Abstract
Purpose
To report a previously poorly recognized process of secondary formation of inflammatory bowel disease (IBD)-like process, specifically Crohn’s-like (CD) changes in pediatric surgery patients who underwent major small bowel and colorectal surgery. We describe potential etiologies, presenting symptoms and treatment approaches.
Methods
Retrospective chart review of patients with history of either chronic, partial gastrointestinal (GI) obstruction or Hirschsprung disease (HD) and subsequent histopathologic findings similar to IBD. Pathology and case histories were reviewed and treatments were compared.
Results
Over the last 20 years, a total of nine patients were identified that had the diagnoses of either HD (n=3) or chronic GI partial obstruction (n=6) with subsequent development of histopathologic changes similar to those seen in IBD. Overall mean time to diagnosis of IBD-like changes after intestinal resection was 7.70 ±5.6 years. Half of the patients were also being managed for short bowel syndrome (SBS), and associated GI symptoms may have prolonged the time to indentifying these IBD-like changes. When SBS patients were excluded, mean time to IBD changes after pull-through for HD was 2.4±0.24 years and after chronic GI partial obstruction was 6.3±2.1 years. Two of the nine patients who underwent a resection of this IBD-like lesion developed a recurrence of this lesion. Anti-TNF-α treatment was used in 3 of the GI partial obstruction cases: 2 with complete relief and 1 with partial response that was supplemented with steroids. Two HD patients were treated with anti-TNF-α and both had marked improvement of symptoms.
Conclusion
We describe IBD-like intestinal changes following intestinal resection in the pediatric age group. We also present the novel finding that these lesions are responsive to anti-IBD treatment, including anti-TNF-α, and recommend it as part of the medical treatment regiment offered for such patients.
Keywords: Hirschsprung Disease, Inflammatory Bowel Disease, Chronic Obstruction, Short Bowel Syndrome, Intestinal Anastomosis
Introduction
The exact etiology of IBD is still being investigated. However, current theories involve an interaction of genetics, host immunity and environmental factors.[1] An increasing appreciation of the pathophysiology suggests that a combination of loss of mucosal barrier function along with an aberrant microbiome may drive IBD development. More specifically, alterations in the interleukin 23 (IL-23) and T helper 17 (Th17) pathway of microbial defense and intestinal immune homeostasis have been recognized as key for IBD development.[2] Thus, processes that result in deranged barrier function may be a predisposing factor for IBD development.
In addition, post-anastomotic ulceration syndrome has been described in both the adult and pediatric populations.[3, 4] Similar to IBD, the exact etiology of this syndrome is not known. There are several theories which involve exposure of colonic bacteria to the small intestine and resultant bacterial overgrowth.[4]
Over the past two decades our surgery practice has observed several children who have undergone major small bowel and colorectal surgery and have secondarily developed IBD-like histologic changes; specifically Crohn’s-like changes in residual intestine. Crohn’s is a clinical diagnosis using a combination of symptoms (diarrhea, recurrent abdominal pain, anorectal lesions, etc.) and imaging/endoscopy. When looking at pathology slides, one can only determine that the changes look similar to the changes seen with Crohn’s. Therefore, we use the term Crohn’s-like throughout this manuscript.
There is a paucity of literature describing this rare association between intestinal resection and IBD-like changes.[5] Such changes are important to recognize due to the long-term implications for pediatric surgery patients. As well, this report heightens the awareness for this disease process as it may be missed if the pediatric surgeon is not aware of this potential secondary process. The purpose of this article is to describe a group of pediatric surgery patients who years following successful intestinal resection present with such IBD-like changes. We describe their presenting symptoms and treatment approaches for these complicated patients, as well as some speculation on potential etiologies.
Methods
After Institutional Review Board approval at C.S. Mott Children’s Hospital in Ann Arbor, MI (#HUM00074945) and at Texas Tech University Health Sciences Center in El Paso, TX (#E14020), a retrospective chart review was done of pediatric surgery patients with a history of either chronic, partial GI obstruction or HD who developed a clinical symptoms and pathologic findings of IBD-like findings in their intestinal biopsies or resections.
