Abstract
Injured peripheral nerves regenerate their lost axons but functional recovery in humans is frequently disappointing. This is so particularly when injuries require regeneration over long distances and/or over long time periods. Fat replacement of chronically denervated muscles, a commonly accepted explanation, does not account for poor functional recovery. Rather, the basis for the poor nerve regeneration is the transient expression of growth-associated genes that accounts for declining regenerative capacity of neurons and the regenerative support of Schwann cells over time. Brief low-frequency electrical stimulation accelerates motor and sensory axon outgrowth across injury sites that, even after delayed surgical repair of injured nerves in animal models and patients, enhances nerve regeneration and target reinnervation. The stimulation elevates neuronal cyclic adenosine monophosphate and, in turn, the expression of neurotrophic factors and other growth-associated genes, including cytoskeletal proteins. Electrical stimulation of denervated muscles immediately after nerve transection and surgical repair also accelerates muscle reinnervation but, at this time, how the daily requirement of long-duration electrical pulses can be delivered to muscles remains a practical issue prior to translation to patients. Finally, the technique of inserting autologous nerve grafts that bridge between a donor nerve and an adjacent recipient denervated nerve stump significantly improves nerve regeneration after delayed nerve repair, the donor nerves sustaining the capacity of the denervated Schwann cells to support nerve regeneration. These reviewed methods to promote nerve regeneration and, in turn, to enhance functional recovery after nerve injury and surgical repair are sufficiently promising for early translation to the clinic.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-015-0415-1) contains supplementary material, which is available to authorized users.
Keywords: Peripheral nerve regeneration, Peripheral nerve injury, Electrical stimulation, Delayed nerve repair, Side-to-side crossbridges
Introduction
Injured nerves in the peripheral nervous system (PNS) regenerate their lost axons in contrast to those nerves in the central nervous system (CNS) that cannot. The Schwann cells within the denervated distal nerve stump provide the essential support for the regeneration of the PNS nerve fibers in contrast to the analogous glial cells of the CNS, the oligodendrocytes [1]. Yet, the recovery of function after human peripheral nerve injuries is frequently disappointing. This is the case particularly for injuries that are sustained at some distance from the denervated targets, requiring that the injured nerves regenerate over long distances and over long periods of time at the established rate of ~1 mm/day [2].
The basis for this poor regeneration is explored in this review before presenting the evidence that brief electrical stimulation is effective in accelerating axon outgrowth across injury sites [3, 4] that, even after delayed surgical repair of injured peripheral nerves, functional recovery is enhanced [5]. Associated with this enhancement, the electrical stimulation upregulates the expression of neurotrophic factors and, in turn, growth-associated genes [6, 7]. Although exogenous application of growth factors is effective in promoting nerve regeneration after delayed nerve repair or through nerve grafts that connect transected nerve stumps [8–11], and even though developments have been made in the delivery of these factors [11], the question remains as to whether the stimulation of endogenous sources of these factors and other as yet unknown mediators of nerve regeneration is more appropriate. Additionally, recent work has indicated an accelerating effect on target reinnervation when denervated muscle is electrically stimulated immediately after nerve transection but, how the daily requirement of long-duration electrical pulses can be delivered to muscles remains a practical question that needs to be addressed prior to clinical application [12].
Finally, a relatively new technique has been introduced that has the potential to prevent the progressive deterioration of the Schwann cell support of regenerating nerves. The technique of inserting nerve autografts through perineurial windows in peripheral nerves to bridge between a donor nerve and a recipient denervated distal nerve stump, encourages the regeneration of axons from the donor nerve into the recipient denervated nerve stump and allows limited axon regeneration proximal and distal to the nerve autografts that bridge the two nerves, the side-to-side cross-bridges. In turn, these cross-bridges allow for greatly improved nerve regeneration through the chronically denervated distal nerve stump when the proximal nerve stump is surgically united with the distal “protected” nerve stump [13–15]. Even when the surgical coaptation of the transected nerve stumps is performed immediately after injury, the long distance of nerve regeneration would otherwise allow for the deterioration of the growth support of the chronically denervated distal nerve stumps. The occupation of some of the denervated endoneurial tubes by the regenerated axons from the donor nerve sustains the growth permissive environment. The distal growth of the axons toward the denervated targets may also prevent the rapid denervation atrophy that normally occurs.
The Window of Opportunity for Nerve Regeneration is Restricted
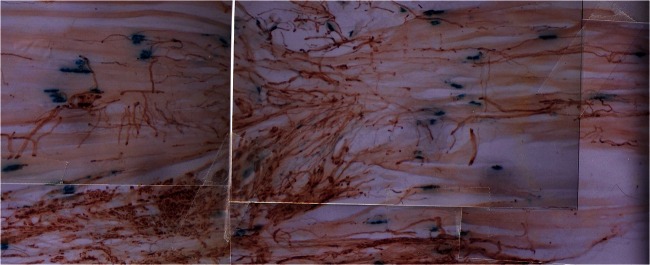
Normally the regeneration of injured nerves is excellent in small rodents, at least with respect to numbers of axons that regenerate and reinnervate distal targets after crush and transection injuries [16, 17]. The axons that are separated from their neuronal cell bodies lose their myelin sheaths and undergo Wallerian degeneration [18–24]. The denervated Schwann cells, in turn, line the endoneurial tubes and cross the surgical gap as the Bands of Bungner that guide regenerating axons across the crush or transection site, through the tubes, and back to the denervated targets [24, 25]. It is often said that, even without surgical repair, axons are emitted from the proximal nerve stump and frequently “find” their way back to the denervated nerve stump [26]. A dramatic illustration of this phenomenon of nerve outgrowth from the proximal nerve stump is the growth of axons along the surface of denervated muscle, the axons frequently re-entering denervated intramuscular nerve stumps (Fig. 1). This growth is associated with the outpouring of Schwann cells from the implanted proximal nerve stump that “support” the regenerating axons from the stump. However, this growth is limited by the distance between the implanted nerve and the denervated intramuscular nerve stump. Additional factors include the number of Schwann cells that can migrate from the proximal nerve stump and the transected intramuscular nerves, and the distance over which the Schwann cells can migrate to reach the intramuscular nerves and/or the denervated endplates (Gordon, unpublished data). Evidence to date indicates that Schwann cells lead and the regenerating axons follow from the proximal nerve stump [27].
Fig. 1.
Outgrowth of regenerating axons from a common peroneal nerve sutured directly to denervated soleus muscle. Silver nitrate-stained axons grow out from the suture site (at the bottom of the micrograph) and follow the muscle fibers to reinnervate many denervated endplates that are identified by their immunostaining with cholinesterase (blue)
The question remains as to why functional outcomes are so poor after delayed nerve surgeries or injuries suffered where long periods of time pass before target reinnervation would be expected. The answer to the question is generally assumed to be that regenerating axons fail to reinnervate target muscles due to irreversible denervation atrophy with ultimate fat replacement [2, 25, 28]. Experimentally, we asked the question of the consequences of the long periods of isolation of the injured neurons that regenerate their lost axons from their denervated targets (chronic axotomy of the neurons), the long periods of denervation of the Schwann cells in the distal nerve stumps (chronic Schwann cell denervation), and, finally, the chronic denervation of the muscles (chronic muscle denervation). Each of these conditions feature in human patients after delayed nerve repair. They also feature after immediate nerve repair for peripheral nerves that are injured close to the spinal cord and the dorsal root ganglia.
