Abstract
Background
There are various definitions and diagnostic criteria for dementia, leading to discrepancies in case ascertainment in both clinical practice and research. We reviewed the different definitions, approaches and measurements used to operationalize dementia in health care studies in German nursing homes with the aim of discussing the implications of different approaches.
Methods
We conducted a systematic search of the MEDLINE and CINAHL databases to identify pre-2016 studies conducted in German nursing homes that focused on residents with dementia or cognitive impairment. In- or exclusion of studies were consented by all authors; data extraction was independently carried out by 2 authors (RP, SJ). The studies’ sampling methods were compared with respect to their inclusion criteria, assessment tools and methods used to identify the study population.
Results
We summarized case ascertainment methods from 64 studies. Study participants were identified based on a diagnosis that was evaluated during the study, or a recorded medical dementia diagnosis, or a recorded medical diagnosis either with additional cognitive screenings or using screening tests exclusively. The descriptions of the diagnostics that were applied to assess a diagnosis of dementia were not fully transparent in most of the studies with respect to either a clear reference definition of dementia or applied diagnostic criteria. If reported, various neuropsychological tests were used, mostly without a clear rationale for their selection.
Conclusion
Pragmatic considerations often determine the sampling strategy; they also may explain the variances we detected in the different studies. Variations in sampling methods impede the comparability of study results. There is a need to consent case ascertainment strategies in dementia studies in health service research in nursing homes. These strategies should consider resource constraints and ethical issues that are related to the vulnerable population of nursing home residents. Additionally, reporting about dementia studies in nursing homes need to be improved. If a diagnosis cannot be evaluated based on either ICD or DSM criteria, the study population may not be reported as having dementia. If a diagnosis is evaluated based on ICD or DSM criteria within the study, there is a need for more transparency of the diagnostic process.
Electronic supplementary material
The online version of this article (doi:10.1186/s12877-016-0249-7) contains supplementary material, which is available to authorized users.
Keywords: Dementia, Cognitive impairment, Diagnosis, Symptom assessment, Nursing home, Health services research
Background
Health care service research aims to evaluate care strategies by obtaining an understanding of the causal factors in improving dementia-related outcomes for individuals living in nursing homes [1]. It is self-evident that a valid method to ascertain a case is a prerequisite for any study [2]. Case ascertainment strategies must rely on an established definition, and diagnostic criteria must be both valid and feasible to conduct.
Dementia may be defined as a clinical syndrome of mental capacity characterized by a substantial global decline in cognitive function that is not attributable to altered consciousness; it consists of a combination of symptoms attributable to various causes or pathological events [3]. Following the Diagnostic and Statistical Manual (DSM) 5th edition [4], dementia is subsumed under the entity of neurocognitive disorders (NCD). The diagnostic criteria for NCD are as follows:
-
i.
Evidence of a significant cognitive decline from a previous level of performance in one or more cognitive domains (complex attention, executive function, learning and memory, language, perceptual-motor or social cognition)
-
ii.
The cognitive deficits interfere with independence in everyday activities (present in major NCD but not in mild NCD)
-
iii.
The cognitive deficits do not occur exclusively in the context of a delirium
-
iv.
The cognitive deficits are not better explained by another mental disorder
Evidence for the cognitive decline should be based on concerns from the individual patient, a knowledgeable informant, or the clinician regarding the presence of a significant decline in cognitive function and a substantial impairment in cognitive performance; ideally, this evidence will be documented by standardized neuropsychological testing. The DSM-V manual notes that both concern and objective evidence are required to establish a diagnosis because they are complementary. If alterations in cognition are only tested utilizing objective measures, a disorder may be underdiagnosed in individuals who demonstrate high-functioning performance and score in the “normal” range but are still substantially impaired relative to their baseline performance. On the other hand, an exclusive reliance on subjective symptoms may result in underdiagnosing individuals who deny or fail to express their impairments. In either case, it is essential to interpret results in comparison with the individual patients’ prior performance.
The International Classification of Diseases (ICD) defines dementia as a decline in both memory and other cognitive abilities such as deterioration in judgment and thinking or the general processing of information. The decline in memory and cognitive abilities should be objectively verified by obtaining a reliable history from an informant and supplemented by neuropsychological tests or quantified cognitive assessments. The diagnosis of mild impairment is based on the degree of memory loss that interferes with everyday activities. A diagnosis of dementia requires, in addition to the previously mentioned symptoms for mild impairment, a decline in emotional control or motivation, changes in social behavior, and the absence of delirium. Furthermore, the described symptoms must be present for at least 6 months [5].
The diagnostic process follows two steps, starting with the initial recognition of the dementia syndrome and ending with the specification of an etiological subtype. The diagnostic criteria for each of the subtypes are specified in the DSM-V, ICD-10 [4, 5] and publications from different disease-related societies or national institutes (e.g., the National Institute of Neurological and Communicative Disorders and Stroke, NINCDS) [6–10]. The subtype differentiation may require biomarker diagnostics such as genetic, blood and liquor testing or diverse imaging procedures.
For more than 3 decades, the history of classification systems for mental disorders has reflected both many changes and a relative emphasis on phenomenology, etiology, and course as defining features. When the DSM-III’s (1980) explicit diagnostic criteria were introduced (Feighner-criteria), several revisions followed: the DSM-III-R (revision) in 1987, the DSM-IV in 1994, and the DSM-V in 2013. The ICD introduced diagnostic criteria in its revised version 9, ICD-9-CM (for clinical modification). Every revision aimed both to reduce inconsistencies and to improve clarity and precision. Different versions included changes in the classification, diagnostic criteria sets, and descriptive texts [11].
Dementia diagnoses pose several challenges. First, the lack of valid biomarkers forces healthcare providers to diagnose dementia based on the presence of specific symptoms and the elimination of other conditions with similar symptoms. To arrive at a clinical diagnosis of dementia, a combination of basic assessments,—including physical, psychopathological and basic neuropsychological examinations—are recommended [12, 13]. For the basic neuropsychological diagnosis, different short screening tests such as the Mini Mental Status Examination (MMSE) [14, 15], the DemTect [16] and the Clock Drawing Test [17] are recommended. In clinical practice, these tests should be administered to every patient with dementia or suspected dementia on a regular basis both to quantify cognitive impairments and to enable providers to supervise the progression of the disease. Comprehensive neuropsychological diagnostics are necessary either if diagnostic findings are not congruent in the early stage of dementia or if an etiological classification is required. A dementia diagnosis should not be based solely on one neuropsychological test but instead must be based on both behavioral symptoms and impairment assessments related to daily function. Additionally, blood tests and imaging diagnostics should be conducted to assure both the diagnosis and its etiology [18].
The reliability, validity and utility of the new diagnostic criteria have discussed by clinicians and researchers since the date of their release; furthermore, a consensus about how to best diagnose dementia has still not been found [19, 20]. One result of this controversy is the methodological differences in epidemiological studies that lead to discrepancies in case ascertainment and varying incidence and prevalence rates [3, 21]. Another limitation of the generalizability of study results on dementia in nursing homes originates from the methodological difficulties of assessing the disease in the oldest old population. The normative data necessary to determine “impairment” for this population are lacking and therefore, aging is constitutionally associated with a decline in both cognition and function. Consequently, pathological processes are difficult to distinguish from normal aging; in addition, alterations in function and sensory impairments may both affect subjects’ cognitive performance and limit an accurate assessment [20].
The quality of a dementia diagnosis in German nursing homes is suboptimal: many studies of nursing home residents show both vast inaccuracies and diagnostics that do not conform to either the ICD or the DSM. Studies from Germany show that between 30 and 40 % of people with dementia living in nursing homes are not accurately diagnosed; for these residents, either an etiological differentiation is missing or there is an inappropriate diagnosis [22–24]. Inaccurate diagnosis has also been found in studies from the United States, Norway, Israel and Ireland [25–29].
Nursing home research is challenged by inaccurate recorded diagnoses. Ignoring inconsistencies in a recorded dementia diagnosis may provoke misclassifications, bias selection and confound study results [30]. However, the evaluation of a diagnosis within a study is resource-intensive and may be ethically questionable because the diagnosis process is reported as burdensome and stigmatizing [31, 32].
Researchers must develop a sampling strategy that eliminates inconsistencies while remaining both reliable and practicable. We assume that in the past, researchers defined dementia differently and applied different methods to identify residents with dementia in nursing homes. Therefore, we investigated the methods used to define dementia and to identify people with dementia in health services research. Although we assume that the delineated problem is obvious in various countries, we limited the scope of our study to research undertaken in a single country (Germany). In Germany, physicians are not constantly present in nursing homes, and nurses are not allowed to assist in diagnostic procedures; resources for dementia diagnostics in primary care are scarce [33]; hence, diagnostics are often superficial, performed rapidly and lacking recommended measures [34, 35]. Due to the free choice of medical practitioners that is guaranteed by law in Germany, residents within one nursing home may be cared for by different practitioners, making it difficult for researchers to reconstruct the diagnostic procedures that were applied by various medical doctors. These conditions require a carefully considered sampling strategy. The narrowed focus on one country ensures the comparability of the studies because the nursing-home conditions are identical. This allows a synthesis and comparison of the various methods used in the included studies.
Because cognitive impairment (CI) is the leading symptom in dementia and the conditions “dementia” and “CI” are sometimes used interchangeably in scientific publications, the scope of our review encompasses studies that do not exclusively focus on residents with dementia but instead include residents with CI. Provided we found differences, we discuss the implications of different methods on the generalizability and comparability of the study findings.
The following research questions were addressed:
How is dementia defined and measured in health services research studies in German nursing homes?
Which implications can be derived for health services research in nursing homes?
Based on the results, we discuss the implications of the different methods and suggest principles to guide future studies.
Methodology of the review
Compared to systematic reviews that aim to answer questions about the effectiveness of clinical procedures, there are few well-defined tools and processes for methodological reviews; not all items of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines are applicable (see PRISMA checklist in the Additional file 1: Table S1) [36]. Unlike a traditional Cochrane-style review, Lilford and colleagues [36] recommend methodological reviewers not to obtain a thorough collection of studies, but to ensure a wide search that provides an overview of the topic. They also recommend various safeguards to reduce potential bias, such as multi-disciplinary teams and peer reviewing of the final report. The methods applied in this review consider these recommendations.
Literature search strategy
We conducted a systematic literature search to identify relevant publications in the MEDLINE [PubMed] and CINAHL [EBSCO] databases in January 2014 (MND) and updated it in December 2015 (RP).These databases were chosen because we considered them as the most relevant to the field of health services research. We detail the search strategy in the Additional file 2: Table S2 and Table S3. The search was not limited to a specific time and study design, but it was limited to the German and English languages. After the initial title and abstract screening, publications were excluded if they were considered irrelevant to the topic or setting (RP, CGGS). The remaining publications were divided to be read in full-text and to be excluded according to predefined criteria by one research team member (RP, SR, MND, CGGS, BH, MH). Publications fulfilling the following criteria were included for review: the research aim or question focused on residents with dementia or CI in German nursing homes and was answered based on empirical data. Each decision to exclude a publication was discussed and consented by the research team.
Data extraction and method of analysis
Two researchers (RP, SJ) read the included articles in full-text and extracted the data: SJ conducted an initial data extraction, and RP checked all of the information for accuracy. In the first step of the data extraction, an overview of the data on the study aim, design and sample size were compiled. In the second step, the publications were sorted according to sample determination procedure. Publications from one study using the same method to identify residents with dementia/CI were summarized; this was the case with publications from large projects with various research aims and questions. To answer the research questions, the following information was extracted: information on the method of sample determination, the definition and criteria used for dementia diagnostics, the screening instrument(s) used, and the qualification and training of the professionals involved. The extracted findings and key characteristics of each method were summarized in an overview, and differing approaches were compared.
Results
The literature search yielded 650 articles. Sixty-four articles were identified to answer our research questions. The results of the literature search can be seen in the flow chart in Fig. 1.
Fig. 1.
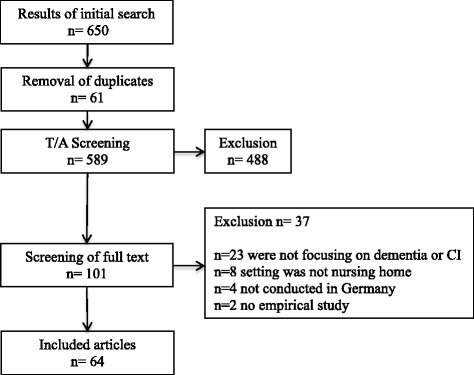
Flow chart of literature search
The characteristics of the included studies are presented in Table 1.
Table 1.
