Abstract
A low-Na+, high-K+ diet (LNaHK) is considered a healthier alternative to the “Western” high-Na+ diet. Because the mechanism for K+ secretion involves Na+ reabsorptive exchange for secreted K+ in the distal nephron, it is not understood how K+ is eliminated with such low Na+ intake. Animals on a LNaHK diet produce an alkaline load, high urinary flows, and markedly elevated plasma ANG II and aldosterone levels to maintain their K+ balance. Recent studies have revealed a potential mechanism involving the actions of alkalosis, urinary flow, elevated ANG II, and aldosterone on two types of K+ channels, renal outer medullary K+ and large-conductance K+ channels, located in principal and intercalated cells. Here, we review these recent advances.
Keywords: Na+-Cl− cotransporter, renal outer medullary K+ channel, angiotensin II, large-conductance K+ channel, epithelial Na+ channel
the majority of the United States population consumes a “Western” diet of high Na+ and low K+. The Western diet produces an acid load (29, 71) and is associated with hypertension, especially in genetically predisposed, Na+-retaining individuals (4, 38, 43, 166, 167). The “ancient” or “Paleo” diet consists of fruits, vegetables, and nuts and produces an alkaline load (29). The Yanomami (no-salt culture) tribe of South America, when studied in the 1970s, gave us a window into the ionic content and renal handling of the ancient diet. The urinary K+ concentration was very high and the Na+ and Cl− concentrations were near zero, indicating the consumption of an exaggerated form of the modern-day equivalent of the ancient diet (95). However, it has been questioned how K+ is eliminated on such a diet of minimal Na+ when Na+ reabsorption in the distal nephron is the driving force for K+ secretion. Moreover, a low Na+ intake may be detrimental to health (93). Nevertheless, a longitudinal study (5) has shown that a diet of fruits and vegetables, known as the dietary approaches to stop hypertension diet, reduced blood pressure in hypertensive subjects, and most studies now agree that the Paleo diet is healthier, antihypertensive, and associated with stroke reduction (4, 13, 23, 23, 51, 84, 95). To understand the beneficial effects of a low-Na+, high-K+ (LNaHK) diet, studies are needed to determine how such a long-term diet is handled by the mammalian kidney.
Very few studies have explored renal handling of the LNaHK diet. However, recent progress has been made in the field of distal renal transport physiology with respect to the regulation of Na+ and K+ homeostasis after consumption of either a low-Na+ or high-K+ diet. With low volume or low Na+ intake, Na+ is reabsorbed by the Na+-Cl− cotransporter (NCC) in the early distal tubule (168). During high K+ intake, Na+ bypasses the NCC and is reabsorbed in the connecting tubules (CNTs) and cortical collecting ducts (CCDs) via the epithelial Na+ channel (ENaC), which is localized in principal cells (PCs) (19). Na+ reabsorbed by amiloride-sensitive ENaC causes an electronegative driving force for the secretion of K+ into the lumen (24, 121, 122). Na+ reabsorbed with Cl− via NCC will restore Na+ and volume (110). Na+ reabsorbed via ENaC will restore Na+ but reduce blood K+ concentration (121, 169). A set of key regulators and signaling networks are involved in distinguishing between these Na+ transporters. The LNaHK diet reveals several interesting findings that can help distinguish these regulatory pathways.
This review will emphasize three major simultaneously occurring events associated with the LNaHK diet that are responsible for maintaining K+ balance and may explain why the LNaHK diet is beneficial. The first is the dietary anionic content, which is responsible for creating an acidic or alkaline load for the kidneys. The second is the role of elevated plasma ANG II concentration (P[ANG II]) and plasma aldosterone (Aldo) concentration (P[Aldo]). The third is the generation and effects of the high distal flow.
The ability of the LNaHK diet to produce an alkaline load appears to be a critical factor for maintaining K+ homeostasis. The urine of the Yanomami people contained near 0 mM Cl−, indicating the production of an alkaline load, as expected from a diet of fruits, vegetables, and nuts. Like the Yanomami, mice maintain their K+ balance with a high dietary K+ of 5%, so long as it is accompanied with alkaline counteranions (30, 169).
Because of the combination of low Na+ and high K+, ANG II, P[Aldo], and plasma K+ concentration (P[K+]) are all elevated in the LNaHK diet. Thus, we will discuss the importance and interactions of K+, ANG II, and Aldo and their importance in influencing K+ secretion to maintain K+ balance on the LNaHK diet. Two studies in particular, one study with mice (31) and the other study with rats (150), have reported renal handling on LNaHK diets of similar Na+ and K+ composition, and one or the other has addressed the roles of ANG II and Aldo in maintaining K+ homeostasis.
