Abstract
Introduction
The combination of independent risk factors for erectile dysfunction, obesity, hypertension, and diabetes are collectively manifested in a condition known as metabolic syndrome X (MSX). However, the regulatory mechanisms responsible for the erectile dysfunction (ED) are not fully understood. Clinical studies suggest that a pleiotropic effect of statin’s ability to enhance vascular relaxation might be through an impact on nitric oxide signaling or through a regulation of RhoA activation.
Aim
We hypothesized that regulatory aspects of short-term statin therapy involve the alteration of the RhoA/Rho-kinase signaling cascade and will reverse the ED seen in a rat model of MSX.
Main Outcome Measures
The magnitude and sensitivity of the voltage-dependent maintenance of intracavernosal blood pressure and mean arterial blood pressure. These responses were correlated with tissue protein and mRNA expression levels of RhoA and Rho kinases.
Methods
Erectile function was evaluated by assessing voltage-dependent stimulation of the cavernosal nerve in 16–20 weeks old lean and obese-diabetic Zucker rats treated with 5 mg/kg/day of rosuvastatin intraperitoneally for 3 days. Cavernosal tissue RhoA and Rho-kinases expression levels were evaluated by real-time reverse transcriptase-polymerase chain reaction, Western blot.
Results
The voltage-dependent erectile responses were suppressed by >30% in the obese-diabetic Zucker rat. The 3-day treatment with rosuvastatin partially restored the erectile response. The Rho-kinase inhibitor, H-1152, dose dependently increased the erectile responses and shifted the voltage sensitivity with statin treatment. Analysis of protein expression levels suggested elevation of RhoA and Rho kinases in obese-diabetics and statin treatment lowering Rho-kinase II. The RhoA and Rho-kinase II mRNA levels were significantly reduced in the rosuvastatin-treated obese-diabetic animals.
Conclusions
These results support a hypothesis that short-term statin therapy may lower RhoA/Rho-kinase expression levels and improve cavernosal blood pressure response to Rho-kinase inhibition and voltage-stimulation, and reversing an augmented vasoconstricted state associated with diabetes and/or hypertension in MSX.
Keywords: Rosuvastatin, Nitric Oxide Synthase, Metabolic Syndrome X
Introduction
Diabetes and the prediabetic state are estimated to affect more than 28 million Americans. The incidence of type II diabetes mellitus (DM) has increased by more than 70% in the last decade and has become increasingly common among children [1]. This situation is rapidly becoming a highly relevant public health issue. In the urology field, it has been recognized that a strong independent link between DM or hypertension and erectile dysfunction (ED) exists. The effects of the diabetic state can be exacerbated when combined with hypertension and obesity in a condition often referred to as metabolic syndrome X (MSX) [2,3]. These risk factors are also known to act as independent risk factors for ED [4–9]. Recent reviews have emphasized the impact of a cohort of independent risk factors in the treatment of ED [10–15], or for ED as a predictor of MSX [16–18].
The use of statins to influence cholesterol metabolism has been a therapeutic approach for treatment of several cardiovascular disorders including hypertension and diabetes [19,20]. However, recent evidence suggests that the pleiotropic effects of statins at doses or durations insufficient to lower plasma lipid levels may include effects regulating critical processes relevant to the control of the erectile response. These would include RhoA/Rho-kinase modulation of vasoconstriction, as well as increased nitric oxide (NO) signaling. One of the more accepted pleiotropic effects include improved expression and/or activity of elements of the endothelial NO signaling process [21–23], whereas an alternative effect includes the regulation of posttranslational modification of proteins associated with the RhoA/Rho-kinase signaling pathway [24,25].
To date, there has been no investigation into the mechanism by which statins resolve the ED in a rat model of metabolic syndrome. We hypothesized that the regulatory mechanisms of short-term statin therapy involve alteration of the RhoA/Rho-kinase signaling cascade and would reverse the ED seen in a rat model of MSX. In this report, we established that rosuvastatin ameliorated the ED seen in the obese-diabetic Zucker rat. This was manifested as an improved voltage-dependent erectile response following dose-dependent Rho-kinase inhibition. Furthermore, we suggested that statin treatment may reduce the mRNA and protein expression level of either RhoA or Rho-kinase in the cavernosal tissue, thus identifying a potential mechanism by which statins might ameliorate ED associated with metabolic syndrome.
Results
Comparison of Voltage-Dependent Erectile Response of Lean and Obese-Diabetic Zucker Rats
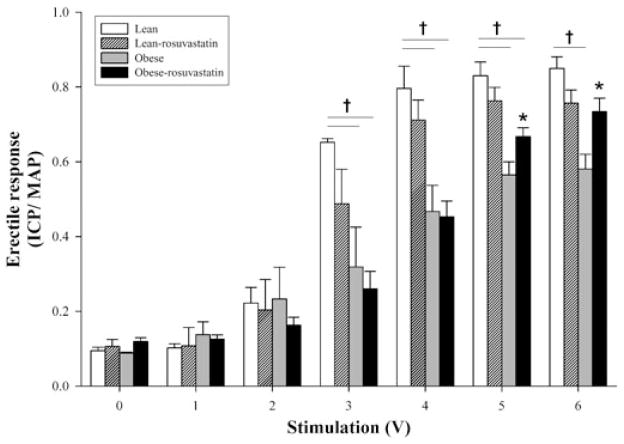
Adult male lean and obese-diabetic Zucker (ZDF/fa/fa) rats 16–20 weeks of age (Charles River Laboratories, Wilmington, MA) were used in these studies. Stimulation of the major pelvic ganglion and cavernosal nerve in the anesthetized rat resulted in voltage-dependent increases in erectile response for both lean and obese-diabetic animals (Figure 1). The erectile response was calculated from the ratio of the average intracavernosal blood pressure (ICP) divided by the average mean arterial blood pressure (MAP) during the duration of the particular level of stimulation. However, the erectile response was significantly depressed (by an average of 32.9 ± 3.6%) at stimulation voltages greater than 2 V in the obese-diabetic animals. The erectile response of the lean animals was not significantly different from the reported voltage-dependent erectile response measured in other rat strains [26,29].