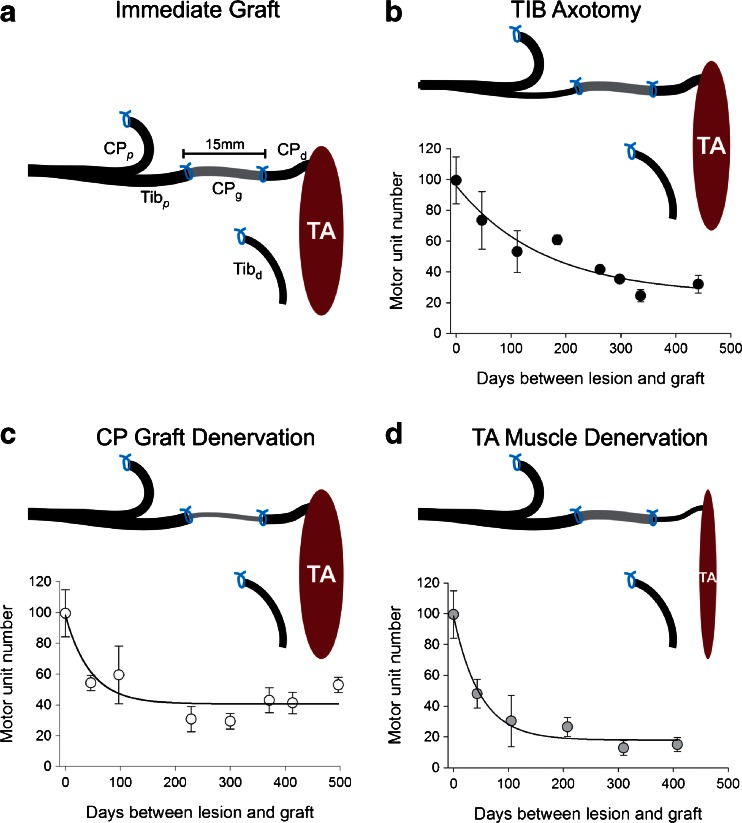
We addressed the question of the basis for poor functional outcomes in a rat model of delayed nerve repair. We used a cross-suture surgical paradigm with and without an autograft in which we progressively prolonged the period of time of chronic axotomy of the neurons, of chronic denervation of the Schwann cells, and of the chronic denervation of the muscles independently of each other (Fig. 2) [5, 8–10, 17, 29–36]. In the first series of experiments, we chronically axotomized the neurons or chronically denervated the distal nerve stump [17, 29]. We prolonged chronic axotomy for up to 12 months before suturing the proximal nerve stump of the chronically axotomized neurons to the distal stump of a freshly transected nerve; after at least 6 months muscle reinnervation was determined. We found that the numbers of nerves that reinnervated the denervated muscle declined exponentially to reach plateau levels of ~35 % of the numbers that reinnervated the denervated muscle after immediate nerve repair [17]. Chronic denervation of the distal nerve stump was even more detrimental to nerve regeneration with progressively fewer freshly transected nerves regenerating and reinnervating the denervated muscles as the chronic denervation was prolonged, the numbers declining to ~10 % [29]. Contrary to the belief that regenerating nerves could not reinnervate chronically denervated muscle because the muscle fibers had degenerated and were replaced by fat, retrograde labeling of those axons that were able to regenerate into the denervated nerve stump revealed that the chronic denervation reduced the capacity of the freshly axotomized neurons to regenerate their axons through the chronically denervated nerve stump [30]. Hence, chronic denervation of the muscle was not responsible for the low percentage of axons that did regenerate through the atrophic Schwann cells. Interestingly, the remaining atrophic Schwann cells were able to myelinate the regenerated axons normally and the regenerated nerves reinnervated as many as 3 times the number of muscle fibers than they normally do to enlarge the muscle units (number of muscle fibers per motoneuron) as much as they do under conditions of partial denervation of freshly denervated muscles [29, 37]. Further analysis of the capacity of chronically denervated Schwann cells to support axonal regeneration was done by encouraging nerve regeneration through a chronically denervated autograft into a freshly denervated muscle, demonstrating the key role that freshly denervated Schwann cells play in supporting the regeneration of axons (Fig. 2) [36]. In these experiments, the tibial nerve was cross-sutured to the distal nerve stump of the common peroneal nerve via a 12-mm common peroneal nerve autograft from the contralateral leg. Either the tibial motoneurons were chronically axotomized, the Schwann cells in the nerve autograft chronically denervated, or the tibialis anterior muscle chronically denervated for periods of up to 500 days to isolate the effects of chronic Schwann cell denervation from chronic muscle denervation (Fig. 2a–d). These experiments confirmed the detrimental effect of chronic axotomy on nerve regeneration as well as differentiating between the rapid detrimental effect of Schwann cell denervation within the autograft and the slower but greater effect of the denervation of the intramuscular nerves and the muscle.
Fig. 2.
Progressive failure of regenerating axons to grow through an autograft into the distal nerve stump and to reinnervate denervated muscles after delayed nerve repair. (a) The experimental protocol was to tease Sprague–Dawley rat ventral roots to evoke and record isometric contractile force from muscle fibers in the reinnervated tibialis anterior (TA) muscle that were reinnervated by single tibial (Tib) axons (motor units) at least 6 months after cross-suturing the proximal tibial (Tibp) nerve stump to the distal common peroneal (CPd) nerve via a CP nerve autograft (CPg) taken from the contralateral hindlimb. The cross-suture took place either (a) immediately or after a delay of up to 500 days, resulting in (b) chronic TIB axotomy, (c) chronic CP graft denervation, or (d) chronic TA muscle denervation. The relative contributions of (b) chronic axotomy, (c) chronic CP nerve denervation, and (d) TA muscle denervation to the decline in the number of regenerating Tib nerves that reinnervated the denervated TA muscle (motor unit number) are seen with each contributing to the progressive failure of nerve regeneration
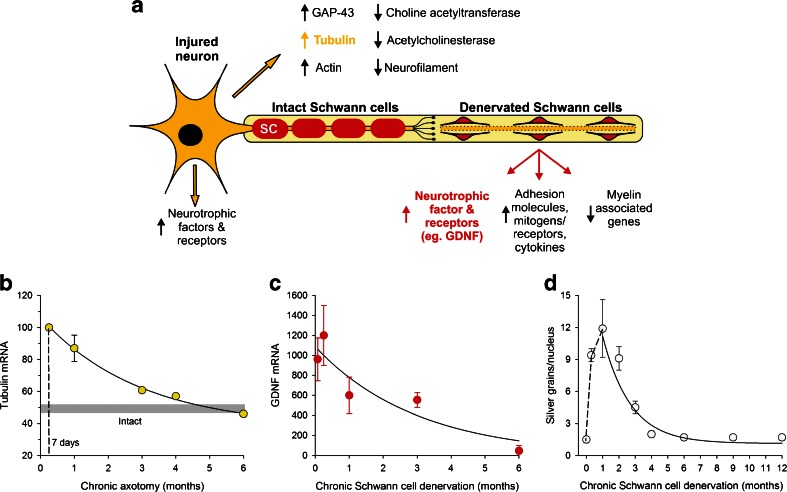
While the basis for the restricted window of opportunity for nerve regeneration over time and/or distance is not yet fully understood, the transient expression of growth-associated genes concomitant with the declining regenerative capacity of the neurons in rat models of delayed nerve repair is likely to be sufficient to account for the progressive failure of effective nerve regeneration. Analysis of the expression of growth-associated genes in motoneurons and within denervated distal nerve stumps revealed that the expression is transient (Fig. 3). There is a rapid upregulation of neurotrophic factors and their receptors in motoneurons and in Schwann cells, but this upregulation is short-lived, declining to baseline levels within a month or more of chronic axotomy and chronic denervation, respectively [31, 39–42]. Examples include the upregulation of neurotrophic factors, galectin-1, and cytoskeletal proteins, including actin and T-α-1 tubulin in the neurons [43–46], and neurotrophic factors and their receptors in the Schwann cells (Fig. 3a) [31, 41, 42, 47–49]. A specific example of upregulation of T-α-1 tubulin in the neurons is shown in Fig. 3b, and of glial cell line-derived neurotrophic factor (GDNF) and the p75 receptor for the neurotrophins in Schwann cells (Fig. 3c, d).
Fig. 3.
The transient expression of regeneration-associated genes in injured neurons after transecting the peripheral nerve. (a) The chromatolytic reaction of an injured motoneuron includes the movement of the nucleus to an eccentric position with expression of several growth-associated genes (GAGs) that include the neurotrophic factors and their receptors. There is a switch from the transmitting to the growth state in the axotomized motoneurons that includes the downregulation of transmitter associated genes (choline acetyltransferase and acetylcholinesterase) and the cytoskeletal protein, neurofilament, concurrent with a decline in the size of the axons proximal to the lesion [38]. The cytoskeletal proteins of tubulin and actin are upregulated, as are several growth-associated proteins (GAPs) that include GAP-43. However, this expression is transient as shown by the examples of the decline in the expression of (b) tubulin in chronically axotomized sciatic motoneurons, (c) glial-derived neurotrophic factor (GDNF), and the p75 receptor for the neurotrophic factors in chronically denervated Schwann cells. Mean ± SE bars are plotted in the graphs and the data points are fitted by exponential lines
Brief Electrical Stimulation Accelerates Axon Outgrowth Across the Nerve Injury Site After Both Immediate and Delayed Nerve Repair
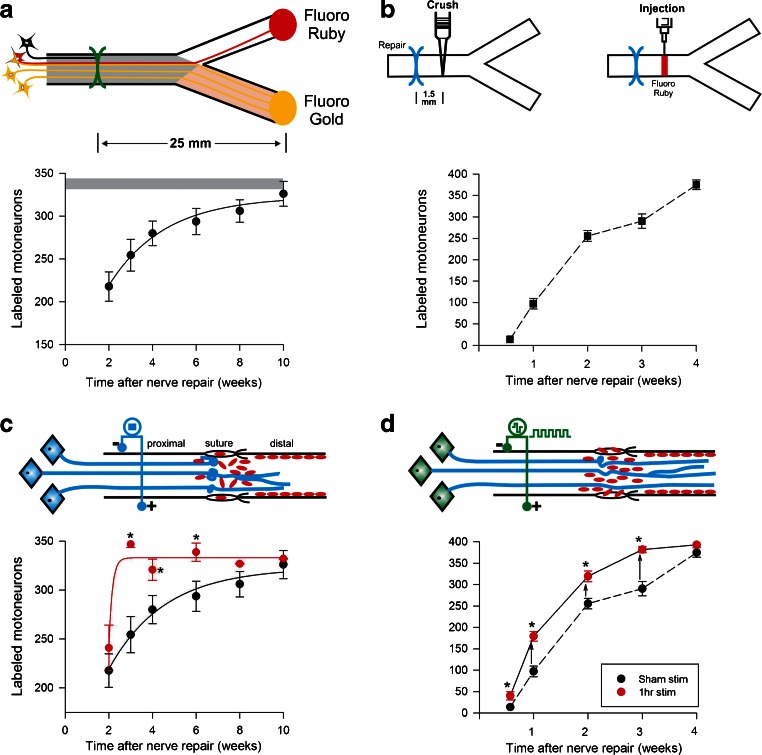
The concept of a latent period that precedes the outgrowth of regenerating axons from a proximal nerve stump into a denervated distal nerve stump was the result of experiments that were designed to examine the rate of nerve regeneration. The latent period of nerve regeneration was determined by extrapolation of the regression line of the distance of nerve regeneration against the days after the nerve injury [50]. Periods of as short as a day or as long as 3 days were described with axons regenerating thereafter at rates of 1–3 mm/day in humans and rats, respectively [50–55]. When motoneurons that regenerated their axons through a site of femoral nerve transection and microsurgical coaptation were back-labeled with fluorescent dyes 25 mm from the repair site, the numbers of these neurons increased to their maximum over a period of 8–10 weeks (Fig. 4a) [4]. This period of time was considerably longer than the predicted 2 weeks of regeneration calculated with a consideration of a latent period of 2–3 days and the reported regeneration rate of 3 mm/day in the rat. We termed this regeneration “staggered” with the prediction that the “staggering” occurs at the injury site where Cajal had described axons wondering at the suture site with some axons even turning to grow back towards the proximal nerve stump [26]. Indeed, this was the case, because back-labeling of the neurons whose regenerated axons had just crossed into the distal nerve stump revealed that a period of 3–4 weeks transpired before all the axons crossed the site of coapted femoral nerve stumps (Fig. 4b) [3], and because Witzel et al. [56], using transgenic mice that expressed fluorescent protein in their axons, demonstrated the “staggering” of regenerating axons as they crossed a suture line.