Characteristics of included studies
| Publication | Author | Study aim | Study design | Sample size | |
|---|---|---|---|---|---|
| NH | P | ||||
| Experimental studies | |||||
| [54] | Bär et al. 2006 | Efficacy of an individual approach in the care of people with dementia to stimulate positive emotions | CT | N/A | 46 |
| [82] | Berg et al. 2010 | Efficacy of snoezelen, structured reminiscence therapy and 10-minute activation on apathy in dementia | C-RCT | N/A | 360 |
| [107] | Dichter et al. 2015 | Testing the effectiveness of Dementia Care Mapping on PwD and caregivers, exploring implementation facilitators and barriers | CT | 9 | 154 |
| [76] | Graessel et al. 2011 | Efficacy of a non-pharmacological intervention on the cognition of residents with dementia | RCT (Follow-up) | 5 | 98 |
| [108] | Halek et al. 2013 | Efficacy of dementia care mapping on the quality of life of residents with dementia | CT | 9 | N/A |
| [70] | Kuske et al. 2009 | Effectiveness of a nursing home training staff for the interaction between residents with dementia and their caregivers | C-RCT | 6 | 298 |
| [77] | Luttenberger et al. 2012 | Efficacy of a non-pharmacological intervention on dementia symptoms and need of care in NH residents with dementia | RCT | 5 | 139 |
| [78] | Luttenberger et al. 2012 | Sustainability of a non-pharmacological intervention after 10 months | RCT (Follow-up) | 5 | 61 |
| [48] | Majic et al. 2013 | Efficacy of animal-assisted therapy on agitation/aggression and depression in nursing home residents with dementia | RCT | 18 | 65 |
| [80] | Pickel et al. 2011 | Efficacy of occupational group therapy in dementia | CT | N/A | 56 |
| [51] | Rapp et al. 2013 | Efficacy of a complex guideline-based intervention on agitation and the use of psychotropic drugs | C-RCT | 18 | 304 |
| [47] | Reuther et al. 2014 | Effect evaluation of dementia-specific case conferences (study protocol) | C-RCT | 12 | 360 |
| [109] | Schäufele et al. 2013 | Efficacy of an interdisciplinary guideline to enhance the mobility of residents with dementia in nursing homes | CT | 31 | 707 |
| [110] | Schäufele et al. 2015 | ||||
| [81] | Treusch et al. 2015 | Effect evaluation of an occupational and sports therapy intervention for PwD in NH | C-RCT | 18 | 117 |
| Observational studies | |||||
| [85]a | Afram et al. 2014 | Exploration of reasons and variations for institutionalization of people with dementia in 8 European countries according to caregivers | Cross-sectional | 3 | 786 |
| [86]a | Alvira et al. 2015 | Description of the association between reactions of informal caregivers of people with dementia and health outcomes from 8 European countries | Cross-sectional | N/A | 119 |
| [61] | Becker et al. 2005 | Development and validation of an instrument to assess quality of life in dementia | Cross-sectional | 11 | 121 |
| [87]a | Beerens et al. 2014 | Exploration of the variance of quality of life and quality of care for people with dementia from 8 European countries | Cross-sectional | N/A | 119 |
| [88] | Beerens et al. 2015 | Assessment of factors that contribute to the change of quality of life of people with dementia recently admitted to an NH from 8 European countries | Longitudinal | N/A | 791 |
| [22] | Brune-Cohrs et al. 2007 | Quality of dementia diagnosis in nursing homes | Cross-sectional | 2 | 200 |
| [89]a | De Mauleon et al. 2014 | Determination of factors associated with the antipsychotic prescription for PwD in 8 European countries | Cross-sectional | N/A | 119 |
| [83] | Dettbarn-Reggentin 2005 | Evaluation of milieu-therapeutic living units on residents with dementia | Longitudinal | 3 | 60 |
| [53] | Dichter et al. 2013 | Validation of the QUALIDEM in nursing homes | Cross-sectional | 43 | 634 |
| [111] | Dichter et al. 2014 | Testing of the inter- and intra-rater reliability of the QUALIDEM instrument to measure quality of life in PwD | Cross-sectional | 9 | 161 |
| [44]a | Foebel et al. 2014 | Description of patterns of antipsychotic drug use in PwD in nursing homes in 7 European countries and Israel | Cross-sectional | 9 | 496 |
| [72] | Geiger-Kabsich and Weyerer 1993 | Validity of the Alters-Konzentrations-Test | Cross-sectional | 2 | 71 |
| [91] | Gietzelt et al. 2014 | Study protocol for an RCT testing the effectiveness of behavioral treatment for mild Alzheimer’s patients | Longitudinal | 1 | 40 |
| [41] | Graessel et al. 2009 | Validation of the Erlangen Test of Activities of Daily Living (E-ADL) | Cross-sectional | 2 | 46 |
| [45] | Gräske et al. 2014 | Examination of variability and associated factors of quality of life ratings | Cross-sectional | 5 | 133 |
| [66] | Jakob et al. 2002 | Prevalence and incidence of dementia in nursing homes compared to private households | Longitudinal | N/A | 192 |
| [69] | Köhler et al. 2007 | Validation of the Dementia Screening Scale (DSS) | Cross-sectional | 20 | 589 |
| [37] | Kölzsch et al. 2012 | Description of pain treatment in nursing home residents with CI | Cross-sectional | 40 | 560 |
| [68] | König et al. 2014 | Comparison of the costs of care for community-dwelling PwD and PwD living in nursing homes | Cross-sectional | N/A | 48 |
| [62] | Lueken et al. 2007 | Development of a short version of the Apathy Evaluation Scale specifically adapted for nursing home residents with dementia | Cross-sectional | N/A | 356 |
| [79] | Luttenberger et al. 2012 | Revalidation of the E-ADL scale | Cross-sectional | 5 | 139 |
| [49] | Majic et al. 2010 | Pharmacotherapy in residents with dementia | Cross-sectional | 18 | 304 |
| [50] | Majic et al. 2012 | Correlation of agitation and depression in nursing home residents with dementia | Cross-sectional | 18 | 304 |
| [46] | Makai et al. 2014 | Validation of the ICECAP-O measure for wellbeing in older PwD in NH and exploration of response-associated factors | Cross-sectional | 1 | 95 |
| [58] | Marquard and Schmieg 2009 | Relationship between architectural characteristics of the nursing home and the residents ability to perform way finding tasks | Cross-sectional | N/A | 450 |
| [59] | |||||
| [75] | Meyer-König et al. 1984 | Examination of nursing home residents with a chronic brain syndrome | Cross-sectional | N/A | 163 |
| [38] | Osterbrink et al. 2012 | Prevalence of pain in nursing home residents with various cognitive functions | Cross-sectional | 13 | 436 |
| [57] | Palm et al. 2013 | Evaluation of the provision of dementia care and identification of resident- and facility-related factors associated with quality of life and behavior | Longitudinal | N/A | N/A |
| [52] | Palm et al. 2015 | Comparison of case conferences between dementia-specialized versus traditional care units | Cross-sectional | 51 | 888 |
| [67] | Riedel et al. 2013 | Prevalence of Parkinson’s disease, associated dementia and depression in Dresden | Cross-sectional | 36 | 195 |
| [24] | Schäufele et al. 2013 | Prevalence of dementia and provision of dementia care in nursing homes | Cross-sectional | 58 | 4481 |
| [65] | Schuler et al. 2007 | Validation study of the “Pain Assessment in Advanced Dementia Scale” (PAINAD-G) in nursing home residents | Cross-sectional | 8 | 99 |
| [39] | Schumacher et al. 1997 | Prevalence of depression and CI in nursing home residents | Cross-sectional | 3 | 380 |
| [23] | Seidl et al. 2007 | Prevalence of non-cognitive symptoms and psychopharmacological treatment in nursing home residents with dementia | Cross-sectional | N/A | 145 |
| [64] | Seidl et al. 2009 | Comparison of neurological soft signs of residents with AD with residents without cognitive impairments | Cross-sectional | N/A | 120 |
| [63] | Seidl et al. 2011 | Description of autobiographical memory deficits in residents with dementia | Cross-sectional | N/A | 239 |
| [84] | Theison et al. 2009 | Association of agitation in the morning and depression | Cross-sectional | 3 | 110 |
| [73] | Weyerer et al. 1990 | Validation of the Brief-Assessment-Interview | Cross-sectional | 1 | 32 |
| [71] | Weyerer et al. 1995 | Prevalence of dementia and depression in nursing home residents from Mannheim and Camden | Cross-sectional | 12 | 542 |
| [56] | Weyerer et al. 2004 | Comparison of residents from day-care centers and nursing homes | Cross-sectional | 47 | 1644 |
| [42] | Weyerer et al. 2005 | Evaluation of special and traditional dementia care in nursing homes | Cross-sectional | 31 | 1644 |
| [43] | Weyerer et al. 2010 | Evaluation of special and traditional dementia care in nursing homes | Cross-sectional | 31 | 1644 |
| [90] | Wubker et al. 2015 | Comparison of costs for PwD receiving home care versus nursing home care in 8 European countries | Cross-sectional | N/A | 76 |
| [40] | Wulff et al. 2012 | Description of perceived autonomy of nursing home residents with and without CI | Cross-sectional | 40 | 560 |
| [93] | Zenthofer et al. 2014 | Comparison of oral hygiene and health status of nursing home residents with and without dementia. | Cross-sectional | N/A | 93 |
| Qualitative studies | |||||
| [55] | Bär et al. 2003 | Identification of characteristic situations accompanied by positive emotions | Qualitative | N/A | 29 |
| [60] | Becker et al. 2006 | Identification and cross-validation of patterns of competence in nursing home residents. | Qualitative | N/A | 362 |
| [92] | Nordheim et al. 2015 | Evaluation of the use of tablet PCs in PwD in NH | Qualitative | 1 | 14 |
AD Alzheimer’s disease, C-RCT Cluster-randomized controlled trial, CT controlled trial, N/A not available, NHs nursing homes, P Participants, PwD People with dementia, RCT Randomized controlled trial
aThe number of participants reported from this international study refers to the German sample only
Methods to identify the study population residents with dementia
All 64 articles focused on the population of nursing home residents with CI. In 60 articles, dementia was specified as the etiology of CI. In these articles, the term “dementia” was used consistently in the title, abstract, key words and research questions. Four articles focused their research question on residents with CI without any specification of etiology, which means they did not clearly define dementia residents as their study population [37–40]. However, in these articles the terminology was not consistent throughout the article: the term dementia and CI were used interchangeably.
The articles focusing on residents with dementia used different methods to determine their study sample. We identified 4 methods that were clearly described.
Nursing home residents with dementia were identified in one of the following ways:
A study diagnosis that was evaluated during the study.
A recorded medical diagnosis.
A recorded medical diagnosis and an additional cognitive screening performed during the study.
A cognitive or accordant screening.
- Study diagnosis
- In 17 articles, a study diagnosis of dementia was used to determine the participant sample (Table 2). The diagnosis was newly assessed either in all residents or in residents with a diagnosis that had previously been documented.
- Recorded medical diagnosis
- In 6 articles, the recorded diagnosis was the criterion for determining the sample participants (Table 3). In the study by Graessel et al. [41], attending physicians confirmed the recorded diagnosis; in the study by Weyerer et al. [42, 43], the diagnosis was used as an indirect inclusion criterion. In this study, the dementia diagnosis was an admission criterion for the care unit that participated in the study (Dementia Special Care Unit). This study does not report whether the diagnosis was confirmed. In 3 articles, the diagnosis was obtained from the residents’ records [44–46]; 1 article reported that the dementia type was evaluated according to the ICD classification (as recorded), and the dementia severity evaluation was guideline-based and performed according to the recommended MMSE cutoff values [46].
- Recorded medical diagnosis and screening
- In 20 articles, the recorded medical diagnosis of dementia was used as a criterion to determine the sample participants, but it was combined with the results of a cognitive screening measure (Table 4). In 19 studies, a resident was included if the result of the MMSE also indicated CI; 1 study included a resident if a diagnosis was recorded and the result of a screening using the Functional Assessment Staging Test (FAST) indicated dementia [47]. One study used a stepwise approach [48–51]: in the case of an incongruity between the diagnosis and the MMSE result, additional diagnostics were performed to decide whether a resident fulfilled the inclusion criteria.
- Screening
- In 10 articles, residents with dementia were identified by 1 screening or a combination of 2 screenings (Table 5). In these studies, an existing diagnosis was recorded, but it was not used as the inclusion criterion. Palm et al. [52] used the place of residence in a Dementia Special Care Unit as an inclusion criteria as well as the results of a cognitive screening to define their sample. One article reported exclusion criteria that were used to differentiate dementia from other psychiatric disorders [53].
- The 3 studies (4 articles) that investigated residents with CI exclusively used screenings to identify their study participants (Table 6).
- Seven publications could not be classified according to the 4 groups explained above (Table 7). In 4 of these publications, all of the residents from a participating nursing home or living unit were included in the study and investigated with respect to various indicators for dementia (dementia diagnosis in the records and results of cognitive screenings) [24, 54–56]. In these studies, the sample was described, but participants were not selected or assigned based on these indicators. One article is a study protocol; the method to define residents with dementia was not determined, only the measurements that were intended to assess dementia [57]. In 2 publications [58, 59], the method to identify residents with dementia was not reported at all.