The final critical event for animals on the LNaHK diet is the generation of a high urinary flow. The beneficial blood pressure-lowering effects of high K+ may be related to this natural diuretic effect (146, 169). The mechanism for generating high flow will be discussed. High flow in the distal nephron stimulates K+ secretion by activating large-conductance Ca2+-activated K+ (BK) channels (75, 178). BK channels are composed of two subunits: pore-forming BK-α and one of four BK-β proteins (β1–β4), which confer differing properties on BK channels (150). CNT cells contain BK-α–BK-β1 (103, 104), and intercalated cells (ICs) contain BK-α–BK-β4 (52). Mice with knockout (KO) of the BK β1-subunit (β1-KO mice) or BK β4-subunit (β4-KO) exhibit attenuated K+ excretion compared with wild-type (WT) mice when given a high-K+ diet.
A variety of K+ channels may be involved in the secretion of K+, depending on dietary intake and distal flow. BK channels and renal outer medullary K+ (ROMK) channels have been the most studied channels that secrete K+ in the CNT/CCD of the Aldo-sensitive distal nephron (ASDN). The ROMK channel mediates K+ secretion in the Aldo-sensitive distal nephron when flow is normal or low (47, 119, 153, 180). Both ROMK (113, 162, 180) and BK (31, 36, 171) channels are regulated by Aldo. However, it has been difficult to discern the functional activity of ROMK channels in the CCD in vivo using ROMK KO mice because the thick ascending limb (TAL) also contains ROMK channels (32, 33, 66, 161). In the TAL, the ROMK channel recycles K+ absorbed via Na+-K+-2Cl− cotransporter (NKCC)2 in the apical membrane. ROMK KO mice exhibit Bartter's syndrome, with flow-stimulated, BK channel-mediated K+ secretion in the distal nephron (9). The small-conductance Ca2+-activated K+ channel (16) also has been ascribed a K+ secretory role in the Aldo-sensitive distal nephron. The small-conductance Ca2+-activated K+ channel is very sensitive to Ca2+ (10) and may also be involved in flow-induced K+ secretion.
This review will not discuss the “with no lysine kinase” (WNK) and SP-related proline/alanine-rich kinase (SPAK) pathways because this topic has been expanding rapidly and would add considerably to the length. These pathways, which will have large roles in sorting the downstream responses to changes in P[K+], P[Aldo], and P[ANG II] in animals on the LNaHK diet, have been extensively studied and reviewed by other groups (2, 53, 54, 57, 140).
Unless otherwise noted in the review, the Na+ and K+ contents of the diets are in percent composition by weight as follows: regular diet = 0.3% Na+ and 0.6% K+; low Na+ = 0.01% Na+ and 0.6% K+; high K+ = 0.3% Na+ and 5% K+; and LNaHK = 0.01% Na+ and 5% K+. The high-K+ and LNaHK diets contain 5% equal parts citrate-carbonate-Cl− as the counteranions to K+. The high-K+-Cl− and LNaHK-Cl− diets contain 5% Cl− as the counteranion to K+. K+ imbalance is defined by P[K+] > 5.5 mM, which is clinically considered “hyperkalemia” (67).
The LNaHK Diet and the Role of Acid/Base
The reciprocal relation between K+ and H+ secretion is well established; however, the ability of high-K+ diets to generate an acid or alkaline load has been seldom considered. The Yanomami study revealed nearly zero urinary Cl−, indicating that their LNaHK diet was very alkaline. The acid load is considerably greater (hyperchloremic acidosis) when Cl− (5%) is associated with 5% K+ in the diet, meaning that renal mechanisms will be required to eliminate the high H+ concentration as well as K+ concentration. The dramatic differences between the effects of an alkaline-producing and acid-producing LNaHK diet are shown in Table 1, which shows different values relating the K+ handling of mice given either a LNaHK diet (alkaline producing), a LNaHK diet plus acidic drinking water (280 mM NH4Cl + 2% sucrose), or a LNaHK-Cl− diet (5% K+ + 5% Cl−). As shown in Table 1, the P[K+] of 4.71 for mice on the LNaHK diet is within the normal range; however, mice become very hyperkalemic (6.8 mM) when the LNaHK diet is accompanied by acidic drinking water. When the diet was an acid-producing LNaHK-Cl− diet, P[K+] increased to 9.3 mM. P[Aldo] of mice on the LNaHK-Cl− diet was very high [12,674 pg/ml (unpublished observations)], showing that the primary defect was the failure to secrete K+ rather than a failure to produce Aldo. In the LNaHK mice, the transtubular K+ gradient, a measure of the passive driving force for K+ across the CNT/CCD, is approximately twofold the value of mice on the LNaHK-Cl− diet. Therefore, these mouse experiments indicate that the ability of the Yanomami diet to produce an alkaline load was key to maintaining K+ balance when consuming a LNaHK diet.
Table 1.
