Abstract
A respiratory load compensation response is characterized by increases in activation of primary respiratory muscles and/or recruitment of accessory respiratory muscles. The contribution of the external intercostal (EI) muscles, which are a primary respiratory muscle group, during normal and loaded breathing remains poorly understood in conscious animals. Consciousness has a significant role on modulation of respiratory activity, as it is required for the integration of behavioral respiratory responses and voluntary control of breathing. Studies of respiratory load compensation have been predominantly focused in anesthetized animals, which make their comparison to conscious load compensation responses challenging. Using our established model of intrinsic transient tracheal occlusions (ITTO), our aim was to evaluate the motor behavior of EI muscles during normal and loaded breathing in conscious rats. We hypothesized that 1) conscious rats exposed to ITTO will recruit the EI muscles with an increased electromyogram (EMG) activation and 2) repeated ITTO for 10 days would potentiate the baseline EMG activity of this muscle in conscious rats. Our results demonstrate that conscious rats exposed to ITTO respond by recruiting the EI muscle with a significantly increased EMG activation. This response to occlusion remained consistent over the 10-day experimental period with little or no effect of repeated ITTO exposure on the baseline ∫EI EMG amplitude activity. The pattern of activation of the EI muscle in response to an ITTO is discussed in detail. The results from the present study demonstrate the importance of EI muscles during unloaded breathing and respiratory load compensation in conscious rats.
Keywords: external intercostal, respiratory neurophysiology, conscious state
the thoracic intercostal muscles are primary respiratory muscles (35) with the external intercostal muscles (EI) primarily inspiratory in action during eupnea (22, 55). The EI muscles also play a prominent role in trunk movements (62), and their activity is dependent on posture (24, 25, 62). The EI muscles act together with other muscles of the chest wall to produce ventilatory pressure changes for breathing (7). However, these observations are based on studies in anesthetized, reduced preparations in cats (51) and dogs (21, 23), sleeping cats (24, 25), or conscious (62) and sleeping (30) healthy humans. The function of intercostal muscles in conscious animals remains poorly understood.
The descending drive to the rat's thoracic spinal cord is an integrated complex combination of direct and indirect inputs from the ventral respiratory group (33, 38), dorsal respiratory group (14), cervical spinal cord (37, 56, 57), and cortical motor tracts (47, 48, 50). Intercostal muscles are modulated by afferent feedback from their muscle spindles (10, 32, 46), tendon organs (4, 5), and joint receptors (16). These afferents act segmentally at the spinal cord level and on medullary inspiratory neurons to inhibit inspiratory activity (4, 5), and at the level of the cortex (13) may modulate intercostal muscle activity. Reflex connections between thoracic spinal segments (27) involving phrenic and intercostal motor neuronal systems (6, 36) have also been identified. Thus intercostal muscle activity is modulated via respiratory muscle afferent feedback on a breath-by-breath basis.
Ventilation is the rhythmic and coordinated activity of primary and accessory respiratory muscles to generate minute ventilation. When internal or external mechanical stimuli act to perturb the system, respiratory load compensation responses act to restore ventilatory homeostasis (9, 64). Respiratory load compensation is a motor response mediated by the primary and accessory muscles of respiration (39), and involves increases in activity of primary respiratory muscles (45, 49) and/or recruitment of accessory respiratory muscles (18). The majority of animal load compensation studies have been performed in anesthetized animals, making the translation to conscious load compensation behavior (such as occurs in humans) difficult. Consciousness significantly influences ventilation and is fundamental for voluntary control of breathing (31) and the integration of behavioral respiratory responses (41).
Intrinsic transient tracheal occlusion (ITTO) is an established model to study respiratory load compensation in conscious rats (44). ITTO allows transient, reversible, and repeatable occlusion of the trachea, thereby activating the respiratory load compensation response in conscious animals. ITTO is applied by inflating a vascular cuff implanted around the extra thoracic trachea with sufficient pressure to close off the lumen of the trachea. Deflating the cuff restores the tracheal lumen to its original state with no evidence of residual damage. Because of the short duration and transient nature of an ITTO stimulus, minimal fluctuations in arterial blood gasses are expected to occur. Respiratory load compensation responses have been reported in conscious rats for the diaphragm (44) but not for the EI muscles. The purpose of the present study is to determine the ITTO load compensation responses of EI muscles in conscious rats.
The multiple sources of descending input as well as muscle afferent feedback, phrenic-intercostal, intercostal-intercostal, and abdominal-intercostal connectivity all contribute to the complex neural control of the intercostal motor neuron system. We hypothesized that conscious rats exposed to ITTO will recruit the EI muscles with an increased electromyogram (EMG) activation. We also hypothesized that repeated ITTO for 10 days would potentiate the baseline EMG activity of this muscle in normal conscious rats.
MATERIALS AND METHODS
Animals.
The Institutional Animal Care and Use Committee at the University of Florida, Gainesville, reviewed and approved all procedures. Twenty-four adult male Sprague-Dawley rats (300-450 g) were studied (Harlan Laboratories, Indianapolis, IN). The animals were housed in a 12-h light/12-h dark cycle with free access to standard rat pellets and water.
Surgical protocol.
Animals were anesthetized with isoflurane anesthesia (3–5% in O2). Subcutaneous injections of carprofen (5 mg/kg body wt) and buprenorphine (0.03 mg/kg body wt) were administered preoperatively for management of pain and discomfort. After an absence of corneal and paw-withdrawal reflexes was confirmed, a 1-in. midline incision was made on the ventral surface of the neck. After isolating the extrathoracic trachea, a saline-filled inflatable cuff (Vivo Metric, Fine Science Tools) was placed around the trachea and the ends of the cuff sutured together. The actuator tube of the cuff was externalized by routing the tube subcutaneously to an incision between the scapulae. Small cutaneous incisions were made bilaterally on the chest wall at level T5-T7. The EI muscles were visualized by blunt dissection. An area between the parasternal and anterior axillary lines was exposed, and bipolar wire electrodes were sutured through the exposed EI muscles (Omnetics Connector, Minneapolis, MN). The EMG wires were also routed subcutaneously to the rats' dorsal incision between the scapulae. The incision in the dorsal scapular surface was closed with sutures, with the externalized actuator tube and connector head accessible for use during experiments. The incisions on the ventral surfaces were sutured. Rats were administered warm normal saline (0.01–0.02 ml/g body wt), penicillin (0.1 ml/kg PenG 30,000 units/ml), and gradually weaned off the isoflurane anesthesia. Postoperative analgesia was administered once every 24 h for 3 days using carprofen (5 mg/kg body wt) and buprenorphine (0.03 mg/kg body wt). Animals recovered for 1 wk before the experimental protocols were initiated.
