Abstract
Inflammasomes activate caspase-1 to produce interleukin (IL)-1β. Activation of the NLRP3 inflammasome is involved in various renal pathological conditions. It remains unknown whether the NLRP3 inflammasome activation participates in the abnormal renal response to high-salt (HS) diet in Dahl salt-sensitive (S) rats. In addition, our lab recently showed that transplantation of mesenchymal stem cells (MSCs) attenuated HS-induced inflammation in the renal medulla in Dahl S rat. However, it is unclear whether the anti-inflammatory action of MSCs is associated with inhibition of the NLRP3 inflammasome. The present study determined the response of the NLRP3 inflammasome to HS intake and the effect of MSC transplantation on the NLRP3 inflammasome in the renal medulla in Dahl S rats. Immunostaining showed that the inflammasome components NLRP3, ASC, and caspase-1 were mainly present in distal tubules and collecting ducts. Interestingly, the renal medullary levels of these inflammasome components were remarkably increased after a HS diet in Dahl S rats, while remaining unchanged in normal rats. This HS-induced activation of the NLRP3 inflammasome was significantly blocked by MSC transplantation into the renal medulla in Dahl S rats. Furthermore, infusion of a caspase-1 inhibitor into the renal medulla significantly attenuated HS-induced hypertension in Dahl S rats. These data suggest that HS-induced activation of the NLRP3 inflammasome may contribute to renal medullary dysfunction in Dahl S rats and that inhibition of inflammasome activation may be one of the mechanisms for the anti-inflammatory and anti-hypertensive effects of stem cells in the renal medulla in Dahl S rats.
Keywords: interleukin-1β, caspase-1, immunoprecipitation, hypertension
inflammasomes are cytosolic machineries consisting of NLRP (NOD-like receptor family, pyrin domain containing) and ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain). Inflammasomes recruit and activate caspase-1. The activated caspase-1 then cleaves prointerleukin (IL)-1β to produce mature IL-1β (28, 31). Thus, inflammasomes control the production of proinflammatory factor IL-1β. Inflammasome activation has been shown to participate in a variety of conditions associated with inflammation (19, 20, 32). Renal inflammation plays a pivotal role in salt-sensitive hypertension (24, 29). However, it remains unknown whether renal inflammasomes are activated in salt-sensitive hypertension. Different inflammasomes containing different NLRP family proteins, such as NLRP1, NLRP2, NLRP3, AIM2, and NLRC4, have been identified. These different inflammasomes are activated in response to different stimuli and mainly detect viral and bacterial pathogens (5, 27). For example, the NLRP1 inflammasome is activated by directly binding to bacterial ligands, such as anthrax lethal toxin and muramyl dipeptide; the AIM2 inflammasome recognizes foreign cytoplasmic double-stranded DNA and is activated in response to viruses; the NLRC4 inflammasome senses Gram-negative bacteria possessing type III or IV secretion systems. The NLRP3 inflammasome, however, is also stimulated by different endogenous/host-derived factors associated with damage, such as ATP, uric acid crystals, and amyloid polypeptide, in addition to recognizing exogenous danger signals (5, 27). The NLRP3 inflammasome has been well-characterized and implicated in the development of chronic diseases (27). The present study therefore determined the expression and function of the NLRP3 inflammasome in response to high-salt diet in the kidneys in Dahl salt-sensitive (S) rats, a common model of salt-sensitive hypertension.
In addition, it has been well-documented that stem cells possess immunomodulatory and anti-inflammatory functions (21, 33). However, whether stem cells regulate the function of inflammasomes is not clear. Our lab recently demonstrated that transplantation of mesenchymal stem cells (MSCs) into the renal medulla significantly attenuates the high salt-induced hypertension in Dahl S rats (17). This attenuation of hypertension is associated with the inhibition of high salt-induced increase of inflammatory factors and with reduced infiltration of immune cells in the renal medulla (17). We therefore hypothesized that the NLRP3 inflammasome is activated in response to high-salt intake and that transplantation of MSCs inhibits high salt-induced activation of the NLRP3 inflammasome in the renal medulla in Dahl S rats. The present study first detected the distributions and levels of the NLRP3 inflammasome components in the kidneys in response to the high-salt challenge and then determined the effect of renal medullary transplantation of MSCs on the expression and function of the NLRP3 inflammasome in the kidneys in Dahl S rats. Our results demonstrated for the first time that the components of the NLRP3 inflammasome were remarkably increased in response to high-salt intake, that high salt-induced activation of the NLRP3 inflammasome was blocked by MSC transplantation in the renal medulla in Dahl S rats, and that infusion of a caspase-1 inhibitor into the renal medulla attenuated salt-sensitive hypertension in Dahl S rats.
MATERIALS AND METHODS
Animals.
Experiments used male Dahl S and SS-13BN rats (Charles River) weighing 250 to 350 g. Animal procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University. SS-13BN rats were chosen because it is one of the best control rat strains for the Dahl S rat (9). SS-13BN is a consomic subcolony of the Dahl S rat, in which chromosome 13 is substituted from that of the Brown Norway (BN) rat. The genotype difference is 1.95% between Dahl S rat and SS-13BN rat. This genotype difference is much smaller than those between the Dahl S rat and other commonly used “control” rat strains: Dahl R 30%, Sprague-Dawley 52%, ACI 57%, and BN 77% (9). Animals were kept on a low-salt diet (0.4% NaCl; Dyets). During experiments, some of the rats were fed with a high-salt diet (8% NaCl; Dyets) as indicated in results.
Immunohistochemistry of NLRP3, ASC, and caspase-1 in the kidneys.
The kidney was fixed in 10% neutral buffered formalin, paraffin-embedded, and cut into 4-μm sections. Immunostaining was performed as we described before (37, 44), using antibodies against rat NLRP3 (Novus Biological), ASC (Santa Cruz), and caspase-1 (BioLegend).
Preparation of tissue homogenate and Western blot analyses for protein levels of NLRP3, ASC, caspase-1, and monocyte chemoattractant protein-1 in the renal medulla.
