Abstract
αKlotho is a circulating protein that originates predominantly from the kidney and exerts cytoprotective effects in distant sites. We previously showed in rodents that the lung is particularly vulnerable to αKlotho deficiency. Because acute lung injury is a common and serious complication of acute kidney injury (AKI), we hypothesized that αKlotho deficiency in AKI contributes to lung injury. To test the hypothesis, we created AKI by renal artery ischemia-reperfusion in rats and observed the development of alveolar interstitial edema and increased pulmonary oxidative damage to DNA, protein, and lipids. Administration of αKlotho-containing conditioned media 6 h post-AKI did not alter plasma creatinine but improved recovery of endogenous αKlotho production 3 days post-AKI, reduced lung edema and oxidative damage, and increased endogenous antioxidative capacity in the lung. Intravenously injected αKlotho rapidly exits alveolar capillaries as a macromolecule, suggesting transcytosis and direct access to the epithelium. To explore the epithelial action of αKlotho, we simulated oxidative stress in vitro by adding hydrogen peroxide to cultured A549 lung epithelial cells. Purified recombinant αKlotho directly protected cells at 20 pM with half-maximal effects at 40-50 pM, which is compatible with circulating αKlotho levels. Addition of recombinant αKlotho activated an antioxidant response element reporter and increased the levels of target proteins of the nuclear factor erythroid-derived 2 related factor system. In summary, αKlotho deficiency in AKI contributes to acute lung injury by reducing endogenous antioxidative capacity and increasing oxidative damage in the lung. αKlotho replacement partially reversed these abnormalities and mitigated pulmonary complications in AKI.
Keywords: oxidative stress, total antioxidant capacity, cytoprotection, antioxidant response element, nuclear factor erythroid-derived 2 transcription factor
acute lung injury culminating in acute respiratory distress syndrome (ARDS) is a life-threatening complication of systemic organ failure such as acute kidney injury (AKI). In general, pulmonary dysfunction in AKI is underappreciated and its complex multifactorial pathophysiology remains incompletely understood. There are a host of “reno-pulmonary” pathways in clinical and experimental settings that can compromise lung function in AKI (8, 10, 42, 47, 55). Septic AKI patients with multiorgan failure are especially prone to developing ARDS. In nonseptic patients, impaired cardiac contractility, metabolic acidosis, and volume overload (26, 33, 34) can all contribute to respiratory failure and a high mortality. Independent from these septic and cardiovascular issues, there are tissue factors such as apoptosis, macrophage-derived mediators, chemokines, and ion channel derangements (12, 28, 29, 44) that have been implicated in the pathogenesis of alveolar hyperpermeability leading to the “inflamed, leaky, and wet” lung with low-pressure pulmonary edema. Patients with AKI develop significant functional impairment of diffusing capacity, forced vital capacity, and maximal ventilation (8, 10, 42, 47, 55). The occurrence of ARDS requiring ventilator support drastically increases AKI mortality from 29% to 81% (6). There is a dire need for strategies to protect the lung against secondary injury in the presence of extrapulmonary organ failure such as AKI.
The αKlotho gene was discovered when its serendipitous disruption led to premature multiorgan failure in mice (31). αKlotho is a single-pass transmembrane protein produced predominantly by the kidney that functions as an obligate coreceptor for fibroblast growth factor 23, which is crucial for mineral metabolism (22). The extracellular domain of αKlotho is released via cleavage by secretases into blood, urine, and cerebrospinal fluid as endocrine-soluble αKlotho (14, 24, 36) exerting multiple effects, including antioxidation, antiapoptosis, and antifibrosis, on distant organs (15, 22). αKlotho is not normally expressed in the lung, but the alveolar capillary bed is constantly exposed to circulating αKlotho via perfusion by the entire cardiac output. Hemizygous genetic αKlotho haplo-insufficient mice are normal in all organs at baseline except the lung, which exhibits age-exacerbated degenerative changes such as air space enlargement and increased compliance (46, 50), suggesting particularly high sensitivity of the lung to αKlotho deficiency. We have shown that administration of conditioned media containing recombinant αKlotho protein protects the lung and lung epithelial cells against hyperoxic damage by increasing endogenous antioxidative capacity in vivo and in vitro (46).
Because circulating soluble αKlotho originates mostly from the kidney, AKI is a state of transient systemic αKlotho deficiency (20, 43). AKI per se is also a state of heightened systemic oxidative stress (40). These observations beg the question of whether αKlotho deficiency in AKI contributes to the development of acute lung injury. We hypothesized that αKlotho deficiency is a mediator of pulmonary damage and dysfunction in AKI. To test this hypothesis, we used a rodent renal ischemia-reperfusion injury (IRI) model of AKI and its associated αKlotho deficiency to characterize and quantify lung injury. To investigate causality, we replenished serum αKlotho in AKI with exogenous αKlotho-containing conditioned medium and queried whether pulmonary abnormalities are alleviated. In addition, we visualized the in vivo transit of circulating αKlotho, a 130-kDa macromolecule, from the alveolar capillary to the interstitium and epithelium. Finally, we simulated oxidative stress in vitro by adding hydrogen peroxide (H2O2) to cultured lung epithelial cells, and tested the antioxidative cytoprotective mechanisms of αKlotho cytoprotection.
METHODS
Animal Models
All animal experiments were conducted following the Guide for the Care and Use of Laboratory Animals by The National Institutes of Health. The Institutional Animal Care and Use Committee at University of Texas Southwestern approved all study protocols. An established IRI model was conducted as previously described (20). Briefly, male Sprague-Dawley rats (250–350 g) were housed in a 12:12-h light-dark cycle with free access to rodent chow and water. Under anesthesia, renal arteries were clamped with arterial clips (30 min), and occlusion was visually verified as blanching of the entire kidney surface. After clips were removed, the kidneys were observed for at least 5 min to ensure reperfusion. Control (Sham) animals underwent laparotomy of the same duration with manual manipulation of the kidneys without arterial clamping. At 3 days following surgery, blood was drawn and stored at −80°C for further processing. Plasma and urine chemistry was analyzed using a Vitros Chemistry Analyzer (Ortho-Clinical Diagnosis, Rochester, NY). Serum αKlotho concentration was measured by immunoprecipitation-immunoblot (1).