Experimental protocol.
Instrumented animals were randomly divided into two groups: ITTO group (n = 16) and control group (n = 8). Conscious animals were placed in a whole body restrainer for the entire experimental trial duration. The EMG signals were amplified (P511 series, Grass Instruments, Quincy, MA) and band-pass filtered (30-1,000 Hz). Analog outputs were digitized at 5 kHz (Model 1401, Cambridge Electronics Design), computer processed (Spike2, Cambridge Electronics Design), and stored for subsequent analyses. Trial and data recording began after allowing the animals sufficient time (2–4 min) to acclimate to the restrainer. For the ITTO conditioning group, rats were placed in the restrainer and left undisturbed for 2 min of baseline recording. After 2 min, the ITTO group animals received 3–5 s of tracheal occlusion via cuff inflation by using saline pressure, followed by 10–15 s of unoccluded breathing. This was repeated for a total of 20 min. Cuff pressure was recorded with a differential pressure transducer connected to the actuator tube and syringe.
A total of ∼35–40 tracheal occlusions were presented over the 20 min. After ITTO trials were completed, the animals were allowed to recover for 2 min post-ITTO while recording EI EMG activity. Control animals were placed in the restrainer, EMG activity recorded, and no ITTOs presented throughout the 24-min trial duration. At the end of the trial, the animals were removed from the restrainer and returned to their cage. This procedure was repeated every day for a total of 10 days. The timing of ITTO trials was maintained throughout the 10 days of trials for every animal. Animals were euthanized after 10 days of experimental trials by overdosing with isoflurane gas anesthesia (5% in O2). After the breathing had stopped the diaphragm was cut to create a pneumothorax.
Data Analyses
Integrated EMG analysis.
Raw EI EMG was finite impulse response digital high-pass filtered (300 Hz; Spike2, Cambridge Electronic Design) to minimize the effect of heart rate and movement artifacts. DC remove, rectification, and smoothing (50 ms) functions were applied to the filtered data. This integrated EI EMG (∫EI EMG) was used for further analyses. Data for days 1, 3, 5, 7, and 10 were analyzed. The ∫EI EMG recording for each of the analyzed days was divided into the following:
Before—2-min baseline activity prior to ITTO exposure.
Onset—initial breath response during each ITTO presentation.
Phasic—all remaining breaths during each ITTO presentation.
Two seconds after—2-s time period immediately after offset of each ITTO presentation.
Between—time period in between each ITTO presentations excluding the 2 s after period.
After—2 min after completion of ITTO exposure.
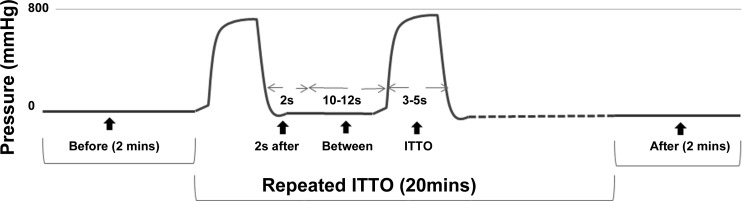
These phases are illustrated in Fig. 1.
Fig. 1.
Occlusion pressure during phases of intrinsic transient tracheal occlusions (ITTO) trial. The trace is the internal tracheal cuff pressure. A minimum of 200 mmHg of cuff pressure is required to fully occlude the tracheal lumen. The phases of ITTO trial are defined on the pressure trace.
Peak ∫EI EMG amplitude and peak-to-peak ∫EI EMG frequency values were obtained from Before and After and a minimum of 5–10 randomly selected ITTO presentations for days 1, 3, 5, 7, and 10 for each animal. Data measures greater than ±3 SD were considered as outliers and eliminated from further analysis (42). Peak ∫EI EMG amplitude were normalized to Before values on 1) day 1 and 2) within each day (day 1, 3, 5, 7, and 10). Based on the Onset response on day 1, ITTO animals were divided into two groups for post hoc analysis: 1) High responders, those that showed an increase in percentage change ∫EI EMG amplitude (n = 7); and 2) Low responders, those that showed a decrease in percentage change ∫EI EMG amplitude (n = 6). Percentage changes in ∫EI EMG amplitude and group data for peak-to-peak ∫EI EMG frequency were analyzed for statistical significance (SigmaPlot 12.5, Systat Software).
Statistical analysis.
Normalized percentage change in ∫EI EMG amplitude and peak-to-peak ∫EI EMG frequency were used for statistical analyses. One-way repeated measures ANOVA was used for within-group analysis across days 1, 3, 5, 7, and 10. One-way ANOVA was used to statistically compare data between ITTO and control groups. Multiple pair-wise comparison procedure using Student-Newman-Keuls method was used to identify group differences. Data were considered significant for P ≤ 0.05. All data are presented as means ± SE (unless noted otherwise).
RESULTS
Results presented here are from the ITTO group (n = 14) and the control group (n = 8). Two animals from the ITTO group with mean amplitude values greater than ± 3 SD were considered as outliers (42) and eliminated from analyses.
Characteristics of applied ITTO stimulus and effect on animal weight.
Presurgical weight (Table 1) of the animals was not significantly different between the ITTO and control groups. Both groups showed significant decreases in body weight on ITTO day 1 compared with presurgical weight: ITTO 316 ± 13.7, P < 0.05, and control 355.4 ± 19.6, P < 0.05. Animals showed continuous increases in body weight on ITTO days 3, 5, and 7, and by ITTO day 10 all rats had regained their body weight and were not significantly different between groups (Table 1). The mean durations and occlusion pressures during applied ITTO (Table 2) were not significantly different across days.
Table 1.
Body weight of the animal groups
| Presurgery | ITTO Day 1 | ITTO Day 3 | ITTO Day 5 | ITTO Day 7 | ITTO Day 10 | |
|---|---|---|---|---|---|---|
| ITTO | 348.4 ± 13.0 | 316.0 ± 13.7* | 325.8 ± 18.0‡ | 324.5 ± 15.3‡ | 333.3 ± 17.0† | 358.0 ± 11.4 |
| Control | 381.4 ± 21.0 | 355.4 ± 19.6* | 359.8 ± 17.5‡ | 366.6 ± 16.4† | 377.4 ± 14.0 | 384.3 ± 13.0 |
Body weight (g) for both intrinsic transient tracheal occlusions (ITTO) and control groups on study days 1, 3, 5, 7, and 10 as means ± SE.
P < 0.05 compared with presurgical weight;
P < 0.05 and
P < 0.01 for body weights compared to ITTO day 10.
Table 2.
