Supplemental Digital Content is available in the text.
Key Words: Endometrium, EMT, β-Catenin, E-cadherin, Sex steroid receptors
Abstract
Epithelial-stroma interactions in the endometrium are known to be responsible for physiological functions and emergence of several pathologic lesions. Periglandular stromal cells act on endometrial cells in a paracrine manner through sex hormones. In this study, we immunohistochemically evaluated the expression of epithelial-mesenchymal transition regulators (SNAIL/SLUG, TWIST, ZEB1), adhesion molecules (β-catenin and E-cadhenin), estrogen (ER)-progesterone (PR) receptor and their correlation with each other in 30 benign, 148 hyperplastic (EH), and 101 endometrioid-type endometrial carcinoma (EC) endometria. In the epithelial component, loss of expression in E-cadherin, ER and PR, and overexpression of TWIST and ZEB1 were significantly higher in EC than in EH (P<0.01). In the periglandular stromal component, β-catenin and SNAIL/SLUG expression were significantly higher in normal endometrium and simple without atypical EH compared with complex atypical EH and EC (P<0.01). In addition, periglandular stromal TWIST expression was significantly higher in EH group compared with EC (P<0.05). There was significantly negative correlation between β-catenin and ER, TWIST and ER, and TWIST and PR in hyperplastic and carcinomatous glandular epithelium, whereas there was a significantly positive correlation between β-catenin and SNAIL-SLUG, β-catenin and TWIST, β-catenin and ER, β-catenin and PR, SNAIL-SLUG and ER, SNAIL-SLUG and PR, TWIST and ER, TWIST and PR, in periglandular/cancer-associated stromal cells (P<0.01). In conclusion, the pattern of positive and negative correlations in the expression of epithelial-mesenchymal transition regulators (SNAIL-SLUG and TWIST), sex hormone receptors (ER and PR), and β-catenin between ECs and hyperplasia, as well as between epithelium and stroma herein, is suggestive of a significant role for these proteins and their underlying molecular processes in the development of endometrial carcinomas.
Endometrial carcinomas are the most prevalent gynecologic malignancies 1. Endometrioid-type endometrial adenocarcinoma (EC), which often arises from a background of endometrial hyperplasia (EH), constitutes approximately 70% to 80% of all endometrial carcinomas and often develops as a result of high estrogen levels 2. The endometrium is composed of glandular epithelium derived from the mesoderm and the supporting mesenchymal stroma 3,4. Endometrial functions, which require interactions between epithelial and stromal components, are cyclically regulated by sex hormones (estrogen and progesterone) and their receptors 5. In addition, ovarian steroids have been reported to act on endometrial stromal steroid receptors rather than the epithelium, mediating physiological endometrial function through paracrine signaling 3,4.
Wnt/β-catenin signaling molecules are tightly regulated in mesenchymal and epithelial cells of the uterus, mediating physiological uterine function. However, abnormal β-catenin expression resulting from CTNNB1 mutation has been shown to be closely related to the development of EC and EH 6,7. Moreover, the β-catenin and steroid hormone signaling pathways have been shown to interact in the endometrium, and both stromal and epithelial factors are necessary for endometrial transcriptional regulation of stromal β-catenin 8. The E-cadherin/β-catenin complex plays a critical role in the maintenance of epithelial cell-to-cell adhesion and normal histologic structure 9. Phosphorylated β-catenin cannot bind to E-cadherin, leading to loss of the epithelial cell-stabilizing function of E-cadherin 10. Loss of E-cadherin and mutations in β-catenin has been shown to induce the epithelial-mesenchymal transition (EMT) in some cancers 11. The EMT is a multistep process involved in the development of biological cells that results in disappearance of intercellular adhesion in the epithelium, acquisition of mesenchymal properties and the migratory phenotype, and increased resistance to apoptosis. Besides its role in embryogenesis, the EMT has also been shown to influence cancer progression and metastasis 12. The first step in induction of the EMT, which also activates mesenchymal genes, is loss of cellular adhesion due to the decreased expression of E-cadherin resulting from the activities of several major transcription factors, including TWIST, ZEB1, SNAIL, and SLUG 13,14. Cells that have undergone the EMT exhibit increased migration and invasion properties in response to estrogen receptor (ER) stimulation. In addition, activation of the Wnt/β-catenin pathway, which initiates nuclear translocation of β-catenin 15, triggers the EMT. However, the expression of EMT-related proteins in EC and EH and their contributions to tumorigenesis are unclear.
In this study, we investigated the expression levels of β-catenin, E-cadherin, ER, and progesterone receptor (PR) in EC and its precursor lesion EH within epithelial components and stromal cells surrounding the gland/tumor to determine their contributions to tumorigenesis.
