Abstract
A number of established and investigational anticancer drugs slow the religation step of DNA topoisomerase I (topo I). These agents induce cytotoxicity by stabilizing topo I-DNA covalent complexes, which in turn interact with advancing replication forks or transcription complexes to generate lethal lesions. Despite the importance of topo I-DNA covalent complexes, it has been difficult to detect these lesions within intact cells and tumors. Here, we report development of a monoclonal antibody that specifically recognizes covalent topo I-DNA complexes, but not free topo I or DNA, by immunoblotting, immunofluorescence or flow cytometry. Utilizing this antibody, we demonstrate readily detectable topo I-DNA covalent complexes after treatment with camptothecins, indenoisoquinolines and cisplatin but not nucleoside analogues. Topotecan-induced topo I-DNA complexes peak at 15–30 min after drug addition and then decrease, whereas indotecan-induced complexes persist for at least 4 h. Interestingly, simultaneous staining for covalent topo I-DNA complexes, phospho-H2AX and Rad51 suggests that topotecan-induced DNA double-strand breaks occur at sites distinct from stabilized topo I-DNA covalent complexes. These studies not only provide new insight into the action of topo I-directed agents, but also illustrate a strategy that can be applied to study additional topoisomerases and their inhibitors in vitro and in vivo.
INTRODUCTION
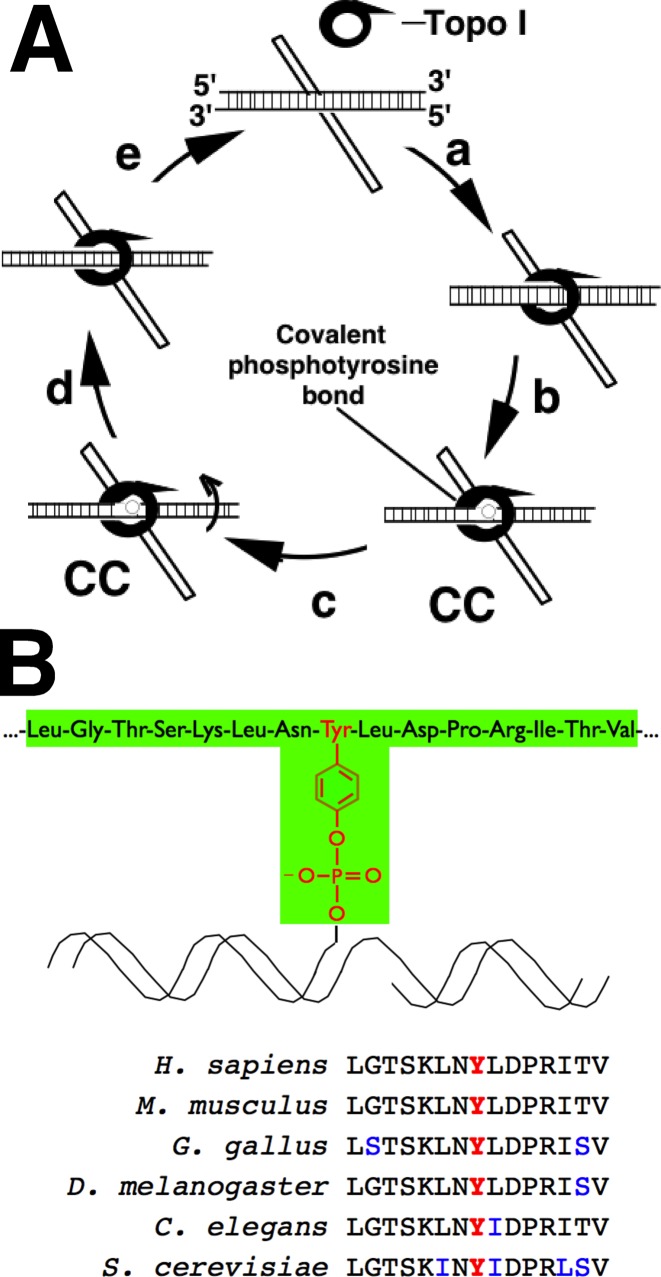
Topoisomerase I (topo I) is an abundant nuclear enzyme that relaxes torsional strain in DNA (1,2). During the course of its normal catalytic cycle, topo I nicks one DNA strand, allows rotation around the intact strand and then reseals the DNA backbone, restoring DNA integrity (Figure 1A). Previous studies have demonstrated roles for this DNA relaxation activity in replication (3), transcription (4–6) and viral integration (7,8).
Figure 1.
Topo I catalytic cycle and antigen used for immunization. (A) Catalytic cycle of topo I. After initially binding to sites of supercoiled DNA (‘a’), topo I creates a nick via a transesterification reaction catalyzed by an active site tyrosine residue (‘b’), resulting in a topo I-DNA covalent complex (‘cc’). After DNA rotates about the nick (‘c’), the enzyme reverses the transesterification reaction, resealing the nicked DNA (‘d’) and disassociating from DNA (‘e’). Camptothecin and its analogues stabilize topo I-DNA covalent complexes. (B) Peptide used to raise α-TopoIcc antibody (shaded in green) and sequence of topo I active site peptides from various species to illustrate protein sequence conservation.
Additional studies have demonstrated that topo I is the target for a class of widely utilized anticancer drugs, the camptothecins, as well as other agents (9–11). Camptothecins intercalate into DNA at the topo I active site, inhibiting the religation step of the enzyme and shifting the equilibrium toward topo I-DNA covalent complexes (Figure 1A) that are formed during DNA nicking (12,13). Collisions of advancing replication forks or transcription complexes with these drug-stabilized topo I-DNA covalent complexes are thought to produce further DNA damage, ultimately leading to cell death (14,15). Consistent with this model, inhibition of replication diminishes the cytotoxicity of topo I poisons (16,17). Moreover, topo I mutants that slow the topo I religation step recapitulate the cytotoxic effects of camptothecins (18).
Camptothecin derivatives are widely used for the treatment of various cancers (19–22). In particular, topotecan (TPT) is approved for the treatment of ovarian, cervical and small cell lung cancer (23) and is currently being investigated for its potential activity in acute myelogenous leukemia (24,25). Irinotecan, another camptothecin derivative, is FDA-approved for the treatment of colorectal cancer (26) and also has activity in non-small cell lung cancer, pancreatic cancer and breast cancer (27). Novel topo I poisons, including camptothecin derivatives and indenoisoquinolines, continue to be identified and developed for clinical use (11,28–33).
Because the toxicities of these agents in normal tissues are substantial, predicting which tumors are likely to respond would be beneficial. Cellular modifications that diminish the number of drug-stabilized topo I-DNA covalent adducts, including impaired drug accumulation or decreased topo I content, are major mechanisms of resistance to these agents (21,34). As a result, there is substantial interest in being able to detect and quantify topo I-DNA covalent complexes in tumor cells.
Previous methods for detecting topo I-DNA covalent complexes have included alkaline elution (35), in vivo complexing of enzyme (ICE) assays (36) and potassium-SDS precipitation assays (37–39). Alkaline elution, which separates nicked from intact DNA by filtration, is time-consuming, needs specialized equipment and typically requires high drug concentrations (>250 nM TPT) to detect covalent topo I-DNA complexes. ICE assays, which involve cell lysis followed by ultracentrifugation to separate covalent topo I-DNA complexes from free protein, are lengthy (20+ h for ultracentrifugation alone) and even less sensitive. Potassium-SDS methods, which involve precipitation of proteins along with any covalently bound DNA, are not specific for topo I-DNA covalent complexes and usually require radiolabeling of DNA as well as reproducible DNA shearing for sensitive, accurate quantitation. A more recently described method that uses chaotropic salts to rapidly denature protein and recover DNA-bound protein (40) has improved sensitivity for topo I-DNA covalent complexes but is limited to immunoblot- or ELISA-based detection and cannot be paired with immunofluorescence or flow cytometry.
To overcome these difficulties, we have developed a monoclonal antibody with specificity for topo I covalently bound to DNA that is capable of detecting topo I-DNA covalent complexes by immunoblotting, immunofluorescence or flow cytometry. Here, we utilize this antibody to detect topo I-DNA covalent complexes in vitro and in vivo, evaluate previous suggestions that various agents are topo I poisons and assess the temporal and special relationships between topo I-DNA covalent complexes and repair proteins.
MATERIALS AND METHODS
Materials
Indenoisoquinolines and TPT were provided by the Drug Synthesis Branch of the National Cancer Institute. Reagents were purchased from the following suppliers: Hoechst 33342, sarkosyl, cytarabine, actinomycin D, camptothecin, cisplatin, etoposide, 2-mercaptotethanol, Freund's adjuvant, p-nitrophenyl phosphate disodium and heat-inactivated fetal bovine serum (FBS) from Sigma-Aldrich (St. Louis, MO, USA); SN-38 and gemcitabine from Tocris Bioscience (Minneapolis, MN); 16% paraformaldehyde from Electron Microscopy Sciences (Hatfield, PA, USA) and PEG1500 from Roche Molecular Biochemicals (Mannheim, Germany). All other reagents were obtained as previously described (41,42).
