Abstract
Transforming growth factor-β (TGF-β) is generally considered as a central mediator of fibrotic diseases. Indeed, much focus has been placed on inhibiting TGF-β and its downstream targets as ideal therapeutic strategies. However, pharmacological blockade of TGF-β has not yet translated into successful therapy for humans, which may be due to pleiotropic effects of TGF-β signaling. Equally, TGF-β signaling as a protective response in kidney injury has been relatively underexplored. An emerging body of evidence from experimental kidney disease models indicates multifunctionality of TGF-β capable of inducing profibrotic and protective effects. This review discusses recent advances highlighting the diverse roles of TGF-β in promoting not only renal fibrosis but also protective responses of TGF-β signaling. We review, in particular, growing evidence that supports protective effects of TGF-β by mechanisms which include inhibiting inflammation and induction of autophagy. Additional detailed studies are required to fully understand the diverse mechanisms of TGF-β actions in renal fibrosis and inflammation that will likely direct toward effective antifibrotic therapies.
Keywords: TGF-β, Smad, BMP, kidney, fibrosis, autophagy, apoptosis, inflammation
transforming growth factor -β (TGF-β) is a multifunctional cytokine that is well recognized to regulate a broad spectrum of cellular processes such as growth, differentiation, apoptosis, wound repair, and the pathogenesis of fibrosis. It is a member of a superfamily of 38 cytokines that include TGF-β, bone morphogenetic proteins (BMP), growth differentiation factors, inhibins, and activins. TGF-β superfamily members, in particular, TGF-β and BMP, have been implicated to play important and diverse roles in chronic kidney disease (CKD) (71, 100). The hallmark of progressive CKD is excessive extracellular matrix (ECM) production leading to renal fibrosis, and TGF-β has been generally considered a potent profibrotic mediator in this process (17, 48).
Mammalian TGF-β includes three major isoforms that are encoded by distinct genes (TGF-β1, TGF-β2, and TGF-β3). The isoforms are synthesized as large precursor proteins (pro-TGF-β) forming dimeric complexes in the endoplasmic reticulum, and are subsequently cleaved near the carboxy terminus to yield mature 112-amino acid polypeptides, which share 60–80% conservation across the three TGF-β isoforms (99). The mature TGF-β dimer remains associated with the cleaved latency peptide portion of the precursor as an inactive latent complex, and dissociation from the complex results in biologically active TGF-β (76). Thus newly synthesized TGF-β bound to the latency-associated peptide (LAP) forming a small latent complex (SLC) is biologically inactive and cannot bind to its receptors. Through the formation of disulfide bonds, this complex loosely binds to a latent TGF-β binding protein (LTBP) to form a large latent complex (LLC). TGF-β is then secreted in a latent state and is stored in the ECM (2, 76). Activation of TGF-β involves release from the latent complex following exposure to a number of different factors, including integrins, proteases, metalloproteinases, reactive oxygen species (ROS), plasmin, and acid, that allow binding to its cell surface receptors for initiation of TGF-β signaling (25, 76, 81, 87).
It is well established that activation of TGF-β leads to pleiotropic cellular responses that are mediated by TGF-β signaling via interactions with TGF-β receptor type I (TβRI) and TGF-β receptor type II (TβRII) and subsequent activation of intracellular Smad- and non-Smad-dependent signaling pathways (13, 16, 57). In this review, we describe an emerging body of evidence from experimental kidney disease models demonstrating the multifunctional nature of TGF-β capable of inducing profibrotic and protective effects. We provide an overview of recent advances highlighting the diverse roles of TGF-β in promoting not only renal fibrosis but also protective responses, focusing on the growing evidence for protective effects of TGF-β by mechanisms which include inhibiting inflammation and induction of autophagy.
TGF-β Isoforms in the Kidney
Studies of human kidney specimens have confirmed that the three major isoforms TGF-β1, TGF-β2, and TGF-β3 are expressed in the kidney (33). While functional redundancy between the TGF-β isoforms has been long recognized, there is a growing body of evidence for the existence of nonredundant functions in inflammation and organ development (76). TGF-β1, the major focus of this review, is the predominant and best-characterized isoform, while TGF-β2 and TGF-β3 are less well known. Some clear differences among the isoforms have been noted. In the normal human adult kidney, glomerular expression of TGF-β2 and TGF-β3 is seen mainly in podocytes, whereas TGF-β1 is primarily detected in the tubules but not in the glomeruli (33). Interestingly, glomerular expression of TGF-β1, generally with TGF-β2 and TGF-β3, was detected in podocytes in kidney biopsy specimens from patients with proliferative glomerulonephritis and in mesangial cells in diabetic nephropathy and IgA nephropathy (33). Moreover, increased expression of TGF-β1 was associated with development of severe glomerulonephritis and glomerulosclerosis (33).
Biological actions of TGF-β isoforms are mediated by ligand binding to its receptors for the initiation of signaling. Both TGF-β1 and TGF-β3 bind directly with TβRII, whereas TGF-β2 requires the presence of a type III TGF-β receptor (TβRIII) for ligand binding to TβRII (99). Given the differences in the expression patterns and the mechanism of ligand binding, together with apparent nonoverlapping phenotypes of the three TGF-β isoform knockout mice, it is not unreasonable that some cellular responses may differ among the TGF-β isoforms.
All three TGF-β isoforms have been shown, in vitro, to induce ECM protein production in various renal cells, including glomerular mesangial cells, renal fibroblasts, and renal tubular epithelial cells (91, 99). While most studies have demonstrated similar profibrotic effects of the TGF-β isoforms, a number of recent studies have suggested that TGF-β2 and TGF-β3 can exert antifibrotic effects (72, 75, 96, 99). Studies in human podocytes demonstrated antifibrotic effects of TGF-β2 through upregulation of sphingosine kinase-1 (SK-1) activity, thereby suppressing profibrotic connective tissue growth factor (CTGF) expression (75). It is important to note that similar antifibrotic effects of TGF-β2 were not observed in other kidney cell types, as all three isoforms equally induced upregulation of CTGF mRNA in cultured mesangial cells and glomerular visceral epithelial cells (33). Moreover, TGF-β2 stimulated the expression of ECM proteins and induced EMT in tubular epithelial cells, whereas neutralizing antibody to TGF-β2 or repression of TGF-β2 expression inhibited renal fibrogenesis (91). Further investigations are warranted to clarify the seemingly opposite findings regarding the antifibrotic roles of TGF-β2 and TGF-β3, which carry important implications for therapeutic targeting strategy.