Fig. 4.
Brief 20-Hz electrical stimulation accelerates axon outgrowth across the surgical site of nerve repair. (a) The rat femoral nerve was transected and microsurgically repaired 20 mm from the branching of the nerves into the motor branch to the quadriceps muscle and the cutaneous saphenous nerve branch. The number of backlabeled motoneurons that regenerated their axons into both the branches and were backlabeled 25 mm from the suture site with fluororuby and fluorogold, increased progressively over an 8–10-week period of time. (b) Staggered axon regeneration was due to the “staggering” of regenerating axons across the suture site over a 4-week period as determined by counting motoneurons that were backlabeled by crushing the femoral nerve stump 1.5 mm from the repair site. (c, d) The “staggered axon regeneration” across the suture site was accelerated significantly (*p < 0.05) by a 1-h period of 20-Hz continuous electrical stimulation such that all motoneurons regenerated their axons over a distance of 25 mm within 3 weeks rather than 8-10 weeks with sham stimulation and motoneurons regenerated their axons significantly more qucikly across the suture site into the distal nerve stump
Electrical stimulation of the transected nerve proximal to the site of transection and surgical repair reduces the staggering of the axons as they regenerate across the suture site: a 2-week period of continuous stimulation at 20 Hz or even 1 h of stimulation promoted the outgrowth of regenerating axons across the suture line with the result that all motoneurons regenerated their axons over the 25-mm distance within 3 weeks (Fig. 4c, d). The electrical stimulation did not affect the rate of axonal transport as determined by injecting radiolabeled thymidine into the neurons [3]. The rationale for stimulating the nerve for 2 weeks was that this period of electrical stimulation after a crush injury of the nerve to the soleus muscle of the rabbit accelerated recovery of contractile forces in the muscle [57]. The finding that electrical stimulation accelerated the recovery of muscle contractile force indicated a positive effect of electrical stimulation without localizing the site of action of the electrical stimulation. Moreover, later findings by Pockett and Gavin [58] demonstrated the return of reflex contractions of ankle extensor muscles after electrical stimulation of the crushed sciatic nerve. Again, though, the site of action of the electrical stimulation was unknown. These investigators reduced the time period of electrical stimulation to record positive effects for durations as short as 15 min. In our study, we found that a 1-h period of electrical stimulation was effective in accelerating axon regeneration, this finding being serendipitous because longer periods of electrical stimulation were ineffective for sensory neurons whose axon outgrowth was also accelerated by 1-h of electrical stimulation but not by electrical stimulation for longer periods of time [59]. Most importantly, we established that the action potentials, generated by the brief electrical stimulation of the axons proximal to the lesion site and transmitted back to the soma of the neurons, were essential for the efficacy of the accelerated axon outgrowth in response to the electrical stimulation. This was because a tetrodotoxin blockade of these potentials obliterated the effect of the electrical stimulation [4].
The findings of accelerated nerve outgrowth after immediate nerve repair have been replicated many times for several different nerves, including the sciatic nerve and its main tributary nerve branches [60–91]. Presently, neither the frequency nor the duration of electrical stimulation have been considered in detail, although the crushed facial nerve was stimulated daily until functional recovery at 20 Hz for 30 min/day, the stimulation commencing a day after the crush injury [67, 81, 82]; the transected sciatic nerve was stimulated for only 20 min after delayed nerve repair [92], and for 10 min after a transection injury that was repaired via a silicone tube filled with a collagen gel [93].
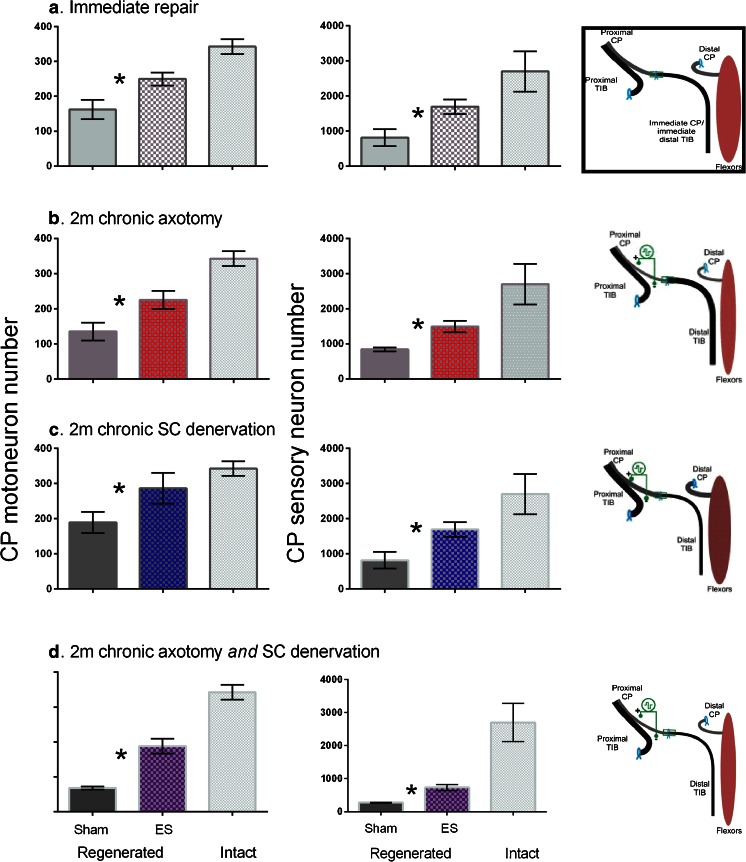
Only 2 studies [5, 92] have addressed the important clinical and unanswered question of whether electrical stimulation is effective under conditions of the chronic axotomy and chronic denervation, conditions that human nerves suffer even after immediate nerve repair as a consequence of long distances between the site of nerve repair and denervated targets. The study of Huang et al. [92] indicated a very small but significant increase of ~10 % and 3 % in the regeneration of axons by motor and sensory neurons, respectively, when the sciatic nerve was subjected to a 20-min period of low-frequency electrical stimulation after a delayed nerve repair via a 5-mm long hollow nerve conduit. Hence, the question of efficacy of the electrical stimulation on chronically injured nerves remained prior to our more recent study in which we again used a cross-suture technique to examine whether the brief 1-h 20-Hz electrical stimulation regimen could promote axon regeneration after chronic axotomy and/or chronic denervation [5]. Indeed, we found that the electrical stimulation was very effective in promoting outgrowth of axons from chronically axotomized motor and sensory neurons, from freshly axotomized motor and sensory neurons that regenerated axons into chronically denervated nerve stumps (compare Fig. 5b and c with A), and from chronically axotomized motor and sensory neurons that regenerated their axons into chronically denervated nerve stumps (Fig. 5d), and, in turn, reinnervated their chronically denervated muscles [5].
Fig. 5.
Brief electrical stimulation accelerates axon outgrowth across the nerve injury site after both immediate and delayed nerve repair. Two months after cross-suture of the common peroneal (CP) proximal nerve stump and the tibial (TIB) distal nerve stump, the numbers of backlabeled CP motor and sensory neurons (left and right set of histograms, respectively) that regenerated their axons 10 mm into the TIB nerve stump was increased significantly (*p < 0.05) when the CP nerve proximal to the repair site was subjected to a 1-h period of electrical stimulation after (a) immediate nerve repair, (b) 2 months (2 m) chronic axotomy of the CP neurons, (c) 2 months after chronic denervation of the TIB distal nerve stump, and (d) after 3 months chronic axotomy and denervation of the CP neurons and the distal TIB nerve stump. SC = Schwann cells
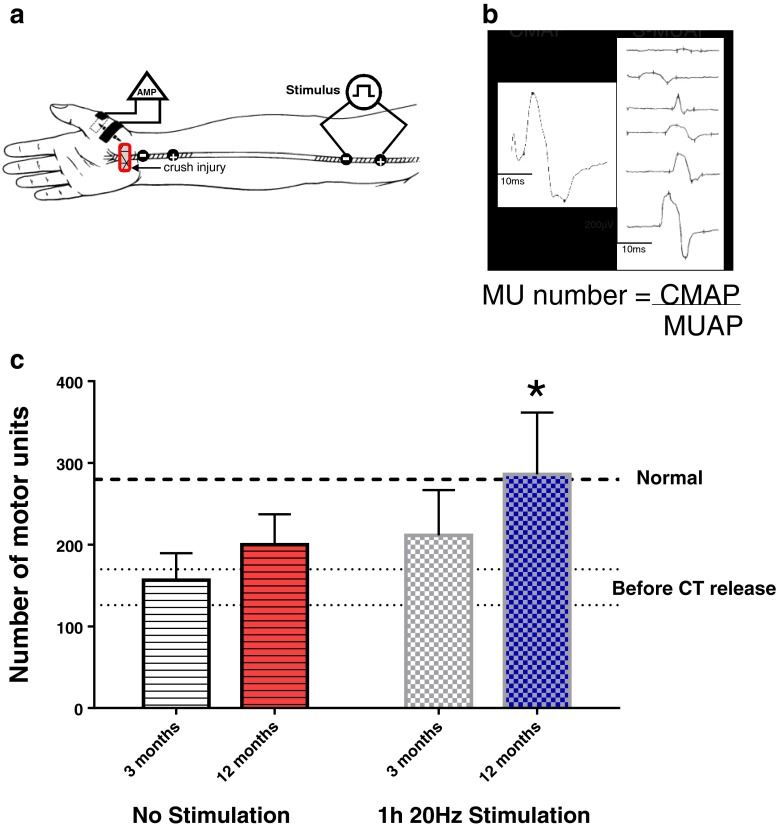
The efficacy of the 1-h 20-Hz electrical stimulation regimen was also demonstrated in patients who underwent carpal tunnel release surgery to promote the regeneration of injured median nerves that were severed by the constriction of the ligament at the wrist [94]. In these patients who suffered severe carpal tunnel syndrome based on several clinical and electrophysiological criteria, it was ascertained that ~50 % of the motor innervation of the thenar eminence was lost by Wallerian degeneration of the isolated axons distal to the lesion (Fig. 6). The numbers of innervated motor units in the thenar eminence increased gradually over a period of 1 year, but the increase was not significant with no stimulation (Fig. 6c). The patients experienced the welcomed release of pain but they were not aware of the minimal reinnervation of the muscles that move the thumb, their long flexor muscles in the forearm being effective in moving the thumb. The number of reinnervated motor units was estimated from the ratio of the compound and single all-or-none motor unit action potential amplitudes recorded in response to stimulation of all and of single nerves innervating the thenar, respectively after eminence musculature and (Fig. 6a, b). In the patients in which the nerve proximal to the carpal tunnel was subjected to brief electrical stimulation immediately after the surgical release, the number of motor units increased progressively, the numbers becoming significantly larger than preoperative values within 6–8 months and all the motor axons reinnervating the musculature within the year (Fig. 6c). As these patients had demonstrated symptoms of denervation for periods of as long as 5 years prior to the release surgery, these data complement the rat data in demonstrating the brief 1-h 20-Hz continuous electrical stimulation is effective in promoting axon regeneration even after chronic nerve injuries.