Mean pressure and duration of ITTO presentations
| ITTO Day 1 | ITTO Day 3 | ITTO Day 5 | ITTO Day 7 | ITTO Day 10 | |
|---|---|---|---|---|---|
| Pressure | 954.2 ± 11.6 | 949.5 ± 15.8 | 950.9 ± 12.4 | 958.2 ± 12.2 | 943.4 ± 29.5 |
| Duration | 4.7 ± 0.3 | 4.4 ± 0.2 | 4.8 ± 0.2 | 4.8 ± 0.2 | 4.9 ± 0.2 |
ITTO tracheal cuff pressure (mmHg) and ITTO duration (seconds) for the trial days as means ± SE. No significant differences were recorded across days.
ITTO-mediated respiratory load compensation in ∫EI EMG muscle of conscious rats.
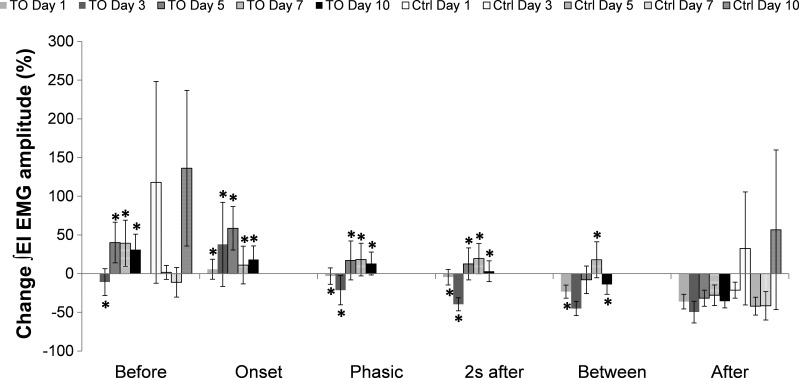
The responses to ITTO of experimental and control animals on days 1, 3, 5, 7, and 10 are presented in Fig. 2. Before data normalized to day 1 of ITTO trial presentation resulted in percentage changes in Before on day 3 (−10.94 ± 17.26%), day 5 (40.18 ± 26.19%), day 7 (39.25 ± 29.87%), and day 10 (31.05 ± 19.89%). The baseline activity (Before) of the EI muscles was increased before ITTO presentation began on trial days 5, 7, and 10 compared with day 1.
Fig. 2.
Percent change in ∫ external intercostal (EI) electromyogram (EMG) amplitude normalized to day 1 Before for each phase of the ITTO (TO) trial. *P ≤ 0.05 significance compared with After values of the same day. No significant differences were found in the control group within days.
Onset and Phasic were the two phases corresponding to responses observed during the application of an ITTO. ITTO animals showed a significant load compensation response to applied ITTO on all days. Percentage changes in Onset response on the time points were: day 1 (5.64 ± 12.92%), day 3 (37.78 ± 54.28%), day 5 (58.57 ± 28.11%), day 7 (11.07 ± 24.40%), and day 10 (18.16 ± 17.66%). Percentage changes in Phasic response were the following: day 1 (−3.20 ± 10.59%), day 3 (−21.46 ± 18.85%), day 5 (17.00 ± 25.15%), day 7 (18.17 ± 21.08%), and day 10 (13.11 ± 14.84%). The Onset and Phasic responses on all days were significantly greater (P ≤ 0.05) than the After response on the same day: day 1 (−36.13 ± 9.49%), day 3 (−49.65 ± 14.00%), day 5 (−31.78 ± 10.48%), day 7 (−28.00 ± 13.30%), and day 10 (−35.68 ± 8.59%).
Percentage changes in 2 s after response were the following: day 1 (−4.56 ± 10.10%), day 3 (−39.69 ± 8.44%), day 5 (12.59 ± 20.64%), day 7 (19.38 ± 19.53%), and day 10 (3.09 ± 13.47%). The 2 s after response on each day was significantly greater (P ≤ 0.05) than the After response on the same day: day 1 (−36.13 ± 9.49%), day 3 (−49.65 ± 14.00%), day 5 (−31.78 ± 10.48%), day 7 (−28.00 ± 13.30%), and day 10 (−35.68 ± 8.59%). The percentage changes in Between response were the following: day 1 (−23.24 ± 8.48%), day 3 (−45.12 ± 9.21%), day 5 (−8.00 ± 17.83%), day 7 (17.85 ± 23.22%), and day 10 (−14.02 ± 12.63%). During this phase, percentage change in ∫EI EMG amplitude was significantly greater (P ≤ 0.05) than that during After on a days 1, 7, and 10; day 1 (−36.13 ± 9.49%), day 3 (−49.65 ± 14.00%), day 5 (−31.78 ± 10.48%), day 7 (−28.00 ± 13.30%) and day 10 (−35.68 ± 8.59%).
Percentage changes in the After response were: day 1 (−36.13 ± 9.49%), day 3 (−49.65 ± 14.00%), day 5 (−31.77 ± 10.48%), day 7 (−28.00 ± 13.30%), and day 10 (−35.68 ± 8.59%). Responses during After were the following: day 3 (−49.65 ± 14.00%), day 5 (−31.78 ± 10.48%), day 7 (−28.00 ± 13.30%), and day 10 (−35.68 ± 8.59%); they were significantly smaller than those during Before: day 3 (−10.94 ± 17.26%), day 5 (40.18 ± 26.19%), day 7 (−39.25 ± 29.87%), and day 10 (31.05 ± 19.89%), on all days (P ≤ 0.05).