Variables of interest were demographic information, primary surgical diagnosis, time after surgical establishment of intestinal continuity until findings of IBD-like changes, complications of operations and primary diagnosis, treatments (including use of corticosteroids, anti-TNF-α, and other anti-inflammatory medications), and results of treatments. Pathology slides of intestinal biopsies and resection specimens were rereviewed by a single pathologist (RR) for confirmation of the histologic findings.
Results
A total of nine children were identified over a 20-year period that had the diagnoses of either HD (n=3) or chronic GI partial obstruction after intestinal surgery (n=6) followed by IBD-like pathologic changes. Table 1 contains a description of the demographic data of these patients. Male patients made up 78% of the study population. None of the patients were positive for Trisomy-21. The majority of children were of Caucasian descent (77.8%). At the time of development of IBD-like lesions, 5 had small intestine ulcerations visualized by endoscopy (cases #2, #3, #6, #7, and #8); and 3 had visualized ulcerations or other pathology only in the large intestine (cases #1, #4, and #5). Case #9 was diagnosed after intestinal resection for intolerance of feeds and bowel obstruction.
Table 1.
Patients with primary chronic partial GI obstruction or HD and secondary IBD
| Case # | Gender | Primary diagnosis | Years to IBD diagnosis after placed in continuity | HD associated complications | Clinical manifestiations |
|---|---|---|---|---|---|
| 1 | F | NEC, chronic partial obstruction | 4.83 | n/a | Abdominal distention, abdominal pain, rectal bleeding, anal skin tags |
| 2 | M | Gastroschisis, chronic partial obstruction, SBS | 7.42 | n/a | Abdominal distention, abdominal pain, GI bleed |
| 3 | M | Total colonic HD, SBS | 20.1 | HAEC | Abdominal pain, abdominal distention, diarrhea |
| 4 | M | Total colonic HD | 2.58 | HAEC | Abdominal pain, abdominal distention, diarrhea, perianal fistulas, aphthous ulcers |
| 5 | M | HD | 2.25 | HAEC, Redo pull-through | Abdominal pain, abdominal distention, diarrhea, aphthous ulcers |
| 6 | F | NEC, chronic partial obstruction, SBS | 7.33 | n/a | Abdominal pain, abdominal distention, GI bleed |
| 7 | M | Omphalocele, cloacal exstrophy, chronic partial obstruction | 7.83 | n/a | Abdominal distention, abdominal pain |
| 8 | M | Ileal atresia, chronic partial obstruction, SBS | 12.4 | n/a | Abdominal distention, abdominal pain, GI bleed |
| 9 | M | Colonic atresia, chronic partial obstruction, SBS | 4.58 | n/a | Abdominal distention, abdominal pain, intolerance of feeds, fistulas |
NEC = necrotizing enterocolitis, SBS = short bowel syndrome, HD = Hirschsprung disease, HAEC = Hirschsprung associated enterocolitis.
With respect to the primary diseases, HD was found in three children. One had recto-sigmoid disease (case #5) complicated by the need for a redo-pullthrough and two had total colonic HD (cases #3 and #4). All three children suffered from multiple episodes of Hirschsprung-associated enterocolitis (HAEC). One total colonic HD child (case #3) also had short bowel syndrome (SBS) and was supplemented with total parental nutrition (TPN) until age six. In addition to HAEC, case #5 had recurrent issues with constipation. He ultimately required a redo rectal pull-through using his proximal sigmoid colon three years after his original pull-through. Pathology after his redo showed a long residual aganglionic cuff (10cm) and ganglion cells without nerve hypertrophy proximal to the prior anastomotic line. Of interest, many granulomas were present in the colonic mucosa on both sides of the anastomosis (Figure 1A). Subsequent to this, two years later he developed intermittent symptoms of abdominal pain, distention, and bloody stools. After a year of multiple treatment regimens for HAEC, he underwent colonoscopy that showed colonic pseudomembranes and proctitis. Biopsies at that time revealed active chronic colitis (Figure 1B) with mild chronic architectural changes and numerous nonnecrotizing epithelioid granulomas suggesting CD-like pathology (Figure 1C). As a result, he was treated with infliximab with a subsequent improvement of his medical course, including resolution of his perianal fistula and clinical symptoms.