Table 2.
Overview of studies that determine study participants with dementia based on a study diagnosis
| Publications | Method of sample determination | Definition and diagnostic criteria used for (new) dementia diagnosis | Screenings performed | Qualification and training of professionals performing screenings/diagnostics |
|---|---|---|---|---|
| [22] | Diagnostics performed for every resident with an existing dementia diagnosis |
▪ Clinical examination ▪ Semi-standardized interview and neuropsychological testing according to ICD-10 criteria ▪ Consolidation of existing diagnostic findings ▪ NINCDS-ADRDA criteria for diagnosis of AD ▪ Consensus criteria for frontotemporal dementia ▪ Petersen criteria for mild cognitive impairment (MCI)a ▪ NINDS-AIREN for vascular dementia |
MMSE, CDR, Behave-AD, BPRS, HDRS 17, B-ADL | Diagnosis: Physician with experience in geriatric psychiatry |
| [23] | Diagnostics performed by physicians from the research team for every resident fulfilling one of the criteria: ▪ Presence of dementia diagnosis in the nursing records ▪ Resident appears forgetful ▪ Resident has problems with orientation within the NH |
▪ NINCDS-ADRDA criteria on the basis of clinical examination, existing assessments of status and progress, existing diagnostic findings (technical investigations) ▪ Dementia was classified into different types: AD, vascular dementia, mixed type, frontotemporal dementia |
MMSE, GDS, Clock Drawing Test, CERAD entire battery, BAGI, AES, NPI | Screening instruments and diagnosis: Experienced geriatric psychiatrist with formal training in the administration and scoring of the respective instruments. |
| [60] | ||||
| [61] | ||||
| [62] | ||||
| [63] | ||||
| [64] | ||||
| [65] | ||||
| [66] | Diagnostics performed for a random sample of nursing home residents |
▪ SIDAM-interview for the assessment of cognitive function; in case of severe physical impairment CDR ▪ Diagnosis of etiological subtype based on the findings from the SIDAM-interview ▪ Diagnosis discussed in an expert conference of physicians and psychologists according to DSM-III-R |
SIDAM, MMSE or CDR | Diagnosis: Physicians and psychologist who received training in conducting structured interviews |
| [67] | Diagnostics performed for a random sample of residents with Parkinson’s disease | ▪ Diagnosis assessed according to DSM-IV-TR criteria using the SIDAM-interview, clinical examination, medical history | SIDAM, MMSE, PANDA (subsample) | Screening instruments and diagnosis: Study monitor with a medical education |
| [69] | Diagnostics in the study was performed for all nursing home residents. |
▪ No definition or diagnostic criteria stated ▪ Diagnosis assessed using the CDR (≥ 1) |
MMSE, BAS-DEM, CDR, DSS, BAI | Diagnosis: Trained clinical psychologist Screening (DSS): Licensed geriatric nurses with frequent contact with the residents during the previous 4 weeks |
| [70] | Diagnostics in the study was performed for every consenting resident. |
▪ No definition or diagnostic criteria stated ▪ Diagnosis assessed using the CDR (≥ 1) |
CDR, MMSE, Barthel-Index | Diagnosis: Determined in multidisciplinary consensus conferences held by psychiatrists, clinical psychologists and health and nursing specialists. Screening instruments: Not specified |
| [71] | Diagnostics performed for all NH residents |
▪ No definition or diagnostic criteria stated ▪ Diagnosis assessed using the BAI (3-8 = mild to severe dementia) |
BAI | Interviews performed by trained NH staff with experience in clinical psychology and psychiatry |
| [72] | Diagnostics performed for NH residents able to be interviewed |
▪ Assessment of diagnosis according to Feighner-criteria ▪ Dementia severity cutoff value (MMSE ≤ 23 minimum mild dementia) |
AKT, BAI, | Diagnosis: NH manager experienced in psychiatry |
| [73] | Diagnostics in the study performed for a non-defined sample of NH residents | ▪ Diagnosis assessed according to the Feighner criteria and compared with a diagnosis assessed with the BAI (BAI 0-2 = most likely no dementia, 3-7 = mild to moderate dementia, 8 = severe dementia) | BAI | Diagnosis (Feighner criteria): experienced NH manager |
| [75] | Diagnostics for organic psycho syndrome (OPS) (dementia) performed in a non-defined sample of NH residents | ▪ Differentiation of OPS severity based on an assessment of cerebral dysfunction and changes in personality | Not specified | Not specified |
| [68] | Diagnostics in the study performed for all included participants |
▪ SIDAM interview was conducted ▪ Diagnosis was based on a consensus between study interviewers and an experienced geriatrician or geriatric psychiatrist according to DSM IV for Alzheimer or ICD-10 or DSM III R criteria for multi-infarct dementia and other etiology ▪ Diagnostic criteria: objective deficits in memory and another cognitive domain, impairment in activities of daily living ▪ Classification of dementia was based on the CDR (≤ 1 = mild, 2 = moderate, 3 = severe) ▪ Assessed data were combined into simple and weighted count scores |
SIDAM, CDR, MMSE, Barthel-Index for ADL impairment, IADL impairment scale, 28 chronic conditions | Trained physicians or psychologists conducted interviews with participants and their caregivers. |
AD Alzheimer’s disease, ADL Activites of daily living, AES Apathy Evaluation Scale, AKT Alters-Konzentrationstest, B-ADL, Bayer-Activities of Daily Living Scale, BAGI Bielefelder Autobiografisches Gedächtnisinventar, BAI Brief Assessment Interview, BAS-Dem Brief Assessment Schedule, Behave-AD Behavioral Pathology in Alzheimer’s Disease, BPRS Brief Psychiatric Rating Scale, CDR Clinical Dementia Rating, CERAD The Consortium to Establish a Registry for Alzheimer’s Disease, DSS Dementia Screening Scale, DSM Diagnostic Statistical Manual, E-ADL Erlangen Test for Activities of Daily Living, GDS Global Deterioration Scale, HDRS 17 Hamilton Depression Scale 17, IADL Instrumental Activities of daily living, MMSE Mini Mental State Examination, NOSGER Nurses’ Observation Scale, NPI Neuropsychiatric Inventory, PANDA Parkinson Neuropsychometric Dementia Assessment, SIDAM Structured Interview for the Diagnosis of Alzheimer’s Dementia, Multi-infarct dementia and dementias of other etiology
aMild Cognitive Impairment is defined as a cognitive disorder that is characterized by impaired memory function and learning abilities. None of the symptoms are severe enough to justify a dementia diagnosis [5]
Table 3.
Overview of studies that defined study participants with dementia based on a recorded diagnosis
| Publications | Method of sample determination | Definition and diagnostic criteria used for (new) dementia diagnosis | Screenings performed | Qualification and training of professionals performing screenings/diagnostics |
|---|---|---|---|---|
| [41] | A suspected dementia diagnosis (by nursing staff) or existing dementia diagnosis confirmed by the attending GP |
▪ ICD-10 criteria ▪ Dementia severity stages: MMSE: 0-9 severe, 10-17 moderate, ≥ 18 mild dementia |
MMSE, GDS, NOSGER, E-ADL, | Not specified |
| [42] | Diagnostics not performed in the study, but the admission criteria for the living unit were used as inclusion criteria (dementia diagnosis, minimum of care level 2, behavioral problems according to the CMAI, and mobility) | ▪ No information given on the diagnostic procedure of the existing diagnosis | DSS | Professional nursing staff, training of raters is not specified |
| [43] | ||||
| [44] | Dementia diagnosis was derived from the interRAI LTCF assessment in the records | ▪ No information given on the diagnostic procedure of the existing diagnosis | InterRAI (LTCF) | Not specified |
| [45] | Residents with a medical diagnosis of dementia were included. | ▪ No information given on the diagnostic procedure of the existing diagnosis | GDS | Measures were assessed by nurses. |
| [46] | Residents with a medical diagnosis of dementia were included. |
▪ Assessment of the dementia type according to the ICD-10 classification (as recorded) ▪ Assessment of dementia severity according to the German guideline for dementia and recommended MMSE cutoff values (0-9 severe, 10-19 moderate, 20-26 mild) |
MMSE | Not specified. |
DSS Dementia Screening Scale, E-ADL Erlangen Activities of Daily Living, GDS Global Deterioration Scale, interRAI LTCF international Resident Assessment Instrument Long Term Care Facility, MMSE Mini Mental State Examination, NOSGER Nurses’ Observation Scale
Table 4.
Overview of studies that defined study participants with dementia based on a recorded diagnosis and additional cognitive screenings
| Publications | Method of sample determination | Definition and criteria used for (existing) dementia diagnostics | Screenings performed to define and describe dementia | Qualification and training of professionals involved |
|---|---|---|---|---|
| [48] | Residents with dementia were identified using a mixed stepwise approach: 1st step: Multiple combined inclusion criteria: ▪ Presence of dementia diagnosis in the nursing and medical records and ▪ MMSE ≤ 24 2nd step: In case of incongruity of diagnosis and MMSE result, dementia diagnostics were performed, diagnostics were also performed for residents with a suspected dementia but no diagnosis ▪ Exclusion criteria: Presence of other neurological/psychiatric diseases that could explain patients’ decline in cognitive function (schizophrenia, bipolar disorders, mental retardation) |
▪ Existing dementia diagnosis performed in 70 % by GPs and 30 % by medical specialists and according to ICD-10 criteria ▪ An ICD-10/DSM-IV conform study diagnosis was assessed based on clinical investigation and MMSE ▪ Dementia severity stages: MMSE: 0-9 severe/very severe; 10-18 moderate; 19-24 = mild dementia |
MMSE, FAST, AES, NPI | Diagnosis: Physician from the research team who was experienced in geriatric psychiatry Screening instruments: Specifically trained raters, including medical students with an advanced academic degree and physicians experienced in geriatric psychiatry |
| [49] | ||||
| [50]a | ||||
| [51] | ||||
| [81]b | ||||
| [76] | Residents with dementia were identified using two combined inclusion criteria: ▪ Confirmed presence of primary degenerative dementia and ▪ MMSE < 24 ▪ Exclusion criteria: presence of other neurological/psychiatric diseases that could explain patients’ decline in cognitive function (such as addiction, major depression, or schizophrenia), high nursing care needs, blindness, deafness |
▪ Dementia diagnosis confirmed according to ICD-10 (F00, F03, or G30), exclusion of vascular (F01) and secondary (F02) dementia ▪ Dementia severity stages: MMSE: 0-9 severe; 10-17 moderate; 18-23 mild dementia |
MMSE, ADAS (cognitive subscale), NOSGER (subscale mood), E-ADL | Diagnosis: confirmed by the attending physician Screening instruments: Psychology students in their final year who had received training |
| [77] | ||||
| [78] | ||||
| [79] | ||||
| [80] | Residents with dementia were identified using two combined inclusion criteria: ▪ Presence of a dementia diagnosis; and ▪ MMSE < 24, GDS stadium 4, 5 or 6 ▪ Exclusion criteria: presence of other neurological/psychiatric diseases that could explain patients’ decline in cognitive function (such as addiction, major depression, or schizophrenia) |
Dementia diagnosis according to ICD-10 in the doctoral records | MMSE, GDS, ADAS (cognitive subscale), NOSGER (subscale mood), E-ADL | Not specified |
| [82] | Residents with dementia were identified using 2 combined inclusion criteria: ▪ Existing diagnosis of dementia and ▪ MMSE ≤ 24 ▪ Exclusion criteria: Korsakoff’s syndrome or CI caused by diseases other than dementia |
No specified information given on the diagnostic procedure of the existing diagnosis | MMSE, CDR, AES | Not specified |
| [83] | Residents with dementia were identified using 2 combined inclusion criteria: ▪ Admission criteria to the living unit were used as inclusion criteria (presence of a dementia diagnosis) and ▪ MMSE < 18 |
No specified information given on the diagnostic procedure of the existing diagnosis | MMSE, NOSGER, GDS | Not specified |
| [84] | Residents with dementia were identified using two combined criteria: ▪ Presence of a dementia diagnosis; and ▪ MMSE ≤ 27 and DemTect (scores of 6-8) for residents with a MMSE score between 24-27 |
▪ No specified information given on the diagnostic procedure of the existing diagnosis ▪ Dementia severity stages: MMSE: ≤ 10 severely demented; 11-19 moderately demented, 20-27 mild dementia |
MMSE, DemTect | Not specified |
| [85] | Residents with dementia were identified using 2 combined criteria: ▪ S-MMSE-Score ≤ 24 ▪ Exclusion criteria: primary psychiatric diagnosis or Korsakov’s syndrome |
▪ No specified information given on the diagnostic procedure of the existing diagnosis ▪ Different dementia severity stages were used |
MMSE, NPI, Katz-Index | Formal diagnosis of dementia as determined by a healthcare professional (physician, psychiatrist, neurologist, geriatrician, general practitioner) |
| [86] | ||||
| [87] | ||||
| [88] | ||||
| [89] | ||||
| [90] | ||||
| [47] (Study Protocol) |
Residents with dementia were identified using 2 combined criteria. ▪ Medical diagnosis of dementia ▪ FAST > 1 |
▪ No specified information given on the diagnostic procedure of the existing Diagnosis | FAST, NPI, PSMS | ▪ Data were assessed by trained study assistants who interview two caregivers simultaneously ▪ Study assistants (mainly students) undergo a 2-day training on the use of the questionnaires and receive a manual ▪ The first data collection was assisted by senior and junior researchers |
AD Alzheimer’s disease, ADAS Alzheimer’s disease Assessment Scale, AES Apathy Evaluation Scale, CDR Clinical Dementia Rating, E-ADL Erlangen Test for Activities of Daily Living, FAST Functional Assessment Staging, GDS Global Deterioration Scale, MMSE Mini Mental State Examination, NOSGER Nurses’ Observation Scale, NPI Neuropsychiatric Inventory
aThe reporting of instruments is not consistent across the publications. Therefore, all instruments used were summarized in this group. The studies also differ also regarding the criteria used to diagnose. Majic 2012 referred to DSM- IV, other publications to ICD-10
bReporting of methods and proceedings slightly deviates from that of the other publications originating from this project (validation of recorded dementia diagnosis is not specified; MMSE cutoff 23; no exclusion criteria are reported)
Table 5.
