Abstract
Urothelial carcinoma is a highly heterogeneous disease that can arise throughout the entire urothelial lining from the renal pelvis to the proximal urethra. Upper tract urothelial carcinoma (UTUC) is rare and while it shares many similarities with urothelial carcinoma of bladder (UCB), there are also significant differences between UTUC and UCB regarding clinical management and outcomes. No major advances have been made recently in the development of new systemic therapies for urothelial carcinoma, partly due to the lack of understanding of underlying molecular pathogenetic mechanisms. In the past decade, the emergence of next-generation sequencing has greatly enabled genomic characterization of tumor samples. Researchers are currently exploring a personalized approach to augment traditional clinical decision-making based on genetic alterations. In the present review, we summarize current genomic advances in UTUC and discuss the potential implications of these developments for developing prognostic and predictive biomarkers.
Keywords: upper tract urothelial carcinoma, genomics, prediction, biomarkers
Introduction
Upper tract urothelial carcinoma (UTUC) can arise from the epithelial lining of the urinary tract from the renal calyces to the ureteric orifices. Compared to urothelial carcinoma of bladder (UCB), UTUC is a rare disease, accounting for approximately 5–10% of all urothelial carcinomas and less than 10% of renal tumors [1]. UTUC may be associated with smoking, arsenic exposure, analgesic abuse, occupational carcinogen exposure, hypertension, long-standing urinary obstruction, infection and Balkan nephropathy. Radical nephroureterectomy (RNU) with excision of the bladder cuff is the gold standard treatment for organ-confined disease. Some patients with low-risk disease may be treated with endoscopic ablation or segmental resections.
Current knowledge about the risk stratification and molecular pathogenesis of UTUC is sparse and often extrapolated from UCB, which is thought to share common pathways of carcinogenesis. UTUC however presents distinct challenges given limitations in accurate pathologic grading and staging at diagnosis with current endoscopic biopsy techniques and imaging technologies. Specifically, decision-making and the prognosis of UTUC relies heavily on TNM stage and pathological grade that is not accurately available until RNU is performed, at which point a significant proportion of patients may be rendered ineligible for adjuvant cisplatin-based chemotherapy. A personalized approach for prediction of oncologic outcomes and therapeutic responses is important, given the variability in disease behavior, the diversity of treatment options, and their impact on kidney function and quality of life. Further understanding of the genetic mechanisms underlying the development of UTUC will certainly help identify biological markers for prognostication and potential therapeutic targets. Intense research efforts are being made to identify and characterize robust molecular and genetic markers. Novel genomic technologies, such as next-generation sequencing, have improved our understanding of the molecular basis of both UCB and UTUC. In this review, we focus on the topic of genetic alterations in UTUC and the value of prognostic genetic markers in the prediction of outcomes and possible response to various therapeutic interventions.
1. Chromosomal aberrations and copy number variation
Comparative genomic hybridization has been used to identify chromosomal aberrations in UTUC. Losses in 9q are present in 50% of cases and high level amplifications are often detected at 1q21~q25, 6p22~p23, 8q21~q22, 8q22~q24.1, 11q13, and 12q14~q21 [2]. One study utilized array-based comparative genomic hybridization to detect frequent copy number gains on chromosomal regions 8p23.1 and 20q13.12, and frequent copy number losses on chromosomal regions 13q21.1, 17p13.1, 6q16.3, and 17p11.2. DNA copy number aberrations occurred more frequently in tumors with lymphovascular invasion (LVI) than in those without LVI [3]. In a cohort of 171 UTUC patients treated with RNU, Sasaki et al. demonstrated 18% (31/171) ERBB2 gene amplification using dual-color in situ hybridization. ERBB2 gene amplification was correlated with HER2 protein overexpression and high-grade histology. HER2 positivity was found to be an independent predictive marker for early intravesical recurrence of urothelial carcinoma [4]. Recently, we examined the landscape of copy number alterations (CNAs) in UTUC and found that TP53/MDM2-altered UTUC tumors possessed a high frequency of CNAs. TP53/MDM2-altered high-grade invasive UTUC tumors had significantly more copy number gains and total CNAs compared with FGFR3/HRAS/KRAS mutant high-grade invasive UTUC tumors. Furthermore, high-grade tumors had more CNAs than low-grade tumors, and invasive tumors had more CNAs than non-invasive tumors [5**].
2. Microsatellite instability
Epidemiological studies have demonstrated a 14-fold increased incidence of developing UTUC and a cumulative lifetime risk of 2.9% in hereditary non-polyposis colorectal cancer (HNPCC) patients compared to general population [6]. HNPCC, also known as Lynch syndrome (LS), is an autosomal-dominant familial cancer syndrome caused by germline mutations in the DNA mismatch repair (MMR) genes. LS patients with MSH2 mutations are at an increased risk for not only UTUC, but also UCB [7]. The MMR genes comprise MLH1, PMS1, PMS2, MSH2, MLH3, and MSH6. Defective MMR function leads to replication errors and frame shift mutations, which may result in aberrations in major cancer gene pathways. Loss of function in the MMR system results in microsatellite instability (MSI) throughout the human genome. MSI is a hallmark feature seen in approximately 85% of LS-associated tumors in mutation carriers [8]. An early study indicated a high level of MSI (46%) in UTUC [9]. High MSI indicates a better prognosis, especially in patients younger than 70 years with stage T2-T3N0M0 compared to low MSI patients [10]. MSI may arise from inactivating germline mutations, MLH1 promoter hypermethylation (10% of sporadic cases of UTUC) [11], or overexpression of upstream miR-155 [12]. García-Tello et al. recently found that the inactivation of PMS2 or MLH1 occurs in a quarter of sporadic UTUC cases and is an independent marker of good prognosis [13].