Topo I peptides were generated in the Mayo Proteomics Core using standard, solid-phase Fmoc chemistry. The phospho-topo I sequence was 716LGTSKLN(phosphoY)LDPRITV730, corresponding to the active site of human topo I with a phosphotyrosine replacing the catalytic tyrosine (Tyr723). Non-phospho-topo I peptide was synthesized with unmodified Tyr723. The synthesis of the phospho-topo I peptide covalently bound to the universal nucleoside 1-(2′-deoxy-β-D-ribofuranosyl)-3-nitropyrrole (43) will be described elsewhere. All three peptides had an additional cysteine residue at the N-terminus for conjugation to keyhole limpet hemocyanin or bovine serum albumin. In addition to the topo I peptides, decoy phosphotyrosine peptide was produced with stoichiometric amounts of random amino acids at positions 1-7 and 9-15, with a phosphotyrosine at residue 8.
Antibodies were obtained from the following sources: Rabbit anti-Rad51 and rabbit anti-Ser139-H2AX from Active Motif (Carlsbad, CA, USA), rabbit anti-Raf1 from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and rabbit monoclonal anti-RPA70 from Abcam (Cambridge, MA, USA). Murine anti-RasGRP1 was generated and characterized as previously described (44). Rabbit anti-53BP1 was a kind gift from Zhenkun Lou (Mayo Clinic, Rochester, MN, USA).
Cell culture
All cells were grown at 37°C in an incubator with 5% CO2 and saturated humidity and were maintained at densities of less than 1 × 106/ml (non-adherent lines). Cells were grown in the following media, all of which contained 10% (v/v) heat-inactivated fetal bovine serum, 100 U/ml penicillin G, 100 μg/ml streptomycin and 2 mM glutamine: FOXNY myeloma cells in Iscove's modified Dulbecco's medium, A549 and K562 cells in RPMI 1640 medium (medium A), HCT116 cells in McCoy's 5A medium, and P388 and P388/CPT cells (kind gifts from Yves Pommier, National Cancer Institute) in medium A supplemented with 100 μM 2-mercaptoethanol and, for the camptothecin-resistant line, 10 μM camptothecin.
Immunization
Murine monoclonal antibodies were generated using the strategy of de St. Grooth and Scheidegger (45). Female BALB/c mice were immunized by subcutaneous injection of 1 mg of phospho-topo I peptide conjugated to keyhole limpet hemocyanin in complete Freund's adjuvant. Test bleeds were assayed for immunoreactivity with the phospho-topo I peptide and non-phosphorylated peptide by enzyme-linked immunosorbent assay (ELISA). Splenocytes (100 million) from the mouse with strongest reactivity with the phospho-topo I peptide were fused with FOXNY cells using PEG1500. Cells were resuspended in adenine-hypoxanthine-aminopterin-thymidine selection medium. At day 10 after fusion, hybridoma supernatants were screened by ELISA and ICE assay, then subcloned by limiting dilution.
ELISA
Ninety-six well plates were coated with 25 ng peptide/well in 100 mM Na2CO3 buffer, washed and blocked with 5% milk in calcium- and magnesium-free Dulbecco's phosphate buffered saline (PBS). To test each hybridoma, 50 μl culture supernatant was applied for 1 h at 20°C. Plates were washed with Na2CO3 buffer, incubated with alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma-Aldrich), washed again and incubated with 100 μl of 1 mg/ml p-nitrophenyl phosphate. Positive clones were assayed by secondary screening using an ICE assay as described below.
ICE assays (36)
A549 cells (40–60% confluent) in 100 mm tissue culture plates were incubated for 60 min at 37°C with DMSO, 10 μM TPT or 10 μM etoposide in serum-free RPMI containing 10 mM HEPES, pH 7.4 (medium B). After treatment, cells were rapidly lysed in 1 ml lysis buffer (10 mM Tris-HCl, pH 8.0, containing 1% (w/v) sarkosyl and 1 mM EDTA). Lysates were layered on a CsCl2 gradient and sedimented at 125 000 x g for 21 h at 20°C. Fractions (0.5 ml) were collected from the bottom of the gradients, assayed for the presence of DNA (detectable in fractions 1-4) and deposited onto nitrocellulose membranes using a slot blotting apparatus.
Band depletion assays
Band depletion assays were performed as described (46). A549 cells (40-60% confluent) in 100 mm dishes were treated with increasing concentrations of TPT or camptothecin for 60 min at 37°C in RPMI 1640 medium containing 10 mM HEPES (pH 7.4 at 20°C). After treatment, protein was harvested by solubilization in buffer consisting of 6 M guanidine hydrochloride containing 250 mM Tris-HCl, pH 8.5 at 20°C, 10 mM EDTA, 1% (v/v) 2-mercaptoethanol and 1 mM freshly added phenylmethylsulfonyl fluoride. After preparation for electrophoresis as described previously (47), aliquots containing 50 μg of protein [assayed by the bicinchoninic acid method] were deposited on slot blots for probing with α-TopoIcc or separated on SDS-polyacrylamide gels, electrophoretically transferred to nitrocellulose and probed with either C-21 anti-topo I or anti-Hsp90β antibody (kind gifts from Y-C. Cheng, Yale University, New Haven, CT and D. Toft, Mayo Clinic, Rochester, MN, USA, respectively).
Immunofluorescence
Immunofluorescence studies were performed using a previously described method (42) with several modifications. In brief, cells grown on nitric acid-etched or autoclaved coverslips were treated for varying times and with varying TPT concentrations in medium A, fixed for 15 min at 4°C in 4% (w/v) paraformaldehyde in PBS and permeabilized with 0.25% (v/v) Triton X-100 in PBS for 15 min at 4°C. To render the DNA-protein crosslinks more accessible to antibody, the coverslips were incubated in 1% (w/v) SDS at 20-22°C for 5 min, washed five times with wash buffer [0.1% (w/v) bovine serum albumin and 0.1% (v/v) Triton X-100 in PBS] and blocked in TSM buffer consisting of 10% (w/v) powdered nonfat milk in 150 mM NaCl and 10 mM Tris-HCl (pH 7.4). After reaction overnight with primary antibody (1-4 μg/ml) in PBS containing 5% (v/v) goat serum at 4°C, cells were rinsed 5-6 times with wash buffer over 20 min; incubated with Alexa Fluor 488- or 568-conjugated secondary antibody (Invitrogen) at 1:1000 in PBS/5% goat serum for 1 h in subdued light; washed 5-6 times with wash buffer over 20 min; stained with 1 μg/ml Hoechst 33258 in PBS; and mounted using ProLong antifade reagent (Invitrogen; Carlsbad, CA, USA).
Alternatively, cells were fixed, permeabilized, reacted with 0.1% SDS as described above, washed three times with PBS and blocked in IF blocking buffer [PBS with 1% (w/v) glycerol, 0.1% (w/v) gelatin from cold water fish, 5% (v/v) normal goat serum, 0.1% (w/v) bovine serum albumin and 0.4% (w/v) sodium azide]. Coverslips were incubated overnight at 4°C in primary antibody in IF blocking buffer, washed extensively with PBS over 20 min and stained with fluorochrome-conjugated secondary antibody diluted 1:1000 in IF blocking buffer for 1 h at 20-22°C. After extensive washes with PBS and counterstaining with 1 μg/ml Hoechst 33258 in PBS, slides were mounted as described above. In this procedure, α-TopoIcc antibody was used at 0.67 μg/ml and anti-topo I human IgG (TopoGEN; Port Orange, FL) at 1:1000.
With either method, images were captured on a LSM 710 scanning confocal microscope (Carl Zeiss AG; Oberkochen, Germany) using a 63X/1.2 W Korr C-Apo objective and processed using Zeiss Zen software and Adobe Photoshop CS3.
Flow cytometry
Aliquots containing one million cells in medium A were treated for 30 min at 37°C with varying concentrations of TPT, centrifuged at 150×g for 5 min, decanted and fixed by incubation in 2% paraformaldehyde in PBS for 15 min at 4°C. After sedimentation at 150×g for 5 min, cells were treated with 0.25% (w/v) Triton X-100 in PBS (15 min, 4°C), sedimented at 150×g, incubated with 1% (w/v) SDS in PBS (5 min, 20-22°C), washed twice with PBS, resuspended in PBS containing 5% (v/v) normal goat serum and incubated with α-TopoIcc antibody (10 μg/ml) for 60 min at 20-22°C. Cells were then washed 4-5 times with PBS, incubated with Alexa Fluor 647 anti-mouse IgG (1:1000) for 30 min at 20-22°C in subdued light, sedimented at 150×g for 5 min, resuspended in PBS and immediately subjected to flow microfluorimetry on a FACSCanto II flow cytometer (BD Biosciences; San Jose, CA, USA) using the FL4 channel (excitation: 633 nm; emission: 660/20 nm). Data were analyzed and overlays created using BD CellQuest software.
