Chronic heart failure (CHF) heightens intact mitochondria H2O2 emission and weakens the diaphragm. Systemic treatment with a mitochondria-targeted antioxidant, starting once CHF was already established, normalized diaphragm mitochondrial H2O2 emission and contractile function. The effects of mitochondria-targeted antioxidant treatment were associated with the maintenance of diaphragm glutathione content. Thus antioxidant strategies specifically targeting the mitochondria could therapeutically benefit respiratory function in CHF.
Keywords: inspiratory muscle, oxidants, mycardial infarction, skeletal muscle, force
Abstract
Diaphragm muscle weakness in chronic heart failure (CHF) is caused by elevated oxidants and exacerbates breathing abnormalities, exercise intolerance, and dyspnea. However, the specific source of oxidants that cause diaphragm weakness is unknown. We examined whether mitochondrial reactive oxygen species (ROS) cause diaphragm weakness in CHF by testing the hypothesis that CHF animals treated with a mitochondria-targeted antioxidant have normal diaphragm function. Rats underwent CHF or sham surgery. Eight weeks after surgeries, we administered a mitochondrial-targeted antioxidant (MitoTEMPO; 1 mg·kg−1·day−1) or sterile saline (Vehicle). Left ventricular dysfunction (echocardiography) pre- and posttreatment and morphological abnormalities were consistent with the presence of CHF. CHF elicited a threefold (P < 0.05) increase in diaphragm mitochondrial H2O2 emission, decreased diaphragm glutathione content by 23%, and also depressed twitch and maximal tetanic force by ∼20% in Vehicle-treated animals compared with Sham (P < 0.05 for all comparisons). Diaphragm mitochondrial H2O2 emission, glutathione content, and twitch and maximal tetanic force were normal in CHF animals receiving MitoTEMPO. Neither CHF nor MitoTEMPO altered the diaphragm protein levels of antioxidant enzymes: superoxide dismutases (CuZn-SOD or MnSOD), glutathione peroxidase, and catalase. In both Vehicle and MitoTEMPO groups, CHF elicited a ∼30% increase in cytochrome c oxidase activity, whereas there were no changes in citrate synthase activity. Our data suggest that elevated mitochondrial H2O2 emission causes diaphragm weakness in CHF. Moreover, changes in protein levels of antioxidant enzymes or mitochondrial content do not seem to mediate the increase in mitochondria H2O2 emission in CHF and protective effects of MitoTEMPO.
NEW & NOTEWORTHY
Chronic heart failure (CHF) heightens intact mitochondria H2O2 emission and weakens the diaphragm. Systemic treatment with a mitochondria-targeted antioxidant, starting once CHF was already established, normalized diaphragm mitochondrial H2O2 emission and contractile function. The effects of mitochondria-targeted antioxidant treatment were associated with the maintenance of diaphragm glutathione content. Thus antioxidant strategies specifically targeting the mitochondria could therapeutically benefit respiratory function in CHF.
chronic heart failure (CHF) is a prominent cause of worldwide morbidity and mortality (55). CHF causes inspiratory muscle weakness that depends on disease severity (3, 33, 36), and CHF-induced inspiratory dysfunction contributes to breathing abnormalities, fatigue, and the overall pathogenesis of the disease (14, 18, 39, 70). The diaphragm is the primary inspiratory muscle and diaphragm weakness has been shown in several animal models of CHF (11, 35, 59, 67). However, the cellular processes leading to diaphragm weakness remain poorly understood.
Excess reactive oxygen species (ROS) are putative mediators of diaphragm weakness (54). For instance, systemic antioxidant treatment prevents diaphragm weakness in a model of CHF induced by myocardial infarction (60). However, the specific ROS source was not defined and the antioxidant treatment started early during the development of CHF (acute phase: 2 wk after myocardial infarction). There are multiple ROS sources in muscle cells (53). Identifying the major source of ROS is important to reveal mediators and possible therapeutic targets to counteract diaphragm weakness in CHF. To date, the effects of CHF on ROS emission by intact mitochondria (in saponin permeabilized fibers) and the specific contribution of mitochondrial ROS to diaphragm weakness in CHF have not been established. Moreover, it is unclear whether an antioxidant intervention is effective against diaphragm weakness once CHF is already established.
Targeted antioxidants are a useful tool for examining the role of mitochondrial ROS emission on cellular abnormalities of several chronic diseases (21, 61), including diaphragm weakness induced by mechanical ventilation (52). Therefore, we tested the hypothesis that animals treated with a mitochondria-targeted antioxidant, starting after the development of CHF, would have normal diaphragm morphology and function. The processes involved in left ventricular remodeling and dysfunction that cause CHF usually stabilize within 8 wk of myocardial infarction in rodents (30, 48, 56). Thus we started the intervention 8 wk postsurgery to test our hypothesis.
METHODS
Animals and interventions.
A total of 41 Wistar rats, aged 12-16 wk, were used in the present study. The animals were housed at the University of Florida Animal Care Facilities under a 12:12-h light-dark cycle and had access to standard chow and water ad libitum. The animals underwent myocardial infarction (MI; n = 29) or Sham surgery (n = 12). Eight weeks postsurgery, rats were treated with intraperitoneal injections of MitoTEMPO (1 mg·kg−1·day−1) or Vehicle solution (sterile saline) for 10–11 wk. MitoTEMPO was from Santa Cruz Biotechnology (sc-221945). Fourteen rats that received MI surgery (∼50%) died before the start of treatment, whereas all Sham animals survived the entire study. We only performed all functional and biochemical measurements in animals from the MI group with fractional shortening (FS) < mean − 2 × SD of the Sham group and endocardial infarct area ≥20% of left ventricle (LV) + septum. These criteria are based on the notion that diaphragm weakness is more prominent in moderate to severe CHF (3, 27, 39), previous studies in rodents (1, 23, 68), and the relationship between infarct area and circumference (28).
Surgeries.
We ligated the coronary artery to cause MI and induce CHF. Our procedures have been described before (23, 25). Briefly, we anesthetized (isoflurane) the rats and intubated for mechanical ventilation. We exposed the heart through a left thoracotomy in the intercostal space, removed the pericardium, and permanently ligated the left anterior descending coronary artery near the left atrium using 6-0 monofilament absorbable PGA suture (Demesorb; Demetec, Miami, FL). After ligation, the lungs were hyperinflated and the thoracic (3-0 PGA suture; Demetec) and skin incisions (3-0 Nylon; Demetec) were closed separately. Sham surgeries mirrored the procedures for MI, except for the ligation of the artery. The animals received bupivacaine and buprenorphine immediately after surgery, and buprenorphine injections continued every 8–12 h for 3 days postsurgery.
Echocardiography.
Echocardiography was performed 8 and ∼16 wk after surgery. Briefly, animals were kept under 1.5–2% isoflurane anesthesia while two-dimensional M-mode ultrasound images were obtained at 7.5 Hz (Aplio; Toshiba America Medical Systems, Tustin, CA) in the parasternal long and short axis view. Measurements were performed using the leading edge-to-leading edge method. The FS was calculated using the following equation: %FS = (LVIDd − LVIDs) × 100/(LVIDd), where LVIDd is LV internal diameter end-diastole and LVIDs is LV internal diameter end-systole.
Terminal experiments.
On the day of the experiment, we anesthetized the animals (isoflurane: 5% induction, 2–3% maintenance) and performed a laparotomy and thoracotomy to collect the diaphragm and heart. The right hemidiaphragm was quickly freed from adipose tissue and frozen in liquid nitrogen, while the left hemidiaphragm was further dissected for assessment of contractile function and mitochondrial H2O2 emission in vitro.