Interestingly, a recent phase 2 study showed that mismatch repair status predicted clinical benefit of immune checkpoint blockade with pembrolizumab [14]. Pembrolizumab was administered intravenously in patients with mismatch repair-deficient colorectal cancers and in patients with mismatch repair-proficient colorectal cancers. The study showed mismatch repair-deficient colorectal cancer patients had significantly better immune-related objective response rate and immune-related progression-free survival rate compared with mismatch repair-proficient colorectal cancer patients. The prolonged progression-free survival in mismatch repair-deficient colorectal cancer patients was associated high somatic mutation loads (a mean of 1782 somatic mutations per tumor in mismatch repair-deficient tumors, as compared with 73 in mismatch repair-proficient tumors). The results from this study suggest the potential utility of immune checkpoint inhibitors in a specific subset of UTUC tumors based on mismatch repair genetic status [14].
3. Mutational landscape and clinically relevant genes
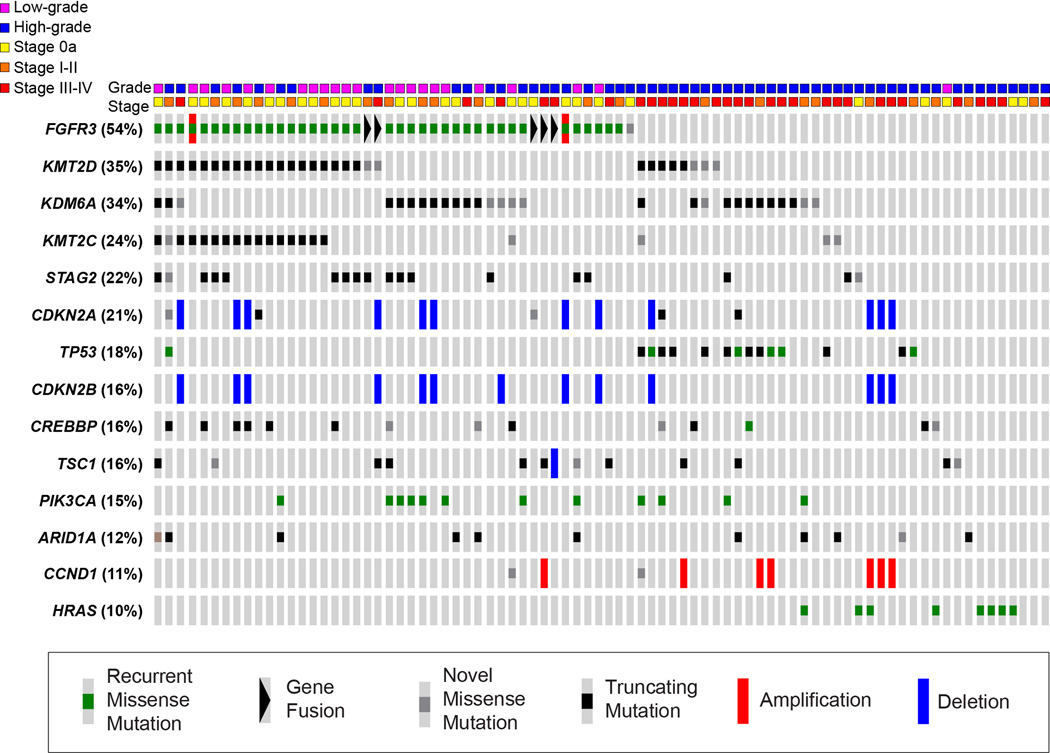
Recently, we comprehensively characterized the spectrum of genomic alterations in UTUC using massively parallel next-generation sequencing [5**]. The most frequently mutated genes in UTUC tumors included those commonly altered in previous studies of urothelial carcinoma of the bladder (UCB), including FGFR3 (54%), KMT2D (35%), KDM6A (34%), STAG2 (22%), CDKN2A (21%), TP53 (18%), PIK3CA (16%) and TSC1 (16%) (Figure 1). Consistent with prior studies, we identified a predominantly mutually exclusive pattern of alterations in the RTK/RAS/MAPK pathway and the p53/MDM2 pathway. The prevalence of specific mutations differed between UTUC and UCB. FGFR3, HRAS and CDKN2B were more frequently altered in UTUC tumors (36.8% vs 21.6%, p=0.042; 14.0% vs. 1.0%, p=0.001; and 15.8% vs. 3.9%, p=0.014, respectively) whereas TP53 and ARID1A were more frequently altered in UCB tumors (57.8% vs. 24.6%, p<0.001 and 27.5% vs. 12.3%, p=0.029, respectively) [5**].
Figure 1.
Representation of the 14 most frequently altered genes in a series of 82 upper tract urothelial carcinoma tumors. Mutations are categorized as missense mutations reported in COSMIC (green), gene fusions (black triangle), novel missense mutations (gray), truncating nonsense mutations or indels (black), amplifications (red), and deletions (blue).
1) p53
The tumor suppressor gene TP53 has been described as “the guardian of the genome” due to its role in conserving stability by preventing genome mutation. Mutations of p53 have been identified in approximately 50% of all human cancers. p53 can activate DNA repair genes to repair DNA damage or can arrest cell growth at the G1/S checkpoint. p53 can initiate apoptosis if DNA damage proves to be irreparable. Among all biomarkers, p53 expression is the most extensively investigated molecular marker in UTUC. Expression of mutant p53 has been found approximately 30–60% of UTUC [15*]. Many studies have demonstrated a correlation between p53 expression and poor survival. In immunohistochemistry (IHC) studies, p53 often lost statistical significance in multivariable analysis or failed to confirm other well established pathologic prognostic markers in multivariable analysis.