To examine the effects of αKlotho replacement in AKI, the rats received intraperitoneal injections of either control medium (100 μl), or αKlotho-containing conditioned medium (100 μl, ∼60–100 pM, n = 8) 6 h after induction of IRI (see Conditioned αKlotho-Containing Medium and Recombinant Soluble αKlotho for preparation of conditioned medium). Seven to eight animals per group were used. Three days after IRI, rats were killed by intraperitoneal overdose injection of Euthasol. The left lung was perfused with sterile PBS and snap-frozen in liquid nitrogen for the assays described later. The right lung was fixed by intratracheal instillation of 4% paraformaldehyde at a constant airway pressure (25 cmH2O). Tissue blocks from the right caudal lobe were sampled, embedded in paraffin, sectioned (4 μm) and stained (trichrome) for histological evaluation.
For immunohistochemistry, lung sections were incubated with anti-FLAG antibody (Sigma-Aldrich, St. Louis, MO) for FLAG-αKlotho, Oregon Green 488 phalloidin (Invitrogen, Carlsbad, CA) for actin, and CD31 antibody (Abcam, Cambridge, U.K.) to outline the endothelium. The sections were incubated with the appropriate secondary antibodies: Alexa fluor 555 (red; FLAG-αKlotho), Alexa fluor 488 (green; CD31), and SYTO 61 (red converted to blue pseudocolor for nuclei; Invitrogen).
To determine whether circulating αKlotho directly reaches alveolar epithelium or acts via other mechanisms on the epithelium, we visualized the fate of intravenously injected FLAG-tagged αKlotho. Three normal C57/BL6 mice were anesthetized with intraperitoneal ketamine (100 mg/kg body wt) and xylazine (10 mg/kg). Through a transverse neck incision (∼0.5 cm), the trachea was cannulated, the right jugular vein was isolated, and a silastic catheter filled with heparinized saline was introduced into the vessel. Recombinant αKlotho (4 nmol) was injected, and, after a predetermined time, the lung was fixed by tracheal instillation of 4% paraformaldehyde at 25 cmH2O pressure. Two control mice received injection of vehicle (saline).
Conditioned αKlotho-Containing Medium and Recombinant Soluble αKlotho
Two types of αKlotho preparations were used. Conditioned media containing αKlotho were prepared as previously described (46) for use in animal experiments. Briefly, Chinese hamster ovary (CHO) cells were stably transfected with soluble αKlotho or empty vector. Serum-free DMEM was added 16 h posttransfection and, after another 16 h, media were collected and stored at −80°C until further processing. The conditioned medium contains recombinant soluble αKlotho along with other secreted proteins from the CHO cells. The control media were obtained from CHO cells transfected with only the expression plasmid.
Purified recombinant αKlotho was used in αKlotho tracking and in cell culture experiments. Murine soluble αKlotho was cloned into pEF1/Myc-His/A vector (Invitrogen) and transfected into suspension of Free-Style 293-F cells (Invitrogen). To establish stable cell lines that expressed recombinant protein, cells were selected in 500 μg/ml G418 (Gibco, Grand Island, NY). Single clones of stable αKlotho-expressing cells were cultured in serum-free medium (Invitrogen) with 100 μg/ml G418 and 10% Pluronic F-68. The condition media were collected at suspension cell density ∼300 × 104 and centrifuged at 15,000 g for 30 min at 4°C to remove cell debris. The supernatant was mixed with Ni-NTA resin (Thermo, Waltham, MA) that was pre-equilibrated with 20 mM phosphate, pH 7.4, 300 mM NaCl. After incubation at 1 h at room temperature, the Ni-NTA resin was packed into the Econo column and washed with the above buffer (35× column volume). Bound αKlotho was eluted by 50–250 mM imidazole. The highest concentration αKlotho protein was eluted at the second fraction by 50 mM imidazole. αKlotho protein quality was checked by SDS-PAGE, Coomassie blue staining of a single band, and immunoblot with specific αKlotho antibodies (data not shown).
Lung Edema Estimation (Na+ to Dry Lung Weight Ratio)
Lung tissue (∼100 mg) was weighed and transferred to a platinum ashing crucible and placed on a hot plate set at 100°C under a heat lamp for 2 h. The dried-sample-containing crucible was weighed to determine the sample dry weight. The crucible was placed overnight in the ash oven set at 600°C, removed, and weighed to determine the ash weight. The ash was dissolved in 2 ml of HCl, and the Na+ content was measured by flame photometry.
Cell Culture
Human A549 lung epithelial cells (American Type Culture Collection, Manassas, VA) were grown in DMEM-nutrient mixture F12 (Life Technologies, Grand Island, NY) with 1% l-glutamine. An in vitro oxidative stress model using H2O2, modified from one employed by Panesso et al. (43) in kidney cells, was used to study the cytoprotective effects of αKlotho. Triplicate experiments and assays were performed.
Biochemical Assays
Cell injury.
A lactate dehydrogenase (LDH) cytotoxicity detection kit (Clontech Laboratories, Mountain View, CA) was used to measure release of cytoplasmic LDH into the culture supernatant. LDH generates NADH from lactate, which reacts with diaphorase to generate formazan dye products (absorbance 490 nm).
Total antioxidant capacity.
Both copper- and iron-based assays were performed. Copper-reducing equivalents were measured using a calorimetric assay (OxiSelect; Cell BioLabs, San Diego, CA). The Trolox antioxidant assay (Sigma-Aldrich) is based on formation of a ferryl myoglobin radical from metmyoglobin and H2O2, which oxidizes 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) to generate the ABTS.+ radical cation, a soluble chromogen (absorbance 405 nm). Trolox, a water-soluble vitamin E analog, serves as a standard control antioxidant.
Antioxidant reporter.
A dual-luciferase assay (Cignal; SA Biosciences, Valencia, CA) was used to measure the activity of Nrf-1 and -2 (nuclear factor E2-related factors 1 and 2) transcription factor activation of tandem antioxidant response element (ARE) regulating genes that protect cells from oxidative damage. The ARE reporter contains an inducible antioxidant responsive firefly luciferase construct and a constitutively expressed Renilla luciferase construct. Upon transfection with the ARE reporter, the activities of the firefly and Renilla luciferases quantify transcriptional activities of the antioxidant response pathway.
Oxidative damage.
8-Hydroxydeoxyguanosine (8-OHdG) was used as a marker of oxidative DNA injury. DNA was extracted using DNAzol (Life Technologies), precipitated in 100% ethanol, washed with 70% ethanol, and suspended in 8 mM NaOH, and the 8-OHdG concentration was determined by ELISA (OxiSelect; Cell BioLabs) compared against a 8-OHdG standard curve. Protein carbonyl was measured by ELISA (OxiSelect; Cell BioLabs) as a surrogate for protein oxidation against a known reduced/oxidized BSA standard curve. 8-Isoprostane was measured by enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) as a marker for lipid oxidation, based on the competitive binding between 8-isoprostane and an 8-isoprostane acetylcholinesterase conjugate (8-isoprostane tracer) for limited specific binding sites.
