Abstract
Neuregulin-1 (NRG-1) is an endothelium-derived growth factor with cardioprotective and antiatherosclerotic properties and is currently being tested in clinical trials as a treatment for systolic heart failure. In clinical practice, heart failure often coexists with renal failure, sharing an overlapping pathophysiological background. In this study, we hypothesized that NRG-1 might protect against cardiomyopathy, atherosclerosis, and nephropathy within one disease process. We tested this hypothesis in a hypercholesterolemic apolipoprotein E-deficient (apoE−/−) type 1 diabetes mouse model prone to the development of cardiomyopathy, atherosclerosis, and nephropathy and compared the effects of NRG-1 with insulin. Upon onset of hyperglycemia induced by streptozotocin, apoE−/− mice were treated with vehicle, insulin, or recombinant human (rh)NRG-1 for 14 wk and were compared with nondiabetic apoE−/− littermates. Vehicle-treated diabetic apoE−/− mice developed left ventricular (LV) dilatation and dysfunction, dense atherosclerotic plaques, and signs of nephropathy. Nephropathy was characterized by abnormalities including hyperfiltration, albuminuria, increased urinary neutrophil gelatinase-associated lipocalin (NGAL), upregulation of renal fibrotic markers, and glomerulosclerosis. rhNRG-1 treatment induced systemic activation of ErbB2 and ErbB4 receptors in both heart and kidneys and prevented LV dilatation, improved LV contractile function, and reduced atherosclerotic plaque size. rhNRG-1 also significantly reduced albuminuria, NGALuria, glomerular fibrosis, and expression of fibrotic markers. Regarding the renal effects of rhNRG-1, further analysis showed that rhNRG-1 inhibited collagen synthesis of glomerular mesangial cells in vitro but did not affect AngII-induced vasoconstriction of glomerular arterioles. In conclusion, systemic administration of rhNRG-1 in hypercholesterolemic type 1 diabetic mice simultaneously protects against complications in the heart, arteries and kidneys.
Keywords: neuregulin-1, type 1 diabetes, heart failure, atherosclerosis, nephropathy
diabetes is a worldwide health problem with high morbidity and mortality rates. About half of the patients with diabetes die as a consequence of cardiovascular disease (29). Although type 2 diabetes is more frequent and more intensively studied, type 1 diabetes also imparts a substantial risk for cardiovascular disease (37). Even though insulin treatment has significantly reduced cardiovascular risk, patients with type 1 diabetes still have a two- to fivefold more chance to develop cardiovascular disease than nondiabetic age-matched patients (31). Another major complication of type 1 diabetes is renal failure, with 30% of type 1 diabetic patients developing kidney disease, often progressing to end-stage renal disease (ESRD) (1). Importantly, the currently available treatment options have failed to sufficiently attenuate the number of patients progressing to ESRD (16).
Neuregulin-1 (NRG-1) is a growth factor of the epidermal growth factor family which acts through ErbB2, ErbB3, and ErbB4 tyrosine kinase receptors in a paracrine and autocrine fashion. The NRG-1/ErbB system is indispensable during embryonic development of the heart and nervous system (13, 19, 27). In postnatal life, NRG-1 released by cardiac endothelial cells has cell-protective, regenerative, and antifibrotic effects in the myocardium (3, 11). In animals, recombinant NRG-1 has beneficial effects on several forms of cardiomyopathy, including ischemic, toxic, viral, and diabetic cardiomyopathy (23, 26). With specific relation to diabetes, endogenous NRG-1 signaling may be impaired in type 1 diabetic cardiomyopathy, while exogenous treatment with NRG-1 has reversed cardiac remodeling in diabetic rats (14, 22, 23, 34). Currently, two forms of recombinant NRG-1 are being examined in clinical trials as potential therapies for heart failure with a reduced left ventricular (LV) ejection fraction (12, 17). In addition to effects on cardiomyopathy, NRG-1 also has arterioprotective effects by suppressing macrophage foam cell formation (44). These effects have been observed in hypercholesterolemic apolipoprotein E-deficient (apoE−/−) mice, and the molecular mechanisms involved reduced endocytosis of acetylated low-density lipoprotein and reduced cholesterol ester formation. These effects were caused by downregulation of scavenger receptor class A and acyl-coenzyme A:cholesterol acyltransferase-1, respectively, and increased apolipoprotein A-I-mediated cholesterol efflux in macrophages.
Besides regulatory effects in the cardiovascular system, NRG-1 may have a more general physiological role, as suggested by the expression of NRG-1 and ErbB receptors in the central nervous system, liver, skeletal muscle, pulmonary cells, intestine, and kidney (5, 9, 20, 33). With specific regard to the kidney, ErbB2, ErbB3, and ErbB4 are expressed in glomeruli and/or tubuli, with restricted expression of glomerular ErbB4 in mesangial cells (45). A more profound role for the ErbB4 receptor in renal physiology has not been shown, but a potential role for the maintenance of renal structure has been suggested by the accelerated development of polycystic kidney disease in absence of this receptor (46). Recently, genome-wide association studies (GWAS) of type 1 diabetes-associated nephropathy showed association with an intronic single-nucleotide polymorphism in the ErbB4 gene (4, 38).
Heart failure is often associated with renal dysfunction, especially in diabetes, and both diseases share risk factors and pathophysiological pathways. For example, chronic heart failure and chronic renal dysfunction respond to treatment with angiotensin inhibitors (10, 15). Interestingly, recent studies show that albuminuria is associated with LV remodeling and worse outcomes of chronic heart failure (18).
In this study, we hypothesized that cardioprotective effects of NRG-1 are associated with nephroprotective effects within one disease process. We tested this hypothesis in atherosclerosis-prone apoE−/− type 1 diabetic mice. As previously shown, these mice develop LV contractile dysfunction, atherosclerosis, and nephropathy (40, 42). We show that recombinant human (rh)NRG-1, in the absence of effects on glycemia and cholesterolemia, attenuates development of LV dysfunction, atherosclerotic plaque formation, and nephropathy. Whereas effects of rhNRG-1 on the development of LV dysfunction and atherosclerosis have been described before, protective effects on nephropathy are novel. We further show that the mechanisms underlying NRG-1-mediated nephroprotection are different from those recruited by inhibitors of the renin-angiotensin system and may include an inhibitory action on transforming growth factor-β1 (TGFβ1)-mediated matrix production by glomerular mesangial cells. These data reinforce the emerging therapeutic potential of NRG-1 in cardiovascular disease and may open new avenues for therapeutic application in nephropathy.
