Abstract
The hepatic tricarboxylic acid (TCA) cycle is central to integrating macronutrient metabolism and is closely coupled to cellular respiration, free radical generation, and inflammation. Oxidative flux through the TCA cycle is induced during hepatic insulin resistance, in mice and humans with simple steatosis, reflecting early compensatory remodeling of mitochondrial energetics. We hypothesized that progressive severity of hepatic insulin resistance and the onset of nonalcoholic steatohepatitis (NASH) would impair oxidative flux through the hepatic TCA cycle. Mice (C57/BL6) were fed a high-trans-fat high-fructose diet (TFD) for 8 wk to induce simple steatosis and NASH by 24 wk. In vivo fasting hepatic mitochondrial fluxes were determined by 13C-nuclear magnetic resonance (NMR)-based isotopomer analysis. Hepatic metabolic intermediates were quantified using mass spectrometry-based targeted metabolomics. Hepatic triglyceride accumulation and insulin resistance preceded alterations in mitochondrial metabolism, since TCA cycle fluxes remained normal during simple steatosis. However, mice with NASH had a twofold induction (P < 0.05) of mitochondrial fluxes (μmol/min) through the TCA cycle (2.6 ± 0.5 vs. 5.4 ± 0.6), anaplerosis (9.1 ± 1.2 vs. 16.9 ± 2.2), and pyruvate cycling (4.9 ± 1.0 vs. 11.1 ± 1.9) compared with their age-matched controls. Induction of the TCA cycle activity during NASH was concurrent with blunted ketogenesis and accumulation of hepatic diacylglycerols (DAGs), ceramides (Cer), and long-chain acylcarnitines, suggesting inefficient oxidation and disposal of excess free fatty acids (FFA). Sustained induction of mitochondrial TCA cycle failed to prevent accretion of “lipotoxic” metabolites in the liver and could hasten inflammation and the metabolic transition to NASH.
Keywords: steatosis, hepatic insulin resistance, mitochondria, nonalcoholic steatohepatitis
over 70% of obese humans and patients with type 2 diabetes mellitus (T2DM) are afflicted with fatty liver disease (25, 30), of which mitochondrial dysfunction is a central feature (19, 42). About 30–40% of patients undergo the transition from simple steatosis to nonalcoholic steatohepatitis (NASH) (1, 11, 38), which is projected to be the most common indication for liver transplantation (8). Uncovering defects in hepatic mitochondrial metabolism assumes great importance toward understanding the transition to NASH and developing future prevention strategies. With progression of hepatic insulin resistance and triglyceride accumulation, there is constant remodeling of mitochondrial oxidative metabolism (19, 35, 41), which includes alterations in enzyme activities and molecular regulation of mitochondrial networks (e.g., mitochondrial β-oxidation, TCA cycle, ketogenesis, mitochondrial respiration, and ATP synthesis). Apart from fueling gluconeogenesis and lipogenesis, these pathways are also tightly coupled to generating reactive oxygen species (ROS) and mediating normal inflammatory responses.
During the pathophysiology of fatty liver disease, triglyceride accumulation is thought to exceed and impede oxidative catabolism of free fatty acid (FFA) (3). Defects in mitochondrial lipid oxidation are shown to occur together with hepatic insulin resistance and simple steatosis (29, 31, 47). Interestingly, recent studies (15, 19, 35, 41) also point to a simultaneous induction of hepatic mitochondrial oxidative metabolism during hepatic insulin resistance and simple steatosis. In the setting of simple steatosis, hepatic tricarboxylic acid cycle (TCA) cycle flux was induced together with high rates of lipid accretion (21) in both mouse models (35) and human subjects (41). Furthermore, simple steatosis was also associated with higher rates of mitochondrial respiration (19, 35), which, under normal physiology, is closely coupled to the generation of reducing equivalents from the TCA cycle. However, progressive severity of hepatic insulin resistance and onset of NASH and/or T2DM is shown to impair mitochondrial respiration and ATP synthesis (19, 36, 44). Taken together, it is not clear whether specific defects in mitochondrial oxidative metabolism are characteristic during the progression of simple steatosis and NASH.
Hepatic insulin resistance and triglyceride accumulation are also associated with accumulation of several “lipotoxic” lipid intermediates, including diacylglycerols (DAGs), ceramides (Cer), and long-chain acylcarnitines (20, 24). These are often considered as products of impaired/incomplete mitochondrial oxidative metabolism and furthermore have the potential to impair insulin signaling and mediate hepatocyte inflammation (13, 39). However, it is not clear whether impaired flux through hepatic TCA cycle occurs concurrently with elevated levels of lipid byproducts and/or altered expression of genes involved in mitochondrial oxidative metabolism in the liver.
We investigated whether impaired mitochondrial TCA cycle activity is a central feature of NASH. By using a combination of metabolic flux analysis, targeted metabolomics, and hepatic gene expression profiles, we demonstrate that the hepatic TCA cycle activity is induced during NASH. However, elevated TCA cycle activity was inadequate to prevent lipotoxicity and incomplete fat oxidation.
RESEARCH DESIGN AND METHODS
Animals and diets.
Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Florida. Male mice (C57BL/6) purchased from Jackson Laboratories (Bar Harbor, ME) at 6–8 wk were fed either a control diet (10% fat calories, no. D09100304; Research Diets) or a high-trans-fat high-fructose diet (TFD, 40% fat calories, no. D09100301; Research Diets) for 8 or 24 wk. The TFD diet was enriched with 40% kcal fat containing Primex partially hydrogenated vegetable oil shortening, fructose (22% by wt), and cholesterol (2% by wt) (10). At 8 wk of TFD feeding, mice had already developed simple steatosis, whereas 24 wk on TFD resulted in development of histological features of NASH as previously reported (Refs. 10 and 46, also see results).
Euglycemic-hyperinsulinemic clamp (insulin stimulation).
Mice (n = 6–7/group) were implanted with indwelling jugular vein catheters 5 days before the experiment. In conscious and unrestrained mice following an overnight fast, insulin was infused at a constant rate (2.5 mU·kg−1·min−1). A mixture of 30% glucose enriched to 2.5% with [U-13C]glucose was infused to maintain euglycemia. Blood glucose was monitored every 10 min using a glucose meter. Following 90 min of euglycemia, the animals were killed, and blood glucose enrichments were determined as previously detailed for determination of endogenous glucose production (EGP) (35). Severity of hepatic insulin resistance was also evaluated from the hepatic insulin sensitivity index, calculated as the product of fasting plasma insulin and fasting EGP (23).
Stable isotope tracer infusions and metabolic studies.
Alterations in liver metabolism were studied following 8 (simple steatosis) and 24 (NASH) wk of dietary challenge. Mice (n = 6–7/group) were implanted with indwelling jugular vein catheters 5 days before metabolic experiments. Following an overnight fast, mice were infused with a mixture containing [13C3]propionate (37.5 mg/ml) and [3,4-13C2]glucose (3.75 mg/ml) as a bolus (0.30 ml/h) for the first 10 min followed by continuous infusion (0.12 ml/h) for the next 80 min. Following 90 min of tracer infusion, mice were anesthetized, and whole blood was collected from the descending aorta. Livers were flash-frozen in liquid nitrogen and stored at −80°C until further analysis.
Analysis of EGP and mitochondrial TCA cycle metabolism by 13C-nuclear magnetic resonance.