Renal medullary tissue homogenates were performed as described previously (46). Primary antibodies used were as above with addition of rabbit polyclonal anti-monocyte chemoattractant protein (MCP)-1 (Abcam). The intensities of the blots were determined using an imaging analysis program (ImageJ, free download from http://rsbweb.nih.gov/ij/).
Confocal microscopic detection of inflammasome protein complexes.
Double immunostaining was used to detect colocalization of the inflammasome components in the kidneys, which indicates the formation of the inflammasome complex (3, 39, 42). Paraffin-embedded kidney tissue slides were incubated with rabbit anti-ASC (1:50) and mouse anti-caspase-1 (1:100), followed by being incubated with either Alexa-488 or Alexa-555-labeled secondary antibodies, and then examined with a confocal laser scanning microscope (Fluoview FV1000, Olympus, Japan). As previously described (4, 42, 47), captured images were analyzed and the correlation coefficients were calculated using a computer program Image Pro Plus (Media Cybernetics, Bethesda, MD).
Co-immunoprecipitation of ASC and caspase-1.
Co-immunoprecipitation (IP) was performed as we described before (3). In brief, the renal medullary proteins were mixed with antibody against ASC and followed by addition of Protein-A beads. The beads were then collected and subject to Western blot analysis with anti-caspase-1 antibodies.
Preparation of rat mesenchymal stem cells and rat renal medullary interstitial cells.
Rat mesenchymal stem cells (MSCs) were provided free by Texas A&M Health Science and cultured as per the instructions. Rat renal medullary interstitial cells (RMICs) were isolated from Sprague-Dawley rats as described previously (18, 36) and cultured the same as MSCs. In total, 5 × 106 cells in 600 μl of 0.9% saline were used as described before (17).
Transplantation of MSCs or RMICs into renal medulla.
Cell suspensions were prepared as above and infused into the renal medulla of the remaining left kidney in uninephrectomized Dahl S rats as we described before (17). RMICs were used in control animals. Animal groups included RMICs + low-salt diet (Ctrl+LS), RMICs + high-salt diet (Ctrl+HS), and MSC+HS.
Chronic renal medullary infusion of caspase-1 inhibitor.
The rats were anesthetized with 2% isoflurane and uninephrectomized. After 1-wk recovery, the rats were implanted with medullary interstitial catheters (tapered tip, 4–5 mm) into the remaining left kidneys. The catheter was made with several circular “pig-tail” bends to prevent the catheter from being pulled out of the kidney during the movement of the rat. The catheter was anchored into place on the kidney surface with Vetbond Tissue Adhesive (3M) and a small piece of fat tissue. The catheter was then tunneled to the back of neck and connected to an osmotic pump (ALZET, model 2ML2) implanted subcutaneously. The osmotic pump contained a caspase-1 inhibitor Ac-YVAD-cmk (Sigma) and the infusion dose was 125 ng/h after a 300-ng/rat bonus injection (15, 16). This technique has been used successfully for chronic infusion into the kidneys in previous studies, including ours (25, 30, 40, 41, 45). At the end of the experiment, kidneys were removed and rapidly dissected into the renal cortex and medulla and then frozen in liquid N2. The precise location of interstitial infusion catheter was determined when the kidney tissue was dissected. No solution remaining in the osmotic pump was also checked and confirmed at the end.
Chronic monitoring of arterial blood pressure in conscious rats.
Mean arterial pressures (MAP) were recorded daily for 3 h using a telemetry system (Data Sciences International) as we described previously (43, 45). After baseline MAP were recorded for 2 days when the animals remained on a low-salt diet, a high-salt diet was given to some rats and the MAP was recorded for an additional 12 days. Animal groups included the following: vehicle + low-salt diet (LS), vehicle + high-salt diet (HS), and caspase-1 inhibitor + HS. At the end of experiment, renal tissues were collected for the assays of caspase-1 activities, IL-1β levels, and MCP-1 levels.
Fluorometric assay of caspase-1 activity and ELISA analysis of IL-1β level in renal medullary tissues.
Caspase-1 activity was measured using a fluorometric assay kit (Enzo Life Sciences, Farmingdale, NY). In brief, the tissue was homogenized in a lysis buffer and a fluorogenic substrate (Z-YVAD-AFC) for caspase-1 incubated with tissue homogenate; the resulting fluorescence, which represents the caspase-1 activity, was quantified using a fluorescence plate reader. For the measurement of IL-1β level, the tissues were homogenized in ice-cold sucrose buffer (pH 7.2) containing (in mmol/l) 20 Tris·HCl, 250 sucrose, and 2 μg/ml protease inhibitor cocktail. After centrifugation of the homogenate at 10,000 g for 10 min at 4°C, the supernatant containing 50 μg protein was subjected for IL-1β assay using an ELISA kit (R&D System, Minneapolis, MN).
Statistics.
Data are presented as means ± SE. The significance of differences in mean values within and between multiple groups was evaluated using an ANOVA followed by a Duncan's multiple range test. Student's t-test was used to evaluate statistical significance of differences between two groups. P < 0.05 was considered statistically significant.
RESULTS
Localization of inflammasome components NLRP3, ASC, and caspoase-1 in the kidney by immunohistochemistry.