MATERIALS AND METHODS
Experimental Animals and Study Design
All experiments were approved by the institutional ethics committee and conform to the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Laboratory-bred male apoE−/− mice were initially obtained from Jackson Laboratories (Strain 002052). Although mostly used in studies of vascular disease, apoE−/− mice also develop accelerated nephropathy (42). Diabetes type 1 was induced at the age of 16 wk with a low-dose streptozotocin (STZ) treatment (apoE−/− STZ mice, dose STZ 60 mg/kg, 5 consecutive days ip, 0.05 M Na citrate, Sigma Aldrich). apoE−/− STZ mice with blood glucose lower than 300 mg/dl at 2 wk after the initial STZ injections or during further follow-up (in absence of insulin treatment) were excluded from the experiment. Nondiabetic apoE−/− control mice were sham treated with citrate buffer and randomized to receive either 1) vehicle (vehicle-treated apoE−/− control mice, n = 12, PBS, 5 days/wk ip) or 2) rhNRG-1 (rhNRG-1-treated apoE−/− control mice, n = 12, rh-heregulin-β1, 20 μg·kg−1·day−1, 5 days/wk ip, PeproTech) over a period of 14 wk. apoE−/− STZ mice were randomized into treatment with 3) vehicle (vehicle-treated apoE−/− STZ mice, n = 21, dosing as above), 4) insulin (insulin-treated apoE−/− STZ mice, n = 22, INS, 0.3 U·kg−1·day−1, Linshin, Canada), or 5) rhNRG-1β (rhNRG-1-treated apoE−/− STZ mice, n = 19, dosing as above) for 14 wk. Mice were monitored weekly for body weight and blood glucose (OneTouch glucose meter).
Blood and Urine Analysis
Twenty-four-hour urine was collected in metabolic cages prior to euthanasia and blood collection. Urinary albumin (Mouse Albumin ELISA kit, Bethyl Laboratories) and NGAL (Neutrophil gelatinase-associated lipocalin, ab119601, Abcam) ELISAs were performed according to the manufacturers' protocols. Urinary creatinine and serum creatinine, triglycerides, LDL (low-density lipoproteins) and HDL (high-density lipoproteins) were measured via autoanalyzer (Siemens Vista 1500). Total cholesterol levels in serum were determined via a colorimetric end-point assay (Randox Laboratories) according to the manufacturer's guidelines.
Measurements of LV Function
Echocardiography.
Echocardiographic measurements were performed using a Toshiba diagnostic ultrasound system (SSA-700A). End-systolic and end-diastolic internal dimensions (ESD and EDD, respectively) were measured, and fractional shortening (FS) was calculated as %FS: [(EDD − ESD)/EDD] × 100. Except for heart rate and %FS, measurements were normalized to individual body mass.
Intracardiac pressure-volume measurements.
Invasive hemodynamic measurements were recorded via cardiac catheterization with Millar pressure catheter transducers. Steady-state measurements were recorded, and LV preload was decreased by occlusion of the inferior vena cava for 5–10 s to derive load-independent parameters of contractility [slope of the end-systolic pressure-volume (ESPVR)], parameters of ventriculoarterial coupling (end-systolic elastance/arterial elastance, Ees/Ea), and diastolic LV stiffness [slope of the end-diastolic pressure-volume relation (EDPVR); Powerlab/4SP, ADInstruments, LabChart 7 Pro Software].
Histological Analysis
The apex of the heart, the brachiocephalic artery, and the left kidney were fixed in 4% buffered formalin and embedded in paraffin. Sections of the heart were stained with Sirius red, Masson's trichrome, TUNEL [terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate (dUTP) nick-end labeling] and collagen type III (T3330G, Campro Scientific). Staining of the arteria brachiocephalica includes hematoxylin and eosin (H&E), mac-3 (M3/84, Santa Cruz Biotechnology) and TUNEL. Quantification of positive staining was normalized to plaque surface. The thoracic aorta underwent Oil red O staining. Sections of the kidneys were stained with periodic acid Shiff-base (PAS), Masson's trichrome, TUNEL, and the podocyte-specific antibody WT-1 (Wilm's Tumor-1, ab89901, Abcam). Per kidney, 20 images of outer cortical glomerular cross-sections where analyzed as previously described (36). Glomerular positivity was expressed as the ratio of the percentage of positive staining to the glomerular tuft area. Presence of the NRG-1 specific receptors ErbB2 to ErbB4 [c-erbB-2 Ab-1 (21N), Neomarkers, HER3/ErbB3, D22C5, Cell Signaling, and ErbB-4 (C-18), Santa Cruz, respectively] within the kidney were determined by immunohistological staining. Negative controls were performed with secondary antibody only. Sections of a mouse thymus were used as a positive control for the TUNEL staining.
Vasoconstriction Studies of Renal Efferent and Afferent Arterioles
Both renal efferent and afferent arterioles were isolated from healthy male C57BL/6N mice (25–30 g). Efferent and afferent arterioles were treated with rhNRG-1 (n = 11, 50 ng/ml, PeproTech) or with vehicle (n = 12) for 20 min, after which rhNRG-1 remained present in the bath solution during measurements. Thereafter, AngII (Sigma-Aldrich) concentration response curves (10−12 to 10−6 mol/l) with a 2-min interval between every application were performed. In efferent arterioles, an additional series of measurements (n = 4) was performed where the AT1 receptor inhibitor ZD7155 (Tocris) was added together with rhNRG-1 during the concentration response for Ang II.
Renal Mesangial Cells
The presence of ErbB receptors in primary mouse (Swiss) renal glomerular mesangial cells (P10628, Innoprot, Spain) was analyzed by Western blotting. To assess collagen synthesis, mesangial cells were stimulated with TGFβ1 (10 ng/ml, PeproTech) in the presence or absence of rhNRG-1β (50 ng/ml, PeproTech).
Western Blotting and Immunoprecipitation
Cardiac tissue, kidneys, or mesangial cells were collected in lysis buffer suitable for Western blotting or immunoprecipitation. Antibodies used for Western blotting were ErbB2 [c-erbB-2 Ab-1 (21N), Neomarkers], ErbB3 (D22C5, Cell Signaling), ErbB-4 (C-18, Santa Cruz Biotechnology), total eNOS (C-20, Santa Cruz Biotechnology), and phospho-eNOS (Ser1177, Cell Signaling). Phosphorylation of ErbB2 and ErbB4 was determined by immunoprecipitation of total tissue protein with anti-ErbB2 [c-erbB-2 Ab-1 (21N), Neomarkers] and anti-ErbB4 (C-18, Santa Cruz Biotechnology) antibody, followed by immunoblotting with anti-phosphotyrosine antibody (P-Tyr-100, Cell Signaling Technologies).
Real-Time Quantitative PCR
Total RNA was isolated from the heart, kidneys, and mesangial cells. Using TaqMan real-time PCR (Life Technologies), TGFβ1 (Mm01178820_m1, tgfb1), procollagen 1a1 (Mm00801666_g1, Col1a1), procollagen 3a1 (Mm01254476_m1, Col3a1), myosin heavy-chain-α (MHCα, Mm00440359_m1, Myh6) and -β (MHCβ, Mm01319006_g1, Myh7) mRNA levels were determined in the heart. TGF-β1, procollagen 4a1 (Mm01210125_m1, Col4a1) and fibroblast-specific protein-1 (FSP-1, Mm01210125_m1, s100a4) mRNA expression was analyzed in whole kidney tissue. In mesangial cells, collagen synthesis was determined by procollagen 4a1 and fibronectin-1 (Mm01256744_m1, Fn-1) expression. Gene expression of the gene of interest was normalized to the expression of β-actin (Mm00607939_s1, Actb).