Figure 1.
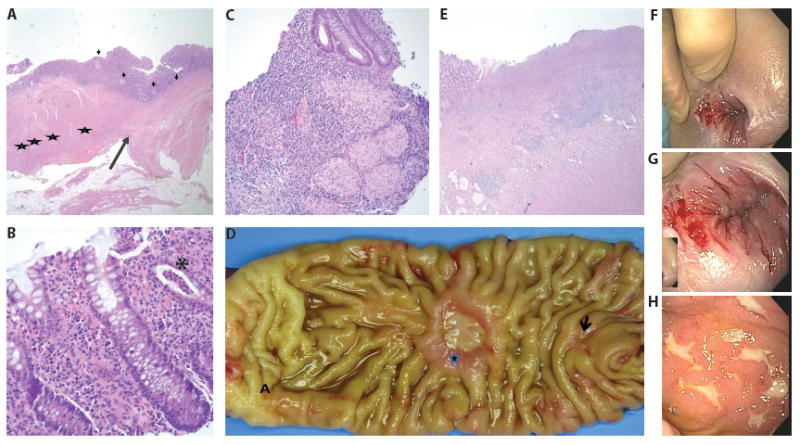
Pathology and Colonoscopy Images
Panels A-C are pathology slides from case #5. Panel A Redo pull through resection. Long arrow marks the anastomotic line. Ganglion cells are noted between the muscular layers in the proximal segment (left, stars). The distal mucosal sleeve is aganglionic (right). Non necrotizing epithelioid granulomas are noted on both sides of anastomosis (short arrow). Panel B Colonic biopsy shows diffuse chronic inflammation of the lamina propria and focal crypt abscess (asterisk). Panel C Numerous epithelioid granulomas seen in the colonic biopsies.
Panels D-E are images from case #6. Panel D Small bowel resection shows two chronic ulcers, small (arrow) and large (star) proximal to the anastomotic site (A). Panel E Deep ulceration and underlying transmural chronic inflammation and fibrosis resembling Chron disease.
Panels F-H are initial colonoscopy images of case #4. Panel F is a gross image of perianal disease prior to anti-TNF-α treatment. Panel G is a closer image of the same perianal lesions. Panel H is an image of his aphthous ulcers.
For the remaining six children, the causes of their initial intestinal resections were varied. Two had an initial diagnosis of necrotizing enterocolitis (NEC) (cases #1 and #6), one had gastroschisis (case #2), one had cloacal exstrophy (case #7), one had ileal atresia (case #8), and one had colonic atresia (case #9). Each of them suffered from symptoms of abdominal pain and abdominal distention at the time of their presentation with IBD-like pathology. Interestingly, none of the six possessed an ileocecal valve at the time of diagnosis of IBD-like pathology. Four of them had clinical documentation of GI hemorrhage(s) requiring transfusion (cases #1, #2, #6, and #8). Three required prolonged use of PN (cases #2, #6, and #8), but were weaned off at the time of presentation with IBD-like changes. Growth was ultimately maintained with enteral nutrition. Case #6 had the shortest intestinal length documented (40 centimeters at five months of age) and required PN supplementation for about six months of life. This was re-measured seven years later at 190 centimeters. Case #8 required PN the longest (seven years) for nutritional supplementation.
Overall mean time to diagnosis of IBD-like changes after intestinal resection was 7.70±5.6 years. When stratified by primary diagnosis, half of the children were also being managed for short bowel syndrome (SBS), and thus associated GI symptoms may have prolonged the time to IBD-like diagnosis. When the SBS children were excluded, the mean time to diagnosis of IBD-like changes after pull-through for HD was 2.4±0.24 years, and after symptoms of chronic GI partial obstruction the mean time to diagnosis was 6.3±2.1 years.