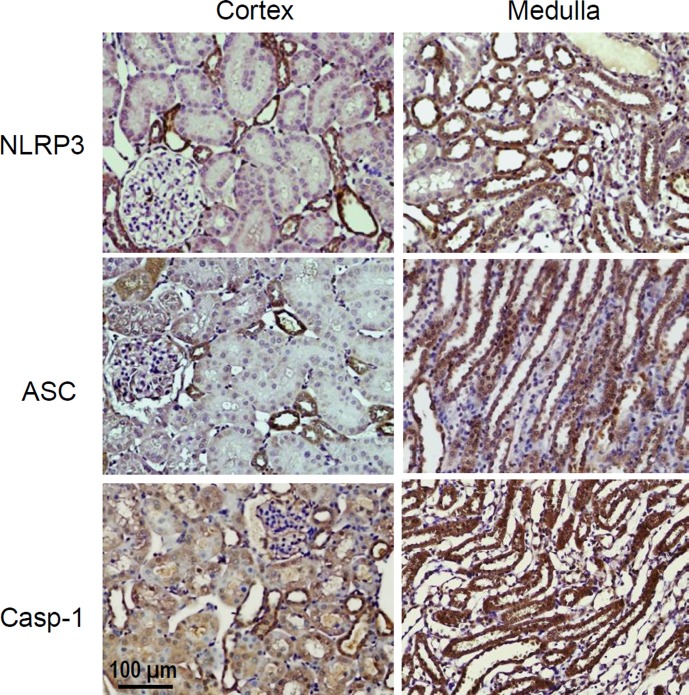
NLRP3, ASC, and caspase-1 were detected in all kidney regions including the cortex and medulla. The immunostaining patterns of these inflammasome components were similar and mainly located in distal tubules and collecting ducts with much stronger staining in the medullary area. Weak staining was observed in proximal tubules and glomeruli. We focused our study on the medullary tissues (Fig. 1).
Fig. 1.
Immunostaining of the NLRP3 inflammasome components NLRP3, ASC, and caspase-1 in the kidneys. Representative photomicrographs from 4 SS-13 Brown Norway (BN) rats. Brown color indicates positive staining.
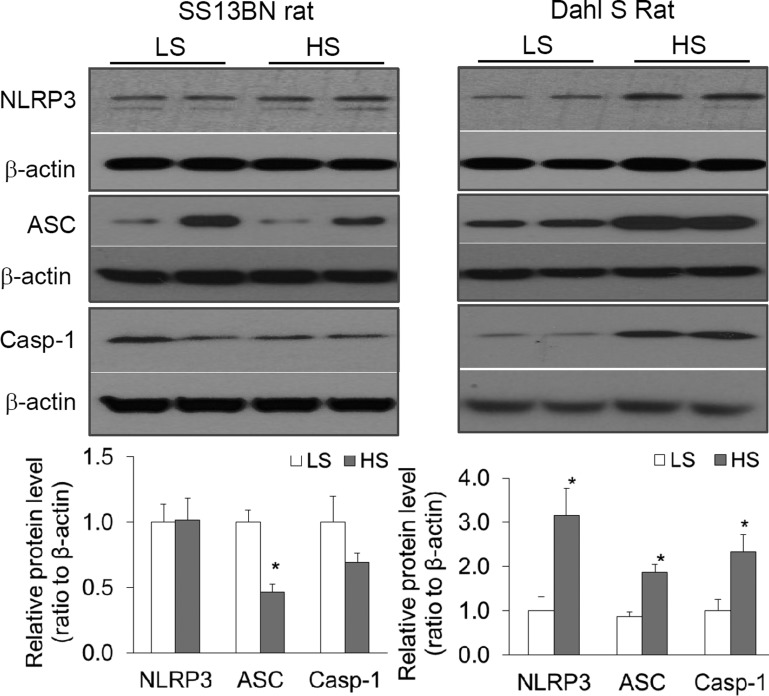
Effects of high-salt intake on the levels of inflammasome components in the renal medulla: a comparison between SS-13BN and Dahl S rats.
Animals were treated with a high-salt diet for 2 wk and the protein levels of the inflammasome components NLRP3, ASC, and caspase-1 in the renal medulla were analyzed by Western blot. As shown in Fig. 2, in SS-13BN rats, the levels of NLRP3 and caspase-1 were similar between animals treated with a low- or a high-salt diet and the levels of ASC were lower in high salt-treated animals than in low salt-treated animals. However, in Dahl S rats, the levels of NLRP3, ASC, and caspase-1 were much higher in high salt-treated animals than in low salt-treated animals (Fig. 2). These data suggest that high-salt challenge significantly activates the inflammasome in the renal medulla in Dahl S, but not in SS-13BN rats.
Fig. 2.
Effects of high-salt intake on the levels of the NLRP3 inflammasome components in the renal medulla: a comparison between SS-13BN and Dahl salt-sensitive (S) rats. Top: representative gel documents. Bottom: summarized band intensities normalized to NLRP3 level from rats fed with a low-salt diet (LS). HS, high-salt diet. *P < 0.05 vs. LS (n = 5–6).
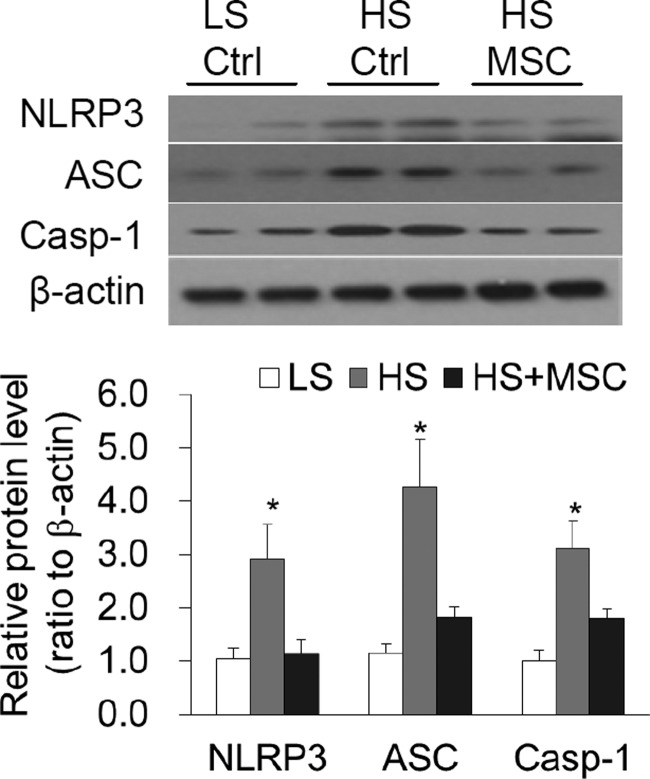
Effect of MSC transplantation on the levels of inflammasome components in the renal medulla in Dahl S rats.
