Abstract
Introduction
It is suggested that erectile dysfunction (ED) may be an early risk factor for cardiovascular disease.
Aim
The goal of this study was to determine whether development of ED precedes the onset of coronary artery endothelial dysfunction in response to a Western diet (WD), thereby establishing whether the WD differentially impacts the endothelium in a time-dependent manner. Additionally, a goal was to determine if diet-induced ED is reversible with intracavernosal sepiapterin treatment.
Methods
Male Sprague-Dawley rats were fed a WD for 4, 8, or 12 weeks, or a control diet for 8 weeks. Erectile function was evaluated by measuring the mean arterial pressure (MAP) and intracavernosal pressure (ICP) in response to electrical field stimulation of the cavernosal nerve near the major pelvic ganglion, in the absence and presence of sepiapterin. Coronary artery endothelial function was evaluated ex vivo with cumulative doses of acetylcholine (ACh) applied to segments of the left anterior descending coronary artery preconstricted with serotonin.
Main Outcome Measures
Erectile function was assessed as the ICP response to electrical field stimulation (EFS), normalized to MAP. Coronary artery endothelial function was assessed as the effective concentration producing 50% of a maximal response (EC50) of the ACh response.
Results
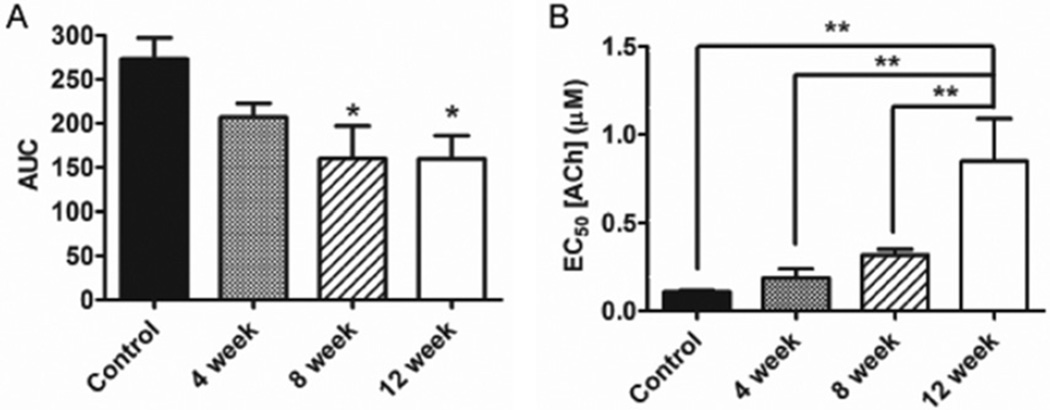
The ICP/MAP response to EFS was significantly attenuated following both 8 and 12 weeks of the WD compared with the control diet (P < 0.05). Sepiapterin treatment augmented the ICP/MAP response in all WD groups (P < 0.05). The coronary artery EC50 of the ACh response was not different from control following 4 or 8 weeks but was significantly elevated following 12 weeks of the WD (P < 0.01).
Conclusions
These data suggest that erectile function is reduced prior to coronary artery endothelial function in response to the WD. Improvement of erectile function with sepiapterin in WD rats indicates that nitric oxide synthase uncoupling is a key mechanism in diet-induced ED.
Keywords: High-Fat High-Sucrose Diet, Coronary Endothelial Function, Erectile Function, Sepiapterin, NOS Uncoupling
Introduction
The prevalence of obesity in the United States has risen from 19% in 1997 to over 33% in 2008 [1]. Epidemiological studies indicate that obesity is a significant, independent risk factor for erectile dysfunction (ED) [2–4]. Reduction in erectile function may be an early indication of cardiovascular risk as patients with ED are twice as likely to develop major adverse cardiac events compared with those without ED [5]. Recognition of reduced erectile function may be an important indicator of future adverse cardiac events, as clinical trial data suggest that the presence of ED in otherwise healthy men may be associated with early, subclinical signs of coronary artery disease (CAD) that may not be detectable during stress testing [6–9]. The time interval between the onset of ED symptoms and the occurrence of CAD symptoms is estimated at 2–3 years and an estimated 3–5 years between onset of ED and a cardiovascular event (myocardial infarction or stroke) [10–12]. Additionally, multiple regression analysis indicates that ED is the most efficient predictor of silent CAD in apparently uncomplicated type 2 diabetic patients among a host of traditional cardiovascular disease (CVD) risk factors [13]. However, differential development patterns of ED and CAD have not previously been investigated in a rodent model.
Electrical field stimulation (EFS) of the major pelvic ganglion (MPG) or cavernosal nerve in rodents provides an in vivo method in which to evaluate erectile function. This method produces information on the quality of erections assessed as an increase in intracavernosal pressure (ICP) relative to mean arterial pressure (MAP) [14]. A reduction in erectile response to EFS has been observed in several rodent models of the metabolic syndrome, including type 1 diabetic models induced by streptozotocin [15,16] and alloxan [17], obesediabetic Zucker rats [18], Goto-Kakizaki diabetic rats [19], as well as LDLR−/− mice [20] and ApoE−/− mice fed a hypercholesterolemic diet [21].
Nitric oxide (NO) is a major mediator of penile erection [22]. A critical requirement for normal NO synthase (NOS) functioning is an adequate presence of the essential cofactor tetrahydrobiopterin (BH4) [23]. Under conditions of elevated oxidative stress, cellular BH4 is depleted, which destabilizes NOS and induces a state of “uncoupling,” where NOS produces a proportionally higher amount of superoxide and lower amount of NO [23]. Administration of sepiapterin stimulates intracellular BH4 production [24,25], providing a potential pharmacological means of reversing NOS uncoupling.
In rodent models, diets rich in saturated fat and sucrose have been used to induce aortic endothelial dysfunction [26,27]. In human populations, the Western diet (WD) has been associated with inflammation and presence of vascular adhesion molecules [28], whereas adipose tissue concentrations of linoleic acid have been positively associated with CAD development [29]. There has been motivation to replace some of the saturated fat in the American diet with polyunsaturated fatty acids (PUFA), resulting in mass consumption of vegetable oils that are rich in linoleic acid [30]. Thus, the purpose of this study was to investigate the time course of development of ED relative to the development of coronary artery endothelial dysfunction in rats fed a WD that is high in saturated fat and omega-6 (n-6) PUFA derived from linoleic acid as well as simple sugars. Additionally, we sought to determine the reversibility of ED with acute, intracavernosal administration of sepiapterin, a BH4 precursor.