MATERIALS AND METHODS
Patients and Samples
A total of 101 cases of EC and 148 cases of EH were included in this study. Of these cases, 74 were simple hyperplasia without atypia, 25 were complex hyperplasia without atypia, 21 were simple atypical hyperplasia, and 28 were complex atypical hyperplasia [according to new terminology described by the World Health Organization (WHO) (16); based on an alternative classification, 99 were hyperplasia without atypia and 49 were atypical hyperplasia]. Diagnoses were made in tissues collected from dilation and curettage between 2007 and 2014 at Istanbul Medeniyet University Göztepe Training and Research Hospital. The Ethical Committee of our institution approved the study protocol (approval number 25-13/2013). Information on tumor grade, patient age, and menopausal state was collected for all samples. As a control group, we also analyzed 30 endometrial tissues with normal histology in the proliferative or secretory phase. Periglandular cells were positive for CD-10, confirming their identity as endometrial stromal cells. The composition of acute and chronic inflammatory cells was evaluated morphologically and represented immunohistochemically using LCA (CD45) and CD38 markers (Figure, Supplemental Digital Content 1, http://links.lww.com/IJGP/A30). Cases with intense inflammatory stromal infiltration were excluded from the study.
Tissue Microarray Construction
For comparison of paraffin blocks and hematoxylin-eosin slides of EC and EH tissues, cylindrical samples (1 cylinder per sample) measuring 4 mm in diameter were obtained. Fourteen cylinders were mapped to produce 1 block for immunohistochemical analysis. This procedure was performed using a manual tissue microarrayer (Quick Ray; Unitma Co. Ltd., Seoul, Korea). The obtained tumor tissue samples were mapped, reembedded in paraffin blocks, and processed for immunohistochemical testing.
Immunohistochemistry and Scoring
Paraffin blocks were obtained by tissue microdissection, and immunohistochemical analysis was performed with a Leica Bond Max Autostainer (Leica Biosystems, NE, UK) using antibodies against β-catenin (224M-18; mouse monoclonal; ready to use; 7 mL; Cell Marque, CA), E-cadherin (clone 36B5; mouse monoclonal; 1:100; 1 mL; Leica Biosystems), SNAIL-SLUG (rabbit polyclonal; 1:100; Abcam, CB, UK), TWIST (R10911; rabbit polyclonal; 1:100; 0.1 mL; Atlas Antibody, Sweden), ZEB1 (D83218; rabbit polyclonal; 1:500; 0.1 mL; Atlas Antibody), ER (clone 1D5; mouse monoclonal; 1:200; 1 mL; Biogenex, Fremont, CA), and PR (clone PR88; mouse monoclonal; 1:200; 1 mL; Biogenex).
For epithelial assessment, the cytoplasmic staining intensity of β-catenin was scored using 3 categories (mild, moderate, and intense). The staining ratio was scored as 0 for no staining, 1 for <10%, 2 for 10% to 50%, and 3 for >50%. Staining was considered positive when the result of multiplication of the ratio and intensity scores was 5 or more. For nuclear reactivity, staining of >20% of cells was evaluated as positive. For E-cadherin, membranous staining of >70% was scored as 0, staining with focal loss of 50% to 70% was scored as 1, total loss of 10% to 50% was scored as 2, and total loss of >50% was scored as 3. Membranous staining with scores of 0 and 1 were evaluated as negative, whereas scores of 2 and 3 were evaluated as positive. For SNAIL-SLUG, TWIST, ZEB1, ER, and PR, nuclear staining of 50% or more was semiquantitatively evaluated as positive. For endometrial stroma, nuclear staining of 10% or more was evaluated as positive/spared, whereas nuclear staining of <10% of cells was evaluated as negative/loss for each biomarker. Tissue microarray results were validated by 3 pathologists (S.S., A.B.C., and A.A.).
Statistical Analysis
Pearson χ2 tests, Yates Continuity Correction, and Fisher Freeman Halton (Monte Karlo) tests were used for comparisons. For determination of the correlation between immunohistochemical variables, Spearman correlation analysis was used. Differences or correlations with P values of <0.05 were considered significant. Statistical analysis was performed with the Number Cruncher Statistical System (NCSS) 2007 and Power Analysis and Sample Size (PASS) 2008 Statistical Software (NCSS LLC, Kaysville, UT).