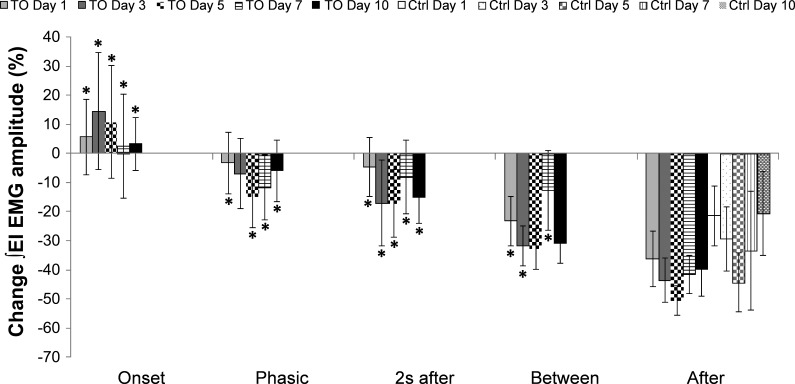
Figure 3 presents the data normalized to Before values on the same day; for example, ∫EI EMG amplitude for Onset response from day 3 was normalized to ∫EI EMG amplitude Before values on day 3. Percentage changes on day 1 during Onset (5.64 ± 12.92%), Phasic (−3.20 ± 10.59%), 2 s after (−4.56 ± 10.10%), and Between (−23.24 ± 8.49%) were significantly greater (P ≤ 0.05) than After (−36.13 ± 9.49%). Percentage changes on day 3 during Onset (14.51 ± 20.18%), 2 s after (−17.04 ± 14.67%), and Between (−31.84 ± 6.83%) were significantly greater (P ≤ 0.05) than After (−42.33 ± 7.46%). Percentage changes on day 5 during Onset (10.92 ± 19.38%), Phasic (−14.90 ± 10.51%), and 2 s after (−17.15 ± 11.73%) were significantly greater (P ≤ 0.05) than After (−50.55 ± 4.95%). Percentage changes on day 7 during Onset (2.58 ± 17.83%), Phasic (−11.53 ± 11.17%), 2 s after (−8.01 ± 12.61%), and Between (−12.67 ± 13.68%) were significantly greater (P ≤ 0.05) than After (−41.59 ± 6.56%). Percentage changes on day 10 during Onset (3.25 ± 9.17%), Phasic (−5.94 ± 10.64%), 2 s after (−14.99 ± 9.05%), and Between (−30.85 ± 6.94%) were significantly greater (P ≤ 0.05) than After (−39.86 ± 9.09%). The After responses for the control group on day 1 (−21.38 ± 10.30%), day 3 (−29.34 ± 11.09%), day 5 (−44.28 ± 10.23%), day 7 (−33.46 ± 20.45%), day 10 (−20.64 ± 14.52%), and for the ITTO group were not significantly different between groups and across days.
Fig. 3.
Percent change in ∫EI EMG amplitude normalized to the same day Before. Each phase of the ITTO trial for the ITTO group and the After phase for the control group on days 1, 3, 5, 7, and 10 were normalized to same day Before values. *P ≤ 0.05 significance compared with After of the same day. No significant differences were found in the control group across all days.
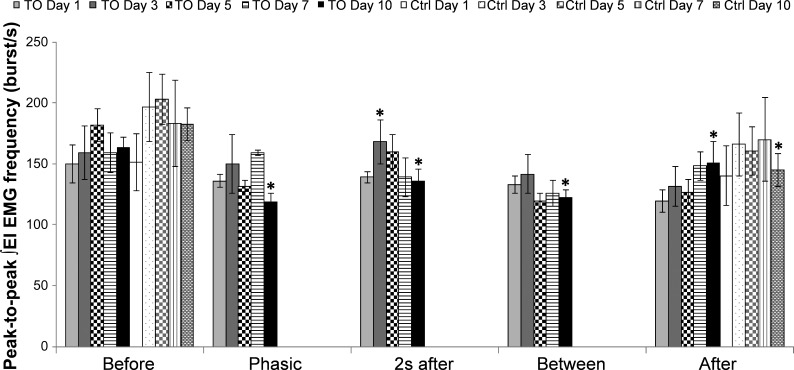
Peak-to-peak frequency of ∫EI EMG.
The peak-to-peak ∫EI EMG frequency during eupneic Before phase of the trial was not significantly different across days and between groups (Fig. 4). During an ITTO trial, changes in peak-to-peak ∫EI EMG frequency were not significantly different on days 1, 5, and 7. On day 3, 2 s after (168.0 ± 18.3 bursts/min) showed a significantly higher (P ≤ 0.05) frequency than After (131.2 ± 16.4 bursts/min) in the ITTO group. On day 10, peak-to-peak ∫EI EMG frequency was significantly decreased (P ≤ 0.05) during Phasic (119.0 ± 6.9 bursts/min), 2s after (136.3 ± 8.9 bursts/min), Between (122.6 ± 5.7 bursts/min), and After (150.6 ± 17.9 bursts/min) compared with Before (163.6 ± 7.9 bursts/min). Peak-to-peak ∫EI EMG frequency in the control animal group remained stable over days 1, 3, 5, and 7. There was a significant decrease (P ≤ 0.05) during After (144.9 ± 13.6 bursts/min) compared with Before (182.1 ± 13.5 bursts/min) on day 10 in the control group.
Fig. 4.
Peak-to-peak ∫EI EMG frequency on days 1, 3, 5, 7, and 10. In ITTO animals on day 3, peak-to-peak frequency of ∫EI EMG during 2 s after was significantly greater than After. In ITTO animals on day 10, peak-to-peak ∫EI EMG frequency during Phasic, 2 s after, Between, and After significantly decreased compared with Before in the experimental group. For control animals on day 10, peak-to-peak ∫EI EMG frequency After was significantly lower than Before. *P ≤ 0.05.
Characteristics of ITTO-mediated ∫EI EMG muscle load compensation response.
The time required to elicit the Onset response when an ITTO was presented is termed the latency-to-onset response. Repeated presentations of ITTO did not alter the pattern of the EI muscle load compensation response. The latency-to-onset response on day 1 (0.5 ± 0.03 s), day 3 (0.4 ± 0.04 s), day 5 (0.6 ± 0.04 s), day 7 (0.5 ± 0.05 s), and day 10 (0.5 ± 0.04 s) and number of phasic breaths on day 1 (6.5 ± 0.5), day 3 (7.5 ± 0.5), day 5 (7.1 ± 0.2), day 7 (7.5 ± 0.4), and day 10 (6.4 ± 0.4) were not significantly different.
Effect of ITTO conditioning on eupneic ∫EI EMG.
Figure 2 presents the percentage changes in ∫EI EMG across days with data normalized to Before values from day 1. The Before value on day 3 (−10.94 ± 17.26%) is a decreased percentage change, and on day 5 (40.18 ± 26.2%), day 7 (39.25 ± 29.87%), and day 10 (31.05 ± 19.89%) are all increased percentage changes in ∫EI EMG. This indicates that ∫EI EMG was increased (days 5, 7, and 10) compared with the Before trial phase on day 1. The After values on day 1 (−36.13 ± 9.49%), day 3 (−49.65 ± 14.01%), day 5 (−31.77 ± 10.48%), day 7 (−28.00 ± 13.30%), and day 10 (−35.68 ± 8.59%) were all decreased percentage changes. Thus the After ∫EI EMG decreased on days 1, 3, 5, 7, and 10 relative to the Before trial phase on day 1. Normalized Before value on each day was significantly greater than After on the same day (P ≤ 0.05).