Fig. 6.
Brief electrical stimulation accelerates reinnervation of muscles after carpal tunnel (CT) release surgery in humans with severe CT syndrome. (a) Electromyographic (EMG) signals were recorded from surface electrodes on the thenar eminence in response to maximal and all-or-none stimulation of the median nerve. (b) The ratio of the compound action potential (CMAP) and mean motor unit potential (MUAP) provided the motor unit number estimation. Examples of MUAPs recorded in response to progressive movement of the stimulating electrodes up the arm [in (a)] are shown in (b) with the CMAP. (c) The standard errors of the mean values before CT release (shown as dotted lines) of motor unit number before the CT release surgery demonstrate that ~50 % of median motoneurons were axotomized by pressure exerted by the ligament that overlies the nerve at the wrist. When the median nerve was not stimulated after the CT release surgery, the trend to increase above the preoperative number of motor units was not significant. In contrast, in those patients whose median nerve was electrically stimulated for 1 h continuously at 20 Hz, the entire thenar muscle was reinnervated with restoration of the normal number of motor units (shown as an interrupted line)
The Effect of Electrical Stimulation on Nerve Regeneration is Mimicked by Pharmacological Elevation of Cyclic Adenosine Monophosphate
The possibility that electrical stimulation mediates its effects by elevating neuronal cyclic adenosine monophosphate (cAMP) was suggested by the early findings of improved nerve regeneration in response to pharmacological elevation of cAMP [95, 96]. However, these findings were not replicated by others, with the authors concluding that cAMP did not enhance the rate of axon regeneration [55, 97, 98]. Findings that rolipram, a type IV inhibitor of the enzyme phosphodiesterase that breaks down cAMP, promoted nerve outgrowth in the CNS inspired our examination of whether local delivery of rolipram might enhance axon regeneration in the PNS [99–101]. Indeed, the rolipram infusion of the surgical site via a miniosmotic pump accelerated axon outgrowth across the surgical suture site and into the distal nerve stump in an analogous manner to the effect of the electrical stimulation in accelerating femoral motoneurons to regenerate their axons across the surgical repair site (Fig. 7a, b). Pharmacological elevation of cAMP in cultured motoneurons was also effective in promoting neurite outgrowth [102].
Fig. 7.
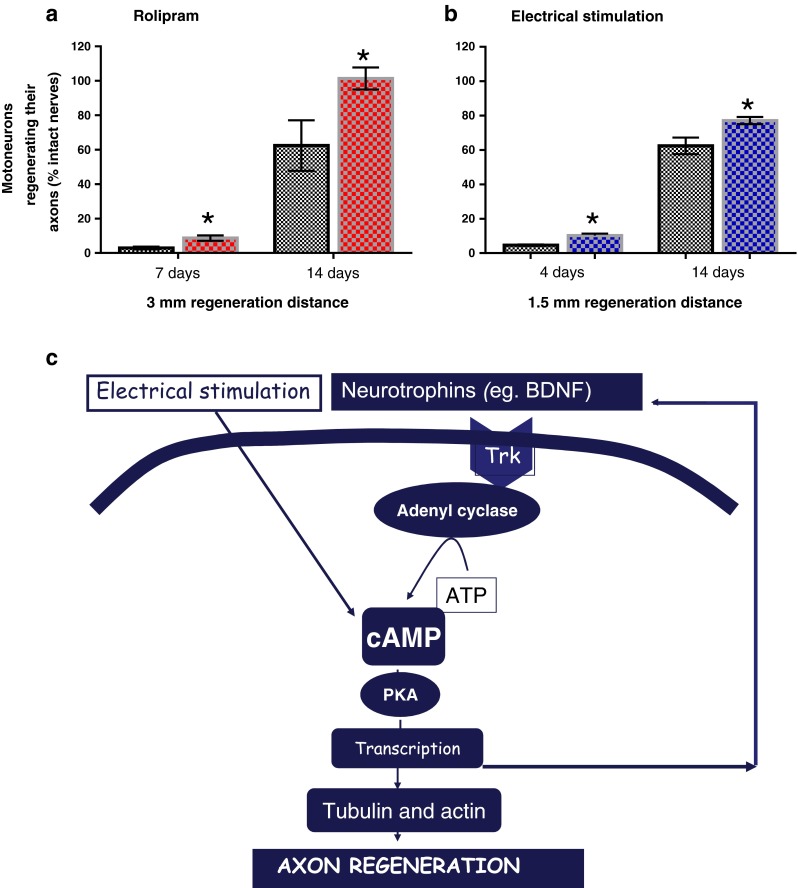
Rolipram, a phosphodiesterase that increases intracellular cyclic adenosine monophosphate (cAMP) in neurons or brief electrical stimulation accelerates axon outgrowth across the suture line of a transected and surgically repaired peripheral nerve in rats. (a) Rolipram administered locally to a surgically repaired common peroneal nerve or (b) 1 h continuous 20-Hz electrical stimulation proximal to the surgical repair site of a transected femoral nerve significantly increases the number of motoneurons that regenerate their axons. (c) Protein kinase A is activated by cAMP to promote transcription of proteins that include the neurotrophic factors that amplify the effects of cAMP in promoting gene transcription of growth associated genes such as tubulin and actin and, in turn, accelerate axon outgrowth (p < 0.05). BDNF = brain-derived neurotrophic factor; ATP = adenosine triphosphate; PKA = protein kinase A
Brief Electrical Stimulation Accelerates Axon Outgrowth Within the CNS
The analogous growth promoting effects of brief low-frequency electrical stimulation and rolipram-induced elevation of neuronal cAMP in the PNS suggested that the electrical stimulation paradigm might also promote nerve outgrowth in the CNS. Indeed, Woolf and colleagues had demonstrated that elevated cAMP in dorsal root ganglion neurons was responsible for the efficacy of a conditioning lesion to the sensory axons in the PNS in promoted outgrowth from transected central axons [103]. The efficacy of the conditioning lesion in promoting regeneration of CNS axons was first demonstrated in 1984 [104]. Because sensory neurons discharge at rates up to 200 Hz, we compared the efficacy of a 1-h period of continuous 200- and 20-Hz electrical stimulation applied to the sciatic nerve immediately after cutting the central axons at the level of T8 in the spinal cord [86]. The 20-Hz electrical stimulation paradigm but not the 200-Hz electrical stimulation paradigm promoted the outgrowth of CNS sensory axons at the level of T8, although to a lesser extent than the conditioning lesion (Fig. 8). This outgrowth was associated with a significant elevation in cAMP in the neurons that were stimulated at 20 Hz but not at 200 Hz, the elevation being the same as after the conditioning lesion. The discrepancy between the elevated cAMP and the efficacy of the electrical stimulation in promoting axon outgrowth suggested additional mechanisms to account for the greater effect of the conditioning lesion than the electrical stimulation [86].
Fig. 8.