Glucose in the mice plasma was converted to the 1,2-isopropylidene glucofuranose derivative (monoacetone glucose) before 13C isotopomer analysis (35). 13C-nuclear magnetic resonance (NMR) isotopomer experiments were conducted on a 600-MHz (14.1 T/5.1 cm) Agilent NMR spectrometer available at the National High Magnetic Field Laboratory, University of Florida, using a probe optimized for 13C detection sensitivity accommodating 1.5-mm tubes with a sample volume of 35–40 μl (28). Peak areas were analyzed using one-dimensional NMR software ACD/Labs 9.0 before metabolic analysis as reported previously (5, 16, 32). Endogenous glucose production was determined for stable isotope dilution of [3,4-13C2]glucose in mouse plasma. Furthermore, isotopomer analysis of the multiples arising from 13C labeling of carbon-2 of glucose was used to determine the functional activity of hepatic TCA cycle metabolism (32, 34). Briefly, [13C3]propionate is taken up by the liver and enters the TCA cycle as succinyl-[1,2,3-13C3]CoA. The 13C multiplets in glucose arising from the incorporation of 13C are reflective of the relative rates of mitochondrial anaplerosis, pyruvate cycling, and TCA cycle flux (17). These relative mitochondrial fluxes were converted to absolute fluxes by normalizing with EGP (16, 35). The [13C3]propionate tracer by itself does not alter EGP or hepatic mitochondrial fluxes (33), and the utility of this method has been extensively validated and discussed (6, 27).
Targeted metabolomics.
Frozen livers were ground to fine powder in liquid nitrogen before a representative aliquot (∼20–25 mg) was weighed out to determine concentrations of individual classes of metabolites, including DAGs, Cer, acylcarnitines, organic acids, and amino acids. Following addition of their respective internal standards and homogenization with ceramic beads, metabolites were extracted and analyzed by mass spectrometry as detailed below for each individual class. Metabolites were quantified by peak area comparison with their respective or a representative internal standard.
Analysis of liver DAG and Cer by LC-MS/MS.
Internal standards [Cer(d18:1/17:0) and a d5-DAGs mix] were prepared in chloroform-methanol (1:2 vol/vol) and added to the liver aliquot before Folch extraction (14). Separation was accomplished with a Sigma Aldrich Supelco C18 column (75 × 2.1 mm, 1.9 μm pore size) following the gradient information described in the following section. Hepatic DAGs and Cer were identified using precursor ion scanning [mass-to-charge ratio (m/z) 264.3] and neutral loss scanning (for fatty acid tails), respectively, on a Thermo TSQ Quantum Access triple-quadrupole mass spectrometer (Thermo Scientific, San Jose, CA) with Accela 1200 pump and heated electrospray ionization (HESI) source (positive ionization). Relative quantitation data were acquired on the Thermo Q-Exactive Orbitrap (Thermo Scientific) combined with a Dionex Ultimate 3000 liquid chromatography instrument. Data analysis was performed with Xcalibur software and expressed as nanomoles of targeted compound per gram of liver protein.
LC-MS (Thermo TSQ triple quadrupole) conditions for DAG and Cer analysis.
Each reconstituted liver sample was injected (2 μl) on a Sigma Aldrich Supelco C18 column (75 × 2.1 mm, 1.9 μm pore size) with the mobile phases as follows: 1) 60:40 acetonitrile-water (mobile phase A) and 2) 90:10 isopropanol-acetonitrile (mobile phase B), with 10 mM ammonium formate and 0.1% formic acid in both phases A and B. The gradient started at 32% phase B and rose to 100% phase B over 16 min, remaining isocratic from 16 to 18 min, before returning to starting conditions after 21 min, at a 500 μl/min flow rate. The MS was operated in positive mode with spray voltage at 3 kV, capillary temperature at 350°C, and vaporizer temperature at 50°C. The precursor ion scan was set to monitor the precursor ions of m/z 264.3, representative of Cer with d18:1 chain. Cer putative assignments were based on fragmentation, presence of [M + H]+ and [M + H − H2O]+ peaks, as well as exact mass comparison of Q-Exactive data and LipidMaps database. DAG assignments included presence of [M + NH4]+ peak, exact mass comparison, and matching fragmentation. The neutral loss scans included 245, 273, 271, 297, 299, 301, and 321, corresponding to losses of common fatty acid chain lengths.
LC-MS (Thermo Q-Exactive Orbitrap) conditions for quantification.
Each sample was analyzed in positive mode full scan with resolving power of 70,000 at m/z 200, spray voltage of 3.5 kV, capillary temperature of 300°C, sheath gas of 30, heater temperature of 350°C, and s-lens RF of 35. Chromatography was the same as on the triple quadrupole; however, the column employed was a Waters Acquity C18 BEH column (50 × 2.1 mm, 1.7 μm pore size) (Milford, MA) at 30°C. Pooled QC and blanks were injected every 12 samples throughout the sequence.
Analysis of liver acylcarnitines by LC-MS/MS.
Liver aliquots were homogenized and deproteinized with cold acetonitrile containing stable isotope-labeled internal standards (Cambridge Isotopes, Andover, MA). Following centrifugation at 13,500 revolutions/min (rpm) for 15 min, the supernatant was dried under nitrogen gas at 30°C and reconstituted in 90:10 methanol-water for LC-MS/MS analysis. Acylcarnitine data were collected using SRM mode on a Thermo TSQ Quantum Access triple-quadrupole mass spectrometer with an Accela 1200 LC pump and HESI source (positive ionization). Reactions fragmenting to m/z 85.3 were monitored following a 5-μl injection on an ACE PFP-C18 column (100 × 2.1 mm, 2 μm particle size) at 40°C, as reported previously (40).
Analysis of liver organic acids by GC-MS.
Liver aliquots were spiked in with stable isotope-labeled organic acid internal standards, deproteinized with sulfosalicylic acid (10% final volume), and centrifuged at 13,500 rpm for 15 min. The supernatant was decanted to clean screw-cap tubes, 5 mmol of hydroxylamine-hydrochloride were added, and the pH was adjusted to seven to eight with 4 M potassium hydroxide. Following sonication (15 min) and incubation (65°C for 1 h), the pH was reduced to two by adding hydrochloric acid (6 M) and saturated with sodium chloride. Organic acids were extracted with ethyl acetate, dried under nitrogen gas, and converted to their tertiary-butyldimethylsilyl (tBDMS) derivatives before separation on a HP-5MS column (30 m × 0.25 mm × 0.25 μm; Agilent) under electron ionization (HP 5973N Mass Selective Detector; Agilent).
Analysis of liver amino acids by GC-MS.
Amino acid concentrations were determined by isotope dilution with GC-MS as previously described (7). To the liver aliquot, a known concentration of internal standard (hydrolyzed [U-13C,15N]algae protein powder) was added. Samples were then deproteinized with sulfosalicylic acid, and the supernatant passed over a cation exchange resin before elution of amino acids with 2.5 M ammonium hydroxide. The lyophilized amino acids were converted to the tBDMS derivatives before separation on a HP-5MS column (30 m × 0.25 mm × 0.25 μm; Agilent) and fragmentation under electron ionization mode to monitor amino acid and internal standard ions (7).
Histology.
Liver from overnight-fasted mice were fixed in 10% neutral buffered formalin for 20–24 h, washed, and stored in 70% ethanol before embedding in paraffin at the Molecular Pathology Core at the University of Florida. The liver sections from the control mice and mice with simple steatosis and NASH were then stained with Masson's Trichrome to visualize collagen fibers. The liver slides were blinded and scored by a veterinary pathologist using a previously published and validated scoring system of liver biopsies (18).