Immunoblot.
Whole cell lysates from A549 cells after treatment were prepared using RIPA buffer (150 mM NaCl, 50 mM Tris·HCl, pH 7.4, 5 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, and 0.1% SDS) containing fresh phosphatase and protease inhibitors and cleared by centrifugation (14,000 g, 4°C, 30 min), and protein content was determined by the method of Bradford. Protein samples (30 µg) were loaded onto SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes. After blocking in 5% nonfat milk, PVDF membranes were probed with primary antibodies against MT (Metallothionein, Santa Cruz Biotechnology, San Diego, CA, sc-11377, 1:500), HOX1 (Heme Oxygenase 1, Abcam, Cambridge, MA, ab13248, 1:500), HOX2 (Heme Oxygenase 2, Abcam, ab90492, 1:1000), and PXDN (Peroxidasin homolog, Abcam, ab179663, 1:500) overnight at 4°C followed by secondary antibodies conjugated with horseradish peroxidase. Specific signal was visualized by enhanced chemiluminescence (Amersham Life Sciences, Pittsburgh, PA) and quantified by densitometry using ImageQuant.
Data Analysis
Measurements from seven to eight animals per group and triplicate independent assays or in vitro experiments were expressed as means ± SD. Statistical analysis used factorial ANOVA with a post hoc Fisher's protected least-significant difference test, and P < 0.05 was considered significant.
RESULTS
Acute Lung Injury in AKI
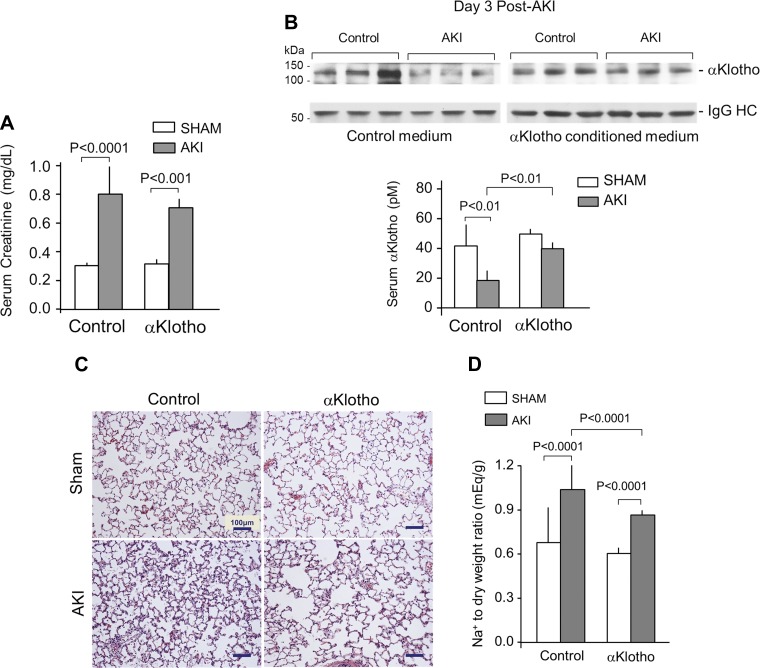
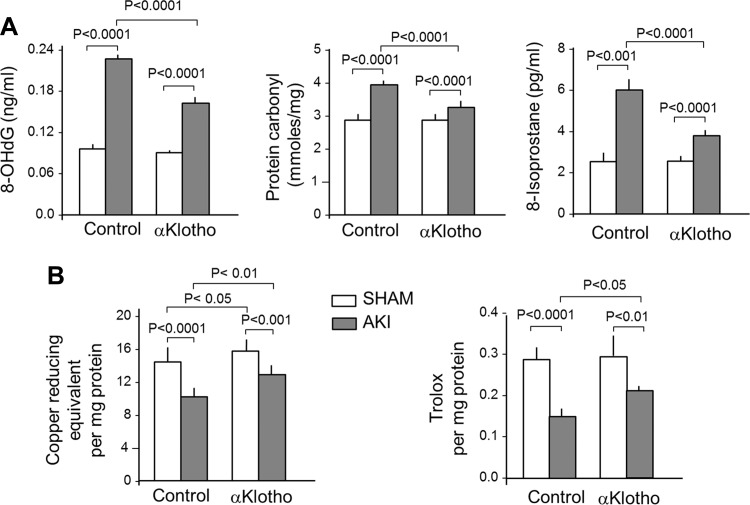
The established model of renal ischemia-reperfusion injury markedly increased serum creatinine, which typically peaks at five to eight times normal control levels at 1–2 days as reported by Hu et al. (20), then declined to two to three times control levels by Day 3 (Fig. 1A), and reduced circulating αKlotho levels by ∼60% (Fig. 1B). In this AKI setting, lung histology showed small crowded distal air spaces, thicker alveolar walls, and cellular exudation into the air spaces (Fig. 1C). The Na+ to dry lung weight ratio was elevated, indicating tissue edema (Fig. 1D). The lungs from AKI animals showed generalized oxidative damage of DNA, protein, and lipids compared with control (sham AKI) lungs (Fig. 2A), which is consistent with heightened oxidative stress to the lungs in AKI in a manner similar to that previously described by Ravikumar et al. (46) in direct lung injury caused by acute hyperoxia exposure. Total antioxidant capacity measured by copper-reducing equivalents and Trolox (a water-soluble vitamin E analog) equivalent assays showed diminished antioxidant capacity with AKI. These results show an association between circulating αKlotho deficiency in AKI and lung damage, which may reflect depletion of endogenous antioxidants in AKI (Fig. 2B).
Fig. 1.
αKlotho deficiency and repletion in acute kidney injury (AKI). Sprague-Dawley rats underwent bilateral ischemia-reperfusion injury (IRI), and lungs were harvested 3 days later. The control group underwent general anesthesia and laparotomy with manual manipulation of the kidneys but without IRI (Sham). Rats received intraperitoneal injection of either αKlotho-containing or control conditioned media 6 h after surgery. A: severity of AKI was measured by serum creatinine level in the animals 3 days after IRI. B: serum αKlotho level was measured by immunoprecipitation-immunoblot 3 days after IRI at the time of lung harvest. Representative blots are shown. IgG-HC, immunoglobulin heavy chain. C: representative lung histology (Trichrome stain). D: Na+ to dry weight ratio as a measure of interstitial edema. Means ± SD (n = 7–8 animals per group). Statistical significance was assessed by ANOVA.