Diaphragm contractile function.
A diaphragm muscle strip was dissected and placed at optimal length for twitch force production (L0). Isometric contractile characteristics were measured at 37°C using a Dual-Mode Muscle Lever System (300C- LR; Aurora Scientific, Aurora, Canada) while the muscle was stimulated supramaximally using a biphasic high-power stimulator (701C; Aurora Scientific). Stimulus frequencies ranged from 1 to 200 Hz (0.25-ms pulse and 0.5-s train durations) in solution containing d-tubocurarine (25 μM). The force-frequency relationships from each animal were analyzed using a four-parameter Hill equation to define the shape of the curve.
Diaphragm fiber cross-sectional area and fiber type distribution.
We froze a diaphragm bundle using Tissue-Tek medium submersed in liquid nitrogen-cooled isopentane and stored the sample at −80°C. The diaphragm bundle was cut in 10-μm sections using a cryotome (Shandon, Pittsburgh, PA) and stained with antibodies against dystrophin and myosin heavy chain I and IIa isoforms, as described previously (52). We acquired images using a Zeiss Axio-ObserverA1 microscope with a ×10 objective and an AxioCam-MRm3 camera (Carl Zeiss Microscopy). To measure fiber CSA, we traced the membrane boundaries established by dystrophin in all visible fibers of each diaphragm section using National Institutes of Health ImageJ software. We determined fiber type distribution from the antibody staining (type I and IIa) and unstained fibers were considered as type IIx/b (52).
Mitochondrial ROS production.
Permeabilization of skeletal limb muscle fiber bundles has been previously described (4, 29), and the technique was adapted to the diaphragm muscle with minor modifications. We dissected small portions (∼10–20 mg) of the costal diaphragm and placed them into a small tissue culture dish filled with ice-cold buffer X containing the following (in mM): 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 0.5 DTT, 20 taurine, 5.7 ATP, 14.3 PCr, 6.56 MgCl2-6H2O, 50 K-MES, 0.5 glutamate, and 0.2 malate (pH 7.1). We peeled and removed connective and fat tissue surrounding the diaphragm and separated individual fibers from small bundles along the longitudinal axis. The bundles were incubated on a rotator for 30 min at 4°C in buffer X containing saponin (30 μg/ml). Following the permeabilization step, bundles were washed 3× 5 min on a rotator in ice-cold wash buffer containing the following (in mM): 105 K-MES, 30 KCl, 10 K2HPO4, 5 MgCl2-6H2O, 0.5 mg/ml BSA, 0.1 EGTA, 0.5 glutamate, and 0.2 malate (pH 7.1).
Hydrogen peroxide emission was measured fluorimetrically (Fluorolog-3; HORIBA Jobin Yvon, Edison, NJ) from the permeabilized diaphragm bundles using an Amplex Ultra Red solution containing the following: 10 μM Amplex Ultra Red (Life Technologies), 25 μM blebbistatin, 105 mM K-MES, 30 mM KCl, 10 mM K2HPO4, 5 mM MgCl2-6H2O, 0.5 mg/ml BSA, 1 mM EGTA, and 1 U/ml horseradish peroxidase. The solution was continuously mixed using a magnetic stir bar and temperature was kept at 37°C. After measurements of baseline fluorescence, we added 10 mM succinate to determine the rate of mitochondrial H2O2 emission (JH2O2). We converted Amplex Ultra Red fluorescence to nanomolar H2O2 via an H2O2 standard curve established on the day of each experiment and under the same substrate conditions as used with the fiber bundles. At the end of each experiment, fiber bundles were washed in distilled water for 5 min and dried in an incubator chamber overnight. We measured the dry bundle weight to calculate the rate of H2O2 emission in pmol·min−1·mg bundle dry wt−1. We also normalized the rate of H2O2 emission to markers of mitochondrial content described below.
Cytochrome c oxidase and citrate synthase activity.
We measured cytochrome c oxidase (COX) and citrate synthase (CS) activities as markers of mitochondrial content. We pulverized the diaphragm in liquid nitrogen and mixed the powdered tissue with 20× volume/wt of extraction buffer (71.4 mM Na2HPO4, 28.6 mM KH2PO4, and 2 mM EDTA, pH 7.2) and sonicated 3× 5 s. The samples were further diluted fourfold for COX activity measurements and added to a solution containing ∼5 mM K2HPO4, 5 mM KH2PO4, 0.2 mM sodium dithionite, and 2 mg/ml cytochrome c (C2506; Sigma). We calculated COX activity based on the maximal oxidation rate of reduced cytochrome c determined from changes in absorbance (550 nm) at 30°C. We measured CS activity using a commercial kit (MitoCheck; Cayman Chemical) based on a colorimetric assay following changes in absorbance (412 nm) at 25°C. The activity assays were done using a multidetection microplate reader (Synergy HT; Biotek Instruments, Winooski, VT).
Glutathione and glutathione disulfide (24, 38).
Diaphragm fiber bundles (≤ 50 mg) were weighed and placed in microcentrifuge tubes containing 0.5 ml of ice-cold 5% perchloric acid with 0.2 M boric acid and 10 μM γ-glutamylglutamate. The sample was sonicated until the solution turned opaque, then centrifuged briefly. The supernatant (300 μl) was mixed with 60 μl of 7.4 mg/ml sodium iodoacetic acid, the pH was adjusted to 8.8–9.2 using KOH/potassium tetraborate. After a 20-min incubation at room temperature, we added 300 μl of 20 mg/ml dansyl chloride and incubated the samples for 18–26 h at room temperature in the dark and then added 500 μl chloroform. The final solution was centrifuged for 2 min at 13,200 rpm and 500 μl was injected into the HPLC (Waters 2695) for fluorescence detection using Waters 2475 (ex: 335 nm; em: 518 nm). These procedures were performed at the Emory Clinical Biomarkers Laboratory (Emory University, Atlanta, GA). Total glutathione content was calculated as glutathione (GSH) + 2[glutathione disulfide (GSSG)]. Values were normalized for muscle wet weight.
SDS-PAGE and Western blot.
Diaphragm samples were homogenized using a Kontess Duall Homogenizer in 1× cell lysis buffer (no. 9803; Cell Signaling Technology) containing the following: 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate 1 mM Na3VO4, 1 μg/ml leupeptin, and 1× protease and phosphatase inhibitor cocktail (no. 5872; Cell Signaling Technology). The lysates were mixed 1:1 with 2× Laemmli sample buffer and heated at 95°C for 5 min. We loaded approximately equal amounts of protein (10–30 μg/lane) into 4–20% stain-free TGX gels (Bio-Rad Laboratories) and performed electrophoresis at 200 V for 50 min on ice. We scanned the gel to quantify total protein (Gel Doc EZ Imager; Bio-Rad Laboratories) and transferred the proteins to a nitrocellulose membrane at 100 mA overnight at 4°C. We blocked the membrane using Li-COR Blocking Buffer (Li-COR, Lincoln, NE) for 1 h at room temperature and subsequently probed the membrane with primary antibodies for CuZn-SOD, MnSOD, glutathione peroxidase (GPX), or catalase. After primary antibody incubation, we washed the membranes in TBS-T (4× 5 min), incubated in secondary antibody (IR Dye, LI-COR; 1:20,000) for 45 min at room temperature, washed in TBS-T, rinsed in 1× TBS, and scanned the membrane using an Odyssey Infrared Imaging system (LI-COR, Lincoln, NE). To determine protein carbonyls, we used the Oxi-Select Protein Carbonyl Immunoblot kit (STa-308, Cell Biolabs) following the manufacturer recommendations then proceeded as described above for primary (1 h at room temperature) and secondary incubations. Signal intensity quantification and data analysis were performed exactly as described previously (1, 2).