Gene expression analysis.
As previously reported (32), quantitative real-time PCR mix contained 25 ng cDNA, 150 nmol/l of each primer, and 5 μl SYBR Green PCR master mix (Bio-Rad). Samples were run in triplicate on a CFX Real Time system (C1000 Touch Thermal Cycler; Bio-Rad). The comparative threshold method was used to determine relative mRNA levels with cyclophilin as the internal control.
Biochemical measurements.
Plasma ketone body and FFA concentrations were determined using an analytical kit (Wako Chemicals, Richmond, VA). Plasma insulin was measured by enzyme-linked immunoassay using the mouse Insulin ELISA kit (Crystal Chem, Downers Grove, IL). Triglyceride concentrations were determined using an analytical kit from Sigma (St. Louis, MO).
Materials and reagents.
[3,4-13C]glucose (98%) was purchased from Omicron Biochemicals (South Bend, IN). [U-13C]propionate and acylcarnitine internal standards were purchased from Cambridge Isotopes. Internal standards, including Cer (d18:1/17:0) and d5-DAGs mix, were purchased from Avanti Lipids (Alabaster, AL). Other common chemicals were obtained from Sigma.
Statistical analysis.
Statistical analyses for the targeted metabolomics data were performed in R version 3.1.2 (45). To test the effects of age and treatment on metabolite levels, we fitted Type III ANOVAs with the fixed effects age, treatment, and the interaction between age and treatment (asking which metabolites changed differently with age in mice on a high-fat diet compared with controls) and ranked the metabolites according to their F-statistic for treatment effect (to illustrate which metabolites showed the strongest effect of treatment). We controlled the experimentwide false discovery rate at Q < 0.05 (2). We next examined overall patterns in classes of metabolites (DAGs, Cer, organic acids, amino acids, and acylcarnitines) by summarizing each class of metabolites using a principal-components analysis on z-scored data (pcaMethods package in R; see Ref. 37). We used the first principal component of variation in each class of metabolites to describe the overall pattern in that class and ran five separate ANOVAs (one for each class of metabolites, with fixed effects as described above plus liver triglycerides as a covariate) on the scores of each of these principal components. All other results were analyzed using ANOVA, and pairwise mean comparisons were performed using a t-test. Results are expressed as means ± SE and were considered significantly different at P ≤ 0.05.
RESULTS
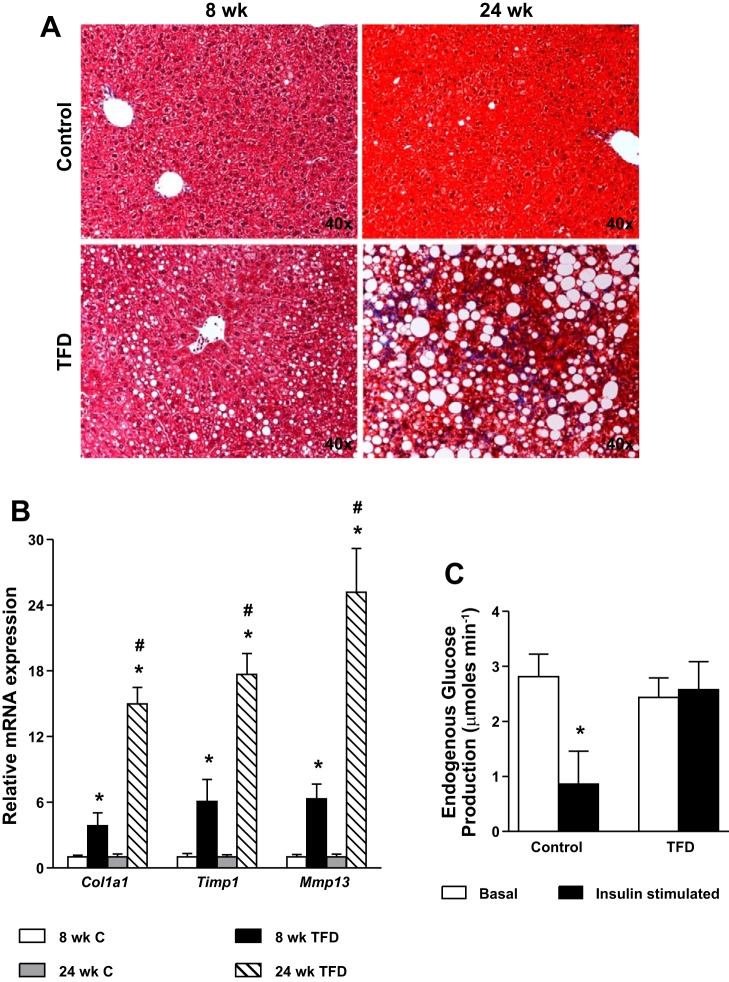
Eight weeks of TFD feeding resulted in simple steatosis that transitioned by 24 wk to NASH.
The metabolic characteristics of the mice are presented in Table 1. Feeding TFD resulted in a mild but significant weight gain by the 8th wk that progressed to obesity by 24 wk of TFD feeding. Liver triglyceride content was significantly elevated by 8 wk of TFD feeding without any histological evidence of inflammation and fibrosis (Fig. 1A) and thus was not scored. Upon prolonged feeding of TFD (24 wk), the phenotype of simple steatosis transitioned to NASH, demonstrated by the presence of inflammation, severe steatosis, and fibrosis in mice with histology scores in the range of six to seven (Fig. 1A and Table 2). The transition to NASH was further evident from the significant induction of genes involved in fibrogenesis after 24 wk of TFD feeding (Fig. 1B). Onset of hepatic insulin resistance was evaluated using euglycemic-hyperinsulinemic clamps following 8 wk of TFD feeding. Insulin stimulation failed to suppress EGP (Fig. 1C) in 8-wk TFD-fed mice, indicating hepatic insulin resistance. The glucose infusion rate required to maintain euglycemia during insulin stimulation was also 70% lower in 8-wk TFD-fed animals (16.7 vs. 53.5 μmol/min), reflecting muscle insulin resistance. Furthermore, hepatic insulin resistance worsened with 24 wk of TFD feeding as evident from the significantly higher values of hepatic insulin resistance index (Table 1). Because mice fed TFD for 24 wk developed histological features of NASH, we selected the 8 vs. 24 wk on TFD to investigate alterations in mitochondrial metabolism associated with progressive disease severity.
Table 1.
Metabolic characteristics of C57Bl/6J mice fed either a control diet or a TFD for 8 and 24 wk
| 8 Weeks |
24 Weeks |
|||
|---|---|---|---|---|
| C | TFD | C | TFD | |
| Body wt, g | 25.0 ± 1.2 | 27.1 ± 0.8* | 30.9 ± 0.7 | 37.2 ± 1.2* |
| Fasting glucose, mg/dl | 103 ± 6 | 99 ± 3 | 99 ± 5 | 104 ± 7 |
| Fasting plasma insulin, ng/ml | 0.22 ± 0.06 | 0.19 ± 0.02 | 0.16 ± 0.01 | 0.30 ± 0.01* |
| Plasma ketones, μM | 1,206 ± 187 | 2,253 ± 255* | 1,087 ± 315 | 1,165 ± 289 |
| Fasting free fatty acids, mM | 0.46 ± 0.08 | 0.37 ± 0.01 | 0.54 ± 0.06 | 0.40 ± 0.03 |
| Hepatic insulin resistance fndex | 0.43 ± 0.08 | 0.50 ± 0.13 | 0.42 ± 0.05 | 1.67 ± 0.34 |
| Liver triglyceride, mg/g liver | 44 ± 15 | 159 ± 20* | 98 ± 13 | 317 ± 13* |
Values are means ± SE; n = 6–7 mice/group.