Almost all cases (88.9%) underwent a resection and were diagnosed with an IBD-like process from the pathology specimen. One child (case #4) did not require resection, as a diagnosis was made by pathology from an endoscopic biopsy. His anastomotic stricture, which may have been contributing to the formation of the IBD-like process, was treated with multiple dilations, botox injections, and an anorectal myectomy. Two children (cases #2 and #8) reformed CD-like changes including ulceration after resection for their IBD-like process. After recurrence, both patients received anti-TNF-α treatment. Case #2 had complete resolution of his ulceration and clinical symptoms. Case #8 had symptom control with steroids after anti-TNF-α treatment.
Histopathologic slides for each case were reviewed. As previously mentioned, one child (case #4) only had colonic biopsies for review as he did not undergo additional resection after his IBD-like diagnosis. In addition to inflammatory changes, epithelioid granulomas were also seen in those colonic biopsies. The remainder of the children had intestinal resections (six small intestine and two colon). All resected specimens showed deep ulcers (Figure 1D). Microscopically transmural, chronic inflammation and fibrosis were noted underneath the ulcerated mucosa (Figure1E). Chronic mucosal architectural changes, prominent pyloric gland metaplasia, neural hypertrophy, and lymphoid aggregates similar to features seen in Crohn’s disease were also noted in every resection specimen.
In total, anti-inflammatory treatments were given to five children (cases #1, #4, #5, #6, and #8). These treatments consisted of combinations of corticosteroids, mesalamine, methotrexate, azathioprine for varying lengths of time. Table 2 lists all of the different anti-inflammatory treatments as well as the lengths of treatment for each.
Table 2.
Anti-inflammatory treatments per case
| Case # | Anti-inflammatory treatment | Length of time given | Anti-TNF-α treatment given? | Anti-TNF-α Results |
|---|---|---|---|---|
| 1 | Steroid enemas | 4 months | Yes, adalimumab | Resolution |
| Mesalamine suppositories | 3 months | |||
|
| ||||
| 2 | None | n/a | Yes, adalimumab | Resolution |
|
| ||||
| 3 | None | n/a | No | n/a |
|
| ||||
| 4 | Steroids | 6 weeks | Yes, adalimumab | Symptoms improved |
| Methotrexate | 1.5 years | |||
| Mesalamine | 1.5 years | |||
|
| ||||
| 5 | Mesalamine | 2 years | Yes, infliximab | Symptoms improved |
| Azathioprine | 2 years | |||
| Steroids | 8 months | |||
|
| ||||
| 6 | Mesalamine | 4 months | No | n/a |
|
| ||||
| 7 | None | n/a | No | n/a |
|
| ||||
| 8 | Steroids | 6 weeks | Yes, adalimumab | Symptoms improved when given with steroids |
| Methotrexate | 1.5 years | |||
| Mesalamine | 1.5 years | |||
|
| ||||
| 9 | None | n/a | No | n/a |
Anti-TNF-α treatment (adalimumab (AbbVie, Chicago, IL) or infliximab) was used in five of the nine cases (cases #1, #2, #4, #5, and #8; Table 2). Partial GI obstruction cases accounted for three instances, and anti-TNF-α was found to be completely effective in two and partially effective in another who was supplemented with steroids. The two HD children without SBS were also treated with anti-TNF-α, and both had marked improvement of symptoms. Anti-TNF-α treatment was given intravenously for 2-4 months in each case. There were no relapses after discontinuation of treatment.