RESULTS
Expression Levels of β-Catenin, E-Cadherin, EMT-related Molecules, and Sex Steroids in Endometrial Tissues: Epithelial Component
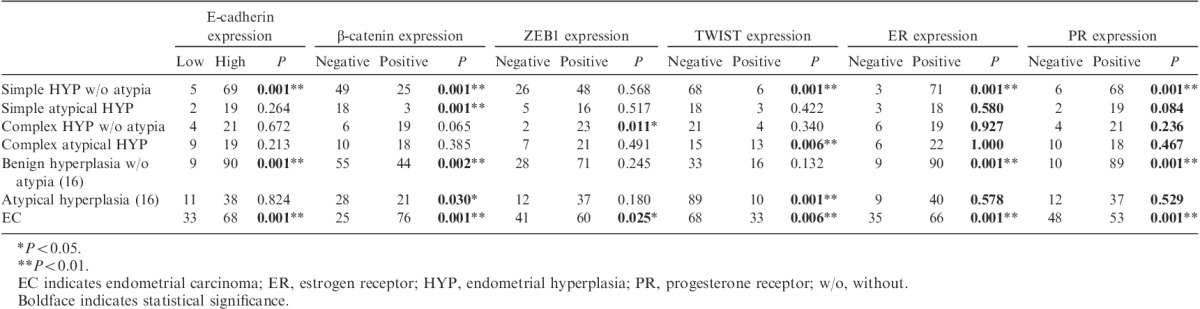
In control endometrium tissue, the expression levels of β-catenin and E-cadherin were mild to moderately positive (Figs. 1A–D), ZEB1 and TWIST were negative (Figs. 1G–M), and SNAIL-SLUG was positive (Fig. 1J). Staining for ER and PR was negative in secretory-phase tissues and positive in proliferative-phase tissues (Figs. 1P–S). In postmenopausal patients, β-catenin expression was significantly increased, whereas E-cadherin expression was significantly decreased (P<0.01 and P<0.05, respectively). Loss of E-cadherin expression was significantly greater in EC than in benign hyperplasia without atypia 16 and simple hyperplasia without atypia (P<0.01; Figs. 1E, F). In EC, β-catenin expression was significantly increased compared with that in benign hyperplasia without atypia 16, simple hyperplasia without atypia, simple atypical hyperplasia (P<0.01), and atypical hyperplasia 16 (P<0.05). Epithelial nuclear TWIST expression was significantly increased in EC, atypical hyperplasia 16, and complex atypical hyperplasia compared with that in simple hyperplasia without atypia (P<0.01; Figs. 1H, I). Epithelial nuclear ZEB1 expression was significantly increased in EC compared with that in EH (P<0.05; Figs. 1N, O). No significant differences in SNAIL-SLUG expression were observed. Significantly decreased expression of ER and PR was observed in EC cases compared with that in EH cases (P<0.01; Figs. 1Q, R, T, U). The results are summarized in Table 1.
FIG. 1.
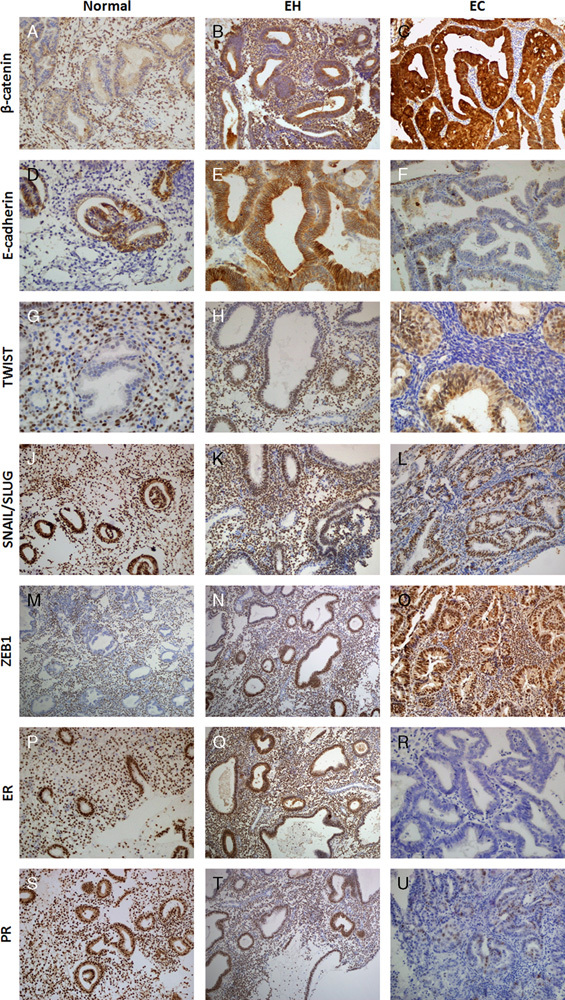
Expression of β-catenin in normal endometrium (400×) (A). Diffuse epithelial and periglandular stromal/mesenchymal cell staining for β-catenin in EH (200×) (B). β-Catenin expression was strongly positive in the epithelium in endometrial carcinoma (EC), but negative in stromal/mesenchymal cells surrounding the tumor (200×) (C). Expression of E-cadherin in normal endometrium (400×) (D). Preserved E-cadherin expression in endometrial hyperplasia (EH) (200×) (E). Loss of E-cadherin expression in EC (200×) (F). Epithelial-negative, stromal-positive TWIST expression in normal secretory endometrium (200×) (G). Stromal TWIST positivity in EH (400×) (H). Nuclear epithelial TWIST