Materials and Methods
Experimental Animals and Diets
Male Sprague-Dawley rats were purchased at 5, 9, or 13 weeks of age (Charles River Laboratories, Wilmington, MA, USA) and housed in the Department of Comparative Medicine, in pairs when possible, in a temperature-controlled room (22 ± 1°C) with a 12 h:12 h light–dark cycle. Rats were fed a WD (Teklad Diets 110365, Harlan Laboratories, Madison, WI, USA) for the final 4 (N = 8), 8 (N = 8), or 12 (N = 5) weeks of life, or a control diet (Teklad Diets 110367, Harlan Laboratories) for the final 8 (N = 5) weeks of life. The WD was designed to be high in fat and sucrose, with an equal proportion of saturated fatty acids (SFA) and PUFA, with a high proportion of n-6 PUFA derived from linoleic acid. The control diet was designed to be a normal-fat, normal-carbohydrate diet with equivalent levels of vitamins, minerals, and protein to the WD when considered on a basis of kcal density (Tables 1 and 2). All rats were sacrificed at 19–20 weeks of age. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals established by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee.
Table 1.
Composition of the Western diet and control diet
| Diet content | Western diet g/kg |
Control diet g/kg |
|---|---|---|
| Casein | 195 | 155 |
| L-Cystine | 3 | 2.3 |
| Sucrose | 340 | 150 |
| Corn starch | 62.46 | 445.19 |
| Maltodextrin | 60 | 100 |
| Anhydrous Milk fat | 120 | 26 |
| Soybean oil | 30 | 18 |
| Safflower oil | 80 | 6 |
| Cellulose | 50 | 50 |
| Mineral mix, AIN-93M-MX | 44 | 35 |
| Vitamin mix, AIN-76A | 12.5 | 10 |
| Choline bitartrate | 3 | 2.5 |
| TBHQ | 0.04 | 0.01 |
TBHQ = tert-butylhydroquinone
Table 2.
Macronutrient composition of the Western and control diets
| Body composition | Western diet | Control diet |
|---|---|---|
| kcal/g | 4.68 | 3.67 |
| Fat, % by weight | 23.2 | 5.2 |
| Carbohydrate, % by weight | 47.6 | 66.3 |
| Protein, % by weight | 17.3 | 13.7 |
| Fat, % of kcal | 44.6 | 12.7 |
| Carbohydrate, % of kcal | 40.7 | 72.4 |
| Protein, % of kcal | 14.8 | 15 |
| SFA, % of total fatty acids | 39 | 40 |
| MUFA, % of total fatty acids | 24 | 26 |
| PUFA, % of total fatty acids | 37 | 33 |
| C18:2 linoleic, % by weight | 8.2 | 1.5 |
| C18:3 linolenic, % by weight | 0.3 | 1.57 |
| n-6 to n-3 | 27 | 9.6 |
| Vitamin E, IU/kg | 62 | 50 |
MUFA = mono-unsaturated fatty acid; PUFA = poly-unsaturated fatty acid; SFA = saturated fatty acid
Glucose, Insulin, and Lipid Profiles
All rats were fasted overnight prior to body composition studies. Approximately 0.3 mL of blood was drawn from the tail vein prior to anesthesia, from which fasting glucose concentration was measured from whole blood (Accu-Check, Roche, Basel, Switzerland). Blood was centrifuged for 20 minutes (4°C, 14,000 rpm), and plasma was separated and frozen in a −80°C freezer until analyzed for insulin concentration with a rat/mouse insulin assay kit (Millipore, Billerica, MA, USA). The homeostatic model of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin concentrations as HOMA-IR = (Insulin [mU/L] * Glucose [mmol/L])/22.5 [31]. Approximately 1.5 mL of blood was drawn through a splitter in the polyethylene (PE) tubing upon placement and stabilization of the carotid cannula, at the onset of the erection assessment surgery, subsequently centrifuged and serum was separated and frozen until analyzed. Serum total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides were measured with a clinical mass analyzer (UniCel DxC 600, Beckman Coulter, Indianapolis, IN, USA).
4-Hydroxynonenal (HNE) Adducts
The relative amount of serum HNE adducts was determined by a modified enzyme-linked immunosorbant assay (ELISA) approach. A standard curve of HNE adducts was established by incubating HNE with predetermined concentrations of bovine serum albumin (BSA). Following an overnight incubation at 37°C, HNE–BSA adducts were added to an Immunolon-coated 96-well assay plate (ThermoScientific, Rochester, NY, USA), along with diluted serum (1:40 in PBS). Samples were incubated overnight at 4°C and subsequently washed with PBS+0.05% Tween-20 and blocked for 2 hours with NB4025 (NOF Corp., Tokyo, Japan). Samples were then incubated with anti-HNE antibody (1 µg/mL in PBS, Oxis Research, Beverly Hills, CA, USA) for 2 hours at 37°C. Samples were washed with PBS+0.05% Tween-20 and incubated with secondary antibody for 2 hours at 27°C (goat anti-mouse HRP, Bio-Rad, Hercules, CA, USA). Following this incubation, samples were washed as before and incubated with TMBZ for 20 minutes. Reactions were quenched with 1M sulfuric acid, and the absorbance of the samples at 450 nm was determined.
Anthropometrics and Body Composition
Body weight was measured prior to surgery with a triple beam balance. Lean mass and fat mass were measured immediately prior to surgery by nuclear magnetic resonance-magnetic resonance imaging (EchoMRI 700, Echo Medical Systems, Houston, TX. USA). Body fat percentage was calculated as (massfat/(masslean + massfat)).
Erectile Response Measurements
All rats were fasted overnight prior to erectile function studies. Rats were anesthetized with an intra-peritoneal injection of 90 mg/kg ketamine and 10 mg/kg xylazine and supplemented with an intramuscular injection as needed. The left carotid artery was cannulated with a 20-gauge needle connected via PE tubing to a pressure transducer, anti-coagulated with 2.0 U/mL heparinized saline allowing for continuous measurement of systemic blood pressure and intra-arterial administration of saline. The shaft of the penis was freed of skin and fascia and the right corpus cavernosum cannulated with a 27-gauge needle connected via PE tubing to a pressure transducer, and the left corpus cavernosum cannulated with a 27-gauge needle connected via PE tubing to a syringe containing heparinized saline. The abdominal cavity was opened, and the right MPG was exposed. Platinum bipolar electrodes attached to a Grass Stimulator (Grass S09, Grass Technologies, West Warwick, RI, USA) were positioned near the MPG on the cavernosal nerve to deliver EFS, consisting of a series of 1–5 volts for 30 seconds at each voltage, delivered in 5-millisecond pulses at a frequency of 13 Hz [18]. MAP and ICP were recorded throughout each voltage series with LabChart 7 software (ADInstruments, Sydney, Australia). Three-voltage series were conducted and averaged as the erectile response. ICP returned to baseline between each series. Following the three-voltage series, 12 µL of 10 µM sepiapterin (Cayman Chemical, Ann Arbor, MI, USA) dissolved in heparinized saline was injected into the left corpus cavernosum. 10 µM sepiapterin has previously been shown to augment endothelial function in aortas of ApoE−/− mice [32]. A voltage series was conducted 30 minutes postinjection where treatment effects were maximal.