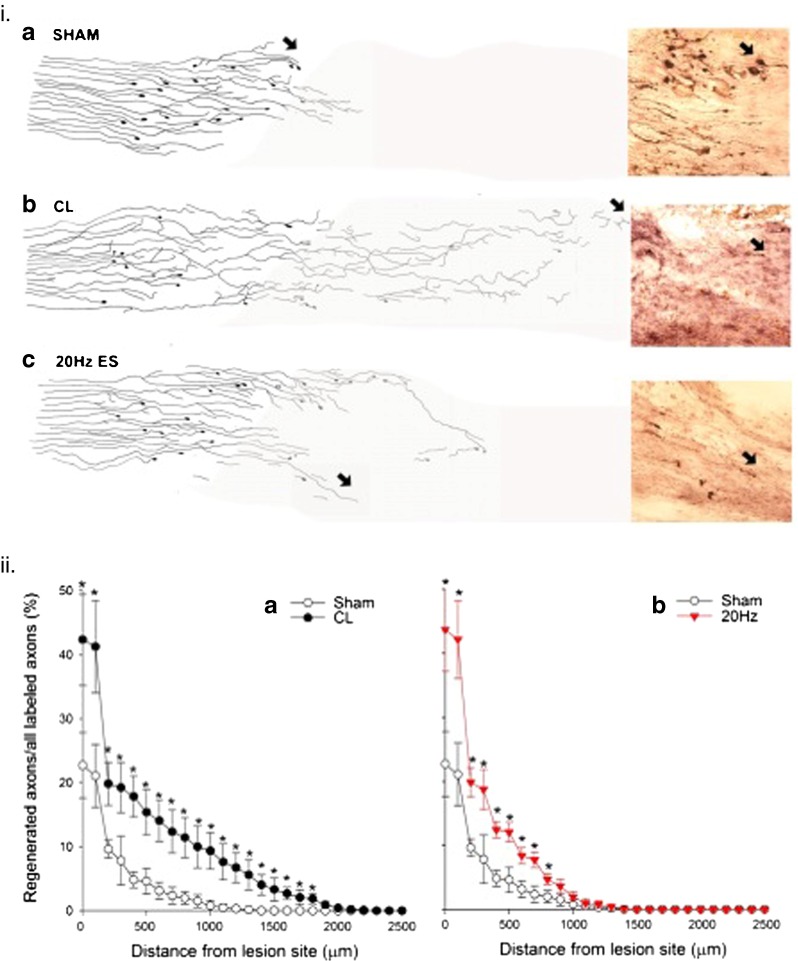
A conditioning lesion and brief electrical stimulation (ES) promotes nerve regeneration within the spinal cord. (ia) Following a spinal lesion at the level of T8, few axons entered the lesion site [camara lucida drawings with example of cholerotoxin (CTX)-stained axons 1 week after injecting CTX into the sciatic nerve] after sham electrical stimulation at 20 Hz for 1 h (or a sham conditional lesion, not shown). (ib) In contrast, a conditioning sciatic crush lesion (CL) resulted in more regeneration of CTX-labeled axons, with (ic) an intermediate number regenerating after 1 h of 20-Hz continuous electrical stimulation of the sciatic nerve. (iia) The significantly (p < 0.05) elevated number of regenerated sciatic nerve axons that grew out and over longer distances from the lesion site after a CL and (iib) the significantly elevated numbers of axons that grew out from the lesion site but did not grow over longer distances after 20Hz electrical stimulation for 1 h. Each point is the mean ± SEM
The suppressed ability of axotomized sensory neurons to upregulate growth-associated proteins (GAPs) when their central axons are injured is 1 of the 2 reasons why the axons do not normally regrow their lost axons, the other being the presence of the oligodendrocytes rather than Schwann cells in the CNS and the proteoglycans that directly inhibit axon extension [105–108]. Only when GAPs are expressed as they are after proximal CNS nerve lesions for example, do the CNS axons regrow [109, 110]. The expression of both GAP-43 and CAP-23, members of a MARCKs-related group of acylated membrane proteins that interact with actin filaments, calmodulin, protein kinase C, and phophoinositides, is prerequisite for CNS nerve regeneration, regeneration occurring only when both proteins are expressed in the injured neurons [111]. Electrical stimulation promoted axon outgrowth in the CNS but the contrasting efficacy of a conditioning lesion to promote both axon outgrowth and extension of the regenerating axons in the spinal cord indicates that electrical stimulation failed to upregulate both GAPs (Fig. 8).
The Role of Neurotrophic Factors and Androgens in the Efficacy of Electrical Stimulation, and Daily Exercise Programs in Promoting Axon Regeneration in the PNS
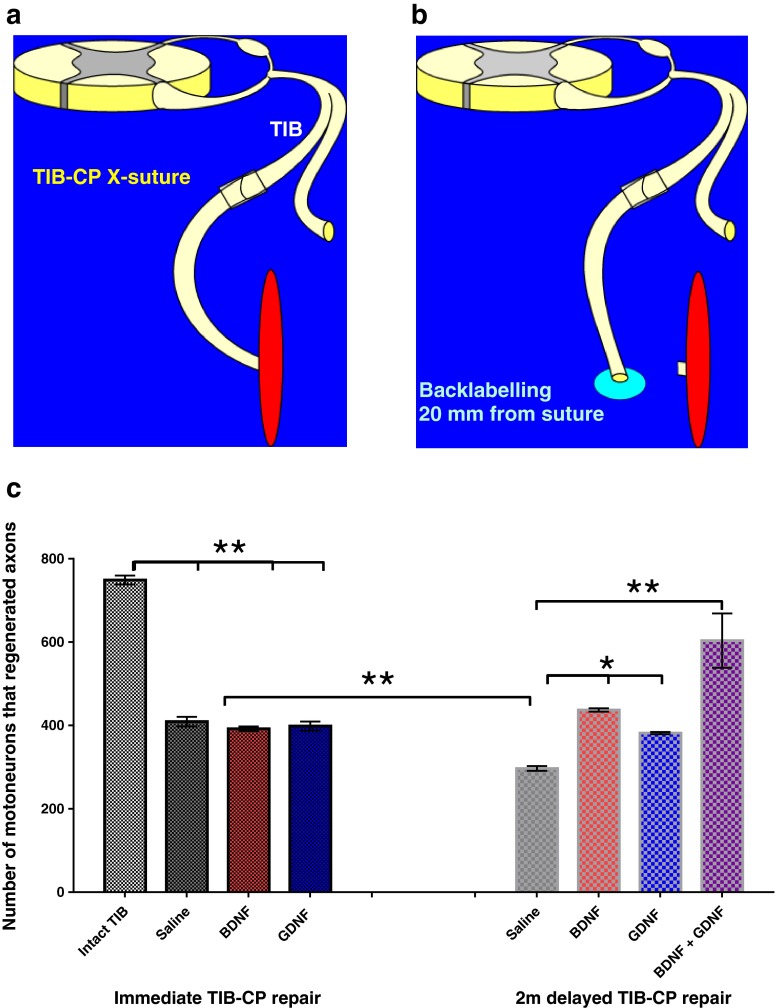
Brief electrical stimulation accelerates the expression of neurotrophic factors and their receptors [6]. Thereafter, the cytoskeletal proteins actin and tubulin, and GAP-43 are upregulated (Fig. 7c) [7]. Using transgenic mice that express yellow fluorescent protein in a small proportion of their axons and by cross-breeding of these mice with mice in which neurotrophic factor expression was deleted, English et al. [66] demonstrated the critical role of the neurotrophic factors brain-derived neurotrophic factor (BDNF) and neurotrophin 4/5 in the efficacy of the electrical stimulation effect in accelerating nerve regeneration [66]. Exogenous administration of either BDNF or GDNF did not increase nerve regeneration after immediate nerve repair (Fig. 9) [9, 10]; however, after a period of chronic axotomy before cross-suturing the proximal tibial nerve stump to a freshly denervated common peroneal distal nerve stump, the administration of either factor alone or together did significantly increase the number of motoneurons that regenerated their axons (Fig. 9c). Normally there is sufficient expression of neurotrophic factors in the neurons and the Schwann cells after nerve injury to sustain nerve regeneration [35]; however, after chronic axotomy, exogenous sources of BDNF and/or GDNF were necessary to promote nerve regeneration (Fig. 9a–c). In contrast to GDNF that acts via its receptors to promote axon regeneration, BDNF has a bimodal effect with low doses acting via trkB receptors to promote axon regeneration, while high doses act via p75 to inhibit axonal regeneration [8–10].
Fig. 9.
Exogenous administration of the neurotrophic factors brain-derived neurotrophic factor (BDNF) and glial cell-line derived neurotrophic factor (GDNF) promotes neuronal regeneration of injured motoneurons after delayed nerve repair. (a) A cross-suture (X-suture) technique to cross-suture the freshly cut tibial (TIB) proximal nerve stump to the distal stump of the freshly cut common peroneal (CP) nerve or to cross-suture the TIB proximal nerve stump that was ligated for 2 months (2 m) previously (to prolong TIB motoneuron axotomy) to a freshly cut CP distal nerve stump. The neurotrophic factors were delivered from a miniosmotic pump to the coaptation site over a 1-month period. (b) Thereafter, the regenerated TIB axons in the CP nerve were exposed to fluorogold to backlabel those TIB motoneurons that regenerated their axons over a 20-mm distance. (c) Saline-treated axons regenerated ~45 % of their axons within the a month of regeneration, consistent with the staggered regeneration demonstrated by Al-Majed et al. [6]. This regeneration was significantly reduced by a 2-month period of chronic axotomy consistent with the data of Fu and Gordon [17]. The decline in motoneuron numbers after 2 months was not only prevented by a month of administration of BDNF (2 μg/day) or GDNF (0.1 μg/day), but also the neurotrophic factors significantly increased the numbers of 2-month chronically axotomized motoneurons that regenerated their axons (* p < 0.05; ** p < 0.01)
Although GDNF has been shown to be efficacious when applied in microspheres around the suture site of injured peripheral nerves with and without an intervening acellular conduit [112–118], the difficulties of titrating effective doses of BDNF illustrate some of the difficulties that may be encountered with exogenous sources of the neurotrophic factors. The biological modulation of the neurotrophic factors is difficult to replicate and problems of excess administration have been described, as, for example, the administration of excess doses of GDNF using retroviral vectors in rats where the findings of axonal coils was attributed to too much GDNF [119, 120] .