Figure 1.
Mean voltage-dependent erectile responses (intracavernosal pressure/mean arterial pressure [ICP/ MAP]) of lean and obese-diabetic Zucker rats with or without rosuvasta-tin treatment. Treatment was 5 mg/kg/day rosuvastatin or saline, as control, for 3 days prior to the assessment of voltage-dependent erectile response. Erectile function was assessed and cavernosal tissue was removed as described previously [26–28]. †Statistical difference from the lean control; *statistical difference from obese-control response, P < 0.05. Reported are means ± standard error of the mean for four to six animals in each group.
Effect of the Rosuvastatin Treatment on the Voltage-Dependent Erectile Response in Lean and Obese-Diabetic Zucker Rats
Three days of 5 mg/kg rosuvastatin (a gift from Astra-Zeneca Corporation) by intraperitoneal administration resulted in a slight but nonsignificant reduction in the voltage-dependent erectile response of lean animals as compared with their control. The statin treatment improved the ICP/MAP ratio at 5- and 6-V stimulations in the obese-diabetic animals from the obese-diabetic control but still remained below that of the lean control (Figure 1).
In Vivo Dose-Dependence of the Voltage-Dependent Erectile Response by Rho-Kinase Inhibition
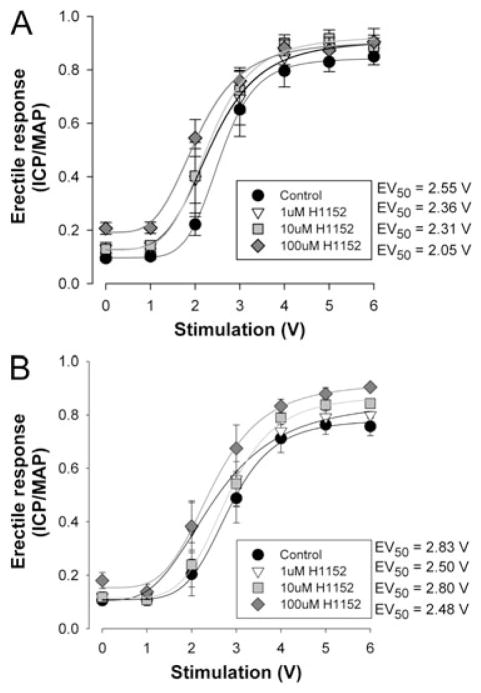
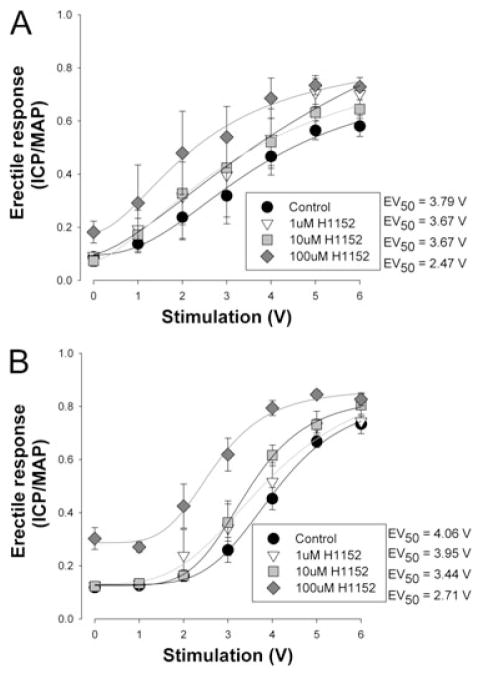
Inhibition of Rho-kinase by 1–100-μM H1152 (Sigma Aldrich, St. Louis, MO, USA) resulted in a dose-dependent elevation in the voltage-dependent erectile response in all treatment groups (Figures 2 and 3). The increase in the ICP/MAP ratio with 100 μM H1152 was greater in the obese (35%) than in the lean (5%) (see Figures 2A and 3A). The increase in maximal response was also associated with a leftward shift in the calculated EV50 (the voltage require to achieve 50% of the maximal response) reflecting increased sensitivity to the voltage stimulation as reported in Table 1. The treatment with rosuvastatin still resulted in dose-dependent improvements in the voltage-dependent erectile response. However, the magnitude of improvement was smaller in the obese (16%) and was reflected in the overall improvement in the ICP/MAP ratio seen in the obese-diabetic animals following the rosuvastatin treatment (Figure 3B).
Figure 2.