Coronary Artery Endothelium-Dependent and Endothelium-Independent Vasodilation
Immediately following the erection surgery, rats were euthanized by exsanguination and double pneumothorax. Hearts were excised, and four segments (0.3–0.8 mm) of the left anterior descending coronary artery were carefully dissected and freed from adhering myocardium. Two, 40-µm diameter, stainless steel wires were passed through each arterial segment and mounted in a multiwire myograph (DMT 620 M, Aarhus, Denmark). Vessel segments equilibrated for 45 minutes bathed in physiological saline solution (PSS) at pH 7.4, 37°C, gassed with medical grade air as described [18]. The relationship between passive wall tension and internal circumference was determined for each segment, from which the internal circumference (L100) corresponding to a transmural pressure of 100 mm Hg for a relaxed vessel in situ was calculated. Vessels were set to an internal circumference equal to 0.9 times L100, at which tension development is maximal in these arteries [33]. Vessels were allowed to equilibrate for another 45 minutes, and then challenged with 109 mM K+ PSS to test vessel viability. Vessels were depolarized for 10 minutes and relaxed with repeated washes of PSS at 10-minute intervals. Vessels were preconstricted with 3.0 µM 5-hydroxytryptamine (5-HT), and endothelial function was tested with cumulative doses of acetylcholine (ACh) (0.001–10.0 µM). Vessels were washed for 30 minutes with repeated washes of PSS at 10-minute intervals, and endothelium-independent relaxation was tested with cumulative doses of sodium nitroprusside (SNP) (0.001–1.0 µM) following 5-HT preconstriction.
Data and Statistical Analysis
The erectile response was calculated from the ratio of the average ICP divided by the average MAP during the final 25 seconds of each 30-second voltage stimulation level. The area-under-the-curve (AUC) of all voltages for each voltage series was calculated by GraphPad Prism (Prism 5.0, GraphPad, San Diego, CA, USA). Coronary artery endothelium-dependent and endothelium-independent vasodilation was assessed by the average stress produced by each vessel in the final 30 seconds of each dose. Vessel stress was calculated by subtracting basal vessel force produced from the force produced at each respective concentration and normalized to vessel surface area. The percent relaxation of 5-HT induced constriction at each concentration was calculated as: ((1 − (stressconcentration/stress5-HT)) * 100). The relaxation responses from the four vessel segments were averaged for each rat and analyzed as a single observation. Concentration-response curves were generated against the logarithm of each concentration, and fit by nonlinear regression to generate EC50 and Hill-slope values to describe each curve (GraphPad Prism v. 5.0). Data were presented as mean ± standard error of the mean. Statistical analyses were performed with GraphPad Prism v. 5.0 or SPSS v. 19.0 (IBM, Armonk, NY, USA), with an alpha level of 0.05. Statistical differences were determined by one-way analysis of variance (anova) with Tukey’s multiple comparisons post hoc analysis or two-way repeated measures anova with Bonferroni’s post hoc analysis where appropriate. Trends were determined by one-way anova with a linear trend posttest in GraphPad Prism v. 5.0.
Results
Metabolic Parameters
There were no statistically significant increases in body weight or composition in response to the WD (Table 3). Fasting blood glucose was significantly elevated in rats fed the WD for 12 weeks compared with all other groups. Despite increased glucose, no significant differences existed for insulin or HOMA-IR between groups (Table 3). There were no apparent differences in serum lipid profiles between the control diet and WD groups (Table 3). There was a trend for increasing serum HNE adducts with increasing diet duration (P = 0.028), suggesting increased lipid peroxidation and systemic oxidative stress in the 8-week and 12-week WD-fed rats (Table 3).
Table 3.
Metabolic parameters
| Parameter | Control | 4 weeks | 8 weeks | 12 weeks | anova P value |
|---|---|---|---|---|---|
| Body weight (g) | 443 ± 12.2 | 478 ± 8.0 | 453 ± 13.6 | 489 ± 12.8 | 0.059 |
| Fat mass (g) | 50.0 ± 1.59 | 56.5 ± 6.0 | 67.8 ± 10.9 | 73.4 ± 4.2 | 0.226 |
| Lean mass (g) | 329 ± 10.8 | 355 ± 2.9 | 326 ± 7.8† | 350 ± 10.3 | 0.018 |
| Body fat % | 13.2 ± 0.47 | 13.6 ± 1.25 | 19.9 ± 2.10 | 17.3 ± 0.94 | 0.241 |
| Glucose (mg/dL) | 115 ± 2.9 | 105 ± 4.8 | 110 ± 3.3 | 131 ± 3.5*,†,‡ | 0.001 |
| Insulin (pmol/L) | 126.7 ± 33.4 | 87.1 ± 15.0 | 159.8 ± 30.4 | 155.8 ± 14.0 | 0.320 |
| HOMA-IR | 5.06 ± 1.36 | 3.19 ± 0.66 | 6.03 ± 1.09 | 7.02 ± 0.62 | 0.207 |
| Triglycerides (mg/dL) | 52.3 ± 14.9 | 45.9 ± 14.1 | 38.8 ± 6.95 | 28.8 ± 6.9 | 0.597 |
| Cholesterol (mg/dL) | 32.3 ± 3.47 | 27.9 ± 2.96 | 38.8 ± 2.76† | 31.4 ± 1.72 | 0.059 |
| HDL-C (mg/dL) | 22.4 ± 2.78 | 21.6 ± 1.61 | 30.9 ± 2.63† | 24.9 ± 1.40 | 0.020 |
| LDL-C (mg/dL) | 7.0 ± 0.45 | 7.7 ± 0.86 | 8.5 ± 0.24 | 8.7 ± 0.55 | 0.202 |
| HNE-adducts | 0.695 ± 0.09 | 0.752 ± 0.23 | 0.976 ± 0.14 | 1.20 ± 0.15 | 0.095 |
Values are means ± SEM, N = 3–8 animals per group.