Consistent with the results in Fig. 2, the levels of NLRP3, ASC, and caspase-1 in high salt-treated rats were significantly higher than those in low salt-treated rats (Fig. 3). However, the levels of these proteins in high-salt plus MSC-treated rats were significantly lower than those in high-salt plus control cell-treated rats (Fig. 3). These data demonstrate that high salt-induced activation of the NLRP3 inflammasome is inhibited by MSC transplantation in the renal medulla in Dahl S rats.
Fig. 3.
Effects of renal medullary transplantation of mesenchymal stem cells (MSCs) on the levels of the NLRP3 inflammasome components NLRP3, ASC, and caspase-1 in Dahl S rats. Top: representative gel documents. Bottom: summarized band intensities normalized to the level from rats fed with a LS diet. Ctrl, control cells. *P < 0.05 vs. other groups (n = 5–6).
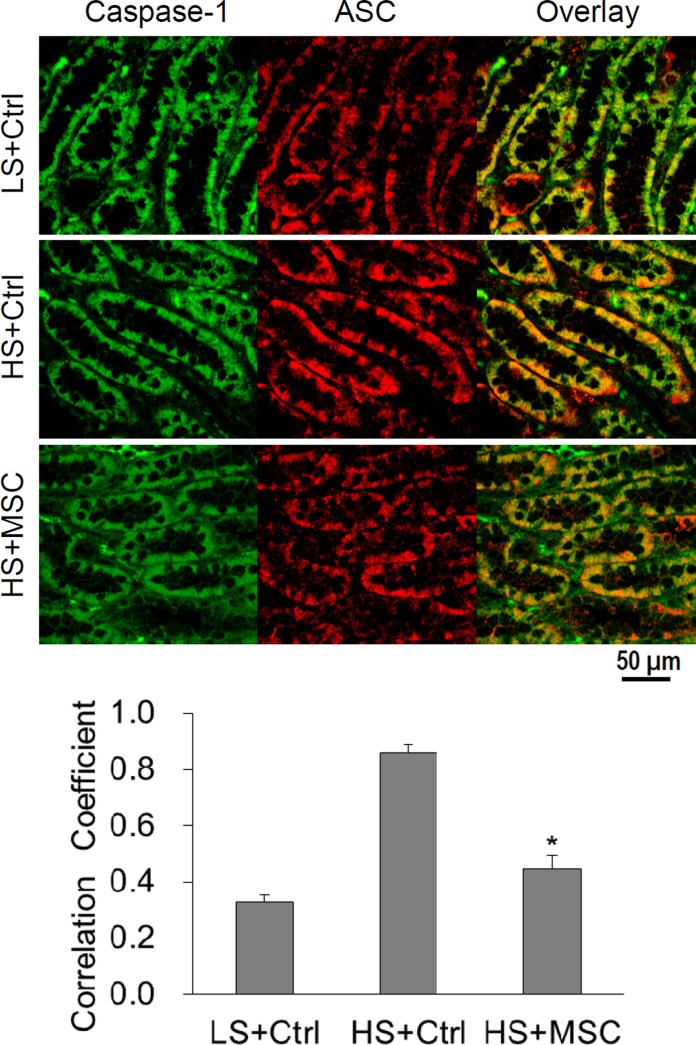
Effect of MSC transplantation on the formation of inflammasome complex in the renal medulla in Dahl S rats.
Double immunostaining of ASC and capase-1 in the renal medulla showed that the colocalization of these two proteins was enhanced as indicated by the significant stronger yellow staining and higher correlation coefficient in the overlaid images in high salt-treated rats (Fig. 4). However, the yellow staining was significantly weaker, and the correlation coefficient lower, in the overlaid images in high-salt plus MSC-treated rats than in high-salt plus RMIC-treated rats (Fig. 4). These results further suggest that high-salt challenge stimulates the aggregation of inflammasome components and the formation of the inflammasome complex, whereas MSC treatment inhibits the high salt-induced activation of the inflammasome in the renal medulla in Dahl S rats.
Fig. 4.
Confocal imaging for the colocalization of ASC and caspase-1 immunostainings in the renal medulla of Dahl S rats. Top: representative photomicrographs showing the immunostaining of caspase-1 (green) and ASC (red) as well as the overlaid images. Yellow color in the overlaid images represents the colocalization of caspase-1 and ASC. Bottom: summarized data showing the colocalization coefficient of caspase-1 and ASC immunostainings analyzed using a computer software Image-Pro Plus. *P < 0.05 vs. other groups, n = 4.
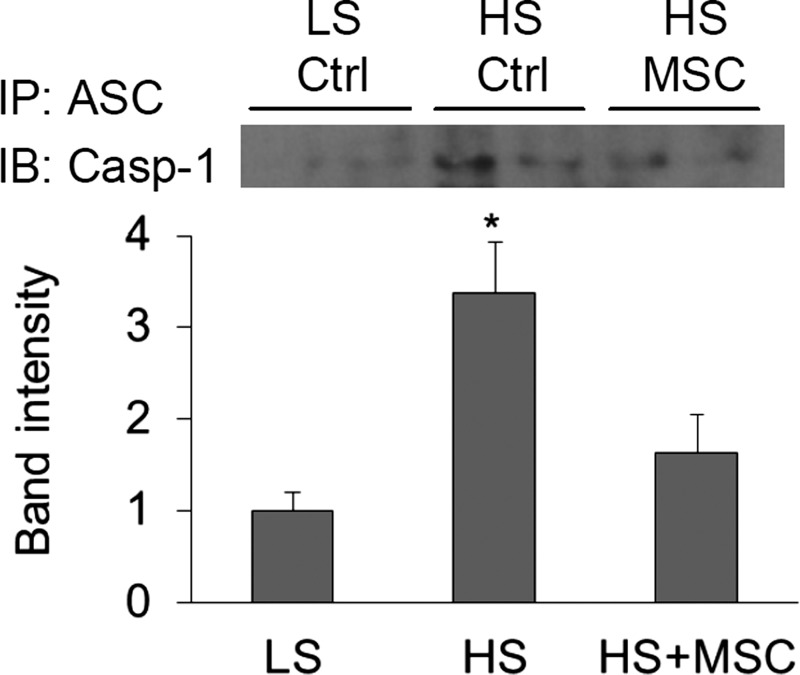
Meanwhile, Co-immunoprecipitation (Co-IP) using ASC antibody produced significantly stronger bands of caspase-1 in high salt-treated rats and weaker bands in high-salt plus MSC-treated rats (Fig. 5). These Co-IP results suggest an increased binding of ASC with caspase-1 in the renal medulla after high-salt challenge. The increased colocalization and binding of ASC with caspase-1 indicated that high-salt intake enhanced the formation of the inflammasome complex, which was inhibited by MSC treatment in the renal medulla in Dahl S rats.