Data Analysis and Statistics
Data are expressed as means ± SE. Differences between groups were analyzed by one-way or two-way ANOVA with Bonferroni corrections for multiple comparisons. Data of the vasoconstriction studies were compared by using the Brunner's test. Western blots were subjected to densitometric analysis using ImageJ v.1.42 software. Statistical significance was defined as P < 0.05. All statistical analyses were done using GraphPad Prism 6, IBM SPSS Statistics 22, and “R” (The R-project, http://www.r-project.org) software.
RESULTS
NRG-1 Has No Effect on Glycemia and Plasma Cholesterol Levels in apoE−/− STZ Mice
Vehicle-treated apoE−/− STZ mice were hyperglycemic and hypercholesterolemic, with a concomitant increase in plasma LDL, displayed reduced body weight despite an increased food and water intake, and increased diuresis (Table 1), all signs of diabetes mellitus. As expected, treatment of apoE−/− STZ mice with insulin significantly attenuated or completely prevented these characteristics. By contrast, treatment of apoE−/− STZ mice with rhNRG-1 did not affect plasma glucose or lipid levels, body weight, diuresis, or food and water intake. Also, rhNRG-1 had no effect on these parameters in apoE−/− control mice.
Table 1.
Physiological parameters
| apoE−/− Control+Vehicle | apoE−/− Control+NRG-1 | apoE−/− STZ+Vehicle | apoE−/− STZ+INS | apoE−/− STZ+NRG-1 | |
|---|---|---|---|---|---|
| Body weight, g | 31.6 ± 1.5 | 31.8 ± 2.3 | 25.8 ± 2.8*** | 28.1 ± 3.8† | 26.7 ± 3.7** |
| Glycemia, mg/dl | 205 ± 32 | 178 ± 11 | 554 ± 46*** | 194 ± 69††† | 536 ± 72*** |
| Cholesterol, mg/dl | 265 ± 42 | 203 ± 10 | 459 ± 58* | 294 ± 18† | 366 ± 48 |
| Triglycerides, mg/dl | 148 ± 22 | 142 ± 6.8 | 196 ± 20 | 169 ± 15 | 167 ± 32 |
| LDL, mg/dl | 66.1 ± 9.8 | 65.7 ± 5.2 | 162 ± 33* | 136 ± 25 | 190 ± 32** |
| HDL, mg/dl | 123 ± 15 | 146 ± 2.7 | 122 ± 16 | 138 ± 11 | 140 ± 8.8 |
| Food intake, g | 3.0 ± 0.46 | 3.4 ± 1.0 | 4.7 ± 1.2*** | 3.3 ± 1.0††† | 4.0 ± 1.4* |
| Water intake, ml | 3.60 ± 1.4 | 3.40 ± 1.7 | 18.6 ± 8.2*** | 6.10 ± 3.5††† | 14.4 ± 9.2*** |
| Urine, ml/24 h | 1.20 ± 0.37 | 1.10 ± 0.53 | 15.1 ± 7.6*** | 2.20 ± 2.2††† | 11.5 ± 8.1*** |
Values represent means ± SE; n = 12. NRG-1, neuregulin-1; STZ, streptozotocin; INS, insulin; LDL, low-density lipoproteins; HDL, high-density lipoproteins.
P < 0.05,
P < 0.01,
P < 0.001 vs. apoE−/− control+vehicle;
P < 0.05,
P < 0.001 vs. apoE−/− STZ+vehicle.
NRG-1 Prevents Diabetes-Induced Cardiac Dysfunction in apoE−/− STZ Mice
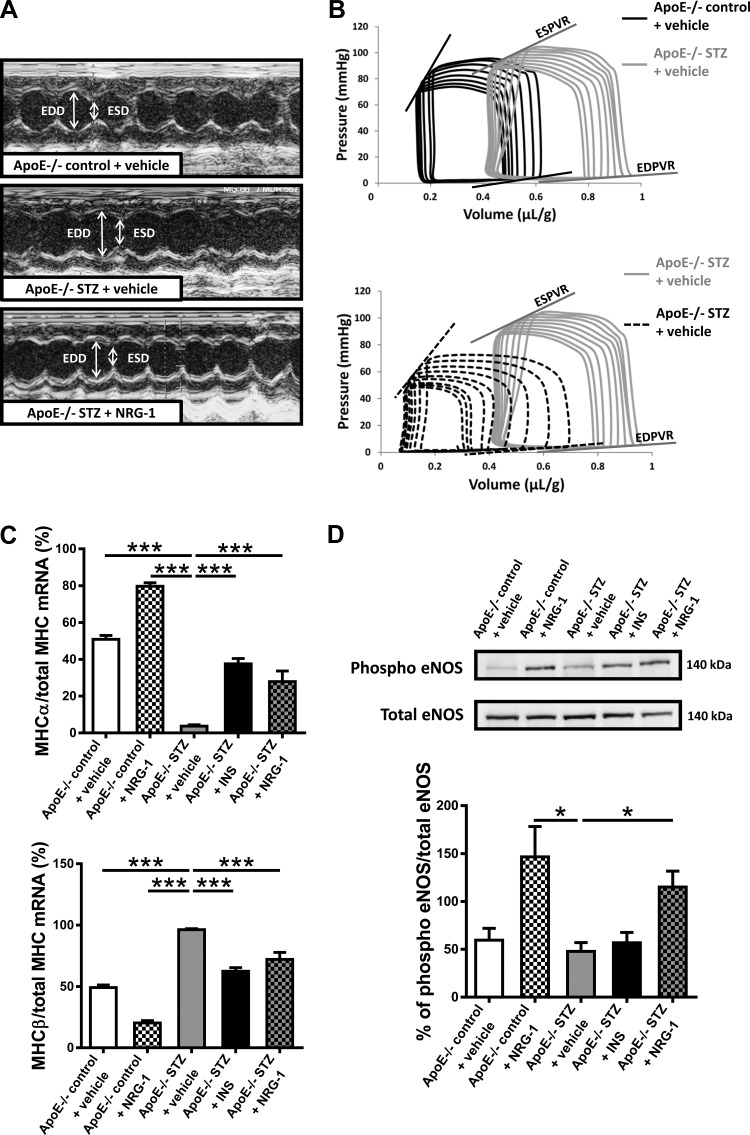
Echocardiographic measurement showed that vehicle-treated apoE−/− STZ mice developed LV dilatation, as shown by the significant increase in EDD (Table 2; representative images in Fig. 1A). These mice also developed severe LV contractile dysfunction, as shown by the marked decrease in the slope of ESPVR and in Ees/Ea (Table 3; representative images in Fig. 1B). LV fractional shortening (%FS), a load-dependent parameter of global pump performance, remained unchanged. The slope of EDPVR, a parameter of diastolic LV stiffness, showed a trend to be reduced in the vehicle-treated apoE−/− STZ mice (P = 0.11), consistent with reduced overall chamber stiffness in this model, as previously described (25). There was no significant difference in the LV end-systolic blood pressure (ESP) among all experimental groups (Table 3). As further shown in Tables 2 and 3, insulin-treated apoE−/− STZ mice showed neither LV dilatation nor contractile dysfunction, indicating that the STZ-induced cardiac changes were related to hyperglycemia and diabetes. Importantly, treatment with rhNRG-1 also significantly prevented LV dilatation and LV contractile dysfunction (Fig. 1, A and B; Tables 2 and 3). Compared with vehicle-treated apoE−/− STZ mice, rhNRG-1-treated apoE−/− STZ mice displayed a significantly lower EDD, higher slope of ESPVR and of EDPVR, and a higher Ees/Aa. rhNRG-1 had no effect on LV function in apoE−/− control mice and did not affect systolic blood pressure in any of the groups (Table 3). Notably, the effects of rhNRG-1 on LV remodeling and function could not be explained by effects on myocardial interstitial fibrosis (Masson's Trichrome, Sirius red, and collagen type III, supporting Fig. 1), which remained negligible in all experimental groups, nor by preventing myocardial apoptosis, which also remained very rare in all experimental groups (TUNEL staining, supporting Fig. 1). Absence of myocardial fibrosis was further confirmed by mRNA analysis of cardiac TGFβ1, Col1a1, and Col3a1, which remained unchanged among the groups (data not shown). Next, quantification of MHCα and MHCβ mRNA expression in the LV showed a significant isoform switch toward MHCβ in vehicle-treated apoE−/− STZ mice compared with vehicle-treated apoE−/− control mice (Fig. 1C). This MHC isoform switch toward fetal cardiac MHCβ typically occurs in diabetes and is associated with heart failure (8, 30). Both insulin and rhNRG-1 treatment significantly prevented this isoform switch. Consistent with previous observations, phosphorylation of LV eNOS in rhNRG-1-treated mice was enhanced, both in apoE−/− control and in apoE−/− STZ mice (Fig. 1D) (22).