Fig. 2.
Acute lung injury in AKI. Sprague-Dawley rats underwent bilateral IRI and lungs were harvested 3 days later. The control group underwent general anesthesia and laparotomy with manual manipulation of the kidneys but without IRI (Sham). Rats received intraperitoneal injection with either αKlotho-containing or control conditioned media 6 h after surgery, and lungs were harvested 3 days later. A: markers of DNA (8-hydroxy-2′-deoxyguanosine; 8OHdG), protein (protein carbonyl), and lipid (8-isoprosane) oxidative damage. B: markers for endogenous antioxidative capacity were assessed by a Cu-based (left) or a Fe-based (right) assay. Bars and error bars are mean and SD (n = 7–8 animals per group). Statistical significance was assessed by ANOVA.
Role of αKlotho in Lung Protection
αKlotho replacement given before or within 1 h after IRI alleviates AKI and accelerates recovery (20), but αKlotho repletion given 4 h or later post-IRI does not affect the peak plasma creatinine (vehicle vs. αKlotho-treated in AKI in a separate cohort of 3 animals each: Day 1: 1.35 ± 0.17 vs. 1.27 ± 0.09 mg/dl; Day 2: 1.25 ± 0.26 vs. 1.13 ± 0.15 mg/dl, means ± SD). Therefore, in this study, by administering αKlotho 6 h after AKI induction by IRI, we did not change the course of AKI as defined by serum creatinine levels on Day 3 of AKI that were similar to that in the untreated group (Fig. 1A). However, exogenous αKlotho administration improved serum endogenous αKlotho levels 3 days after AKI (Fig. 1B). The higher αKlotho levels 3 days post-IRI are unlikely to be attributed to the exogenous αKlotho, because the half-life of αKlotho even in the complete anephric state is only 24 h (21); rather, this observation reflects improved endogenous αKlotho production. Exogenous αKlotho also increases native renal αKlotho expression after two forms of AKI including bilateral IRI (48).
Exogenous αKlotho administration significantly ameliorated alveolar septal crowding and exudation of cells and debris into the air space (Fig. 1B); alveolar edema (Fig. 1C); and oxidative damage to DNA, protein, and lipids in the lungs of animals with AKI (Fig. 2A). The reduction of oxidative damage with αKlotho repletion was associated with a higher endogenous oxidative capacity measured by two independent assays (Fig. 2B). These effects occurred with no change in serum creatinine (Fig. 1A), thus dissociating the benefit on the lung from the improvement of renal function by αKlotho. In control (sham) animals, exogenous αKlotho modestly increased total antioxidant capacity measured by copper-reducing equivalents but had no effect on Trolox total antioxidant capacity or any of the oxidative damage parameters. These interventional data strongly support αKlotho deficiency as a pathogenic factor in the development of acute lung injury in the setting of AKI.
Circulating αKlotho Can Directly Access Pulmonary Epithelial Cells
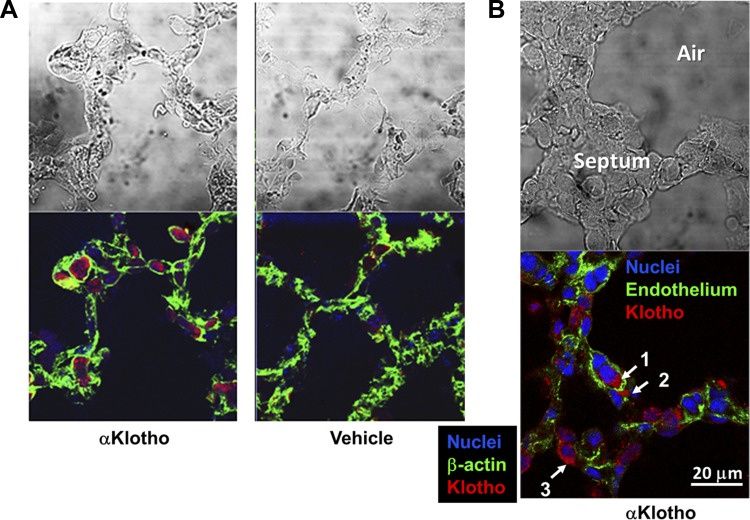
While the animal experiments offer important proof-of-principle data that αKlotho deficiency exacerbates and αKlotho repletion ameliorates lung injury, the complexities of the intact organism preclude examination of the direct actions of αKlotho on pulmonary epithelia. To understand the mechanisms of αKlotho cytoprotection, we first tracked whether this 130-kDa macromolecule can exit the pulmonary vasculature to access the alveolar epithelium. In the kidney it is known that αKlotho cannot traverse the glomerular capillary, but it can selectively cross the peritubular capillary freely, to be taken up by the tubular cells in a surprisingly short time (21). To test whether rapid transport occurs in pulmonary capillaries, we injected FLAG-tagged αKlotho intravenously in mice. Within 20–30 min after injection, the FLAG label was seen inside alveolar capillaries (Fig. 3, A and B), and also clearly detectable in the septal interstitial space with extracapillary cellular uptake, including possibly by alveolar type II cells (Fig. 3B). Thus circulating αKlotho can rapidly exit the capillary to access and directly act on the alveolar epithelium.
Fig. 3.
Tracking soluble αKlotho transfer in the lung. COOH-terminal FLAG-tagged purified recombinant αKlotho (4 nmol in 200 ml) was injected intravenously into 3 normal C57/BL6 mice, and the lungs were harvested 20–30 min after injection. Two control mice received injection of vehicle (saline). Exogenous αKlotho was stained with anti-FLAG antibody. A: 20 min after injection with FLAG-αKlotho or vehicle. Representative images of FLAG-αKlotho (anti-FLAG with Alexa fluor 555-coupled secondary antibody; red), β-actin (Oregon Green 488-coupled phalloidin; green), and nuclei (SYTO 61; red digitally converted to blue) are shown. B: 30 min after injection with FLAG-αKlotho. Representative images of FLAG-αKlotho (anti-FLAG with Alexa fluor 555-coupled secondary antibody; red), endothelium (anti-CD31 with Alexa fluor 488-coupled secondary antibody; green), and nuclei (SYTO 61; red digitally converted to blue) are shown. Arrows, localization of FLAG-αKlotho: intracapillary (1); interstitium (2); and epithelium (3), possibly an alveolar type 2 cell.