Statistical analysis.
Statistical analyses were performed using Sigmaplot v.13.0 (Systat Software). We used two-way ANOVA and, where appropriate, Student-Newman-Keuls test for post hoc comparisons. A set of data from echocardiography failed the normality test and was log transformed before using ANOVA. Differences were considered statistically significant when P < 0.05.
RESULTS
Animals.
Overall, the number of animals and treatment duration in our study were as follows: Sham Vehicle (n = 6; 75 ± 3 days), CHF Vehicle (n = 4; 76 ± 3 days), Sham MitoTEMPO (n = 6, 70 ± 2 days), and CHF MitoTEMPO (n = 6; 71 ± 3 days).
Heart failure induced by MI.
Animals from Vehicle and MitoTEMPO groups that received coronary ligation had similar infarct area with echocardiography variables and ventricular abnormalities consistent with the presence of hypertrophy and CHF (Table 1). Pretreatment echocardiography data were similar for Sham or CHF when comparing Vehicle and MitoTEMPO treatments. The Δ(post-pre) in FS for CHF rats was −2 ± 3% in the Vehicle group and 7 ± 1% (P < 0.05) in animals receiving MitoTEMPO. Left ventricular internal dimensions were unchanged from pre to post in CHF Vehicle, while MitoTEMPO elicited a 5–13% decrease in left ventricular internal dimensions. Posterior wall thickness decreased in CHF Vehicle, whereas CHF animals in the MitoTEMPO group showed a 15–20% increase in posterior and septum wall thickness from pre to post. In Sham rats, MitoTEMPO had no effect on ventricular weights or echocardiography variables.
Table 1.
Animal characteristics and echocardiography variables
| Vehicle |
MitoTEMPO |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sham |
CHF |
Sham |
CHF |
|||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Infarct area, % | n/a | 38 ± 3 | n/a | 34 ± 3 | ||||
| Body weight, g | 561 ± 4 | 593 ± 22α | 557 ± 30 | 587 ± 27† | 596 ± 17 | 608 ± 23† | 532 ± 19 | 570 ± 17† |
| LV weight, mg | — | 922 ± 37 | — | 1,019 ± 30 | — | 981 ± 39 | — | 1,108 ± 57* |
| RV weight, mg | — | 237 ± 6 | — | 386 ± 89* | — | 246 ± 11 | — | 276 ± 26 |
| Tibial length, mm | — | 45 ± 1 | — | 44 ± 1 | — | 45 ± 1 | — | 44 ± 1 |
| LV wt/TL, mg/mm | — | 21 ± 1 | — | 23 ± 1 | — | 22 ± 1 | — | 24 ± 1* |
| RV wt/TL, mg/mm | — | 5.3 ± 0.1 | — | 9 ± 2* | — | 5.5 ± 0.2 | — | 6.3 ± 0.2 |
| LVIDd, mm | 7.4 ± 0.3 | 7.8 ± 0.2 | 9.8 ± 0.5* | 10.5 ± 0.4* | 7.8 ± 0.2 | 8.2 ± 0.2 | 9.8 ± 0.3* | 9.3 ± 0.4*‡ |
| LVIDs, mm | 3.9 ± 0.2 | 4.2 ± 0.2 | 7.6 ± 0.9* | 8.4 ± 0.8* | 4.3 ± 0.2 | 4.5 ± 0.1 | 7.4 ± 0.3* | 6.4 ± 0.3*†‡ |
| FS, % | 47 ± 1 | 46 ± 1 | 23 ± 6* | 21 ± 4* | 45 ± 2 | 45 ± 1 | 25 ± 1* | 31 ± 1*†‡ |
| SWTd, mm | 1.7 ± 0.04 | 1.5 ± 0.05 | 1.4 ± 0.1* | 1.3 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.1 ± 0.1* | 1.3 ± 0.04*† |
| SWTs, mm | 2.9 ± 0.1 | 2.8 ± 0.1 | 2.1 ± 0.3* | 2.0 ± 0.3* | 2.9 ± 0.1 | 2.9 ± 0.1 | 1.8 ± 0.2* | 2.0 ± 0.2* |
| PWTd, mm | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.4 | 1.5 ± 0.3† | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 | 1.8 ± 0.2† |
| PWTs, mm | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.3 ± 0.3 | 2.1 ± 0.2* | 2.8 ± 0.2 | 2.8 ± 0.2 | 2.2 ± 0.1* | 2.6 ± 0.1† |
| HR, beats/min | 402 ± 17 | 372 ± 15 | 384 ± 11 | 376 ± 29 | 397 ± 24 | 372 ± 21 | 420 ± 17 | 392 ± 9 |
Values are means ± SE. CHF, congestive heart failure; LV, left ventricle; RV, right ventricle; TL, tibial length; LVID, left ventricular internal diameter (d, end-diastole; s, end-systole); FS, fractional shortening; SWT, septum wall thickness; PWT; posterior wall thickness; HR, heart rate.
Significantly different (P < 0.05) from Sham (for same time and treatment).
Significantly different (P < 0.05) from pre (for same group and treatment).
Significantly different (P < 0.05) from respective Vehicle (for same time and group).
Diaphragm oxidants, antioxidants, and protein oxidation.
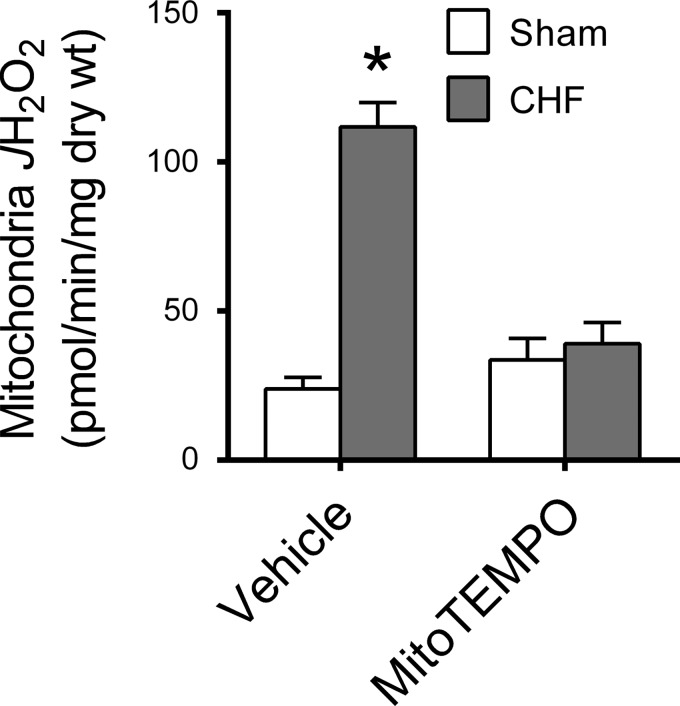
CHF caused an approximate threefold increase in diaphragm mitochondrial H2O2 emission in Vehicle-treated animals (Fig. 1). Diaphragm mitochondrial H2O2 in CHF animals treated with MitoTEMPO was lower than in CHF Vehicle, whereas there was no treatment effect on Sham animals. We measured COX and CS activities as indicators of mitochondrial content and found that CHF increased COX (∼30%) but not CS activity (Table 2). These responses were not modified by MitoTEMPO. The increase in H2O2 emission elicited by CHF persisted after normalizing the data for markers of mitochondrial content (Table 2).
Fig. 1.
Diaphragm mitochondrial H2O2 emission (JH2O2) in saponin-permeabilized fibers. Data are rate of H2O2 emission stimulated by addition of succinate (10 mM) to reaction buffer. *Significantly different (P < 0.05) from all other groups.