Fig. 5.
Effects of renal medullary transplantation of MSCs on the coimmunoprecipitation of ASC and caspase-1 in the renal medulla in Dahl S rats. Top: representative gel documents. Bottom: summarized band intensities normalized to the level from rats fed with a LS diet. Immunoprecipitation (IP) with anti-ASC antibodies and immunoblotting (IB) with anti-coaspase-1 antibodies. *P < 0.05 vs. other groups (n = 5).
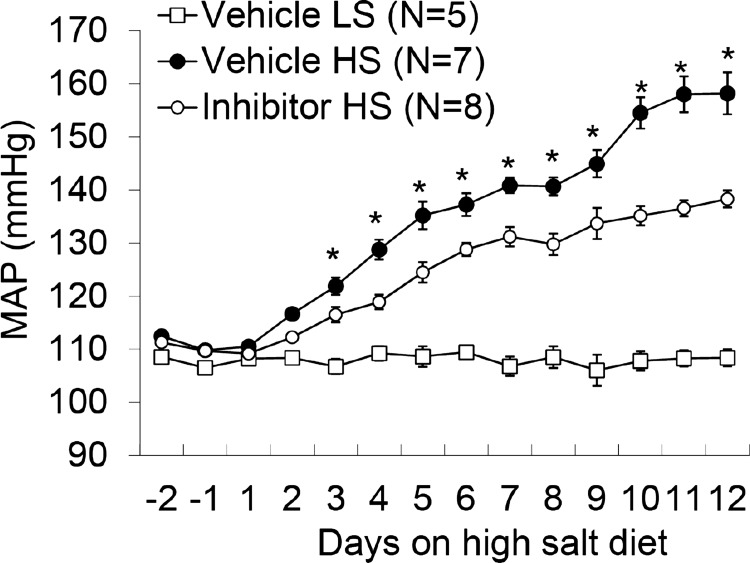
Effect of infusion of caspase-1 inhibitor into the renal medulla on salt-sensitive hypertension in Dahl S rats.
MAP were much higher in Vehicle + HS rats than those in Vehicle + LS rats. However, the MAP in caspase-1 inhibitor + HS rats were significantly lower than those in Vehicle + HS rats (Fig. 6). These results demonstrated that inhibition of caspase-1 in the renal medulla attenuated the salt-sensitive hypertension in Dahl S rats.
Fig. 6.
Effects of renal medullary infusion of caspase-1 inhibitor on mean arterial pressure (MAP) in Dahl S rats. *P < 0.05 vs. others.
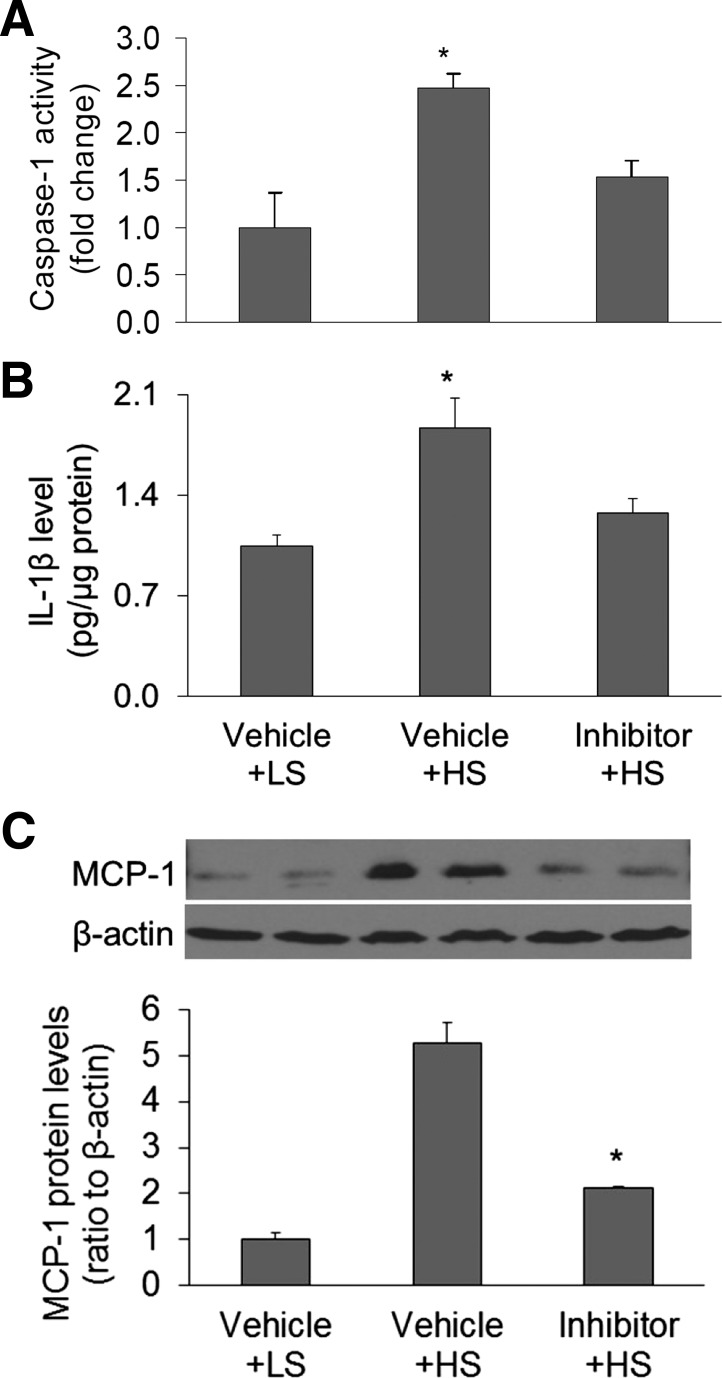
Effect of infusion of caspase-1 inhibitor on the activity of caspase-1 and levels of IL-1β and MCP-1 in the renal medulla.