Activation of TGF-β1 Signaling
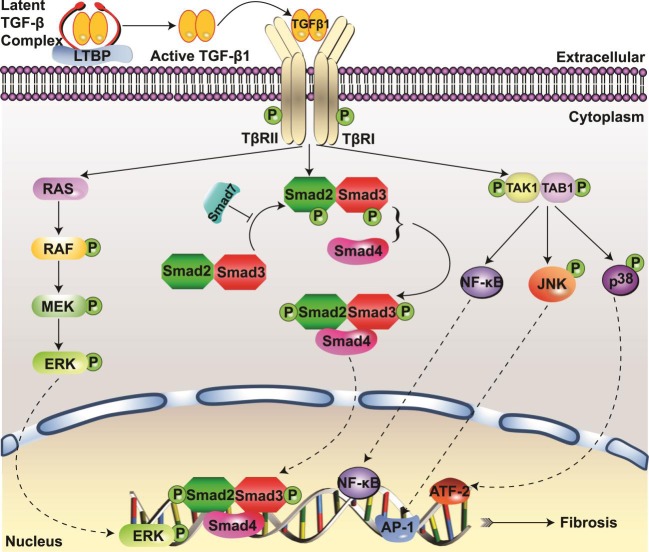
TGF-β1 signaling involves activation of complex intracellular networks to regulate pleiotropic biological functions. As depicted in Fig. 1, the active form of TGF-β1 transmits signals through type I and type II receptors, TβRI and TβRII. These cell surface receptors contain serine/threonine kinases that phosphorylate transcription factor proteins called Smads to initiate the canonical TGF-β signaling pathway. The TGF-β1 ligand assembles a heteromeric complex through binding to TβRII, which then phosphorylates the kinase domain of TβRI and leads to the downstream activation of the receptor-activated or regulatory Smads (R-Smads), namely, Smad2 and Smad3. The activated R-Smads form an oligomeric complex with the common mediator Smad4 and undergo nuclear translocation to regulate transcription of target genes (57). On the other hand, inhibitory Smads (Smad6 and Smad7) bind to activated TβRI and interfere with R-Smad activation. Smad7 has also been shown to negatively regulate TGF-β signaling through recruitment of E3 ubiquitin ligases Smurf1 and Smurf2, which target TGF-β receptors and Smad7 to undergo degradation via proteasomal and lysosomal pathways (22, 36).
Fig. 1.
Smad and non-Smad transforming growth factor-β1 (TGF-β1) signaling pathways. A: TGF-β1 activation occurs with the release from latent TGF-β binding protein (LTBP) complex by proteases. TGF-β1 signaling is initiated upon binding of active TGF-β1 with TGF-β receptor type II (TβRII) and forming the TβRI-TβRII heteromeric complex, leading to phosphorylation of Smad2/3, oligomerization with Smad4, and subsequent nuclear translocation to regulate the transcription of ECM genes. Smad7 serves as a negative regulator of TGF-β1 signaling. TGF-β1 also activates Smad-independent signaling such as the MAPK, mediated by the Ras-Raf-MEK-ERK pathway, and TGF-β-activated kinase 1 (TAK1), mediated by the TAK1-TAK1-binding protein 1 (TAB1) pathway. TGF-β1-induced TAK1 activation leads to signaling via MKK4-JNK and MKK3-p38 pathways and activation of transcription factors activator protein-1 (AP-1) and activating transcription factor 2 (ATF-2), respectively, and activation of NF-κB to mediate profibrotic responses.
Additionally, TGF-β1 signaling is mediated via noncanonical, non-Smad pathways (Fig. 1) including the MAPKs, mediated by Ras-Raf-activating MEK (also called MAPKK) and ERK (16, 68, 90), or the TGF-β-activated kinase 1 (TAK1) pathway, the upstream MAPK kinase kinase (MKKK) activating MKK3-p38 and MKK4-JNK (c-Jun N-terminal kinase) signaling cascades (13, 37, 38, 69). TGF-β1 signaling also activates Rho family small GTPases Rho, Rac, and Cdc42 as well as phosphatidylinositol 3-kinase (PI3K)/AKT and integrin-linked kinase (ILK) (3, 23, 50, 66, 95, 98).
Although the precise mechanisms underlying progressive renal fibrosis are not completely understood, studies have shown that TGF-β1, mediated via its canonical Smad signaling pathway, drives development of renal fibrosis (77). Recent investigations have also demonstrated that the noncanonical, non-Smad pathways can also mediate TGF-β1 fibrogenic responses (13, 38). However, new findings show that blocking TGF-β1 signaling in renal cells in vitro or in experimental animal models in vivo does not necessarily reduce renal fibrosis.
Anti-TGF-β Therapy in Renal Fibrosis
Based on significant preclinical evidence showing TGF-β1 as a key mediator of fibrotic diseases, much effort had been placed on inhibiting TGF-β1 as a therapeutic strategy. Several recent clinical trials using pharmacological blockade of TGF-β in fibrotic kidney diseases are summarized in Table 1. A therapeutic approach using a neutralizing anti-TGF-β antibody has been explored in models of experimental diabetes induced by streptozotocin (STZ) and Leprdb/db diabetic mice, demonstrating significant reduction in TGF-β1 levels and inhibition of glomerular mesangial matrix expansion with suppression of collagen and fibronectin expression (10, 80, 102). However, the recent multicenter phase II trial sponsored by Eli Lilly using LY2382770, a humanized neutralizing monoclonal antibody against TGF-β1 for the treatment of diabetic nephropathy, had to be prematurely terminated due to futility in efficacy (clinicaltrials.gov: NCT01113801). Fresolimumab (GC-1008, Genzyme) is a human monoclonal antibody that neutralizes all three isoforms (TGF-β1, 2, and 3). A single-dose infusion was shown to be relatively safe and well tolerated in a phase I multicenter, open-label study conducted in patients with treatment-resistant primary focal segmental glomerulosclerosis (FSGS). A subsequent phase II multicenter, double-blind, randomized study of fresolimumab in patients with steroid-resistant primary FSGS was recently completed, but no study results have been reported to date (clinicaltrials.gov: NCT01665391).
Table 1.
Recent clinical trials using anti-TGF-β therapy in fibrotic kidney diseases
| Compound | Target | Company | Phase | Disease | Reference or NCT Identifier |
|---|---|---|---|---|---|
| LY2382770 | TGF-β1 | Eli Lilly | Phase II | Diabetic nephropathy | NCT01113801 |
| Fresolimumab (GC1008) | TGF-β1, 2, 3 | Genzyme | Phase I | Focal segmental glomerulosclerosis | 89 |
| Phase II | NCT01665391 | ||||
| Pirfenidone | TGF-β1, 2, 3 | InterMune | Phase II | Focal segmental glomerulosclerosis, | NCT00001959 11 |
| Phase I/II | Diabetic nephropathy | NCT00063583 79 |
TGF, transforming growth factor.
Reference is shown in bold.
Pirfenidone is a small-molecular weight synthetic compound that has attracted much attention as an orally active antifibrotic agent recently approved by the US Food and Drug Administration for the treatment of idiopathic pulmonary fibrosis (35). Pirfenidone's effects are, in part, mediated via inhibition of TGF-β1 synthesis (78). In the kidney, the effects of pirfenidone in suppressing renal fibrosis have been shown in several experimental models including diabetic nephropathy, unilateral ureteral obstruction (UUO), and subtotal nephrectomy (9, 74, 82). An open-label, single-center pilot study demonstrated that pirfenidone significantly slowed renal function decline rate in patients with FSGS, with a median improvement of 25% in patients who have moderate to severe CKD and are already being treated with angiotensin antagonists (11). However, the phase II trial failed to show an improvement in proteinuria (clinicaltrials.gov: NCT00001959). Limitations of the trial included the small-scale, exploratory nature of the study and the lack of a control group. A phase I/II randomized, placebo-controlled trial conducted in 77 patients with overt diabetic nephropathy who had albuminuria and reduced estimated glomerular filtration rate (eGFR; 20–75 ml·min−1·1.73 m−2) demonstrated encouraging results of pirfenidone in improving kidney function, but without improvement in proteinuria (clinicaltrials.gov: NCT00063583). The mean eGFR increased in the pirfenidone (1,200 mg/day) group (+3.3 ± 8.5 ml·min−1·1.73 m−2), whereas, the mean eGFR decreased in the placebo control group (−2.2 ± 4.8 ml·min−1·1.73 m−2) (79).