Dose-dependent effect of Rho-kinase inhibition on voltage-dependent erectile responses of lean Zucker rats following rosuvastatin treatment. Panel A represents the mean voltage-dependent erectile response in untreated lean Zuckers to increasing dosages of H1152 administrated intracavernously. Panel B represents the mean voltage-dependent erectile response in rosuvastatin-treated lean Zuckers to increasing dosages of H1152 administered intracavernously. Animals were administered saline (untreated) or 5 mg/kg rosuvastatin daily for 3 days by intra-peritoneal injection. The Rho-kinase inhibitor H1152 was delivered intracavernously 2 minutes prior to the testing of the voltage-dependent erectile response. Erectile function was assessed and cavernosal tissue was removed as described previously [26–28]. Reported are means ± standard error of the mean for four to six animals in each group. Lines and EV50 represent Hill fits to the means data. EV50 = voltage require to achieve 50% of the maximal response; ICP/MAP = intracavernosal blood pressure/mean arterial blood pressure.
Figure 3.
Dose-dependent effect of Rho-kinase inhibition on voltage-dependent erectile responses of obese-diabetic Zucker rats following rosuvastatin treatment. Panel A represents the mean voltage-dependent erectile response in untreated obese-diabetic Zuckers to increasing dosages of H1152 administrated intracavernously. Panel B represents the mean voltage-dependent erectile response in rosuvastatin treated obese-diabetic Zuckers to increasing dosages of H1152 administered intracavernously. Animals were administered saline (untreated) or 5 mg/kg rosuvastatin daily for 3 days by intraperitoneal injection. The Rho-kinase inhibitor H1152 was delivered intracavernously 2 minutes prior to the testing of the voltage-dependent erectile response. Erectile function was assessed and cavernosal tissue was removed as described previously [26–28]. Reported are means ± standard error of the mean for four to six animals in each group. Lines and EV50 represent Hill fits to the means data. EV50 = voltage require to achieve 50% of the maximal response; ICP/MAP = intracavernosal blood pressure/mean arterial blood pressure.
Table 1.
Mean erectile responses at 0 and 6 V stimulation and calculated EV50 for control or with Rho-kinase inhibition in lean and obese-diabetic Zucker rats
| Group | Control
|
100 μM H1152
|
N | ||||
|---|---|---|---|---|---|---|---|
| 0 V ICP/MAP | 6 V ICP/MAP | EV50 (V) | 0 V ICP/MAP | 6 V ICP/MAP | EV50 (V) | ||
| Lean | 0.095 ± 0.009 | 0.860 ± 0.032 | 2.59 ± 0.21 | 0.295 ± 0.083 | 0.901 ± 0.049 | 2.13 ± 0.26 | 4 |
| Obese | 0.090 ± 0.003 | 0.572 ± 0.015* | 4.25 ± 0.25* | 0.177 ± 0.044 | 0.775 ± 0.030*‡ | 2.88 ± 0.43‡ | 4 |
| Lean–rosuvastatin | 0.107 ± 0.019 | 0.775 ± 0.038† | 2.91 ± 0.24† | 0.154 ± 0.028 | 0.903 ± 0.020†‡ | 2.51 ± 0.23 | 6 |
| Obese–rosuvastatin | 0.126 ± 0.010 | 0.818 ± 0.052† | 3.95 ± 0.32* | 0.283 ± 0.034‡ | 0.845 ± 0.016 | 2.74 ± 0.31‡ | 6 |
Different from Lean in group.
Different from Obese in group.
Different from Control by analysis of variance with Holm–Sidak method.
EV50 = voltage require to achieve 50% of the maximal response; ICP/MAP = intracavernosal blood pressure/mean arterial blood pressure.
Expression of RhoA, Rho-Kinase in Lean and Obese-Diabetic Cavernosal Tissues
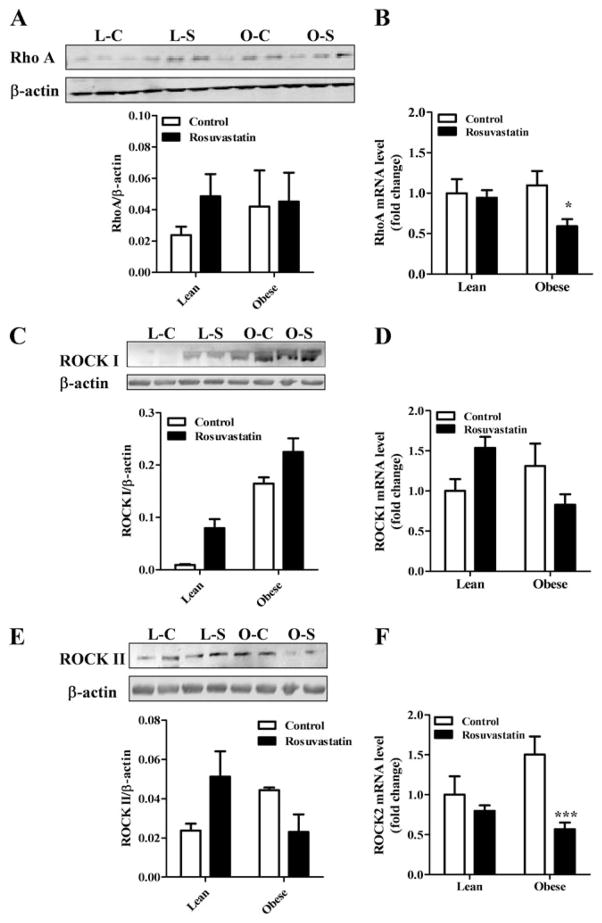
As Rho-kinase is presumed to have a significant role in the maintenance of the vasoconstricted state of the rat penile vasculature [26,27,30], we looked to see if any alteration in the expression of the RhoA or Rho-kinase isoforms were seen in the tissues from the obese-diabetic animals. As shown in Figure 4, RhoA protein expression showed a small nonsignificant elevation in obese-diabetic as compared with the leans, and the rosuvastatin treatment was without effect in the obese-diabetic tissue (Figure 4A). However, the protein expression levels for Rho-kinase isoforms showed a doubling of values in obese-diabetic tissues relative to leans and the rosuvastatin treatment resulted in nearly a 50% lowering of their elevated expression of ROCK II in the obese-diabetic tissues from their controls (Figure 4C, E). Statin treatment in leans animal showed an elevation of expression for RhoA ROCK I and II but did not achieve statistical significance.