Table 2.
LV echocardiographic measurements
| apoE−/− Control+Vehicle | apoE−/− Control+NRG-1 | apoE−/− STZ+Vehicle | apoE−/− STZ+INS | apoE−/− STZ+NRG-1 | |
|---|---|---|---|---|---|
| Heart rate, bpm | 486 ± 41 | 490 ± 40 | 434 ± 33 | 471 ± 28 | 446 ± 28 |
| ESD, 10−2 mm/g | 7.0 ± 0.88 | 6.6 ± 1.0 | 7.8 ± 1.2 | 8.7 ± 0.65 | 5.6 ± 1.6††† |
| EDD, 10−2 mm/g | 11.2 ± 0.49 | 12.0 ± 0.74 | 13.6 ± 0.91*** | 12.8 ± 0.84 | 11.3 ± 1.6††† |
| %FS | 37.9 ± 2.2 | 45.9 ± 1.7 | 39.5 ± 2.3 | 35.0 ± 2.5 | 49.1 ± 2.3**††† |
Values represent means ± SE; n = 12. LV, left ventricular; ESD, end-systolic diameter; EDD, end-diastolic diameter; %FS, fractional shortening.
P < 0.01,
P < 0.001 vs. apoE−/− control+vehicle.
P < 0.001 vs. apoE−/− STZ+vehicle.
Fig. 1.
Representative images of echocardiographic and hemodynamic measurements and the effect of NRG-1 on cardiac molecular markers. A: representative images of LV echocardiographic measurements. Comparison of apoE−/− control + vehicle, apoE−/− STZ + vehicle, and apoE−/− STZ + NRG-1. B: representative images of LV hemodynamic pressure-volume measurements. Comparison of apoE−/− control + vehicle, apoE−/− STZ + vehicle, and apoE−/− STZ + NRG-1. C: mRNA expression of cardiac MHC isozymes, normalized to total MHC and expressed as fold induction to apoE−/− control + vehicle mice. D: Western blots on myocardial tissue for phospho- and total eNOS. NRG-1, neuregulin-1; STZ, streptozotocin; INS, insulin; ESD, end-systolic diameter; EDD, end-diastolic diameter; ESPVR, end-systolic pressure-volume relationship; EDPVR, end-diastolic pressure-volume relationship; eNOS, endothelial nitric oxide synthase; MHCα/β, myosin heavy chain-α/β; H&E, hematoxylin and eosin. *P < 0.05, ***P < 0.001 vs. apoE−/− STZ + vehicle.
Table 3.
LV systolic and diastolic function
| apoE−/− Control+Vehicle | apoE−/− Control+NRG-1 | apoE−/− STZ+Vehicle | apoE−/− STZ+INS | apoE−/− STZ+NRG-1 | |
|---|---|---|---|---|---|
| Slope ESPVR, mmHg/μl | 16.0 ± 7.8 | 11.5 ± 8.0 | 4.42 ± 2.6*** | 14.1 ± 5.7†† | 14.8 ± 9.2†† |
| Slope EDPVR, mmHg/μl | 0.28 ± 0.18 | 0.27 ± 0.15 | 0.13 ± 0.10 | 0.33 ± 0.21† | 0.43 ± 0.33†† |
| Ees/Ea | 2.6 ± 1.2 | 2.0 ± 1.2 | 0.99 ± 0.74** | 1.8 ± 0.69 | 2.9 ± 2.3†† |
| ESP, mmHg | 78.9 ± 4.1 | 72.5 ± 6.2 | 87.4 ± 8.0 | 72.8 ± 4.5 | 75.7 ± 8.6 |
Values represent means ± SE; n = 12. ESPVR, end-systolic pressure-volume relationship; EDPVR, end-diastolic pressure-volume relationship; ESP, end-systolic pressure; Ees/Ea, end-systolic elastance/arterial elastance.
P < 0.01,
P < 0.001 vs. apoE−/− control + vehicle;
P < 0.05,
P < 0.01 vs. apoE−/− STZ + vehicle.
NRG-1 Attenuates Atherosclerotic Plaque Formation in apoE−/− STZ Mice
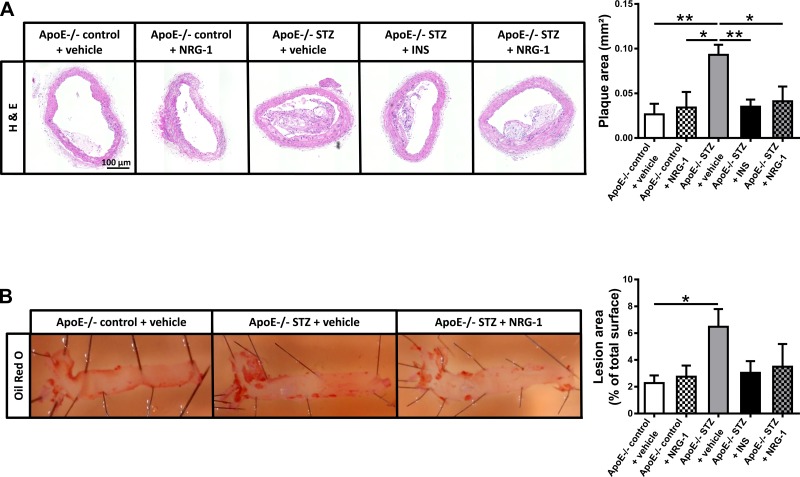
Besides cardiac dysfunction, apoE−/− STZ mice also develop atherosclerosis. As shown in Fig. 2, analyses of the arteria brachiocephalica and the thoracic aorta revealed dense plaque formation in vehicle-treated apoE−/− STZ mice, with significant increases in transverse plaque area and total en face lesion area. Insulin significantly reduced transverse plaque area in the arteria brachiocephalica of apoE−/− STZ mice, and again, rhNRG-1 was equally effective (Fig. 2A). In addition, in both insulin-treated and rhNRG-1-treated apoE−/− STZ mice, total en face area was not increased (Fig. 2B). Plaque composition, including relative size of the necrotic core, number of macrophages, and incidence of apoptotic nuclei remained, however, unaffected (data not shown).