The results from these clinical trials portend some promise of pirfenidone as an antifibrotic therapy to improve or slow the decline in kidney function associated with CKD. It should be noted, however, that in the above studies the assessment of kidney function was by eGFR using the Modification of Diet in Renal Disease (MDRD) equation, and not by direct measurement of GFR. In addition, no serial kidney biopsies were performed to confirm histological evidence of regression of renal fibrosis. Optimal dosage also may need to be further identified as well.
Hence anti-TGF-β therapy has not yet translated into successful treatment in CKD patients, and unfortunately many of the clinical trials that have been completed to date have been small and/or uncontrolled, and conclusive evidence regarding efficacy is limited and disappointing. Below, we describe studies that provide evidence for profibrotic effects as well as evidence that supports the protective effects of TGF-β1.
Profibrotic Effects of TGF-β1
Renal fibrosis is characterized by excessive production and accumulation of ECM proteins in the kidney that leads to parenchymal scarring, renal dysfunction, and ultimately end-stage kidney disease. TGF-β1 is the most extensively studied prototype member of the TGF-β superfamily in the context of fibrosis. TGF-β1 is well known as a central mediator of renal fibrosis and has long been considered to play potent roles in the progression of CKD (7, 17, 48). Increased expression of TGF-β1 mRNA and protein is seen in patients with fibrotic kidney diseases, including IgA nephropathy, focal and segmental glomerulonephritis, lupus nephritis, and diabetic and human immunodeficiency virus-associated nephropathy (5). Moreover, urinary TGF-β1 excretion is significantly elevated in patients with glomerular disease and heavy proteinuria (24).
The overexpression of TGF-β1 in transgenic mice with increased levels of circulating active TGF-β1 induces progressive renal disease characterized by mesangial expansion, accumulation of ECM proteins, and development of glomerulosclerosis and tubulointerstitial fibrosis (44). The accumulation of ECM proteins is stimulated by TGF-β1 acting as both an inducer of ECM synthesis and an inhibitor of ECM degradation by reduced synthesis of matrix metalloproteinases and increased synthesis of proteinase inhibitors, such as plasminogen activator inhibitor-1 (PAI-1).
It has been shown that TGF-β1 activation is required for its biological actions and important in the development of renal fibrosis. Active TGF-β1 is released when the latent complex anchored to ECM is cleaved through proteolysis, following exposure to factors such as integrins (2). Integrin αvβ6 is a heterodimeric matrix receptor expressed in renal epithelia which binds and activates latent TGF-β1. Studies using an in vivo model of progressive renal fibrosis induced by UUO in β6 integrin-null mice have demonstrated that blockade of αvβ6-mediated TGF-β1 activation was associated with lower expression of type I and type III collagen, PAI-1, and collagen content and protected against tubulointerstitial fibrosis (56). Thus these studies provide evidence that local activation of TGF-β1 is important in the development of renal fibrosis.
A significant body of evidence exists in the literature that various approaches that disrupt TGF-β1 signaling protect against renal fibrosis. Inhibition of TGF-β1 by using the proteoglycan decorin, which is a known inhibitor of TGF-β1 or anti-TGF-β antibodies, abrogated development of renal fibrosis (32, 62). BMP-7 is a TGF-β superfamily member that has been shown to play a protective role in renal fibrosis through anti-inflammatory, antioxidative, and antifibrotic effects by counteracting the effects of TGF-β1 (100). The BMP-7 signaling pathway and its role in fibrosis have been the subject of several recent reviews (6, 48, 71). In murine mesangial cells, BMP-7 decreases Smad3 accumulation and inhibits the transcriptional upregulation of Smad3 targets such as PAI-1, thereby suppresses the profibrotic effects of TGF-β1 (93). Administration of exogenous recombinant human BMP-7 reduced renal fibrosis in experimental models of renal injury in mice, including UUO and STZ-induced diabetic kidney disease (64, 84, 92). Thus BMP-7 opposes TGF-β1/Smad3-mediated renal fibrosis. Studies in Smad3-null mice revealed that targeted deletion of Smad3 signaling resulted in reduced ECM protein accumulation and attenuation of tubulointerstitial fibrosis following UUO injury (31, 73, 77). They also showed that the numbers of myofibroblasts, macrophages, and CD4/CD8 T cells, and monocyte influx into the kidney after UUO were significantly decreased in the obstructed kidneys of Smad3-null mice (31, 77). These findings suggest that TGF-β1/Smad3 signaling mediates development of renal fibrosis and inflammation.
TGF-β1 signaling via the non-Smad pathways also participates in mediating profibrotic responses. Data from in vitro studies indicate that TGF-β1-stimulated collagen expression in mesangial cells is mediated via the TAK1/MKK3/p38 signaling cascade and fibronectin expression in fibroblasts (13, 38, 69). Moreover, investigations using pharmacological and genetic blockade in in vivo models of glomerular and tubulointerstitial injury in mice have shown that the TAK1/MKK3/p38 and JNK pathways promote renal fibrosis (13, 39). Both p38 and JNK are downstream targets of TAK1 activation. Conditional Tak1 gene deletion in mice suppressed interstitial myofibroblast accumulation, collagen deposition, and expression of profibrotic molecules following UUO injury (55). These studies strongly suggest that the non-Smad signaling pathway via TAK1 plays a critical role in ECM production and the pathogenesis of kidney fibrosis.
Protective Effects of TGF-β1
Given the abundance of evidence from the preclinical studies, targeting TGF-β signaling pathways seems logical to attenuate the development of renal fibrosis and progression of CKD. However, contrary to highly anticipated results, data from clinical trials to date have not been as strong as was hoped, and anti-TGF-β1 treatment directed at pharmacological blockade of TGF-β1 has not yet translated into successful therapy for humans. Recently emerging evidence suggests that it may be due, at least in part, to the fact that TGF-β1 is capable of inducing not only profibrotic effects but also protective effects (Table 2).
Table 2.
Protective roles of TGF-β signaling in chronic kidney diseases
| Strategy and Results | Disease Model | Reference(s) |
|---|---|---|
| Conditional deletion of TβRII in renal interstitial cells significantly reduces collagen production, but does not ameliorate overall renal fibrosis. | UUO, aristolochic acid-induced nephropathy | 67 |
| Conditional deletion of Smad2 in renal tubular epithelial cells enhances renal fibrosis. | UUO | 58 |
| Overexpression of latent TGF-β1 in keratinocytes reduces renal fibrosis | UUO | 28 |
| Overexpression of latent TGF-β1 in keratinocytes protects against glomerular crescentic formation and severe tubulointerstitial damage. | Crescentic glomerulonephritis | 29 |
| Overexpression of Smad7 suppresses renal fibrosis, whereas deficiency of Smad7 aggravates the severity of renal fibrosis. | Diabetic nephropathy | 8 |
| Smad7 overexpression in kidneys suppresses renal fibrosis, and disruption of Smad7 enhances renal fibrosis. | UUO | 14, 46 |
| Smad 7 deficiency aggravates angiotensin II-mediated renal fibrosis, whereas Smad7 treatment prevents progressive renal injury. | Hypertensive nephropathy | 51, 52 |
| Smad7 disruption exacerbates nephropathy, whereas Smad7 overexpression in kidneys attenuates nephropathy. | Aristolochic acid-induced nephropathy | 15 |
| Conditional deletion of TβRII in renal tubular epithelial cells and renal fibroblasts enhances NF-κB signaling and renal inflammation. | UUO | 59 |
| Conditional deletion of Smad4 in renal tubular epithelial cells enhances renal inflammation. | UUO | 60 |
| Treatment with autophagy inhibitor 3-methyladenine enhances renal tubular cell apoptosis and tubulointerstitial fibrosis. | UUO | 41 |
| Heterozygous deletion in Beclin 1- and LC3b-null mice enhances collagen deposition. | UUO | 19, 40 |
| Conditional deletion of Atg5 in podocytes increases susceptibility to renal injury. | Puromycin, adriamycin, LPS | 27 |
UUO, unilateral ureteral obstruction.