Xenografts
Once subcutaneous xenografts of A549 cells in nu/nu mice (Harlan labs) reached 0.7 cm in their longest axis, mice were treated with 50 mg/kg irinotecan intraperitoneally. Tumors harvested before or 2-6 h after treatment were snap frozen in liquid nitrogen, embedded in OTC embedding medium, stored at -80°C, sectioned onto -20°C slides and immediately fixed and prepared for immunostaining using conditions described above for tissue culture cells.
RESULTS
To test the hypothesis that topo I covalently attached to DNA could be selectively detected immunologically, monoclonal antibodies were raised against a peptide corresponding to the active site of the topo I with a phosphorylated Tyr723 residue (Figure 1B). Of 726 wells screened, one hybridoma was found to react with the immunizing peptide in ELISA assays and preferentially recognize topo I-DNA complexes by immunoblotting upon secondary screening (Supplementary Figure S1). That antibody, termed ‘α-TopoIcc,’ was purified from hybridoma supernatants and used for further studies.
Detection via immunoblotting
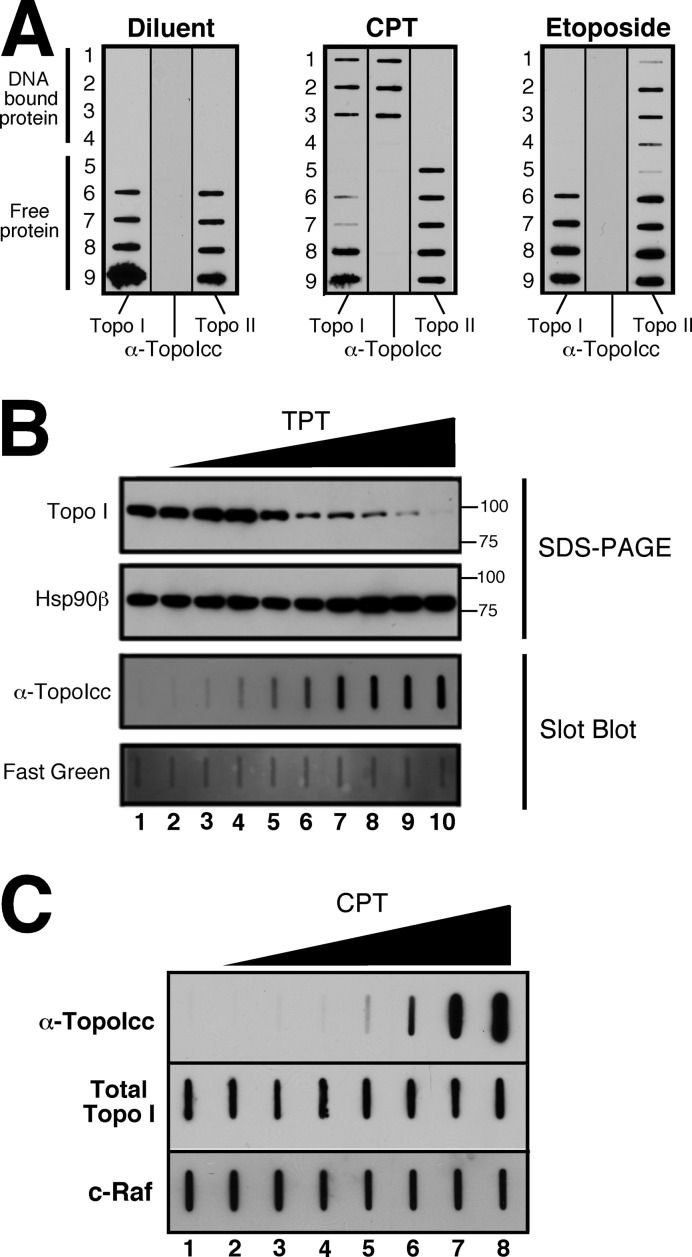
Slot blotting demonstrated that α-TopoIcc detects topo I-DNA covalent complexes in cell lysates with high specificity. Following treatment with topoisomerase poisons, cell lysates were prepared under denaturing conditions and subjected to cesium chloride centrifugation to separate DNA-bound and free proteins. Upon subsequent immunoblotting, α-TopoIcc detected topo I that co-migrated with DNA in lysates from TPT-treated cells (Figure 2A). Importantly, α-TopoIcc did not detect topo II-DNA complexes after etoposide treatment or free topo I protein in cell fractions after diluent or drug treatment, demonstrating specificity for covalent topo I-DNA complexes.
Figure 2.
Detection of topo I-DNA covalent complexes by immunoblotting. (A) ICE assays were used to separate topo I-DNA covalent complexes from free topo I in A549 cell lysates. Fractions were immobilized on nitrocellulose and probed with either anti-topo I antibody (left), the α-TopoIcc antibody (middle) or anti-topo IIα antibody (right). (B) A band depletion assay was performed by treating A549 cell with diluent (lane 1) or 2-fold serial dilutions up to 100 μM TPT (lanes 2-10). Aliquots containing 50 μg of protein were either run on an SDS-polyacrylamide gel and probed with antibodies to topo I and Hsp90β (top two panels), or slot-blotted onto membranes and probed for topo I-DNA covalent complexes (bottom two panels). (C) After HCT116 cells were treated for 20 min with diluent (0.1% DMSO, lane 1) or 1, 3, 10, 30, 100, 1000 and 10 000 nM CPT (lanes 2-8), aliquots containing 50 μg of protein were probed as indicated.
When A549 cells were treated with increasing TPT concentrations, topo I-DNA covalent complexes failed to migrate into SDS-polyacrylamide gels due to the size of the attached DNA (46), resulting in a graduated decrease in topo I signal at 100 kDa (Figure 2B, top panel). After deposition of the same lysates on a slot blot, α-TopoIcc demonstrated a dose-dependent increase in signal (Figure 2B, bottom panel).
To confirm that these results were not unique to TPT or A549 cells, HCT116 cells were treated for 20 min with varying concentrations of the parent drug camptothecin, then lysed and subjected to blotting. Topo I-DNA covalent complexes were readily detectable at 30 nM camptothecin and faintly detectable above background at 10 nM (Figure 2C, wells 4 and 5). Extended ELISA studies were performed to investigate the basis for this selectivity but could not further elucidate the epitope recognized by α-TopoIcc (Supplementary Figure S2).
Detection of topo I-DNA covalent complexes by flow microfluorimetry
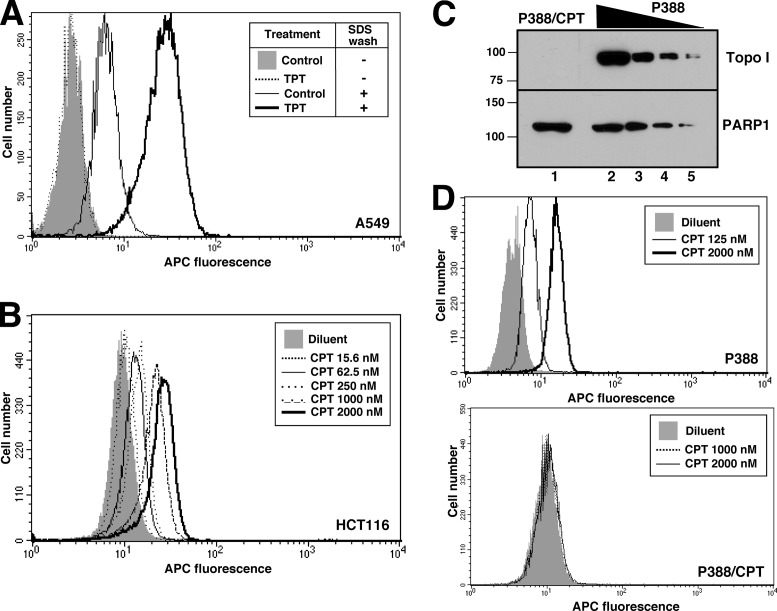
Flow cytometry provides an alternative approach for immunodetection. Fixation and permeabilization with neutral detergent did not yield detectable staining of TPT-treated cells by α-TopoIcc; however, inclusion of an SDS incubation step prior to addition of α-TopoIcc allowed facile detection of an increased signal for topo I-DNA covalent complexes (Figure 3A). This signal was visible as a rightward shift in histogram peaks and was observed in multiple cell lines treated with TPT or camptothecin (Figure 3A and B, and Supplementary Figure S3). This increase in fluorescence was detectable at camptothecin concentrations as low as 16 nM (Figure 3B). To confirm the specificity of staining, the mouse lymphoma line P388 and the camptothecin-resistant subline P388/CPT (48), which lacks detectable topo I protein (Figure 3C), were also stained. Increased binding of α-TopoIcc was detectable following camptothecin treatment of parental P388 cells (Figure 3D, top panel) but not P388/CPT cells (Figure 3D, bottom panel), consistent with the specificity of α-TopoIcc for topo I-DNA covalent complexes.