P < 0.05 vs. Control,
P < 0.05 vs. 4 weeks,
‡P < 0.05 vs. 8 weeks.
HOMA-IR = homeostatic model of insulin resistance; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; HNE = 4-hydroxynonenal
Voltage-Dependent Erectile Response
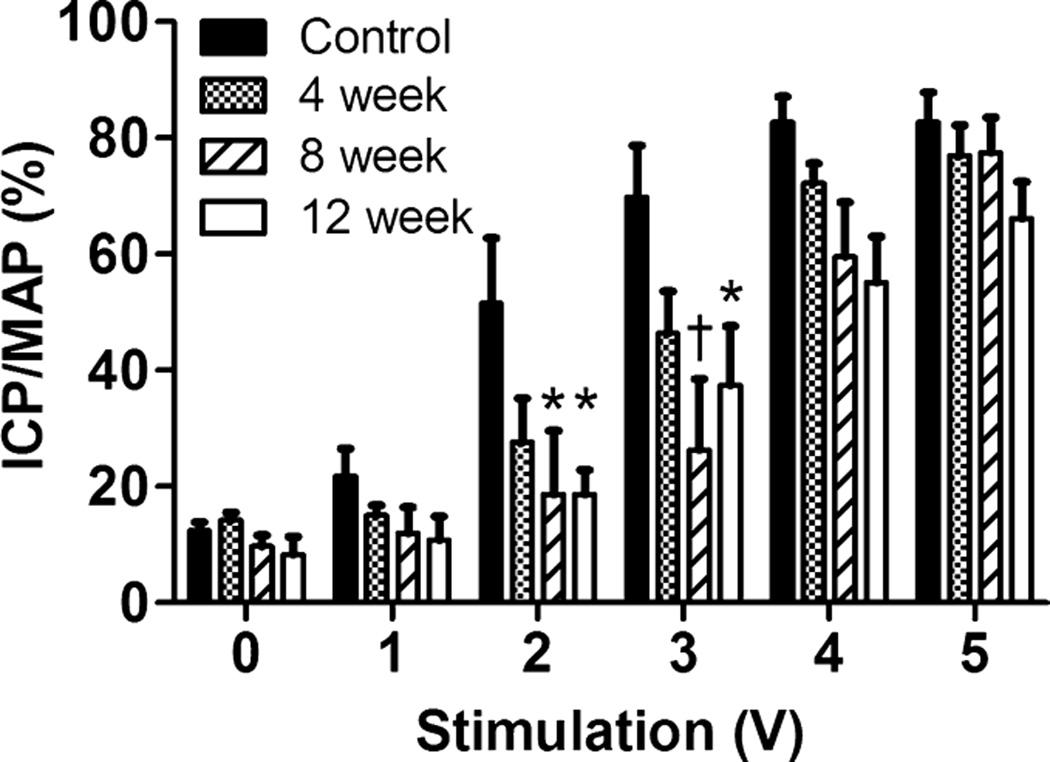
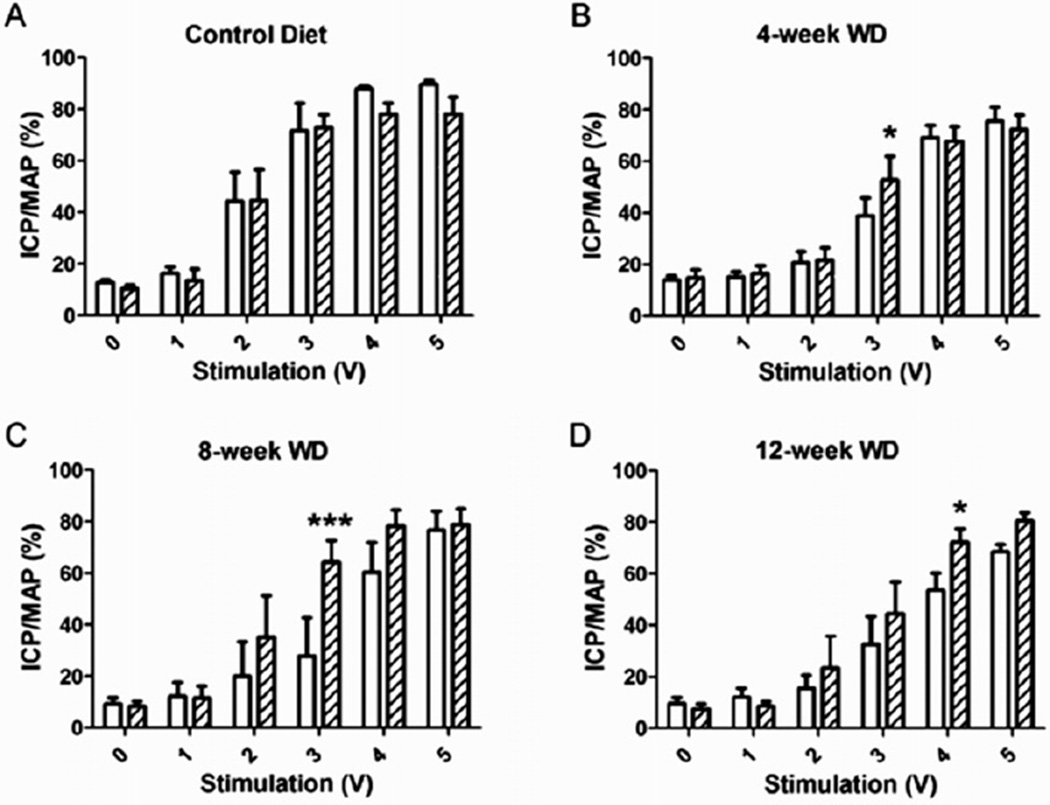
Erectile function was assessed by measuring the AUC of the voltage-dependent erectile response, suggestive of the ability to achieve and maintain an erection upon EFS. The AUC was significantly depressed following 8 weeks of the WD and remained similarly depressed following 12 weeks of the WD (Figure 1A). The ICP/MAP in response to the intermediate voltages (2V and 3V) of stimulation were significantly attenuated following 8 and 12 weeks of the WD (Figure 2). Intracavernosal treatment with sepiapterin had no effect on the voltage-dependent erectile response of control diet rats, but it significantly augmented the erectile response of all groups of WD-fed rats (Figure 3). The ICP/MAP following sepiapterin treatment was increased in response to 3V of stimulation in 4-week (Figure 3B) and 8-week (Figure 3C) WD-fed rats and in response to 4V of stimulation in 12-week WD-fed rats (Figure 3D).
Figure 1.