Figure 4.
Protein and mRNA expression levels of RhoA ROCK I, and ROCK II in lean and obese-diabetic corpus cavernosal tissues following rosuvastatin or saline treatment. Representative protein blots and mean expression levels of RhoA (A), ROCK I (C), and ROCK II (E) in lean and obese-diabetic penile tissue as detected by western blot. Frozen corpus cavernosal tissue was homogenized in lysis buffer supplemented with protease and phosphatase inhibitor cocktails. Proteins were resolved by polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked with Tris-buffered saline containing 0.1% Tween 20 and 4% BSA. Incubation with specific primary antibodies (ROCK I and II from BD Transduction Labs [Franklin Lakes, NJ, USA], and RhoA from Cell Signaling Technology [Beverly, MA, USA]) and then incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulin G. Immunoreactive bands were visualized using ECL Plus and image acquisition was performed on a Typhoon 9410 workstation (Amersham Bioscience, Piscataway, NJ, USA) and analyzed using UN-SCAN-IT™ gel version 5.1. Reported are means ± standard error of the mean (SEM) for three animals in each group. Mean mRNA expression of RhoA (B), Rho-kinase I (D), and II (F) as detected by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) in lean and obese-diabetic corpus cavernosal tissues following rosuvastatin treatment. Total RNA was extracted from harvested cavernosal tissue using TriReagent (Sigma-Aldrich) and cDNA was generated using the High Capacity cDNA kit from Applied Biosystems (Foster City, CA, USA). Real-time RT-PCR was performed using TaqMan primers and probes mixes using glyceraldehyde-3-phosphate dehydrogenase as the reference gene on an iCycler iQ (Bio-Rad Laboratories, Hercules, CA, USA). Reported are means ± SEM for four to six animals in each group. Statistical difference from control within group *P < 0.05 or ***P < 0.001. L C = lean control; L S = lean statin; O C = obese control; O S = obese statin.
The mRNA expression levels for RhoA, ROCK I, and ROCK II in obese-diabetic corporal tissues when compared with the leans were not significantly different. The mRNA expression levels for RhoA and Rho kinase II were decreased by more than 50% in obese-diabetic corpus cavernosum when animals were treated for 3 days with rosuvastatin (Figure 4B, F). The expression of the other isoform of Rho kinase I was also reduced but was not statistically significant from the obese-diabetic nontreated condition (Figure 4D). The expression patterns in mRNA seen in the lean animals following the rosuvastatin treatment were without significant changes in RhoA and Rho kinase II, and a slight elevation in Rho kinase I.
Discussion
Our observations are the first to show that the voltage-dependent erectile response in the Zucker rat, a model of MSX, may be ameliorated by a short duration-statin therapy. Our data, although low in statistical power, support conclusions drawn from clinical and animal studies that show a positive effect of statin therapy on resolving ED. We report a depressed erectile response to voltage-dependent stimulation of the cavernosal nerve in the obese-diabetic ZDF rat that was improved following a 3-day, 5 mg/kg rosuvastatin treatment. The intracavernosal administration of the Rho-kinase inhibitor H-1152 provided an additional augmentation of voltage-dependent erectile response that was greater in the obese-diabetes after statin treatment than for the leans. We have previously shown that the obese-diabetic condition had increased expression of RhoA, and the Rho kinase and now report that 3 days of rosuvastatin may be capable of lowering Rho-kinase expression in the obese diabetics corpora cavernosum. The depressed erectile response seen in this obese-diabetic model may be associated with RhoA/Rho-kinase pathway increasing the vaso-constricted state, as well as the reduction in NO generation and the pleiotropic effects of statin treatment may influence the Rho signaling pathway to aid in resolving the ED.
Vascular Dynamics of Erectile Function
Penile erection occurs as a result of a change in the contractile state of the smooth muscle cells lining the cavernosal sinuses and arterioles that allow blood to enter the erectile tissue and becomes trapped. A variety of signaling agents have been implicated in the regulation of the cavernosal and arteriolar smooth muscle tone, and perturbation in either vasoconstriction or relaxation mechanisms can result in altered erectile responses [31]. Receptor-ligand based signaling is known to activate several pro-constrictor pathways that can regulate the Ca2+-sensitivity of smooth muscle contraction [32,33]. The regulation of the RhoA/Rho-kinase pathway, integral to Ca2+-sensitivity, also appears to rely on the actions of guanosine triphosphatase proteins and the associated post-translational modification by farnesyl or geranyl transferases [34]. The relevance of this may be born out in an apparent synergist effect of the Rho-kinase inhibition and statin treatment on the voltage-dependent erectile responses seen in the obese-diabetic animals and not in the leans (Table 1). The intracavernosal injection of 100-μM H1152 further lowered the calculated EV50 in the statin treated obese-diabetics but not in the statin treated leans. This response occurred when there was a modest reduction in the mRNA expression of Rho and ROCKS and reduction in ROCK II protein expression in obese-diabetics. These data would support a hypothesis that statins may contribute to improvement of the erectile response through a lowering of the vasoconstrictive actions of the RhoA/Rho kinase pathway.