Overview of studies that defined study participants with dementia based on cognitive screenings
| Publications | Method of sample determination | Information about dementia diagnosis | Screenings performed to define and describe dementia | Qualification and training of professionals involved |
|---|---|---|---|---|
| [108] [53]a [111] [107] |
Residents with dementia were identified using one criteria ▪ FAST ≥ 2 ▪ Exclusion criteria: documented diagnosis of schizophrenia or other psychotic disorders |
Existing diagnosis of dementia recorded but not used as an inclusion criteria | MMSEa, FAST, NPI, PSMS | Screening instruments: Caregivers who were familiar with the resident were interviewed from a trained external research assistant (registered nurses and students in health care study programs) |
| [52] | Residents were included using 2 criteria: ▪ Place of residence is a Dementia Special Care Unit ▪ Cognitive impairment according to DSS > 2 |
Existing diagnosis of dementia recorded but not used as an inclusion criteria | DSS, NPI, PSMS | Screening instruments were completed by nurses familiar with the resident; questionnaires were accompanied by a manual to support assessment |
| [109]b
[110] |
Residents with dementia were identified using 2 combined criteria ▪ Dementia according to DSS; and ▪ Mobile according to Rivermead Mobility Index |
Existing diagnosis of dementia recorded but not used as an inclusion criteria; no information is given on existing diagnosis | DSS, NPI, Barthel-Index | Screening instrument: registered nurses who are familiar with the resident (primary nurse) |
| [91] | Residents with dementia were identified based on the MMSE screening < 24 | No information given on existing diagnosis | MMSE, Barthel-Index | Not specified |
| [92] | Residents with dementia were identified based on the MMSE screening < 24 ▪ Dementia severity stages: MMSE ≤ 10 severe, 11-17 moderate, ≥ 18 mild |
No information given on existing diagnosis | MMSE, Barthel-Index | Not specified |
| [93] | Residents with dementia were identified based on the MMSE screening ▪ MMSE cutoff ≤ 20 |
No information given on existing diagnosis | MMSE | MMSE was performed by three psychologists. |
DSS Dementia Screening Scale, FAST Functional Assessment Staging, MMSE Mini Mental State Examination, NOSGER Nurses’ Observation Scale, NPI Neuropsychiatric Inventory, PANDA Parkinson Neuropsychometric Dementia Assessment, PSMS Physical Self Maintenance Scale
aMMSE values are assessed only in a subsample in the study from Dichter et al. (2013) and recalculated into FAST values
bThe publication is a short report/letter; therefore, we additionally reviewed the official study report for more information
Table 6.
Overview of studies that investigated study participants with cognitive impairment
| Publications | Method of sample determination | Screenings performed to define and describe CI | Qualification and training of professionals involved |
|---|---|---|---|
| [37] | Assessment of CI performed for a random sample of NH residents. | MMSE, Barthel-Index | Data were collected through face-to-face interviews by trained research personnel |
| [40] | ▪ MMSE: 0-17 = severe CI; 18-23 = moderate CI; 24-30 = no or mild CI | ||
| [38] | Assessment of CI was performed for NH residents > 65 years without verbal impairments. | MMSE | Trained study assistants (licensed nurses or students in health care programs) who received a comprehensive training in using the MMSE |
| ▪ MMSE: 0-9 severe CI; 10-17 moderate CI; 18-30 = no/mild CI | |||
| [39] | Assessment of CI was performed for all residents of the participating NHs. | MMSE, GDS | Not specified |
|
▪ MMSE: < 15 severe CI, 16-20 relevant CI ▪ GDS: 2-3 mild CI, 4-5 moderate CI, 6-7 severe CI |
CI cognitive impairment, GDS Global Deterioration Scale, MMSE Mini Mental State Examination
Table 7.
Overview of studies that did not clearly define participants with dementia
| Publications | Method of sample determination | Information about dementia diagnosis | Screenings performed to define and describe dementia | Qualification and training of professionals involved |
|---|---|---|---|---|
| [24] | Every resident on the living unit enclosed in the study and described with respect to dementia-related characteristics | Medical diagnosis is derived from the medical records and coded according to ICD-10. | Presence of dementia diagnosis in the nursing records, DSS (rating according to CDR: mild (CDR 1), severe (CDR 2), very severe (CDR 3) | Screening instruments: Nurses who are familiar with the resident performed the ratings; oral and written instructions were provided by the research team |
| [54] | Every resident on the living unit included in the study and described with respect to dementia-related characteristics | Not specified | GDS (> 1 beginning dementia, > 3 moderate dementia, > 5 severe dementia), MMSE, CERAD Verbal Fluency Test, CERAD Boston Naming test | Not specified |
| [55] | ||||
| [56] | Every resident on the living unit is enclosed in the study and described with respect to dementia-related characteristics | No information is given on the diagnostic procedures of the existing diagnosis. | Presence of dementia diagnosis in the nursing records, DSS | Screening instruments: Professional nursing staff that were familiar with the resident |
| [57] (Study protocol) | Every resident on the living unit included in the study and described with respect to dementia-related characteristics | Medical diagnosis is derived from the nursing records. | Presence of dementia diagnosis in the nursing records, DSS, FAST, MMSE as recorded in the nursing records | Screening instruments: Nurses who are familiar with the residents and received training or supervision by a trained study coordinator (NH staff) |
| [58] | Procedure to identify residents with dementia not reported | Not specified | Not specified | Not specified |
| [59] |
CDR Clinical Dementia Rating, CERAD The Consortium to establish a Registry for Alzheimer Disease’s, DSS Dementia Screening Scale, FAST Functional Assessment Staging, GDS Global Deterioration Scale, MMSE Mini Mental State Examination
Reporting about dementia diagnostics within the study
Fifteen articles reporting on studies with a newly assessed diagnosis detailed diagnostic criteria and gave information on the diagnostic process or referred to related articles that described the diagnostic process [22, 23, 48–51, 60–68]. As shown in Table 2, these studies referred to the definition and diagnostic criteria for Alzheimer’s disease and vascular dementia from the ICD, DSM, or NINCDS. Studies that referred to the diagnostic criteria for dementia subtypes reported a more comprehensive diagnostic procedure [22, 60–68] than those reported in studies that did not refer to dementia subtypes. These diagnostic procedures were composed of clinical examinations, interviews, neuropsychological testing and a review of existing diagnostic findings from technical investigations. The articles examined did not specify the number or types of findings available from technical investigations or how missing findings were accommodated.
Three studies did not refer to any definition or diagnostic criteria of dementia, but solely reported the instrument on which the diagnosis was based [69–71]. Two studies conducted prior to 2000 [72, 73], assessed a diagnosis on the basis of the Feighner criteria [74]. Neither of those studies provided any detailed information about the diagnostic process or about which instruments were used to assess the criteria. One pre-1990 study did not report the definition, the diagnostic criteria, or any instrument that was used [75].
The majority of studies in which the diagnosis was newly assessed within the study indicated that a battery of neuropsychological tests was used [22, 23, 48–51, 60–70, 72]. Two of these studies indicated that the final diagnosis was based on the Clinical Dementia Rating (CDR) [69, 70]; in another study, this instrument was used for staging [68].
The compilation of psychological tests differed in the studies with regard to the assessment of cognition, behavior and function. For assessing cognition, the MMSE was the only instrument that was used in all of these studies. Studies using a battery of neuropsychological tests included different tests for cognitive function: the MMSE was combined with the Global Deterioration Scale (GDS), the CDR, the Clock Drawing Test, the Brief Assessment Schedule (BAS-Dem) and the Parkinson Neuropsychometric Dementia Assessment (PANDA). With exception of the PANDA (used for a subsample of residents with Parkinson’s disease) [67] and the CDR (used in cases of severe physical impairment) [66], the studies did not specify whether all of the instruments were administered to each resident or whether any particular instrument was used for residents with certain characteristics. For assessing behavior and function, the researchers also used different instruments, which can be viewed in Tables 2, 3, 4, 5 and 6.
The scope and the rationale of each instrument’s use in the diagnostic process were not clearly documented in most studies. In 10 studies, it was not clear which instruments were essential to the decision about the diagnosis because no cutoff values were reported [22, 23, 60–67]. Moreover, it was not clearly stated which instruments were used for diagnostics and which were used for study purposes only (including the description of the study sample), as an outcome measure, or for validity testing. Five studies reported cutoff values [69–73]; 2 of these studies used the CDR with a cutoff ≥ 1 to assess the dementia diagnosis [69, 70]; 3 studies reported cutoff values for the MMSE and the BAI to stage dementia severity [71–73].
The assessment process for the diagnosis was described briefly in 5 articles: 3 articles reported that the administration of instruments was carried out with the help of interviewers [22, 66, 67], whereas 3 studies reported that the diagnosis was agreed to in a multidisciplinary conference [66, 68, 70]. In all of the studies that assessed a new diagnosis of dementia medical staff with geriatric experience, primarily physicians, performed the diagnostics.
Reporting about dementia diagnostics prior to the study
Altogether, in 26 articles, the recorded diagnosis was used as an inclusion criterion with or without additional screenings (Tables 3 and 4). Twelve articles reported that existing diagnoses used to determine the sample were performed or confirmed according to ICD [41, 46, 48–51, 76–81]. None of these articles gave more explicit information about the pre-study diagnostic procedure or about whether an existing diagnosis was confirmed by the attending physician. In 14 articles, neither the definition of dementia and/or its diagnostic criteria were reported; in addition, no information was provided about the diagnostic procedure that had been undertaken prior to the study [42–45, 47, 82–90].
In 17 articles, it was reported that in addition to the recorded diagnosis or performed screenings, exclusion criteria were also used in the study [48–51, 53, 76–80, 82, 85–90]. These comprised other neurological or psychiatric diseases that could cause CI.
Reporting about screening of cognitive impairment
CI was measured in all studies, but with different instruments and cutoff values.
The use of the MMSE was reported in 47 articles and represents the instrument most often used to identify residents with dementia and the stage of its severity. A broad range of cutoff scores for dementia severity was reported for the MMSE. Seven studies referred to an MMSE of < 25 [37, 40, 48–51], 6 studies used a cutoff of ≤ 24 [85–90], 8 articles referred to a cutoff of < 24 to detect mild dementia [76–80, 82, 91, 92], and 4 articles referred to a more conservative cutoff. [39, 41, 83, 93] One article specified a higher cutoff value of ≤ 27 but conducted confirmatory testing with the Geriatric Depression Scale (GDS) for residents with an MMSE between 24 and 27 [84]. One article use a cutoff value of < 27 without additional testing [46]. The studies also used different cutoff values for staging dementia (Table 8).
Table 8.
MMSE cutoff values for dementia staginga
| Publication | Severe | Moderate | Mild |
|---|---|---|---|
| [38, 41] | 0-9 | 10-17 | 18-30b |
| [76–79] | 0-9 | 10-17 | 18-23 |
| [48–51] | 0-9 | 10-18 | 19-24 |
| [84] | 0-10 | 11-19 | 20-27 |
| [37, 40] | 0-17 | 18-23 | 24-30b |
| [89] | 0-9 | 10-21 | > 21c |
| [46] | 0-9 | 10-19 | 20-26 |
| [92] | 0-10 | 11-17 | > 17 |
| [90] | 0-9 | 10-20 | > 20c |
aThis table only lists publications on studies that used the MMSE for a staging of dementia/CI
bRange of values also cover people with no CI/dementia
cParticipants with a MMSE > 24 were excluded
Thirteen articles did not report a cutoff value for the MMSE but instead reported mean scores for the study sample [22, 23, 60–70].