positivity, stromal cell TWIST negativity in EC (200×) (I). Stromal and epithelial SNAIL-SLUG positivity in normal endometrium (200×) (J). Positive expression of stromal SNAIL-SLUG in EH (200×) (K). Lack of stromal SNAIL-SLUG expression in EC (200×) (L). Lack of epithelial nuclear ZEB1 expression in secretory endometrium (200×) (M). Epithelial focal ZEB1 immunoreactivity in EH (200×) (N). Positive epithelial diffuse nuclear expression of ZEB1 in EC (200×) (O). Diffuse stromal and epithelial estrogen receptor (ER) immunoreactivity in proliferative endometrium (200×) (P). Diffuse epithelial and stromal ER immunoreactivity in EH (200×) (Q). Loss of ER expression in the stroma and epithelium in EC (400×) (R). Diffuse stromal and epithelial progesterone receptor (PR) immunoreactivity in proliferative endometrium (200×) (S). Diffuse epithelial and stromal PR immunoreactivity in EH (200×) (T). Loss of PR expression in the stroma and epithelium in EC (400×) (U).
TABLE 1.
Comparison of β-catenin, E-cadherin, EMT, ER, and PR expressions with epithelial components and clinicopathologic data of endometrial diseases
Expression Levels of β-Catenin, E-Cadherin, EMT-related Molecules, and Sex Steroids in Endometrial Tissues: Stromal Component
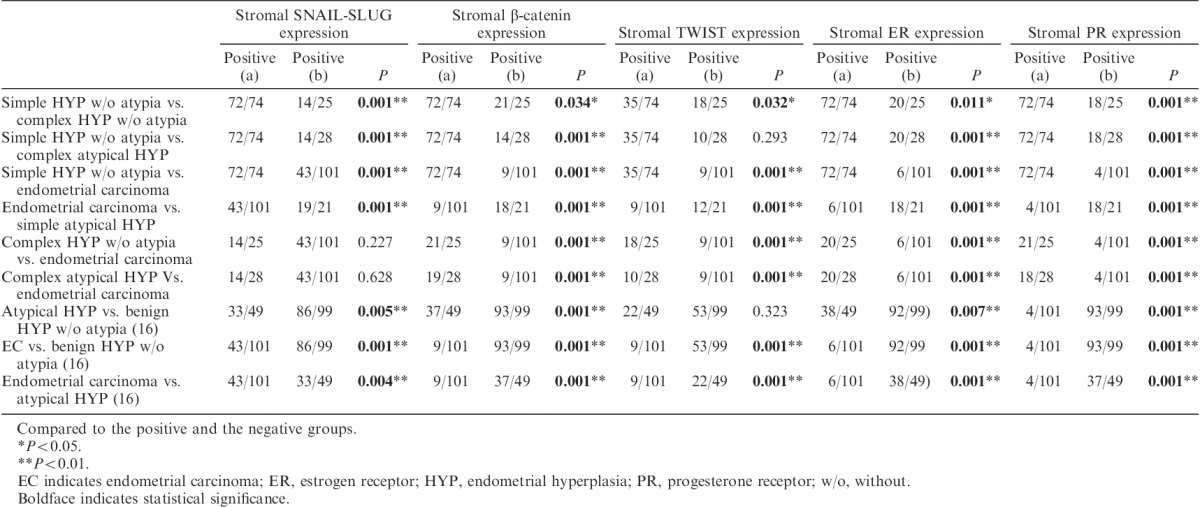
In the control group, all biomarkers except E-cadherin were expressed in stromal cells surrounding the endometrial gland. SNAIL-SLUG, TWIST, ER, PR, and β-catenin expression levels were significantly lower in periglandular stromal cells in postmenopausal patients than those in premenopausal patients (P<0.01). β-Catenin expression was significantly higher in periglandular stromal cells of hyperplasia without atypia 16 than in periglandular stromal cells of complex hyperplasia without atypia, atypical hyperplasia 16, and EC-associated stromal cells (P<0.01; Figs. 1B, C). Similarly, stromal β-catenin expression was significantly higher in complex hyperplasia without atypia and complex atypical hyperplasia than in EC-associated stromal cells (P<0.01). Furthermore, stromal TWIST expression was higher in hyperplasia without atypia 16 than in EC (P<0.01; Figs. 1H, I). TWIST expression in periglandular stromal cells was significantly increased in complex hyperplasia without atypia and complex atypical hyperplasia compared with that in EC (P<0.05). Moreover, SNAIL-SLUG expression was