Discussion
Inflammatory bowel disease (IBD) encompasses a wide variety of nonspecific intestinal inflammatory disorders that can be difficult to manage, especially in the pediatric population.[6] The two main types are ulcerative colitis (UC) with contiguous mucosal and submucosal inflammation and Crohn’s disease (CD) with noncontiguous transmural inflammation.[7] IBD results in malabsorption of nutrients, failure to thrive, abdominal pain, GI bleeding and extraintestinal manifestations.[8]
Current IBD etiologic theories involve an interaction of genetics, host immunity and environmental factors.[1] Genetic factors are more influential than exogenous factors in children.[9] Mutations in barrier function, T cell signaling, and cytokine-receptor interactions seem to be most closely linked to IBD development.[2] Since linking mutations in NOD2/CARD15 to CD, genome-wide association studies have identified more than 160 IBD-associated loci.[2]
An emerging hypothesis is that intestinal dysbiosis (microbial imbalance) may be a trigger for IBD. In children, both the mucosal immune system and intestinal flora are still in the developmental stage.[10] The normal adult human microbiota consist of around 1014 bacterial cells and up to an estimated 1,000 different bacterial species.[10] Patients with active CD have decreased intestinal diversity of beneficial bacterial families, such as Bacteroides and Firmicutes, when compared with those in remission state and healthy individuals.[9] In addition, genera like Roseburia, which normally increase the production of T regulatory cells and therefore anti-inflammatory cytokines, are decreased.[2] Alterations like these may lead to the dysregulation of host-microbe interactions.
The normal immune regulatory function of the intestinal microbiota consists of priming the mucosal immune system and maintenance of intestinal epithelial homeostasis.[10] Dysregulation of host-microbe interactions can lead to inadequately controlled or hyperinflammatory reactions. In a healthy individual, intestinal epithelial cells provide a physical barrier between the luminal microbes and the intestinal tissues.[2] Microbial sensing (and killing) is mediated through Toll-like receptors (TLRs) and nucleotide-binding domain and leucine-rich repeat-containing receptors (NLRs).[9] Overgrowth of aggressive commensal microbes increases the number of antigens that bind TLRs and NLRs to induce pathogenic immune responses that result in an increase mucosal permeability as well as pathogenic innate and T-cell immune responses.[9] In other words, a breakdown in the normal barrier between a potentially aberrant intraluminal microbial flora drives IBD changes.[11]
In the present paper, we propose that the chronic, partial GI obstruction in our group of patients potentially leads to a shift in the microbiome due to intestinal stasis similar to what is seen with small intestinal bacterial overgrowth (SIBO).[12] Historically, SIBO is diagnosed based on bacterial overgrowth in the context of abnormal or postsurgical intestinal anatomy and can be caused by obstruction, motility disorders, and CD to name a few.[12] As a result of the shift in the microbiome, there is activation of the TLR and NLR signals, which result in cytokine production, inflammation, and breakdown in the mucosal integrity. If unregulated and untreated, this may then lead to development of an IBD-like process. It is interesting that all of the initial resections were performed in the newborn period. It is possible that this may represent a uniquely susceptible population to this particular process.
Ileocolonic anastomotic ulcer syndrome has been described in the literature, especially in the pediatric population who underwent bowel resection as a neonate.[3] Similar to IBD patients, these patients have recurrent episodes of bleeding, abdominal distention, and bacterial overgrowth. A proposed etiology of ulcer development involves bacterial contamination of the small intestine from the adjacent colon.[3, 4] The resultant bacterial overgrowth likely causes similar changes in intestinal stasis and barrier function as described above for IBD.
Strikingly, in our patient population there was dramatic improvement in ileocolonic anastomotic ulcerations and CD-like symptoms with anti-TNF-α and steroid treatment regardless of initial reason for intestinal resection, history of HD, or SBS diagnosis. This is potentially consistent with our working theory because we believe the secondary development of ulcerations and CD is caused by a combination of disturbed intestinal defense mechanisms along with a potential dysregulation of the microbiome. While a mainstay of aberrant bacterial overgrowth is anti-microbial therapy, oral antibiotics failed to change the clinical course in over half of our children, suggesting that once this inflammatory process is initiated, more aggressive treatment may be needed.