Figure 3.
Detection of topo I-DNA covalent complexes by flow cytometry. (A and B) A549 cells (A) or HCT116 cells (B) were incubated with 5 μM TPT (A) or the indicated TPT concentration (B) for 1 h, sedimented, lysed, fixed, stained with α-TopoIcc antibody followed by Alexa Fluor 647-conjugated secondary antibody and subjected to flow microfluorimetry. (C) Aliquots containing 50 µg of whole cell lysate from the P388/CPT and parental P388 mouse lymphoma lines (lanes 1 and 2) or serial 2-fold dilutions (lanes 3-5) were subjected to SDS-PAGE, probed for topo I and, as a loading control, poly(ADP-ribose) polymerase 1 (PARP1). (D) Detection of topo I-DNA covalent complexes in P388 (top) and P388/CPT cells (bottom) by flow microfluorimetry.
Detection of topo I-DNA covalent complexes by fluorescence microscopy
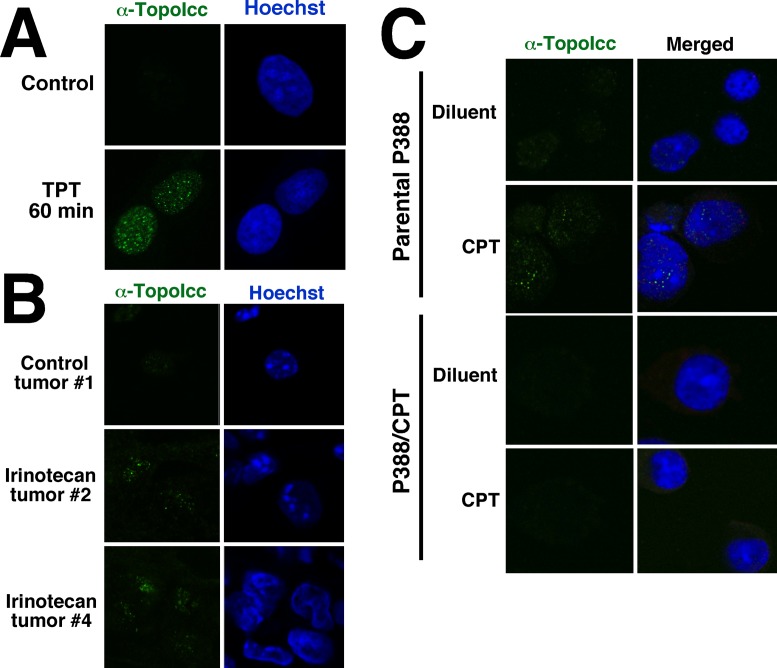
α-TopoIcc also allowed visualization of topo I-DNA complexes by indirect immunofluorescence. A549 cells treated with 1 μM TPT displayed small areas of punctate staining (foci) throughout the nucleus (Figure 4A). This staining increased in a dose-dependent manner (Supplementary Figure S4A) and could be quantitated as average number of foci per cell (Supplementary Figure S4B) or maximal mean fluorescence in a confocal slice (Supplementary Figure S4C). Simultaneous staining with anti-topo I antibody and α-TopoIcc revealed that marginal staining with α-TopoIcc is detectable within nucleoli of untreated cells, but TPT treatment causes a dramatic increase in non-nucleolar staining of topo I-DNA complexes (Supplementary Figure S4D) consistent with previously observed migration of topo I out of nucleoli following TPT treatment (49). Staining was also observed in A549 xenografts after mice were treated with irinotecan, the pro-drug for the topo I poison 7-ethyl-10-hydroxycamptothecin (SN-38), but not in control xenografts (Figure 4B). Importantly, complexes could also be detected in parental P388 cells but not topo I-deficient P388/CPT cells (Figure 4C), demonstrating specificity of the staining for topo I-DNA covalent complexes.
Figure 4.
Detection of topo I-DNA covalent complexes by fluorescence microscopy. (A) After treatment for 1 h with 1 μM TPT, A549 cells were fixed, permeabilized, incubated with SDS and stained with α-TopoIcc antibody (green) and Hoechst 33258 (blue). (B) Cryostat sections of A549 xenografts harvested 0 (top), 2 (middle) and 4 h (bottom) after irinotecan administration were stained with α-TopoIcc and Hoechst 33258. (C) After treatment of P388 or P388/CPT cells with 1 μM camptothecin for 30 min, cells were prepared and stained with α-TopoIcc.
Examination of putative topo I poisons using α-TopoIcc
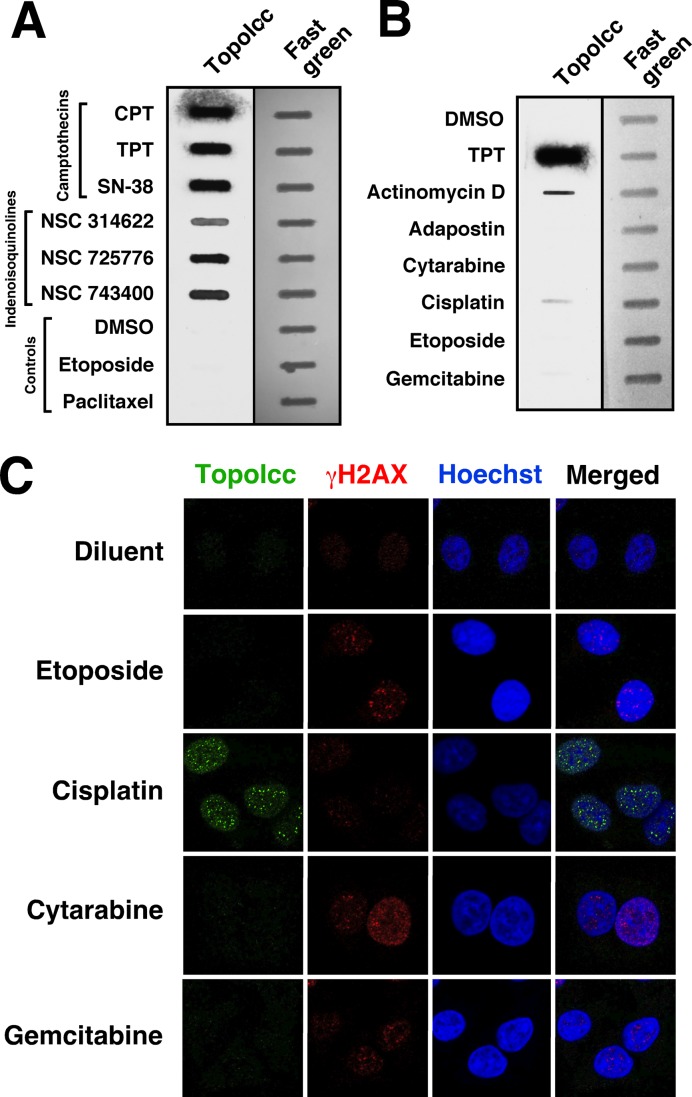
In addition to camptothecins, a number of agents, including a series of indenoisoquinolines (50), actinomycin D (51), cytarabine (52), gemcitabine (53), cisplatin (54) and Hoechst 33342 (55), have been reported to trap topo I-DNA covalent complexes. Most of the studies reporting stabilization of topo I-DNA covalent complexes have relied on alkaline elution or ICE assays. The ability of α-TopoIcc to detect topo I-DNA covalent complexes provided the opportunity to independently assess the action of these compounds. Slot-blotting readily demonstrated that the indenoisoquinolines NSC 314622, NSC 725776 and NSC 743400 (indotecan), like camptothecins, stabilize topo I-DNA covalent complexes (Figure 5A). Increased topo I-DNA covalent complexes were also readily detected after treatment with actinomycin D (Figure 5B), cisplatin (Figure 5B, C) or Hoechst 33342 (not shown). In contrast, α-TopoIcc binding was not detectable above background after treatment with cytarabine or gemcitabine for 1 h (Figure 5B) or 6 h (Figure 5C and data not shown). Thus, the ability to directly detect topo I-DNA complexes provides a new opportunity for assessing the mechanism of action of various antineoplastic agents in situ.
Figure 5.
Examination of topo I-DNA covalent complexes after treatment with various therapeutic agents. After A549 cells were treated for 1 h (A and B) or 6 h (C) with camptothecin (CPT), TPT, 7-ethyl-10-hydroxycamptothecin (SN-38), the indicated indenoisoquinoline or etoposide at 10 μM; paclitaxel, actinomycin D, cytarabine or gemcitabine at 1 μM; adaphostin at 20 μM or cisplatin at 40 μM, lysates were prepared for slot blotting so that aliquots containing 50 μg of total cellular protein could be probed with α-TopoIcc (A and B) or cells were fixed and stained with α-TopoIcc and anti-phospho-Ser139-H2AX (γH2AX; C).