The initial p53 IHC study examined the prognostic role of p53 expression in 83 UTUC patients. The authors showed that the overexpression of p53 was significantly associated with tumor aggressiveness and worse patient survival [16]. Tumor stage, grade, and p53 expression were all significantly associated with outcomes on univariate analyses. However, tumor grade was not an independent predictor in a multivariable analysis whereas p53 expression status remained significant. A Japanese single-center study (n = 66) [17] and a European single-center study (n = 53) [18] showed p53 was a prognostic factor in univariable analyses but did not emerge as an independent prognostic factor after adjustment for clinical and pathologic characteristics. The most recent IHC study examined p53 expression in 112 UTUC patients and found high p53 expression was an independent predictor of poor progression-free (hazard ratio [HR] =3.74, p=0.025) and cancer-specific (HR=5.87, p=0.030) survival [19].
Other investigators have studied p53 protein expression and DNA mutation analysis simultaneously, albeit with only a minority of patient samples suitable for sequencing analysis. The first sequencing study of TP53 point mutations in exons 4 through 9 in UTUC demonstrated TP53 mutations in 7 of 26 cases, 6 of which were also positive for p53 expression. Overexpressed p53 was frequently detected in invasive and high-grade tumors [20]. Another study identified p53 point mutations in 6 of 21 cases, 5 of which were positive for p53 protein expression [21]. Bagrodia et al. prospectively evaluated the utility of a tissue biomarker panel of cell cycle regulators (p53, p21, p27, cyclin E) and a proliferative marker (Ki-67) in patients with UTUC treated with RNU [22]. The number of altered biomarkers was categorized as favorable (≤2 altered markers) or unfavorable (> two altered markers). An unfavorable tissue biomarker score was associated with advanced pathologic T stage, non-organ-confined disease, LVI, and inferior cancer-specific survival in RNU patients.
2) FGFR3
Fibroblast growth factor receptor 3 (FGFR3) is expressed in normal urothelium. Mutation of FGFR3 in bladder cancer is strongly associated with low tumor grade and stage. van Oers et al. examined FGFR3 mutations using the SNaPshot method. They found that FGFR3 mutations occurred with the same frequency in bladder (48%) and UTUC (46%). FGFR3 mutations were associated with low-stage tumors and a milder disease course in UTUC and invasive UTUCs with FGFR3 mutations have a more favorable prognosis [23]. Recently, Lyle et al. identified FGFR3 mutations in 40% of UTUC tumors using real-time polymerase chain reaction. FGFR3 mutations were predominantly associated with non-invasive tumors and overall better survival compared with tumors with wild-type FGFR3 [24].
In our genomic landscape study of UTUC, fifteen patients (18.3%) had TP53 mutations, 6 (7.3%) had mutually exclusive MDM2 amplifications, and 43 (52.4%) had FGFR3 mutations [5]. Mutation in TP53 (HR 3.13, 95% CI 1.44–6.80, p=0.002), TP53/MDM2 alteration (HR 3.66, 95% CI 1.77-7.57, p<0.001), CCND1 (HR 5.19, 95% CI 2.04–13.22, p<0.001), and ERBB3 (HR 3.93, 95% CI 1.18–13.10, p=0.016) significantly increased the risk of distant recurrence after RNU whereas mutation in FGFR3 (HR 0.15, 95% CI 0.06–0.37, p<0.001), RTK/Ras/MAPK pathway (HR 0.39, 95% CI 0.19–0.79, p=0.006), KMT2C (HR 0.29, 95% CI 0.09-0.94, p=0.029), and STAG2 (HR 0.22, 95% CI 0.05-0.92, p=0.022) significantly decreased the risk for distant recurrence (Table 1).TP53/MDM2 alterations were associated with adverse clinicopathologic outcomes whereas FGFR3 mutations were associated with favorable outcomes. We created a risk score including TP53/MDM2 and FGFR3 based on these data. The risk score was assigned as follows: 0=normal TP53/MDM2 and altered FGFR3, 1=normal TP53/MDM2 and normal FGFR3, 2=altered TP53/MDM2 and normal FGFR3. On univariable logistic regression, risk score was significantly associated with grade (p=0.002), stage (p<0.001), and organ-confined status (p<0.001). When we limited our analyses to high-grade patients, risk score remained significantly associated with stage (OR 3.01, 95% CI 1.41-6.40, p=0.004) and organ-confined status (OR 2.62, 95% CI 1.26-5.44, p=0.01). These associations also held among high-grade patients after adjusting for location of tumor. Increasing risk score was associated with both worse recurrence-free and cancer-specific survival (Table 1). On univariable Cox regression, limited to high-grade patients, risk score was marginally associated with cancer-specific survival (HR 1.76, 95% CI 1.00–3.11, p=0.05) and remained significantly associated with recurrence (HR 1.95, 95% CI 1.21–3.12, p=0.006).
Table 1.