Control animals were not exposed to ITTO and, therefore, data from corresponding Before and After are used for analyses. Percentage changes increased for control animals Before responses on day 3 (117.96 ± 130.29%), day 5 (1.65 ± 9.06%), day 7 (−11.18 ± 19.14%), and day 10 (136.24 ± 100.55%). The percentage changes in the After ∫EI EMG responses decreased on day 1 (−21.38 ± 10.30%), day 5 (−42.04 ± 11.50%), and day 7 (−41.60 ± 18.28%), but increased on day 3 (32.47 ± 73.01%) and day 10 (56.64 ± 103.12%). Percentage changes in the After ∫EI EMG amplitude response were not significantly different, compared with Before, across the study days in control animals.
Variability of EI muscle EMG responses to ITTO in conscious rats.
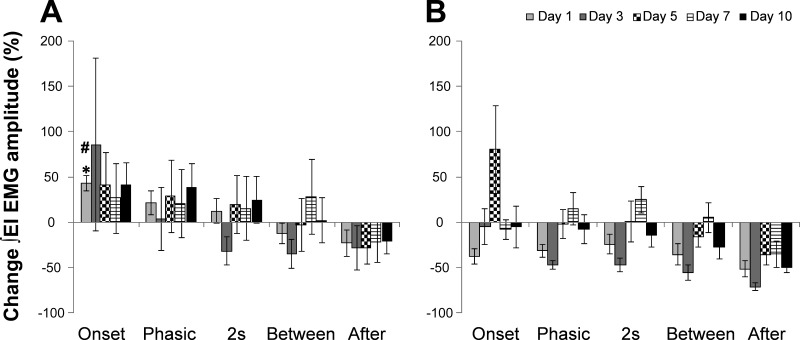
To assess the variability in responses to ITTO observed, post hoc analyses were performed (Fig. 5). Animals were separated into High responders (Fig. 5A) and Low responders (Fig. 5B) based on their Onset responses on day 1. Seven animals were High responders and the remaining six were Low responders on ITTO day 1. This segregation of animals into High and Low responder groups remained throughout the 10 days of ITTO trials. In the High-responder group on ITTO day 1, the Onset response (42.85 ± 8.38%) was significantly greater (P ≤ 0.05) than the After (−23.01 ± 14.60%) response. The Onset response on ITTO day 1 for the High-responder group (42.85 ± 8.38%) was significantly greater (P ≤ 0.001) than the Low responder group (−37.77 ± 8.36%). No other significant differences were found between these groups and across time points in this study.
Fig. 5.
Percent change in ∫EI EMG amplitude on days 1, 3, 5, 7, and 10 normalized to ITTO day 1 Before values. A: High-responder animals had an increase in ∫EI EMG amplitude (%) during Onset on ITTO day 1. B: Low responder animals had a decrease in ∫EI EMG amplitude (%) during Onset on ITTO day 1. *P ≤ 0.05 for Onset compared with After on ITTO day 1, and #P ≤0.001 for Onset response in High vs. Low responders on ITTO day 1.
Our results demonstrate that conscious rats exposed to ITTO respond by recruiting the EI muscle with an increased EMG activation. This response to occlusion remained consistent over the 10 days of ITTO trials with little or no conditioning effect on the baseline EMG activity of EI muscles. In addition, 50% of conscious rats responded with an increase in ∫EI EMG immediately upon presentation with an ITTO. The remaining half initially decreased ∫EI EMG, but subsequently increased ∫EI EMG as ITTO was sustained over a few breaths.
DISCUSSION
The results from this study demonstrate that conscious rats consistently recruit the EI muscles in their load compensation response to ITTO. Increased EI activity has been previously demonstrated as a part of the respiratory load compensation response in rabbits (11), dogs (6, 15, 18–20), and humans (2, 17, 61, 62). The present study also demonstrates the motor pattern of EI muscle load compensation responses in conscious rats. Consistent with our hypothesis, the EI muscles of conscious rats were repeatedly recruited in response to an increased respiratory drive during an applied ITTO. It is likely that the EI muscle recruitment acts to increase ventilator pump forces by stabilizing the thoracic wall, thereby increasing the mechanical function of diaphragmatic contraction. The net effect of the conscious load compensation is to increase respiratory muscle output to attempt to maintain minute ventilation despite increased ventilatory mechanical load.
Presentation of an ITTO resulted in recruitment and increased EMG activation of the EI muscles on days 1, 3, 5, 7, and 10. The percentage change in ∫EI EMG amplitude was consistently and repeatedly increased during an ITTO presentation compared with the baseline activity. Thus repeated presentations of ITTO, both in a single trial and over 10 days, recruited the EI muscles with an increased ∫EI EMG amplitude activity, with no evidence of habituation. The pattern of the responses to an individual ITTO, latency-to-onset response, and the peak-to-peak ∫EI EMG frequency during Phasic activity did not change across trial days. This suggests that repeated exposure to ITTO did not cause neuromuscular potentiation (increased activity over days) or habituation (decreased activity over days). However, Smith et al. (53), using a similar ITTO conditioning paradigm, reported an over a 20% increase in intercostal muscle cross-sectional area. This suggests that 10 days of ITTO conditioning may result in increased respiratory muscle contraction force as a result of hypertrophy of the intercostal muscles. Thus the neuromuscular drive to the EI muscles increased to compensate for the increased respiratory load without the need for additional increases to neural drive for mechanical load compensation response, possibly due to ITTO-mediated EI muscle hypertrophy by day 10.
The activities of the EI muscles are dependent on their segmental level and lung volume. The EI muscles of the upper rib cage are reported to be inspiratory in action (26) at volumes encompassing the vital capacity range. On the other hand, EI muscles of the caudal rib cage are reported to have an expiratory action as lung volume increases (21). In the present study, the EI muscles between thoracic segments five and seven, a transition between cranial and caudal rib cage, were studied. The neurophysiological characteristics of these EI muscles in conscious rats during eupneic and load compensation breathing are likely different from anesthetized rats and intermediate between cranial and caudal EI muscles. In addition, no attempts were made to differentiate between inspiratory and expiratory function of the EI muscle activity. Future studies with corresponding diaphragm (40) and abdominal (1) muscle activity correlated with ventilatory volume changes (60) are required to determine breath phase-dependent functional characteristics of EI muscle load compensation responses in conscious rats.
The response to ITTO was characterized by an increase in ∫EI EMG amplitude with no change in the peak-to-peak ∫EI EMG frequency across days. However, after 10 days of ITTO conditioning, the ITTO animals showed a significant reduction in peak-to-peak ∫EI EMG frequency during all phases of the trial compared with day 10 Before values. This was also the case for the control animals in our study. Both the ITTO and control animals were restrained at all times during the trial. Restraint is a form of immobilization, which is a stress stimulus and influences the control of ventilatory pattern (34). Therefore, one possible explanation for this result could be that after 10 days of restraint the acute restraint effects at the beginning of the trial that caused the animals to breathe with a higher frequency habituated by day 10. As the trial progressed, this frequency increase was not maintained, and no further significant differences were noted.