The ZDF Rat as Model of Metabolic Syndrome for the Study of Erectile Function
MSX is a combination of disorders linking exacerbated non-insulin-dependent diabetes with obesity and hypertension [3,6]. A potential animal model for this syndrome is the Zucker obese-diabetic rat. The characteristics of this model are caused by a deficiency in the leptin receptor gene that in animals fed ad libitum, elevates food intake, leading to the development of obesity, type II diabetes mellitus, and hypertension [35]. Vascular complications are recognized in the Zucker rat model of MSX [27,36,37]. The erectile response in the penis is dependent on the reactive state of the smooth muscle of the cavernosal sinuses and of the arteries supplying blood to those sinuses. Recent work on understanding the signaling mechanism of vasoconstrictors in penile circulation and cavernosal smooth muscle have identified an important role for the RhoA/Rho-kinase signaling pathway, a pathway associated with Ca2+-sensitization [27,38,39] and is discussed in recent reviews [31,40–42].
Previously we have demonstrated in the obese-diabetic Zucker rat that there was depressed erectile response associated with a slight increase in responsiveness to both endothelin-1 and phenylephrine, as well as increased expression of proteins associated with the RhoA/Rho-kinase pathway [27]. In these studies we demonstrated that the isolated tissue responses to agonist-mediated constriction were exaggerated in the obese-diabetic whereas the relaxation by Rho-kinase inhibition was attenuated. We suggested that the model of metabolic syndrome in the Zucker rat had an impaired erectile response that was mediated in part through Rho/Rho-kinase signaling possibly linked to Ca2+ senitization of the corporal smooth muscle. Our data presented here are in agreement with our previous findings regarding the protein expression of RhoA and Rho kinase in cavernosal tissues [27]. Our data appear to show that in corpus cavernosum from the obese-diabetic animals there were small elevations in mRNA and protein expression level of the isoforms of Rho kinase as well as a tendency towards an increased expression level of RhoA in the obese-diabetic tissues. Our data might then allow one to suggest that short-term statin therapy may lower the mRNA and protein expression levels for Rho signaling in the corpus cavernosum of the obese-diabetic animals. If these changes prove robust they would favor the tumescent state and may underlie the improved erectile response we measured during the voltage stimulation following the statin administration.
Pleiotropic Effect of Statins
The literature contains numerous reports of the effectiveness of statin therapy for treating lipid disorders, and the subsequent or associated improvement in vascular response also observed. With in those reports are those that demonstrate the beneficial effects of a statin therapy on erectile function. Yet little is know about the underlying mechanism by which statin is providing this effect.
The 3-hydroxyl-3-methylglutayl (HMG-CoA) reductase inhibitors, also referred to as statins, are strong inhibitors of cholesterol synthesis. The beneficial effects of statins on vascular biology include mechanisms beyond the effect on serum cholesterol levels. These effects include: (i) an improved endothelial cell function via up regulation of eNOS expression and activity; (ii) a reduction in isoprenelation of RhoA leading to an accumulation of its inactive form in the cytoplasm; and (iii) a decreased ROS production via reduction in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunit assembly. Recent studies have identified an additional mechanism that include the inhibition of L-mevalonate synthesis, thus preventing the formation of geranylgeranylation and farnesol pyrophosphates. These products are both important elements in the posttranslation modification (lipidation) of the Rho-associated proteins as well as regulation of NOS enzymes [43–47].
The recent work by Fibbi et al. provides some critical insight as to how some of the actions of statin mentioned earlier might be acting when treating depressed erectile response in animal model by influencing Rho signaling in the cavernosal smooth muscle [48]. In their study, a 2-week treatment with atorvastatin improved the voltage-dependent erectile response of the spontaneously hypertensive rat when used in conjunction with sildenafil. The statin treatment lowered the mRNA expression of the RhoA and Rho kinase II in the rat tissue. Furthermore, their work on isolated human penile smooth muscle cells showed that atorvastatin treatment would prevent the membrane association of RhoA, an essential step in its activation. Based on the conclusion of their work and our findings presented here, in a different model of ED and statin, it is suggested that the statin affect maybe through lowering mRNA and protein expression of elements of Rho signaling. These results are in agreement with reports in the literature regarding the action of statins [43–45].
Additionally, endothelial NOS plays an important role in the erectile process [49,50] and statins have been shown to improve eNOS activity in other vascular beds [51]. Thus, the use of statins in the treatment of ED associated with MSX may have multiple benefits, by interfering with vaso-constrictor signaling while also improving NO signaling. The work of Nangle reported that rosuvastatin could reverse a large portion of the depressed nerve-mediated relaxation of cavernosal strips from a diabetic mouse model through an NO mechanism [52]. However, Fibbi did examine the NOS isoform mRNA expression in the corpora cavernosal from the SHR and WKY rat, and reported no difference between control WKY animals, SHR, or SHR treated with atrovastatin suggesting that the statin may be having a stronger effect on expression of Rho signaling elements rather than NOS [48]. The effect of short-duration statin therapy on the NOS signaling in the model of metabolic syndrome remains to be thoroughly investigated. Our choice concentration duration of day treatment was based on the positive effects reported in several articles utilizing a 3- to 7-day duration statin treatment in vivo in protecting cardiac, brain, and liver from perfusion injuries [53–55]. Although our study reports only a single dosage and brief duration of treatment, it revealed moderate beneficial effects. Because statin therapy is often longer duration the potential impact of longer treatments might strengthen the effects reported here and is the subject of ongoing experiments. Based on our current and previous work [27] and the work of Fibbi [48] and Nagle [52], we believe that the restoration in the erectile response seen in the obese-diabetic treated with statin was likely associated with changes in the RhoA/Rho-kinase signaling cascade.