Rats developed erectile dysfunction prior to coronary artery endothelial dysfunction in response to a Western diet. (A) The area under the curve (AUC) of all voltages of the voltage-dependent erectile response was attenuated 8 weeks into the Western diet. (B) ACh-stimulated vasodilation of the coronary artery was attenuated 12 weeks following initiation of the Western diet (elevated EC50). *P < 0.05 vs. Control group, **P < 0.01. Reported are means ± SEM for 5–7 animals in each group. ACh = acetylcholine; SEM = standard error of the mean; EC50 = effective concentration producing 50% of a maximal response.
Figure 2.
Voltage-dependent erectile response following control diet or Western diet for 4, 8, or 12 weeks. ICP/MAP was reduced at 2 and 3 volts in the 8-week (hatched bars) and 12-week (open bars) Western diet-fed rats compared with control diet-fed rats (black bars). Reported are means ± SEM for 5–7 animals in each group; *P < 0.05, †P < 0.01 vs. Control. ICP = intracavernosal pressure; MAP = mean arterial pressure; SEM = standard error of the mean.
Figure 3.
Effect of sepiapterin on voltage-dependent erectile response. Following the untreated voltage series (open bars), 10 µM sepiapterin was injected intracavernosally, and a voltage series was applied 30-minutes postinjection (hatched bars). No treatment effects were observed in rats fed the control diet (A), whereas rats fed the WD for 4 weeks (B), 8 weeks (C), and 12 weeks (D) demonstrated an enhanced erectile response with sepiapterin treatment. *P < 0.05, ***P < 0.001. Reported are means ± SEM for 4–6 animals in each group. ICP = intracavernosal pressure; MAP = mean arterial pressure; SEM = standard error of the mean; WD = Western diet.
Coronary Artery Endothelium-Dependent and Endothelium-Independent Relaxation
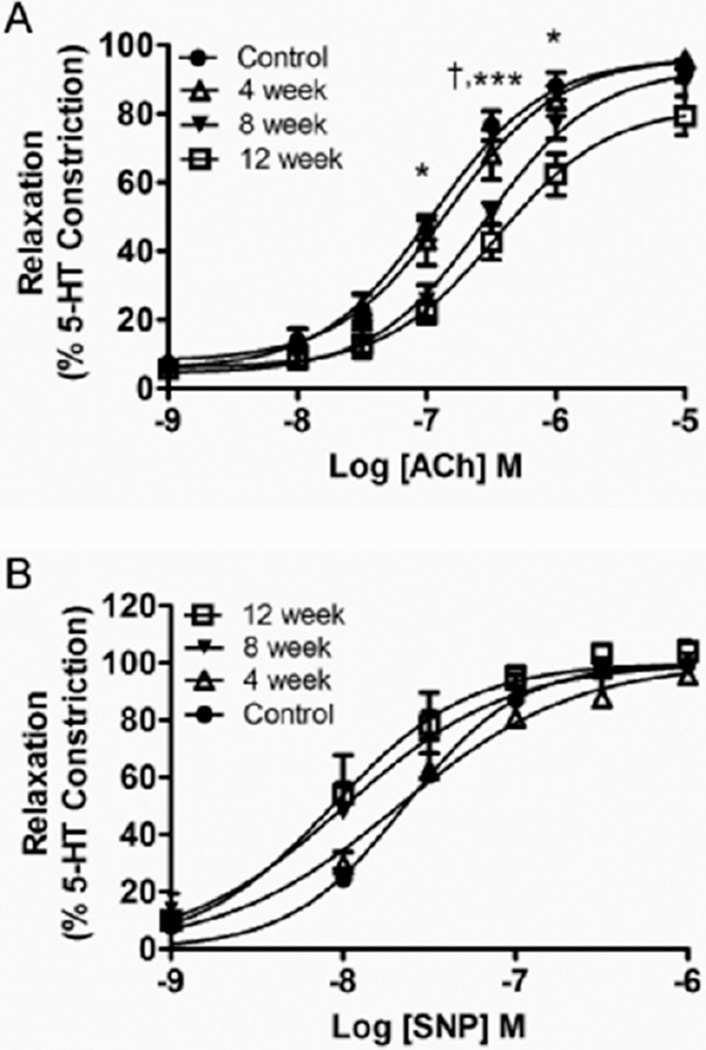
Coronary artery endothelial function was assessed from the concentration-response to the endothelium-dependent agonist ACh. The effective concentration producing 50% of a maximal relaxation response (EC50) following preconstriction was used to assess sensitivity to ACh, and thus the ability of the endothelium to produce NO. The EC50 of the ACh response was significantly elevated in 12-week WD-fed rats compared with all other groups (Figure 1B). The ACh-induced relaxation curves are plotted in Figure 4A, where the relaxation profile was significantly attenuated at a single concentration for the 8-week WD rats and at three concentrations for the 12-week WD rats when compared with the relaxation profiles of control diet rats. There were no significant differences in the Hill-slope for the ACh response (Control: 1.01 ± 0.23; 4 weeks: 0.96 ± 0.16; 8 weeks: 1.01 ± 0.21; 12 weeks: 0.64 ± 0.09; P = 0.522). Coronary artery endothelium-independent relaxation was assessed from the concentration-response profile to the NO donor SNP. There were no significant differences between groups for the relaxation response to SNP (Figure 4B), indicating no diet-induced differences in vessel response to NO. There were no significant differences for the EC50 (Control: 25.1 ± 0.23 nM; 4 weeks: 21.7 ± 6.8 nM; 8 weeks: 19.9 ± 8.6 nM; 12 weeks: 14.5 ± 6.0 nM; P = 0.821) or the Hill-slope (Control: 1.48 ± 0.18; 4 weeks: 1.45 ± 0.24; 8 weeks: 1.47 ± 0.24; 12 weeks: 1.54 ± 0.16; P = 0.994) values for the SNP response.
Figure 4.
Mean concentration-response of left-anterior descending coronary artery segments following 3.0 µM 5-HT pre-constriction to (A) acetylcholine (ACh) stimulation (0.001–10.0 µM) or (B) sodium nitroprusside stimulation (SNP) (0.001–1.0 µM) in rats exposed to the control diet or Western diet for 4, 8, or 12 weeks. Reported are means ± SEM for 5–7 animals in each group. †P < 0.05 8-week vs. Control; *P < 0.05 12-week vs. Control; ***P < 0.001 12-week vs. Control. 5-HT = 5-hydroxytryptamine; SEM = standard error of the mean.