C, control diet; TFD, high-fructose high-trans-fat diet.
P ≤ 0.05 between C and TFD-fed mice.
Fig. 1.
High-fructose high-trans-fat diet (TFD)-fed mice develop hepatic insulin resistance and simple steatosis by 8 wk that then transitions to nonalcoholic steatohepatitis (NASH) by 24 wk. A: trichrome staining of liver slices reveals normal hepatocyte morphology in mice fed a control diet for 8 or 24 wk. Liver histology of mice fed TFD for 8 wk reveals panacinar steatosis. Liver histology of mice fed TFD for 24 wk reveals microvesicular and macrovesicular steatosis with stage 1b fibrosis. B: expression of genes involved in fibrogenesis was significantly higher in the 24-wk TFD-fed mice compared with their 8-wk counterparts. C: endogenous glucose production fails to get suppressed in 8-wk TFD-fed mice during euglycemic-hyperinsulinemic clamp (insulin stimulation), indicating hepatic insulin resistance. Values are means ± SE (n = 5–7 mice/group). *P ≤ 0.05 between control (C) vs. TFD-fed mice or C vs. C insulin stimulated. #P ≤ 0.05 between 8-wk TFD and 24-wk TFD-fed mice.
Table 2.
Histological scoring of mice livers for NASH after 24 wk of TFD feeding
| Animal | Steatosis (0–3)/Location | Lobular Inflammation (0–3) | Ballooning (0–2) | Summation Score (0–8) | Fibrosis Stage/Microgranuloma/Microsteatosis (Present-Absent) |
|---|---|---|---|---|---|
| C 1 | 0 | 1 | 0 | 1 | 0/Absent/absent |
| C 2 | 0 | 0 | 0 | 0 | 0/Absent/absent |
| C 3 | 0 | 0 | 0 | 0 | 0/Absent/absent |
| C 4 | 0 | 0 | 0 | 0 | 0/Absent/absent |
| C 6 | 0 | 0 | 0 | 0 | 0/Absent/absent |
| TFD 2 | 3, Panacinar | 1 | 2 | 6 | 1b/Present/present |
| TFD 3 | 3, Panacinar | 1 | 2 | 6 | 1b/Present/present |
| TFD 4 | 3, Panacinar | 1 | 2 | 6 | 1b/Present/present |
| TFD 5 | 3, Panacinar | 1 | 2 | 6 | 1b/Present/present |
| TFD 6 | 3, Panacinar | 0 | 2 | 5 | 1b/Present/present |
| TFD 7 | 3, Panacinar | 2 | 2 | 7 | 1b/Present/present |
| TFD 8 | 3, Panacinar | 2 | 2 | 7 | 2/Present/present |
| TFD 9 | 3, Panacinar | 2 | 2 | 7 | 1b/Present/present |
| TFD 10 | 3, Panacinar | 1 | 2 | 6 | 1b/Present/present |
The scoring system was adapted from Kleiner et al. (18). Steatosis: grade 0, ≤5%; grade 1, 5–33%; grade 2, 34–66%; grade 3, >67%. Lobular inflammation: grade 0, 0/200X; grade 1, <2/200X; grade 2, 2–4/200X; grade 3, >4/200X. Ballooning: grade 0, 0; grade 1, few; grade 2, many. Fibrosis/stage: 1a, zone 3/mild; 1b, zone 3/dense; 1c, portal only; 2, perisinusoidal and portal/periportal.
Mitochondrial TCA cycle activity was induced in mice with NASH.
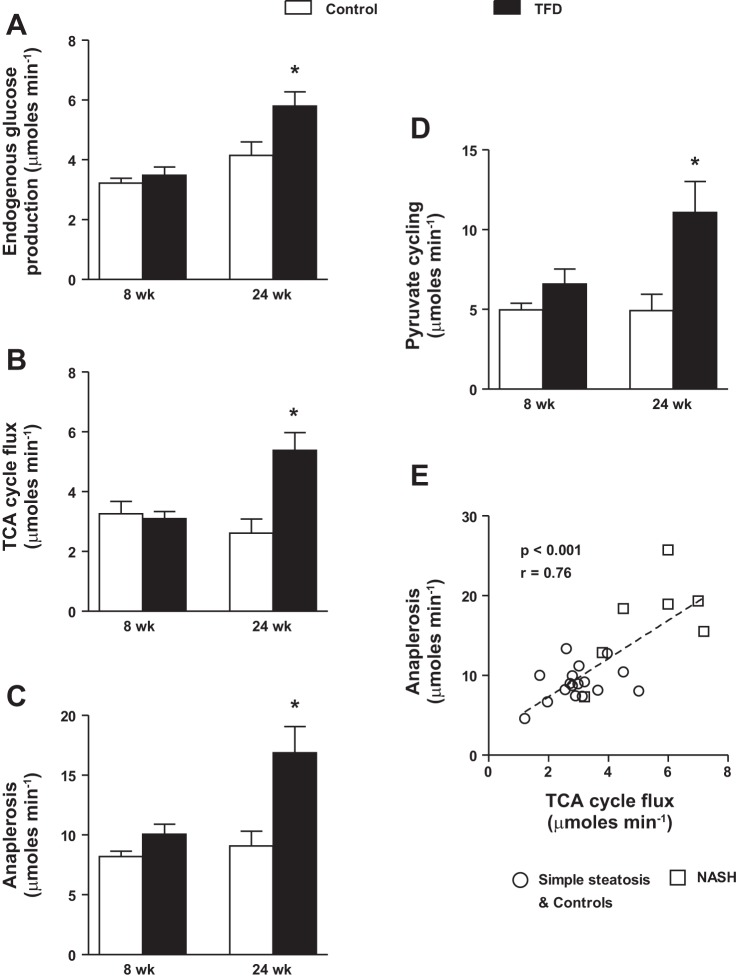
As elaborated above, 13C-NMR-based isotopomer analysis allowed us to determine the activity of multiple pathways through the hepatic mitochondrial TCA cycle, during the transition from simple steatosis to NASH. In spite of hepatic insulin resistance, EGP (Fig. 2A), absolute TCA cycle flux (Fig. 2B), total anaplerosis (Fig. 2C), and pyruvate cycling (Fig. 2D) remained unchanged after 8 wk of TFD. Thus, during early stages of diet-induced insulin resistance and simple steatosis, mitochondrial fluxes could remain apparently normal (35). We then tested the hypothesis that onset of NASH will impair mitochondrial fluxes through the TCA cycle. As expected, onset of NASH was accompanied by higher rates of EGP (Fig. 2A). Interestingly, mitochondrial TCA cycle metabolism was upregulated in mice with NASH. There was an approximate twofold induction in absolute TCA cycle flux (Fig. 2B), total anaplerosis (Fig. 2C), and pyruvate cycling (Fig. 2D) in mice with NASH, relative to their age-matched controls. Furthermore, a robust correlation between TCA cycle flux and anaplerosis was evident, indicating the dependence of anabolic pathways of glucose production on TCA cycle flux (Fig. 2E).
Fig. 2.