Other mechanisms of action of statins still remain to be fully investigated in their ability to preserving or restoring erectile response in disease models. One alternative explanation for the effect of rosuvastatin can be one that is mediated through inhibition of the NADPH oxidase [44,45,56,57]. Although we observed an improved voltage-dependent erectile response these data suggest NO is still present and the statin treatment may be improving ROS levels and reducing NO scavenging [58–60]. Any improved NO production might then secondarily reduce RhoA expression through cyclic guanosine monophosphate-mediated signaling and contribute to an improvement in the erectile response [61] as also seen in the hypertensive animals with the addition of the phosphodiesterase type 5 inhibitor [48]. This is an interesting potential mechanism for the statin effect and one that has not yet been explored in MSX model.
Conclusions
This work in the Zucker rat is the first to investigate the pleiotropic action of rosuvastatin in a model of metabolic syndrome examining the voltage-dependent erectile response; it provides unique evidence that parallels clinical/epidemiological and animal model evidence for the beneficial effects of statins. Our data suggest that short-term statin therapy can reverse some of the pro-vasoconstriction conditions contributing to impaired voltage-dependent erectile responses in the rat model of MSX. The mechanisms by which statins may accomplish this include alterations in the expression of RhoA/Rho kinase and improved cavernosal relaxation with Rho-kinase inhibitors. These results support a hypothesis that statin therapy may be beneficial for preventing the augmented vasoconstricted state associated with diabetes and/or hypertension in MSX.
Acknowledgments
This work was supported in part by an ECU Research Development Award to CJW and Dean’s Summer fellowship to L. Wilkinson.
Footnotes
Conflict of Interest: None declared.
References
- 1.Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS. Diabetes trends in the U.S. : 1990–1998. Diabetes Care. 2000;23:1278–83. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Reilly MP, Rader DJ. The metabolic syndrome: More than the sum of its parts? Circulation. 2003;108:1546–51. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 4.Heaton JP, Adams MA. Causes of erectile dysfunction. Endocrine. 2004;23:119–23. doi: 10.1385/ENDO:23:2-3:119. [DOI] [PubMed] [Google Scholar]
- 5.Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol. 2004;171:2341–5. doi: 10.1097/01.ju.0000125198.32936.38. [DOI] [PubMed] [Google Scholar]
- 6.Shabbir M, Mikhailidis DM, Morgan RJ. Erectile dysfunction: An underdiagnosed condition associated with multiple risk factors. Curr Med Res Opin. 2004;20:603–6. doi: 10.1185/030079904125003458. [DOI] [PubMed] [Google Scholar]
- 7.Yeh HC, Wang CJ, Lee YC, Hsiao HL, Wu WJ, Chou YH, Huang CH. Association among metabolic syndrome, testosterone level and severity of erectile dysfunction. Kaohsiung J Med Sci. 2008;24:240–7. doi: 10.1016/S1607-551X(08)70148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corona G, Mannucci E, Petrone L, Schulman C, Balercia G, Fisher AD, Chiarini V, Forti G, Maggi M. A comparison of NCEP-ATPIII and IDF metabolic syndrome definitions with relation to metabolic syndrome-associated sexual dysfunction. J Sex Med. 2007;4:789–96. doi: 10.1111/j.1743-6109.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 9.Corona G, Mannucci E, Petrone L, Balercia G, Paggi F, Fisher AD, Lotti F, Chiarini V, Fedele D, Forti G, Maggi M. NCEP-ATPIII-defined metabolic syndrome, type 2 diabetes mellitus, and prevalence of hypogonadism in male patients with sexual dysfunction. J Sex Med. 2007;4:1038–45. doi: 10.1111/j.1743-6109.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AL. Metabolic syndrome, endothelial dysfunction, and erectile dysfunction: Association and management. Curr Urol Rep. 2005;6:470–5. doi: 10.1007/s11934-005-0043-0. [DOI] [PubMed] [Google Scholar]
- 11.Matfin G, Jawa A, Fonseca VA. Erectile dysfunction: Interrelationship with the metabolic syndrome. Curr Diab Rep. 2005;5:64–9. doi: 10.1007/s11892-005-0070-8. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca V, Jawa A. Endothelial and erectile dysfunction, diabetes mellitus, and the metabolic syndrome: Common pathways and treatments? Am J Cardiol. 2005;96:13M–18M. doi: 10.1016/j.amjcard.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Shabsigh R, Arver S, Channer KS, Eardley I, Fabbri A, Gooren L, Heufelder A, Jones H, Meryn S, Zitzmann M. The triad of erectile dysfunction, hypogonadism and the metabolic syndrome. Int J Clin Pract. 2008;62:791–8. doi: 10.1111/j.1742-1241.2008.01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billups KL, Bank AJ, Padma-Nathan H, Katz SD, Williams RA. Erectile dysfunction as a harbinger for increased cardiometabolic risk. Int J Impot Res. 2008;20:236–42. doi: 10.1038/sj.ijir.3901634. [DOI] [PubMed] [Google Scholar]