Testing the proposed mechanism of killing by topo I poisons
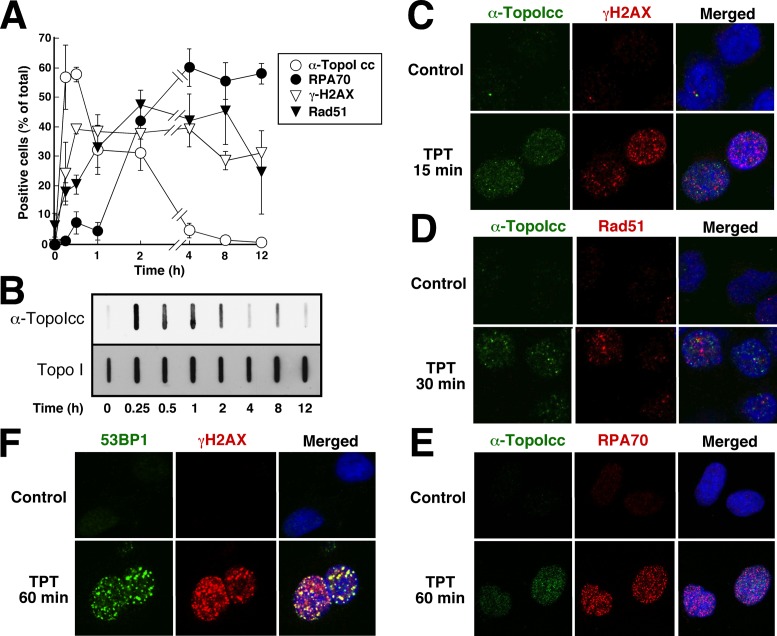
Further experiments examined the relationship between topo I-DNA covalent complexes and events involved in the response to these lesions. According to current understanding, topo I-DNA covalent complexes, like other bulky adducts, cause stalling of DNA replication forks followed by replication fork collapse and formation of DNA double-strand breaks (1,15,16,56,57). Based on this model, one would expect to observe sequential formation of topo I-DNA covalent complexes, replication protein A (RPA) foci, phospho-Ser139-H2AX (γH2AX) foci and Rad51 foci, reflecting the primary DNA lesion, stalled replication forks, DNA double-strand breaks and double-strand break repair, respectively. Time-course experiments after treatment with 1 μM TPT, a concentration used in previous mechanistic experiments, yielded a result that differed from this prediction in a number of ways. First, the percentage of cells with detectable topo I-DNA covalent complexes was greatest at 15–60 min after addition of TPT and then diminished (Figure 6A), a result that was confirmed by slot blotting (Figure 6B). This decrease in complexes occurred despite persistence of total topo I content at baseline levels for at least several hours (Figure 6B). Second, γH2AX foci, which reflect activation of ATM, ATR and/or DNA-PK, were detected in a maximal number of cells by 30 min and remained detectable for at least 12 h despite the diminished topo I-DNA covalent complexes (Figure 6A). Rad51 foci, which are thought to reflect ongoing double-strand break repair through the homologous recombination pathway, appeared more slowly than γH2AX foci and remained detectable for at least 8 h, which is consistent with the predicted order. RPA70 foci, on the other hand, accumulated in a substantial fraction of cells only after γH2AX and Rad51 foci (Figure 6A).
Figure 6.
γH2AX foci accumulate before RPA foci after stabilization of topo I-DNA covalent complexes. (A) A549 cells grown on coverslips were treated with 1 μM TPT for the indicated length of time, fixed and prepared for staining with the indicated antibodies. Cells were considered positive if they contained >10 foci. Error bars, ±sd of 3 independent experiments. (B) A549 cells were treated with 1 μM TPT for the indicated length of time, solubilized in 6 M guanidine hydrochloride under reducing conditions and prepared for blotting. Aliquots containing 50 μg of total cellular protein were deposited on nitrocellulose by slot blotting and probed with the indicated antibody. (C–E) A549 cells treated with diluent or 1 μM TPT were stained with α-TopoIcc and rabbit anti-γH2AX (C), anti-Rad51 (D) or anti-RPA70 (E) followed by fluorochrome-labeled secondary antibody and Hoechst 33258. (F) To establish ability to detect colocalization when present, A549 cells treated with 1 μM TPT for 1 h were stained with anti-γH2AX and anti-53BP1.
When these DNA damage-induced events were further examined by double-label immunofluorescence, α-TopoIcc foci were distinct from foci of RPA, γH2AX and Rad51 even at the earliest time points (Figure 6C-E). The readily detectable colocalization of other DNA repair proteins such as γH2AX and 53BP1 in the same cells (Figure 6F) ruled out technical problems with the colocalization strategy. When cells were treated with TPT concentrations as low as 25 nM, γH2AX foci, Rad51 foci and RPA70 foci again failed to colocalize with topo I-DNA covalent complexes (Supplementary Figure S5), indicating that the failure of the topo I-DNA covalent complexes to colocalize was not a reflection of the concentrations chosen.
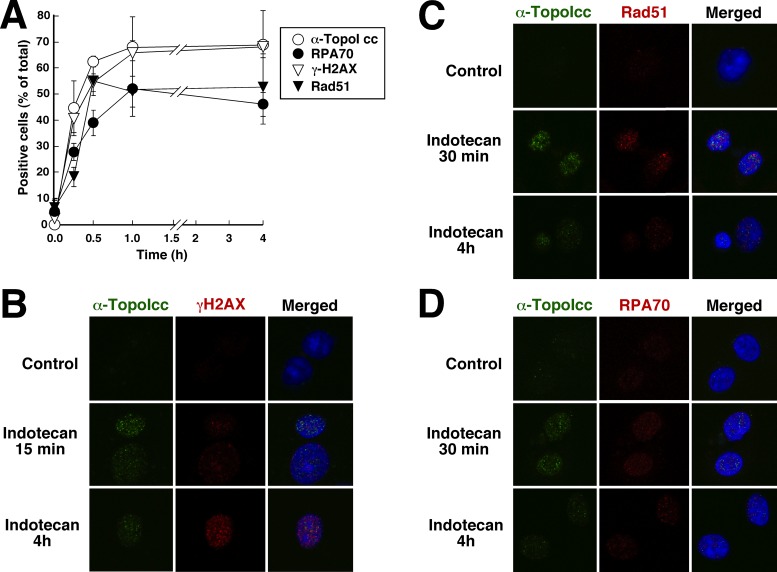
When cells were treated with indotecan, an indenoisoquinoline that lacks a hydrolyzable lactone ring, results differed in two respects (Figure 7). First, the number of nuclei with detectable topo I-DNA covalent complexes reached a peak within 30 min and did not decrease thereafter (Figure 7A). Second, RPA foci were detectable within 15 min (Figure 7A and D). Despite these differences, the overwhelming majority of topo I-DNA covalent complexes visualized with α-TopoIcc still failed to colocalize with phospho-Ser139-H2AX, Rad51 and RPA foci (Figure 7B–D).
Figure 7.
Indenoisoquinoline-stabilized topo I-DNA covalent complexes persist for at least 4 h. (A) A549 cells grown on coverslips were treated with 1 μM indotecan for the indicated length of time, fixed and prepared for staining with the indicated antibodies. Cells were considered positive if they contained >10 foci. Error bars, ±sd of three independent experiments. (B–D) A549 cells treated with diluent or 1 μM indotecan were stained with α-TopoIcc and rabbit anti-γH2AX (B), anti-Rad51 (C) or anti-RPA70 (D) followed by fluorochrome-labeled secondary antibody and Hoechst 33258.
DISCUSSION
In the present study, we describe the development and characterization of a monoclonal antibody that specifically detects DNA-protein covalent complexes containing topo I. This antibody, which is the first of its kind, has several potential uses and serves as a paradigm for a new class of reagent to monitor widely used anticancer drugs.
Previous studies have shown that antibodies can be highly sensitive and specific probes for various types of DNA alterations, including platinum-DNA adducts (58,59) and oxidative damage (60,61). Based on these prior results, along with the exquisite selectivity of many phosphorylation site-specific antibodies, we surmised that it might be possible to generate an antibody specific for topo I-DNA covalent complexes. After multiple unsuccessful attempts at producing rabbit polyclonal antisera, we generated the mouse monoclonal antibody characterized here. Even though protein-DNA covalent complexes are formed during the course of catalysis by several enzymes, including eukaryotic topo I, topo II, topo III and TDP1 as well as viral topo I and bacterial gyrase, to our knowledge this is the first antibody to selectively detect these types of covalent protein-DNA adducts.