While electrical stimulation upregulates the expression of neurotrophic factors and their receptors, this expression is transient, declining within days [6]. The administration of androgens in conjunction with electrical stimulation, however, sustains their upregulation [81, 82]. Over the course of evaluating the extent of activity-mediated axonal regeneration, Sabatier et al. [121] and Wood et al. [122] discovered a sex difference in the effectiveness of treadmill training when comparing 2 treadmill training paradigms, continuous and interval training. A daily slow training protocol at 10 m/min for 1 h resulted in a marked increase in the length of regenerating axons in male mice 2 weeks after nerve transection and repair, but the protocol had no effect on regeneration in female mice [85, 122]. On the other hand, when the female mice were exposed to a faster 20 m/min training protocol for 2-min intervals 4 times daily, an impressive enhancement in axonal regeneration was seen. No enhancement was appreciated in the male mice exposed to the same interval training protocol. The studies from English’s laboratory provide evidence that sex steroid hormones, particularly testosterone, mediate this sex difference in exercised mice [122]. They also demonstrate that androgens are also critical for the efficacy of low-frequency electrical stimulation in promoting axon outgrowth [85]. In the former case, castration of male rats eliminated the enhanced axon regeneration by daily interval training, and treating unexercised female mice with an aromatase inhibitor, anastrozole, to block the conversion of testosterone or its precursors into estradiol, enhanced axonal regeneration [122]. Subsequent experiments demonstrated that treating mice with flutamide, an androgen receptor blocker, inhibited the effect of both exercise and electrical stimulation in both sexes [85]. As treadmill training can be readily applied to humans, the translational potential of this modality in combination with electrical stimulation in peripheral nerve injuries is promising, while activity-dependent therapies also simultaneously empower patients to assume responsibility for their own recovery.
Conclusions
The sluggish crossing of regenerating axons across injury sites, whether or not the endoneurial tubes are disrupted by transection of peripheral nerves, compounds the problems of the slow rate of axon regeneration of 1 mm/day in humans and of 3 mm/day in animals [3, 123]. As a result, functional recovery after nerve injuries is commonly recognized to be disappointing [124]. The efficacy of brief low-frequency electrical stimulation in accelerating axon outgrowth across the injury site in both animal and human studies results in accerelated and improved functional recovery. This improvement was demonstrated in a number of published and ongoing studies of human nerve injuries [94, 125, and Chan, unpublished data]. Importantly, the electrical stimulation regimen is effective after delayed nerve repair in animals and humans [5, 94]. These very promising findings anticipate further studies that may form the basis for the adoption of technique of intraoperative brief electrical stimulation at the time of surgical repair of injured nerves to become the standard of practice in management of peripheral nerve injuries. The ability of training programs after surgical repair of peripheral nerves to accelerate nerve regeneration in animal studies also holds considerable promise for the management of peripheral nerve injuries [85, 122]. Whilst movement is usually restricted after surgical repair of injured nerves, possibilities such as the adoption of imagined movement in the early stages of recovery followed by adoption of active programs of activity may be explored in the future.
Finally, there are several other surgical and pharmacological strategies that are being explored to promote regeneration and to counteract the negative effects of chronic nerve injuries [126]. These include the placement of end-to-side or side-to-side nerve autografts between a donor nerve and a recipient denervated distal nerve stump [14, 127], and the localized administration of FK506 and neurotrophic factors to the surgical site [11]. In the former case, the ingrowth of axons into a denervated nerve stump that proceeds both proximal and distal to the insertion site of the autograft into the recipient denervated stump significantly improves the regeneration of axons after surgical coaptation of the proximal and distal nerve stumps through the distal nerve stump into which the donor axons had grown. Local administration of FK506 to the coaptation site of a transected nerve was also very effective in promoting nerve regeneration. Hence, a combinational approach to surgical repair of transected nerves has enormous potential for greatly improving nerve regeneration and, in turn, functional recovery after peripheral nerve injuries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1224 kb)
Acknowledgments
I thank all my colleagues who contributed to the several published papers that are included in this review, and the Canadian Institutes of Research who provided the grant funding to carry out the work published in the papers.
Compliance with Ethical Standards
Required Author Forms Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Fenrich K, Gordon T. Canadian Association of Neuroscience review: axonal regeneration in the peripheral and central nervous systems-current issues and advances. Neurol Sci 2004;31:142--156. [DOI] [PubMed]
- 2.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 3.Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–38. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elzinga K, Tyreman N, Ladak A, Savaryn B, Olson J, Gordon T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp Neurol. 2015;269:142–153. doi: 10.1016/j.expneurol.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12:4381–4390. [PubMed] [Google Scholar]
- 7.Al-Majed AA, Tam SL, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol. 2004;24:379–402. doi: 10.1023/B:CEMN.0000022770.66463.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd JG, Gordon T. The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J Neurobiol. 2001;49:314–325. doi: 10.1002/neu.10013. [DOI] [PubMed] [Google Scholar]
- 9.Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- 10.Boyd JG, Gordon T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol. 2003;183:610–619. doi: 10.1016/S0014-4886(03)00183-3. [DOI] [PubMed] [Google Scholar]
- 11.Tajdaran K. Enhancement of peripheral nerve regeneration with controlled release of glial cell line-derived neurotrophic factor (GDNF). MSc thesis, University of Toronto, 2015.
- 12.Willand MP, Chiang CD, Zhang JJ, Kemp SW, Borschel GH, Gordon T. Daily electrical muscle stimulation enhances functional recovery following nerve transection and repair in rats. Neurorehabil Neural Repair. 2015;29:690–700. doi: 10.1177/1545968314562117. [DOI] [PubMed] [Google Scholar]
- 13.Ladak A, Tyreman N, Schembri P, Udina E, Olson J, Gordon T. Application of side-to-side nerve grafts to sustain chronically denervated nerve pathways and in turn, to encourage axon regeneration and improved reinnervation [abstract]. Sunderland Society 2009;19. [DOI] [PubMed]
- 14.Gordon T, Hendry M, Lafontaine CA, Cartar H, Zhang JJ, Borschel GH. Nerve cross-bridging to enhance nerve regeneration in a rat model of delayed nerve repair. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0127397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendry JM, Alvarez-Veronesi MC, Snyder-Warwick A, Gordon T, Borschel GH. Side-to-side nerve bridges support donor axon regeneration into chronically denervated nerves and are associated with characteristic changes in Schwann cell phenotype. Neurosurgery. 2015;77:803–813. doi: 10.1227/NEU.0000000000000898. [DOI] [PubMed] [Google Scholar]
- 16.Wood MD, Kemp SW, Weber C, Borschel GH, Gordon T. Outcome measures of peripheral nerve regeneration. Ann Anat. 2011;193:321–333. doi: 10.1016/j.aanat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abercrombie M, Johnson ML. Quantitative histology of Wallerian degeneration 1). Nuclear population in rabbit sciatic nerve. J Anat. 1946;80:37–50. [PubMed] [Google Scholar]
- 19.Beuche W, Friede RL. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984;13:767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- 20.Scheidt P, Friede RL. Myelin phagocytosis in Wallerian degeneration. Properties of millipore diffusion chambers and immunohistochemical identification of cell populations. Acta Neuropathol. 1987;75:77–84. doi: 10.1007/BF00686796. [DOI] [PubMed] [Google Scholar]
- 21.Stoll G, Griffin JW, Li CY, Trapp BD. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989;18:671–683. doi: 10.1007/BF01187086. [DOI] [PubMed] [Google Scholar]
- 22.Bruck W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997;7:741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9:313–325. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon T. The biology, limits, and promotion of peripheral nerve regeneration in rats and human. Nerves And Nerve Injuries Eds: Tubbs RS, Rizk E, Shoja M, Loukas M, and Spinner RJ. Elsevier, 2015, Vol. 2 Ch 61, pp. 993–1019.
- 26.Cajal SR. Degeneration and regeneration of the nervous system (RM May, transl.). Oxford University Press, New York, 1928.