The primary functions of the EI muscles are to maintain mechanical stability of the chest wall and to aid in the upward and outward movement of the rib cage in concert with contraction of the diaphragm (22, 28). In the present study, the EI muscles were always recruited with each ITTO presentation. This suggests that in conscious animals the EI muscles are activated to maintain chest wall stability as part of the respiratory load compensation response. However, the magnitude of EI muscle activation was variable, indicating that ∫EI EMG activity in response to ITTO may be dependent on the animals' conscious and affective state, posture, phase of breath during which ITTO was applied, and modulation via afferent feedback.
Modulation by conscious and affective state.
Consciousness significantly influences ventilatory activity by incorporating voluntary control (31) and integration of different behaviors (41) with breathing. Thus conscious state influences the sensory motor responses to internal and external respiratory stimuli. Sniffing, vocalization, and whole body movements affected the results from this study with conscious rats. In addition, ITTO is an aversive stimulus eliciting escape behavior that may account for the variable responses of EI muscle activity. Observations during the trials elicited these behaviors, and the corresponding EI data could not be used for respiratory pattern analyses. Hence, activity of EI muscles of conscious animals are behaviorally unique and dynamically modulated compared with anesthetized rats.
From previous studies in our lab, it is known that ITTO conditioning increases stress, anxiety, and associated neural changes in conscious rats (44). Afferent processing of respiratory stimuli is modality specific (63), and changes in affective state modulate sensory gating and cortical processing of conscious ventilatory sensations (8). Our lab has shown that ITTO conditioning in conscious rats causes anxiety and associated changes in gene expression in the thalamus (3). These in turn may influence respiratory activity pattern of the EI and other primary and accessory respiratory muscles. The possibility of learned responses to ITTO, however, is also likely, but may be masked by the current study use of restrained vs. freely moving rats. ITTO is an unexpected, inescapable stimulus. In freely moving rats, it has been suggested to elicit learned helplessness (43). For stimuli that result in progressive difficulty to breathe, habituation does not occur and respiratory awareness is heightened (59). This random and repeated application of an ITTO may have contributed to the variability in the data in conscious respiratory load compensation responses and may have differentiated the rats into High and Low responders.
Breath phase and lung volume.
Each ITTO presented was of a consistent duration, and the cuff occlusion pressure was also maintained relatively constant within each ITTO trial and across days. No significant differences were observed in these parameters. However, we did not selectively apply the ITTO to a breath phase. Respiratory rhythm is cyclic in nature (58), and application of a load during inspiratory or expiratory phases modulates the immediate and subsequent breaths differently (9, 64). This modulation is dependent on activity of vagal afferents, which in turn is dependent on lung volume during respiratory loading (12, 64). Future studies with corresponding breath phase differentiation will aid in the understanding of modulation of EI muscle activity by lung volume during respiratory loading in conscious rats.
Effect of afferent modulation on ∫EI EMG ITTO responses.
Afferent feedback from intercostal muscle spindles (10, 32, 46), tendon organs (4, 5), and joint receptors (16) modulates EI muscle activity. These afferents act segmentally (4, 5) and at the level of the cortex (13) to drive appropriate EI muscle activity to maintain ventilatory homeostasis. Modulation from reflex pathways between intercostal segments (27) and those involving phrenic and intercostal systems (6, 36) further ensure that EI muscle activity is modulated on a breath-by-breath basis. The results from the present study do not allow the evaluation of influences from each of these afferent mechanisms on these conscious animals.
Postural influences on ∫EI EMG responses to ITTO.
EI muscles have both respiratory and postural (62) functions. Dick et al. (25) demonstrated in cats sleeping in a curled, semiprone posture that EI muscle activity on the upward side was always greater than the opposite downward side. These authors also reported that the activity of the inspiratory intercostal muscles variably changed from awake to non-REM sleep states in supine restrained cats (24). This is contrary to reports in healthy adolescent human subjects where intercostal muscle activity increased during non-REM sleep in supine position (54). Therefore, although EI muscle activity is modulated by changes in posture, species differences in the pattern and type of modulation also exists. In the present study, the animals were restrained and in the prone position. The effect of this posture on baseline activity is likely present but could not be specifically differentiated from respiratory activity. Further, the EI muscle activity recorded in the present study was in conscious (nonsleeping) rats, which is also different from many previous conscious animal studies. Thus because of the need for restraint and the conscious state of the animal, influences from postural component of EI muscle activity are likely to contribute to the present outcome measures.
Effect of body weight on ∫EI EMG activity.
A change in body weight and associated changes in muscle fiber mass would affect recruitment of EI muscle and therefore the ∫EI EMG activity (40). The weight of the animals in our study was significantly decreased 1 wk after surgical instrumentation procedure. However, both groups of animals consistently gained weight over the study time period, no significant differences in body weight were observed between groups, and the body weights returned to presurgical levels by the end of our study. Therefore, although the potential effect of changed body weight cannot be neglected, the variability in ∫EI EMG responses observed in our data may not be due to loss of body weight. However, there may have been an increase in intercostal muscle fiber cross-sectional area and related EI motor unit output in these animals, similar to the hypertrophy effects previously reported (53).
A combination of the conscious state, afferent feedback modulation, restraint, posture, and EI muscle hypertrophy potentially influenced the results of the present study. We propose that these factors modulate the brainstem load compensation response not normally observed in anesthetized animals, resulting in variability of ∫EI EMG activity in conscious rats. To understand the distribution of the variability in the observed responses, we divided the ITTO animals into High and Low responders based on their Onset responses to an ITTO presentation. Approximately 50% percent of conscious rats were High responders with an increase in ∫EI EMG immediately upon presentation with an ITTO. Approximately 50% of conscious rats were Low responders with decreased ∫EI EMG at the Onset of the ITTO but subsequently increased as ITTO was sustained. This pattern was observed on the day 1 trial and persisted throughout the 10 days of ITTO. The between animal, within species/strain difference in Onset response is consistent with anxiety measures (29) with high- and low-anxiety rats within a strain and is usually only observed in conscious studies. The subgroups of High and Low responders may potentially result from this segregation in anxiety responses and further increase the inherent variability of ∫EI EMG activity (24) and load compensation responses in the conscious state.
Technical considerations.