Numerous in vitro studies have shown that TGF-β1 acts as a potent stimulator of ECM production, including the synthesis of type I collagen and fibronectin. However, a recent report by Neelisetty and colleagues (67) demonstrated surprising findings that blocking TGF-β signaling through deletion of TβRII in matrix-producing renal interstitial cells failed to suppress renal fibrosis in mouse models kidney injury induced by UUO or aristolochic acid. The studies showed that TβRII-deleted renal interstitial cells had significantly reduced type I collagen production, but the overall renal fibrosis following kidney injury was not decreased in the conditional knockout mice (67). These findings indicate that inhibiting TGF-β signaling in matrix-producing renal interstitial cells is not sufficient to protect against development of fibrosis after kidney injury.
Interestingly, mice overexpressing the latent (inactive) form of TGF-β1 in keratinocytes demonstrated protection against renal fibrosis in experimental obstructive kidney disease and crescentic glomerulonephritis (28, 29). These mice had increased levels of latent, but not active, TGF-β1 in plasma and kidney tissue, and upregulation of renal Smad7. The observed protective effects in the latent TGF-β1 transgenic mice may be due, at least in part, to Smad7-mediated inhibition of NF-κB as well as inhibition of TGF-β1 activation, consequently protecting the kidney against inflammation and fibrosis.
Taken together, these studies suggest that TGF-β1 is capable of exerting protective effects by opposing the profibrotic pathway via negative feedback effects of Smad2 and Smad7. The maintenance of balance through this negative self-regulation of profibrotic effects may be important for tissue homeostasis. Under pathological conditions, it is possible that the balance tips toward the profibrotic pathway through dysregulation of the inhibitory mechanisms to cause renal fibrosis, rather than solely through excess TGF-β1, and that this may perhaps account for the disappointing results of targeting TGF-β in humans. Furthermore, there is emerging evidence for the protective effects of TGF-β by mechanisms that include inhibiting inflammation and induction of autophagy, which will be discussed in the following sections.
TGF-β Signaling and Renal Inflammation
Renal inflammation is implicated as a critical event in the initiation and progression of renal fibrosis in CKD. The inflammatory response to renal injury involves infiltrating immune cells as well as activated resident renal cells, which stimulate the production and release of profibrotic cytokines and growth factors and drive the profibrotic process. Interestingly, despite the established proinflammatory role, TGF-β1 also possesses anti-inflammatory effects (49). Its anti-inflammatory properties are evidenced by findings that targeted deletion of the TGF-β1 gene results in profound multifocal inflammatory disease in mice (48, 83). Tgf-β1-null mice develop a rapid wasting syndrome and early death by 3–4 wk of age and display excessive inflammatory responses with massive infiltration of lymphocytes and macrophages in many organs (45). These data indicate that TGF-β1 is a potent anti-inflammatory molecule.
It has been proposed that the reason therapies directed against TGF-β1 in general have yielded disappointing results is that blockade of TGF-β1 signaling not only abrogates its profibrotic effects but also inhibits its anti-inflammatory effects, and thereby can promote renal fibrosis. Indeed, in vivo studies in mice have confirmed that conditional deletion of TβRII from kidney tubular epithelial cells enhanced NF-κB signaling and renal inflammation, with upregulation of proinflammatory cytokines such as IL-1β and TNF-α in the kidney following UUO injury (59). Similarly, disruption of TβRII in cultured renal tubular epithelial cells and fibroblasts resulted in impairment of the anti-inflammatory effect of TGF-β1 (59). Furthermore, conditional Smad4 knockout mice in which Smad4 was specifically deleted from kidney tubular epithelial cells displayed significantly enhanced renal inflammation as evidenced by increased infiltration of CD45+ leukocytes and F4/80+ macrophages and upregulation of proinflammatory cytokines IL-1β, TNF-α, monocyte chemoattractant protein-1 (MCP-1), as well as intercellular adhesion molecule-1 (ICAM-1) in the UUO kidney (60). Thus inhibition of TGF-β signaling through disruption of either TβRII or Smad4 resulted in enhanced renal inflammation, suggesting that TGF-β possesses anti-inflammatory effects in the kidney.
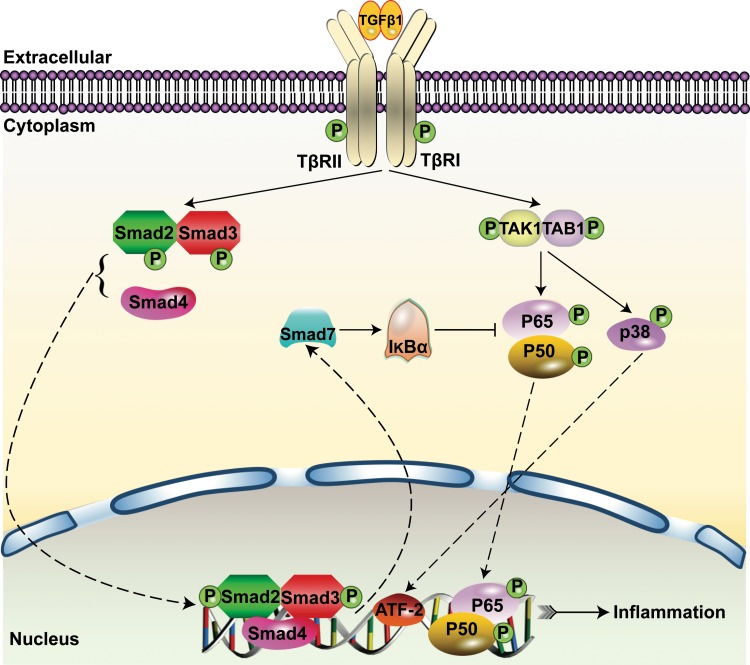
Further evidence indicates that loss of Smad4 represses Smad7 transcription, which leads to decreased nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor α (IκBα) expression but enhanced NF-κB activation in the tubulointerstitium after UUO, thereby promoting renal inflammation (60). Moreover, Smad7 knockout mice display enhanced renal inflammation with increased NF-κB/p65 phosphorylation and increased macrophage infiltration in models of UUO and STZ-induced diabetes (8, 14). In contrast, Smad7 gene transfer into the kidney of STZ-induced diabetic rats using an ultrasound microbubble-mediated technique significantly attenuated NF-kB/p65-driven renal inflammation and macrophage infiltration in diabetic rats (8). Latent TGF-β1 overexpression in mice also protected against renal inflammation, via upregulation of renal Smad7 and IκBα and suppression of NF-κB activation in models of UUO and crescentic glomerulonephritis (29, 94). Thus Smad7-mediated inhibition of NF-κB activation via the induction of IκBα may be a central mechanism in abrogating renal inflammation (Fig. 2).
Fig. 2.