- 27.Webber C, Zochodne D. The nerve regenerative microenvironment: Early behavior and partnership of axons and Schwann cells. Exp Neurol. 2010;223:51–59. doi: 10.1016/j.expneurol.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci 2015, pp1--15 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 29.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulaiman OAR, Gordon T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 2000;32:234–246. doi: 10.1002/1098-1136(200012)32:3<234::AID-GLIA40>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Hoke A, Gordon T, Zochodne DW, Sulaiman OAR. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- 32.Sulaiman OAR, Voda J, Gold BG, Gordon T. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic Schwann cell denervation. Exp Neurol. 2002;175:127–137. doi: 10.1006/exnr.2002.7878. [DOI] [PubMed] [Google Scholar]
- 33.Sulaiman OAR, Gordon T. Transforming growth factor-beta and forskolin attenuate the adverse effects of long-term Schwann cell denervation on peripheral nerve regeneration in vivo. Glia. 2002;37:206–218. doi: 10.1002/glia.10022. [DOI] [PubMed] [Google Scholar]
- 34.Gordon T, Sulaiman OAR, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236–250. doi: 10.1111/j.1085-9489.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- 35.Furey MJ, Midha R, Xu QG, Belkas J, Gordon T. Prolonged target deprivation reduces the capacity of injured motoneurons to regenerate. Neurosurgery. 2007;60:723–732. doi: 10.1227/01.NEU.0000255412.63184.CC. [DOI] [PubMed] [Google Scholar]
- 36.Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011;31:5325–5334. doi: 10.1523/JNEUROSCI.6156-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafuse VF, Gordon T, Orozco R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. J Neurophysiol. 1992;68:1261–1275. doi: 10.1152/jn.1992.68.4.1261. [DOI] [PubMed] [Google Scholar]
- 38.Gordon T, Stein RB. Time course and extent of recovery in reinnervated motor units of cat triceps surae muscles. J Physiol. 1982;323:307–323. doi: 10.1113/jphysiol.1982.sp014074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You S, Petrov T, Chung PH, Gordon T. The expression of the low affinity nerve growth factor receptor in long-term denervated Schwann cells. Glia. 1997;20:87–100. doi: 10.1002/(SICI)1098-1136(199706)20:2<87::AID-GLIA1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 41.Brushart TM, Aspalter M, Griffin JW, et al. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp Neurol. 2013;247C:272–281. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoke A, Redett R, Hameed H, et al. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGraw J, McPhail LT, Oschipok LW, et al. Galectin-1 in regenerating motoneurons. Eur J Neurosci. 2004;20:2872–2880. doi: 10.1111/j.1460-9568.2004.03802.x. [DOI] [PubMed] [Google Scholar]
- 44.Bisby MA, Tetzlaff W. Changes in cytoskeletal protein synthesis following axon injury and during axon regeneration. Mol Neurobiol. 1992;6:107–123. doi: 10.1007/BF02780547. [DOI] [PubMed] [Google Scholar]
- 45.Tetzlaff W, Alexander SW, Miller FD, Bisby MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tetzlaff W, Bisby MA, Kreutzberg GW. Changes in cytoskeletal proteins in the rat facial nucleus following axotomy. J Neurosci. 1988;8:3181–3189. doi: 10.1523/JNEUROSCI.08-09-03181.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eggers R, Tannemaat MR, Ehlert EM, Verhaagen J. A spatio-temporal analysis of motoneuron survival, axonal regeneration and neurotrophic factor expression after lumbar ventral root avulsion and implantation. Exp Neurol. 2010;223:207–220. doi: 10.1016/j.expneurol.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 48.Gordon T, You S, Cassar SL, Tetzlaff W. Reduced expression of regeneration associated genes in chronically axotomized facial motoneurons. Exp Neurol. 2014;264:26–32. doi: 10.1016/j.expneurol.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 49.Mi R, Chen W, Hoke A. Pleiotrophin is a neurotrophic factor for spinal motor neurons. Proc Natl ACad Sci 2007;104:4664--4669. [DOI] [PMC free article] [PubMed]
- 50.Gutmann E, Guttmann L, Medawar PB, Young JZ. The rate of regeneration of nerve. J Exp Biol. 1942;19:14–44. [Google Scholar]
- 51.Sunderland S. Rate of regeneration of motor fibers in the ulnar and sciatic nerves. Arch Neurol Psychiatry. 1947;58:7–13. doi: 10.1001/archneurpsyc.1947.02300300017002. [DOI] [PubMed] [Google Scholar]
- 52.Sunderland S. Rate of regeneration of sensory nerve fibers. Arch Neurol Psychiatry. 1947;58:1–5. doi: 10.1001/archneurpsyc.1947.02300300011001. [DOI] [PubMed] [Google Scholar]
- 53.Sunderland S. Rate of regeneration in human peripheral nerves: analysis of interval between injury and onset of recovery. Arch Neurol Psychiat. 1947;58:251–295. doi: 10.1001/archneurpsyc.1947.02300320002001. [DOI] [PubMed] [Google Scholar]
- 54.Danielsen N, Lundborg G, Frizell M. Nerve repair and axonal transport: outgrowth delay and regeneration rate after transection and repair of rabbit hypoglossal nerve. Brain Res. 1986;376:125–132. doi: 10.1016/0006-8993(86)90906-6. [DOI] [PubMed] [Google Scholar]
- 55.Black MM, Lasek RJ. Slowing of the rate of axonal regeneration during growth and maturation. Exp Neurol. 1979;63:108–119. doi: 10.1016/0014-4886(79)90188-2. [DOI] [PubMed] [Google Scholar]
- 56.Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J Comp Neurol. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- 57.Nix WA, Hopf HC. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 1983;272:21–25. doi: 10.1016/0006-8993(83)90360-8. [DOI] [PubMed] [Google Scholar]
- 58.Pockett S, Gavin RM. Acceleration of peripheral nerve regeneration after crush injury in rat. Neurosci Lett. 1985;59:221–224. doi: 10.1016/0304-3940(85)90203-4. [DOI] [PubMed] [Google Scholar]
- 59.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205:347–359. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 60.Ahlborn P, Schachner M, Irintchev A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol. 2007;208:137–144. doi: 10.1016/j.expneurol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Alrashdan MS, Park JC, Sung MA, et al. Thirty minutes of low intensity electrical stimulation promotes nerve regeneration after sciatic nerve crush injury in a rat model. Acta Neurol Belg. 2010;110:168–179. [PubMed] [Google Scholar]
- 62.Alrashdan MS, Sung MA, Kwon YK, Chung HJ, Kim SJ, Lee JH. Effects of combining electrical stimulation with BDNF gene transfer on the regeneration of crushed rat sciatic nerve. Acta Neurochir (Wien) 2011;153:2021–2029. doi: 10.1007/s00701-011-1054-x. [DOI] [PubMed] [Google Scholar]
- 63.Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 64.Beaumont E, Cloutier FC, Atlan M, Rouleau DM, Beaumont PH. Chondroitinase ABC and acute electrical stimulation are beneficial for muscle reinnervation after sciatic nerve transection in rats. Restor Neurol Neurosci. 2009;27:297–305. doi: 10.3233/RNN-2009-0479. [DOI] [PubMed] [Google Scholar]
- 65.Cavalcante Miranda de AD, Martins LE, Teixeira GB, et al. The parameters of transcutaneous electrical nerve stimulation are critical to its regenerative effects when applied just after a sciatic crush lesion in mice. Biomed Res Int 2014;2014:572949. [DOI] [PMC free article] [PubMed]
- 66.English AW, Schwartz G, Meador W, Sabatier MJ, Mulligan A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol. 2007;67:158–172. doi: 10.1002/dneu.20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foecking EM, Fargo KN, Coughlin LM, Kim JT, Marzo SJ, Jones KJ. Single session of brief electrical stimulation immediately following crush injury enhances functional recovery of rat facial nerve. J Rehabil Res Dev. 2012;49:451–458. doi: 10.1682/JRRD.2011.03.0033. [DOI] [PubMed] [Google Scholar]
- 68.Franz CK, Rutishauser U, Rafuse VF. Intrinsic neuronal properties control selective targeting of regenerating motoneurons. Brain. 2008;131:1492–1505. doi: 10.1093/brain/awn039. [DOI] [PubMed] [Google Scholar]
- 69.Haastert-Talini K, Schmitte R, Korte N, Klode D, Ratzka A, Grothe C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma. 2011;28:661–674. doi: 10.1089/neu.2010.1637. [DOI] [PubMed] [Google Scholar]
- 70.Haastert-Talini K, Grothe C. Electrical stimulation for promoting peripheral nerve regeneration. Int Rev Neurobiol. 2013;109:111–124. doi: 10.1016/B978-0-12-420045-6.00005-5. [DOI] [PubMed] [Google Scholar]
- 71.Hetzler LE, Sharma N, Tanzer L, et al. Accelerating functional recovery after rat facial nerve injury: Effects of gonadal steroids and electrical stimulation. Otolaryngol Head Neck Surg. 2008;139:62–67. doi: 10.1016/j.otohns.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Huang J, Hu X, Lu L, Ye Z, Zhang Q, Luo Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J Biomed Mater Res A. 2010;93:164–174. doi: 10.1002/jbm.a.32511. [DOI] [PubMed] [Google Scholar]
- 73.Huang J, Lu L, Hu X, et al. Electrical stimulation accelerates motor functional recovery in the rat model of 15-mm sciatic nerve gap bridged by scaffolds with longitudinally oriented microchannels. Neurorehabil Neural Repair. 2010;24:736–745. doi: 10.1177/1545968310368686. [DOI] [PubMed] [Google Scholar]
- 74.Huang J, Lu L, Zhang J, et al. Electrical stimulation to conductive scaffold promotes axonal regeneration and remyelination in a rat model of large nerve defect. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0039526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koppes AN, Zaccor NW, Rivet CJ, et al. Neurite outgrowth on electrospun PLLA fibers is enhanced by exogenous electrical stimulation. J Neural Eng. 2014;11:046002. doi: 10.1088/1741-2560/11/4/046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu MC, Ho CY, Hsu SF, et al. Effects of electrical stimulation at different frequencies on regeneration of transected peripheral nerve. Neurorehabil Neural Repair. 2008;22:367–373. doi: 10.1177/1545968307313507. [DOI] [PubMed] [Google Scholar]
- 77.McDevitt L, Fortner P, Pomeranz B. Application of weak electric field to the hindpaw enhances sciatic motor nerve regeneration in the adult rat. Brain Res. 1987;416:308–314. doi: 10.1016/0006-8993(87)90911-5. [DOI] [PubMed] [Google Scholar]
- 78.Monaco GN, Brown TJ, Burgette RC, et al. Electrical stimulation and testosterone enhance recovery from recurrent laryngeal nerve crush. Restor Neurol Neurosci. 2015;33:571–578. doi: 10.3233/RNN-130334. [DOI] [PubMed] [Google Scholar]