Fig. 2.
Effect of NRG-1 on atherosclerotic plaque formation. A: representative images of plaque size in arteria brachiocephalica and bar graphs showing quantification of plaque area. B: representative images of en face lesion area in thoracic aorta. Comparison of apoE−/− control + vehicle, apoE−/− STZ + vehicle, and apoE−/− STZ + NRG-1 and bar graphs showing quantification of lesion area in all experimental groups. *P < 0.05, **P < 0.01 vs. apoE−/− STZ + vehicle.
NRG-1 Inhibits the Development of Diabetic Nephropathy in apoE−/− STZ Mice
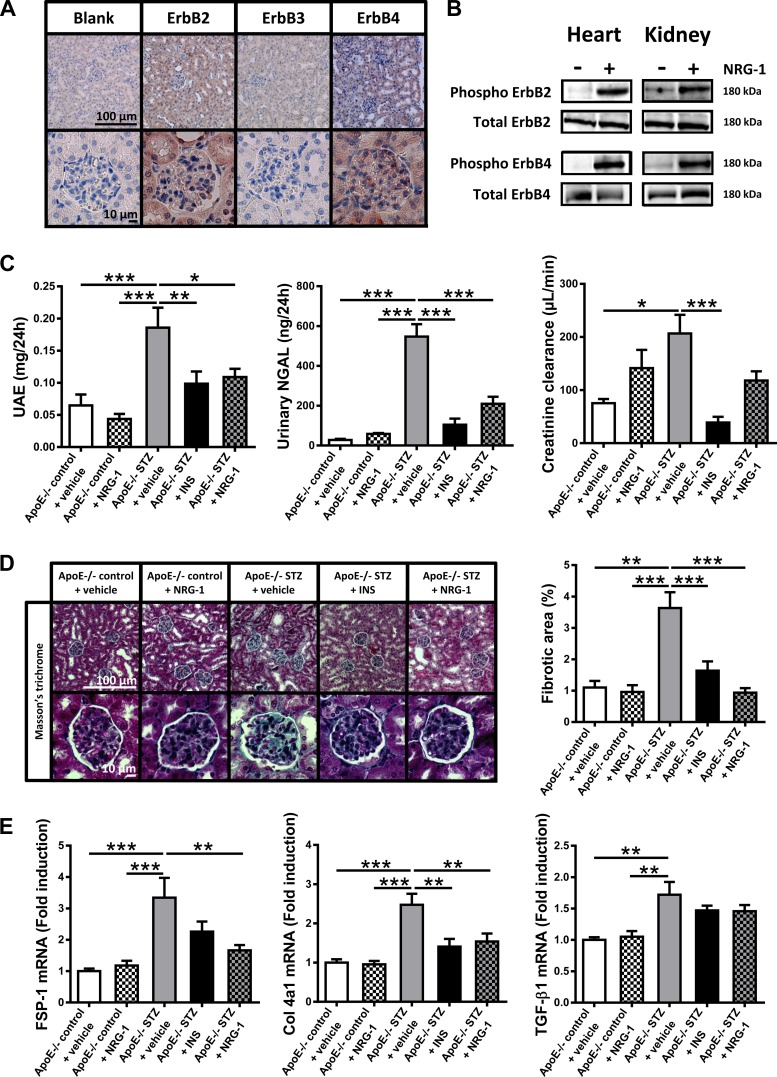
As shown in Figure 3A, histological analysis showed that ErbB2 and ErbB4 were abundantly present in glomerular cells and in tubular epithelial cells, whereas expression of ErbB3 was nearly absent. Systemic administration of rhNRG-1 by intraperitoneal injection induced phosphorylation of ErbB2 and ErbB4 receptors in peripheral organs, including the heart and kidneys (Fig. 3B).
Fig. 3.
Presence and activation of ErbB receptors and effect of NRG-1 on urinary markers, glomerulosclerosis, and renal fibrotic markers. A: immunohistochemical staining of a mouse kidney for the presence of ErbB2, ErbB3, and ErbB4 tyrosine kinase receptors in renal tubular and glomerular cells. B: levels of phosphorylated ErbB2 and ErbB4 in cardiac and renal tissue of C57Bl/6 mice treated with either vehicle (−) or rhNRG-1 (+). GAPDH abundancy in equal amounts of samples used for immunoprecipitation is shown below. C: kidney function markers. Bar graphs showing urinary albumin and urinary NGAL concentrations, and creatinine clearance. D: representative images and quantification of renal glomerular fibrotic area. E: bar graphs showing mRNA expression in whole kidney tissue of TGFβ1, FSP-1, and Col4a1, expressed as fold induction to apoE−/− control + vehicle mice. FSP-1, fibroblast-specific protein-1; Col4a1, procollagen 4a1; TGFβ1, transforming growth factor-β1; UAE, urinary albumin excretion; NGAL, neutrophil gelatinase-associated lipocalin. *P < 0.05, **P < 0.01, ***P < 0.001 vs. apoE−/− STZ + vehicle.
Figure 3C shows protective effects of insulin and rhNRG-1 on the development of nephropathy in apoE−/− STZ mice. Signs of nephropathy in vehicle-treated apoE−/− STZ mice consisted of significant increases in albuminuria (3-fold increase vs. vehicle-treated apoE−/− control mice), NGALuria (20-fold increase vs. vehicle-treated apoE−/− control mice), and glomerular hyperfiltration (increased creatinine clearance, 2.5-fold increased vs. vehicle-treated apoE−/− control mice). Each of these changes remained at control levels during treatment with insulin, indicating that the nephropathy in apoE−/− STZ mice was related to hyperglycemia and diabetes. Strikingly, also treatment with rhNRG-1 significantly prevented albuminuria and NGALuria, reaching the same level of efficacy as insulin. By contrast, diabetic glomerular hyperfiltration was less sensitive to rhNRG-1, with no significant effect on creatinine clearance.
Histological analysis revealed marked glomerulosclerosis (Fig. 3D; a significant, nearly 4-fold increase of fibrotic area within the glomerular tuft area vs. vehicle-treated apoE−/− control mice) and a trend toward a decrease in podocyte positivity (P = 0.083 vs. vehicle-treated apoE−/− control mice, Supporting Fig. 2) in vehicle-treated apoE−/− STZ mice. Mesangial matrix expansion, hypercellularity, glomerular basement membrane thickening, and apoptosis (Supporting Fig. 2), which are signs of nephropathy described in some, but not all diabetic models, were not detected. Glomerulosclerosis (Fig. 3D), but not podocyte loss, was significantly prevented by insulin treatment. Strikingly, also, rhNRG-1 significantly attenuated glomerulosclerosis to the same extent as insulin (Fig. 3D). In addition, rhNRG-1 prevented the upregulation of FSP-1 and Col4a1 mRNA expression in renal tissue (Fig. 3E), which is consistent with antifibrotic effects of rhNRG-1 in the diabetic kidney. Expression of TGFβ1 (Fig. 3E), a mediator of glomerular fibrosis that was only slightly upregulated in vehicle-treated apoE−/− STZ mice, remained unaffected by both insulin and rhNRG-1 treatment.