The activities of caspase-1 were higher in Vehicle + HS rats than in Vehicle + LS rats, indicating that high-salt intake enhances the activity of inflammasomes. The activities of caspase-1 were significantly lower in inhibitor + HS rats, indicating a successful inhibition of the caspase-1 activity in the renal medulla (Fig. 7A). The levels of IL-1β showed the same pattern as the activity of caspase-1: both were increased in HS-treated rats and reduced in caspase-1 inhibitor + HS-treated rats, suggesting a functional inhibition of the caspase-1 by the inhibitor (Fig. 7B). The levels of an additional inflammatory factor MCP-1, which can be induced by IL-1β (6), were also measured by Western blot. The levels of MCP-1 were higher in HS-treated rats when compared with LS-treated rats. The MCP-1 levels were lower in caspase-1 inhibitor + HS-treated rats when compared with HS-treated rats (Fig. 7C), further suggesting that inhibition of caspase-1 activity reduces the inflammatory response to HS intake in the renal medulla of Dahl S rats.
Fig. 7.
Effect of renal medullary infusion of caspase-1 inhibitor on the caspase-1 activity, levels of IL-1β and MCP-1 in the renal medulla in Dahl S rats. A: caspase-1 activity by fluorometric assay. B: IL-1β levels by ELISA assay. C: MCP-1 levels by Western blot analysis. *P < 0.05 vs. other groups (n = 5–8).
DISCUSSION
The results from the present study demonstrated that inflammasome components NLRP3, ASC, and caspase-1 were mainly expressed in the distal tubules and collecting ducts in the kidneys; the level and assembly of these inflammasome components were significantly increased in response to high-salt intake in the renal medulla in Dahl S rats, but not in normotensive rats; transplantation of MSCs inhibited high salt-induced increase in these inflammasome components and blocked the assembly of the NLRP3 inflammasome complex; and infusion of a caspase-1 inhibitor in the renal medulla attenuated salt-sensitive hypertension in Dahl S rats. These data for the first time suggest that high-salt challenge induces activation of the NLRP3 inflammasome and that transplantation of MSCs blocks the high salt-induced activation of the NLRP3 inflammasome, which may contribute to the anti-inflammatory and anti-hypertensive effects of stem cells in the renal medulla in Dahl S rats.
It has been demonstrated that renal inflammation plays a pivotal role in salt-sensitive hypertension (24, 29) and that activation of the NLRP3 inflammasome participates in a variety of conditions associated with inflammation (19, 20, 32). However, it is not clear whether the NLRP3 inflammasome is activated in the kidneys in salt-sensitive hypertension. We first detected the localization of the NLRP3 inflammasome in the kidney and found that the NLRP3 inflammasome components were mainly expressed in distal tubules and collecting ducts with strong immunostaining in the renal medulla and weak staining in cortex. It is well-known that the renal medulla plays an important role in the regulation of sodium excretion and long-term blood pressure regulation (8, 22). Dahl S rat is a widely used genetic model of human salt-sensitive hypertension. Renal medullary dysfunction has long been recognized as one of the major mechanisms for the development of hypertension in Dahl S rats (2, 22). High expression of the NLRP3 inflammasome components in the renal medulla may indicate a possible involvement of the NLPR3 inflammasome in the inflammatory response to high-salt intake in the renal medulla in this hypertension model. We then compared the levels of the NLRP3 inflammasome components after high-salt challenge in the renal medulla in normotensive and Dahl S rats. Our data demonstrated that high-salt intake activates the NLRP3 inflammasome in the renal medulla in Dahl S rats but not in normotensive rats, suggesting that the NLRP3 inflammasome activation may participate in the inflammatory response to high-salt challenge, in renal medullary dysfunction, and in salt-sensitive hypertension in this animal model.
Regarding the mechanisms by which high-salt challenge activates the NLRP3 inflammasome in the renal medulla in Dahl S rats, stem cell dysfunction might be one of the potential mechanisms accountable for it. Our previous study suggested that there is a defect in stem cells in the renal medulla and that correction of the stem cell deficiency inhibits the inflammatory response to high-salt challenge in the renal medulla and attenuates salt-sensitive hypertension in Dahl S rats (17). Therefore, normal stem cell behavior may preserve a well-maintained anti-inflammatory mechanism. In contrast, deficient stem cell function that impairs the stem cell-mediated anti-inflammatory mechanisms in the renal medulla may result in the incapacity to counterbalance the proinflammatory stimulation of high-salt challenge, consequently causing inflammation in the renal medulla in Dahl S rats. As IL-1β, a product of activated inflammasomes, has been shown to strikingly enhance the immune cell responses (1), activation of the NLRP3 inflammasome to produce proinflammatory factors may serve as an early step to initiate and amplify the inflammatory response. We therefore hypothesized that high salt-induced activation of the NLRP3 inflammasome observed in the present study was also associated with the stem cell defects in the renal medulla in Dahl S rats. If so, correction of stem cell deficiency would inhibit the high salt-induced activation of the NLRP3 inflammasome in this rat model.