Table 2.
Mitochondrial enzyme activity and intrinsic H2O2 emission
| Vehicle |
MitoTEMPO |
|||
|---|---|---|---|---|
| Sham | CHF | Sham | CHF | |
| COX activity, U/mg protein | 708 ± 26 | 902 ± 52* | 649 ± 30 | 907 ± 56* |
| CS activity, AU·min−1·mg protein−1 | 4.0 ± 0.2 | 3.9 ± 0.4 | 4.2 ± 0.5 | 4.2 ± 0.3 |
| Mitochondria JH2O2, pmol·min−1·COX activity −1 | 34 ± 6 | 130 ± 11† | 52 ± 12 | 43 ± 10 |
| Mitochondria JH2O2, pmol·min−1·CS activity −1 | 6.4 ± 1.2 | 31 ± 5.6† | 8.0 ± 2.3 | 8.8 ± 1.6 |
Values are means ± SE. COX, cytochrome c oxidase; CS, citrate synthase; AU, arbitrary units. Intrinsic H2O2 emission (JH2O2) calculated as mitochondria JH2O2 (Fig. 1) divided by COX or CS activities.
P < 0.05 vs. Sham within treatment.
P < 0.05 vs. all other groups.
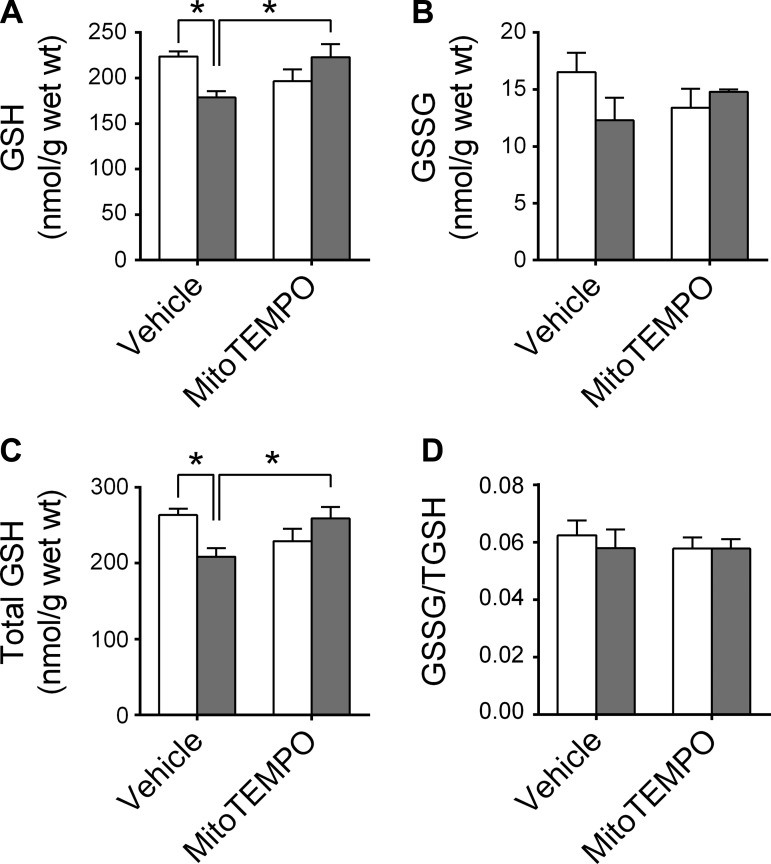
CHF and MitoTEMPO also affected glutathione metabolism.
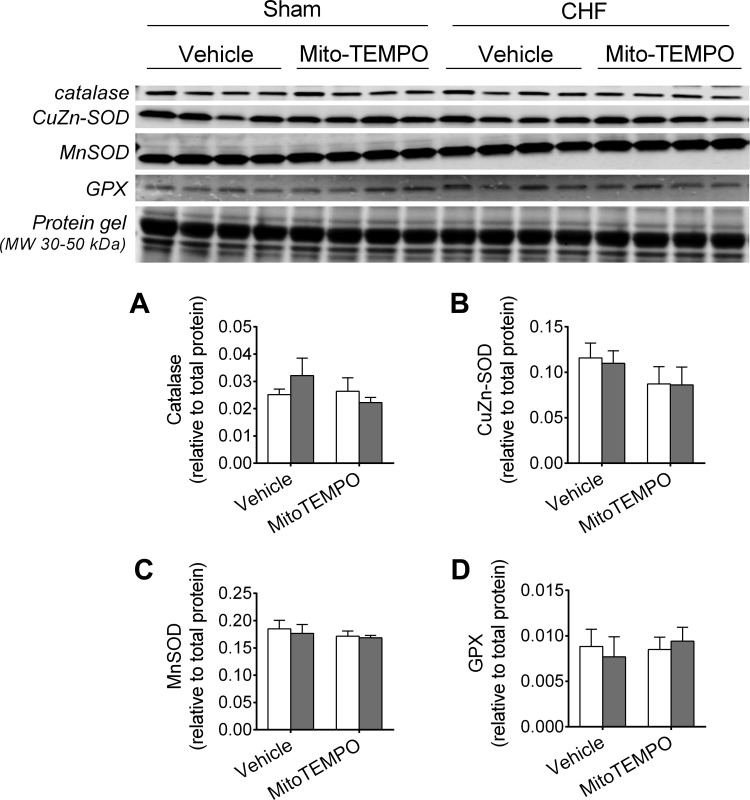
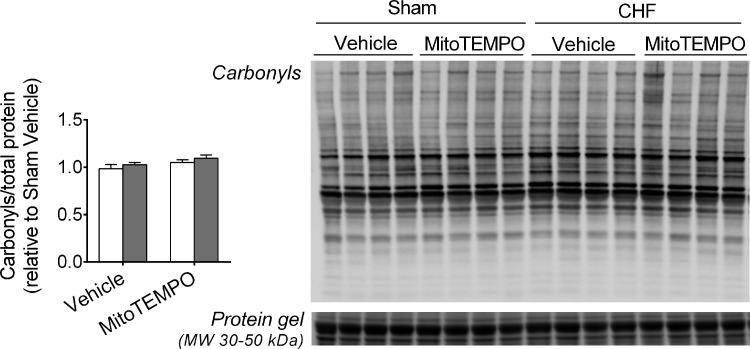
Specifically, CHF animals in the Vehicle group had decreased GSH and total GSH, and this effect was not present in the MitoTEMPO group (Fig. 2). There were no changes in GSSG/total GSH with CHF or MitoTEMPO. Neither CHF nor MitoTEMPO altered the diaphragm protein levels of catalase, CuZn-SOD, MnSOD, or GPX (Fig. 3). There were no changes in total protein carbonyls with CHF or MitoTEMPO (Fig. 4).
Fig. 2.
Diaphragm glutathione (GSH), glutathione disulfide (GSSG), and total glutathione content. Data are measured by high-performance liquid chromatography and normalized to tissue wet weight. Sham and CHF are shown as in Fig. 1. *Significant difference (P < 0.05).
Fig. 3.
Protein levels of antioxidant enzymes. SOD, superoxide dismutase; GPX, glutathione peroxidase. Protein levels were determined by Western blots (sample images shown in rows 1–4) and normalized to total protein loaded onto gel (sample image shown in row 5). Sham and CHF are shown as in Fig. 1.
Fig. 4.
Diaphragm protein carbonyls. Data are ratio of carbonyl to total protein. Immunoblot (top right) of carbonyls and total protein from stain-free gel (bottom right). Total protein shown is only for molecular weight (MW) range of ∼30 to 50 kDa for clarity.