Discussion
In the present study, we investigated the time course of development of ED and coronary artery endothelial dysfunction in response to a WD that is high in sucrose and fat derived from saturated fatty acids and n-6 PUFA. Rats demonstrated an impaired erectile response to EFS of the cavernosal nerve near the MPG following 8 weeks of the WD, where an impaired coronary artery relaxation response to ACh followed at 12 weeks. Additionally, sepiapterin treatment improved the erectile response in all groups of rats fed the WD. These findings indicate that uncoupled NOS is an important mechanism of WD-induced ED. Rats on this WD did not demonstrate severe increases in body weight or adiposity. The 12-week WD-fed rats developed significantly higher fasting blood glucose levels; however, there were no severe elevations of insulin resistance or evidence of dyslipidemia. The 8-week and 12-week WD-fed rats developed a trend for higher serum concentrations of HNE adducts, suggesting elevated systemic oxidative stress. These findings indicate that chronic poor nutrition may supersede many of the traditional metabolic risk factors at predicting risk of ED and CAD development in this rodent model.
Consistent with the hypothesis that ED is an early indicator of future CAD, it is likely that a reduction in erectile function occurs prior to the onset of coronary endothelial dysfunction in the Zucker rat model, where obese Zucker rats (OZR) become diabetic and severely hyperlipidemic [18,34,35]. In 17- to 18-week-old lean Zucker rats (LZR) and OZR, there is a fourfold increase in the media : lumen ratio in the penile arteries of OZR compared with LZR, where no difference was observed in the coronary arteries [35]. This finding indicates that atherosclerotic development occurs in the penile artery prior to the coronary artery and reflects the state of endothelial function as the vasodilatory response to ACh in the OZR is attenuated in the penile artery but not the coronary artery [35]. Oltman et al. [34] found similar results as there was no difference in the coronary vasodilatory response to ACh between LZR and OZR at 16–24 weeks of age, where an impaired coronary vasodilatory response to ACh was apparent in OZR at 28–36 weeks of age. It is also likely that the reduction in penile artery endothelial dysfunction corresponds to ED as Wingard et al. [18] observed a reduction in ICP/MAP in OZR at 16–20 weeks of age. These findings in Zucker rats may translate to other models of obesity, as 10 weeks of a 61.6% fat, lard-based diet attenuates ACh-stimulated vasodilatory response in the penile artery of Wistar rats [36] but not the coronary artery [37]. In the present study, there were no substantial diet-induced differences in the coronary relaxation response to SNP, a direct NO donor and activator of the cyclic guanosine monophosphate (cGMP)-mediated relaxation pathway. These findings are in contrast to those where the coronary dilatory response to SNP is significantly augmented in response to a high-fat diet [37], suggestive of a sensitization to NO in diet-induced obesity. Considering the SNP response, the impaired relaxation response to ACh in the 12-week WD-fed rats is likely a dysfunction of the endothelium via impaired NO production as the smooth muscle retains a normal relaxation response to NO stimulation.
Various high-fat or hypercholesterolemic diets have previously been employed to investigate erectile function, cavernosum dynamics, and biochemical characteristics. Feeding Sprague-Dawley rats a 2% cholesterol, 10% lard diet for 5–6 months has previously shown to attenuate the voltage-dependent erectile response, in addition to induction of cavernosal smooth muscle hyperplasia and a reduction in cavernosal endothelial cell content and neuronal nitric oxide synthase (nNOS) positive nerve fibers [38–40]. In a design similar to the present study, Xie et al. [41] fed C57Bl6 mice a 45% fat diet for progressive durations and observed a progressive decline in ACh-mediated relaxation of cavernosal strips and cavernosal endothelial cell content with increased diet duration. Similarly, ApoE−/− mice fed a hypercholesterolemic diet for progressive durations displayed a progressive decline in cavernosal nNOS content, eNOS Ser1177 phosphorylation, capillary density, and ACh-mediated relaxation of cavernosal strips with increased diet duration [42]. In addition to decreased cavernosal strip endothelium-dependent dilation, feeding ApoE−/− mice a hypercholesterolemic diet has promoted increased lipid peroxidation and superoxide production as well as decreased NO production from cavernosal tissue [43]. Consistent with the findings that the metabolic syndrome promotes an enhanced oxidative burden in the penis, feeding Yucatan miniature swine a 46% fat diet for 24 weeks has shown to increase penile thiobarbituric acid reactive substances and decrease the penile eNOS dimer : monomer ratio, an indicator of uncoupled eNOS [44]. Similarly, LDLR−/− mice fed a hypercholesterolemic diet demonstrate an impaired voltage-dependent erectile response, increased penile HNE adducts and a decreased eNOS dimer : monomer ratio [20]. In the present study, there was a trend for increased serum HNE adducts with increased WD duration, which coincided with an impaired voltage-dependent erectile response. Furthermore, intracavernosal exposure to sepiapterin augmented the erectile response in WD fed rats. This is the first study to demonstrate reversal of diet-induced ED with sepiapterin treatment; however, four days of IP injection of sepiapterin augmented erectile function in aged rats [45], indicating that oxidative stress and uncoupled NOS are commonly associated with diet and aging-associated ED. It should be noted that sepiapterin stimulates intracellular BH4 production, which may act as an antioxidant [23]. Thus, in the absence of NOS dimer : monomer measurements, we cannot be certain that the reversal of ED with sepiapterin treatment is driven by enhanced NOS coupling rather than oxidant scavenging.
ED and CAD both have the common underpinnings of endothelial dysfunction. This study supports the notion that ED may be an early marker of cardiovascular risk, as has been proposed in several review articles [46–49]. In a large clinical study, Thompson et al. [50] found that the association between ED and CVD was nearly identical to the risk associated with current smoking or family history of myocardial infarction, both of which have long been established as CVD risk factors. Presentation of ED may provide an opportune time-point for clinician intervention. Long-term weight loss interventions through increased physical activity and/or caloric restriction have proven successful at improving erectile function in obese men [51,52]. Although it is logical that such lifestyle interventions initiated at the onset of ED would postpone, or perhaps prevent CAD development, future studies are necessary to establish these relationships.
Conclusion
Taken together, the findings of this study show that rats fed a high-fat, high-sucrose Western pattern diet demonstrated a depressed voltage-dependent erectile response prior to a depression in Ach-stimulated vasodilation of the left anterior descending coronary artery. A strength of this study is the use of nongenetically modified rodents that demonstrated modest characteristics of the metabolic syndrome with prolonged exposure to the WD, in contrast to studies that utilize overtly diabetic or hypercholesterolemic models. Additionally, acute cavernosal exposure to sepiapterin improved erectile function in rats exposed to each duration of the WD, suggesting that restoration of the coupled state of NOS is a mechanism by which diet-induced ED may be reversible.