Compared to earlier methods for detecting topo I-DNA complexes or the associated ‘concealed’ DNA strand breaks, assays using α-TopoIcc are more specific and more sensitive. Specificity of the antibody was demonstrated by its inability to detect free topo I by immunoblotting (Figure 2A), lack of reactivity with topo I-deficient P388/CPT cells (Figures 3D and 4C) and lack of reactivity with etoposide-stabilized covalent topo II-DNA adducts (Figures 2A and 5A-C). Importantly, this antibody was able to detect increased topo I-DNA complexes at camptothecin concentrations as low as 10-30 nM by immunoblotting (Figure 2C) and 25 nM or below by immunofluorescence (Supplementary Figure S5 and data not shown).
Previous X-ray crystallography has indicated that the covalent bond linking the topo I active site tyrosine to the 3′-phosphate of the DNA backbone (Figure 1A) is located inside the clamp-like topo I catalytic domain (62). Consistent with these results, α-TopoIcc was unable to detect Topo I-DNA complexes in native chromatin, e.g. by flow cytometry (Figure 3A) or immunofluorescence in the absence of SDS (not shown). Instead, denaturation with SDS was required in order to detect topo I-DNA complexes in situ. This is not unlike detection of BrdU incorporation into double-stranded DNA, where denaturation of the DNA is required to facilitate access of anti-BrdU antibodies for immunodetection. Immunoflorescent detection of topo I-DNA complexes, in particular, provides a valuable approach for future study. In addition to the colocalization results described below, we observed heterogeneity in the size of foci stained by α-TopoIcc. Because this was observed within individual experiments, it is unlikely that it merely reflects day to day variation in reagents or technique. Further study is required to understand this phenomenon, which might reflect stabilization of topo I-DNA covalent complexes in areas where topo I molecules are closely spaced because of particularly high torsional strain.
The ability of α-TopoIcc to detect topo I-DNA covalent complexes by slot blotting was utilized to reassess the mechanism of action of selected agents. These studies confirmed that SN-38 and TPT, as well as the indenoisoquinolines NSC 314622, NSC 725776 and indotecan, all rapidly stabilize topo I-DNA covalent complexes (Figure 5A). The previously reported ability of actinomycin D (51) or cisplatin (54) to stabilize topo I-DNA covalent complexes was also confirmed (Figure 5B and C). In contrast, topo I-DNA covalent complexes did not detectably increase during treatment with cytarabine or gemcitabine (Figure 5B and C), suggesting that stabilization of topo I-covalent complex occurs much more weakly, if at all, in viable cells treated with these agents.
In further experiments, we utilized α-TopoIcc to examine the relationship between topo I-DNA covalent complexes and other signs of DNA damage-induced signaling. Time-course experiments demonstrated that TPT-induced complexes were maximal at 15-60 min and then decreased, likely reflecting hydrolysis of the TPT lactone ring after 30 min (63) rather than topo I destruction (Figure 6B). Consistent with this hypothesis, indenoisoquinoline-induced topo I-DNA covalent complexes were observed to persist for at least 4 h (Figure 7).
Comparison of topo I-DNA covalent complexes with other markers of the DNA damage response produced unpredicted results. Although topo I-DNA covalent complexes are thought to act like other bulky DNA adducts, stalling replication forks and leading to replication fork collapse with accompanying DNA double-strand breaks, several of our observations do not appear to fit this model. In particular, γH2AX foci and Rad51 foci formed before RPA foci during TPT treatment (Figure 6A). Second, γH2AX foci, Rad51 foci and RPA foci are spatially distinct from topo I-DNA covalent complexes (Figures 6C-E and 7B-D).
There are several potential explanations for the lack of co-localization of foci detected by α-TopoIcc and antibodies to RPA70, Rad51 and phospho-Ser139-H2AX. First, it is possible that topo I-DNA covalent complexes are processed or removed, e.g. by the action of endonucleases such as XPF/ERCC1 (64), phosphodiesterases such as tyrosyl-DNA phosphodiesterase 1 (65,66) or proteases analogous to the yeast metalloproteinase Wss1 (67,68), to a point where they are no longer recognized by α-TopoIcc before DNA double-strand breaks become manifest, a DNA damage response is initiated and homologous recombination ensues. Alternatively, it is possible that other effects of topo I poisons, e.g. the ability of TPT to inhibit topo I-mediated relaxation of positively supercoiled DNA in front of advancing replication forks (69), might contribute to generation of DNA double strand breaks at sites distinct from covalent topo I-DNA covalent complexes detected by α-TopoIcc. While further studies are required to distinguish between these possibilities, α-TopoIcc provides an important new tool for studying these processes.
In addition to its research uses, it is possible that α-TopoIcc might also be useful for therapeutic monitoring of topo I poisons in clinical scenarios. Stabilization of topo I-DNA covalent adducts clearly plays a critical role in killing by these agents, as demonstrated by genetic studies in yeast and biochemical experiments mammalian cells showing that decreases in topo I-DNA covalent adducts are associated with diminished CPT-induced killing (1,21,70,71). Over the past several years, assays for total topo I levels and H2AX phosphorylation have been credentialed for use in the clinical setting (72,73), but these assays only provide an indirect measure of the ability of topo I poisons to stabilize topo I-DNA covalent complexes in the clinical setting. The present study detected topo I-DNA complexes in tumors after irinotecan administration in vivo (Figure 4B). Further studies are needed to determine whether α-TopoIcc can be utilized to detect topo I-DNA covalent complexes in circulating solid tumor cells or leukemic blasts after initial treatment with a topo I poison and whether the amount of complex formation correlates with clinical outcome or not.
In summary, the ability to directly visualize topo I-DNA covalent complexes by immunoblotting, flow cytometry and immunofluorescence provides new insight into the ability of various agents to stabilize topo I-DNA complexes and the signaling that is initiated by those complexes.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge gifts of reagents from Y-C. Cheng, David Toft and Yves Pommier; seminal conversations with Ralph Parchment; encouragement of members of the Kaufmann lab; and editorial assistance of Deb Strauss.
Footnotes
Present address: Anand G. Patel, St. Louis Children's Hospital, St. Louis, MO, USA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institutes of Health (NIH) [R01 CA73709 to S.H.K., T32 GM65841 to A.G.P. and T32 GM072474 to A.G.P.]; Minnesota Partnership for Biotechnology and Medical Genomics [#10.03 to D.H. and S.H.K.]; and a predoctoral fellowship from the Mayo Foundation for Education and Research (to A.G.P.). Funding for open access charge: Institutional.
Conflict of interest statement. Mayo Clinic, along with Dr Patel and Dr Kaufmann, holds a patent on the generation and use of antibodies that detect covalent protein–DNA complexes.