Associations with genomic alterations and clinical outcomes in 82 patients with upper tract urothelial carcinoma treated with radical nephroureterectomy.
| Distant Recurrence (n=31 events) |
Cancer-Specific Mortality (n=23 events) |
|||||
|---|---|---|---|---|---|---|
| Gene | HR (95% CI) | p* | HG only p* (n=59) |
HR (95% CI) | p* | HG only p* (n=59) |
| TP53/MDM2 | 3.66 (1.77, 7.57) | <0.001 | 0.048 | 3.43 (1.46, 8.08) | 0.003 | 0.092 |
| TP53 | 3.13 (1.44, 6.80) | 0.002 | 0.114 | 3.25 (1.29, 8.21) | 0.008 | 0.117 |
| MDM2 | 2.64 (0.92, 7.59) | 0.060 | 0.339 | 2.05 (0.61, 6.96) | 0.239 | 0.619 |
| Rb pathway | 1.31 (0.64, 2.67) | 0.458 | 0.908 | 1.20 (0.52, 2.77) | 0.673 | 0.835 |
| CCND1 | 5.19 (2.04, 13.22) | <0.001 | 0.011 | 3.50 (1.14, 10.72) | 0.020 | 0.132 |
| CCNE1 | <NA> | <NA> | <NA> | <NA> | <NA> | <NA> |
| CDKN2A | 1.20 (0.52, 2.79) | 0.666 | 0.613 | 1.58 (0.62, 4.02) | 0.334 | 0.193 |
| CDKN2B | 1.22 (0.50, 2.97) | 0.667 | 0.438 | 1.69 (0.67, 4.30) | 0.263 | 0.126 |
| CDKN1A | 0.41 (0.06, 3.03) | 0.369 | 0.295 | 0.53 (0.07, 3.91) | 0.523 | 0.490 |
| E2F3 | 2.18 (0.30, 16.06) | 0.434 | 0.747 | <NA> | <NA> | <NA> |
| PI3K pathway | 0.72 (0.31, 1.67) | 0.442 | 0.892 | 0.58 (0.20, 1.72) | 0.324 | 0.782 |
| PIK3CA | 0.32 (0.08, 1.34) | 0.099 | 0.348 | 0.22 (0.03, 1.64) | 0.105 | 0.300 |
| PTEN | <NA> | <NA> | <NA> | <NA> | <NA> | <NA> |
| TSC1 | 1.12 (0.39, 3.22) | 0.830 | 0.708 | 0.85 (0.20, 3.66) | 0.828 | 0.930 |
| TSC2 | 2.26 (0.53, 9.66) | 0.259 | 0.156 | 1.83 (0.24, 14.08) | 0.557 | 0.576 |
| RTK/RAS/MAPK pathway | 0.39 (0.19, 0.79) | 0.006 | 0.301 | 0.48 (0.21, 1.09) | 0.074 | 0.537 |
| FGFR3 | 0.15 (0.06, 0.37) | <0.001 | 0.012 | 0.22 (0.08, 0.60) | 0.001 | 0.116 |
| ERBB2 | 2.77 (0.65, 11.80) | 0.150 | 0.420 | 3.21 (0.74, 13.91) | 0.099 | 0.292 |
| ERBB3 | 3.93 (1.18, 13.10) | 0.016 | 0.126 | 1.93 (0.25, 14.94) | 0.524 | 0.786 |
| HRAS | 2.15 (0.75, 6.17) | 0.144 | 0.595 | 2.10 (0.71, 6.19) | 0.169 | 0.589 |
| KRAS | 1.32 (0.31, 5.53) | 0.706 | 0.804 | 0.83 (0.11, 6.16) | 0.854 | 0.553 |
| BRAF | <NA> | <NA> | <NA> | <NA> | <NA> | <NA> |
| RAF1 | 0.90 (0.12, 6.60) | 0.915 | 0.562 | 1.04 (0.14, 7.75) | 0.971 | 0.710 |
| NF1 | 0.48 (0.06, 3.49) | 0.454 | 0.407 | 0.60 (0.08, 4.46) | 0.614 | 0.622 |
| Any Chromatin-Modifying Gene | 0.69 (0.33, 1.44) | 0.321 | 0.604 | 0.72 (0.31, 1.66) | 0.433 | 0.569 |
| ARID1A or SMARCA4 | 0.86 (0.35, 2.09) | 0.731 | 0.801 | 0.55 (0.16, 1.86) | 0.331 | 0.336 |
| ARID1A | 0.56 (0.17, 1.84) | 0.333 | 0.292 | <NA> | <NA> | <NA> |
| SMARCA4 | 1.35 (0.41, 4.47) | 0.622 | 0.157 | 3.33 (0.90, 12.25) | 0.055 | 0.021 |
| CREBBP or EP300 | 0.37 (0.11, 1.22) | 0.089 | 0.435 | 0.18 (0.02, 1.31) | 0.055 | 0.235 |
| CREBBP | 0.35 (0.08, 1.46) | 0.132 | 0.446 | 0.27 (0.04, 2.01) | 0.170 | 0.469 |
| EP300 | 0.37 (0.05, 2.69) | 0.303 | 0.691 | <NA> | <NA> | <NA> |
| KDM6A | 0.76 (0.35, 1.65) | 0.487 | 0.762 | 0.98 (0.40, 2.39) | 0.965 | 0.829 |
| KMT2A (MLL) | 5.09 (0.67, 38.72) | 0.080 | 0.229 | <NA> | <NA> | <NA> |
| KMT2D (MLL2) | 0.50 (0.21, 1.16) | 0.098 | 0.316 | 0.59 (0.22, 1.59) | 0.289 | 0.690 |
| KMT2C (MLL3) | 0.29 (0.09, 0.94) | 0.029 | 0.189 | 0.29 (0.07, 1.24) | 0.075 | 0.255 |
| STAG2 | 0.22 (0.05, 0.92) | 0.022 | 0.221 | 0.35 (0.08, 1.51) | 0.143 | 0.578 |
p values reflect logrank test.