- 15.Miner M, Billups KL. Erectile dysfunction and dyslipidemia: Relevance and role of phosphodiesterase type-5 inhibitors and statins. J Sex Med. 2008;5:1066–78. doi: 10.1111/j.1743-6109.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 16.Kupelian V, Shabsigh R, Araujo AB, O’Donnell AB, McKinlay JB. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: Results from the Massachusetts male aging study. J Urol. 2006;176:222–6. doi: 10.1016/S0022-5347(06)00503-9. [DOI] [PubMed] [Google Scholar]
- 17.Demir T, Demir O, Kefi A, Comlekci A, Yesil S, Esen A. Prevalence of erectile dysfunction in patients with metabolic syndrome. Int J Urol. 2006;13:385–8. doi: 10.1111/j.1442-2042.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 18.Shabsigh R, Shah M, Sand M. Erectile dysfunction and men’s health: Developing a comorbidity risk calculator. J Sex Med. 2008;5:1237–43. doi: 10.1111/j.1743-6109.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM. Statin therapy for the treatment of diabetic dyslipidemia. Diabetes Metab Res Rev. 2003;19:280–7. doi: 10.1002/dmrr.393. [DOI] [PubMed] [Google Scholar]
- 20.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–13. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 21.Endres M, Laufs U. Effects of statins on endothelium and signaling mechanisms. Stroke. 2004;35(11 1 suppl):2708–11. doi: 10.1161/01.STR.0000143319.73503.38. [DOI] [PubMed] [Google Scholar]
- 22.Sessa WC. Can modulation of endothelial nitric oxide synthase explain the vasculoprotective actions of statins? Trends Mol Med. 2001;7:189–91. doi: 10.1016/s1471-4914(01)01985-2. [DOI] [PubMed] [Google Scholar]
- 23.Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23:729–36. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- 24.Kato T, Hashikabe H, Iwata C, Akimoto K, Hattori Y. Statin blocks Rho/Rho-kinase signalling and disrupts the actin cytoskeleton: Relationship to enhancement of LPS-mediated nitric oxide synthesis in vascular smooth muscle cells. Biochim Biophys Acta. 2004;1689:267–72. doi: 10.1016/j.bbadis.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Laufs U, Endres M, Custodis F, Gertz K, Nickenig G, Liao JK, Bohm M. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102:3104–10. doi: 10.1161/01.cir.102.25.3104. [DOI] [PubMed] [Google Scholar]
- 26.Wingard CJ, Johnson JA, Holmes A, Prikosh A. Improved erectile function following Rho-kinase inhibition in a rat castrate model of erectile dysfunction. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1572–9. doi: 10.1152/ajpregu.00041.2003. [DOI] [PubMed] [Google Scholar]
- 27.Wingard C, Fulton D, Husain S. Altered penile vascular reactivity and erection in the Zucker obese-diabetic rat. J Sex Med. 2007;4:348–62. doi: 10.1111/j.1743-6109.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 28.Husain S, Young D, Wingard CJ. Role of PKCalpha and PKC iota in phenylephrine-induced contraction of rat corpora cavernosa. Int J Impot Res. 2004;16:325–33. doi: 10.1038/sj.ijir.3901164. [DOI] [PubMed] [Google Scholar]
- 29.Mills TM, Chitaley K, Wingard CJ, Lewis RW, Webb RC. Effect of Rho-kinase inhibition on vaso-constriction in the penile circulation. J Appl Physiol. 2001;91:1269–73. doi: 10.1152/jappl.2001.91.3.1269. [DOI] [PubMed] [Google Scholar]
- 30.Mills TM, Lewis RW, Wingard CJ, Linder AE, Jin L, Webb RC. Vasoconstriction RhoA/Rho-kinase and the erectile response. Int J Impot Res. 2003;15(5 suppl):S20–4. doi: 10.1038/sj.ijir.3901068. [DOI] [PubMed] [Google Scholar]
- 31.Saenz dTI, Angulo J, Cellek S, Gonzalez-Cadavid N, Heaton J, Pickard R, Simonsen U. Pathophysiology of erectile dysfunction. J Sex Med. 2005;2:26–39. doi: 10.1111/j.1743-6109.2005.20103.x. [DOI] [PubMed] [Google Scholar]
- 32.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II. Modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 33.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 34.Chardin PGT. Pase regulation: Getting aRnd rock and rho inhibition. Curr Biol. 2003;13:R702–4. doi: 10.1016/j.cub.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 35.Frisbee JC. Remodeling of the skeletal muscle microcirculation increases resistance to perfusion in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2003;285:H104–11. doi: 10.1152/ajpheart.00118.2003. [DOI] [PubMed] [Google Scholar]
- 36.Frisbee JC. Enhanced arteriolar alpha-adrenergic constriction impairs dilator responses and skeletal muscle perfusion in obese Zucker rats. J Appl Physiol. 2004;97:764–72. doi: 10.1152/japplphysiol.01216.2003. [DOI] [PubMed] [Google Scholar]
- 37.Oltman CL, Davidson EP, Coppey LJ, Klein-schmidt TL, Lund DD, Yorek MA. Attenuation of vascular/neural dysfunction in Zucker rats treated with enalapril or rosuvastatin. Obesity (Silver Spring) 2008;16:82–9. doi: 10.1038/oby.2007.19. [DOI] [PubMed] [Google Scholar]
- 38.Vignozzi L, Morelli A, Filippi S, Ambrosini S, Mancina R, Luconi M, Mungai S, Vannelli GB, Zhang XH, Forti G, Maggi M. Testosterone regulates RhoA/Rho-kinase signaling in two distinct animal models of chemical diabetes. J Sex Med. 2007;4:620–30. doi: 10.1111/j.1743-6109.2007.00440.x. [DOI] [PubMed] [Google Scholar]