Activity from underlying internal intercostal muscles may contribute to the EI EMG recordings, especially during ITTO. We used fine wire electrodes and surgical techniques to minimize contact with internal intercostal muscles, but electrical activity from the internal intercostal muscles can be conducted to the electrodes. Placement of wire electrodes in the EI muscle was confirmed postmortem, and results presented in this study are from successful EI muscle implants only. It is also important to consider movement of electrodes over time as a possible change in recording site (40). By suturing the electrode wires in place, we minimized the possibility of differences in recordings across days due to electrode displacement. Also, normalized EMG data analyses allow for statistical comparisons between animals and within the same animal over days. We normalized the data to two different time points: ITTO day 1 (Fig. 2) and to each day (Fig. 3) to decrease between- and within-animal variability. Postmortem macroscopic analysis of EI muscle surrounding electrode placement site confirmed the stability of electrode implant sites and an absence of fibrosis.
In summary, we have determined the respiratory load compensation responses of the EI muscles in response to ITTO in conscious rats. Activity of the EI muscles is not only dependent on descending drive (22, 28) but is also influenced by lung volume (21), breath phase (64), afferent modulation (52), and conscious state (24, 41). The present investigation focused on the intercostal spaces located between thoracic levels five and seven. These EI muscles are intermediate between the cranial and caudal thoracic levels and have different load compensation responses in the anesthetized state. The conscious rats had a respiratory load compensation response characterized by increased EI muscle activity on presentation of ITTO. There was significant variability in the load compensation response that is likely due to conscious state and responder subgroups within the same strain of rats used in this study. Further studies are required to understand the load compensation response of the EI muscles at all the thoracic levels and their integrated, functional relationship with other respiratory muscles that are critical for sustaining ventilation during increased respiratory mechanical load. The results from the present study demonstrate the importance of EI muscles during unloaded breathing and respiratory load compensation in conscious rats.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant R01 HL-109025.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.B.J. and P.W.D. conception and design of research; P.B.J. performed experiments; P.B.J. analyzed data; P.B.J. and P.W.D. interpreted results of experiments; P.B.J. prepared figures; P.B.J. drafted manuscript; P.B.J. and P.W.D. edited and revised manuscript; P.B.J. and P.W.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Poonam B. Jaiswal is currently a Postdoctoral Fellow at Emory University, Atlanta, GA.
REFERENCES
- 1.Abe T, Kusuhara N, Yoshimura N, Tomita T, Easton PA. Differential respiratory activity of four abdominal muscles in humans. J Appl Physiol 80: 1379–1389, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Altose MD, Kelsen SG, Stanley NN, Levinson RS, Cherniack NS, Fishman AP. Effects of hypercapnia on mouth pressure during airway occlusion in conscious man. J Appl Physiol 40: 338–344, 1976. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt V, Hotchkiss MT, Garcia-Reyero N, Escalon BL, Denslow N, Davenport PW. Tracheal occlusion conditioning in conscious rats modulates gene expression profile of medial thalamus. Front Physiol 2: 24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolser DC, Lindsey BG, Shannon R. Medullary inspiratory activity: influence of intercostal tendon organs and muscle spindle endings. J Appl Physiol 62: 1046–1056, 1987. [DOI] [PubMed] [Google Scholar]
- 5.Bolser DC, Remmers JE. Synaptic effects of intercostal tendon organs on membrane potentials of medullary respiratory neurons. J Neurophysiol 61: 918–926, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Brichant JF, De Troyer A. On the intercostal muscle compensation for diaphragmatic paralysis in the dog. J Physiol 500 (Pt 1): 245–253, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappello M, de Troyer A. Interaction between left and right intercostal muscles in airway pressure generation. J Appl Physiol (1985) 88: 817–820, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Chan PY, von Leupoldt A, Bradley MM, Lang PJ, Davenport PW. The effect of anxiety on respiratory sensory gating measured by respiratory-related evoked potentials. Biol Psychol 91: 185–189, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark FJ, von Euler C. On the regulation of depth and rate of breathing. J Physiol 222: 267–295, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Critchlow V, von Euler C. Intercostal muscle spindle activity and its gamma motor control. J Physiol 168: 820–847, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Angelo E, Monaco A, Pecchiari M. Motor control of the diaphragm in anesthetized rabbits. Respir Physiol Neurobiol 170: 141–149, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Davenport PW, Freed AN, Rex KA. The effect of sulfur dioxide on the response of rabbits to expiratory loads. Respir Physiol 56: 359–368, 1984. [DOI] [PubMed] [Google Scholar]
- 13.Davenport PW, Shannon R, Mercak A, Reep RL, Lindsey BG. Cerebral cortical evoked potentials elicited by cat intercostal muscle mechanoreceptors. J Appl Physiol 74: 799–804, 1993. [DOI] [PubMed] [Google Scholar]
- 14.de Castro D, Lipski J, Kanjhan R. Electrophysiological study of dorsal respiratory neurons in the medulla oblongata of the rat. Brain Res 639: 49–56, 1994. [DOI] [PubMed] [Google Scholar]
- 15.De Troyer A. Differential control of the inspiratory intercostal muscles during airway occlusion in the dog. J Physiol 439: 73–88, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Troyer A. Role of joint receptors in modulation of inspiratory intercostal activity by rib motion in dogs. J Physiol 503 (Pt 2): 445–453, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Troyer A, Estenne M. Coordination between rib cage muscles and diaphragm during quiet breathing in humans. J Appl Physiol 57: 899–906, 1984. [DOI] [PubMed] [Google Scholar]
- 18.De Troyer A, Farkas GA. Inspiratory function of the levator costae and external intercostal muscles in the dog. J Appl Physiol 67: 2614–2621, 1989. [DOI] [PubMed] [Google Scholar]
- 19.De Troyer A, Farkas GA. Linkage between parasternals and external intercostals during resting breathing. J Appl Physiol (1985) 69: 509–516, 1990. [DOI] [PubMed] [Google Scholar]
- 20.De Troyer A, Farkas GA, Ninane V. Mechanics of the parasternal intercostals during occluded breaths in dogs. J Appl Physiol 64: 1546–1553, 1988. [DOI] [PubMed] [Google Scholar]
- 21.De Troyer A, Kelly S, Macklem PT, Zin WA. Mechanics of intercostal space and actions of external and internal intercostal muscles. J Clin Invest 75: 850–857, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev 85: 717–756, 2005. [DOI] [PubMed] [Google Scholar]
- 23.De Troyer A, Ninane V. Respiratory function of intercostal muscles in supine dog: an electromyographic study. J Appl Physiol 60: 1692–1699, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Dick TE, Parmeggiani PL, Orem J. Intercostal muscle activity during sleep in the cat: an augmentation of expiratory activity. Respir Physiol 50: 255–265, 1982. [DOI] [PubMed] [Google Scholar]