HR = hazard ratio; CI = confidence interval; HG = high-grade
4. Single nucleotide polymorphisms (SNP)
Researchers from Taiwan have performed molecular epidemiological studies to evaluate the single nucleotide polymorphism of different genes in UTUC. Lin et al. examined the cyclin D1 (CCND1) genotypes of 170 patients and 249 control subjects. They found that C allele of the cell cycle regulator CCND1 C1722G polymorphism may be a potential predictive and prognostic biomarker for advanced UTUC [25]. Chang et al. examined six CAV1 polymorphic genotypes, C521A (rs1997623), G14713A (rs3807987), G21985A (rs12672038), T28608A (rs3757733), T29107A (rs7804372), and G32124A (rs3807992) in 218 UTUC patients and 580 healthy controls using polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP). The haplotype analysis showed the A allele of CAV1 rs3807987 and T allele of CAV1 rs7804372 might become potential biomarkers for the early screening and risk prediction of UUTC [26]. Another group from France demonstrated an association between a T/T rs9642880 genotype on chromosome 8q24 and aggressive UTUC tumors [27]. Recently, the G allele of COX2 G-765C and A allele of COX2 intron 5 were found to be genomic risk factors predictive biomarkers for UTUC in Taiwan [28]. Although these SNP studies have suggested that information regarding genetic variation may improve risk prediction, they are probably not sufficient to identify all potentially causative SNPs and to develop a full understanding of these genetic variations in UTUC.
5. Epigenetic biomarkers
Epigenetic changes affect the spatial conformation of DNA and its transcriptional activity and lead to changes in phenotype without changing the sequence of DNA bases. Researchers have observed that many tumors show an aberrant epigenetic modification pattern, affecting a variety of cancer-related genes. Epigenetic regulation occurs on several mechanistic levels, including DNA methylation, covalent histone modifications, and small regulatory RNAs [29]. In our cohort of UTUC tumors profiled with next-generation sequencing, mutations in chromatin-modifying genes (CMGs) were highly prevalent in UTUC (KDM6A 34%, ARID1A 12%, KMT2D 35%, CREBBP 16%) (Figure 1) [5**].
Promoter methylation of tumor suppressors is a frequent and early event in tumor development. Aberrant promoter hypermethylation has been investigated in different panels of genes in UCB, including tumor suppressor genes, oncogenes, genes involved in cell adhesion, and genes of cell cycle regulation [30]. Promoter methylation was first demonstrated in 86% of urothelial carcinomas and occurred both more frequently and more extensively in UTUC (94%) than in bladder tumors (76%). Methylation was associated with advanced tumor stage and higher tumor progression and mortality rates, when compared with tumors without methylation. Methylation at the RASSF1A and DAPK loci, in addition to tumor stage and grade, was associated with disease progression [11]. Importantly, DNA methylation assays can be performed on small biopsies, archival frozen or paraffin-embedded samples, as well as on the soluble genomic DNA found in peripheral blood and voided urine samples. A panel of epigenetic biomarkers (GDF15, TMEFF2 and VIM promoter methylation) was tested in 57 UTUC tumors, 36 normal upper tract urothelial samples, 22 urines from UTUC patients and 20 urines from controls. This panel identified UTUC with 100% and 91% sensitivity in tissue and urine samples, respectively, and 100% specificity in both samples. Low VIM promoter methylation levels independently predicted poor disease-specific survival in UTUC patients [31].
Xiong et al. conducted methylation-sensitive polymerase chain reaction for the promoter regions of ten genes (ABCC6, BRCA1, CDH1, GDF15, HSPA2, RASSF1A, SALL3, THBS1, TMEFF2, and VIM) in 687 UTUC patients to correlate methylation status with prognosis. Among ten genes, only methylated TMEFF2 promoter and BRCA1 promoter were significantly associated with CSS [32]. Although aberrant DNA methylation patterns in UTUC have emerged as potential biomarkers that are detectable in the serum or voided urine of UTUC patients, none have been validated, nor have they reached a sufficient level of accuracy. Clinically relevant methylation assays still await more validation studies, testing on large cohorts of patients and healthy controls, and functional validation of aberrant methylation patterns.
6. Potential biomarkers from gene expression profiling
Most previous biomarker studies in UTUC have focused on immunohistochemical analysis of protein expression. Numerous studies investigated the prognostic impact of various tissue-based molecular markers that are related to cellular processes such as cell adhesion (Metalloproteinase-9, E-cadherin [33], ParvB [34], snail [35], b-catenin[36]), cell signaling (EGFR[37], EMP3 [38], HER2 [4], PI3K/AKT [39, 40], IGFBP-5 [41], mTOR [42]), angiogenesis (Hypoxia-inducible factor-1[43]), cell proliferation (Ki67[44], p27 [45],cyclin D[46], NF- κ B [47], Aurora-A[48]), cell transport (GRP78 [49]) and apoptosis (bcl-2, survivin [50]). Recently, Wu et al. compared the genome-wide mRNA expression profile using digital gene expression sequencing of tumors and matched normal tissues in 10 UTUC patients. They identified 3431 to 7702 significantly deregulated genes, mainly characterized by abnormal cell proliferation, and metabolism. Further IHC study showed that low protein expression of ALDH2 and high CCNE1/SMAD3 were associated with lower overall survival in a cohort of 103 patients [51]. Comparative proteomic analysis of urine has recently been applied in UTUC. Lu et al. identified 55 differential proteins among totally 1028 protein spots in the urine of 13 UTUC patients compared with the urine of 20 healthy control adults using two-dimensional gel electrophoresis. Three proteins (CALR, annexin A2 and annexin A3) were found to be essential secreting proteins in the urine from UTUC tumor tissues, suggesting their potential role as a panel of biomarkers [52]. However, the major limitations shared by these studies were their retrospective nature and small sample sizes.