- 39.Luttrell IP, Swee M, Starcher B, Parks WC, Chitaley K. Erectile dysfunction in the type II diabetic db/db mouse: Impaired venoocclusion with altered cavernosal vasoreactivity and matrix. Am J Physiol Heart Circ Physiol. 2008;294:H2204–11. doi: 10.1152/ajpheart.00027.2008. [DOI] [PubMed] [Google Scholar]
- 40.Jin L, Burnett AL. RhoA/Rho-kinase in erectile tissue: Mechanisms of disease and therapeutic insights. Clin Sci (Lond) 2006;110:153–65. doi: 10.1042/CS20050255. [DOI] [PubMed] [Google Scholar]
- 41.Christ G, Wingard C. Calcium sensitization as a pharmacological target in vascular smooth-muscle regulation. Curr Opin Investig Drugs. 2005;6:920–33. [PubMed] [Google Scholar]
- 42.Gonzalez-Cadavid NF, Rajfer J. Therapy of erectile dysfunction: Potential future treatments. Endocrine. 2004;23:167–76. doi: 10.1385/ENDO:23:2-3:167. [DOI] [PubMed] [Google Scholar]
- 43.Mason JC. Statins and their role in vascular protection. Clin Sci (Lond) 2003;105:251–66. doi: 10.1042/CS20030148. [DOI] [PubMed] [Google Scholar]
- 44.Mason RP. Molecular basis of differences among statins and a comparison with antioxidant vitamins. Am J Cardiol. 2006;98:4P–41P. doi: 10.1016/j.amjcard.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Mason RP, Walter MF, Day CA, Jacob RF. Intermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors contribute to distinct pharmacologic and pleiotropic actions. Am J Cardiol. 2005;96:11F–23F. doi: 10.1016/j.amjcard.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 47.Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–5. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 48.Fibbi B, Morelli A, Marini M, Zhang XH, Mancina R, Vignozzi L, Filippi S, Chavalmane A, Silvestrini E, Colli E, Adorini L, Vannelli GB, Maggi M. Atorvastatin but not elocalcitol increases sildenafil responsiveness in spontaneously hypertensive rats by regulating the RhoA/ROCK pathway. J Androl. 2008;29:70–84. doi: 10.2164/jandrol.107.003152. [DOI] [PubMed] [Google Scholar]
- 49.Burnett AL. Role of nitric oxide in the physiology of erection. Biol Reprod. 1995;52:485–9. doi: 10.1095/biolreprod52.3.485. [DOI] [PubMed] [Google Scholar]
- 50.Burnett AL. Novel nitric oxide signaling mechanisms regulate the erectile response. Int J Impot Res. 2004;16(1 suppl):S15–9. doi: 10.1038/sj.ijir.3901209. [DOI] [PubMed] [Google Scholar]
- 51.Rikitake Y, Liao JK. ROCKs as therapeutic targets in cardiovascular diseases. Expert Rev Cardiovasc Ther. 2005;3:441–51. doi: 10.1586/14779072.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nangle MR, Cotter MA, Cameron NE. Effects of rosuvastatin on nitric oxide-dependent function in aorta and corpus cavernosum of diabetic mice: Relationship to cholesterol biosynthesis pathway inhibition and lipid lowering. Diabetes. 2003;52:2396–402. doi: 10.2337/diabetes.52.9.2396. [DOI] [PubMed] [Google Scholar]
- 53.Jones SP, Gibson MF, Rimmer DM, III, Gibson TM, Sharp BR, Lefer DJ. Direct vascular and cardioprotective effects of rosuvastatin, a new HMG-CoA reductase inhibitor. J Am Coll Cardiol. 2002;40:1172–8. doi: 10.1016/s0735-1097(02)02115-0. [DOI] [PubMed] [Google Scholar]
- 54.Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1998;95:8880–5. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, Nevens F, Sauerbruch T, Heller J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–53. doi: 10.1002/hep.21673. [DOI] [PubMed] [Google Scholar]
- 56.Erdos B, Snipes JA, Tulbert CD, Katakam P, Miller AW, Busija DW. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am J Physiol Heart Circ Physiol. 2006;290:H1264–70. doi: 10.1152/ajpheart.00804.2005. [DOI] [PubMed] [Google Scholar]
- 57.Shinozaki K, Nishio Y, Ayajiki K, Yoshida Y, Masada M, Kashiwagi A, Okamura T. Pitavastatin restores vascular dysfunction in insulin-resistant state by inhibiting NAD(P)H oxidase activity and uncoupled endothelial nitric oxide synthase-dependent superoxide production. J Cardiovasc Pharmacol. 2007;49:122–30. doi: 10.1097/FJC.0b013e31802f5895. [DOI] [PubMed] [Google Scholar]
- 58.Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J, Thum T, Bauersachs J, Ertl G, Zou MH, Forstermann U, Munzel T. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198:65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol. 2008;295:L575–83. doi: 10.1152/ajplung.00428.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sicard P, Acar N, Gregoire S, Lauzier B, Bron AM, Creuzot-Garcher C, Bretillon L, Vergely C, Rochette L. Influence of rosuvastatin on the NAD(P)H oxidase activity in the retina and electroretino-graphic response of spontaneously hypertensive rats. Br J Pharmacol. 2007;151:979–86. doi: 10.1038/sj.bjp.0707322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chitaley K, Wingard CJ, Clinton W, Branam H, Stopper VS, Lewis RW, Mills TM. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7:119–22. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]