significantly downregulated in the periglandular stroma of EC-associated stromal cells, complex hyperplasia without atypia, and complex atypical hyperplasia compared with that in hyperplasia without atypia and simple hyperplasia without atypica (P<0.01; Figs. 1K, L). ER expression in periglandular stromal cells was significantly upregulated in benign hyperplasia without atypia and simple hyperplasia without atypia compared with that in complex atypical hyperplasia, EC-associated stromal cells, and complex hyperplasia without atypia (P<0.01, P<0.01, and P<0.05, respectively; Figs. 1Q, R). In addition, ER expression in periglandular stromal cells was significantly upregulated in complex hyperplasia without atypia and complex atypical hyperplasia compared with that in EC-associated stromal cells (P<0.01). Stromal PR expression was similar to that of ER, and loss of PR expression in EC-associated stromal cells was significantly greater than that in periglandular stromal cells in all EH groups (P<0.01; Figs. 1T, U). The results are summarized in Tables 2 and 3.
TABLE 2.
Comparison of EMT, β-catenin, ER, and PR expressions with stromal components and clinicopathologic data of endometrial diseases
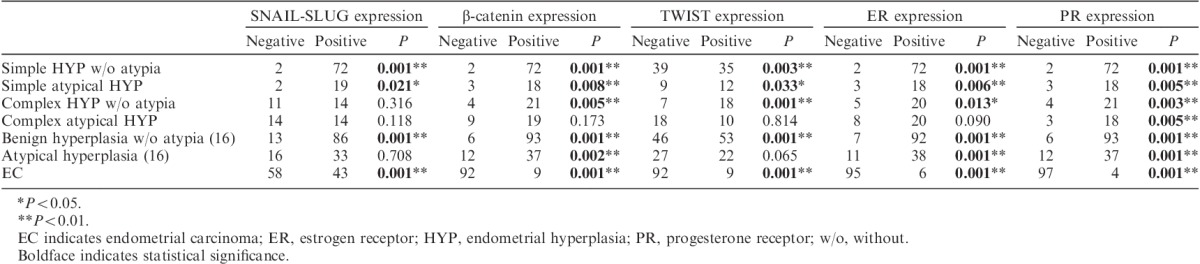
TABLE 3.
Comparison of significant stromal expressions of SNAIL-SLUG, β-catenin, TWIST, ER, and PR with endometrial pathology groups
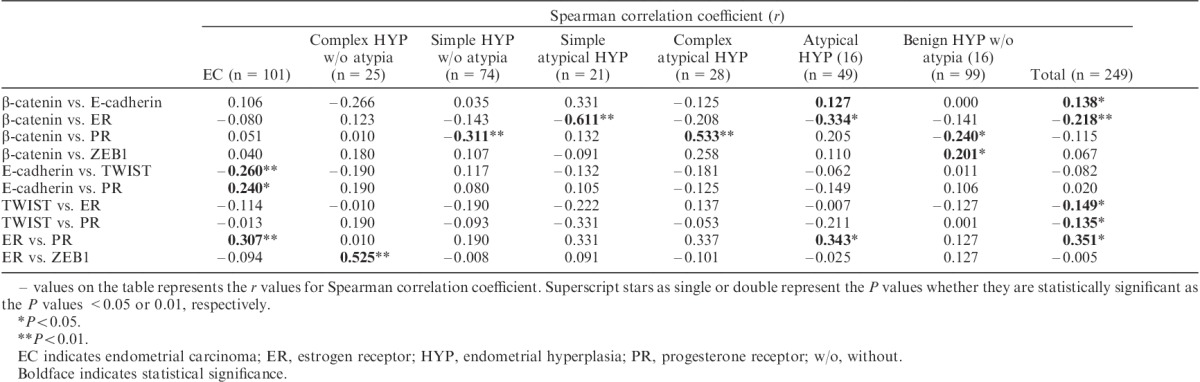
Correlations Between β-Catenin, E-Cadherin, EMT-related Molecules, and Sex Steroid Markers: Epithelial Component
A significant negative correlation between E-cadherin and TWIST (r=−0.260, P<0.01) and a significant positive association between E-cadherin and PR (r=0.240, P<0.05) were observed in EC. Similarly, significant positive correlations were observed between ER and PR (r=0.307, P<0.01) and between β-catenin and PR in complex atypical hyperplasia (r=0.533, P<0.01). Moreover, a significant negative correlation was observed between β-catenin and PR in simple hyperplasia without atypia (r=−0.311, P<0.01). When EC and EH were evaluated together, there were significant negative correlations between β-catenin and ER, TWIST and ER, and TWIST and PR (r=−0.218, P<0.01; r=−0.149, P<0.05; and r=−0.135, P<0.05, respectively), and a significant positive correlation was found between ER and PR (r=0.351, P<0.05). The results are summarized in Table 4.