7. Implication of genomics in UTUC: Potential therapeutic targets and prediction of response
Major advances have recently been made in contributing to the understanding of the genetics underlying the potential pathogenesis of both UCB and UTUC. These advances may lead to identification of new biomarkers and potential therapeutic targets for UTUC. In the near future, personalized genetic profiling of primary or metastatic tumor cells may become readily available for routine clinical decision-making, potentially allowing for identification of patients that are likely to respond to systemic therapy [53]. Our recent genomic study not only revealed the molecular landscape of UTUC, but also identified several currently targetable genetic changes in oncogenic pathways of UTUC, such as FGFR3 (54% mutated), CDKN2B (21% altered), TSC1 (16% altered), and PIK3CA (15% altered). Researchers may shift the focus of investigation from chemotherapy to targeted therapies, either in combination with cytotoxic agents, or as single agents.
Although there is no current clinical trial of targeted therapy for UTUC due to the rarity of the disease, numerous ongoing and developing clinical trials are testing the efficacy of targeted therapies for UCB in multiple molecular pathways, including antiangiogenic agents, anti-EGFR/HER2 therapy, anti-PI3K/AKT/mTOR therapy, and immune checkpoint inhibition [54**]. Genomic characterization before treatment is now being implemented in novel clinical trial designs in order to allocate patients based on predictive biomarkers for targeted molecular therapy [54]. MATCH-UP (Molecular Allocation Trial to CHoose therapy for metastatic Urothelial carcinoma following Platinum-based chemo-therapy) is a phase II trial designed to prospectively screen tumor tissues for specific molecular mutations, including FGFR3 fusion/mutation/amplification, RB1 mutation, PI3K mutation, AKT1 mutation/amplification, mTOR mutation, TSC1 deletion/mutation, PTEN deletion/mutation, ERBB2 mutation/fusion/amplification, EGFR amplification, and histone acetyltransferase mutation[54].
Molecular characterization of tumor tissue may also predict an individual patient’s likelihood of responding to a specific chemotherapy regimen. A novel gene expression algorithm Co-eXpression ExtrapolatioN (COXEN) is derived by comparing gene expression signatures between 60 cancer cell lines (NCI-60) that are sensitive or resistant to a number of FDA-approved drugs. A COXEN “score” using a gene expression model allows an prior analysis of urothelial tumor responsiveness to anticancer agents and translate drug sensitivity of carcinoma cell lines into prediction of clinical response for a patient [55]. A Southwest Oncology Group clinical trial is currently recruiting patients to explore the role of COXEN score for predicting chemotherapy response in patients with urothelial cancer. Kothari et al. recently reported that COXEN accurately predicted drug sensitivity in 9/10 (90%) pts with response and 2/5 (40%) pts with resistance to therapy. The COXEN algorithm might have a role in urothelial cancer to select a “next best therapy” in the perioperative or metastatic setting [56].
Conclusions
UTUC is a highly heterogeneous disease with the majority of tumors harboring numerous concurrent genetic alterations. Currently, tumor stage and grade remain the best established predictors of prognosis in patients with UTUC. Recent molecular investigations of UTUC have led to an improved understanding of the genetic landscape, possible biomarkers, and identification of potentially actionable targets. In the near future, personalized genetic profiling of primary or metastatic UTUC tumors may become readily available for routine clinical decision-making. It is critical to explore, integrate and validate genetic data toward clinically meaningful outcomes for UTUC patients. The integration of molecular with clinical factors may improve our ability to determine prognosis, predict treatment response, and to select UTUC patients for targeted treatment options or clinical trials. More importantly, genetic data should be validated in future prospective multi-institutional studies and used in clinical practice for optimal decision-making toward the design of personalized therapies. Developing reliable prognostic biomarkers and promising drugs based on genomic alterations should translate into better clinical care and improved outcomes for UTUC patients.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Roupret M, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63(6):1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Rigola MA, et al. Comparative genomic hybridization analysis of transitional cell carcinomas of the renal pelvis. Cancer Genet Cytogenet. 2001;127(1):59–63. doi: 10.1016/s0165-4608(00)00426-x. [DOI] [PubMed] [Google Scholar]
- 3.Misumi T, et al. DNA copy number aberrations associated with lymphovascular invasion in upper urinary tract urothelial carcinoma. Cancer Genet. 2012;205(6):313–318. doi: 10.1016/j.cancergen.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki Y, et al. HER2 protein overexpression and gene amplification in upper urinary tract urothelial carcinoma-an analysis of 171 patients. Int J Clin Exp Pathol. 2014;7(2):699–708. [PMC free article] [PubMed] [Google Scholar]
- 5. Sfakianos JP, et al. Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.07.039. This manuscript is the largest series of UTUC tumors profiled using next- generation sequencing and provided the molecular landscape of UTUC and potential actionable genomic targets in UTUC.