An overview of Smad signaling and inflammatory pathways of TGF-β1 signaling. Binding of Smad4 to phosphorylated Smad2/3 leads to the nuclear translocation of the Smad complex to regulate gene transcription, including Smad7. Smad7 induces IκBα expression, which inhibits phosphorylation of p65/p50 proteins of the NF-κB signaling complex to prevent NF-κB-driven renal inflammation.
Recent investigations have also explored the role of TGF-β signaling and macrophage differentiation in inflammation. Kidney injury stimulates the recruitment and induction of macrophages to undergo classic activation and differentiate into proinflammatory M1 and anti-inflammatory M2 macrophages (1, 47). More recently, M2 macrophages have been further subdivided into three subsets, namely, M2a, M2b, and M2c macrophages. Regulatory M2c macrophages are induced by TGF-β or IL-10, and recent evidence has reported that this particular subset displayed protection against renal fibrosis (54, 65). While the M2c phenotype is induced by TGF-β and generally considered to be anti-inflammatory, M2c macrophages in turn produce TGF-β, and it has been proposed that this might be a mechanism by which macrophages drive the development and progression of fibrosis (54). However, a recent study using conditional deletion of TGF-β1 in macrophages suggests that macrophage-derived TGF-β may not serve as a functionally important source of TGF-β for renal fibrosis (30).
TGF-β Signaling and Autophagy
Recent evidence proposes a new mechanism by which TGF-β1 can provide cytoprotective effects via induction of a highly conserved cellular process known as macroautophagy, hereafter referred to as autophagy. It has long been known that autophagy is an intracellular process that occurs in all eukaryotes and results in the degradation of sequestered cytoplasmic elements in lysosomes (12). The execution of autophagy begins with the establishment of protein complexes that function in the formation of the phagophore, also known as the isolation membrane. The next major step, elongation of the autophagosome, includes two ubiquitin-like protein complexes, namely, autophagy-related gene (ATG)5–ATG12 and microtubule-associated protein 1 light chain 3 (LC3) conjugation systems. These two major steps are sequentially followed by autophagosome-lysosomal fusion, lysosomal degradation, and release of free amino acids and fatty acids (12, 85, 86).
Accumulating data implicate critical roles of autophagy in health and kidney disease (17). TGF-β1 stimulation induced the accumulation of autophagosomes and conversion of LC3 to the lipidated form, LC3-II, and upregulated autophagy-related genes ATG5, ATG7, LC3, and Beclin 1 in renal tubular epithelial cells (19, 42, 97). We also recently reported that TGF-β1 induced autophagy in glomerular mesangial cells (18, 40). Hence these studies provide strong evidence that TGF-β1 acts as an inducer of autophagy.
Important advances have also been made in our understanding of the mechanisms of autophagy activation by TGF-β1 signaling pathways (18, 40, 88). In primary mesangial cells, we further showed evidence that TGF-β1 induces autophagy through the TAK1-MKK3-p38 signaling pathway, and autophagy promotes intracellular degradation of collagen and aggregated, insoluble procollagen (40). Moreover, TGF-β1 protects against serum deprivation-induced mesangial cell apoptosis through the induction of autophagy via TAK1 and AKT activation (18). The role of the AKT-mammalian target of rapamycin signaling pathway has also been demonstrated to mediate the activation of autophagy in vivo following UUO injury in rats (41).
Autophagy is increasingly recognized as an important cellular defense against various stress stimuli, and dysregulated autophagy is implicated in diseases characterized by progressive kidney fibrosis (17). Upregulation of autophagy expression was noted in human kidney biopsies of patients with acquired proteinuric diseases such as FSGS (27). We and others have demonstrated induction of autophagy following UUO-induced kidney injury (19, 40, 41). Using GFP-LC3 transgenic mice, we confirmed that autophagy is induced in renal tubular epithelial cells of obstructed kidneys after UUO (19). Autophagy deficiency led to enhanced kidney fibrosis following UUO injury. For instance, mice deficient in autophagic protein Beclin 1 by heterozygous deletion of Beclin 1 displayed increased collagen deposition in the kidney (40). Both Lc3b-null mice and Beclin 1-heterozygous mice displayed enhanced collagen deposition in the obstructed kidneys after UUO (19). Treatment with autophagy inhibitor 3-methyladenine (3-MA) also enhanced renal tubular cell apoptosis and tubulointerstitial fibrosis in the obstructed kidneys after UUO in rats (41).
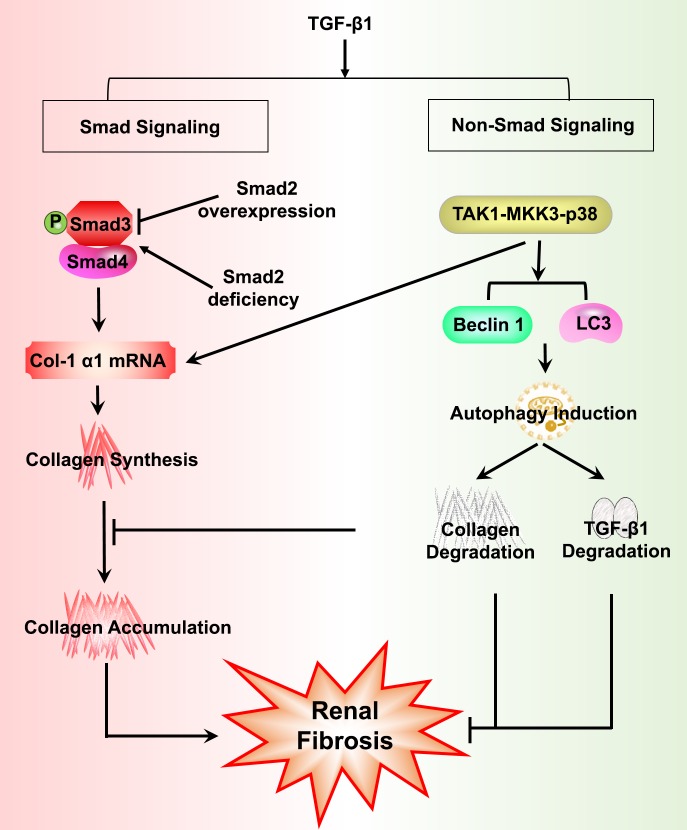
Podocytes exhibit a high basal level of autophagy, important in cell survival, given that they are terminally differentiated and have a very limited capacity for cell division and replacement (27). Podocyte-specific deletion of the Atg5 gene resulted in increased susceptibility to injury with more severe albuminuria, foot process effacement, loss of podocytes, and glomerulosclerosis, following exposure to inducers of proteinuric glomerular injury, such as puromycin aminonucleoside, adriamycin, or LPS (27). Induction of autophagy has also been shown to be protective against cyclosporine-induced renal tubular cell death (70). Collectively, these findings provide evidence that TGF-β1-induced autophagy provides cytoprotective effects that promote renal cell survival and negatively regulate and limit ECM accumulation in the kidney, thereby protecting against kidney fibrosis (Fig. 3).
Fig. 3.
An overview of profibrotic and protective pathways of TGF-β1 signaling. Activation of TGF-β1 in response to kidney injury can signal both profibrotic and protective effects via Smad and non-Smad signaling pathways. Overexpression of Smad2 attenuates TGF-β1-induced Smad3 phosphorylation and type I collagen (Col-1α1) expression, whereas inhibition of Smad2 promotes renal fibrosis via enhanced TGF-β1/Smad3 signaling. TGF-β1-induced autophagy negatively regulates TGF-β1-stimulated collagen accumulation in the kidney by promoting collagen degradation. Autophagy also negatively regulates mature TGF-β1 expression, thereby protecting against kidney fibrosis.