Hepatic mitochondrial tricarboxylic acid (TCA) cycle activity is induced in mice with NASH. Overnight fasting basal levels of endogenous glucose production (A), TCA cycle flux (B), anaplerosis (C), and pyruvate cycling (D), determined using 13C-nuclear magnetic resonance (NMR)-based isotopomer analysis, were all elevated in mice fed a TFD for 24 wk compared with their age-matched control counterparts. E: robust relationship between hepatic mitochondrial TCA cycle flux and mitochondrial anaplerosis. Values are means ± SE (n = 6–7/group). *P ≤ 0.05 between C and TFD-fed mice.
Lipotoxic byproducts of fat oxidation, including Cer and DAGs, increased concurrently with hepatic TCA cycle activity.
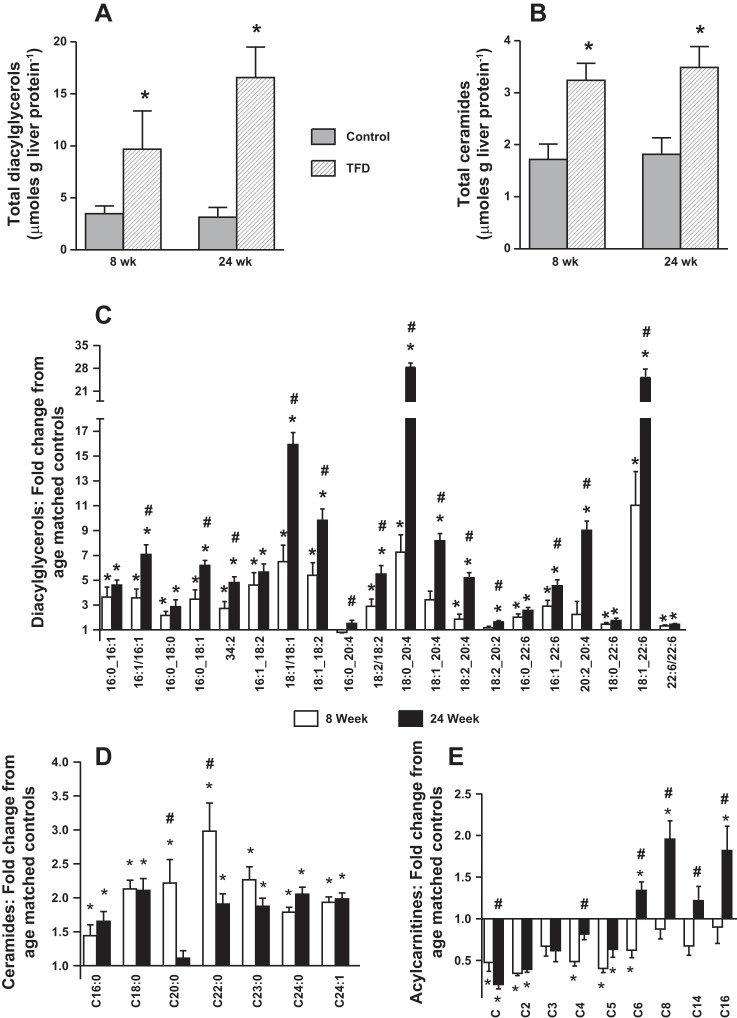
Lipid byproducts, including DAGs and Cer, showed significant increases in both the 8- and 24-wk TFD mice (Fig. 3, A and B). DAGs were elevated in mice with simple steatosis by 8 wk, and the magnitude of this elevation increased markedly by 24 wk concurrent with the onset of NASH (Figs. 3A, 4A, and 4C). Cer also increased in TFD-fed mice compared with controls (Fig. 3B), but, unlike DAGs, this elevation was no more pronounced in NASH compared with simple steatosis (Fig. 4, B and D). However, the patterns were far less consistent in the Cer compared with the DAGs, with elevation of some metabolites increasing with increasing severity of disease (e.g., Cer16:0, Cer24:0), whereas elevation of others was mitigated with disease progression (e.g., Cer22:0, Cer23:0; Fig. 4D). Taken together, the concurrent induction of hepatic TCA cycle metabolism and elevated Cer and DAGs suggest inefficient and incomplete fat oxidation during NASH.
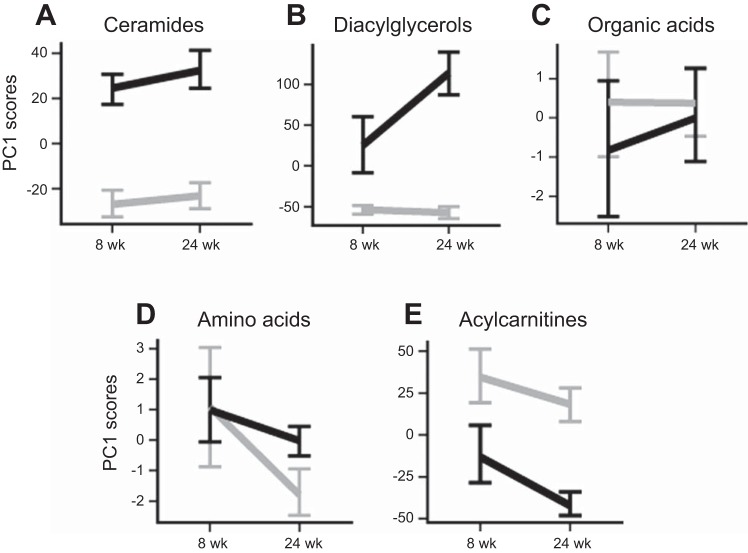
Fig. 3.
Principal component analysis provides a snapshot of the overall metabolite profiles during the transition from simple steatosis to NASH. Summary of hepatic profiles of diacylglycerols (A), ceramides (B), acylcarnitines (C), organic acids (D), and amino acids (E) represented as a function of age (8 or 24 wk) and diet (black, TFD; gray, C). Data are means ± SE of metabolite scores from the first principal component (PC1) of variation in each metabolite class.
Fig. 4.
Elevated levels of ceramides and diacylglycerols (DAGs) together with altered acylcarnitine profile in the liver of TFD mice indicate inefficient fat oxidation. Total hepatic DAG content (A), total hepatic ceramide content (B), fold changes in individual hepatic DAGs (C), fold changes in individual hepatic ceramides (D), and fold changes in individual hepatic acylcarnitines (E) relative to their respective age-matched controls. Mice were fed a TFD for either 8 or 24 wk. Values are means ± SE (n = 6–7/group). *P ≤ 0.05 between C vs. TFD-fed mice. #P ≤ 0.05 between 8- vs. 24-wk dietary treatments.
Altered acylcarnitine levels and hepatic gene expression profiles substantiate inefficient fat oxidation during NASH.
In contrast to the general pattern of accumulation of DAGs and Cer, acylcarnitines were lower in TFD-fed mice (Fig. 3C), although this was driven partly by increased liver triglycerides rather than directly by the effects of diet. However, examination of individual metabolites showed a striking dichotomy in the responses of long-chain and short-chain acylcarnitines: while all acylcarnitines decreased or showed a trend toward decreasing in mice with simple steatosis, long-chain acylcarnitines accumulated markedly in the liver concurrent with the transition to NASH (Fig. 4E). Hepatic gene expression also revealed a consistent pattern of dysregulated mitochondrial fat metabolism wherein expression of several genes (Pc, Pgc1a, Ppara, Cpt1a, Lcad, and Mcad) was downregulated with onset of NASH (Table 2). This occurred together with an induction on genes involved in pathways of inflammation (Il6 and Tnfa) during NASH. Taken together, elevated levels of metabolic byproducts of lipid metabolism and altered hepatic mitochondrial gene expression point to incomplete and inefficient disposal of FFA by hepatic mitochondria during simple steatosis and NASH.