- 25.Dick TE, Parmeggiani PL, Orem JM. Intercostal muscle activity of the cat in the curled, semiprone sleeping posture. Respir Physiol 56: 385–394, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Dimarco AF, Romaniuk JR, Supinski GS. Mechanical action of the interosseous intercostal muscles as a function of lung volume. Am Rev Respir Dis 142: 1041–1046, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Downman CB, Hussain A. Spinal tracts and supraspinal centres influencing visceromotor and allied reflexes in cats. J Physiol 141: 489–499, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldman JL. Neurophysiology of breathing in mammals. In: Handbook of Physiology. The Nervous System. Bethesda, MD: Am. Physiol. Soc, 1986, p. 463–524. [Google Scholar]
- 29.Gomes Vde C, Hassan W, Maisonnette S, Johnson LR, Ramos A, Landeira-Fernandez J. Behavioral evaluation of eight rat lines selected for high and low anxiety-related responses. Behav Brain Res 257: 39–48, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Henke KG, Badr MS, Skatrud JB, Dempsey JA. Load compensation and respiratory muscle function during sleep. J Appl Physiol (1985) 72: 1221–1234, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Hlastala MP, Berger AJ. Physiology of Respiration. Seattle, WA: Oxford University Press, 2001. [Google Scholar]
- 32.Holt GA, Johnson RD, Davenport PW. The transduction properties of intercostal muscle mechanoreceptors. BMC Physiol 2: 16, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanjhan R, Lipski J, Kruszewska B, Rong W. A comparative study of pre-sympathetic and Botzinger neurons in the rostral ventrolateral medulla (RVLM) of the rat. Brain Res 699: 19–32, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Kinkead R, Dupenloup L, Valois N, Gulemetova R. Stress-induced attenuation of the hypercapnic ventilatory response in awake rats. J Appl Physiol (1985) 90: 1729–1735, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol 179: 3–13, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Leduc D, de Troyer A. Effect of chest wall vibration on the canine diaphragm during breathing. Eur Respir J 19: 429–433, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Lipski J, Duffin J, Kruszewska B, Zhang X. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res 95: 477–487, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640: 171–184, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Lopata M, Onal E, Ginzburg AS. Respiratory muscle function during CO2 rebreathing with inspiratory flow-resistive loading. J Appl Physiol 54: 475–482, 1983. [DOI] [PubMed] [Google Scholar]
- 40.Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol 177: 176–182, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orem J, Netick A. Behavioral control of breathing in the cat. Brain Res 366: 238–253, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Osborne JW, Overbay A. The power of outliers (and why researchers should always check for them). Pract Assess Res Eval 9: 9, 2004. [Google Scholar]
- 43.Pate KM. Respiratory load compenstaion responses in concious animals. Doctoral dissertation retrieved from University of Florida library catalog. In: Physiological Sciences. Gainesville, FL: Univ. of Florida, 2010, p. 144. [Google Scholar]
- 44.Pate KM, Davenport PW. Tracheal occlusion conditioning causes stress, anxiety and neural state changes in conscious rats. Exp Physiol 98: 819–829, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pate KM, Davenport PW. Tracheal occlusions evoke respiratory load compensation and neural activation in anesthetized rats. J Appl Physiol 112: 435–442, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Remmers JE. Inhibition of inspiratory activity by intercostal muscle afferents. Respir Physiol 10: 358–383, 1970. [DOI] [PubMed] [Google Scholar]
- 47.Rikard-Bell GC, Bystrzycka EK, Nail BS. Cells of origin of corticospinal projections to phrenic and thoracic respiratory motoneurones in the cat as shown by retrograde transport of HRP. Brain Res Bull 14: 39–47, 1985. [DOI] [PubMed] [Google Scholar]
- 48.Rikard-Bell GC, Bystrzycka EK, Nail BS. The identification of brainstem neurones projecting to thoracic respiratory motoneurones in the cat as demonstrated by retrograde transport of HRP. Brain Res Bull 14: 25–37, 1985. [DOI] [PubMed] [Google Scholar]
- 49.Romaniuk JR, Supinski G, DiMarco AF. Relationship between parasternal and external intercostal muscle length and load compensatory responses in dogs. J Physiol 449: 441–455, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saji M, Miura M. Thoracic expiratory motor neurons of the rat: localization and sites of origin of their premotor neurons. Brain Res 507: 247–253, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Sears TA. The slow potentials of thoracic respiratory motoneurones and their relation to breathing. J Physiol 175: 404–424, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shannon R. Effects of thoracic dorsal rhizotomies on the respiratory pattern in anesthetized cats. J Appl Physiol Respir Environ Exerc Physiol 43: 20–26, 1977. [DOI] [PubMed] [Google Scholar]
- 53.Smith BK, Mathur S, Ye F, Martin A, Truelson SA, Vandenborne K, Davenport PW. Intrinsic transient tracheal occlusion training and myogenic remodeling of rodent parasternal intercostal fibers. J Rehabil Res Dev 51: 841–854, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabachnik E, Muller NL, Bryan AC, Levison H. Changes in ventilation and chest wall mechanics during sleep in normal adolescents. J Appl Physiol Respir Environ Exerc Physiol 51: 557–564, 1981. [DOI] [PubMed] [Google Scholar]
- 55.Taylor A. The contribution of the intercostal muscles to the effort of respiration in man. J Physiol 151: 390–402, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian GF, Duffin J. Connections from upper cervical inspiratory neurons to phrenic and intercostal motoneurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 110: 196–204, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 111: 178–186, 1996. [DOI] [PubMed] [Google Scholar]
- 58.von Euler C. The functional organization of the respiratory phase-switching mechanisms. Fed Proc 36: 2375–2380, 1977. [PubMed] [Google Scholar]
- 59.von Leupoldt A, Vovk A, Bradley MM, Lang PJ, Davenport PW. Habituation in neural processing and subjective perception of respiratory sensations. Psychophysiology 48: 808–812, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker JK, Lawson BL, Jennings DB. Breath timing, volume and drive to breathe in conscious rats: comparative aspects. Respir Physiol 107: 241–250, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Whitelaw WA, Derenne JP, Milic-Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol 23: 181–199, 1975. [DOI] [PubMed] [Google Scholar]
- 62.Whitelaw WA, Ford GT, Rimmer KP, De Troyer A. Intercostal muscles are used during rotation of the thorax in humans. J Appl Physiol 72: 1940–1944, 1992. [DOI] [PubMed] [Google Scholar]
- 63.Zechman FW, Davenport PW. Temporal differences in the detection of resistive and elastic loads to breathing. Respir Physiol 34: 267–277, 1978. [DOI] [PubMed] [Google Scholar]
- 64.Zechman FW, Frazier DT, Lally DA. Respiratory volume-time relationships during resistive loading in the cat. J Appl Physiol 40: 177–183, 1976. [DOI] [PubMed] [Google Scholar]