TABLE 4.
Correlations between β-catenin, E-cadherin, ER, PR, and EMT markers in pathologic endometrial epithelium
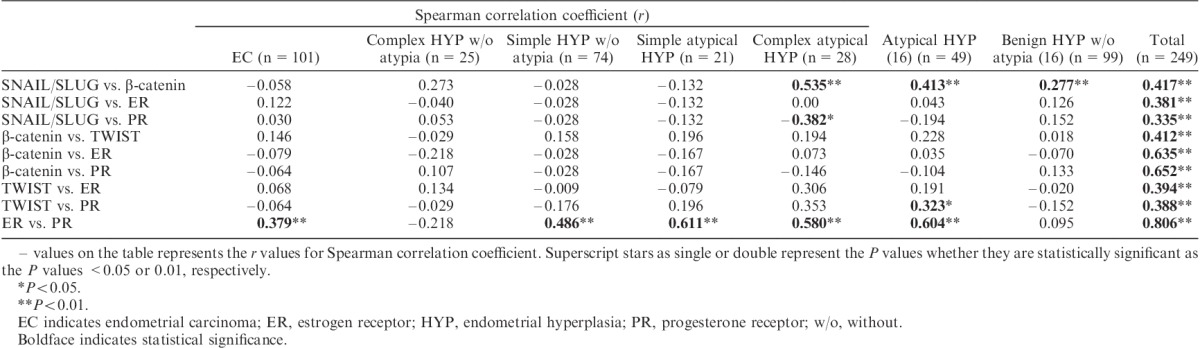
Correlations Between β-Catenin, E-Cadherin, EMT-related Molecules, and Sex Steroid Markers: Stromal Component
There was a significant correlation between ER and PR in EC-associated stromal cells (r=0.379, P<0.01). In periglandular stromal cells of complex atypical hyperplasia, there was a statistically significant negative correlation between SNAIL/SLUG and PR and a positive correlation between SNAIL/SLUG and β-catenin (r=−0.382, P<0.05; and r=0.535, P<0.01, respectively). When EC and EH were evaluated, there were highly significant positive correlations between β-catenin and SNAIL-SLUG, β-catenin and TWIST, β-catenin and ER, β-catenin and PR, SNAIL-SLUG and ER, SNAIL-SLUG and PR, TWIST and ER, TWIST and PR, and ER and PR (P<0.01). The results are summarized in Table 5.
TABLE 5.
Correlations between β-catenin, ER, PR, and EMT markers in the stroma of the endometrial pathologies
DISCUSSION
In this study, we examined the expression levels of various proteins related to the EMT within epithelial components and stromal cells surrounding the gland/tumor in the progression of EH to EC. We found variations in the expression levels of β-catenin, E-cadherin, TWIST, SNAIL-SLUG, ER, and PR in various types of EC. Our results provide important insights into the possible mechanisms mediating progression/invasion in EC.
Mesenchymal cells have important roles in epithelial function, differentiation, growth, and development. In addition, the stroma can regulate epithelial development and reprogram epithelial differentiation in the female reproductive tract 3,17,18. Similarly, in vitro studies have shown that endometrial stromal and glandular cells are able to communicate with each other, and endometrial stromal cells regulate the differentiation and growth of endometrial epithelial cells 19. In recent mutagenic studies, researchers demonstrated that stromal ER is essential for the management of endometrial epithelial cell proliferation and functions via paracrine stromal signals, regardless of epithelial ER activation 3,5. In addition, the transcriptional coactivator β-catenin, which interacts with ER and PR in the uterine epithelia 7,8, is necessary and important for endometrial stromal cell differentiation 6,20,21. Deletion of β-catenin (CTNNB1) in the endometrial stromal compartment, but not in the epithelium, results in deterioration of several endometrial functions, such as decidualization and adenomyosis, supporting the function of β-catenin in mediating the effects of steroid hormones in the endometrium 20,22. Furthermore, several studies have demonstrated that β-catenin contributes to carcinogenesis through stromal/mesenchymal cells rather than the epithelium 23,24. Our results suggest that loss of β-catenin in periglandular stromal cells could contribute to the transitions from hyperplasia without atypia to atypical hyperplasia and from atypical hyperplasia to EC. Moreover, we found that loss of β-catenin expression was associated with loss of ER and PR expression in tumor-associated stroma.