When all of the metabolites were ranked by statistical significance (F-statistic) of the effect of diet, it revealed a unanimous trend predominated by DAGs and Cer (Table 3). In summary, while most of the organic acids and amino acids, which are substrates fueling the hepatic TCA cycle, remained unchanged during the transition to NASH (Fig. 3, D and E), Cer, DAGs, and acylcarnitines were strongly affected by the TFD treatment.
Table 3.
Expression of genes related to carbohydrate, lipid, and mitochondrial metabolism in liver of overnight-fasted C57Bl/6J mice fed TFD for 8 and 24 wk compared with their age-matched control counterparts
| TFD |
||
|---|---|---|
| 8 wk | 24 wk | |
| Mitochondrial Metabolism | ||
| Pc | 0.9 ± 0.04 | 0.7 ± 0.02* |
| Pgc1a/Ppargc1a | 0.4 ± 0.06* | 0.5 ± 0.06* |
| Ppara | 1.4 ± 0.15* | 0.7 ± 0.03* |
| Cpt1a | 0.8 ± 0.10 | 0.5 ± 0.03* |
| Lcad/Acadl | 0.9 ± 0.11 | 0.7 ± 0.06* |
| Mcad/Acadm | 1.2 ± 0.15 | 0.8 ± 0.05* |
| Hmgcs2 | 0.6 ± 0.15 | 0.7 ± 0.07 |
| Fgf21 | 2.2 ± 0.51* | 1.6 ± 0.20* |
| Cs | 1.2 ± 0.12 | 0.9 ± 0.07 |
| Cycs | 0.8 ± 0.08 | 0.9 ± 0.04 |
| Ucp2 | 1.2 ± 0.16 | 2.5 ± 0.19* |
| Lipogenesis | ||
| Srebp1c/Srebf1 | 4.3 ± 0.54* | 2.0 ± 0.32* |
| Acc1/Acaca | 1.0 ± 0.06 | 1.2 ± 0.06* |
| Fasn | 1.0 ± 0.05 | 1.0 ± 0.07 |
| Inflammation | ||
| Il6 | 0.8 ± 0.09 | 1.6 ± 0.38 |
| Tnfa | 1.9 ± 0.53 | 2.0 ± 0.23* |
Values are means ± SE; n = 5–6 mice/group.
P ≤ 0.05 between C and TFD groups.
DISCUSSION
Dysfunctional hepatic mitochondrial energetics in the setting of obesity and hepatic insulin resistance plays a key role in the development of simple steatosis. However, it is unknown whether the pathogenic combination of high rates of triglyceride accumulation, inflammation, and fibrosis, observed during NASH, is accompanied with impaired oxidative capacity of the mitochondrial TCA cycle. Our results demonstrate for the first time that the oxidative capacity of hepatic mitochondrial TCA cycle is twofold elevated during NASH. The upregulation of TCA cycle activity persisted in spite of clear impairments in disposal/storage mechanisms of FFA, as evident from accumulation of a variety of lipid intermediates, blunted ketogenesis, and altered mitochondrial gene expression. A combination of upregulated oxidative flux and accretion of lipotoxic metabolites could provide a potent mitochondrial milieu during NASH to fuel ROS production and hasten inflammation.
Hepatic metabolism constantly adapts and remodels with progressive severity of insulin resistance and substrate overload. A key initial aspect of this adaptation is the rapid accretion of triglycerides in the liver (simple steatosis), from plasma or dietary FFA and de novo lipid synthesis (12, 21). Depending on the ability of the hepatocyte to buffer and store excess lipids, the initial stages of adaptation may not be accompanied by any apparent alterations in mitochondrial oxidative metabolism (35). In fact, hepatic insulin resistance and simple steatosis were evident in our mice fed TFD for 8 wk (Fig. 1D) even before alterations in mitochondrial TCA cycle metabolism were apparent (Fig. 2), as previously reported (35). However, the 8-wk TFD-fed mice with simple steatosis also exhibited a twofold elevation in plasma ketones (Table 1) along with upregulation of Ppara and Fgf21 gene expression. This suggests an early onset of hepatic compensatory mechanisms to dispose acetyl-coA units via ketogenesis. Fasting ketogenesis can account for up to two-thirds of the total fat oxidation in the liver, and further diversion of acetyl-coA toward ketones prevents oxidative burden on the hepatic TCA cycle. Thus, consistent with previous observations in both diet-induced (35, 43) and genetic rodent models of hepatic insulin resistance (32), upregulated ketogenesis could serve to efficiently channel FFAs away from the TCA cycle. Interestingly, the apparently normal TCA cycle metabolism and elevated plasma ketones during simple steatosis occurred together with significantly higher levels of hepatic DAGs and Cer. This could be an early indication of the failure of lipid storage mechanisms to optimally buffer and store excess FFAs in the form of hepatic triglycerides.
Transition from simple steatosis to NASH combines additional factors, including cellular inflammation, ballooning, and fibrosis, together with steatosis, thus aggravating hepatocyte injury. Do these additional insults responsible for the transition to NASH impair mitochondrial oxidative metabolism? In this regard, mitochondrial oxidative metabolism encompasses several central pathways, including β-oxidation, hepatic TCA cycle, ketogenesis, respiratory chain activity, and ATP synthesis, all of which work in concert to maintain cellular homeostasis. Consistent with the idea of an early compensatory induction of mitochondrial oxidative metabolism, β-oxidation (15, 22), ketogenesis, and TCA cycle activity (32, 35, 41) have all been reported to be induced in several mice models and in human subjects with simple steatosis. However, prolonged nutritional overload and progressive severity of liver disease (e.g., onset of NASH, T2DM) are associated with impairments in ketogenesis (32, 35), mitochondrial respiratory chain activity (19), and rates of ATP synthesis (36, 44). In spite of the above reports, our results indicate that hepatic TCA cycle metabolism remains upregulated during NASH. Because generation of acetyl-coA through β-oxidation and its terminal oxidation through hepatic TCA cycle are major sources of energy generation, induction of these networks could be an obligatory phenomenon for synchronizing the energy-demanding processes (e.g., gluconeogenesis, lipogenesis) during states of substrate overload, such as obesity, simple steatosis, and NASH.
Consistent with the idea that impaired mitochondrial TCA cycle could be deleterious to cellular energy demand, all three discrete pathways through mitochondrial TCA cycle metabolism, including absolute TCA cycle flux, anaplerosis, and pyruvate cycling, were all elevated in mice with NASH. Elevated TCA cycle flux provides evidence for the higher activity of citrate synthase toward terminal oxidation of acetyl-coA. Furthermore, high rates of anaplerosis are reflective of the activity of pyruvate carboxylase enzyme, which is closely coupled to phosphoenolpyruvate carboxykinase and cataplerosis to regulate biosynthetic pathways of gluconeogenesis and lipogenesis (26). Pyruvate cycling, which involves carboxylation/decarboxylation of pyruvate, has a significant role in lipid biosynthesis. Here, acetyl-coA generated from the cleavage of citrate by ATP-citrate lyase is used for lipogenesis, whereas the oxaloacetate (OAA) generated is returned back to the mitochondrial TCA cycle (by converting it to malate and then pyruvate and back to OAA) (4). Taken together, the coordinated induction of mitochondrial TCA cycle fluxes helps to sustain the bioenergetics of the insulin-resistant liver during simple steatosis (35, 41) and NASH.