Diaphragm contractile properties, fiber type, and CSA.
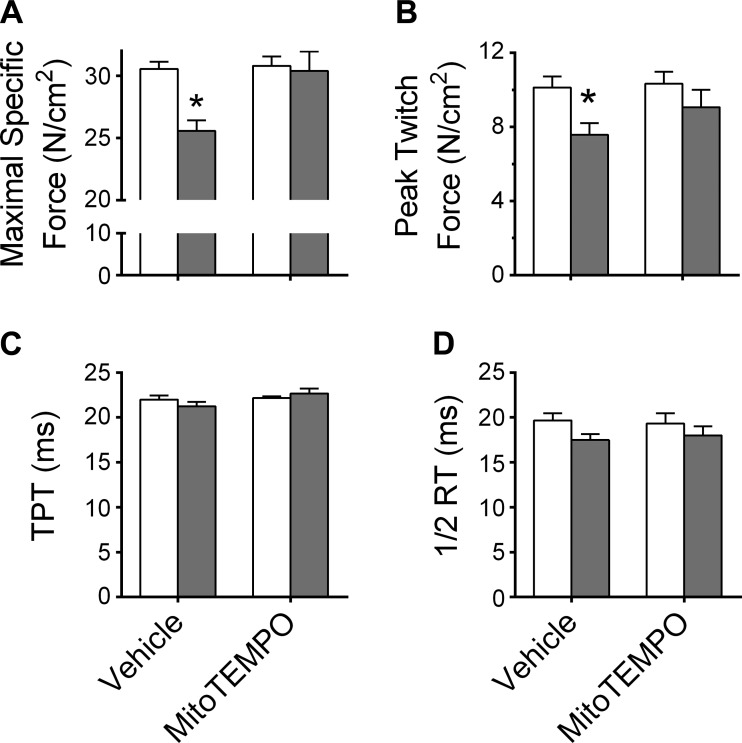
The force-frequency relationship of each group is shown in Fig. 5. CHF decreased maximal tetanic force by ∼20% in Vehicle (P < 0.05; Fig. 6) but not MitoTEMPO-treated CHF animals. Regarding twitch force, an ANOVA revealed a group effect (P = 0.021), but no significant treatment effect or interaction. Post hoc comparisons of twitch force with Student-Newman-Keuls test were not significant. However, unpaired t-tests with Sidak-Bonferroni correction for multiple comparisons revealed differences in twitch force between Sham-Vehicle and CHF-Vehicle (P < 0.05; Fig. 6). Twitch kinetics (time to peak tension and half-relaxation time) were similar among groups and treatments.
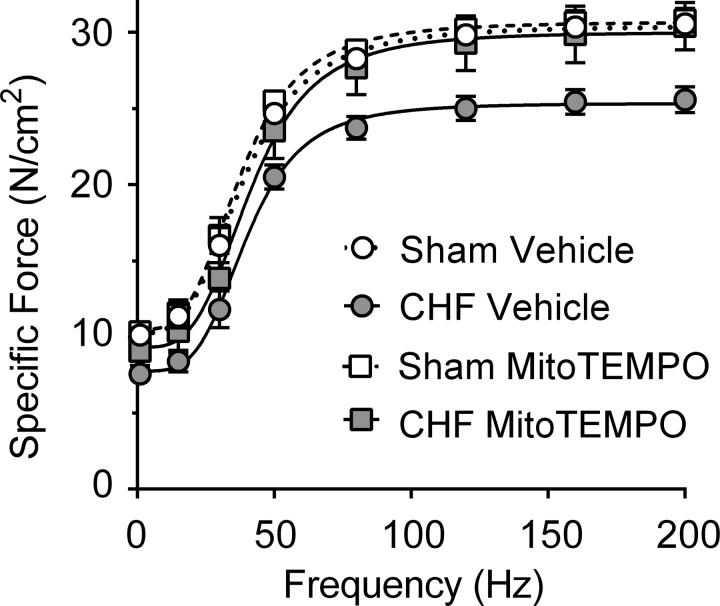
Fig. 5.
Diaphragm force-frequency relationship. Data shown are specific forces (in N/cm2), with force normalized for estimated cross-sectional area (CSA). Lines represent best fit using sigmoidal Hill equation. Sham and CHF are shown as in Fig. 1. Significant differences are indicated in Fig. 6 for clarity.
Fig. 6.
Diaphragm isometric contractile properties. Maximal and twitch forces are normalized to bundle CSA (specific force, N/cm2). TPT, time to peak tension; ½ RT, one-half relaxation time. Specific force was normal in CHF rats receiving MitoTEMPO. Sham and CHF are shown as in Fig. 1. Twitch kinetics (TPT and ½ RT) were unchanged with CHF or MitoTEMPO. *Significantly different (P < 0.05) from all other groups.
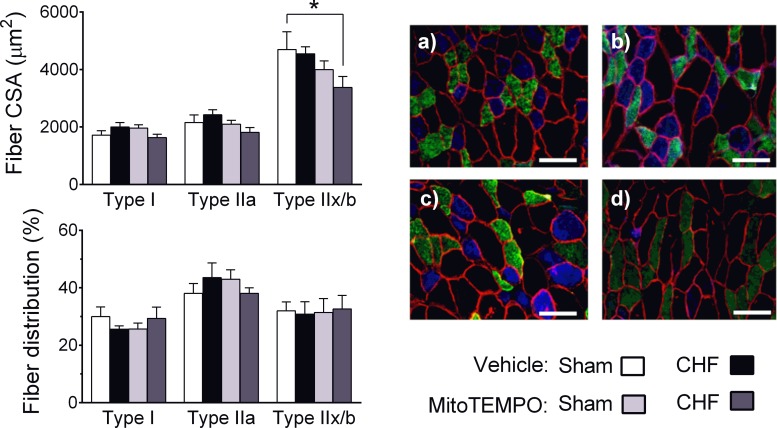
Regarding fiber size and distribution, CHF did not affect fiber CSA in Vehicle-treated animals (Fig. 7). However, the type IIx/b fiber CSA was lower in CHF animals receiving MitoTEMPO compared with Sham or CHF Vehicle. There were no differences in fiber type distribution among groups.
Fig. 7.
Diaphragm fiber CSA and fiber type distribution. Sample images are from Sham Vehicle (A), CHF Vehicle (B), Sham MitoTEMPO (C), and CHF MitoTEMPO (D) groups. Scale (white) bar = 100 μm. Blue: fiber type I; green: type IIa; black: type IIx/b.
DISCUSSION
Our main findings were that elevations in intact mitochondria H2O2 emission and associated loss of specific force in the diaphragm of CHF rats were not present after systemic treatment with MitoTEMPO. CHF also caused a decrease in diaphragm glutathione content that was not present in the MitoTEMPO group. Changes in antioxidant protein levels or fiber type distribution do not seem to account for the effects of CHF or MitoTEMPO on diaphragm mitochondria H2O2 emission, glutathione content, or specific force.
CHF effects on diaphragm mitochondrial oxidants, levels of antioxidant enzymes, and redox balance.
Mitochondria are an important source of elevated ROS in the diaphragm during CHF (60). We observed that CHF-induced elevation of diaphragm mitochondrial H2O2 emission was not evident after MitoTEMPO treatment. Our finding of heightened mitochondria ROS emission in the diaphragm during CHF is in accordance with a previous study in mitochondria isolated from the diaphragm (60). An important difference is that we tested intact mitochondria in saponin-permeabilized fibers, which are free of confounding factors induced by mitochondrial isolation procedures (50). The finding that systemic treatment with MitoTEMPO decreased diaphragm mitochondrial H2O2 emission in CHF is new. These observations are consistent with MitoTEMPO effects on endothelial cells (21) and heart (43). The effect of MitoTEMPO on mitochondrial H2O2 emission may appear counterintuitive because the compound is a superoxide dismutase mimetic that catalyzes conversion of superoxide to H2O2. However, MitoTEMPO may have acted by inhibiting accumulation of superoxide or superoxide-derivatives (e.g., peroxynitrite) (17, 21), which impair mitochondrial enzyme function and result in heightened H2O2 (19, 51).