Increasing evidence has indicated that multiple reciprocal interactions between E-cadherin/β-catenin and EMT-inducing transcriptional repressors function to stabilize the invasive mesenchymal phenotype in epithelial tumor cells 25. The EMT is regulated by interactions between carcinoma and stromal cells and is crucial for the progression of several types of cancers 26. In several studies, ER-α-negative endometrial carcinomas have been shown to be associated with increased EMT and decreased E-cadherin expression 27,28, consistent with our current results. In addition, high expression of SNAIL, SLUG, ZEB1, and TWIST in EC compared with that in normal endometrium has been shown to be correlated with loss of E-cadherin expression, potentially leading to loss of E-cadherin-mediated cellular adhesion, acquisition of mesenchymal properties, and motility in epithelial cells, thus promoting carcinogenesis and metastasis/invasion 28,29. In our study, E-cadherin expression decreased in EC, consistent with previous reports, and this phenomenon was accompanied by overexpression of TWIST in the epithelium. Moreover, loss of TWIST and SNAIL-SLUG expression was found to be significantly higher in periglandular stroma of EC than in EH and normal endometrium. A negative correlation between TWIST and E-cadherin was also observed in both the EC and EH. The presence of significant correlations between β-catenin and SNAIL-SLUG and between β-catenin and TWIST supports the idea that β-catenin may have critical and complementary roles in the EMT, consistent with several recent studies 9,11,23.
In our study, we found that TWIST, SNAIL-SLUG, and β-catenin expression levels were increased, whereas E-cadherin expression was decreased in EC epithelium compared with that in EH and normal endometrial tissue. In addition, stromal β-catenin, SNAIL-SLUG, TWIST, ER, and PR expression levels were significantly lower in EC than in EH and normal endometrium. These results suggested that EMT and adhesion molecules may have regulatory effects in stromal mesenchymal cells and epithelial cells; in cases in which ER expression is dysregulated, this effect could be disrupted, leading to the development of EH and EC. In other words, imbalances in the regulation of sex steroids, EMT-related proteins, and β-catenin, which are known to affect mesenchymal/stromal cells, could have roles in EC development. In addition, decreases in SNAIL-SLUG, TWIST, ER, PR, and β-catenin expression levels in periglandular stromal cells in postmenopausal patients compared with those in premenopausal patients, patients with postmenopausal hormonal irregularities, and patients with decreased ER/PR expression suggested that the regulatory functions of EMT molecules and β-catenin in the epithelium via stromal paracrine signaling were disrupted, leading to EH and EC. Loss of β-catenin, TWIST, and ER expression was observed in EC-associated stromal cells compared with those in all EH samples except complex atypical hyperplasia. In addition, significant loss of ER and PR expression was observed in EC compared with that in simple hyperplasia without atypia and the normal proliferative phase of the endometrium. Thus, our data suggested that loss of ER and PR expression in periglandular stromal cells may be an important marker for atypical hyperplasia and EC.
Consistent with similar studies 3–5,17–24, the results of our study supported the hypothesis that glandular cell pathologies resulting in development of either EH or EC could arise as a result of disruption of the regulatory functions of the aforementioned biomolecules in periglandular stromal cells via paracrine signaling. Thus, our results suggest that interactions or loss of activity of β-catenin, EMT-related molecules, and sex steroids in the stroma could result in endometrial glandular pathologies via paracrine signaling. In contrast, stromal expression of SNAIL-SLUG, TWIST, and β-catenin was decreased in postmenopausal cases compared with that in premenopausal cases, suggesting that decreasing ER and PR expression caused disorganization in the EMT and that β-catenin acted as a transcriptional cofactor for steroid hormone signaling pathways, consistent with reports in the literature 7,20. The presence of significant correlations between stromal β-catenin and SNAIL-SLUG and between stromal β-catenin and TWIST suggested that the Wnt/β-catenin pathway and EMT act as coinducers for tumorigenesis or act in a synchronous manner.
In conclusion, our data suggest that sex steroids, EMT-related molecules, and β-catenin may play a role in the development of EC by acting on stromal cells and the endometrial epithelium through loss of regulatory function and expression. Our results indicate that loss of β-catenin, TWIST, SNAIL-SLUG, ER, and PR in the peritumoral endometrial stroma may be an important marker of the transition from hyperplasia without atypia to atypical hyperplasia and from atypical hyperplasia to malignancy. Further studies, including molecular genetic analyses, are needed to confirm our findings.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.intjgynpathology.com.