A current prevailing hypothesis dictates that, during hepatic insulin resistance, long-chain acylcarnitines are diverted from mitochondrial oxidation to biosynthesis of hepatic triglycerides, DAGs, and Cer (9, 13). While this was indeed the case in our study, it is important to note that accumulation of liver triglycerides, DAGs, and Cer occurred together with hepatic insulin resistance and simple steatosis when no alterations in mitochondrial TCA cycle fluxes were evident. Furthermore, during NASH, elevated levels of these lipotoxic intermediates coexisted with a twofold induction in mitochondrial oxidative TCA cycle flux. Taken together, incomplete fat oxidation (20, 24, 29) may be an early hallmark of hepatic insulin resistance. Several hepatic DAGs and long-chain acylcarnitines continued to increase with triglyceride accumulation and high rates of TCA cycle activity in NASH. This suggests that mitochondrial fat oxidation is becoming increasingly inefficient at disposing excess FFA. The plot of scores from the first principal component of variation for each metabolite class (Fig. 3) together with the statistical ranking of individual metabolites (Table 4) illustrates the high predictive power of DAGs and Cer in distinguishing NASH from simple steatosis.
Table 4.
Effect of the dietary regime (TFD or C) and age (8 or 24 wk) on metabolite concentrations
| Diet |
Age |
Diet × Age |
||||
|---|---|---|---|---|---|---|
| Metabolite | F | Q | F | Q | F | Q |
| DAG (18:0_20:4) | 299.1* | <0.001* | 59.1* | <0.001* | 80.7* | <0.001* |
| Cer (d18:1/24:1) | 214.7* | <0.001* | 1.3 | 0.396 | 0.4 | 0.668 |
| DAG (18:1/18:1) | 183.1* | <0.001* | 28* | <0.001* | 35.7* | <0.001* |
| DAG (18:1_20:4) | 135.8* | <0.001* | 20.1 | 0.002* | 27.9* | <0.001* |
| Cer (d18:1/24:0) | 133.8* | <0.001* | 2.9 | 0.268 | 2.9 | 0.281 |
| DAG (18:1_18:2) | 110.5* | <0.001* | 9.1* | 0.039* | 11.7* | 0.023* |
| DAG (16:0_18:1) | 100.5* | <0.001* | 13.3* | 0.014* | 14.9* | 0.01* |
| Cer (d18:1/23:0) | 95.3* | <0.001* | 0.1 | 0.724 | 1.8 | 0.362 |
| DAG (16:1_22:6) | 75.9* | <0.001* | 6.1 | 0.091 | 7.2 | 0.072 |
| DAG (16:1_16:1) | 73.7* | <0.001* | 5.9 | 0.091 | 9.1* | 0.047* |
| Cer (d18:1/18:0) | 68.8* | <0.001* | 1.3 | 0.396 | 0.1 | 0.812 |
| DAG (18:2_20:4) | 68.8* | <0.001* | 9.0* | 0.039* | 21.6* | 0.002* |
| DAG (16:0_16:1) | 66.7* | <0.001* | 10.4* | 0.028* | 6.9 | 0.078 |
| DAG (34:2) | 65.8* | <0.001* | 5.1 | 0.119 | 7.8 | 0.071 |
| DAG (18:2/18:2) | 56.2* | <0.001* | 0.6 | 0.539 | 3.4 | 0.247 |
| DAG (16:1_18:2) | 54.5* | <0.001* | 3.8 | 0.204 | 3.0 | 0.281 |
| DAG (16:0_22:6) | 53.2* | <0.001* | 1.3 | 0.396 | 2.1 | 0.334 |
| Carnitine | 51.6* | <0.001* | 10.7 | 0.028 | 1.4 | 0.405 |
| Cer (d18:1/22:0) | 51.2* | <0.001* | 3.1 | 0.268 | 5.8 | 0.097 |
| DAG (18:1_22:6) | 49.3* | <0.001* | 1.3 | 0.396 | 6.1 | 0.094 |
| Alanine | 26.3* | <0.001* | 1.7 | 0.372 | 0.01 | 0.923 |
| Cer (d18:1/16:0) | 25.9* | <0.001* | 0.5 | 0.547 | 0.9 | 0.492 |
| Acetylcarnitine | 25.9* | <0.001* | 2.5 | 0.312 | 0.02 | 0.909 |
| DAG (20:2_20:4) | 24.5* | <0.001* | 1.8 | 0.372 | 6.7 | 0.078 |
| Succinate | 23.1* | <0.001* | 74.5* | <0.001* | 7.2 | 0.072 |
| DAG (16:0_18:0) | 22.5* | <0.001* | 1.2 | 0.421 | 1.3 | 0.405 |
| DAG (18:0_22:6) | 21.8* | <0.001* | 1.7 | 0.372 | 1.5 | 0.398 |
| DAG (22:6/22:6) | 19.4* | <0.001* | 1.3 | 0.396 | 0.3 | 0.675 |
| α-Ketoglutarate | 14.4* | 0.002* | 0.3 | 0.604 | 0.02 | 0.909 |
| Cer (d18:1/20:0) | 13.2* | 0.003* | 2.9 | 0.268 | 9.6 | 0.043 |
| DAG (18:2_20:2) | 12.1* | 0.004* | 1.3 | 0.396 | 3.8 | 0.224 |
| n-Butyrylcarnitine | 10.7* | 0.006* | 0.4 | 0.59 | 0.07 | 0.857 |
| Isovalerylcarnitine | 8.7* | 0.012* | 4.4 | 0.157 | 1.8 | 0.362 |
| Glutamate | 6.0* | 0.038* | 5.3 | 0.114 | 1.5 | 0.398 |
DAG, diacylglycerol; Cer, ceramide.
F and Q are F-statistics and false discovery rate-corrected P values, respectively, from linear models (see text). Metabolites are ranked by statistical significance of the effect of diet.
Significant effects.
With prolonged TFD feeding, hepatic ketogenesis was blunted in mice with NASH, indicating impaired flexibility of this pathway to adapt to excess substrate supply (35). This is evident because the compensatory induction in plasma ketones in mice with simple steatosis was completely abolished with NASH. Furthermore, several genes associated with mitochondrial oxidative metabolism were downregulated in mice with NASH, suggesting an inability to adapt and compensate in spite of elevated nutrient flux. Taken together with the elevated levels of lipotoxic intermediates in NASH, these data indicate that the flux through β-oxidation could be considered “impaired” (29, 47), since it is inefficient to handle the chronic FFA overload during NASH. Even then, considering the twofold induction in the hepatic TCA cycle, the FFA flux through β-oxidation, or from other nonlipid sources (40), could be high enough to generate enough acetyl-coA (15, 35, 41). This acetyl-coA is then being selectively partitioned into mitochondrial TCA cycle for complete oxidation during simple steatosis (35, 41) and NASH, fueling biosynthetic pathways in the liver.