NRG-1 Does Not Affect AngII-Induced Vasocontriction of Glomerular Arterioles
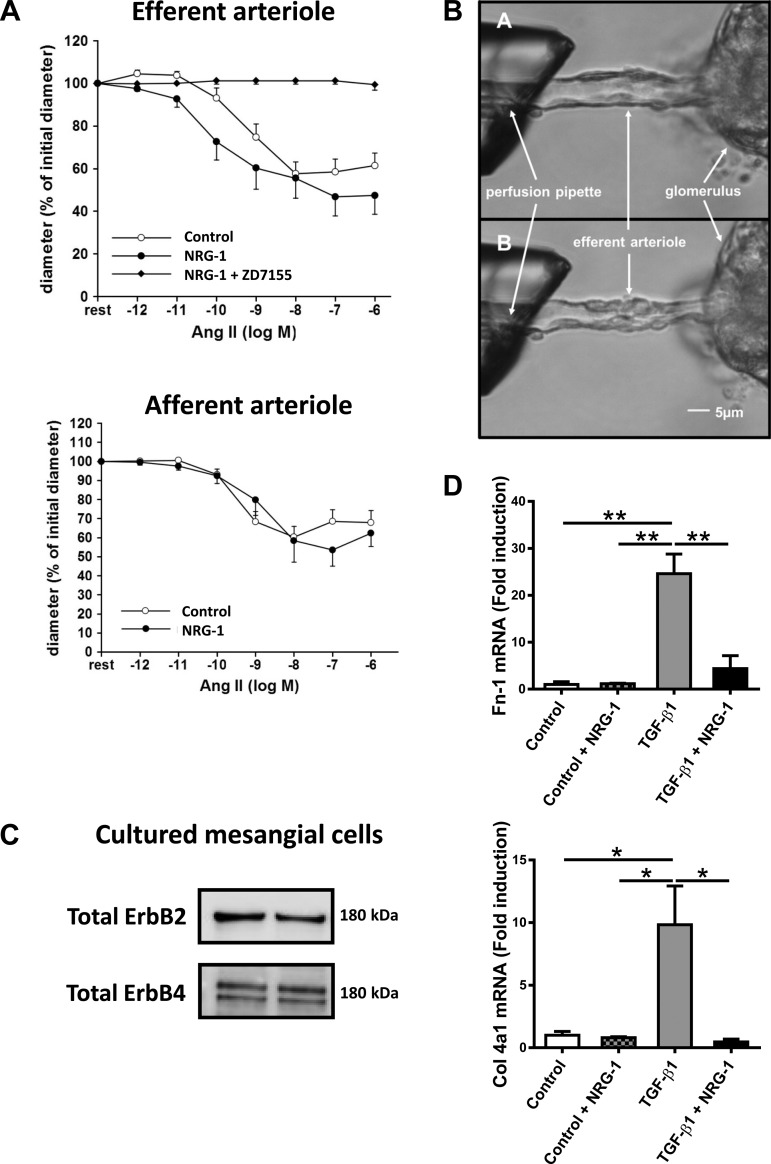
Development of diabetic nephropathy is linked to increased renal activity of AngII, vasoconstriction of glomerular efferent arterioles, and increased glomerular filtration pressure (28). Figure 4A shows that rhNRG-1, in contrast to the selective angiotensin type 1A receptor antagonist ZD7155, did not inhibit AngII-induced vasoconstriction of glomerular arterioles.
Fig. 4.
Direct effect of NRG-1 on glomerular arterioles and renal glomerular mesangial cells. A: graphs showing the effect of AngII on vasoconstriction in glomerular arterioles in the absence and the presence of rhNRG-1. The selective angiotensin type 1A receptor antagonist ZD7155 served as positive control for inhibition of AngII-induced vasoconstriction. B: digital microscopic images of a mouse efferent arteriole. The free end of the arteriole is aspirated into a pipette, visible on the left. A: basal situation of efferent arteriole. B: application of rhNRG-1 (50 ng/ml) + AngII (10−12 to 10−6 mol/l). C: Western blot analysis confirming the presence of ErbB2 and ErbB4 receptors in mesangial cells. D: bar graphs showing TGFβ1-induced mRNA expression of Fn-1 and Col4a1 in the absence and the presence of rhNRG-1 as fold induction to control. AngII, angiotensin II; Fn-1, fibronectin-1. *P < 0.05, **P < 0.01 vs. apoE−/− STZ + vehicle.
NRG-1 Inhibits TGFβ1-Induced Fibrotic Responses of Glomerular Cells In Vitro
Cultured mesangial cells are the main source of extracellular matrix in the glomerulus. In view of the observation that rhNRG-1 reduced glomerular fibrosis, we studied the direct effects of rhNRG-1 on mesangial cells. Figure 4C shows expression of ErbB2 and ErbB4 receptors in these cells. Figure 4D shows that treatment of mesangial cells with rhNRG-1 robustly attenuated TGFβ1-induced upregulation of Fn-1 and Col4a1 mRNA synthesis, implying a direct inhibitory effect of rhNRG-1 on mesangial cells.
DISCUSSION
This study demonstrates preventive therapeutic effects of rhNRG-1 in different organs of hypercholesterolemic diabetic mice, prone to development of dilated cardiomyopathy, atherosclerosis, and nephropathy. Although beneficial effects on the failing heart and on atherosclerosis have been described before, effects of rhNRG-1 on glomerulosclerosis and nephropathy are entirely novel. The NRG-1/ErbB system is a cell growth and cell differentiation promoting system with a remarkable diversity of functions throughout life. During embryogenesis, this system is indispensable for several processes including development of the heart, mammary tissue, and nervous system, participating in ventricular trabeculation, cardiac valve formation, and axonal myelination by Schwann cells (27). In postnatal/adult life, the NRG-1/ErbB system is expressed in many tissues (5). Regulatory effects have been shown in the brain cardiovascular control system, skeletal muscle, neurons, atherosclerotic vessel wall, myocardium, etc. (20, 33). In this study, we have confirmed that rhNRG-1 suppresses the formation of atherosclerotic lesions in apoE-deficient mice (44).
We also confirmed that rhNRG-1 attenuates the development of LV dilatation and of LV contractile dysfunction in type 1 diabetes. The protective characteristics of rhNRG-1 against LV dilatation have already been described in several models of cardiomyopathy and heart failure (26). The precise mechanisms underlying the cardioprotective effects of NRG-1/ErbB signaling in vivo, however, are still incompletely understood and may be circumstance dependent. Upon acute myocardial injury, NRG-1 may desensitize the myocardium for adrenergic stimulation and increase cardiomyocyte threshold for apoptosis and ischemic cell death (21). In a chronic stabilized condition, NRG-1 may induce cardiomyocyte mitosis and promote myocardial regeneration (3). Also, protection against diabetic cardiomyopathy in type 1 diabetes has been described (22, 23). Consistent with previous data, rhNRG-1 reversed cardiac MHCα downregulation and enhanced LV eNOS phosphorylation, presumably contributing to improved contractile function and reduced LV remodeling (22, 24).