For the CDR, a consistent cutoff value was reported (≥ 1) in 3 studies [68–70]; 3 articles did not report any cutoff values for the CDR [22, 66, 82]. For the GDS, consistent cutoff values were most often reported. Schumacher et al. and Bär et al. [39, 54, 55] specified mild CI as corresponding to a GDS of 2-3, moderate CI as corresponding to a GDS of 4-5, and severe CI as corresponding to a GDS of 6-7. Pickel et al. [80]. set a cutoff value of > 3 for mild, moderate or severe dementia.
In addition to differences concerning the use of instruments, we also identified differences regarding the professionals who administered the instruments to assess CI. Studies using the assessments to state a dementia diagnosis primarily used physicians. Studies assessing CI without making a statement on the dementia diagnosis used physicians, psychologists, nurses, medical students and students from other health care programs (see Tables 2, 3, 4, 5 and 6). Some studies stated that the assessors were experienced in the field and when nurses were assessors, it was stated that they were familiar with the residents. Training for raters who performed the cognitive assessments was conducted in numerous studies (see Tables 2, 3, 4, 5 and 6); however, the duration of training, training concepts and training evaluation were not specified.
Discussion
This review reveals a sampling challenge for dementia studies in German nursing homes in health services research: uncertainty related to existing dementia diagnoses, constrained resources and ethical dilemmas concerning accurate diagnostic procedures and the achievement of study validity. When designing a sampling plan, the advantages of convenient and efficient access and ethical considerations related to the residents must be weighed against participant-related validity concerns.
In our review, we found 4 sampling methods to identify residents with dementia:
A study diagnosis;
A recorded medical diagnosis;
A recorded medical diagnosis and additional cognitive screening; or
Cognitive or accordant screening.
Each method has to be discussed with regard to its practicability and validity.
A guideline- and criteria-based diagnosis of dementia is the most valid criterion to select study participants when the aim of the study is to target people with dementia. The clear benefits of this method are a thorough assessment of dementia-related conditions and a sound differential diagnosis to ensure that the CI is not caused by conditions other than dementia. Challenges related to this approach are the resources required in terms of time, costs and availability of physicians.
The performance of diagnostics by a member of the research team enhances study validity by ensuring conformity to the criteria. As demonstrated in several studies [22, 23, 48–51, 60–68], criteria conformity can be achieved in the evaluation of a dementia syndrome. In these studies, both subjective and objective information on the patient’s cognitive decline were thoroughly assessed. However, the validity of the dementia subtype diagnosis, as performed in a few of these studies [22, 23, 60–65], was questionable. In particular, during the late stage of dementia, a differentiation between dementia subtypes is difficult and requires the use of disease progression measures such as repetitive cognitive tests. It can be assumed that this information was not readily available for a large number of residents because the implementation of diagnostic measures, other than the collection of subjective information, is infrequent in the German primary care sector. A survey of 23 German primary care physicians regarding the use of diagnostic measures showed that only 3 physicians used neuropsychological tests as a component of their diagnosis [34]. There is also evidence showing that in primary care, imaging techniques and other diagnostic features are seldom used; misclassifications that lead to an overestimation of vascular dementia were frequently discovered in several studies [34, 94, 95].
With respect to the reporting of the diagnostic performance, the studies lacked detailed information. Several studies did not report which instrument was used to assess which criterion, whether normative data were used to determine CI, which cutoffs were used and how missing data were addressed. It is recommended that this information be provided when a dementia diagnosis in the oldest-old population is evaluated [20]. Additionally, information on training for the raters who conducted the cognitive assessments was missing in all of the studies. In our opinion, this is a crucial issue because 1 study showed that even physicians who underwent training in cognitive assessments may still fail to use these diagnostic instruments correctly [34].
However, evaluating a study diagnosis also lacks feasibility when resources are constrained. Imaging diagnostics are critical because nursing home residents are often too frail to transport, and nursing homes are often not affiliated with a clinic. Consequently, appropriate technical devices may not be available. As we can see in the studies that evaluated dementia subtypes, only existing findings were used to conduct a subtype diagnosis. Thus, the diagnosis must be made based on neuropsychological and functional tests, clinical examinations and interviews that are conducted by either geriatric psychiatrists or clinical psychologists; this process requires time, experienced assessors and willing participants. The study with the largest sample size involved 589 residents from twenty nursing homes in a German city [69]. Thus, performing dementia diagnostics within a study seems feasible for a sample size up to 600 people who live in a single city. However, we assume that the evaluation of a dementia diagnosis may have a negative impact on the willingness to participate in a study if the assessment is overly burdening or involves invasive procedures [96]. For example, in 1 study, 20 of 113 eligible residents were unwilling to submit to an MMSE [93]. For the majority of the nursing home population, legal guardians decide whether the resident will participate. In our experience from studies in the field, legal guardians are hesitant to permit participation because studies involve measures that will burden the resident.
The use of a recorded medical diagnosis as an inclusion criteria may be appealing, especially in studies with large samples and with a geographically widely dispersed population. However, the well-documented inaccuracy of recorded dementia diagnoses indicates that in a research situation, this approach must be questioned [6, 30]. If researchers cannot be sure which criteria have been used and how they were measured, we must assume that the diagnosis may not correspond to the established ICD- or DSM-criteria. Consequently, misclassification threatens the internal validity of study results. In experimental trials, diagnostic discrepancies may lead to heterogeneity in the intervention and control group that may have an impact on treatment effects. The inclusion of false-positive participants may explain the failure to show treatment effects, and the exclusion of false-negative participants both prolongs the recruitment process and may cause a selection bias.
The third sampling method that combines a recorded diagnosis with a cognitive screening is more feasible than evaluating a diagnosis following defined criteria and recommended procedures. Cognitive tests are comparatively easy and quick to administer and therefore, their administration can be delegated to nursing-home staff members who are more easily accessible than geriatricians or psychologists, such as nurses or medical students. This method may ensure a more homogenous sample with respect to cognition, but it misses other information about functioning and decline in functionality and cognition. Without this information, it is impossible to verify the diagnosis according to established diagnostic criteria [30, 97]. The risk of including false-positives can be reduced, but not eliminated. In this respect, exclusion criteria are relevant. Several studies reported that residents were excluded when diseases other than dementia explained their cognitive decline. However, the combination of a recorded diagnosis, a cognitive screening and exclusion criteria still does not reduce potential selection bias resulting from systematically missing false negatives. Furthermore, it is necessary to decide what to do when combined inclusion criteria contradict each other. In 1 study, these concerns were addressed. Majic and colleagues report that participants were included with a recorded diagnosis of dementia, an MMSE ≤ 24 and a duration of CI of > 6 months, but diagnostics were performed in case of incongruity [48]. They report elsewhere that additional diagnostics were performed in residents if the nursing home staff suspected dementia, but there was no recorded diagnosis [49]. Other studies did not sufficiently report how they addressed incongruity in the combined inclusion criteria and whether they performed any measures to rule out the outlined problems.
The fourth method that was identified in this review is the use of a single test result. The instruments FAST, DSS and the MMSE were used for this purpose.
The FAST staging procedure is used as a diagnostic measure for dementia and relies on the assessment of functional impairments that are attributable to dementia [98]. This instrument was tested in its original language on a sample that was composed of 16 participants who had different levels of cognitive impairment and numerous possible underlying diseases. The testing was conducted by physicians who were in their postdoctoral training phase; their results revealed excellent values for inter- and intra-rater reliability and concurrent validity [99]. Unfortunately, data on internal consistency and discriminant validity are absent [100] as well as the results from the German version of the FAST. To our knowledge, this instrument has not been evaluated for its sensitivity or specificity when being used to detect dementia. In the reviewed studies, it was administered by health personnel, not physicians. Additionally, there are no studies that compare the accuracy of this instrument depending on if the instrument is administered by physicians versus other health personnel.
The DSS is a 7-item scale that assesses both memory and orientation at the time of assessment [69]. The instrument was originally developed in the German language, tested in 589 nursing home residents, and administered by nurses [69]. It was validated against the MMSE, BAS-DEM and the CDR. Utilizing the CDR as the gold standard, the DSS was able to correctly classify more than 80 % of residents; however, it showed small inaccuracies when compared to the MMSE and BAS-DEM. Because the scale is fast and easy to administer, this approach can be considered to be feasible in health services research, even though its validity must be critically discussed. The scientific literature concordantly states that the assessment of single domains of cognition, especially a single cognitive test, cannot accurately diagnose dementia and should not be used to substitute systematic evaluations, examinations and laboratory tests [101]. Because the DSS does not assess changes in cognitive functions, it is not possible to determine if the present cognitive deficits had declined over the past 6 months. Hence, the DSS will misclassify residents who have acute cognitive deficits that are due to infection or dehydration. The same misclassification can occur in residents who have chronic cognitive impairments due to other diseases.
In our review, the MMSE was the instrument that was most often chosen to define the inclusion criteria. The rationale for using the MMSE in the studies was primarily its degree of popularity and widespread utilization. A meta-analysis on the accuracy of the MMSE confirms this choice: in high prevalence settings, the MMSE shows a sensitivity and specificity of 77 and 90 %, respectively [15]. The notable shortcomings of the MMSE in the context of nursing home research were rarely discussed in the studies, although those shortcomings threatened the internal validity of the study results. The MMSE shows a floor effect in very severe score ranges, people with little formal education, and people with severe language problems. [101] Several items are strongly influenced by age and education, which implies a need to adjust MMSE scores when establishing thresholds. We found a variety of MMSE thresholds for staging dementia in the reviewed studies - a result that was also reported in other reviews [12, 20]. This result may be a consequence of missing normative data for the age group of the oldest-old and standardized age-adjusted cutoff values. The authors of a meta-analysis on dementia screening and case-finding tool validation studies suggest alternatives to the MMSE such as the Mini-Cog [102]. However, the suitability of these screening tools for nursing home research needs to be investigated.
In this respect, an associated question must be discussed in health services research in nursing homes: is a valid diagnosis of dementia necessary to define the study population? If the aim of the study is to prove the benefit of an intervention that should be provided exclusively to residents with dementia, a valid diagnosis is essential to prove the benefit for this population. If the aim of the study is to prove a benefit for residents with cognitive and functional impairments independent of their etiology, a dementia diagnosis is dispensable. The same question must be considered by clinicians because in the absence of disease-modifying treatments, the primary advantage of a diagnosis for the oldest-old population has to be determined to justify resource-intensive diagnostics [20]. The need to perform diagnostics in the nursing home population must also be ethically justified because psychological tests can be significantly burdensome to people with CIs.
We also found 4 articles that aimed to investigate residents who had CI but did not explicitly have dementia. To us, it was unclear why some studies focused on the population of people with CI but not on people with dementia because the authors did not elaborate on the question whether the etiology of CI played a role with respect to their research focus. In 2 publications, the terms “dementia” and “CI” were used interchangeably [39, 40]. If the authors did not explain why they defined their population based on a disease or a symptom and later failed to distinguish between the 2 conditions, one may ask whether the question was sufficiently addressed.
In addition to the variability of case ascertainment strategies in dementia studies, this review mirrors the developments in knowledge, concepts, differentiations and diagnostic criteria related to “dementia” that have occurred during the last 30 years. With each decade of dementia studies in German nursing home research, the diagnostic procedures were refined and reflect methodological innovations. Therefore, the definition and assessment of dementia and CI must be considered against the respective state of knowledge at the time a particular study was conducted. For example, in 1 study from 1993 [72], the Feighner criteria were applied; these criteria had been introduced in the DSM-III and became available in the German language with the German translation of the DSM-III in 1984. On the contrary, another study [75] was published the same year; it is obvious that those researchers could not apply today’s diagnostic criteria because they were not available at the time the study was designed and conducted.
An overview of the advantages and disadvantages of the different case ascertainment strategies is summarized in Table 9.
Table 9.