In summary, hepatic insulin resistance during NASH presents a unique environment where high rates of triglyceride synthesis/storage coexist with high rates of mitochondrial TCA cycle activity. Considering the tight relationship between hepatic TCA cycle flux and mitochondrial anaplerosis (Fig. 2E and Refs. 32 and 41) to maintain gluconeogenesis, impairment at the level of mitochondrial TCA cycle metabolism could be implausible during NASH. Even then, sustained induction of hepatic mitochondrial TCA cycle during NASH could be reflecting combinations of 1) early compensatory response to FFA overload and 2) a progressive “inflexibility” of mitochondrial metabolism to insulin action and macronutrients, with severity of the disease. Constitutive induction of mitochondrial TCA cycle together with inefficient disposal/storage of FFA in the liver can be a chronic source of ROS and could hasten inflammation and fibrosis during NASH. Targeted manipulation of hepatic oxidative TCA cycle metabolism could provide an attractive prospective to alleviate mitochondrial dysfunction during simple steatosis and NASH.
GRANTS
This work was supported by a University of Florida Research Opportunity Seed Fund Award (00089467; to N. E. Sunny), a Southeast Center for Integrated Metabolomics National Institute of Diabetes and Digestive and Kidney Diseases Grant (U24-DK-097209), and a National Institutes of Health and National Center for Research Resources Clinical and Translational Science Award to the University of Florida (UL1-TR-00064).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.E.P., S.K., M.N., J.T.M., J.M., D.J.M., J.R.R., and N.E.S. performed experiments; R.E.P., S.K., C.M.W., M.N., J.T.M., J.M., M.K.R., D.J.M., T.J.G., and N.E.S. analyzed data; R.E.P., S.K., C.M.W., and N.E.S. prepared figures; R.E.P., S.K., K.C., and N.E.S. drafted manuscript; R.E.P., S.K., C.M.W., M.N., R.A.Y., K.C., T.J.G., and N.E.S. edited and revised manuscript; M.K.R., R.A.Y., K.C., T.J.G., and N.E.S. interpreted results of experiments; T.J.G. and N.E.S. approved final version of manuscript; N.E.S. conception and design of research.
REFERENCES
- 1.Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, Lavine J, Tonascia J, Loomba R. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: guidelines for referral in NAFLD. Diabetes Care 38: 1347–1355, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple hypothesis testing. J Royal Stat Soc Ser B 57: 289–300, 1995. [Google Scholar]
- 3.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 59: 713–723, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem 279: 48941–48949, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Burgess SC, Jeffrey FMH, Storey C, Milde A, Hausler N, Merritt ME, Mulder H, Holm C, Sherry AD, Malloy CR. Effect of murine strain on metabolic pathways of glucose production after brief or prolonged fasting. Am J Physiol Endocrinol Metab 289: E53–E61, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Burgess SC, Merritt ME, Jones JG, Browning JD, Sherry AD, Malloy CR. Limitations of detection of anaplerosis and pyruvate cycling from metabolism of [1-(13)C] acetate. Nat Med 21: 108–109, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder AG, Garden KE, Anderson SE, Lobley GE. Quantitation of blood and plasma amino acids using isotope dilution electron impact gas chromatography/mass spectrometry with U-(13)C amino acids as internal standards. Rapid Commun Mass Spectrom 13: 2080–2083, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 141: 1249–1253, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 15: 585–594, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Clapper JR, Hendricks MD, Gu G, Wittmer C, Dolman CS, Herich J, Athanacio J, Villescaz C, Ghosh SS, Heilig JS, Lowe C, Roth JD. Diet-induced mouse model of fatty liver disease and nonalcoholic steatohepatitis reflecting clinical disease progression and methods of assessment. Am J Physiol Gastrointest Liver Physiol 305: G483–G495, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology 122: 1649–1657, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med 16: 400–402, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folch-Pi J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissue. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 15.Iozzo P, Bucci M, Roivainen A, Nagren K, Jarvisalo MJ, Kiss J, Guiducci L, Fielding B, Naum AG, Borra R, Virtanen K, Savunen T, Salvadori PA, Ferrannini E, Knuuti J, Nuutila P. Fatty acid metabolism in the liver, measured by positron emission tomography, is increased in obese individuals. Gastroenterology 139: 846–856, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Jin ES, Jones JG, Merritt M, Burgess SC, Malloy CR, Sherry AD. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal Biochem 327: 149–155, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Jones JG, Naidoo R, Sherry AD, Jeffrey FMH, Cottam GL, Malloy CR. Measurement of gluconeogenesis and pyruvate recycling in the rat liver: a simple analysis of glucose and glutamate isotopomers during metabolism of [1,2,3-13C3]propionate. FEBS Lett 412: 131–137, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 21: 739–746, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146: 726–735, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazarin Mde O, Ishii-Iwamoto EL, Yamamoto NS, Constantin RP, Garcia RF, da Costa CE, Vitoriano Ade S, de Oliveira MC, Salgueiro-Pagadigorria CL. Liver mitochondrial function and redox status in an experimental model of non-alcoholic fatty liver disease induced by monosodium L-glutamate in rats. Exp Mol Pathol 91: 687–694, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55: 1389–1397, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Muoio DM, Newgard CB. Mechanisms of disease:Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9: 193–205, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 43: 617–649, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277: 30409–30412, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Previs SF, Kelley DE. Tracer-based assessments of hepatic anaplerotic and TCA cycle flux: practicality, stoichiometry, and hidden assumptions. Am J Physiol Endocrinol Metab 309: E727–E735, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Ramaswamy V, Hooker JW, Withers RS, Nast RE, Brey WW, Edison AS. Development of a (1)(3)C-optimized 1.5-mm high temperature superconducting NMR probe. J Magnet Res 235: 58–65, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. J Am Vet Med Assoc 313: 2263–2273, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279: 32345–32353, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Satapati S, He T, Inagaki T, Potthoff M, Merritt ME, Esser V, Mangelsdorf DJ, Kliewer SA, Browning JD, Burgess SC. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes 57: 2012–2021, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston K, Fu X, Merritt ME, Sherry AD, Malloy CR, Shelton JM, Lambert J, Parks EJ, Corbin I, Magnuson MA, Browning JD, Burgess SC. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest 125: 4447–4462, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Mendez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res 53: 1080–1092, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Mendez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res 53: 1080–1092, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid AI, Szendroedi J, Chmelik M, Krssak M, Moser E, Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care 34: 448–453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stacklies W, Redestig H, Scholz M, Walther D, Selbig J. pcaMethods: a bioconductor package providing PCA methods for incomplete data. Bioinformatics 23: 1164–1167, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 51: 1820–1832, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45: 42–72, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Sunny NE, Kalavalapalli S, Bril F, Garrett TJ, Nautiyal M, Mathew JT, Williams CM, Cusi K. Cross-talk between branched chain amino acids and hepatic mitochondria is compromised in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab 309: E311–E319, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunny NE, Parks Elizabeth J, Browning Jeffrey D, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14: 804–810, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14: 804–810, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunny NE, Satapati S, Fu X, He T, Mehdibeigi R, Spring-Robinson C, Duarte J, Potthoff MJ, Browning JD, Burgess SC. Progressive adaptation of hepatic ketogenesis in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 298: E1226–E1235, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szendroedi J, Chmelik M, Schmid AI, Nowotny P, Brehm A, Krssak M, Moser E, Roden M. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 50: 1079–1086, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. [Google Scholar]
- 46.Trevaskis JL, Griffin PS, Wittmer C, Neuschwander-Tetri BA, Brunt EM, Dolman CS, Erickson MR, Napora J, Parkes DG, Roth JD. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol 302: G762–G772, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 104: 17075–17080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]