Acknowledgments
This work was supported in part by a trainee grant from the Sexual Medicine Society of North America awarded to JDL.
Footnotes
Conflict of Interest:
Justin D. La Favor: None.
Ethan J. Anderson: None.
Robert C. Hickner: None.
Christopher J. Wingard: None.
- Category 1
-
Conception and DesignJustin D. La Favor; Ethan J. Anderson; Robert C. Hickner; Christopher J. Wingard
-
Acquisition of DataJustin D. La Favor; Ethan J. Anderson
-
Analysis and Interpretation of DataJustin D. La Favor; Christopher J. Wingard
-
- Category 2
-
Drafting the ArticleJustin D. La Favor; Ethan J. Anderson
-
Revising It for Intellectual ContentChristopher J. Wingard; Robert C. Hickner
-
- Category 3
-
Final Approval of the Completed ArticleJustin D. La Favor; Ethan J. Anderson; Robert C. Hickner; Christopher J. Wingard
-
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Andersen I, Heitmann BL, Wagner G. Obesity and sexual dysfunction in younger Danish men. J Sex Med. 2008;5:2053–2060. doi: 10.1111/j.1743-6109.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 3.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: Results from the health professionals follow-up study. Ann Intern Med. 2003;139:161–168. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Fung MM, Bettencourt R, Barrett-Connor E. Heart disease risk factors predict erectile dysfunction 25 years later: The Rancho Bernardo Study. J Am Coll Cardiol. 2004;43:1405–1411. doi: 10.1016/j.jacc.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 5.Gazzaruso C, Solerte SB, Pujia A, Coppola A, Vezzoli M, Salvucci F, Valenti C, Giustina A, Garzaniti A. Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease: A potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol. 2008;51:2040–2044. doi: 10.1016/j.jacc.2007.10.069. [DOI] [PubMed] [Google Scholar]
- 6.Borgquist R, Gudmundsson P, Winter R, Nilsson P, Willenheimer R. Erectile dysfunction in healthy subjects predicts reduced coronary flow velocity reserve. Int J Cardiol. 2006;112:166–170. doi: 10.1016/j.ijcard.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Jackson G, Padley S. Erectile dysfunction and silent coronary artery disease: Abnormal computed tomography coronary angiogram in the presence of normal exercise ECGs. Int J Clin Pract. 2008;62:973–976. doi: 10.1111/j.1742-1241.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 8.Montorsi F, Briganti A, Salonia A, Rigatti P, Margonato A, Macchi A, Galli S, Ravagnani PM, Montorsi P. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44:360–364. doi: 10.1016/s0302-2838(03)00305-1. [DOI] [PubMed] [Google Scholar]
- 9.Montorsi P, Montorsi F, Schulman CC. Is erectile dysfunction the “tip of the iceberg” of a systemic vascular disorder? Eur Urol. 2003;44:352–354. doi: 10.1016/s0302-2838(03)00307-5. [DOI] [PubMed] [Google Scholar]
- 10.Baumhakel M, Bohm M. Erectile dysfunction correlates with left ventricular function and precedes cardiovascular events in cardiovascular high-risk patients. Int J Clin Pract. 2007;61:361–366. doi: 10.1111/j.1742-1241.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- 11.Hodges LD, Kirby M, Solanki J, O’Donnell J, Brodie DA. The temporal relationship between erectile dysfunction and cardiovascular disease. Int J Clin Pract. 2007;61:2019–2025. doi: 10.1111/j.1742-1241.2007.01629.x. [DOI] [PubMed] [Google Scholar]
- 12.Montorsi P, Ravagnani PM, Galli S, Rotatori F, Veglia F, Briganti A, Salonia A, Deho F, Rigatti P, Montorsi F, Fiorentini C. Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: The COBRA trial. Eur Heart J. 2006;27:2632–2639. doi: 10.1093/eurheartj/ehl142. [DOI] [PubMed] [Google Scholar]
- 13.Gazzaruso C, Giordanetti S, De Amici E, Bertone G, Falcone C, Geroldi D, Fratino P, Solerte SB, Garzaniti A. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. Circulation. 2004;110:22–26. doi: 10.1161/01.CIR.0000133278.81226.C9. [DOI] [PubMed] [Google Scholar]
- 14.Mehta N, Sikka S, Rajasekaran M. Rat as an animal model for male erectile function evaluation in sexual medicine research. J Sex Med. 2008;5:1278–1283. doi: 10.1111/j.1743-6109.2008.00854.x. [DOI] [PubMed] [Google Scholar]
- 15.Bivalacqua TJ, Usta MF, Kendirci M, Pradhan L, Alvarez X, Champion HC, Kadowitz PJ, Hellstrom WJG. Superoxide anion production in the rat penis impairs erectile function in diabetes: Influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med. 2005;2:187–198. doi: 10.1111/j.1743-6109.2005.20228_1.x. [DOI] [PubMed] [Google Scholar]
- 16.Christ GJ, Hsieh Y, Zhao W, Schenk G, Venkateswarlu K, Wang HZ, Tar MT, Melman A. Effects of streptozotocin-induced diabetes on bladder and erectile (dys)function in the same rat in vivo. BJU Int. 2006;97:1076–1082. doi: 10.1111/j.1464-410X.2006.06058.x. [DOI] [PubMed] [Google Scholar]
- 17.Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2005;102:11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingard C, Fulton D, Husain S. Altered penile vascular reactivity and erection in the Zucker obese-diabetic rat. J Sex Med. 2007;4:348–363. doi: 10.1111/j.1743-6109.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 19.Carneiro FS, Giachini FRC, Carneiro ZN, Lima VV, Ergul A, Webb RC, Tostes RC. Erectile dysfunction in young non-obese type II diabetic Goto-Kakizaki rats is associated with decreased eNOS phosphorylation at Ser1177. J Sex Med. 2010;7:3620–3634. doi: 10.1111/j.1743-6109.2010.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musicki B, Liu T, Lagoda GA, Strong TD, Sezen SF, Johnson JM, Burnett AL. Hypercholesterolemia-induced erectile dysfunction: Endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J Sex Med. 2010;7:3023–3032. doi: 10.1111/j.1743-6109.2010.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behr-Roussel D, Darblade B, Oudot A, Compagnie S, Bernabe J, Alexandre L, Giuliano F. Erectile dysfunction in hypercholesterolemic atherosclerotic apolipoprotein E knockout mice. J Sex Med. 2006;3:596–603. doi: 10.1111/j.1743-6109.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 22.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: A physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 23.Channon KM. Tetrahydrobiopterin: Regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med. 2004;14:323–327. doi: 10.1016/j.tcm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25:81–88. doi: 10.1016/j.niox.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: Implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol. 2002;22:1655–1661. doi: 10.1161/01.atv.0000029122.79665.d9. [DOI] [PubMed] [Google Scholar]