Case ascertainment strategies in comparison (summary)
| Case ascertainment strategy | Advantages | Disadvantages |
|---|---|---|
| Study diagnosis | ▪ The most valid inclusion criterion if recommended diagnostic procedures are followed |
▪ Requires intense resources ▪ Burdens the resident ▪ Is ethically questionable in the nursing home population ▪ Decreases willingness to participate |
| Recorded diagnosis |
▪ Requires little resources ▪ Easy and quick to assess ▪ No burden for the resident ▪ Increases willingness to participate |
▪ Validity of the diagnosis cannot be assured ▪ Residents without a recorded diagnosis are systematically excluded ▪ Potential inclusion of false-positives ▪ Differential diagnosis is often missing |
| Recorded diagnosis and screening result |
▪ Requires little resources ▪ Easy and quick to assess ▪ No burden for the resident if proxy-ratings are used ▪ Increases willingness to participate if the resident is not burdened with assessment procedures |
▪ Validity of the diagnosis cannot thoroughly be assured, but with the help of screening results false-positives can be detected and verified ▪ Residents without a recorded diagnosis are systematically excluded, unless residents with a probable diagnosis are also screened and a new diagnosis is evaluated ▪ Validity of the recorded diagnosis cannot be assured ▪ Differential diagnosis is often missing |
| Screening result |
▪ Requires little resources ▪ Easy and quick to assess ▪ No burden for the resident if proxy-ratings are used ▪ Increases willingness to participate if the resident is not burdened with assessment procedures |
▪ The declaration of the existence of a dementia is not entirely possible ▪ Enables the selection of residents that are homogenous with regard to the screened condition but cannot prevent heterogeneity of other conditions |
Strengths and limitations
The problem of inconsistent management of methodological challenges in dementia research has already been recognized and has led to an initiative on how to improve the reporting of these challenges in clinical studies [103]. However, studies of the outlined problem of inconsistent case ascertainment strategies in nursing home research have been lacking to date. We consider this as a prerequisite for improving dementia research in the nursing home sector and outline this as the major strength of this study.
The generalizability of the displayed results is constrained to one country (Germany). However, we assume that the outlined problem can also be demonstrated in other countries, but this must be proven in a later review. Perhaps in countries in which physicians are employed in nursing homes and adherence to diagnostic standards can be guaranteed, the reliance on a recorded diagnosis can be received less critically.
As discussed, the reporting of diagnostic procedures was partially insufficient; in particular, different publications from a single study provided varied information about their case ascertainment. In some studies, it was not clear whether the assessments were used exclusively for study purposes, for diagnosis, or both. In particular, the scope of assessments with respect to behavior and function was not described clearly, making it unclear whether these instruments were also used for diagnostic procedures. In an effort to rule out this lack of clarity, we unsuccessfully attempted to contact the authors. The short reporting made it also impossible to assess the quality of diagnostic procedures or the associated risk of bias.
Because of the descriptive objective of the review, it has not been registered in a review register.
Conclusions
Considering the findings of our review, we suggest the following principles to improve the validity and comparability of study results on dementia in nursing homes.
Investigators must clearly distinguish whether their research addresses residents with CIs or residents with dementia with an appropriate etiology. If residents with dementia are addressed, it should be in accordance with the study aim. Studies that address the population of nursing home residents with dementia should report in detail which method of case ascertainment was used and should discuss the limitations associated with each method. In our opinion, a criterion-based diagnosis is essential when a study addresses explicitly residents with dementia. If residents are included in studies based on diagnoses that do not conform to established criteria, the results from different studies are not comparable. To ensure that a diagnosis conforms to criteria, clinicians who are members of the research team should evaluate a diagnosis during the research project. When financial or human capacities are constrained, one should consider alternative methods to a face-to-face consultation by a geriatric physician, psychologist or psychiatrist. Several methods utilizing an expert panel are described for the diagnosis of dementia [104]. The method by Magaziner et al. [30] appears promising for research purposes: 2 clinicians decide whether dementia is present based on the resident’s history and neuropsychological tests performed by lay evaluators. Compared to direct clinical evaluation, agreement was satisfactory (76 %). Another promising approach is a nurse-administered diagnosis. Studies showed a high agreement between a nurse-administered diagnosis and a multidisciplinary team diagnosis in determining mild CI in primary care [105]; in addition, there is agreement between a Memory Clinic diagnosis [106] and a moderate agreement with the ICD-10 diagnosis (ibd.). To our knowledge, a nurse-administered diagnostic procedure for dementia in nursing home residents has not yet been developed and tested.
Regardless of which method is used to evaluate a diagnosis of dementia within a study, reporting requires details regarding the domains that are tested, the rationale behind instrument use for each domain examined, the cutoff values set for each domain and the management of missing data. Transparency in reporting missing data is needed because approaches for adequately managing these gaps can differ and produce varying effect estimates [103].
If a dementia diagnosis is considered as dispensable or a criteria-based diagnostic procedure cannot be applied due to constrained resources, the study should not target the dementia population, but instead clearly state which population they are addressing.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All data are contained within the manuscript and its supplementary files.
Acknowledgement
We would like to thank Prof. Stefan Teipel and Dr. Martin Berwig for their constructive feedback on the manuscript.
Funding
The German Center for Neurodegenerative Diseases funded the study.
Abbreviations
- AD
Alzheimer’s disease
- ADL
activites of daily living
- AES
Apathy Evaluation Scale
- AKT
Alters-Konzentrationstest
- B-ADL
Bayer-Activities of Daily Living Scale
- BAGI
Bielefelder Autobiografisches Gedächtnisinventar
- BAI
Brief Assessment Interview
- BAS-Dem
Brief Assessment Schedule
- Behave-AD
Behavioral Pathology in Alzheimer’s Disease
- BPRS
Brief Psychiatric Rating Scale
- CDR
Clinical Dementia Rating
- CERAD
The Consortium to Establish a Registry for Alzheimer’s Disease
- CI
Cognitive Impairment
- DSM
Diagnostic Statistical Manual
- DSS
Dementia Screening Scale
- E-ADL
Erlangen Test for Activities of Daily Living
- FAST
Functional Assessment Staging
- GDS
Global Deterioration Scale
- HDRS 17
Hamilton Depression Scale 17
- IADL
Instrumental Activities of daily living
- ICD
International Classification of Diseases
- interRAI LTCF
International Resident Assessment Instrument Long Term Care Facility
- MCI
Mild Cognitive Impairment
- MMSE
Mini Mental State Examination
- NINCDS
National Institute of Neurological and Communicative Disorders and Stroke
- NOSGER
Nurses’ Observation Scale
- NPI
Neuropsychiatric Inventory
- PANDA
Parkinson Neuropsychometric Dementia Assessment
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSMS
Physical Self Maintenance Scale
- SIDAM
Structured Interview for the Diagnosis of Alzheimer’s Dementia, Multi-infarct dementia and dementias of other etiology
Additional file
Table S1. PRISMA checklist. (DOCX 63 kb)
Table S2. Search strategy in MEDLINE. Table S3. Search strategy in CINAHL. (DOCX 17 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors contributed in the conceptualization of the article and revised the final version for intellectual content. MND and RP conducted the literature search, RP, CGGS and MND screened title/abstracts, all authors contributed to the selection of studies based on full-texts, RP and SJ extracted and summarized the data. RP drafted the manuscript and is responsible for final compilation. MH supervised the whole process. All authors have read and approved the final version of the manuscript.
Contributor Information
Rebecca Palm, Phone: 0049-2302-926-224, Email: rebecca.palm@dzne.de.
Saskia Jünger, Email: juenger.saskia@mh-hannover.de.
Sven Reuther, Email: sven.reuther@dzne.de.
Christian G. G. Schwab, Email: christian.schwab@dzne.de
Martin N. Dichter, Email: martin.dichter@dzne.de
Bernhard Holle, Email: bernhard.holle@dzne.de.
Margareta Halek, Email: margareta.halek@dzne.de.
References
- 1.Bartholomeyczik S, Holle B, Riesner C, Halek M, Vollmar HC. Versorgungsnahe Demenzforschung ermöglichen - Fragestellungen im Deutschen Zentrum fur Neurodegenerative Erkrankungen an der Universität Witten/Herdecke. Z Evid Fortbild Qual Gesundhwes. 2010;104(10):744–753. doi: 10.1016/j.zefq.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 2.Ragin CC, Becker HS. What is a case? Exploring the foundations of social inquiry. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 3.Breitner JC. Dementia-epidemiological considerations, nomenclature, and a tacit consensus definition. J Geriatr Psychiatry Neurol. 2006;19(3):129–136. doi: 10.1177/0891988706291081. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association, editor. DSM-5. Diagnostic and statistical manual of mental disorders. 5. Washington/DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 5.World Health Organization (WHO). International statistical classification of diseases and related health problems, vol. 10th revision Geneva: WHO; 2011. http://apps.who.int/classifications/icd10/browse/2016/en.
- 6.Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 7.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/WNL.43.2.250. [DOI] [PubMed] [Google Scholar]
- 8.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/WNL.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 9.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 10.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 Suppl):417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association: DSM: History of the Manual [http://www.psychiatry.org/practice/dsm/dsm-history-of-the-manual], Accessed 09.03.2015.
- 12.Holsinger T, Deveau J, Boustani M, Williams JW., Jr Does this patient have dementia? JAMA. 2007;297(21):2391–2404. doi: 10.1001/jama.297.21.2391. [DOI] [PubMed] [Google Scholar]
- 13.American Psychological Association Guidelines for the evaluation of dementia and age-related cognitive change. Am Psychol. 2012;67(1):1–9. doi: 10.1037/a0024643. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411–431. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Kalbe E, Kessler J, Calabrese P, Smith R, Passmore AP, Brand M, Bullock R. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry. 2004;19(2):136–143. doi: 10.1002/gps.1042. [DOI] [PubMed] [Google Scholar]
- 17.Cullen B, O’Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78(8):790–799. doi: 10.1136/jnnp.2006.095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deutsche Gesellschaft für Psychiatrie Psychotherapie und Nervenheilkunde (DGPPN) Deutsche Gesellschaft für Neurologie (DGN) Diagnose- und Behandlungsleitlinie Demenz. Heidelberg: Springer Verlag; 2010. [Google Scholar]
- 19.Dalal PK, Sivakumar T. Moving towards ICD-11 and DSM-V: Concept and evolution of psychiatric classification. Indian J Psychiatry. 2009;51(4):310–319. doi: 10.4103/0019-5545.58302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slavin MJ, Brodaty H, Sachdev PS. Challenges of diagnosing dementia in the oldest old population. J Gerontol A Biol Sci Med Sci. 2013;68(9):1103–1111. doi: 10.1093/gerona/glt051. [DOI] [PubMed] [Google Scholar]