Negative Feedback Regulation of TGF-β Signaling
The diverse roles of TGF-β1 necessitate that its signaling is tightly regulated at various levels. TGF-β1 induces Smad7, which is an inhibitory Smad that acts in a negative feedback loop to negatively regulate TGF-β1 (22). Overexpression of Smad7 has been shown to oppose TGF-β1-mediated renal fibrosis following UUO or STZ-induced diabetes, whereas deficiency of Smad7 aggravated the severity of renal fibrosis (8, 14, 46). Smad7-null mice display enhanced TGF-β/Smad3 signaling and worsened progressive renal fibrosis in a model of angiotensin II-mediated hypertensive nephropathy (51), whereas treatment with Smad7 prevented angiotensin II-induced hypertensive nephropathy (52). Disruption of Smad7 also exacerbated chronic aristolochic acid nephropathy (AAN) in mice, a progressive CKD related to herbal medicine (15). Furthermore, overexpression of Smad7 in the kidneys with established chronic AAN attenuated progression of chronic AAN through inactivation of TGF-β/Smad3 signaling (15).
Similarly, studies utilizing other approaches to inhibit TGF-β signaling, downstream of the cell surface-expressed TGF-β receptors, have also shown failure to protect against kidney fibrosis. Findings in mice with targeted conditional deletion of Smad2 in the kidney show that blockade of the Smad2 signaling pathway not only failed to protect against the development of fibrosis after UUO injury but actually enhanced renal fibrosis (58). Moreover, knockdown of Smad2 in cultured renal tubular epithelial cells and fibroblasts resulted in enhanced ECM production in response to TGF-β1 stimulation, with increased expression of type I and type III collagen and TIMP-1, and decreased expression of MMP-2, a major matrix-degrading enzyme (58). Smad2 deletion was associated with increases in Smad3 phosphorylation, nuclear translocation, and Smad3 binding to a collagen promoter (COL1A2), and autoinduction of TGF-β1 (58). These studies indicate that inhibition of Smad2 promotes renal fibrosis via enhanced Smad3 signaling. On the other hand, overexpression of Smad2 resulted in attenuation of TGF-β1-induced Smad3 phosphorylation and type I collagen production in renal tubular epithelial cells (58). Taken together, these findings suggest that Smad2 functions to protect against development of renal fibrosis through countering the profibrotic function of Smad3 signaling.
Our recent investigations have uncovered a novel mechanism whereby autophagy regulates TGF-β1 expression and suppresses kidney fibrosis induced by UUO. Increased levels of mature TGF-β1 were seen in the obstructed kidneys of LC3-deficient mice upon UUO injury (19). Similarly, mature TGF-β1 levels were increased in LC3-deficient renal tubular epithelial cells, and in cells treated with the autophagy inhibitor bafilomycin A1, without alterations in TGF-β1 mRNA (19). These findings suggest a novel feedback mechanism to limit TGF-β signaling through autophagic degradation and suppress kidney fibrosis.
A number of endogenous modulators of TGF-β signaling has been described, such as secreted Klotho, which may serve as a promising therapeutic target against renal fibrosis. Klotho is a transmembrane protein expressed in renal tubular epithelial cells, and its extracellular domain is secreted by ectodomain shedding (4). Secreted Klotho inhibited TGF-β1-induced EMT in cultured cells, and administration of secreted Klotho protein to mice suppressed renal fibrosis induced by UUO (20). Interestingly, it has been suggested that the Klotho-mediated renoprotective effects may be due, at least in part, to upregulated autophagy flux (4). Secreted Klotho protein directly binds to TβRII, thereby inhibiting TGF-β1 binding to cell surface receptors, and inhibit TGF-β1 signaling (20). In contrast, high plasma levels of lipoprotein(a) [Lp(a)], an LDL-like particle, can inhibit the activation of latent TGF-β1 by competing with the binding of plasminogen to cell or matrix surfaces (26, 43). The proteoglycan decorin is another natural antagonist of TGF-β1 that binds to active TGF-β1 and can neutralize the biological effects of TGF-β1 (32). Retinoic acid, particularly all-trans retinoic acid (ATRA), has also been shown to suppress renal expression of TGF-β1 and TβRII, leading to decreased ECM accumulation and inhibit progression of renal fibrosis (53, 63, 101). Other studies have indicated that ATRA exacerbates kidney injury with increased glomerular ECM, mesangial cell activation, glomerular macrophage influx, and immune complex deposition in a model of membranoproliferative glomerulonephritis in mice (34, 101). Thus the therapeutic effects of retinoic acid in fibrotic kidney disease remain somewhat controversial, suggesting that caution is needed in applying retinoid therapy to human disease. No published clinical studies using soluble Kotho protein or ATRA administration in patients with CKD have been reported to date, despite the preclinical data supporting their therapeutic potential. Recently, a phase I/II trial has been initiated to evaluate the safety and efficacy of isotretinoin (13-cis retinoic acid) treatment in patients with biopsy-proven FSGS, minimal change disease, or collapsing glomerulopathy (clinicaltrials.gov: NCT00098020). The results of this clinical study will be of great interest.
Concluding Remarks
Renal fibrosis represents the common pathway in kidney injury of the most progressive CKD, leading to renal failure and end-stage kidney disease. Unfortunately, there are no effective therapies that prevent progressive renal fibrosis. Current treatment for patients with CKD primarily relies on inhibition of the renin-angiotensin-aldosterone system (RAAS) by angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) (61). However, the magnitude of the renoprotective effects of RAAS blockade is estimated to be only about a 20% risk reduction of progression (21).
TGF-β1 remains an attractive target for treating fibrotic kidney diseases, to counter its potent profibrotic effects. However, as this review highlights its opposing protective effects, indiscriminate complete blockade of TGF-β functions is not sufficient to reduce fibrosis and may actually aggravate the disease in some pathological settings. Perhaps, there are also interspecies differences suggesting that results derived from preclinical studies in mice and in vitro may not necessarily translate to humans. TGF-β1 actions depend on multiple factors, including the involved cell type and context, the dose, isoform, and the distinct Smad and non-Smad signaling pathways. Therefore, therapeutic targeting of TGF-β1 requires more optimal strategies such as those selectively directed at specific signaling pathway(s), and future investigations to better understand the precise molecular mechanisms of TGF-β1 signaling are warranted.
GRANTS
This work was supported in part by National Institutes of Health Grants R01 DK57661 and R01 HL079904 to M. E. Choi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.S. prepared figures; A.S., S.A.M., and M.E.C. drafted manuscript; A.S., S.A.M., and M.E.C. edited and revised manuscript; A.S., S.A.M., and M.E.C. approved final version of manuscript.