- 6.Sijmons RH, et al. Urinary tract cancer and hereditary nonpolyposis colorectal cancer: risks and screening options. J Urol. 1998;160(2):466–470. [PubMed] [Google Scholar]
- 7.Skeldon SC, et al. Patients with Lynch syndrome mismatch repair gene mutations are at higher risk for not only upper tract urothelial cancer but also bladder cancer. Eur Urol. 2013;63(2):379–385. doi: 10.1016/j.eururo.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 8.Umar A, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amira N, et al. Microsatellite instability in urothelial carcinoma of the upper urinary tract. J Urol. 2003;170(4 Pt 1):1151–1154. doi: 10.1097/01.ju.0000086551.22844.cd. [DOI] [PubMed] [Google Scholar]
- 10.Roupret M, et al. Microsatellite instability as predictor of survival in patients with invasive upper urinary tract transitional cell carcinoma. Urology. 2005;65(6):1233–1237. doi: 10.1016/j.urology.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Catto JW, et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J Clin Oncol. 2005;23(13):2903–2910. doi: 10.1200/JCO.2005.03.163. [DOI] [PubMed] [Google Scholar]
- 12.Valeri N, et al. Modulation of mismatch repair and genomic stability by miR-155. Proc Natl Acad Sci U S A. 2010;107(15):6982–6987. doi: 10.1073/pnas.1002472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Tello A, et al. DNA repair genes and prognosis in sporadic forms of urothelial carcinoma of the upper urinary tract. Actas Urol Esp. 2014;38(9):600–607. doi: 10.1016/j.acuro.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Le DT, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell S, Mayer E, Patel A. Expression of p53 in upper urinary tract urothelial carcinoma. Nat Rev Urol. 2011;8(9):516–522. doi: 10.1038/nrurol.2011.92. This article critically summarizes previous studies of p53 expression in UTUC and highlights the limitations of sample size, methods to detect p53 expression and statistical analyses.
- 16.Rey A, et al. Overexpression of p53 in transitional cell carcinoma of the renal pelvis and ureter. Relation to tumor proliferation and survival. Cancer. 1997;79(11):2178–2185. doi: 10.1002/(sici)1097-0142(19970601)79:11<2178::aid-cncr16>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto H, et al. Roles of p53 and MDM2 in tumor proliferation and determination of the prognosis of transitional cell carcinoma of the renal pelvis and ureter. Int J Urol. 2000;7(12):457–463. doi: 10.1046/j.1442-2042.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 18.Zigeuner R, et al. Prognostic impact of p63 and p53 expression in upper urinary tract transitional cell carcinoma. Urology. 2004;63(6):1079–1083. doi: 10.1016/j.urology.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Lee YC, et al. Prognostic value of p53 protein overexpression in upper tract urothelial carcinomas in Taiwan. Anticancer Res. 2013;33(3):1091–1098. [PubMed] [Google Scholar]
- 20.Furihata M, et al. p53 and human papillomavirus DNA in renal pelvic and ureteral carcinoma including dysplastic lesions. Int J Cancer. 1995;64(5):298–303. doi: 10.1002/ijc.2910640503. [DOI] [PubMed] [Google Scholar]
- 21.Furihata M, et al. Detection of p53 and bcl-2 protein in carcinoma of the renal pelvis and ureter including dysplasia. J Pathol. 1996;178(2):133–139. doi: 10.1002/(SICI)1096-9896(199602)178:2<133::AID-PATH455>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Bagrodia A, et al. Prospective evaluation of molecular markers for the staging and prognosis of upper tract urothelial carcinoma. Eur Urol. 2012;62(1):e27–e29. doi: 10.1016/j.eururo.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 23.van Oers JM, et al. FGFR3 mutations indicate better survival in invasive upper urinary tract and bladder tumours. Eur Urol. 2009;55(3):650–657. doi: 10.1016/j.eururo.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Lyle SR, et al. Molecular grading of tumors of the upper urothelial tract using FGFR3 mutation status identifies patients with favorable prognosis. Res Rep Urol. 2012;4:65–69. doi: 10.2147/RRU.S37355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin HH, et al. CCND1 1722 polymorphism and potential relevance to upper tract urothelial cancer. Anticancer Res. 2011;31(3):1043–1047. [PubMed] [Google Scholar]
- 26.Chang WS, et al. Significant association of caveolin-1 (CAV1) genotypes with upper urothelial tract cancer. Anticancer Res. 2013;33(11):4907–4912. [PubMed] [Google Scholar]
- 27.Roupret M, et al. Genetic variability in 8q24 confers susceptibility to urothelial carcinoma of the upper urinary tract and is linked with patterns of disease aggressiveness at diagnosis. J Urol. 2012;187(2):424–428. doi: 10.1016/j.juro.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 28.Chang WS, et al. Significant Association of Cyclo-oxygenase 2 Genotypes with Upper Tract Urothelial Cancer. Anticancer Res. 2015;35(5):2725–2730. [PubMed] [Google Scholar]
- 29.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8(4):286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 30.Phe V, Cussenot O, Roupret M. Interest of methylated genes as biomarkers in urothelial cell carcinomas of the urinary tract. BJU Int. 2009;104(7):896–901. doi: 10.1111/j.1464-410X.2009.08696.x. [DOI] [PubMed] [Google Scholar]
- 31.Monteiro-Reis S, et al. Accurate detection of upper tract urothelial carcinoma in tissue and urine by means of quantitative GDF15, TMEFF2 and VIM promoter methylation. Eur J Cancer. 2014;50(1):226–233. doi: 10.1016/j.ejca.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Xiong G, et al. Prognostic and predictive value of epigenetic biomarkers and clinical factors in upper tract urothelial carcinoma. Epigenomics. 2015:1–12. doi: 10.2217/epi.15.34. [DOI] [PubMed] [Google Scholar]
- 33.Inoue K, et al. The prognostic value of angiogenesis and metastasis-related genes for progression of transitional cell carcinoma of the renal pelvis and ureter. Clin Cancer Res. 2002;8(6):1863–1870. [PubMed] [Google Scholar]