A very similar process was recently described in eight children with a previous diagnosis of HD.[5] Levin, et al. performed a 20-year analysis and estimated an incidence rate of about 1% (8 in 700) among HD patients. Most of these cases (75%) involved distal colon or rectum. Only one had total colonic HD. In this report, three of the eight children were noted to have Trisomy-21, however, none of our patients were positive for Trisomy-21. Also distinct from the present study, none of the patients in Levin, et al. received anti-TNF-α treatment. One patient could not tolerate any of the antiinflammatory medications and is suffering from pain, fatigue, and frequent mucous stools with blood. Two had resolution of symptoms with oral mesalazine or azathioprine alone. The other five remained symptomatic on individually designed treatment regimens. One required anastomotic revision for stricture. One was still diverted with a colostomy at the time of analysis.
In conclusion, we propose that partial GI obstruction after several common pediatric surgical procedures leads in some pediatric patients to the secondary development of Crohn’s disease-like pathology. Interestingly, this process is responsive to classic surgical treatments (e.g. resection) if the process is highly localized, however, more extensive disease can be very responsive to anti-Crohn’s medical treatments including anti-TNF-α and steroids.
References
- 1.Rabizadeh S, D M. Update in Pediatric Inflammatory Bowel Disease. Rheumatic Disease Clinics of North America. 2013;39:789–99. doi: 10.1016/j.rdc.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knights D, L K, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505–10. doi: 10.1136/gutjnl-2012-303954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sondheimer JM, S R, Narkewicz MR, Tyson RW. Anastomotic ulceration: a late complication of ileocolonic anastomosis. J Pediatr. 1995;127(2):225–30. doi: 10.1016/s0022-3476(95)70299-7. [DOI] [PubMed] [Google Scholar]
- 4.Chari ST, K R. Ileocolonic anastomotic ulcers: a case series and review of the literature. Am J Gastroenterol. 2000;95:1239–43. doi: 10.1111/j.1572-0241.2000.02016.x. [DOI] [PubMed] [Google Scholar]
- 5.Levin DN, M M, Rintala RJ, Jacobson D, Langer JC. Inflammatory Bowel Disease Manifesting After Surgical Treatment for Hirschsprung Disease. JPGN. 55(3):272–7. doi: 10.1097/MPG.0b013e31824f617a. [DOI] [PubMed] [Google Scholar]
- 6.Kwon YH, K Y. Pre-diagnostic Clinical Presentations and Medical History Prior to the Diagnosis of Inflammatory Bowel Disease in Children. Pediatr Gastroenterol Hepatol Nutr. 2013;16(3):178–84. doi: 10.5223/pghn.2013.16.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler J, Coran AG, Teitelbaum DH. In: Ulcerative Colitis, in Pediatric Surgery. Coran AG, editor. Elsevier Saunders; Philadelphia: 2012. [Google Scholar]
- 8.Quach P, N G, Benchimol EI. Quality improvement in pediatric inflammatory bowel disease: Moving forward to improve outcomes. World J Gastroenterol. 19(38):6367–74. doi: 10.3748/wjg.v19.i38.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shim JO. Gut Microbiota in Inflammatory Bowel Disease. Pediatr Gastroenterol Hepatol Nutr. 2013;2013(16):17–21. doi: 10.5223/pghn.2013.16.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comito D, R C. Dysbiosis in the Pathogenesis of Pediatric Inflammatory Bowel Disease. Intl J of Inflammation. 2012;2012:1–7. doi: 10.1155/2012/687143. Article ID 687143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tysk C, J G. Has smoking changed the epidemiology of ulcerative colitis? Scand J Gastroenterol. 1992;27(6):508–12. doi: 10.3109/00365529209000113. [DOI] [PubMed] [Google Scholar]
- 12.Sachdev AH, P M. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther Adv Chronic Dis. 2013;4(5):223–31. doi: 10.1177/2040622313496126. [DOI] [PMC free article] [PubMed] [Google Scholar]