Glutathione is one of the main endogenous antioxidants and its depletion heightens mitochondrial ROS emission and causes loss of muscle force (26, 29, 34, 47). Glutathione is the substrate of the glutathione peroxidase reaction that scavenges H2O2 and glutathione depletion sensitizes the pyruvate dehydrogenase complex or enzymes of the electron transport chain to elevate ROS production (29, 34). Thus decreased glutathione levels will contribute to heighten mitochondrial H2O2 emission. We found that diaphragm glutathione content was diminished in CHF rats in the Vehicle group, whereas glutathione content in CHF animals receiving MitoTEMPO was similar to Sham. Neither CHF nor MitoTEMPO altered the glutathione disulfide-to-total glutathione ratio, whereas total glutathione levels (GSH + GSSG) was decreased in CHF. GSSG is typically used as a marker of redox imbalance in the cell. However, GSSG is one of the many forms of oxidized glutathione. Glutathione can also react with thiol groups in proteins or free cysteine. Our measurements reflect solely the levels of free GSSG, while the total GSH signal incorporates all forms of free (nonprotein bound) GSH (38). Therefore, decreases in total GSH that we observed can reflect, and contribute to, a redox imbalance.
An increase in mitochondrial content or decrease in endogenous antioxidant enzymes could also elevate mitochondrial H2O2. CHF increases breathing frequency, minute ventilation, and the work of breathing (12, 16), which can promote diaphragm mitochondrial biogenesis. We measured diaphragm COX and CS activity as mitochondrial content markers. The activity of COX was increased in CHF and unaffected by MitoTEMPO, whereas CS activity was unaffected by either CHF or MitoTEMPO. The two enzymes are encoded and localize differently. COX, a multiunit protein complex encoded by both nuclear and mitochondrial DNA, localizes in the inner membrane. CS, encoded solely by nuclear DNA, localizes in the matrix. These data suggest potential changes in diaphragm mitochondria structure with CHF. However, neither COX nor CS activity account for the increase in mitochondria H2O2. Specifically, intrinsic emission (H2O2 normalized to COX or CS activity) were elevated in CHF vehicle and similar to Sham in CHF animals receiving MitoTEMPO.
The protein levels of antioxidant enzymes CuZn-SOD, MnSOD, catalase, and GPX were not affected by CHF or MitoTEMPO in our study. This contrasts data from Mangner et al. (45), who showed unchanged COX activity and increased MnSOD and GPX activity in diaphragm of CHF rats. The exact source of discrepancy between studies is not readily apparent and may be related to differences on time of experiments postsurgery (12 vs. 16 wk), strain, infarct size, and methods employed to determine antioxidant enzymes (activity assay vs. Western blot). However, our findings are consistent with other studies showing that CHF increased 1) markers of diaphragm mitochondrial content in animals and patients (35, 62); and 2) ROS emission in the diaphragm (1, 60). Thus our data suggest that changes in mitochondrial content and endogenous antioxidant enzyme levels do not explain the effect of CHF and MitoTEMPO on diaphragm mitochondrial H2O2 emission.
MitoTEMPO effects on CHF-induced diaphragm weakness.
Assessment of inspiratory pressure in patients suggests that inspiratory muscles are weakened in CHF (3, 27, 33). The diaphragm is the primary inspiratory muscle and diaphragm weakness has been shown in several animal models of CHF (11, 35, 59, 67). Several studies have implicated a role of ROS in diaphragm weakness in CHF (1, 11, 60). Supinski et al. (60) have shown that systemic administration of SOD conjugated to polyethylene glycol (to promote cell permeability) prevented the decrease in submaximal diaphragm force in CHF rats, indicating a causative role of oxidants on diaphragm weakness. Our study shows that both submaximal and maximal diaphragm forces were normal in CHF rats treated with an SOD mimetic conjugated to triphenylphosphonium to target the mitochondria. It is also important that we administered the compound to Sham rats and are, therefore, able to show that effects were specific to CHF at the dose tested. Exposure to exogenous H2O2 at physiological levels in vitro depresses submaximal and maximal muscle force (5). As the increase in mitochondrial H2O2 emission was not present in CHF rats treated with MitoTEMPO, we consider that mitochondrial oxidants, likely H2O2, mediate diaphragm weakness induced by CHF. This notion is supported by a recent study showing that overexpression of mitochondrial catalase prevents weakness in limb muscles of aged mice (65).
A recent study from our group (1) suggested that NAD(P)H oxidase is also involved in the oxidant-mediated diaphragm weakness in CHF. Combined, our current and previous studies suggest a cross talk between oxidants from mitochondria and NAD(P)H oxidase(s). In this setting, oxidants produced by NAD(P)H oxidase can lead to mitochondrial dysfunction and heightened H2O2 release, or elevated mitochondrial H2O2 triggers the activation of NAD(P)H oxidases. The oxidant-mediated cross talk between mitochondria and NAD(P)H oxidase has been shown in smooth muscle and endothelial cells (20, 69) and has been reviewed in detail by others (13, 19).
CHF and MitoTEMPO effects on diaphragm fiber CSA and fiber type distribution.
Inspiratory muscle abnormalities in CHF patients do not necessarily involve diaphragm fiber atrophy or a shift in fiber type distribution. In one study in humans, diaphragm fiber dimensions were similar in controls and CHF patients (42). Some animal studies have shown that CHF causes diaphragm fiber atrophy (9, 22, 35, 37, 58), whereas others have found no difference between CHF and Sham animals in fiber CSA (41, 68). Our data from Vehicle treated animals is in agreement with the latter. However, the CSA of type IIx/b fibers was decreased in CHF rats receiving MitoTEMPO. This was an unexpected finding as mitochondrial oxidants cause diaphragm atrophy in mechanically ventilated rats (52). It is possible that MitoTEMPO elicited off-target effects on protein synthesis/degradation. However, this is unlikely as such effect would have caused a decrease in CSA for all fiber types. The implication of loss of type IIx/b fiber area is that MitoTEMPO would not be beneficial to improve diaphragm force development during expulsive behaviors (e.g., coughing and sneezing) that involve the recruitment of type IIx/b fibers (46). If confirmed, such effect poses a problem for clinical application of mitochondria-targeted antioxidants in CHF. However, our observation of decreased type IIx/b fiber CSA cannot, at this stage, be generalized to all types of mitochondria-targeted antioxidants. Further testing of other approaches will be required to define the clinical relevance of mitochondria-targeted antioxidant interventions to treat the diaphragm in CHF.
Regarding diaphragm fiber type distribution, there are also conflicting reports on the effects of CHF in animals or humans. Some studies have reported a shift toward higher percentage of type I/slow oxidative fibers and lower type II/fast glycolytic fibers (9, 35, 41, 63), whereas others showed no effect of CHF on fiber type distribution (23, 42). In our study, neither CHF nor MitoTEMPO affected diaphragm fiber type distribution, which suggests that changes in the percentage of fiber types do not explain the decrease in specific force with CHF or the protection elicited by MitoTEMPO.
Effects of CHF and MitoTEMPO on protein carbonyls.