- 26.Bourgoin F, Bachelard H, Badeau M, Melancon S, Pitre M, Lariviere R, Nadeau A. Endothelial and vascular dysfunctions and insulin resistance in rats fed a high-fat, high-sucrose diet. Am J Physiol Heart Circ Physiol. 2008;295:H1044–H1055. doi: 10.1152/ajpheart.00516.2008. [DOI] [PubMed] [Google Scholar]
- 27.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. A high-fat, refined-carbohydrate diet induces endothelial dysfunction and oxidant/antioxidant imbalance and depresses NOS protein expression. J Appl Physiol. 2005;98:203–210. doi: 10.1152/japplphysiol.00463.2004. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 29.Hodgson JM, Wahlqvist ML, Boxall JA, Balazs ND. Can linoleic acid contribute to coronary artery disease. Am J Clin Nutr. 1993;58:228–234. doi: 10.1093/ajcn/58.2.228. [DOI] [PubMed] [Google Scholar]
- 30.Hennig B, Toborek M, McClain CJ. High-energy diets, fatty acids and endothelial cell function: Implications for atherosclerosis. J Am Coll Nutr. 2001;20:97–105. doi: 10.1080/07315724.2001.10719021. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in ApoE-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 33.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;291:H1780–H1787. doi: 10.1152/ajpheart.01297.2005. [DOI] [PubMed] [Google Scholar]
- 35.Villalba N, Martinez P, Briones AM, Sanchez A, Salaices M, Garcia-Sacristan A, Hernandez M, Benedito S, Prieto D. Differential structural and functional changes in penile and coronary arteries from obese Zucker rats. Am J Physiol Heart Circ Physiol. 2009;297:H696–H707. doi: 10.1152/ajpheart.01308.2008. [DOI] [PubMed] [Google Scholar]
- 36.Prieto D, Kaminski PM, Bagi Z, Ahmad M, Wolin MS. Hypoxic relaxation of penile arteries: Involvement of endothelial nitric oxide and modulation by reactive oxygen species. Am J Physiol Heart Circ Physiol. 2010;299:H915–H924. doi: 10.1152/ajpheart.00382.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jebelovszki E, Kiraly C, Erdei N, Feher A, Pasztor ET, Rutkai I, Forster T, Edes I, Koller A, Bagi Z. High-fat diet-induced obesity leads to increased NO sensitivity of rat coronary arterioles: Role of soluble guanylate cyclase activation. Am J Physiol Heart Circ Physiol. 2008;294:H2558–H2564. doi: 10.1152/ajpheart.01198.2007. [DOI] [PubMed] [Google Scholar]
- 38.Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, Lue TF, Lin CS. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7:1391–1400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu X, Fandel TM, Lin G, Huang YC, Dai YT, Lue TF, Lin CS. Cavernous smooth muscle hyperplasia in a rat model of hyperlipidaemia-associated erectile dysfunction. BJU Int. 2011;108:1866–1872. doi: 10.1111/j.1464-410X.2011.10162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman NU, Phonsombat S, Bochinski D, Carrion RE, Nunes L, Lue TF. An animal model to study lower urinary tract symptoms and erectile dysfunction: The hyperlipidaemic rat. BJU Int. 2007;100:658–663. doi: 10.1111/j.1464-410X.2007.07069.x. [DOI] [PubMed] [Google Scholar]
- 41.Xie D, Odronic SI, Wu F, Pippen A, Donatucci CF, Annex BH. Mouse model of erectile dysfunction due to diet-induced diabetes mellitus. Urology. 2007;70:196–201. doi: 10.1016/j.urology.2007.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie D, Odronic SI, Wu F, Pippen A, Donatucci CF, Annex BH. A mouse model of hypercholesterolemia-induced erectile dysfunction. J Sex Med. 2007;4:898–907. doi: 10.1111/j.1743-6109.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 43.Baumhakel M, Custodis F, Schlimmer N, Laufs U, Bohm M. Improvement of endothelial function of the corpus cavernosum in apolipoprotein E knockout mice treated with irbesartan. J Pharmacol Exp Ther. 2008;327:692–698. doi: 10.1124/jpet.108.140533. [DOI] [PubMed] [Google Scholar]
- 44.Musicki B, Liu T, Strong T, Jin L, Laughlin MH, Turk JR, Burnett AL. Low-fat diet and exercise preserve eNOS regulation and endothelial function in the penis of early atherosclerotic pigs: A molecular analysis. J Sex Med. 2008;5:552–561. doi: 10.1111/j.1743-6109.2007.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson JM, Bivalacqua TJ, Lagoda GA, Burnett AL, Musicki B. eNOS-uncoupling in age-related erectile dysfunction. Int J Impot Res. 2011;23:43–48. doi: 10.1038/ijir.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esposito K, Giugliano D. Obesity, the metabolic syndrome, and sexual dysfunction. Int J Impot Res. 2005;17:391–398. doi: 10.1038/sj.ijir.3901333. [DOI] [PubMed] [Google Scholar]
- 47.Hale TM, Hannan JL, Heaton JP, Adams MA. Common therapeutic strategies in the management of sexual dysfunction and cardiovascular disease. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:185–195. doi: 10.2174/1568006043586143. [DOI] [PubMed] [Google Scholar]
- 48.Jackson G, Boon N, Eardley I, Kirby M, Dean J, Hackett G, Montorsi P, Montorsi F, Vlachopoulos C, Kloner R, Sharlip I, Miner M. Erectile dysfunction and coronary artery disease prediction: Evidence-based guidance and consensus. Int J Clin Pract. 2010;64:848–857. doi: 10.1111/j.1742-1241.2010.02410.x. [DOI] [PubMed] [Google Scholar]
- 49.Jackson G, Montorsi P, Adams MA, Anis T, El-Sakka A, Miner M, Vlachopoulos C, Kim E. Cardiovascular aspects of sexual medicine. J Sex Med. 2010;7:1608–1626. doi: 10.1111/j.1743-6109.2010.01779.x. [DOI] [PubMed] [Google Scholar]
- 50.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpoiur CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 51.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D’Andrea F, D’Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men. JAMA. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 52.Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, Worthley MI, Lange K, Wittert GA. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868–2875. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]