- 21.Mayeux R, Reitz C, Brickman AM, Haan MN, Manly JJ, Glymour MM, Weiss CC, Yaffe K, Middleton L, Hendrie HC, et al. Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment-Part 1. Alzheimers Dement. 2011;7(1):15–34. doi: 10.1016/j.jalz.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brune-Cohrs U, Juckel G, Schröder SG. Qualität der Demenzdiagnostik im Seniorenheim. Z Arztl Fortbild Qualitatssich. 2007;101(9):611–615. doi: 10.1016/j.zgesun.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Seidl U, Lueken U, Volker L, Re S, Becker S, Kruse A, Schröder J. Nicht-kognitive Symptome und psychopharmakologische Behandlung bei demenzkranken Heimbewohnern. Fortschr Neurol Psychiatr. 2007;75(12):720–727. doi: 10.1055/s-2007-959211. [DOI] [PubMed] [Google Scholar]
- 24.Schäufele M, Köhler L, Hendlmeier I, Hoell A, Weyerer S. Prävalenz von Demenzen und ärztliche Versorgung in deutschen Pflegeheimen: eine bundesweite repräsentative Studie. Psychiatr Prax. 2013;40(04):200–206. doi: 10.1055/s-0033-1343141. [DOI] [PubMed] [Google Scholar]
- 25.Mansdorf IJ, Harrington M, Lund J, Wohl N. Neuropsychological testing in skilled nursing facilities: the failure to confirm diagnoses of dementia. J Am Med Dir Assoc. 2008;9(4):271–274. doi: 10.1016/j.jamda.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Nygaard HA, Ruths S. Missing the diagnosis: senile dementia in patients admitted to nursing homes. Scand J Prim Health Care. 2003;21(3):148–152. doi: 10.1080/02813430310001798. [DOI] [PubMed] [Google Scholar]
- 27.Feldman H, Clarfield AM, Brodsky J, King Y, Dwolatzky T. An estimate of the prevalence of dementia among residents of long-term care geriatric institutions in the Jerusalem area. Int Psychogeriatr. 2006;18(4):643–652. doi: 10.1017/S1041610205003091. [DOI] [PubMed] [Google Scholar]
- 28.Magsi H, Malloy T. Underrecognition of cognitive impairment in assisted living facilities. J Am Geriatr Soc. 2005;53(2):295–298. doi: 10.1111/j.1532-5415.2005.53117.x. [DOI] [PubMed] [Google Scholar]
- 29.Lithgow S, Jackson GA, Browne D. Estimating the prevalence of dementia: cognitive screening in Glasgow nursing homes. Int J Geriatr Psychiatry. 2012;27(8):785–791. doi: 10.1002/gps.2784. [DOI] [PubMed] [Google Scholar]
- 30.Magaziner J, Zimmerman SI, German PS, Kuhn K, May C, Hooper F, Cox D, Hebel JR, Kittner S, Burton L, et al. Ascertaining dementia by expert panel in epidemiologic studies of nursing home residents. Ann Epidemiol. 1996;6(5):431–437. doi: 10.1016/S1047-2797(96)00065-8. [DOI] [PubMed] [Google Scholar]
- 31.Hellstrom I, Nolan M, Nordenfelt L, Lundh U. Ethical and methodological issues in interviewing persons with dementia. Nurs Ethics. 2007;14(5):608–619. doi: 10.1177/0969733007080206. [DOI] [PubMed] [Google Scholar]
- 32.Boustani M, Watson L, Fultz B, Perkins AJ, Druckenbrod R. Acceptance of dementia screening in continuous care retirement communities: a mailed survey. Int J Geriatr Psychiatry. 2003;18(9):780–786. doi: 10.1002/gps.918. [DOI] [PubMed] [Google Scholar]
- 33.Jessen F, Wiese B, Bickel H, Eifflander-Gorfer S, Fuchs A, Kaduszkiewicz H, Kohler M, Luck T, Mosch E, Pentzek M, et al. Prediction of dementia in primary care patients. PLoS One. 2011;6(2):e16852. doi: 10.1371/journal.pone.0016852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pentzek M, Fuchs A, Wiese B, Abholz HH. Welche Informationen nutzen Hausarzte zur Einschatzung des kognitiven Status alterer nicht dementer Patienten? Psychiatr Prax. 2010;37(8):377–383. doi: 10.1055/s-0030-1248487. [DOI] [PubMed] [Google Scholar]
- 35.van den Bussche H, Wiese B, Koller D, Eisele M, Kaduszkiewicz H, Maier W, Glaeske G, Steinmann S, Wegscheider K, Schon G. Specialist involvement and referral patterns in ambulatory medical care for patients with dementia in Germany: results of a claims data based case-control study. BMC Health Serv Res. 2011;11:148. doi: 10.1186/1472-6963-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, Hutton JL. Issues in methodological research: perspectives from researchers and commissioners. Health Technol Assess. 2001;5(8):1–57. doi: 10.3310/hta5080. [DOI] [PubMed] [Google Scholar]
- 37.Kolzsch M, Wulff I, Ellert S, Fischer T, Kopke K, Kalinowski S, Drager D, Kreutz R. Deficits in pain treatment in nursing homes in Germany: a cross-sectional study. Eur J Pain. 2012;16(3):439–446. doi: 10.1002/j.1532-2149.2011.00029.x. [DOI] [PubMed] [Google Scholar]
- 38.Osterbrink J, Hufnagel M, Mitterlehner B, Krüger C, Bauer Z, Aschauer W, Weichbold M, Sirsch E, Drebenstedt C, Perrar KM, et al. Die Schmerzsituation von Bewohnern in der stationären Altenhilfe. Ergebnisse einer Studie in Münster. Schmerz. 2012;1:9. doi: 10.1007/s00482-011-1127-z. [DOI] [PubMed] [Google Scholar]
- 39.Schumacher J, Zedlick D, Frenzel G. Depressivität und kognitive Beeinträchtigungen bei Altenpflegeheim-Bewohnern. Z Gerontol Geriatr. 1997;30(1):46–53. [PubMed] [Google Scholar]
- 40.Wulff I, Kölzsch M, Kalinowski S, Kopke K, Fischer T, Kreutz R, Dräger D. Perceived enactment of autonomy of nursing home residents: A German cross-sectional study. Nurs Health Sci. 2012;15:186–193. doi: 10.1111/nhs.12016. [DOI] [PubMed] [Google Scholar]
- 41.Graessel E, Viegas R, Stemmer R, Kuchly B, Kornhuber J, Donath C. The Erlangen Test of Activities of Daily Living: first results on reliability and validity of a short performance test to measure fundamental activities of daily living in dementia patients. Int Psychogeriatr. 2009;21(1):103–112. doi: 10.1017/S1041610208007710. [DOI] [PubMed] [Google Scholar]
- 42.Weyerer S, Schäufele M, Hendlmeier I. Besondere und traditionelle stationäre Betreuung demenzkranker Menschen im Vergleich. Z Gerontol Geriatr. 2005;38(2):85–94. doi: 10.1007/s00391-005-0293-y. [DOI] [PubMed] [Google Scholar]
- 43.Weyerer S, Schäufele M, Hendlmeier I. Evaluation of special and traditional dementia care in nursing homes: results from a cross-sectional study in Germany. Int J Geriatr Psychiatry. 2010;25(11):1159–1167. doi: 10.1002/gps.2455. [DOI] [PubMed] [Google Scholar]
- 44.Foebel AD, Liperoti R, Onder G, Finne-Soveri H, Henrard JC, Lukas A, Denkinger MD, Gambassi G, Bernabei R. Use of antipsychotic drugs among residents with dementia in European long-term care facilities: results from the SHELTER study. J Am Med Dir Assoc. 2014;15(12):911–917. doi: 10.1016/j.jamda.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Gräske J, Meyer S, Wolf-Ostermann K. Quality of life ratings in dementia care--a cross-sectional study to identify factors associated with proxy-ratings. Health Qual Life Outcomes. 2014;12:177. doi: 10.1186/s12955-014-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makai P, Beckebans F, van Exel J, Brouwer WB. Quality of life of nursing home residents with dementia: validation of the German version of the ICECAP-O. PLoS One. 2014;9(3):e92016. doi: 10.1371/journal.pone.0092016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reuther S, Holle D, Buscher I, Dortmann O, Müller R, Bartholomeyczik S, Halek M. Effect evaluation of two types of dementia-specific case conferences in German nursing homes (FallDem) using a stepped-wedge design: study protocol for a randomized controlled trial. Trials. 2014;15:319. doi: 10.1186/1745-6215-15-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majic T, Gutzmann H, Heinz A, Lang UE, Rapp MA. Animal-Assisted Therapy and Agitation and Depression in Nursing Home Residents with Dementia: A Matched Case-Control Trial. Am J Geriatr Psychiatry. 2013;21(11):1052–1059. doi: 10.1016/j.jagp.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Majic T, Pluta JP, Mell T, Aichberger MC, Treusch Y, Gutzmann H, Heinz A, Rapp MA. Pharmakotherapie von neuropsychiatrischen Symptomen bei Demenz. Querschnitterhebung in 18 Berliner Seniorenwohnheimen. Dtsch Arztebl Int. 2010;107(18):320–327. doi: 10.3238/arztebl.2010.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majic T, Pluta JP, Mell T, Treusch Y, Gutzmann H, Rapp MA. Correlates of agitation and depression in nursing home residents with dementia. Int Psychogeriatr. 2012;24(11):1779–1789. doi: 10.1017/S104161021200066X. [DOI] [PubMed] [Google Scholar]
- 51.Rapp MA, Mell T, Majic T, Treusch Y, Nordheim J, Niemann-Mirmehdi M, Gutzmann H, Heinz A. Agitation in nursing home residents with dementia (VIDEANT trial): effects of a cluster-randomized, controlled, guideline implementation trial. J Am Med Dir Assoc. 2013;14(9):690–695. doi: 10.1016/j.jamda.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 52.Palm R, Trutschel D, Simon M, Bartholomeyczik S, Holle B. Differences in Case Conferences in Dementia Specific vs Traditional Care Units in German Nursing Homes: Results from a Cross-Sectional Study. J Am Med Dir Assoc 2015. [DOI] [PubMed]
- 53.Dichter MN, Dortmann O, Halek M, Meyer G, Holle D, Nordheim J, Bartholomeyczik S. Scalability and internal consistency of the German version of the dementia-specific quality of life instrument QUALIDEM in nursing homes - a secondary data analysis. Health Qual Life Outcomes. 2013;11:91. doi: 10.1186/1477-7525-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bär M, Böggemann M, Kaspar R, Re S, Berendonk C, Seidl U, Kruse A, Schröder J. Demenzkranke Menschen in individuell bedeutsamen Alltagssituationen. Erste Ergebnisse eines Projekts zur Förderung der Lebensqualität durch Schaffung positiver Anregungsmöglichkeiten. Z Gerontol Geriatr. 2006;39(3):173–182. doi: 10.1007/s00391-006-0384-4. [DOI] [PubMed] [Google Scholar]
- 55.Bär M, Kruse A, Re S. Emotional bedeutsame Situationen im Alltag demenzkranker Heimbewohner. Z Gerontol Geriatr. 2003;36(6):454–462. doi: 10.1007/s00391-003-0191-0. [DOI] [PubMed] [Google Scholar]
- 56.Weyerer S, Schäufele M, Schrag A, Zimber A. Demenzielle Störungen, Verhaltensauffälligkeiten und Versorgung von Klienten in Einrichtungen der Altentagespflege im Vergleich mit Heimbewohnern: Eine Querschnittsstudie in acht badischen Städten. Psychiatr Prax. 2004;31(7):339–345. doi: 10.1055/s-2004-828384. [DOI] [PubMed] [Google Scholar]
- 57.Palm R, Köhler K, Schwab CG, Bartholomeyczik S, Holle B. Longitudinal evaluation of dementia care in German nursing homes: the “DemenzMonitor” study protocol. BMC Geriatr. 2013;13:123. doi: 10.1186/1471-2318-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marquardt G, Schmieg P. Demenzfreundliche Architektur. Möglichkeiten zur Unterstützung der räumlichen Orientierung in stationären Altenpflegeeinrichtungen. Z Gerontol Geriatr. 2009;42(5):402–407. doi: 10.1007/s00391-008-0029-x. [DOI] [PubMed] [Google Scholar]
- 59.Marquardt G, Schmieg P. Dementia-friendly architecture: environments that facilitate wayfinding in nursing homes. Am J Alzheimers Dis Other Demen. 2009;24(4):333–340. doi: 10.1177/1533317509334959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker S, Kaspar R, Kruse A. Die Bedeutung unterschiedlicher Referenzgruppen für die Beurteilung der Lebensqualität demenzkranker Menschen - Kompetenzgruppenbestimmung mit HILDE. Z Gerontol Geriatr. 2006;39(5):350–357. doi: 10.1007/s00391-006-0408-0. [DOI] [PubMed] [Google Scholar]
- 61.Becker S, Kruse A, Schroder J, Seidl U. Das Heidelberger Instrument zur Erfassung von Lebensqualität bei Demenz (H.I.L.DE.). Dimensionen von Lebensqualität und deren Operationalisierung. Z Gerontol Geriatr. 2005;38(2):108–121. doi: 10.1007/s00391-005-0297-7. [DOI] [PubMed] [Google Scholar]
- 62.Lueken U, Seidl U, Volker L, Schweiger E, Kruse A, Schröder J. Development of a short version of the Apathy Evaluation Scale specifically adapted for demented nursing home residents. Am J Geriatr Psychiatry. 2007;15(5):376–385. doi: 10.1097/JGP.0b013e3180437db3. [DOI] [PubMed] [Google Scholar]
- 63.Seidl U, Lueken U, Thomann PA, Geider J, Schröder J. Autobiographical memory deficits in Alzheimer’s disease. J Alzheimers Dis. 2011;27(3):567–574. doi: 10.3233/JAD-2011-110014. [DOI] [PubMed] [Google Scholar]
- 64.Seidl U, Thomann PA, Schröder J. Neurological soft signs in nursing home residents with Alzheimer’s disease. J Alzheimers Dis. 2009;18(3):525–532. doi: 10.3233/JAD-2009-1159. [DOI] [PubMed] [Google Scholar]
- 65.Schuler MS, Becker S, Kaspar R, Nikolaus T, Kruse A, Basler HD. Psychometric properties of the German “Pain Assessment in Advanced Dementia Scale” (PAINAD-G) in nursing home residents. J Am Med Dir Assoc. 2007;8(6):388–395. doi: 10.1016/j.jamda.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Jakob A, Busse A, Riedel-Heller SG, Pavlicek M, Angermeyer MC. Prävalenz und Inzidenz von Demenzerkrankungen in Alten- und Altenpflegeheimen im Vergleich mit Privathaushalten. Z Gerontol Geriatr. 2002;35(5):474–481. doi: 10.1007/s00391-002-0066-9. [DOI] [PubMed] [Google Scholar]