We assessed the levels of protein carbonyls, which can impair myofibrillar protein function and lead to weakness when elevated in CHF (11). However, we did not find changes in protein carbonyls due to CHF or MitoTEMPO. These findings are in agreement with our recent study (1) but contrast studies by others in CHF post-MI (8, 60). We cannot determine the reason(s) for different outcomes between our study and others. The lack of changes in protein carbonyls suggest that the altered redox homeostasis due to enhanced mitochondrial H2O2 emission was not sufficient to enhance carbonyls or that elevations in carbonyls were transient. Carbonylated proteins are turned over at a faster rate than noncarbonylated proteins (32, 57). Because our measurements are in a chronic state of redox imbalance we may have missed the acute phase when carbonyls are accumulating. Alternatively, standard Western blot may not be sufficiently sensitive to detect carbonyls during the later stages of CHF. CHF induced by MI heightens protein carbonyls at earlier stages of the disease (3 days to 6 wk; Refs. 8, 60). In the later stages of CHF, thiol oxidation or oxidant-mediated activation of the ubiquitin-proteasome pathway might account for the loss of diaphragm force (5, 40, 66).
Model of CHF, MitoTEMPO effects on the heart, and implications for data interpretation.
Mortality in the infarct model of CHF in our study is fairly consistent with the literature in rodents (15, 16, 31, 49). Most animals die within 72 h of surgery and, although the cause of death is multifactorial, arrhythmia is one of the main factors in the early stages (15). In any model of CHF, investigators are susceptible to selectively studying more resistant animals or those with moderate degree of cardiac dysfunction. We studied an inbred strain and any resistance due to genetic differences among animals is unlikely. It appears that most long-term studies in rodents, like ours, are in animals with moderate CHF. This may result in underestimation of the impact of severe CHF on peripheral abnormalities and limited understanding of mechanisms or treatments thereof.
The processes involved in left ventricular remodeling and dysfunction that lead to CHF in rodents usually stabilize within 8 wk of MI (30, 48, 56). Thus, we opted to start the pharmacological intervention 8 wk postsurgery to avoid interfering with the development of cardiac abnormalities per se and, therefore, determine whether the mitochondrial antioxidant would be effective once heart failure was already established. Our echocardiography data suggest that we accomplished our goal and CHF animals in the Vehicle and MitoTEMPO groups had similar levels of cardiac dysfunction at the start of the interventions and similar infarct sizes determined posttreatment. Nonetheless, echo measurements posttreatment suggest that MitoTEMPO blunted further cardiac remodeling and improved cardiac function from 8 to 16 wk postinfarct. Specifically, there was a slight increase in FS and wall thickness in CHF animals receiving MitoTEMPO compared with Vehicle. Our findings are in agreement with the role of oxidants on cardiomyocyte remodeling and heart failure (7, 64). The apparently small cardioprotective effects of MitoTEMPO might be a consequence of the delayed onset of treatment (8 wk) compared with other reports where antioxidant interventions started immediately or 2–4 wk after MI (e.g., Refs. 6, 10, 43). However, our experiments were designed to test the role of mitochondrial ROS on diaphragm dysfunction. In this regard, the cardiac responses to MitoTEMPO do not seem to fully explain the protective effects we observed in the diaphragm. In CHF patients, inspiratory muscle weakness is unrelated to left ventricular ejection fraction (3) and is not resolved by a heart transplant (44). Improvements in cardiac function per se are unlikely to account for the effects of MitoTEMPO on diaphragm function. Nevertheless, we cannot exclude a contribution of improved cardiac function to amelioration of diaphragm abnormalities.
Conclusion.
Our data suggest that elevated mitochondrial H2O2 emission causes diaphragm weakness in CHF. The increase in mitochondria H2O2 emission induced by CHF and protective effects of MitoTEMPO do not seem to be mediated by changes in protein levels of antioxidant enzymes or mitochondrial content.
The protection against elevation in H2O2 emission and diaphragm weakness conferred by mitochondria-targeted antioxidant treatment might be critically linked to the maintenance of diaphragm glutathione content. Thus antioxidant strategies specifically targeting the mitochondria or the decrease in diaphragm glutathione content could have therapeutic benefits in CHF. The counterbalance to functional benefits of MitoTEMPO on the diaphragm in CHF is a decrease in type IIx/b fiber CSA that can compromise nonventilatory expulsive behaviors. Hence, alternative approaches to MitoTEMPO would be necessary to achieve full benefits of mitochondrial-targeted antioxidant therapy in the clinical setting.
GRANTS
The study was funded by American Heart Association Grant 13GRNT17160000. L. F. Ferreira was also supported by National Heart, Lung, and Blood Institute Grant R00-HL-098453. O. Laitano was supported by a postdoctoral fellowship from Brazil (CNPq No. 249094/2013-4) and Universidade Federal do Vale do São Francisco (Petrolina, PE).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: O.L., B.S.A., N.P., P.D.C., A.J.S., J.-K.Y., and L.F.F. performed experiments; O.L., B.S.A., N.P., P.D.C., J.-K.Y., D.D.C., P.A., and L.F.F. analyzed data; O.L., B.S.A., N.P., P.D.C., A.J.S., D.D.C., P.A., and L.F.F. interpreted results of experiments; O.L. drafted manuscript; O.L., B.S.A., N.P., P.D.C., A.J.S., J.-K.Y., D.D.C., P.A., and L.F.F. edited and revised manuscript; O.L., B.S.A., N.P., P.D.C., A.J.S., J.-K.Y., D.D.C., P.A., and L.F.F. approved final version of manuscript; P.D.C. and L.F.F. prepared figures; L.F.F. conception and design of research.