Cytoprotection by αKlotho via Antioxidation
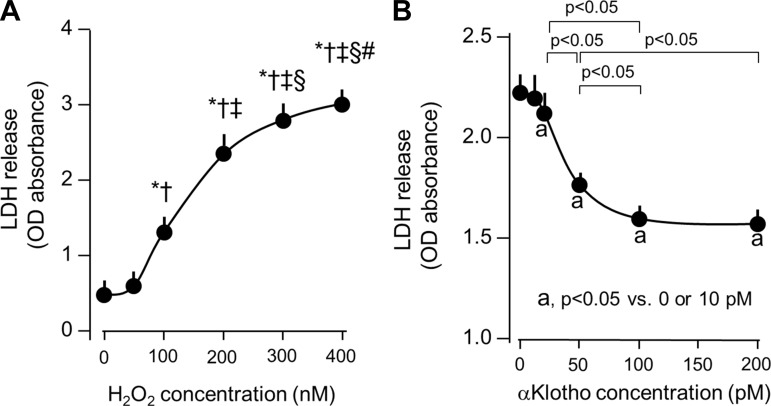
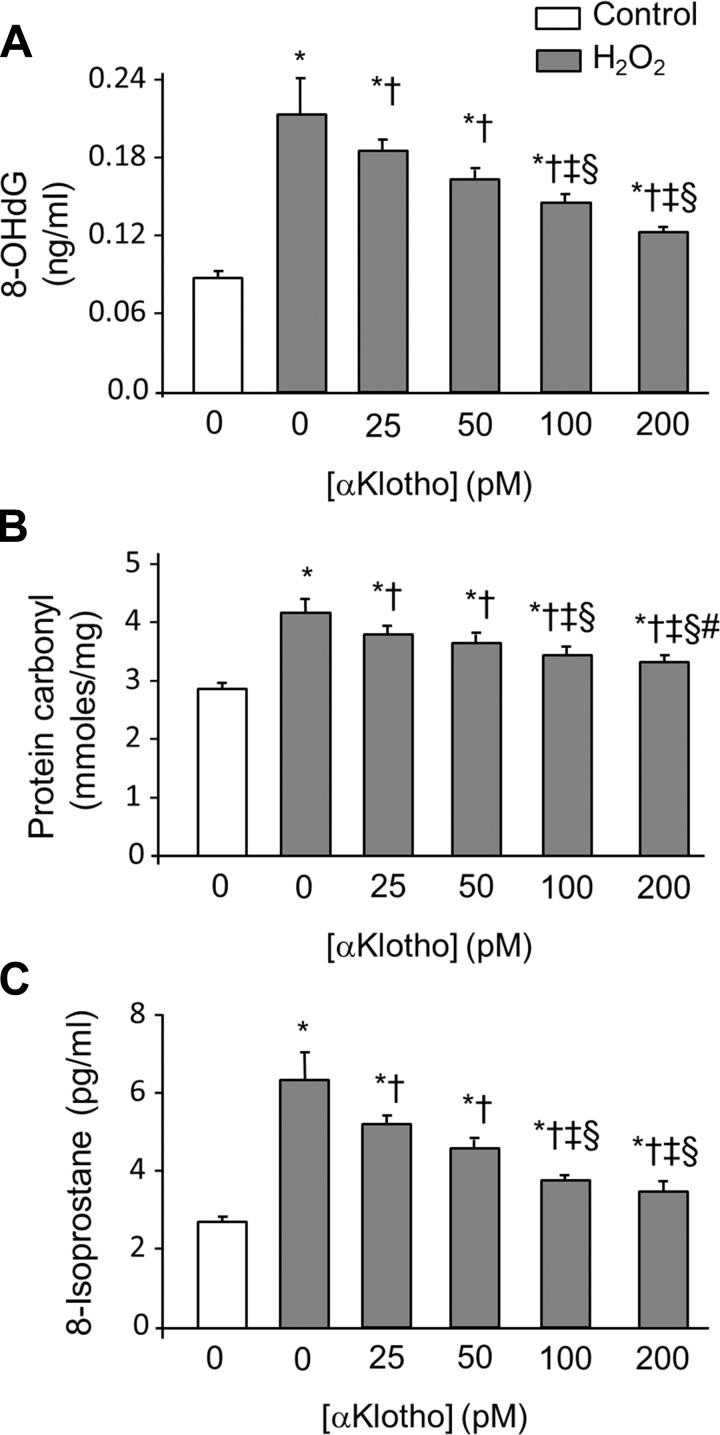
To examine whether αKlotho ameliorates cellular oxidative damage, H2O2 was added to cultured A549 human lung epithelial cells to simulate the post-AKI milieu of oxidative stress. We observed that H2O2 induced cellular damage measured by LDH release in a dose-dependent fashion (Fig. 4A). We chose a H2O2 concentration of 200 nM to induce moderate oxidative stress with measurable cell harm to enable the evaluation of cytoprotection. Previously, we found that αKlotho transfection and the addition of αKlotho-conditioned media prevented A549 cell death from hyperoxic insult (46), but that system did not permit determination of the αKlotho concentration required for cytoprotection and whether it is comparable to the circulating αKlotho concentration. Here we used purified recombinant αKlotho protein to define the dose range of αKlotho required for cytoprotection. A decline in H2O2-induced LDH release was detectable at a αKlotho concentration of 25 pM, and maximal response was attained at ∼200 pM (Fig. 4B). The range where cytoprotective efficacy is observed is in the same order of magnitude as the circulating αKlotho concentration in humans and rodents (1, 21). Consistent with its effect on LDH release, αKlotho addition ameliorated the increase in oxidative damage to DNA, protein, and lipids in a dose-dependent fashion in A549 cells exposed to H2O2 (Fig. 5, A–C).
Fig. 4.
Cytoprotection by αKlotho in an in vitro hydrogen peroxide (H2O2) model of oxidative damage. A: dose-response of H2O2 on lactate dehydrogenase (LDH) release as a marker of cell death. A549 lung epithelial cells were incubated with the indicated H2O2 dose for 4 h. *P < 0.0001 vs. 0 nmol/l H2O2, †P < 0.0001 vs. 50 nmol/l H2O2, ‡P < 0.001 vs. 100 nmol/l H2O2, §P < 0.05 vs. 200 nmol/l H2O2, #P < 0.05 vs. 300 nmol/l H2O2. B: dose-response of purified recombinant αKlotho on H2O2-induced A549 cell death. aP < 0.05 vs. 0 or 10 pM αKlotho concentration. Average of 3 independent experiments (means ± SD). Statistical significance was assessed by ANOVA.