- 67.Riedel O, Schneider C, Klotsche J, Reichmann H, Storch A, Wittchen HU. Die Epidemiologie des idiopathischen Parkinson-Syndroms und assoziierter Demenz und Depression in Dresden. Fortschr Neurol Psychiatr. 2013;81(2):81–87. doi: 10.1055/s-0032-1330278. [DOI] [PubMed] [Google Scholar]
- 68.König HH, Leicht H, Brettschneider C, Bachmann C, Bickel H, Fuchs A, Jessen F, Kohler M, Luppa M, Mosch E, et al. The costs of dementia from the societal perspective: is care provided in the community really cheaper than nursing home care? J Am Med Dir Assoc. 2014;15(2):117–126. doi: 10.1016/j.jamda.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Köhler L, Weyerer S, Schaufele M. Proxy screening tools improve the recognition of dementia in old-age homes: results of a validation study. Age Ageing. 2007;36(5):549–554. doi: 10.1093/ageing/afm108. [DOI] [PubMed] [Google Scholar]
- 70.Kuske B, Luck T, Hanns S, Matschinger H, Angermeyer MC, Behrens J, Riedel-Heller SG. Training in dementia care: a cluster-randomized controlled trial of a training program for nursing home staff in Germany. Int Psychogeriatr. 2009;21(2):295–308. doi: 10.1017/S1041610208008387. [DOI] [PubMed] [Google Scholar]
- 71.Weyerer S, Mann AH, Ames D. Prävalenz von Depression und Demenz bei Altenheimbewohnern in Mannheim und Camden (London) Z Gerontol Geriatr. 1995;28(3):169–178. [PubMed] [Google Scholar]
- 72.Geiger-Kabisch C, Weyerer S. Der Alters-Konzentrations-Test. Ergebnisse einer Studie bei über 65jährigen in Mannheim. Z Gerontol Geriatr. 1993;26(2):81–85. [PubMed] [Google Scholar]
- 73.Weyerer S, Geiger-Kabisch C, Kroper C, Denzinger R, Platz S. Die Erfassung von Demenz und Depression mit Hilfe des Brief-Assessment-Interviews (BAI): Ergebnisse einer Reliabilitäts- und Validitätsstudie bei Altenheimbewohnern in Mannheim. Z Gerontol. 1990;23(4):205–210. [PubMed] [Google Scholar]
- 74.Feighner JP, Robins E, Guze SB, Woodruff RA, Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26(1):57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- 75.Meyer-König E, Riederer M, Schunk W. Zur Psychopathologie der senilen Demenzen bei Pflegeheimbewohnern. Z Gerontol. 1984;17(3):113–116. [PubMed] [Google Scholar]
- 76.Graessel E, Stemmer R, Eichenseer B, Pickel S, Donath C, Kornhuber J, Luttenberger K. Non-pharmacological, multicomponent group therapy in patients with degenerative dementia: a 12-month randomizied, controlled trial. BMC Med. 2011;9:129. doi: 10.1186/1741-7015-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luttenberger K, Donath C, Uter W, Graessel E. Effects of multimodal nondrug therapy on dementia symptoms and need for care in nursing home residents with degenerative dementia: a randomized-controlled study with 6-month follow-up. J Am Geriatr Soc. 2012;60(5):830–840. doi: 10.1111/j.1532-5415.2012.03938.x. [DOI] [PubMed] [Google Scholar]
- 78.Luttenberger K, Hofner B, Graessel E. Are the effects of a non-drug multimodal activation therapy of dementia sustainable? Follow-up study 10 months after completion of a randomised controlled trial. BMC Neurol. 2012;12:151. doi: 10.1186/1471-2377-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luttenberger K, Schmiedeberg A, Grassel E. Activities of daily living in dementia: revalidation of the E-ADL Test and suggestions for further development. BMC Psychiatry. 2012;12:208. doi: 10.1186/1471-244X-12-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pickel S, Grassel E, Luttenberger K. Wirksamkeit eines beschäftigungstherapeutischen Gruppenangebots bei degenerativen Demenzen: Eine kontrollierte Verlaufsstudie im Pflegeheim. Psychiatr Prax. 2011;38(8):389–396. doi: 10.1055/s-0030-1266137. [DOI] [PubMed] [Google Scholar]
- 81.Treusch Y, Majic T, Page J, Gutzmann H, Heinz A, Rapp MA. Apathy in nursing home residents with dementia: results from a cluster-randomized controlled trial. Eur Psychiatry. 2015;30(2):251–257. doi: 10.1016/j.eurpsy.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 82.Berg A, Sadowski K, Beyrodt M, Hanns S, Zimmermann M, Langer G, Becker C, Lautenschlager C, Behrens J. Snoezelen, structured reminiscence therapy and 10-minutes activation in long term care residents with dementia (WISDE): study protocol of a cluster randomized controlled trial. BMC Geriatr. 2010;10:5. doi: 10.1186/1471-2318-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dettbarn-Reggentin J. Studie zum Einfluss von Wohngruppenmilieus auf demenziell Erkrankte in stationären Einrichtungen. Z Gerontol Geriatr. 2005;38(2):95–100. doi: 10.1007/s00391-005-0294-x. [DOI] [PubMed] [Google Scholar]
- 84.Theison AK, Geisthoff UW, Forstl H, Schroder SG. Agitation in the morning: symptom of depression in dementia? Int J Geriatr Psychiatry. 2009;24(4):335–340. doi: 10.1002/gps.2108. [DOI] [PubMed] [Google Scholar]
- 85.Afram B, Stephan A, Verbeek H, Bleijlevens MH, Suhonen R, Sutcliffe C, Raamat K, Cabrera E, Soto ME, Hallberg IR, et al. Reasons for institutionalization of people with dementia: informal caregiver reports from 8 European countries. J Am Med Dir Assoc. 2014;15(2):108–116. doi: 10.1016/j.jamda.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 86.Alvira MC, Risco E, Cabrera E, Farre M, Rahm Hallberg I, Bleijlevens MH, Meyer G, Koskenniemi J, Soto ME, Zabalegui A. The association between positive-negative reactions of informal caregivers of people with dementia and health outcomes in eight European countries: a cross-sectional study. J Adv Nurs. 2015;71(6):1417–1434. doi: 10.1111/jan.12528. [DOI] [PubMed] [Google Scholar]
- 87.Beerens HC, Sutcliffe C, Renom-Guiteras A, Soto ME, Suhonen R, Zabalegui A, Bokberg C, Saks K, Hamers JP. Quality of life and quality of care for people with dementia receiving long term institutional care or professional home care: the European RightTimePlaceCare study. J Am Med Dir Assoc. 2014;15(1):54–61. doi: 10.1016/j.jamda.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 88.Beerens HC, Zwakhalen SM, Verbeek H, Ruwaard D, Ambergen AW, Leino-Kilpi H, Stephan A, Zabalegui A, Soto M, Saks K, et al. Change in quality of life of people with dementia recently admitted to long-term care facilities. J Adv Nurs. 2015;71(6):1435–1447. doi: 10.1111/jan.12570. [DOI] [PubMed] [Google Scholar]
- 89.de Mauleon A, Sourdet S, Renom-Guiteras A, Gillette-Guyonnet S, Leino-Kilpi H, Karlsson S, Bleijlevens M, Zabategui A, Saks K, Vellas B, et al. Associated factors with antipsychotic use in long-term institutional care in eight European countries: Results from the RightTimePlaceCare study. J Am Med Dir Assoc. 2014;15(11):812–818. doi: 10.1016/j.jamda.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 90.Wubker A, Zwakhalen SM, Challis D, Suhonen R, Karlsson S, Zabalegui A, Soto M, Saks K, Sauerland D. Costs of care for people with dementia just before and after nursing home placement: primary data from eight European countries. Eur J Health Econ. 2015;16(7):689–707. doi: 10.1007/s10198-014-0620-6. [DOI] [PubMed] [Google Scholar]
- 91.Gietzelt M, Feldwieser F, Govercin M, Steinhagen-Thiessen E, Marschollek M. A prospective field study for sensor-based identification of fall risk in older people with dementia. Inform Health Soc Care. 2014;39(3-4):249–261. doi: 10.3109/17538157.2014.931851. [DOI] [PubMed] [Google Scholar]
- 92.Nordheim J, Hamm S, Kuhlmey A, Suhr R. Tablet computers and their benefits for nursing home residents with dementia: Results of a qualitative pilot study. Z Gerontol Geriatr. 2015;48(6):543–549. doi: 10.1007/s00391-014-0832-5. [DOI] [PubMed] [Google Scholar]
- 93.Zenthofer A, Schroder J, Cabrera T, Rammelsberg P, Hassel AJ. Comparison of oral health among older people with and without dementia. Community Dent Health. 2014;31(1):27–31. [PubMed] [Google Scholar]
- 94.Donath C, Graessel E, Grossfeld-Schmitz M, Haag C, Kornhuber J, Neubauer S. Diagnostik und Therapie von Demenzerkrankungen in der hausrztlichen Praxis: ein Stadt-Land-Vergleich. Psychiatr Prax. 2008;35(3):142–145. doi: 10.1055/s-2007-986312. [DOI] [PubMed] [Google Scholar]
- 95.Riedel-Heller SG, Schork A, Fromm N, Angermeyer MC. Demenzkranke in der Hausarztpraxis-Ergebnisse einer Befragung. Z Gerontol Geriatr. 2000;33(4):300–306. doi: 10.1007/s003910070050. [DOI] [PubMed] [Google Scholar]
- 96.Kolanowski A, Mulhall P, Yevchak A, Hill N, Fick D. The triple challenge of recruiting older adults with dementia and high medical acuity in skilled nursing facilities. J Nurs Scholarsh. 2013;45(4):397–404. doi: 10.1111/jnu.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Larner AJ. Cognitive Sceening Instruments. Heidelberg, New York, London: Springer; 2013. [Google Scholar]
- 98.Reisberg B. Functional assessment staging (FAST) Psychopharmacol Bull. 1988;24(4):653–659. [PubMed] [Google Scholar]
- 99.Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. International psychogeriatrics/IPA 1992. 1992;4(Suppl 1):55–69. doi: 10.1017/S1041610292001157. [DOI] [PubMed] [Google Scholar]
- 100.Olde Rikkert MGM, Tona KD, Janssen L, Burns A, Lobo A, Robert P, Sartorius N, Stoppe G, Waldemar G. Validity, Reliability, and Feasibility of Clinical Staging Scales in Dementia: A Systematic Review. Am J Alzheimers Dis Other Demen. 2011;26(5):357–365. doi: 10.1177/1533317511418954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davies R, Larner AJ. MMSE Variants and subcores. In: Larner AJ, editor. Cognitive Screening Instruments. Heidelberg: Springer; 2013. pp. 47–60. [Google Scholar]
- 102.Mitchell AJ, Malladi S. Screening and case finding tools for the detection of dementia. Part I: evidence-based meta-analysis of multidomain tests. Am J Geriatr Psychiatry. 2010;18(9):759–782. doi: 10.1097/JGP.0b013e3181cdecb8. [DOI] [PubMed] [Google Scholar]
- 103.Weuve J, Proust-Lima C, Power MC, Gross AL, Hofer SM, Thiebaut R, Chene G, Glymour MM, Dufouil C, Initiative M. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement. 2015;11(9):1098–1109. doi: 10.1016/j.jalz.2015.06.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bertens LC, Broekhuizen BD, Naaktgeboren CA, Rutten FH, Hoes AW, van Mourik Y, Moons KG, Reitsma JB. Use of expert panels to define the reference standard in diagnostic research: a systematic review of published methods and reporting. PLoS Med. 2013;10(10):e1001531. doi: 10.1371/journal.pmed.1001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Page S, Hope K, Bee P, Burns A. Nurses making a diagnosis of dementia--a potential change in practice? Int J Geriatr Psychiatry. 2008;23(1):27–33. doi: 10.1002/gps.1831. [DOI] [PubMed] [Google Scholar]
- 106.Dennis M, Furness L, Lindesay J, Wright N. Assessment of patients with memory problems using a nurse-administered instrument to detect early dementia and dementia subtypes. Int J Geriatr Psychiatry. 1998;13(6):405–409. doi: 10.1002/(SICI)1099-1166(199806)13:6<405::AID-GPS785>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 107.Dichter MN, Quasdorf T, Schwab CG, Trutschel D, Haastert B, Riesner C, Bartholomeyczik S, Halek M. Dementia care mapping: effects on residents’ quality of life and challenging behavior in German nursing homes. A quasi-experimental trial. Int Psychogeriatr. 2015;27(11):1875–1892. doi: 10.1017/S1041610215000927. [DOI] [PubMed] [Google Scholar]
- 108.Halek M, Dichter MN, Quasdorf T, Riesner C, Bartholomeyczik S. The effects of dementia care mapping on nursing home residents’ quality of life and staff attitudes: design of the quasi-experimental study Leben-QD II. BMC Geriatr. 2013;13:53. doi: 10.1186/1471-2318-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schäufele M, Hoell A, Hendlmeier I, Kohler L, Weyerer S. Primärpravention von Mobilitätseinschränkungen bei Menschen mit Demenz in Pflegeheimen. Gesundheitswesen 2013. DOI: 10.1055/s-0032-1331249. [DOI] [PubMed]
- 110.Schäufele M, Hoell A, Hendlmeier I, Kohler L, Weyerer S. Primary Prevention of Impairments of Mobility Among Nursing Home Residents with Dementia. Gesundheitswesen. 2015;77(Suppl 1):105–106. doi: 10.1055/s-0032-1331249. [DOI] [PubMed] [Google Scholar]
- 111.Dichter MN, Schwab CGG, Meyer G, Bartholomeyczik S, Dortmann O, Halek M. Measuring the quality of life in mild to very severe dementia: Testing the inter-rater and intra-rater reliability of the German version of the QUALIDEM. Int Psychogeriatr. 2014;26(5):825–836. doi: 10.1017/S1041610214000052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the manuscript and its supplementary files.