REFERENCES
- 1.Ahn B, Beharry AW, Frye GS, Judge AR, Ferreira LF. NAD(P)H oxidase subunit p47phox is elevated, and p47phox knockout prevents diaphragm contractile dysfunction in heart failure. Am J Physiol Lung Cell Mol Physiol 309: L497–L505, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn B, Empinado HM, Al-Rajhi M, Judge AR, Ferreira LF. Diaphragm atrophy and contractile dysfunction in a murine model of pulmonary hypertension. PLoS One 8: e62702, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosino N, Opasich C, Crotti P, Cobelli F, Tavazzi L, Rampulla C. Breathing pattern, ventilatory drive and respiratory muscle strength in patients with chronic heart failure. Eur Respir J 7: 17–22, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andre L, Fauconnier J, Reboul C, Feillet-Coudray C, Meschin P, Farah C, Fouret G, Richard S, Lacampagne A, Cazorla O. Subendocardial increase in reactive oxygen species production affects regional contractile function in ischemic heart failure. Antioxid Redox Signal 18: 1009–1020, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol 61: 599–610, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen TS, Mangner N, Werner S, Glaser S, Kullnick Y, Schrepper A, Doenst T, Oberbach A, Linke A, Steil L, Schuler G, Adams V. Diaphragm muscle weakness in mice is early-onset post-myocardial infarction and associated with elevated protein oxidation. J Appl Physiol (1985) 118: 11–19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowen TS, Rolim NP, Fischer T, Baekkerud FH, Medeiros A, Werner S, Bronstad E, Rognmo O, Mangner N, Linke A, Schuler G, Silva GJ, Wisloff U, Adams V. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail 17: 263–272, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis 18: 215–220, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Coirault C, Guellich A, Barbry T, Samuel JL, Riou B, Lecarpentier Y. Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 292: H1009–H1017, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Cross TJ, Sabapathy S, Beck KC, Morris NR, Johnson BD. The resistive and elastic work of breathing during exercise in patients with chronic heart failure. Eur Respir J 39: 1449–1457, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 1797: 897–906, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Dall'ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol 47: 757–763, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62: 2422–2430, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Rio R, Marcus NJ, Schultz HD. Inhibition of hydrogen sulfide restores normal breathing stability and improves autonomic control during experimental heart failure. J Appl Physiol (1985) 114: 1141–1150, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, Kalyanaraman B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med 39: 567–583, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Di Lisa F, De Tullio R, Salamino F, Barbato R, Melloni E, Siliprandi N, Schiaffino S, Pontremoli S. Specific degradation of troponin T and I by mu-calpain and its modulation by substrate phosphorylation. Biochem J 308: 57–61, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 20: 281–294, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominguez JF, Howell S. Compartmental analysis of steady-state diaphragm Ca2+ kinetics in chronic congestive heart failure. Cell Calcium 33: 163–174, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Empinado HM, Deevska GM, Nikolova-Karakashian M, Yoo JK, Christou DD, Ferreira LF. Diaphragm dysfunction in heart failure is accompanied by increases in neutral sphingomyelinase activity and ceramide content. Eur J Heart Fail 16: 519–525, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira LF, Gilliam LA, Reid MB. l-2-oxothiazolidine-4-carboxylate reverses glutathione oxidation and delays fatigue of skeletal muscle in vitro. J Appl Physiol 107: 211–216, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 188: 3–13, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol 104: 853–860, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Filusch A, Ewert R, Altesellmeier M, Zugck C, Hetzer R, Borst MM, Katus HA, Meyer FJ. Respiratory muscle dysfunction in congestive heart failure–the role of pulmonary hypertension. Int J Cardiol 150: 182–185, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Finsen AV, Christensen G, Sjaastad I. Echocardiographic parameters discriminating myocardial infarction with pulmonary congestion from myocardial infarction without congestion in the mouse. J Appl Physiol (1985) 98: 680–689, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Fisher-Wellman KH, Gilliam LA, Lin CT, Cathey BL, Lark DS, Neufer PD. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic Biol Med 65: 1201–1208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol 281: R1734–R1745, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res 107: 1445–1453, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun 305: 709–718, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Hammond MD, Bauer KA, Sharp JT, Rocha RD. Respiratory muscle strength in congestive heart failure. Chest 98: 1091–1094, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Han D, Canali R, Rettori D, Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol 64: 1136–1144, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Howell S, Maarek JM, Fournier M, Sullivan K, Zhan WZ, Sieck GC. Congestive heart failure: differential adaptation of the diaphragm and latissimus dorsi. J Appl Physiol 79: 389–397, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Hughes PD, Polkey MI, Harrus ML, Coats AJ, Moxham J, Green M. Diaphragm strength in chronic heart failure. Am J Respir Crit Care Med 160: 529–534, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Hwee DT, Kennedy AR, Hartman JJ, Ryans J, Durham N, Malik FI, Jasper JR. The small-molecule fast skeletal troponin activator, CK-2127107, improves exercise tolerance in a rat model of heart failure. J Pharmacol Exp Ther 353: 159–168, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 348: 93–112, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Kasahara Y, Izawa KP, Watanabe S, Osada N, Omiya K. The relation of respiratory muscle strength to disease severity and abnormal ventilation during exercise in chronic heart failure patients. Res Cardiovasc Med 4: e228944, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol 285: C806–C812, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Lima AR, Martinez PF, Damatto RL, Cezar MD, Guizoni DM, Bonomo C, Oliveira SA Jr, Dal-Pai Silva M, Zornoff LA, Okoshi K, and Okoshi MP. Heart failure-induced diaphragm myopathy. Cell Physiol Biochem 34: 333–345, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Lindsay DC, Lovegrove CA, Dunn MJ, Bennett JG, Pepper JR, Yacoub MH, Poole-Wilson PA. Histological abnormalities of muscle from limb, thorax and diaphragm in chronic heart failure. Eur Heart J 17: 1239–1250, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest 123: 1262–1274, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancini DM, LaManca JJ, Donchez LJ, Levine S, Henson DJ. Diminished respiratory muscle endurance persists after cardiac transplantation. Am J Cardiol 75: 418–421, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Mangner N, Weikert B, Bowen TS, Sandri M, Hollriegel R, Erbs S, Hambrecht R, Schuler G, Linke A, Gielen S, Adams V. Skeletal muscle alterations in chronic heart failure: differential effects on quadriceps and diaphragm. J Cachexia Sarcopenia Muscle 6: 381–390, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol Heart Circ Physiol 260: H1406–H1414, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res 44: 503–512, 1979. [DOI] [PubMed] [Google Scholar]
- 50.Picard M, Taivassalo T, Gouspillou G, Hepple RT. Mitochondria: isolation, structure and function. J Physiol 589: 4413–4421, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell CS, Jackson RM. Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am J Physiol Lung Cell Mol Physiol 285: L189–L198, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 39: 1749–1759, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powers SK, Ji LL, Kavazis AN, Jackson MJ. Reactive oxygen species: impact on skeletal muscle. Compr Physiol 1: 941–969, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shioura KM, Geenen DL, Goldspink PH. Assessment of cardiac function with the pressure-volume conductance system following myocardial infarction in mice. Am J Physiol Heart Circ Physiol 293: H2870–H2877, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Smuder AJ, Kavazis AN, Hudson MB, Nelson WB, Powers SK. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic Biol Med 49: 1152–1160, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stassijns G, Gayan-Ramirez G, De Leyn P, Verhoeven G, Herijgers P, de Bock V, Dom R, Lysens R, Decramer M. Systolic ventricular dysfunction causes selective diaphragm atrophy in rats. Am J Respir Crit Care Med 158: 1963–1967, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Supinski G, DiMarco A, Dibner-Dunlap M. Alterations in diaphragm strength and fatiguability in congestive heart failure. J Appl Physiol 76: 2707–2713, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Supinski GS, Callahan LA. Diaphragmatic free radical generation increases in an animal model of heart failure. J Appl Physiol 99: 1078–1084, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Szeto HH, Schiller PW. Novel therapies targeting inner mitochondrial membrane-from discovery to clinical development. Pharm Res 28: 2669–2679, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation 95: 910–916, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Tikunov BA, Mancini D, Levine S. Changes in myofibrillar protein composition of human diaphragm elicited by congestive heart failure. J Mol Cell Cardiol 28: 2537–2541, 1996. [DOI] [PubMed] [Google Scholar]
- 64.Tsutsui H, Kinugawa S, Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res 81: 449–456, 2009. [DOI] [PubMed] [Google Scholar]
- 65.Umanskaya A, Santulli G, Xie W, Andersson DC, Reiken SR, Marks AR. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci USA 111: 15250–15255, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Hees HW, Li YP, Ottenheijm CA, Jin B, Pigmans CJ, Linkels M, Dekhuijzen PN, Heunks LM. Proteasome inhibition improves diaphragm function in congestive heart failure rats. Am J Physiol Lung Cell Mol Physiol 294: L1260–L1268, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Hees HW, van der Heijden HF, Hafmans T, Ennen L, Heunks LM, Verheugt FW, Dekhuijzen PN. Impaired isotonic contractility and structural abnormalities in the diaphragm of congestive heart failure rats. Int J Cardiol 128: 326–335, 2008. [DOI] [PubMed] [Google Scholar]
- 68.van Hees HW, van der Heijden HF, Ottenheijm CA, Heunks LM, Pigmans CJ, Verheugt FW, Brouwer RM, Dekhuijzen PN. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol 293: H819–H828, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Munzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal 10: 1435–1447, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Woods PR, Olson TP, Frantz RP, Johnson BD. Causes of breathing inefficiency during exercise in heart failure. J Card Fail 16: 835–842, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]