Fig. 5.
Markers of oxidative damage in cultured lung epithelial cells and protection by αKlotho protein. A549 cells were treated with 200 nM H2O2 for 3 h with different concentrations of recombinant αKlotho. A: DNA (8-OHdG). B: protein (protein carbonyl). C: lipid (8-isoprostane) oxidative damage. Average of 3 independent experiments (means ± SD). Statistical significance was evaluated by ANOVA. *P < 0.0001 vs. untreated cells, †P < 0.0001 vs. cells treated with 200 nmol/l H2O2 and 0 pM αKlotho, ‡P < 0.001 vs. cells treated with 200 nmol/l H2O2 and 25 pM αKlotho, §P < 0.05 vs. cells treated with 200 nmol/l H2O2 and 50 pM αKlotho, #P < 0.0001 vs. cells treated with 200 nmol/l H2O2 and 100 pM αKlotho.
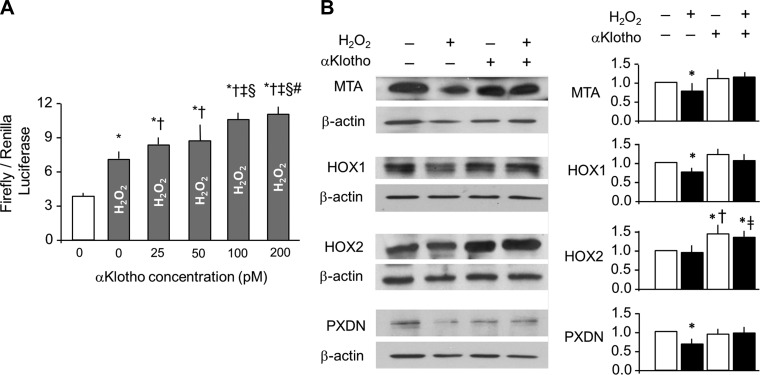
αKlotho-conditioned media contain many cell-derived substances that can potentially activate the Nrf2 pathway (46). Here we used purified recombinant αKlotho to show that it activates Nrf2 in a dose-dependent fashion in A549 cells challenged with H2O2 (Fig. 6A). We screened an array of downstream effectors of Nrf2 and found that αKlotho increased the protein levels of methalothionine (MTA), heme-oxygenase 1 and 2 (HOX1 and HOX2), and peroxidasin (PXDN; Fig. 6B).
Fig. 6.
Activation of nuclear-factor erythroid derived 2 transcription factor (Nrf2) pathway in lung epithelial cells by αKlotho. A: A549 cells were transfected with an antioxidant responsive element (ARE)-luciferase reporter and were treated with the stated concentration of recombinant αKlotho. Activation of ARE is expressed as firefly luciferase luminescence ratio to the control Renilla. Average of 3 independent experiments (means ± SD). Statistical significance was assessed by ANOVA. *P < 0.0001 vs. untreated cells, †P < 0.001 vs. cells treated with 200 nM H2O2 and 0 pM αKlotho, ‡P < 0.0001 vs. cells treated with 200 nmol/l H2O2 and 25 pM αKlotho, §P < 0.0001 vs. cells treated with 200 nM H2O2 and 50 pM αKlotho, #P < 0.05 vs. cells treated with 200 nM H2O2 and 100 pM αKlotho. B, left: selected antioxidant protein expressions were quantified by immunoblot. MTA, methalothionine; HOX1 and HOX2, heme oxygenase 1 and 2; PXDN, peroxidasin. β-Actin served as loading control. B, right: densitometry of antioxidant levels was normalized to β-actin and expressed as a ratio to control (no H2O2, no αKlotho). Average of 3 independent experiments (means ± SD). Statistical significance was assessed by ANOVA. *P < 0.05 vs. control, †P < 0.05 vs. 200 mM H2O2 and no αKlotho, ‡P = 0.065 compared with 200 mM H2O2 and no αKlotho.
DISCUSSION
Summary of the Rationale and Major Findings
This is the first report to examine the role of αKlotho in acute lung injury resulting from extrapulmonary organ failure. We previously showed that the lung exhibits heightened oxidative damage in mice with genetic αKlotho insufficiency and that exogenous αKlotho-containing conditioned media protect the lung against hyperoxia-induced acute damage; protection is at least partly mediated via increasing endogenous antioxidant capacity (46). We also previously showed that the rodent IRI model of AKI represents a state of acute systemic αKlotho deficiency (20), while the reperfusion phase post-renal ischemia is a state of high oxidative stress (51). Thus the AKI model represents double oxidative insults on the lung and provides the rationale for our hypothesis that the acute αKlotho deficiency in AKI contributes to lung damage.
Our findings support the following hypothesis: 1) acute lung injury characterized by interstitial edema and tissue oxidative damage is detectable in this rodent model of endogenous αKlotho deficiency caused by AKI; 2) repletion of αKlotho 6 h post-AKI does not alter the severity of AKI as measured by serum creatinine levels but significantly reduces the severity of acute lung injury; 3) circulating αKlotho exits the alveolar microvasculature rapidly to reach the alveolar interstitium and epithelium; and 4) αKlotho directly alleviates oxidative damage of pulmonary epithelia in cell culture by augmenting endogenous antioxidative capacity at least partly via activation of Nrf2 effectors. In concert, these findings support the notion that endogenous αKlotho deficiency in AKI contributes to development of acute lung injury and suggest the potential for therapeutic replacement.
Cytoprotection by αKlotho
αKlotho is the first member of the three-member KLOTHO gene family (22). αKlotho was touted as an antiaging protein due to the premature multiorgan failure that results from its serendipitous disruption (31) and the life span extension by its ectopic overexpression (32). The extracellular domain of αKlotho is proteolytically cleaved and released into the circulation, cerebrospinal fluid, and urine (4, 5, 18, 24), and exerts a wide array of effects on many target organs (15, 22). The kidney expresses the highest level of αKlotho, and the circulating (soluble) αKlotho comes primarily from the kidney (21, 35). It is not surprising that circulating levels of αKlotho plummets in AKI, with trough levels usually reached around Day 1 (20, 21, 43). The potential diagnostic, prognostic, and therapeutic role of αKlotho in AKI has been summarized elsewhere (16). In general the cytoprotective effects of Klotho have been attributed to its antiapoptotic, antifibrotic, and antioxidative properties (22, 30).