Additionally, although it has been well-recognized that stem cells modulate immune response and execute anti-inflammatory function (21, 33), the detailed mechanism of the anti-inflammatory function by stem cells is not clear. Activation of the NLRP3 inflammasome has been shown to be the early initiative step leading to sterile inflammation (7, 12, 38). It is not clear whether the anti-inflammatory action of stem cells involves the inhibition of the NLRP3 inflammasome activation. We thus determined the effect of stem cell transplantation on the activation of the NLRP3 inflammasome, which will help to elucidate the mechanism of stem cell-mediated anti-inflammatory function. Indeed, our results showed that in MSC-treated Dahl S rats, high salt-induced increases in the levels of inflammasome components NLRP3, ASC, and caspase-1 were significantly inhibited, demonstrating that inhibition of the NLRP3 inflammasome may contribute to MSC-mediated anti-inflammatory functions.
Furthermore, our results from the experiments of coimmunostaining and coimmunoprecipitation showed that the aggregation/assembling of these inflammasome components was also activated by high-salt intake and that MSC treatment significantly reduced the high salt-induced aggregation/assembling of the NLRP3 inflammasome components. The finding that MSCs inhibited high salt-induced activation of the NLRP3 inflammasome in the present study was consistent with our previous results that IL-1β, a product of activated inflammasomes, was increased in response to high-salt challenge and that MSCs blocked the high salt-induced production of IL-1β in Dahl S rats (17). All these data suggest that there is an association between stem cell dysfunction and inflammasome activation in the renal medulla after high-salt intake and that correction of the stem cell defect by MSC transplantation reduces the formation of functional machinery of the NLRP3 inflammasome, which may be one of the mechanisms for stem cells to achieve anti-inflammatory function.
Our previous study showed that transplantation of MSCs into the renal medulla attenuated high salt-induced hypertension in Dahl S rats. Our current study showed that transplantation of MSCs inhibited the activation of the NLRP3 inflammasome in the renal medulla in Dahl S rats. As the major function of the NLRP3 inflammasome is to activate caspase-1, we infused a caspase-1 inhibitor into the renal medulla to determine whether inhibition of caspase-1 would achieve a similar anti-hypertensive effect as MSCs would in Dahl S rats. The results from this experiment using caspase-1 inhibitor would further clarify whether MSC-induced inhibition of the NLRP3 inflammasome activation mediates the anti-hypertensive effect of MSCs. Our results for the first time showed that inhibition of caspase-1 in the renal medulla significantly attenuated salt-sensitive hypertension in Dahl S rats. Meanwhile, a caspase-1 inhibitor reduced HS-induced increases of both IL-1β and MCP-1, which was consistent with the fact that IL-1β induces MCP-1 (6). Our previous study showed that stem cell therapy also inhibited high salt-induced increases in both IL-1β and MCP-1 in the renal medulla in Dahl S rats (17). Taken together, these results indicate that stem cell therapy may share similar anti-inflammatory mechanisms to those by the caspase-1 inhibitor, which further supports the notion that inhibition of the NLRP3 inflammasome contributes to the anti-inflammatory, and thereby anti-hypertensive, functions of MSCs.
MCP-1 is one of the key chemokines that recruit monocyte infiltration into inflamed tissues and an important inflammatory mediator (11). Increased MCP-1 levels in the kidneys have been associated with renal inflammation and hypertension (10, 13, 14, 34). The MCP-1 levels in renal tubular cells have also been closely associated with tubulointerstitial inflammation (14, 26). It has been well-recognized that renal infiltration with immune cells significantly contributes to salt-sensitive hypertension (23, 29). Thus, high salt-induced activation of the NLRP3 inflammasome may increase the production of IL-1β and upregulate MCP-1 to attract immune cells into the renal medulla. This NLRP3 inflammasome-initiated inflammatory response is probably one of the mechanisms for salt-sensitive hypertension in Dahl S rats. The abundant expression of the NLRP3 inflammasome in the renal medullary tubules is in line with the fact that renal tubular cells are a rich source of MCP-1 in tubulointerstitial inflammation (35). Inhibiting the activation of the NLRP3 inflammasome or caspase-1 to reduce the production of IL-1β and MCP-1 may consequently prevent the infiltration of immune cells into the renal medulla (17), preserving renal function via anti-inflammation, which could be one of the mechanisms for MSCs to attenuate high salt-induced hypertension in Dahl S rats.
It is concluded that high salt-induced activation of renal medullary inflammasome may contribute to the inflammatory response to high-salt intake in the renal medulla and that inhibition of inflammasome activation may be one of the mechanisms for the ant-inflammatory and anti-hypertensive effects of stem cells in the renal medulla in Dahl S rats. How MSCs inhibit the activation of the NLRP3 inflammasome after high-salt challenge requires further investigation.
GRANTS
This work was supported by National Institutes of Health Grants HL-89563 and HL-106042.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Q.Z., X.-X.L., W.W., J.H., P.-L.L., and N.L. conception and design of research; Q.Z., X.-X.L., W.W., J.H., and S.M.C. performed experiments; Q.Z., X.-X.L., W.W., J.H., S.M.C., and N.L. analyzed data; Q.Z., X.-X.L., W.W., J.H., P.-L.L., S.M.C., and N.L. interpreted results of experiments; Q.Z., X.-X.L., W.W., J.H., and S.M.C. prepared figures; Q.Z. drafted manuscript; Q.Z., X.-X.L., W.W., J.H., P.-L.L., S.M.C., and N.L. approved final version of manuscript; N.L. edited and revised manuscript.