REFERENCES
- 1.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 80: 915–925, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 116: 217–224, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bian A, Neyra JA, Zhan M, Hu MC. Klotho, stem cells, aging. Clin Interv Aging 10: 1233–1243, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med 342: 1350–1358, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Boon MR, van der Horst G, van der Pluijm G, Tamsma JT, Smit JW, Rensen PC. Bone morphogenetic protein 7: a broad-spectrum growth factor with multiple target therapeutic potency. Cytokine Growth Factor Rev 22: 221–229, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Chen HY, Huang XR, Wang W, Li JH, Heuchel RL, Chung AC, Lan HY. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes 60: 590–601, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JF, Ni HF, Pan MM, Liu H, Xu M, Zhang MH, Liu BC. Pirfenidone inhibits macrophage infiltration in ⅚ nephrectomized rats. Am J Physiol Renal Physiol 304: F676–F685, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Iglesias-de la Cruz MC, Jim B, Hong SW, Isono M, Ziyadeh FN. Reversibility of established diabetic glomerulopathy by anti-TGF-beta antibodies in db/db mice. Biochem Biophys Res Commun 300: 16–22, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2: 906–913, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 368: 651–662, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Choi ME, Ding Y, Kim SI. TGF-beta signaling via TAK1 pathway: role in kidney fibrosis. Semin Nephrol 32: 244–252, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung AC, Huang XR, Zhou L, Heuchel R, Lai KN, Lan HY. Disruption of the Smad7 gene promotes renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) in mice. Nephrol Dial Transplant 24: 1443–1454, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Dai XY, Zhou L, Huang XR, Fu P, Lan HY. Smad7 protects against chronic aristolochic acid nephropathy in mice. Oncotarget 20: 11930–11944, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Ding Y, Choi ME. Regulation of autophagy by TGF-beta: emerging role in kidney fibrosis. Semin Nephrol 34: 62–71, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Y, Kim JK, Kim SI, Na HJ, Jun SY, Lee SJ, Choi ME. TGF-beta1 protects against mesangial cell apoptosis via induction of autophagy. J Biol Chem 285: 37909–37919, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Y, Kim SL, Lee SY, Koo JK, Wang Z, Choi ME. Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol 25: 2835–2846, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drawz PE, Rosenberg ME. Slowing progression of chronic kidney disease. Kidney Int Suppl 3: 372–376, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem 276: 12477–12480, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell 13: 902–914, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goumenos DS, Tsakas S, El Nahas AM, Alexandri S, Oldroyd S, Kalliakmani P, Vlachojannis JG. Transforming growth factor-beta(1) in the kidney and urine of patients with glomerular disease and proteinuria. Nephrol Dial Transplant 17: 2145–2152, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Grabias BM, Konstantopoulos K. The physical basis of renal fibrosis: effects of altered hydrodynamic forces on kidney homeostasis. Am J Physiol Renal Physiol 306: F473–F485, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Grainger DJ, Kirschenlohr HL, Metcalfe JC, Weissberg PL, Wade DP, Lawn RM. Proliferation of human smooth muscle cells promoted by lipoprotein(a). Science 260: 1655–1658, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstadt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang XR, Chung AC, Wang XJ, Lai KN, Lan HY. Mice overexpressing latent TGF-β1 are protected against renal fibrosis in obstructive kidney disease. Am J Physiol Renal Physiol 295: F118–F127, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang XR, Chung AC, Zhou L, Wang XJ, Lan HY. Latent TGF-beta1 protects against crescentic glomerulonephritis. J Am Soc Nephrol 19: 233–242, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huen SC, Moeckel GW, Cantley LG. Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am J Physiol Renal Physiol 305: F477–F484, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inazaki K, Kanamaru Y, Kojima Y, Sueyoshi N, Okumura K, Kaneko K, Yamashiro Y, Ogawa H, Nakao A. Smad3 deficiency attenuates renal fibrosis, inflammation, and apoptosis after unilateral ureteral obstruction. Kidney Int 66: 597–604, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, Border WA. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med 2: 418–423, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Ito Y, Goldschmeding R, Kasuga H, Claessen N, Nakayama M, Yuzawa Y, Sawai A, Matsuo S, Weening JJ, Aten J. Expression patterns of connective tissue growth factor and of TGF-β isoforms during glomerular injury recapitulate glomerulogenesis. Am J Physiol Renal Physiol 299: F545–F558, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Iyoda M, Hudkins KL, Wietecha TA, Banas MC, Guo S, Liu G, Wang L, Kowalewska J, Alpers CE. All-trans-retinoic acid aggravates cryoglobulin-associated membranoproliferative glomerulonephritis in mice. Nephrol Dial Transplant 22: 3451–3461, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis–FDA review of pirfenidone and nintedanib. N Engl J Med 372: 1189–1191, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell 6: 1365–1375, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Kim SI, Kwak JH, Na HJ, Kim JK, Ding Y, Choi ME. Transforming growth factor-beta (TGF-beta1) activates TAK1 via TAB1-mediated autophosphorylation, independent of TGF-beta receptor kinase activity in mesangial cells. J Biol Chem 284: 22285–22296, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SI, Kwak JH, Zachariah M, He Y, Wang L, Choi ME. TGF-β-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-β1-induced MKK3-p38 MAPK activation and stimulation of type I collagen. Am J Physiol Renal Physiol 292: F1471–F1478, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Kim SI, Lee SY, Wang Z, Ding Y, Haque N, Zhang J, Zhou J, Choi ME. TGF-beta-activated kinase 1 is crucial in podocyte differentiation and glomerular capillary formation. J Am Soc Nephrol 25: 1966–1978, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ, Choi ME. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J Biol Chem 287: 11677–11688, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim WY, Nam SA, Song HC, Ko JS, Park SH, Kim HL, Choi EJ, Kim YS, Kim J, Kim YK. The role of autophagy in unilateral ureteral obstruction rat model. Nephrology (Carlton) 17: 148–159, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, Glick AB, Hahnel B, Hosser H, Grone HJ, Kriz W. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol 177: 632–643, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kojima S, Harpel PC, Rifkin DB. Lipoprotein (a) inhibits the generation of transforming growth factor beta: an endogenous inhibitor of smooth muscle cell migration. J Cell Biol 113: 1439–1445, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopp JB, Factor VM, Mozes M, Nagy P, Sanderson N, Bottinger EP, Klotman PE, Thorgeirsson SS. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest 74: 991–1003, 1996. [PubMed] [Google Scholar]
- 45.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 90: 770–774, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14: 1535–1548, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SY, Kim SI, Choi ME. Therapeutic targets for treating fibrotic kidney diseases. Transl Res 165: 512–530, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24: 99–146, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Tan X, Dai C, Stolz DB, Wang D, Liu Y. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol 20: 1907–1918, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu GX, Li YQ, Huang XR, Wei L, Chen HY, Shi YJ, Heuchel RL, Lan HY. Disruption of Smad7 promotes ANG II-mediated renal inflammation and fibrosis via Sp1-TGF-beta/Smad3-NFkappaB-dependent mechanisms in mice. PLoS One 8: e53573, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu GX, Li YQ, Huang XR, Wei LH, Zhang Y, Feng M, Meng XM, Chen HY, Shi YJ, Lan HY. Smad7 inhibits AngII-mediated hypertensive nephropathy in a mouse model of hypertension. Clin Sci (Lond) 127: 195–208, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Long YB, Qin YH, Zhou TB, Lei FY. Association of retinoic acid receptors with extracellular matrix accumulation in rats with renal interstitial fibrosis disease. Int J Mol Sci 13: 14073–14085, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu J, Cao Q, Zheng D, Sun Y, Wang C, Yu X, Wang Y, Lee VW, Zheng G, Tan TK, Wang X, Alexander SI, Harris DC, Wang Y. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int 84: 745–755, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Ma FY, Tesch GH, Ozols E, Xie M, Schneider MD, Nikolic-Paterson DJ. TGF-β1-activated kinase-1 regulates inflammation and fibrosis in the obstructed kidney. Am J Physiol Renal Physiol 300: F1410–F1421, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol 163: 1261–1273, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol 13: 616–630, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, Ju W, Bottinger EP, Lan HY. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol 21: 1477–1487, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng XM, Huang XR, Xiao J, Chen HY, Zhong X, Chung AC, Lan HY. Diverse roles of TGF-beta receptor II in renal fibrosis and inflammation in vivo and in vitro. J Pathol 227: 175–188, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Meng XM, Huang XR, Xiao J, Chung AC, Qin W, Chen HY, Lan HY. Disruption of Smad4 impairs TGF-beta/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro. Kidney Int 81: 266–279, 2012. [DOI] [PubMed] [Google Scholar]