REFERENCES
- 1.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 2.Wang J.C. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell. Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 3.Yang L., Wold M.S., Li J.J., Kelly T.J., Liu L.F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc. Natl. Acad. Sci. U.S.A. 1987;84:950–954. doi: 10.1073/pnas.84.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg L.C., DiAngelo S., Jacob S.T. Role of DNA topoisomerase I in the transcription of supercoiled rRNA gene. Proc. Natl. Acad. Sci. U.S.A. 1987;84:3185–3188. doi: 10.1073/pnas.84.10.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merino A., Madden K.R., Lane W.S., Champoux J.J., Reinberg D. DNA topoisomerase I is involved in both repression and activation of transcription. Nature. 1993;365:227–232. doi: 10.1038/365227a0. [DOI] [PubMed] [Google Scholar]
- 6.Wu H.Y., Liu L.F. DNA looping alters local DNA conformation during transcription. J. Mol. Biol. 1991;219:615–622. doi: 10.1016/0022-2836(91)90658-s. [DOI] [PubMed] [Google Scholar]
- 7.Wang H.P., Rogler C.E. Topoisomerase I-mediated integration of hepadnavirus DNA in vitro. J. Virol. 1991;65:2381–2392. doi: 10.1128/jvi.65.5.2381-2392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng C., Shuman S. Recombinogenic flap ligation pathway for intrinsic repair of topoisomerase IB-induced double-strand breaks. Mol. Cell. Biol. 2000;20:8059–8068. doi: 10.1128/mcb.20.21.8059-8068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailly C. Topoisomerase I poisons and suppressors as anticancer drugs. Curr. Med Chem. 2000;7:39–58. doi: 10.2174/0929867003375489. [DOI] [PubMed] [Google Scholar]
- 10.Pommier Y., Leo E., Zhang H., Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beretta G.L., Zuco V., Perego P., Zaffaroni N. Targeting DNA topoisomerase I with non-camptothecin poisons. Curr. Med. Chem. 2012;19:1238–1257. doi: 10.2174/092986712799320529. [DOI] [PubMed] [Google Scholar]
- 12.Staker B.L., Hjerrild K., Feese M.D., Behnke C.A., Burgin A.B., Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15387–15392. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staker B.L., Feese M.D., Cushman M., Pommier Y., Zembower D., Stewart L., Burgin A.B. Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J. Med. Chem. 2005;48:2336–2345. doi: 10.1021/jm049146p. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann S.H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989;49:5870–5878. [PubMed] [Google Scholar]
- 15.Li T.K., Liu L.F. Tumor cell death induced by topoisomerase-targeting drugs. Ann. Rev. Pharmacol. Toxicol. 2001;41:53–77. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- 16.Hsiang Y.-H., Lihou M.G., Liu L.F. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a Mechanism of Cell Killing by Camptothecin. Cancer Res. 1989;49:5077–5082. [PubMed] [Google Scholar]
- 17.Jaxel C., Capranico G., Kerrigan D., Kohn K.W., Pommier Y. Effect of local DNA sequence on topoisomerase I cleavage in the presence or absence of camptothecin. J. Biol. Chem. 1991;266:20418–20423. [PubMed] [Google Scholar]
- 18.Fiorani P., Amatruda J.F., Silvestri A., Butler R.H., Bjornsti M.A., Benedetti P. Domain interactions affecting human DNA topoisomerase I catalysis and camptothecin sensitivity. Mol. Pharm. 1999;56:1105–1115. doi: 10.1124/mol.56.6.1105. [DOI] [PubMed] [Google Scholar]
- 19.Khadka D.B., Cho W.J. Topoisomerase inhibitors as anticancer agents: a patent update. Expert Opin. Ther. Pat. 2013;23:1033–1056. doi: 10.1517/13543776.2013.790958. [DOI] [PubMed] [Google Scholar]
- 20.Kumler I., Brunner N., Stenvang J., Balslev E., Nielsen D.L. A systematic review on topoisomerase 1 inhibition in the treatment of metastatic breast cancer. Breast Cancer Res. Treat. 2013;138:347–358. doi: 10.1007/s10549-013-2476-3. [DOI] [PubMed] [Google Scholar]
- 21.Beretta G.L., Gatti L., Perego P., Zaffaroni N. Camptothecin resistance in cancer: insights into the molecular mechanisms of a DNA-damaging drug. Curr. Med. Chem. 2013;20:1541–1565. doi: 10.2174/0929867311320120006. [DOI] [PubMed] [Google Scholar]
- 22.Moukharskaya J., Verschraegen C. Topoisomerase 1 inhibitors and cancer therapy. Hematol. Oncol. Clin. North Am. 2012;26:507–525. doi: 10.1016/j.hoc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Riemsma R., Simons J.P., Bashir Z., Gooch C.L., Kleijnen J. Systematic review of topotecan (Hycamtin) in relapsed small cell lung cancer. BMC Cancer. 2010;10:436. doi: 10.1186/1471-2407-10-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratz K.W., Kaufmann S.H., Litzow M.R., Ji J., Chen A., Rudek M.A., Karp J.E. Phase I trial of the oral poly(ADP-ribose) polymerase inhibitor veliparib combined with topotecan and carboplatin for adults with relapsed and refractory actue leukemias. Blood. 2011;118:3634. [Google Scholar]
- 25.Kaufmann S.H., Karp J.E., Letendre L., Kottke T.J., Safgren S., Greer J., Gojo I., Atherton P., Svingen P.A., Loegering D., et al. Phase I and pharmacological study of infusional topotecan and carboplatin in relapsed and refractory leukemia. Clin. Cancer Res. 2005;11:6641–6649. doi: 10.1158/1078-0432.CCR-05-0817. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert D.C., Chalmers A.J., El-Khamisy S.F. Topoisomerase I inhibition in colorectal cancer: biomarkers and therapeutic targets. Br. J. Cancer. 2012;106:18–24. doi: 10.1038/bjc.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparreboom A., Zamboni W.C. In: Cancer Chemotherapy and Biotherapy. 4th edn. Chabner BA, Longo DL, editors. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 371–413. [Google Scholar]
- 28.Sheng C., Miao Z., Zhang W. New strategies in the discovery of novel non-camptothecin topoisomerase I inhibitors. Curr. Med. Chem. 2011;18:4389–4409. doi: 10.2174/092986711797200453. [DOI] [PubMed] [Google Scholar]
- 29.Munster P.N., Daud A.I. Preclinical and clinical activity of the topoisomerase I inhibitor, karenitecin, in melanoma. Expert Opin. Investig. Drugs. 2011;20:1565–1574. doi: 10.1517/13543784.2011.617740. [DOI] [PubMed] [Google Scholar]
- 30.Venditto V.J., Simanek E.E. Cancer therapies utilizing the camptothecins: a review of the in vivo literature. Mol. Pharm. 2010;7:307–349. doi: 10.1021/mp900243b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santi D.V., Schneider E.L., Ashley G.W. Macromolecular prodrug that provides the irinotecan (CPT-11) active-metabolite SN-38 with ultralong half-life, low C(max), and low glucuronide formation. J. Med. Chem. 2014;57:2303–2314. doi: 10.1021/jm401644v. [DOI] [PubMed] [Google Scholar]
- 32.Yurkovetskiy A.V., Fram R.J. XMT-1001, a novel polymeric camptothecin pro-drug in clinical development for patients with advanced cancer. Adv. Drug Deliv. Rev. 2009;61:1193–1202. doi: 10.1016/j.addr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Basili S., Moro S. Novel camptothecin derivatives as topoisomerase I inhibitors. Expert Opin. Ther. Pat. 2009;19:555–574. doi: 10.1517/13543770902773437. [DOI] [PubMed] [Google Scholar]
- 34.Tomicic M.T., Kaina B. Topoisomerase degradation, DSB repair, p53 and IAPs in cancer cell resistance to camptothecin-like topoisomerase I inhibitors. Biochim. Biophys. Acta. 2013;1835:11–27. doi: 10.1016/j.bbcan.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Hsiang Y.-H., Liu L.F., Wall M.E., Wani M.C., Nicholas A.W., Manikumar G., Kirschenbaum S., Silber R., Potmesil M. DNA topoisomerase I-mediated DNA cleavage and cytotoxicity of camptothecin analogues. Cancer Res. 1989;49:4385–4389. [PubMed] [Google Scholar]
- 36.Subramanian D., Furbee C.S., Muller M.T. ICE Bioassay: Isolating In Vivo Complexes of Enzyme to DNA. In: Bjornsti M-A, Osheroff N, editors. DNA Topoisomerase Protoc. Vol. 2. 2001. pp. 137–147. [DOI] [PubMed] [Google Scholar]
- 37.Liu L.F., Rowe T.C., Yang L., Tewey K.M., Chen G.L. Cleavage of DNA by mammalian DNA topoisomerase II. J. Biol. Chem. 1983;258:15365–15370. [PubMed] [Google Scholar]
- 38.Muller M.T., Bolles C.S., Parris D.S. Association of type I DNA topoisomerase with herpes simplex virus. J. Gen. Virol. 1985;66:1565–1574. doi: 10.1099/0022-1317-66-7-1565. [DOI] [PubMed] [Google Scholar]
- 39.Li C.J., Averboukh L., Pardee A.B. beta-Lapachone, a novel DNA topoisomerase I inhibitor with a mode of action different from camptothecin. J. Biol. Chem. 1993;268:22463–22468. [PubMed] [Google Scholar]