Supported by the Research Fund of Istanbul Medeniyet University (Project Number: TSA-2013-401).
The authors declare no conflict of interest.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 2.Ellenson LH, Ronnett BM, Soslow RA, et al. Kurman RJ, Ellenson LH, Ronnett BM. Endometrial carcinoma. Blaustein’s Pathology of the Female Genital Tract. New York: Springer; 2011:395–452. [Google Scholar]
- 3.Cooke PS, Buchanan DL, Young P, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurita T, Young P, Brody JR, et al. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–13. [DOI] [PubMed] [Google Scholar]
- 5.Winuthayanon W, Hewitt SC, Orvis GD, et al. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA. 2010;107:19272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh SJ, Shin JH, Kim TH, et al. beta-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition. J Pathol. 2013;231:210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rider V, Isuzugawa K, Twarog M, et al. Progesterone initiates Wnt-beta-catenin signaling but estradiol is required for nuclear activation and synchronous proliferation of rat uterine stromal cells. J Endocrinol. 2006;191:537–48. [DOI] [PubMed] [Google Scholar]
- 8.Hou X, Tan Y, Li M, et al. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18:3035–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih HC, Shiozawa T, Miyamoto T, et al. Immunohistochemical expression of E-cadherin and beta-catenin in the normal and malignant human endometrium: an inverse correlation between E-cadherin and nuclear beta-catenin expression. Anticancer Res. 2004;24:3843–50. [PubMed] [Google Scholar]
- 10.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–65. [DOI] [PubMed] [Google Scholar]
- 11.Montserrat N, Mozos A, Llobet D, et al. Epithelial to mesenchymal transition in early stage endometrioid endometrial carcinoma. Hum Pathol. 2012;43:632–43. [DOI] [PubMed] [Google Scholar]
- 12.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. [DOI] [PubMed] [Google Scholar]
- 13.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. [DOI] [PubMed] [Google Scholar]
- 14.Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg SG. Tumours of the uterin corpus; Epithelial tumours and related lesions. Lyon: IARC Press, 2014. (Kurman RJ, ed. WHO Classification of Tumours of the Female Reproductive Organs). [Google Scholar]
- 17.Donjacour AA, Cunha GR. Stromal regulation of epithelial function. Cancer Treat Res. 1991;53:335–64. [DOI] [PubMed] [Google Scholar]
- 18.Cooke PS, Uchima FD, Fujii DK, et al. Restoration of normal morphology and estrogen responsiveness in cultured vaginal and uterine epithelia transplanted with stroma. Proc Natl Acad Sci USA. 1986;83:2109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold JT, Kaufman DG, Seppala M, et al. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16:836–45. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Patterson AL, Teixeira JM, et al. Endometrial stromal beta-catenin is required for steroid-dependent mesenchymal-epithelial cross talk and decidualization. Reprod Biol Endocrinol. 2012;10:75. doi: 10.1186/1477-7827-10-75. PubMed: 22958837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanwar PS, Zhang L, Roberts DJ, et al. Stromal deletion of the APC tumor suppressor in mice triggers development of endometrial cancer. Cancer Res. 2011;71:1584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanwar PS, Lee HJ, Zhang L, et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brabletz T, Jung A, Reu S, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–66. [DOI] [PubMed] [Google Scholar]
- 26.Schulte J, Weidig M, Balzer P, et al. Expression of the E-cadherin repressors Snail, Slug and Zeb1 in urothelial carcinoma of the urinary bladder: relation to stromal fibroblast activation and invasive behaviour of carcinoma cells. Histochem Cell Biol. 2012;138:847–60. [DOI] [PubMed] [Google Scholar]
- 27.Wik E, Raeder MB, Krakstad C, et al. Lack of estrogen receptor-alpha is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin Cancer Res. 2013;19:1094–105. [DOI] [PubMed] [Google Scholar]
- 28.Blechschmidt K, Kremmer E, Hollweck R, et al. The E-cadherin repressor snail plays a role in tumor progression of endometrioid adenocarcinomas. Diagn Mol Pathol. 2007;16:222–8. [DOI] [PubMed] [Google Scholar]
- 29.Feng Z, Gan H, Cai Z, et al. Aberrant expression of hypoxia-inducible factor 1alpha, TWIST and E-cadherin is associated with aggressive tumor phenotypes in endometrioid endometrial carcinoma. Jpn J Clin Oncol. 2013;43:396–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