- 61.Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension 38: 635–638, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Morath C, Dechow C, Lehrke I, Haxsen V, Waldherr R, Floege J, Ritz E, Wagner J. Effects of retinoids on the TGF-beta system and extracellular matrix in experimental glomerulonephritis. J Am Soc Nephrol 12: 2300–2309, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Morrissey J, Hruska K, Guo G, Wang S, Chen Q, Klahr S. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol 13, Suppl 1: S14–S21, 2002. [PubMed] [Google Scholar]
- 65.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mucsi I, Skorecki KL, Goldberg HJ. Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor-beta1 on gene expression. J Biol Chem 271: 16567–16572, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Neelisetty S, Alford C, Reynolds K, Woodbury L, Nlandu-Khodo S, Yang H, Fogo AB, Hao CM, Harris RC, Zent R, Gewin L. Renal fibrosis is not reduced by blocking transforming growth factor-beta signaling in matrix-producing interstitial cells. Kidney Int 88: 503–514, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem 275: 3577–3582, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Ono K, Ohtomo T, Ninomiya-Tsuji J, Tsuchiya M. A dominant negative TAK1 inhibits cellular fibrotic responses induced by TGF-beta. Biochem Biophys Res Commun 307: 332–337, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Pallet N, Bouvier N, Legendre C, Gilleron J, Codogno P, Beaune P, Thervet E, Anglicheau D. Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy 4: 783–791, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Patel SR, Dressler GR. BMP7 signaling in renal development and disease. Trends Mol Med 11: 512–518, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Prelog M, Scheidegger P, Peter S, Gershwin ME, Wick G, Sgonc R. Diminished transforming growth factor beta2 production leads to increased expression of a profibrotic procollagen alpha2 type I messenger RNA variant in embryonic fibroblasts of UCD-200 chickens, a model for systemic sclerosis. Arthritis Rheum 52: 1804–1811, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 22: 1462–1474, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.RamachandraRao SP, Zhu Y, Ravasi T, McGowan TA, Toh I, Dunn SR, Okada S, Shaw MA, Sharma K. Pirfenidone is renoprotective in diabetic kidney disease. J Am Soc Nephrol 20: 1765–1775, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ren S, Babelova A, Moreth K, Xin C, Eberhardt W, Doller A, Pavenstadt H, Schaefer L, Pfeilschifter J, Huwiler A. Transforming growth factor-beta2 upregulates sphingosine kinase-1 activity, which in turn attenuates the fibrotic response to TGF-beta2 by impeding CTGF expression. Kidney Int 76: 857–867, 2009. [DOI] [PubMed] [Google Scholar]
- 76.Robertson IB, Rifkin DB. Unchaining the beast; insights from structural and evolutionary studies on TGFbeta secretion, sequestration, and activation. Cytokine Growth Factor Rev 24: 355–372, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev 20: 85–97, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, Donohue M, Ramachandrarao S, Xu R, Fervenza FC, Kopp JB. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol 22: 1144–1151, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 45: 522–530, 1996. [DOI] [PubMed] [Google Scholar]
- 81.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature 474: 343–349, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimizu T, Kuroda T, Hata S, Fukagawa M, Margolin SB, Kurokawa K. Pirfenidone improves renal function and fibrosis in the post-obstructed kidney. Kidney Int 54: 99–109, 1998. [DOI] [PubMed] [Google Scholar]
- 83.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359: 693–699, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sugimoto H, Grahovac G, Zeisberg M, Kalluri R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 56: 1825–1833, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Sureshbabu A, Bhandari V. Targeting mitochondrial dysfunction in lung diseases: emphasis on mitophagy. Front Physiol 4: 384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sureshbabu A, Ryter SW, Choi ME. Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol 4: 208–214, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sureshbabu A, Tonner E, Allan GJ, Flint DJ. Relative roles of TGF-beta and IGFBP-5 in idiopathic pulmonary fibrosis. Pulm Med 2011: 517687, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suzuki HI, Kiyono K, Miyazono K. Regulation of autophagy by transforming growth factor-beta (TGF-beta) signaling. Autophagy 6: 645–647, 2010. [DOI] [PubMed] [Google Scholar]
- 89.Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, Rota S, Remuzzi G, Rump LC, Sellin LK, Heaton JP, Streisand JB, Hard ML, Ledbetter SR, Vincenti F. A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 79: 1236–1243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsou PS, Haak AJ, Khanna D, Neubig RR. Cellular mechanisms of tissue fibrosis. 8. Current and future drug targets in fibrosis: focus on Rho GTPase-regulated gene transcription. Am J Physiol Cell Physiol 307: C2–C13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang B, Koh P, Winbanks C, Coughlan MT, McClelland A, Watson A, Jandeleit-Dahm K, Burns WC, Thomas MC, Cooper ME, Kantharidis P. miR-200a Prevents renal fibrogenesis through repression of TGF-beta2 expression. Diabetes 60: 280–287, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang S, Chen Q, Simon TC, Strebeck F, Chaudhary L, Morrissey J, Liapis H, Klahr S, Hruska KA. Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int 63: 2037–2049, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Wang S, Hirschberg R. Bone morphogenetic protein-7 signals opposing transforming growth factor beta in mesangial cells. J Biol Chem 279: 23200–23206, 2004. [DOI] [PubMed] [Google Scholar]
- 94.Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, Wang XJ, Lan HY. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol 16: 1371–1383, 2005. [DOI] [PubMed] [Google Scholar]
- 95.Wilkes MC, Mitchell H, Penheiter SG, Dore JJ, Suzuki K, Edens M, Sharma DK, Pagano RE, Leof EB. Transforming growth factor-beta activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res 65: 10431–10440, 2005. [DOI] [PubMed] [Google Scholar]
- 96.Xu L, Xiong S, Guo R, Yang Z, Wang Q, Xiao F, Wang H, Pan X, Zhu M. Transforming growth factor beta3 attenuates the development of radiation-induced pulmonary fibrosis in mice by decreasing fibrocyte recruitment and regulating IFN-gamma/IL-4 balance. Immunol Lett 162: 27–33, 2014. [DOI] [PubMed] [Google Scholar]
- 97.Xu Y, Yang S, Huang J, Ruan S, Zheng Z, Lin J. Tgf-beta1 induces autophagy and promotes apoptosis in renal tubular epithelial cells. Int J Mol Med 29: 781–790, 2012. [DOI] [PubMed] [Google Scholar]
- 98.Yi JY, Shin I, Arteaga CL. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem 280: 10870–10876, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Yu L, Border WA, Huang Y, Noble NA. TGF-beta isoforms in renal fibrogenesis. Kidney Int 64: 844–856, 2003. [DOI] [PubMed] [Google Scholar]
- 100.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968, 2003. [DOI] [PubMed] [Google Scholar]
- 101.Zhou TB, Drummen GP, Qin YH. The controversial role of retinoic acid in fibrotic diseases: analysis of involved signaling pathways. Int J Mol Sci 14: 226–243, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]