- 79.Rozman J, Zorko B, Seliskar A. Regeneration of the radial nerve in a dog influenced by electrical stimulation. Pflugers Arch. 2000;439:R184–R186. doi: 10.1007/s004240000139. [DOI] [PubMed] [Google Scholar]
- 80.Sharma N, Coughlin L, Porter RG, et al. Effects of electrical stimulation and gonadal steroids on rat facial nerve regenerative properties. Restor Neurol Neurosci. 2009;27:633–644. doi: 10.3233/RNN-2009-0489. [DOI] [PubMed] [Google Scholar]
- 81.Sharma N, Moeller CW, Marzo SJ, Jones KJ, Foecking EM. Combinatorial treatments enhance recovery following facial nerve crush. Laryngoscope. 2010;120:1523–1530. doi: 10.1002/lary.20997. [DOI] [PubMed] [Google Scholar]
- 82.Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol. 2010;223:183–191. doi: 10.1016/j.expneurol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 83.Singh B, Xu QG, Franz CK, et al. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm. J Neurosurg. 2012;116:498–512. doi: 10.3171/2011.10.JNS11612. [DOI] [PubMed] [Google Scholar]
- 84.Tagami Y, Kurimoto T, Miyoshi T, Morimoto T, Sawai H, Mimura O. Axonal regeneration induced by repetitive electrical stimulation of crushed optic nerve in adult rats. Jpn J Ophthalmol. 2009;53:257–266. doi: 10.1007/s10384-009-0657-8. [DOI] [PubMed] [Google Scholar]
- 85.Thompson NJ, Sengelaub DR, English AW. Enhancement of peripheral nerve regeneration due to treadmill training and electrical stimulation is dependent on androgen receptor signaling. Dev Neurobiol. 2014;74:531–540. doi: 10.1002/dneu.22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Udina E, Furey M, Busch S, Silver J, Gordon T, Fouad K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol. 2008;210:238–247. doi: 10.1016/j.expneurol.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 87.Wan L, Zhang S, Xia R, Ding W. Short-term low-frequency electrical stimulation enhanced remyelination of injured peripheral nerves by inducing the promyelination effect of brain-derived neurotrophic factor on Schwann cell polarization. J Neurosci Res. 2010;88:2578–2587. doi: 10.1002/jnr.22426. [DOI] [PubMed] [Google Scholar]
- 88.Wang WJ, Zhu H, Li F, Wan LD, Li HC, Ding WL. Electrical stimulation promotes motor nerve regeneration selectivity regardless of end-organ connection. J Neurotrauma. 2009;26:641–649. doi: 10.1089/neu.2008.0758. [DOI] [PubMed] [Google Scholar]
- 89.Xu C, Kou Y, Zhang P, et al. Electrical stimulation promotes regeneration of defective peripheral nerves after delayed repair intervals lasting under one month. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0105045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao CH, Chang RL, Chang SL, Tsai CC, Tsai FJ, Chen YS. Electrical stimulation improves peripheral nerve regeneration in streptozotocin-induced diabetic rats. J Trauma Acute Care Surg. 2012;72:199–205. doi: 10.1097/TA.0b013e31822d233c. [DOI] [PubMed] [Google Scholar]
- 91.Yeh CC, Lin YC, Tsai FJ, Huang CY, Yao CH, Chen YS. Timing of applying electrical stimulation is an important factor deciding the success rate and maturity of regenerating rat sciatic nerves. Neurorehabil Neural Repair. 2010;24:730–735. doi: 10.1177/1545968310376758. [DOI] [PubMed] [Google Scholar]
- 92.Huang J, Zhang Y, Lu L, Hu X, Luo Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur J Neurosci. 2013;38:3691–3701. doi: 10.1111/ejn.12370. [DOI] [PubMed] [Google Scholar]
- 93.Calvey C, Zhou W, Stakleff KS, et al. Short-term electrical stimulation to promote nerve repair and functional recovery in a rat model. J Hand Surg Am. 2015;40:314–322. doi: 10.1016/j.jhsa.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 94.Gordon T, Amirjani N, Edwards DC, Chan KM. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol. 2010;223:192–202. doi: 10.1016/j.expneurol.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 95.Kilmer SL, Carlsen RC. Chronic infusion of agents that increase cyclic AMP concentration enhances the regeneration of mammalian peripheral nerves in vivo. Exp Neurol. 1987;95:357–367. doi: 10.1016/0014-4886(87)90144-0. [DOI] [PubMed] [Google Scholar]
- 96.Pichichero M, Beer B, Clody DE. Effects of dibutyryl cyclic AMP on restoration of function of damaged sciatic nerve in rats. Science. 1973;182:724–725. doi: 10.1126/science.182.4113.724. [DOI] [PubMed] [Google Scholar]
- 97.McQuarrie IG, Grafstein B, Gershon MD. Axonal regeneration in the rat sciatic nerve: effect of a conditioning lesion and of dbcAMP. Brain Res. 1977;132:443–453. doi: 10.1016/0006-8993(77)90193-7. [DOI] [PubMed] [Google Scholar]
- 98.Han PJ, Shukla S, Subramanian PS, Hoffman PN. Cyclic AMP elevates tubulin expression without increasing intrinsic axon growth capacity. Exp Neurol. 2004;189:293–302. doi: 10.1016/j.expneurol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 99.Flora G, Joseph G, Patel S, et al. Combining neurotrophin-transduced schwann cells and rolipram to promote functional recovery from subacute spinal cord injury. Cell Transplant. 2013;22:2203–2217. doi: 10.3727/096368912X658872. [DOI] [PubMed] [Google Scholar]
- 100.Pearse DD, Pereira FC, Marcillo AE, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 101.Udina E, Ladak A, Furey M, Brushart T, Tyreman N, Gordon T. Rolipram-induced elevation of cAMP or chondroitinase ABC breakdown of inhibitory proteoglycans in the extracellular matrix promotes peripheral nerve regeneration. Exp Neurol. 2010;223:143–152. doi: 10.1016/j.expneurol.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aglah C, Gordon T, Posse De Chaves EI. cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology. 2008;55:8–17. doi: 10.1016/j.neuropharm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 103.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/S0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 104.Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- 105.Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qiu J, Cai D, Filbin MT. Glial inhibition of nerve regeneration in the mature mammalian CNS. Glia. 2000;29:166–174. doi: 10.1002/(SICI)1098-1136(20000115)29:2<166::AID-GLIA10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 107.Goldberg JL, Barres BA. Nogo in nerve regeneration. Nature. 2000;403:369–370. doi: 10.1038/35000309. [DOI] [PubMed] [Google Scholar]
- 108.Zhao RR, Fawcett,JW. Combination treatment with chondroitinase ABC in spinal cord injury-breaking the barrier. Neurosci Bull 2013;4:477--483. [DOI] [PMC free article] [PubMed]
- 109.Campbell G, Anderson PN, Turmaine M, Lieberman AR. GAP-43 in the axons of mammalian CNS neurons regenerating into peripheral nerve grafts. Exp Brain Res. 1991;87:67–74. doi: 10.1007/BF00228507. [DOI] [PubMed] [Google Scholar]
- 110.Woolf CJ. Turbocharging neurons for growth: accelerating regeneration in the adult CNS. Nat Neurosci. 2001;4:7–9. doi: 10.1038/82921. [DOI] [PubMed] [Google Scholar]
- 111.Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci. 2001;4:38–43. doi: 10.1038/82881. [DOI] [PubMed] [Google Scholar]
- 112.Wood MD, Moore AM, Hunter DA, et al. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomater. 2009;5:959–968. doi: 10.1016/j.actbio.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wood MD, Borschel GH, Sakiyama-Elbert SE. Controlled release of glial-derived neurotrophic factor from fibrin matrices containing an affinity-based delivery system. J Biomed Mater Res A. 2009;89:909–918. doi: 10.1002/jbm.a.32043. [DOI] [PubMed] [Google Scholar]
- 114.Moore AM, Wood MD, Chenard K, et al. Controlled delivery of glial cell line-derived neurotrophic factor enhances motor nerve regeneration. J Hand Surg Am. 2010;35:2008–2017. doi: 10.1016/j.jhsa.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 115.Wood MD, Macewan MR, French AR, et al. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol Bioeng. 2010;106:970–979. doi: 10.1002/bit.22766. [DOI] [PubMed] [Google Scholar]
- 116.Wood MD, Kim H, Bilbily A, et al. GDNF released from microspheres enhances nerve regeneration after delayed repair. Muscle Nerve. 2012;46:122–124. doi: 10.1002/mus.23295. [DOI] [PubMed] [Google Scholar]
- 117.Wood MD, Gordon T, Kim H, et al. Fibrin gels containing GDNF microspheres increase axonal regeneration after delayed peripheral nerve repair. Regen Med. 2013;8:27–37. doi: 10.2217/rme.12.105. [DOI] [PubMed] [Google Scholar]
- 118.Wood MD, Gordon T, Kemp SW, et al. Functional motor recovery is improved due to local placement of GDNF microspheres after delayed nerve repair. Biotechnol Bioeng. 2013;110:1272–1281. doi: 10.1002/bit.24800. [DOI] [PubMed] [Google Scholar]
- 119.Eggers R, De WF, Hoyng SA, et al. Lentiviral vector-mediated gradients of GDNF in the injured peripheral nerve: Effects on nerve coil formation. Schwann cell maturation and myelination. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0071076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Santosa KB, Jesuraj NJ, Viader A, et al. Nerve allografts supplemented with schwann cells overexpressing glial-cell-line-derived neurotrophic factor. Muscle Nerve. 2013;47:213–223. doi: 10.1002/mus.23490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wood K, Wilhelm JC, Sabatier MJ, Liu K, Gu J, English AW. Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev Neurobiol. 2012;72:688–698. doi: 10.1002/dneu.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Redett R, Jari R, Crawford T, Chen YG, Rohde C, Brushart TM. Peripheral pathways regulate motoneuron collateral dynamics. J Neurosci. 2005;25:9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sunderland S. Nerve and nerve injuries. Edinburgh: Livingstone; 1978. [Google Scholar]
- 125.Wong JN, Olson JL, Morhart MJ, Chan KM. Electrical stimulation enhances sensory recovery: A randomized control trial. Ann Neurol. 2015;77:996–1006. doi: 10.1002/ana.24397. [DOI] [PubMed] [Google Scholar]
- 126.Chan KM, Gordon T, Zochodne DW, Power HA. Improving peripheral nerve regeneration: from molecular mechanisms to potential therapeutic targets. Exp Neurol. 2014;261:826–835. doi: 10.1016/j.expneurol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 127.Placheta E, Wood MD, Lafontaine C, et al. Enhancement of facial nerve motoneuron regeneration through cross-face nerve grafts by adding end-to-side sensory axons. Plast Reconstr Surg. 2015;135:460–471. doi: 10.1097/PRS.0000000000000893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)