Interestingly, in this study, the beneficial effects of rhNRG-1 on LV remodeling and dysfunction in diabetes occurred in the absence of increased myocardial fibrosis (which was consistent with the reduced LV stiffness in vehicle-treated apoE−/− STZ mice) and apoptosis, implicating fibrosis- and apoptosis-independent protective pathways. These observations may be different from previous studies with rhNRG-1 in diabetic models (22, 23). In this study, absence of increased myocardial fibrosis was confirmed by three immunohistological staining methods and by mRNA analysis of fibrotic markers. Although various studies have shown myocardial fibrosis and apoptosis in different models of diabetic cardiomyopathy, other investigators have observed the absence of fibrosis and apoptosis (32). Variability in the degree of myocardial fibrosis and apoptosis in diabetic rodent models has been explained by differences in animal species (2), differences in mouse strains (41), variable levels of hyperglycemia (39), differences in compensatory upregulations of metalloproteinases (43), and differences in the effects of STZ (7), the last of which may cause nonspecific toxicity besides pancreatic injury and includes tissue apoptosis. Importantly, in our study, all cardiac changes in apoE−/− mice were prevented by insulin, supporting that hyperglycemia, rather than nonspecific STZ toxicity, was the responsible factor.
The most important and novel finding of this study was the protective effect of rhNRG-1 on the development of diabetic nephropathy. More specifically, we found that rhNRG-1 attenuated albuminuria and NGALuria, respective biomarkers of glomerular and tubular injury, and protected against histological glomerulosclerosis. Glomerulosclerosis is a complex process induced by a dysregulated metabolic milieu (including hyperlipidemia and hyperglycemia) and is characterized by a combination of factors including glomerular basement membrane thickening, endothelial dysfunction, podocyte loss, mesangial expansion, and accumulation of extracellular matrix (35). Some, but not all, of these characteristics were present in our model, suggesting that the level of nephropathy was rather mild. Strikingly, accumulation of glomerular matrix in apoE−/− STZ mice was completely prevented by rhNRG-1 to the same extent as insulin. Accumulation of glomerular matrix is a final common pathway during glomerulosclerosis, resulting from the activation of mesangial cells by TGFβ1 (47). An inhibitory effect of rhNRG-1 on matrix formation was recapitulated on cultured mesangial cells in vitro. Direct inhibition of mesangial cells may thus contribute to the nephroprotective effects of rhNRG-1 in apoE−/− STZ mice. We do not exclude the possibility, however, that other direct actions of rhNRG-1 on renal tissue contribute or that indirect effects, coupled to the attenuation of atherosclerosis and/or LV dysfunction, play a role.
Despite the introduction of renoprotective therapy, mostly consisting of inhibitors of the renin-angiotensin system, the incidence of diabetic kidney disease is increasing, and the prognosis of albuminuria remains poor, frequently progressing to ESRD (16). Therefore, the current observation of renoprotective effects of rhNRG-1, a protein already introduced in clinical trials with concomitant protective effects on atherosclerosis and heart failure, is a fascinating discovery with translational potential. Interestingly, studies in isolated glomerular arterioles showed that rhNRG-1 did not inhibit AngII-induced vasoconstriction. These observations indicate that the protective mechanisms of rhNRG-1 on nephropathy differ from those of inhibitors of the renin-angiotensin system, in the sense that at least the renal hemodynamic component of rhNRG-1 is clearly different.
This study, together with the recent GWAS in diabetic nephropathy, should encourage further studies on the function of the NRG-1/ErbB system in the kidney and on the potential therapeutic application of rhNRG-1 in diabetic nephropathy (4, 38). This study uncovers renoprotective effects of rhNRG-1, preventively administrated in a model of rather mild nephropathy from onset of type 1 diabetes, hence, before initiation of renal disease. Obviously, it would be interesting to confirm these observations in more severe and standardized models of diabetic nephropathy that more utterly recapitulate the features of human diabetic nephropathy and to initiate therapy after onset of renal disease, e.g., upon detection of albuminuria, a regimen that may be more clinically relevant (6).
In conclusion, in a diabetes type 1 mouse model with a high cardiovascular risk, this proof-of-concept study shows that systemic delivery of rhNRG-1 exerts a combined cardioprotective, antiatherosclerotic and nephroprotective effect in the absence of effects on blood glucose or cholesterol levels. In particular, this study has uncovered that rhNRG-1 may protect against nephropathy, occurring by mechanistic pathways that at least partly diverge from renin-angiotensin inhibitors and potentially involve direct inhibitory actions on renal mesangial cells. On the basis of these data, the nephroprotective effects of rhNRG-1 should be further explored as a potential novel therapeutic avenue for kidney disease.
GRANTS
This work was supported by a research grant from the “Fonds voor Wetenschappelijk Onderzoek” Vlaanderen (Application number, G0C5214), by a grant from the European Commission (FP7-HEALTH-F2-2010-261409), by an IOF POC [Industrieel Onderzoeksfonds (Industrial Research Fund) Proof-Of-Concept] of the University of Antwerp, project #FFI150002, by a DeHousse mandaat of the University of Antwerp (Z. Vermeulen) and by an Amidila Postdoctoral fellowship from Erasmus Mundus (S. Boimvaser).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.V. and G.W.D.K. conception and design of research; L.V., Z.V., Z.Z.L., and S.B. performed experiments; L.V. analyzed data; L.V., A.P., and G.W.D.K. interpreted results of experiments; L.V. prepared figures; L.V., V.F.S., and G.W.D.K. drafted manuscript; L.V., A.P., V.F.S., and G.W.D.K. edited and revised manuscript; L.V., A.P., V.F.S., and G.W.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Katrien Demol and Rita Van Den Bossche for excellent technical support.
