and hypotensive therapy in patients with newly diagnosed diabetes. A multicenter study. Hypertension. 1985;7(6):8–13. doi: 10.1161/01.hyp.7.6_pt_2.ii8. [DOI] [PubMed] [Google Scholar]
- 86.Gatling W, Guzder RN, Turnbull JC, Budd S, Mullee MA, Poole Diabetes S The Poole Diabetes Study: how many cases of Type 2 diabetes are diagnosed each year during normal health care in a defined community? Diab Res Clin Pract. 2001;53(2):107–112. doi: 10.1016/S0168-8227(01)00245-5. [DOI] [PubMed] [Google Scholar]
- 87.Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ. 2008;336(7642):491–495. doi: 10.1136/bmj.39474.922025.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonetti S, Trombetta M, Malerba G, Boselli L, Trabetti E, Muggeo M, et al. Variants and haplotypes of TCF7L2 are associated with beta-cell function in patients with newly diagnosed type 2 diabetes: the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS) 1. J Clin Endocrinol Metab. 2011;96(2):E389–E393. doi: 10.1210/jc.2010-1677. [DOI] [PubMed] [Google Scholar]
- 89.Hartwig S, Kluttig A, Tiller D, Fricke J, Muller G, Schipf S, et al. Anthropometric markers and their association with incident type 2 diabetes mellitus: which marker is best for prediction? Pooled analysis of four German population-based cohort studies and comparison with a nationwide cohort study. BMJ Open. 2016;6(1):e009266. doi: 10.1136/bmjopen-2015-009266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Eng J Med. 1988;319(10):599–604. doi: 10.1056/NEJM198809083191002. [DOI] [PubMed] [Google Scholar]
- 91.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54(6):1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Eng J Med. 2002;346(22):1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 93.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Eng J Med. 2005;352(25):2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 94.Weber KS, Nowotny B, Strassburger K, Pacini G, Mussig K, Szendroedi J, et al. The role of markers of low-grade inflammation for the early time course of glycemic control, glucose disappearance rate, and beta-cell function in recently diagnosed type 1 and type 2 diabetes. Diabetes Care. 2015;38(9):1758–1767. doi: 10.2337/dc15-0169. [DOI] [PubMed] [Google Scholar]
- 95.Perseghin G, Lattuada G, De Cobelli F, Esposito A, Costantino F, Canu T, et al. Reduced intrahepatic fat content is associated with increased whole-body lipid oxidation in patients with type 1 diabetes. Diabetologia. 2005;48(12):2615–2621. doi: 10.1007/s00125-005-0014-5. [DOI] [PubMed] [Google Scholar]
- 96.Rossetti L. Glucose toxicity: the implications of hyperglycemia in the pathophysiology of diabetes mellitus. Clin Invest Med. 1995;18(4):255–260. [PubMed] [Google Scholar]
- 97.Jelenik T, Sequaris G, Kaul K, Ouwens DM, Phielix E, Kotzka J, et al. Tissue-specific differences in the development of insulin resistance in a mouse model for type 1 diabetes. Diabetes. 2014;63(11):3856–3867. doi: 10.2337/db13-1794. [DOI] [PubMed] [Google Scholar]
- 98.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 99.Leite SAO, Monk AM, Upham PA, Chacra AR, Bergenstal RM. Low cardiorespiratory fitness in people at risk for type 2 diabetes: early marker for insulin resistance. Diabetol Metab Syndr. 2009;1:8. doi: 10.1186/1758-5996-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haufe S, Engeli S, Budziarek P, Utz W, Schulz-Menger J, Hermsdorf M, et al. Cardiorespiratory fitness and insulin sensitivity in overweight or obese subjects may be linked through intrahepatic lipid content. Diabetes. 2010;59(7):1640–1647. doi: 10.2337/db09-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kullo IJ, Khaleghi M, Hensrud DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol. 2007;102(4):1374–1379. doi: 10.1152/japplphysiol.01028.2006. [DOI] [PubMed] [Google Scholar]