REFERENCES
- 1.Ben-Sasson SZ, Wang K, Cohen J, Paul WE. IL-1beta strikingly enhances antigen-driven CD4 and CD8 T-cell responses. Cold Spring Harb Symp Quant Biol 78: 117–124, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom G, Evans RG. Mechanisms underlying the antihypertensive functions of the renal medulla. Acta Physiol Scand 181: 475–486, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Boini KM, Xia M, Abais JM, Li G, Pitzer AL, Gehr TW, Zhang Y, Li PL. Activation of inflammasomes in podocyte injury of mice on the high fat diet: effects of ASC gene deletion and silencing. Biochim Biophys Acta 1843: 836–845, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boini KM, Xia M, Xiong J, Li C, Payne LP, Li PL. Implication of CD38 gene in podocyte epithelial-to-mesenchymal transition and glomerular sclerosis. J Cell Mol Med 16: 1674–1685, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev 243: 174–190, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang MC, Tsai YL, Chang HH, Lee SY, Lee MS, Chang CW, Chan CP, Yeh CY, Cheng RH, Jeng JH. IL-1beta-induced MCP-1 expression and secretion of human dental pulp cells is related to TAK1, MEK/ERK, and PI3K/Akt signaling pathways. Arch Oral Biol 61: 16–22, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10: 826–837, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowley MD AW Jr, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension 25: 663–673, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Cowley AW., Jr Genomics and homeostasis. Am J Physiol Regul Integr Comp Physiol 284: R611–R627, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55: 99–108, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29: 313–326, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmarakby AA, Faulkner J, Posey SP, Sullivan JC. Induction of hemeoxygenase-1 attenuates the hypertension and renal inflammation in spontaneously hypertensive rats. Pharmacol Res 62: 400–407, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Fujii S, Zhang L, Kosaka H. Albuminuria, expression of nicotinamide adenine dinucleotide phosphate oxidase and monocyte chemoattractant protein-1 in the renal tubules of hypertensive Dahl salt-sensitive rats. Hypertens Res 30: 991–998, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur J Neurosci 26: 2795–2803, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci 22: 1751–1756, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Zhu Q, Xia M, Guo T, Wang Z, Li PL, Han WQ, Yi F, Li N. Transplantation of mesenchymal stem cells into the renal medulla attenuated salt-sensitive hypertension in Dahl S rat. J Mol Med 92: 1139–1145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li PL. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol 292: F207–F216, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Deroide N, Mallat Z. The role of the inflammasome in cardiovascular diseases. J Mol Med 92: 307–319, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Chan C. The role of inflammasome in Alzheimer's disease. Ageing Res Rev 15: 6–15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariani E, Facchini A. Clinical applications and biosafety of human adult mesenchymal stem cells. Curr Pharm Des 18: 1821–1845, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 284: R13–R27, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 307: F499–F508, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Moore AF, Heiderstadt NT, Huang E, Howell NL, Wang ZQ, Siragy HM, Carey RM. Selective inhibition of the renal angiotensin type 2 receptor increases blood pressure in conscious rats. Hypertension 37: 1285–1291, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Morii T, Fujita H, Narita T, Shimotomai T, Fujishima H, Yoshioka N, Imai H, Kakei M, Ito S. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications 17: 11–15, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res 8: 15–27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol 21: 10–16, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Iturbe B, Quiroz Y, Herrera-Acosta J, Johnson RJ, Pons HA. The role of immune cells infiltrating the kidney in the pathogenesis of salt-sensitive hypertension. J Hypertens Suppl 20: S9–S14, 2002. [PubMed] [Google Scholar]
- 30.Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension 47: 1131–1139, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Schroder K, Tschopp J. The inflammasomes. Cell 140: 821–832, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Skeldon AM, Faraj M, Saleh M. Caspases and inflammasomes in metabolic inflammation. Immunol Cell Biol 92: 304–313, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Souidi N, Stolk M, Seifert M. Ischemia-reperfusion injury: beneficial effects of mesenchymal stromal cells. Curr Opin Organ Transplant 18: 34–43, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Takai S, Jin D, Sakonjo H, Miyazaki M. Combination therapy with irbesartan and efonidipine for attenuation of proteinuria in Dahl salt-sensitive rats. Hypertens Res 33: 953–959, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Viedt C, Dechend R, Fei J, Hänsch GM, Kreuzer J, Orth SR. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-κB and activating protein-1. J Am Soc Nephrol 13: 1534–1547, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li PL, Li N. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int 79: 300–310, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, Li N. Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt intake to increase hypoxia inducible factor 1alpha levels in the renal medulla. Hypertension 55: 1129–1136, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases–did Warburg miss inflammation? Nat Immunol 13: 352–357, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia M, Conley SM, Li G, Li PL, Boini KM. Inhibition of hyperhomocysteinemia-induced inflammasome activation and glomerular sclerosis by NLRP3 gene deletion. Cell Physiol Biochem 34: 829–841, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada M, Katsuma S, Adachi T, Hirasawa A, Shiojima S, Kadowaki T, Okuno Y, Koshimizu TA, Fujii S, Sekiya Y, Miyamoto Y, Tamura M, Yumura W, Nihei H, Kobayashi M, Tsujimoto G. Inhibition of protein kinase CK2 prevents the progression of glomerulonephritis. Proc Natl Acad Sci USA 102: 7736–7741, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoneda M, Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Sasaki M, Katoh T, Watanabe T, Andrews PM, Jose PA, Felder RA. Differential effects of angiotensin II type-1 receptor antisense oligonucleotides on renal function in spontaneously hypertensive rats. Hypertension 46: 58–65, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Boini KM, Xia M, Abais JM, Li X, Liu Q, Li PL. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension 60: 154–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Q, Hu J, Han WQ, Zhang F, Li PL, Wang Z, Li N. Silencing of HIF prolyl-hydroxylase 2 gene in the renal medulla attenuates salt-sensitive hypertension in Dahl S rats. Am J Hypertens 27: 107–113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Q, Wang Z, Xia M, Li PL, Van Tassell BW, Abbate A, Dhaduk R, Li N. Silencing of hypoxia-inducible factor-1α gene attenuated angiotensin II-induced renal injury in Sprague-Dawley rats. Hypertension 58: 657–664, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Q, Wang Z, Xia M, Li PL, Zhang F, Li N. Overexpression of HIF-1alpha transgene in the renal medulla attenuated salt sensitive hypertension in Dahl S rats. Biochim Biophys Acta 1822: 936–941, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Q, Xia M, Wang Z, Li PL, Li N. A novel lipid natriuretic factor in the renal medulla: sphingosine-1-phosphate. Am J Physiol Renal Physiol 301: F35–F41, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zinchuk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem Cytochem 40: 101–111, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]