- 40.Kiianitsa K., Maizels N. Ultrasensitive isolation, identification and quantification of DNA-protein adducts by ELISA-based RADAR assay. Nucleic Acids Res. 2014;42:e108. doi: 10.1093/nar/gku490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel A., Sarkaria J., Kaufmann S.H. Nonhomologous end-joining drives PARP inhibitor synthetic lethality in homologous recombination-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel A.G., Flatten K.S., Schneider P.A., Dai N.T., McDonald J.S., Poirier G.G., Kaufmann S.H. Enhanced killing of cancer cells by poly(ADP-ribose) polymerase inhibitors and topoisomerase inhibitors reflects poisoning of both enzymes. J. Biol. Chem. 2012;287:4198–4210. doi: 10.1074/jbc.M111.296475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergstrom D.E., Zhang P., Toma P.H., Andrews P.C., Nichols R. Synthesis, structure, and deoxyribonucleic-acid sequencing with a universal nucleoside -1-(2′-deoxy-beta-D-ribofuranosyl)-3-nitropyrrole. J. Am. Chem. Soc. 1995;117:1201–1209. [Google Scholar]
- 44.Ding H., Hackbarth J., Schneider P.A., Peterson K.L., Meng X.W., Dai H., Witzig T.E., Kaufmann S.H. Cytotoxicity of Farnesyltransferase inhibitors in lymphoid cells mediated by MAPK pathway inhibition and bim upregulation. Blood. 2011;118:4872–4881. doi: 10.1182/blood-2011-02-334870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de StGroth S.F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J. Immunol. Methods. 1980;35:1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- 46.Kaufmann S.H., Svingen P.A. Immunoblot analysis and band depletion assays. Methods Mol. Biol. (Clifton, N.J) 1999;94:253–268. doi: 10.1385/1-59259-259-7:253. [DOI] [PubMed] [Google Scholar]
- 47.Kaufmann S.H., Svingen P.A., Gore S.D., Armstrong D.K., Cheng Y.C., Rowinsky E.K. Altered formation of topotecan-stabilized topoisomerase I-DNA adducts in human leukemia cells. Blood. 1997;89:2098–2104. [PubMed] [Google Scholar]
- 48.Eng W.K., McCabe F.L., Tan K.B., Mattern M.R., Hofmann G.A., Woessner R.D., Hertzberg R.P., Johnson R.K. Development of a stable camptothecin-resistant subline of P388 leukemia with reduced topoisomerase I content. Mol. Pharm. 1990;38:471–480. [PubMed] [Google Scholar]
- 49.Buckwalter C.A., Lin A.H., Tanizawa A., Pommier Y.G., Cheng Y.C., Kaufmann S.H. RNA synthesis inhibitors alter the subnuclear distribution of DNA topoisomerase I. Cancer Res. 1996;56:1674–1681. [PubMed] [Google Scholar]
- 50.Antony S., Kohlhagen G., Agama K., Jayaraman M., Cao S., Durrani F.A., Rustum Y.M., Cushman M., Pommier Y. Cellular topoisomerase I inhibition and antiproliferative activity by MJ-III-65 (NSC 706744), an indenoisoquinoline topoisomerase I poison. Mol. Pharm. 2005;67:523–530. doi: 10.1124/mol.104.003889. [DOI] [PubMed] [Google Scholar]
- 51.Trask D.K., Muller M.T. Stabilization of type I topoisomerase-DNA covalent complexes by actinomycin D. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1417–1421. doi: 10.1073/pnas.85.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pourquier P., Takebayashi Y., Urasaki Y., Gioffre C., Kohlhagen G., Pommier Y. Induction of topoisomerase I cleavage complexes by 1-beta-D-arabinofuranosylcytosine (Ara-C) in vitro and in Ara-C-treated cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1885–1890. doi: 10.1073/pnas.97.4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pourquier P., Gioffre C., Kohlhagen G., Urasaki Y., Goldwasser F., Hertel L.W., Yu S., Pon R.T., Gmeiner W.H., Pommier Y. Gemcitabine (2′,2′-difluoro-2′-deoxycytidine), an antimetabolite that poisons topoisomerase I. Clin. Cancer Res. 2002;8:2499–2504. [PubMed] [Google Scholar]
- 54.van Waardenburg R.C., de Jong L.A., van Eijndhoven M.A., Verseyden C., Pluim D., Jansen L.E., Bjornsti M.A., Schellens J.H. Platinated DNA adducts enhance poisoning of DNA topoisomerase I by camptothecin. J. Biol. Chem. 2004;279:54502–54509. doi: 10.1074/jbc.M410103200. [DOI] [PubMed] [Google Scholar]
- 55.Chen A.Y., Yu C., Bodley A., Peng L.F., Liu L.F. A new mammalian DNA topoisomerase I poison Hoechst 33342: cytotoxicity and drug resistance in human cell cultures. Cancer Res. 1993;53:1332–1337. [PubMed] [Google Scholar]
- 56.Cliby W.A., Lewis K.A., Lilly K.K., Kaufmann S.H. S phase and G2 arrests induced by topoisomerase I poisons are dependent on ATR kinase function. J. Biol. Chem. 2002;277:1599–1606. doi: 10.1074/jbc.M106287200. [DOI] [PubMed] [Google Scholar]
- 57.Loegering D., Arlander S.A.H., Hackbarth J., Vroman B., Lieberman H.B., Karnitz L.M., Kaufmann S.H. Rad9 protects cells from topoisomerase poison-induced cell death. J. Biol. Chem. 2004;279:18641–18647. doi: 10.1074/jbc.M313536200. [DOI] [PubMed] [Google Scholar]
- 58.Meijer C., de Vries E.G., Dam W.A., Wilkinson M.H., Hollema H., Hoekstra H.J., Mulder N.H. Immunocytochemical analysis of cisplatin-induced platinum-DNA adducts with double-fluorescence video microscopy. Br. J. Cancer. 1997;76:290–298. doi: 10.1038/bjc.1997.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terheggen P.M., Floot B.G., Lempers E.L., van Tellingen O., Begg A.C., den Engelse L. Antibodies against cisplatin-modified DNA and cisplatin-modified (di)nucleotides. Cancer Chemother. Pharmacol. 1991;28:185–191. doi: 10.1007/BF00685507. [DOI] [PubMed] [Google Scholar]
- 60.Degan P., Shigenaga M.K., Park E.M., Alperin P.E., Ames B.N. Immunoaffinity isolation of urinary 8-hydroxy-2′-deoxyguanosine and 8-hydroxyguanine and quantitation of 8-hydroxy-2′-deoxyguanosine in DNA by polyclonal antibodies. Carcinogenesis. 1991;12:865–871. doi: 10.1093/carcin/12.5.865. [DOI] [PubMed] [Google Scholar]
- 61.Lee Y.S., Lee H.S., Park M.K., Hwang E.S., Park E.M., Kasai H., Chung M.H. Identification of 8-hydroxyguanine glycosylase activity in mammalian tissues using 8-hydroxyguanine specific monoclonal antibody. Biochem. Biophys. Res. Commun. 1993;196:1545–1551. doi: 10.1006/bbrc.1993.2427. [DOI] [PubMed] [Google Scholar]
- 62.Redinbo M.R., Stewart L., Kuhn P., Champoux J.J., Hol W.G. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998;279:1504–1513. doi: 10.1126/science.279.5356.1504. [DOI] [PubMed] [Google Scholar]
- 63.Danks M.K., Pawlik C.A., Whipple D.O., Wolverton J.S. Intermittent exposure of medulloblastoma cells to topotecan produces growth inhibition equivalent to continuous exposure. Clin. Cancer Res. 1997;3:1731–1738. [PubMed] [Google Scholar]
- 64.Zhang Y.W., Regairaz M., Seiler J.A., Agama K.K., Doroshow J.H., Pommier Y. Poly(ADP-ribose) polymerase and XPF-ERCC1 participate in distinct pathways for the repair of topoisomerase I-induced DNA damage in mammalian cells. Nucleic Acids Res. 2011;39:3607–3620. doi: 10.1093/nar/gkq1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Comeaux E.Q., van Waardenburg R.C. Tyrosyl-DNA phosphodiesterase I resolves both naturally and chemically induced DNA adducts and its potential as a therapeutic target. Drug Metab. Rev. 2014;46:494–507. doi: 10.3109/03602532.2014.971957. [DOI] [PubMed] [Google Scholar]
- 66.Pommier Y., Huang S.Y., Gao R., Das B.B., Murai J., Marchand C. Tyrosyl-DNA-phosphodiesterases (TDP1 and TDP2) DNA Repair (Amst) 2014;19:114–129. doi: 10.1016/j.dnarep.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stingele J., Schwarz M.S., Bloemeke N., Wolf P.G., Jentsch S. A DNA-dependent protease involved in DNA-protein crosslink repair. Cell. 2014;158:327–338. doi: 10.1016/j.cell.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 68.Stingele J., Habermann B., Jentsch S. DNA-protein crosslink repair: proteases as DNA repair enzymes. Trends Biochem. Sci. 2015;40:67–71. doi: 10.1016/j.tibs.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Koster D.A., Palle K., Bot E.S., Bjornsti M.A., Dekker N.H. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature. 2007;448:213–217. doi: 10.1038/nature05938. [DOI] [PubMed] [Google Scholar]
- 70.Liu L.F. DNA Topoisomerase Poisons as Antitumor Drugs. Annu. Rev. Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- 71.Slichenmyer W.J., Rowinsky E.K., Donehower R.C., Kaufmann S.H. The current status of camptothecin analogues as antitumor agents. J. Natl. Cancer Inst. 1993;85:271–291. doi: 10.1093/jnci/85.4.271. [DOI] [PubMed] [Google Scholar]
- 72.Kinders R.J., Hollingshead M., Lawrence S., Ji J., Tabb B., Bonner W.M., Pommier Y., Rubinstein L., Evrard Y.A., Parchment R.E., et al. Development of a validated immunofluorescence assay for gammaH2AX as a pharmacodynamic marker of topoisomerase I inhibitor activity. Clin. Cancer Res. 2010;16:5447–5457. doi: 10.1158/1078-0432.CCR-09-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfister T.D., Hollingshead M., Kinders R.J., Zhang Y., Evrard Y.A., Ji J., Khin S.A., Borgel S., Stotler H., Carter J., et al. Development and validation of an immunoassay for quantification of topoisomerase I in solid tumor tissues. PloS One. 2012;7:e50494. doi: 10.1371/journal.pone.0050494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.