- 102.Jae SY, Heffernan KS, Lee MK, Fernhall B, Park WH. Relation of cardiorespiratory fitness to inflammatory markers, fibrinolytic factors, and lipoprotein(a) in patients with type 2 diabetes mellitus. Am J Cardiol. 2008;102(6):700–703. doi: 10.1016/j.amjcard.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 103.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94(9):3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 104.Tan KC, Chow WS, Tam SC, Ai VH, Lam CH, Lam KS. Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2002;87(2):563–568. doi: 10.1210/jcem.87.2.8249. [DOI] [PubMed] [Google Scholar]
- 105.Zhang L, Gong D, Li S, Zhou X. Meta-analysis of the effects of statin therapy on endothelial function in patients with diabetes mellitus. Atherosclerosis. 2012;223(1):78–85. doi: 10.1016/j.atherosclerosis.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 106.Menart-Houtermans B, Rutter R, Nowotny B, Rosenbauer J, Koliaki C, Kahl S, et al. Leukocyte profiles differ between type 1 and type 2 diabetes and are associated with metabolic phenotypes: results from the German Diabetes Study (GDS) Diabetes Care. 2014;37(8):2326–2333. doi: 10.2337/dc14-0316. [DOI] [PubMed] [Google Scholar]
- 107.Nowotny B, Nowotny PJ, Strassburger K, Roden M. Precision and accuracy of blood glucose measurements using three different instruments. Diabet Med. 2012;29(2):260–265. doi: 10.1111/j.1464-5491.2011.03406.x. [DOI] [PubMed] [Google Scholar]
- 108.Nowotny B, Zahiragic L, Bierwagen A, Kabisch S, Groener JB, Nowotny PJ, et al. Low-energy diets differing in fibre, red meat and coffee intake equally improve insulin sensitivity in type 2 diabetes: a randomised feasibility trial. Diabetologia. 2015;58(2):255–264. doi: 10.1007/s00125-014-3457-8. [DOI] [PubMed] [Google Scholar]
- 109.Begovatz P, Koliaki C, Weber K, Strassburger K, Nowotny B, Nowotny P, et al. Pancreatic adipose tissue infiltration, parenchymal steatosis and beta cell function in humans. Diabetologia. 2015;58:1646–1655. doi: 10.1007/s00125-015-3544-5. [DOI] [PubMed] [Google Scholar]
- 110.Livingstone RS, Begovatz P, Kahl S, Nowotny B, Strassburger K, Giani G, et al. Initial clinical application of modified Dixon with flexible echo times: hepatic and pancreatic fat assessments in comparison with (1)H MRS. Magma. 2014;27(5):397–405. doi: 10.1007/s10334-013-0421-4. [DOI] [PubMed] [Google Scholar]
- 111.Ziegler D SA, Brüggemann J, et al. Overexpression of cutaneous mitochondrial superoxide dismutase in recent-onset type 2 diabetes. Diabetologia. 2015; in press. [DOI] [PubMed]
- 112.Szendroedi J, Chmelik M, Schmid AI, Nowotny P, Brehm A, Krssak M, et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology. 2009;50(4):1079–1086. doi: 10.1002/hep.23093. [DOI] [PubMed] [Google Scholar]
- 113.Zoppini G, Cacciatori V, Raimondo D, Gemma M, Trombetta M, Dauriz M, et al. Prevalence of Cardiovascular Autonomic Neuropathy in a Cohort of Patients With Newly Diagnosed Type 2 Diabetes: the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS) Diabetes Care. 2015;38(8):1487–1493. doi: 10.2337/dc15-0081. [DOI] [PubMed] [Google Scholar]
- 114.Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014;126:3–22. doi: 10.1016/B978-0-444-53480-4.00001-1. [DOI] [PubMed] [Google Scholar]
- 115.Schamarek I, Herder C, Nowotny B, Carstensen-Kirberg M, Strassburger K, Nowotny P et al. Adiponectin, markers of subclinical inflammation and nerve conduction in individuals with recently diagnosed type 1 and type 2 diabetes. Eur J Endocrinol/Eur Feder Endocr Soc. 2016. [DOI] [PubMed]
- 116.Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17(14):1623–1634. doi: 10.1002/(SICI)1097-0258(19980730)17:14<1623::AID-SIM871>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]