- 34.Wu CF, et al. Expression of parvin-beta is a prognostic factor for patients with urothelial cell carcinoma of the upper urinary tract. Br J Cancer. 2010;103(6):852–860. doi: 10.1038/sj.bjc.6605835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosaka T, et al. Expression of snail in upper urinary tract urothelial carcinoma: prognostic significance and implications for tumor invasion. Clin Cancer Res. 2010;16(23):5814–5823. doi: 10.1158/1078-0432.CCR-10-0230. [DOI] [PubMed] [Google Scholar]
- 36.Izquierdo L, et al. Adhesion molecules alpha, beta and gamma-catenin as prognostic factors of tumour progression in upper urinary tract urothelial tumours: the role of AKT-P/GSK-3beta/beta-catenin pathway. BJU Int. 2009;104(1):100–106. doi: 10.1111/j.1464-410X.2008.08292.x. [DOI] [PubMed] [Google Scholar]
- 37.Leibl S, et al. EGFR expression in urothelial carcinoma of the upper urinary tract is associated with disease progression and metaplastic morphology. APMIS. 2008;116(1):27–32. doi: 10.1111/j.1600-0463.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang YW, et al. Potential significance of EMP3 in patients with upper urinary tract urothelial carcinoma: crosstalk with ErbB2-PI3K-Akt pathway. J Urol. 2014;192(1):242–251. doi: 10.1016/j.juro.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Izquierdo L, et al. HER-2/AKT expression in upper urinary tract urothelial carcinoma: prognostic implications. Anticancer Res. 2010;30(6):2439–2445. [PubMed] [Google Scholar]
- 40.Qian CN, et al. Activation of the PI3K/AKT pathway induces urothelial carcinoma of the renal pelvis: identification in human tumors and confirmation in animal models. Cancer Res. 2009;69(21):8256–8264. doi: 10.1158/0008-5472.CAN-09-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang PI, et al. IGFBP-5 overexpression as a poor prognostic factor in patients with urothelial carcinomas of upper urinary tracts and urinary bladder. J Clin Pathol. 2013;66(7):573–582. doi: 10.1136/jclinpath-2012-201278. [DOI] [PubMed] [Google Scholar]
- 42.Munari E, et al. Dysregulation of mammalian target of rapamycin pathway in upper tract urothelial carcinoma. Hum Pathol. 2013;44(12):2668–2676. doi: 10.1016/j.humpath.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Nakanishi K, et al. Expression of hypoxia-inducible factor-1alpha protein predicts survival in patients with transitional cell carcinoma of the upper urinary tract. Clin Cancer Res. 2005;11(7):2583–2590. doi: 10.1158/1078-0432.CCR-04-1685. [DOI] [PubMed] [Google Scholar]
- 44.Krabbe LM, et al. Prospective analysis of Ki-67 as an independent predictor of oncologic outcomes in patients with high grade upper tract urothelial carcinoma. J Urol. 2014;191(1):28–34. doi: 10.1016/j.juro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Kamai T, et al. Prognostic significance of p27Kip1 and Ki-67 expression in carcinoma of the renal pelvis and ureter. BJU Int. 2000;86(1):14–19. doi: 10.1046/j.1464-410x.2000.00726.x. [DOI] [PubMed] [Google Scholar]
- 46.Bagrodia A, et al. Evaluation of the prognostic significance of altered mammalian target of rapamycin pathway biomarkers in upper tract urothelial carcinoma. Urology. 2014;84(5):1134–1140. doi: 10.1016/j.urology.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 47.Yeh HC, et al. Nuclear factor-kappaB activation predicts an unfavourable outcome in human upper urinary tract urothelial carcinoma. BJU Int. 2010;106(8):1223–1229. doi: 10.1111/j.1464-410X.2010.09210.x. [DOI] [PubMed] [Google Scholar]
- 48.Scarpini S, et al. Impact of the expression of Aurora-A, p53, and MIB-1 on the prognosis of urothelial carcinomas of the upper urinary tract. Urol Oncol. 2012;30(2):182–187. doi: 10.1016/j.urolonc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Uematsu K, et al. Glucose-regulated protein 78 expression in urothelial carcinoma of the upper urinary tract. BJU Int. 2010;106(6):873–878. doi: 10.1111/j.1464-410X.2009.09144.x. [DOI] [PubMed] [Google Scholar]
- 50.Jeong IG, et al. Prognostic value of apoptosis-related markers in urothelial cancer of the upper urinary tract. Hum Pathol. 2009;40(5):668–677. doi: 10.1016/j.humpath.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Wu S, et al. Global gene expression profiling identifies ALDH2, CCNE1 and SMAD3 as potential prognostic markers in upper tract urothelial carcinoma. BMC Cancer. 2014;14:836. doi: 10.1186/1471-2407-14-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu CM, et al. A panel of tumor markers, calreticulin, annexin A2, and annexin A3 in upper tract urothelial carcinoma identified by proteomic and immunological analysis. BMC Cancer. 2014;14:363. doi: 10.1186/1471-2407-14-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iyer G, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338(6104):221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kurtoglu M, et al. Elevating the Horizon: Emerging Molecular and Genomic Targets in the Treatment of Advanced Urothelial Carcinoma. Clin Genitourin Cancer. 2015;13(5):410–420. doi: 10.1016/j.clgc.2015.02.009. This article reviewed emerging genomic targets, most promising targeted therapies and novel clinical trial designs for urothelial carcinoma of bladder.
- 55.Smith SC, et al. The COXEN principle: translating signatures of in vitro chemosensitivity into tools for clinical outcome prediction and drug discovery in cancer. Cancer Res. 2010;70(5):1753–1758. doi: 10.1158/0008-5472.CAN-09-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shalin Kothari DG, Killian Keith, Costello James, Edelman Daniel C, Walling Jennifer, Meltzer Paul S, Theodorescu Dan, Borghese Andrea. COXEN prediction of antineoplastic drug sensitivity in bladder cancer patients. J Clin Oncol. 2015;33(suppl) abstr e15533. [Google Scholar]