Pulmonary Involvement and Mechanisms of Acute Lung Injury in AKI
Pulmonary impairment in AKI is usually attributed to extrapulmonary factors such as sepsis, cardiac dysfunction, volume overload, and metabolic acidosis (8, 10, 42, 55). Of the twelve comorbid variables examined in one study, severe pulmonary involvement requiring mechanical ventilation conferred the worst prognosis elevating mortality from 29% to 81% even after multivariate adjustment (6). Because current treatment modalities for AKI cannot alter the natural course of kidney injury, the prevention or mitigation of complications associated with AKI should be prime therapeutic targets, as they will likely improve clinical outcomes. The possibility that AKI depletes a natural cytoprotective factor thereby predisposing to lung injury had not been examined.
The mechanism of acute lung injury in AKI is complex and multifactorial. αKlotho deficiency is one potentially important pathogenic factor. Circumstantial observations prompted us to formulate the following hypothesis that αKlotho deficiency contributes to pulmonary dysfunction in AKI: 1) AKI is accompanied by systemic αKlotho deficiency in rodents (20, 43); 2) AKI is a state of heightened oxidative stress (51); 3) the lung is particularly susceptible to αKlotho deficiency and relies on αKlotho to maintain its native antioxidative capacity (46); and 4) αKlotho is protective against oxidative stress in general (30). Unlike most organs that manifest no pathology at baseline in heterozygous hypomorphic αKlotho haplo-insufficient mice, the lungs show age-related morphological abnormalities and increased oxidative DNA damage when the circulating αKlotho level is ∼50% of normal (46). If one lowers the circulating αKlotho level further and contemporaneously increases the oxidative stress challenge such as in AKI, it is conceivable that the lungs would be rendered even more susceptible to damage. Using an interventional approach, we showed that post-AKI restoration of circulating αKlotho levels partially reversed the lung damage without altering peak AKI severity. These results support our hypothesis.
Mechanisms of αKlotho Protection in the Lung
The finding of partial reversal of lung damage by repletion of circulating αKlotho agrees with earlier findings where αKlotho prevents and ameliorates ischemic and nephrotoxic kidney damage (17, 20, 43). Soluble αKlotho functions as an endocrine substance, although a cognate receptor in its target organs has not been identified. One enigma is how this 130-kDa protein transits through the vasculature and reaches its targets. In AKI, pulmonary capillaries are leaky (2, 28), which can theoretically enhance the exit of macromolecules, but circulating levels of αKlotho are low in AKI (20) so increased permeability alone will not suffice. In the kidney, exogenously injected αKlotho crosses the peritubular capillary readily and reaches the urinary tubular lumen via tandem transcytosis across the peritubular capillary and tubular epithelium, but αKlotho does not traverse the glomerular capillary, indicating selective permeability and suggesting differential endothelial transcytosis (21). Now we demonstrate that αKlotho exits the pulmonary capillary to reach the interstitium and alveolar epithelium. The transport of this large protein across the capillary may occur via caveolae- or clathrin-mediated endocytosis in the capillaries (27, 37, 49) or via vesicular vacuolar organelles toward the venules (9, 11); elucidating these mechanisms will require further investigation.
We previously showed that transfection of either the transmembrane or the extracellular domain of αKlotho increases the endogenous antioxidative capacity of cultured human alveolar type I epithelial cells and A549 lung cell lines (46). Transfection does not replicate the in vivo exposure to circulating αKlotho or allow examination of dose-response relationships. Therefore, we synthesized, purified, and added recombinant αKlotho to cultured A549 cells to test the direct effects conferred by αKlotho and to establish a dose response of αKlotho action. Purified αKlotho exerts protection in concentrations compatible with its circulating levels, although we do not know the interstitial αKlotho concentration in the lung. A single injection of recombinant αKlotho, albeit at a pharmacologic concentration, led rapidly to detectable levels in the pulmonary interstitium. The clearance of αKlotho from the circulation is largely via the kidneys (21), but the mechanism of αKlotho clearance from tissue is unclear. It is conceivable that there may be accumulation of αKlotho in the lung interstitium to levels higher than that in plasma.
The Cap and Collar family of transcription factors comprises four related factors: Nrf1, Nrf2, Nrf3, and p45 NF-E2 (3). Nrf1 is important for counteracting steady-state stress under basal homeostatic states while Nrf2 is crucial for maintaining cell health in response to severe stressors such as reactive oxygen species, inflammatory cytokines, and endoplasmic reticulum stress (7, 41, 53). The ARE binds to Nrf2 and small Maf proteins. The small Maf proteins, originally described in musculoaponeurotic fibrosarcoma, are leucine zipper-containing transcriptional cofactors that function cooperatively with Nrf2 in vivo (38). The AREs are found in the transcriptional regulatory regions of many antioxidant and xenobiotic-metabolizing enzyme genes (3, 39) and are thought to account for the concerted upregulation of these genes during oxidative stress. Several lines of evidence support antioxidation via the Nrf2 pathway as a mechanism underlying αKlotho cytoprotection. The stimulation of many antioxidant and detoxification enzyme genes is severely impaired in Nrf2-null mutant mice (25), while αKlotho-overexpressing mice are more resistant to paraquat-induced oxidative stress (54). αKlotho overexpression drives Nrf2 localization to the nucleus in cells (13). Pretreatment of hippocampal neurons with recombinant αKlotho increases expression of the thioredoxin/peroxiredoxin system (56), which is a known downstream effector of the Nrf2 pathway (52). Our findings of increased expression of antioxidant targets of the Nrf2 pathway (MTA, HOX1, HOX2, and PXDN) following αKlotho administration in AKI are consistent with this mechanism of action. In addition to Nrf2, αKlotho also activates the FoxO family of transcriptional factors and induces superoxide dismutase 2 (SOD2) expression (23, 54), and αKlotho-containing conditioned media increase SOD2 expression and nitric oxide production in human umbilical vein endothelial cells (45). Additional mechanisms of αKlotho action such as antiapoptosis may also play a role.