REFERENCES
- 1.Atkins RC. The epidemiology of chronic kidney disease. Kidney Int Suppl S14–S18, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 293: H1883–H1891, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138: 257–270, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Boger CA, Sedor JR. GWAS of diabetic nephropathy: is the GENIE out of the bottle? PLoS Genet 8: e1002989, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol 190: 1–65, 2007. [PubMed] [Google Scholar]
- 6.Brosius FC III, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T. Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL, Kudva YC. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim 45: 131–140, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillmann WH. Diabetes mellitus induces changes in cardiac myosin of the rat. Diabetes 29: 579–582, 1980. [DOI] [PubMed] [Google Scholar]
- 9.Ennequin G, Boisseau N, Caillaud K, Chavanelle V, Etienne M, Li X, Sirvent P. Neuregulin 1 Improves Glucose Tolerance in db/db Mice. PLoS One 10: e0130568, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erhardt L, MacLean A, Ilgenfritz J, Gelperin K, Blumenthal M. Fosinopril attenuates clinical deterioration and improves exercise tolerance in patients with heart failure. Fosinopril Efficacy/Safety Trial (FEST) Study Group. Eur Heart J 16: 1892–1899, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Galindo CL, Kasasbeh E, Murphy A, Ryzhov S, Lenihan S, Ahmad FA, Williams P, Nunnally A, Adcock J, Song Y, Harrell FE, Tran TL, Parry TJ, Iaci J, Ganguly A, Feoktistov I, Stephenson MK, Caggiano AO, Sawyer DB, Cleator JH. Anti-remodeling and anti-fibrotic effects of the neuregulin-1beta glial growth factor 2 in a large animal model of heart failure. J Am Heart Assoc 3: e000773, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y, Li X, Zhou M. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol 55: 1907–1914, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378: 390–394, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Gui C, Zhu L, Hu M, Lei L, Long Q. Neuregulin-1/ErbB signaling is impaired in the rat model of diabetic cardiomyopathy. Cardiovasc Pathol 21: 414–420, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Heeg JE, de Jong PE, van der Hem GK, de ZD. Efficacy and variability of the antiproteinuric effect of ACE inhibition by lisinopril. Kidney Int 36: 272–279, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Himmelfarb J, Tuttle KR. New therapies for diabetic kidney disease. N Engl J Med 369: 2549–2550, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, Amor R, Liu X, Li XY, Zhou MD, Graham RM, Macdonald PS. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail 13: 83–92, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Katz DH, Burns JA, Aguilar FG, Beussink L, Shah SJ. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail 2: 586–596, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378: 394–398, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation 116: 954–960, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Lemmens K, Segers VF, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem 281: 19469–19477, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Xiao J, Li Y, Zhang J, Zeng M. Gene transfer of human neuregulin-1 attenuates ventricular remodeling in diabetic cardiomyopathy rats. Exp Ther Med 6: 1105–1112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Zheng Z, Wei Y, Wang M, Peng J, Kang T, Huang X, Xiao J, Li Y, Li Z. Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc Diabetol 10: 69, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Gu XH, Duan JC, Zeng L, Li Y, Wang L. [Effects of recombined human neuregulin on the contractibility of cardiac muscles of rhesus monkeys with pacing-induced heart failure]. Sichuan Da Xue Xue Bao Yi Xue Ban 38: 105–108, 2007. [PubMed] [Google Scholar]
- 25.Litwin SE, Raya TE, Anderson PG, Daugherty S, Goldman S. Abnormal cardiac function in the streptozotocin-diabetic rat. Changes in active and passive properties of the left ventricle. J Clin Invest 86: 481–488, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Gu X, Li Z, Li X, Li H, Chang J, Chen P, Jin J, Xi B, Chen D, Lai D, Graham RM, Zhou M. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol 48: 1438–1447, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature 378: 386–390, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Mezzano S, Droguett A, Burgos ME, Ardiles LG, Flores CA, Aros CA, Caorsi I, Vio CP, Ruiz-Ortega M, Egido J. Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int Suppl S64–S70, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 44, Suppl 2: S14–S21, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA. Myosin heavy chain gene expression in human heart failure. J Clin Invest 100: 2362–2370, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353: 2643–2653, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation 93: 1905–1912, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res 111: 1376–1385, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odiete O, Konik EA, Sawyer DB, Hill MF. Type 1 diabetes mellitus abrogates compensatory augmentation of myocardial neuregulin-1beta/ErbB in response to myocardial infarction resulting in worsening heart failure. Cardiovasc Diabetol 12: 52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC III. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 57: 1439–1445, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangan GK, Tesch GH. Quantification of renal pathology by image analysis. Nephrology (Carlton) 12: 553–558, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Retnakaran R, Zinman B. Type 1 diabetes, hyperglycaemia, and the heart. Lancet 371: 1790–1799, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Sandholm N, Salem RM, McKnight AJ, Brennan EP, Forsblom C, Isakova T, McKay GJ, Williams WW, Sadlier DM, Makinen VP, Swan EJ, Palmer C, Boright AP, Ahlqvist E, Deshmukh HA, Keller BJ, Huang H, Ahola AJ, Fagerholm E, Gordin D, Harjutsalo V, He B, Heikkila O, Hietala K, Kyto J, Lahermo P, Lehto M, Lithovius R, Osterholm AM, Parkkonen M, Pitkaniemi J, Rosengard-Barlund M, Saraheimo M, Sarti C, Soderlund J, Soro-Paavonen A, Syreeni A, Thorn LM, Tikkanen H, Tolonen N, Tryggvason K, Tuomilehto J, Waden J, Gill GV, Prior S, Guiducci C, Mirel DB, Taylor A, Hosseini SM, Parving HH, Rossing P, Tarnow L, Ladenvall C, Alhenc-Gelas F, Lefebvre P, Rigalleau V, Roussel R, Tregouet DA, Maestroni A, Maestroni S, Falhammar H, Gu T, Mollsten A, Cimponeriu D, Ioana M, Mota M, Mota E, Serafinceanu C, Stavarachi M, Hanson RL, Nelson RG, Kretzler M, Colhoun HM, Panduru NM, Gu HF, Brismar K, Zerbini G, Hadjadj S, Marre M, Groop L, Lajer M, Bull SB, Waggott D, Paterson AD, Savage DA, Bain SC, Martin F, Hirschhorn JN, Godson C, Florez JC, Groop PH, Maxwell AP. New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet 8: e1002921, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 57: 3297–3306, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasquez EC, Peotta VA, Gava AL, Pereira TM, Meyrelles SS. Cardiac and vascular phenotypes in the apolipoprotein E-deficient mouse. J Biomed Sci 19: 22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walkin L, Herrick SE, Summers A, Brenchley PE, Hoff CM, Korstanje R, Margetts PJ. The role of mouse strain differences in the susceptibility to fibrosis: a systematic review. Fibrogen Tissue Repair 6: 18, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen M, Segerer S, Dantas M, Brown PA, Hudkins KL, Goodpaster T, Kirk E, LeBoeuf RC, Alpers CE. Renal injury in apolipoprotein E-deficient mice. Lab Invest 82: 999–1006, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Westermann D, Rutschow S, Jager S, Linderer A, Anker S, Riad A, Unger T, Schultheiss HP, Pauschinger M, Tschope C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes 56: 641–646, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Xu G, Watanabe T, Iso Y, Koba S, Sakai T, Nagashima M, Arita S, Hongo S, Ota H, Kobayashi Y, Miyazaki A, Hirano T. Preventive effects of heregulin-beta1 on macrophage foam cell formation and atherosclerosis. Circ Res 105: 500–510, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res 315: 602–610, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng F, Miyazawa T, Kloepfer LA, Harris RC. Deletion of ErbB4 accelerates polycystic kidney disease progression in cpk mice. Kidney Int 86: 538–547, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziyadeh FN, Han DC, Cohen JA, Guo J, Cohen MP. Glycated albumin stimulates fibronectin gene expression in glomerular mesangial cells: involvement of the transforming growth factor-beta system. Kidney Int 53: 631–638, 1998. [DOI] [PubMed] [Google Scholar]