In conclusion, acute lung injury is a life-threatening complication in AKI that significantly escalates morbidity and mortality (6). The pathogenesis of acute lung injury in AKI involves diverse multisystem interactions including cardiac, extracellular fluid volume, metabolic, cytokine, and sometimes sepsis-related factors. We provide evidence that αKlotho secreted from the kidney into the circulation reaches and directly acts on the alveolar epithelia, and its deficiency in AKI is an important contributing factor to the loss of endogenous antioxidative capacity of the pulmonary epithelium. The diminished αKlotho-mediated antioxidative cytoprotection occurs in a setting of heightened oxidative stress, which can compound and amplify the deleterious effects of all the other pathogenic intermediates that contribute to the development of lung injury and ARDS in the setting of AKI. These data suggest that prevention or amelioration of lung damage by αKlotho replacement holds substantial promise as a therapeutic intervention in AKI to prevent or mitigate secondary pulmonary complications. While αKlotho replacement reduced lung damage in the absence of an improvement in serum creatinine, endogenous αKlotho production also improved. Thus the observed cytoprotective effects of αKlotho arose from a combination of direct pulmonary and indirect systemic actions.
GRANTS
The research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK091392 and DK092461 (OWM, MK, MCH), National Heart, Lung, and Blood Institute Grants R01 HL40070 and U01 HL111146 (CCWH), National Institute of Aging Grant R01 AG019712 (MK), the Ruth L. Kirschstein National Research Service Award F32 HL103043 (PR), the Division of Nephrology Training Grant T32-DK007257 (PR), the O'Brien Kidney Research Center (NIH P30DK-07938; OM), the Simmons Family Foundation, and the Charles and Jane Pak Foundation (OM). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.R., M.K., O.W.M., and C.C.W.H. conception and design of research; P.R., L.L., J.Y., M.S., M.T., and J.Z. performed experiments; P.R. and C.C.W.H. analyzed data; P.R., M.C.H., O.W.M., and C.C.W.H. interpreted results of experiments; P.R. and C.C.W.H. prepared figures; P.R., O.W.M., and C.C.W.H. drafted manuscript; P.R., O.W.M., and C.C.W.H. edited and revised manuscript; P.R. and C.C.W.H. approved final version of manuscript.
REFERENCES
- 1.Barker SL, Pastor J, Carranza D, Quinones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 30: 223–233, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu RK, Wheeler D. Effects of ischemic acute kidney injury on lung water balance: nephrogenic pulmonary edema? Pulmon Med 2011: 414253, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blank V. Small Maf proteins in mammalian gene control: mere dimerization partners or dynamic transcriptional regulators? J Mol Biol 376: 913–925, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583: 3221–3224, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104: 19796–19801, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med 155: 1505–1511, 1995. [PubMed] [Google Scholar]
- 7.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 23: 7198–7209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi K, Ishizu T, Fujita T, Noiri E. Lung injury following acute kidney injury: kidney-lung crosstalk. Clin Exp Nephrol 15: 464–470, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem 49: 419–432, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis 15: 284–296, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Feng D, Nagy JA, Dvorak HF, Dvorak AM. Ultrastructural studies define soluble macromolecular, particulate, and cellular transendothelial cell pathways in venules, lymphatic vessels, and tumor-associated microvessels in man and animals. Microsc Res Tech 57: 289–326, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Hassoun HT, Lie ML, Grigoryev DN, Liu M, Tuder RM, Rabb H. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am J Physiol Renal Physiol 297: F125–F137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh CC, Kuro-o M, Rosenblatt KP, Brobey R, Papaconstantinou J. The ASK1-signalosome regulates p38 MAPK activity in response to levels of endogenous oxidative stress in the Klotho mouse models of aging. Aging (Albany NY) 2: 597–611, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu MC, Kuro-o M, Moe OW. Secreted klotho and chronic kidney disease. Adv Exp Med Biol 728: 126–157, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol 33: 118–129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nature Rev Nephrol 8: 423–429, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Shi M, Cho HJ, Zhang J, Pavlenco A, Liu S, Sidhu S, Huang LJ, Moe OW. The erythropoietin receptor is a downstream effector of Klotho-induced cytoprotection. Kidney Int 84: 468–481, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu MC, Shi MJ, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Sidhu S, Kuro-o M, Moe OW. Renal production, uptake, and handling of circulating alpha-Klotho. J Am Soc Nephrol. 27: 79–90, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun 339: 827–832, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for posttranslational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14: 1549–1558, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, Pagano RE, Hancock JF, Mayor S, Parton RG. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol 168: 465–476, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int 74: 901–909, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int 55: 2362–2367, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. J Biol Chem 389: 233–241, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 275: 1489–1494, 1996. [PubMed] [Google Scholar]
- 34.Levy LA. Severe hypophosphatemia as a complication of the treatment of hypothermia. Arch Intern Med 140: 128–129, 1980. [PubMed] [Google Scholar]
- 35.Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Ostman Wernerson A, Lanske B, Olauson H, Larsson TE. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 25: 2169–2175, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242: 626–630, 1998. [DOI] [PubMed] [Google Scholar]
- 37.McNiven MA. Dynamin in disease. Nat Genet 37: 215–216, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Motohashi H, Katsuoka F, Engel JD, Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci USA 101: 6379–6384, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motohashi H, O'Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 294: 1–12, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med 109: 665–678, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Osburn WO, Karim B, Dolan PM, Liu G, Yamamoto M, Huso DL, Kensler TW. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer 121: 1883–1891, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Paladino JD, Hotchkiss JR, Rabb H. Acute kidney injury and lung dysfunction: a paradigm for remote organ effects of kidney disease? Microvasc Res 77: 8–12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 85: 855–870, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int 63: 600–606, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, Maekawa Y, Kida I, Miyazaki J, Ogihara T. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine 31: 82–87, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, Hsia CC, Moe OW. α-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol 307: L566–L575, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seeley EJ. Updates in the management of acute lung injury: a focus on the overlap between AKI and ARDS. Adv Chronic Kidney Dis 20: 14–20, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC. Alpha-Klotho mitigates progression of acute kidney injury to chronic kidney disease via activation of autophagy. J Am Soc Nephrol. In press, DOI 10.1681/ASN.2015060613 [DOI] [PMC free article] [PubMed]
- 49.Stan RV. Endocytosis pathways in endothelium: how many? Am J Physiol Lung Cell Mol Physiol 290: L806–L808, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Suga T, Kurabayashi M, Sando Y, Ohyama Y, Maeno T, Maeno Y, Aizawa H, Matsumura Y, Kuwaki T, Kuro OM, Nabeshima Y, Nagai R. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am J Respir Cell Mol Biol 22: 26–33, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Sureshbabu A, Ryter SW, Choi ME. Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol 4: 208–214, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med 42: 1838–1850, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun 351: 883–889, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280: 38029–38034, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology 116: 1139–1148, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, Harris DA, Abraham CR. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem 289: 24700–24715, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]