Differences in K+ handling with alkaline versus acidic LNaHK diets
| Diet | Anion | Urine pH | Plasma K+ Concentration, mM | Transtubular K+ Gradient | |
|---|---|---|---|---|---|
| Ref. 169 | LNaHK | Carb/Citr/Cl− | 8.59 | 4.71 | 22.9 |
| Ref. 30 | LNaHK-AW | Carb/Citr/Cl− | 6.63 | 6.8 | 15.1 |
| Ref. 169 | LNaHK | Cl− | 5.89 | 9.3 | 10.2 |
All diets were given for 7–10 days. LNaHK-AW = low-Na+, high-K+ diet (LNaHK) with acid (280 mM NH4Cl plus 2% sucrose) drinking water. Carb/Citr/Cl, equal parts carbonate, citrate, and Cl− anions with K+.
Considerable in vivo and in vitro work has illustrated the competition between the transport pathways for eliminating K+ versus H+ in the distal nephron (6, 21, 69). However, the recently described link between ENaC-mediated Na+ reabsorption and pendrin-mediated HCO3− secretion (64, 100, 101, 158) is an explanation for why an alkaline-producing diet facilitates K+ secretion. HCO3− increases the activity of ENaC in cultured cells (100), and pendrin KO mice exhibit diminished apical expression of ENaC (101). The converse, inhibition of ENaC-mediated Na+ absorption promotes H+ secretion, has also been demonstrated (91). Therefore, K+ secretion could be linked to pendrin activity based on ties between K+ secretion and ENaC activity. Such a mechanism could account for the fact that an alkaline LNaHK diet, which generates systemic HCO3−, promotes a high rate of K+ secretion, and the K+ balance is maintained.
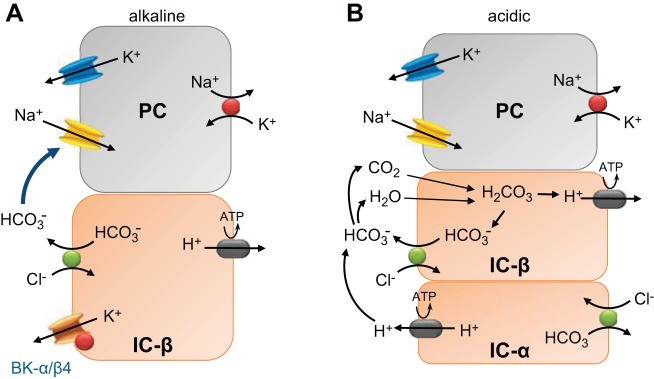
The driving force for K+ secretion not only depends on Na+ reabsorption but also on the resistance for luminal anions to traverse the paracellular pathway. As shown in Fig. 1, IC-β are predominant in the CNT (89, 147), particularly in alkaline conditions. With predominant IC-β, Cl− reabsorbs via pendrin in exchange with secreted HCO3−. Secreted luminal HCO3− is a larger anion than Cl−. Similar to the large anions gluconate and sulfate, HCO3− is more resistant to paracellular reabsorption than Cl−, thereby enhancing the electronegative transepithelial voltage (28, 44, 109, 118). This principal for HCO3− was first demonstrated with microperfused distal tubules of rats on low-Na+ diets (28). The transepithelial voltage was significantly more negative by 6 mV when the distal tubule was perfused with NaHCO3 compared with NaCl. Therefore, with a LNaHK diet that produces an alkaline load, reabsorbing NaCl is tightly exchanged for secreted KHCO3.
Fig. 1.
Illustration of the effects of acidic or alkaline loading on coordinated transport between prinicpal cells (PCs) and intercalated cells (ICs) of mice on a low-Na+, high-K+ (LNaHK) diet. A: mechanism of HCO3−-promoted K+ secretion in predominant IC-β in the connecting tubule (CNT) of mice on a LNaHK diet. With only IC-β, the reabsorbed Cl− is exchanged for secreted HCO3− via pendrin and the lumen negative potential from Na+ reabsorbed via epithelial Na+ channels (ENaC) drives the secretion of K+ from PCs and ICs. B: mechanism of K+ secretion with the LNaHK-Cl− diet. As the load becomes more acidic, IC-β are converted to IC-α (108, 126) in the CNT and initial cortical collecting duct (CCD). Cl− is reabsorbed via pendrin in exchange for secreted HCO3−, which binds to H+, which is pumped into the lumen from IC-α. CO2 recycles back through IC-β. H2O would recycle back via aquaporin 5, which is localized on IC-β (105), and its apical membrane insertion is linked with K+ intake (106). CO2 and H2O would combine to generate HCO3−, which would be secreted, and H+, which would be actively transported into the plasma via basolateral H+-ATPase. The result is Na+ reabsorption with Cl− rather than exchange with K+. The reduced driving force for K+ secretion is reflected by the lower transtubular K+ gradient and subsequent rise in plasma K+ concentration (P[K+])for mice on an acidic LNaHK diet (Table 1). The urine is acidified in the inner medullary collecting ducts, where the predominating cells are acid secreting IC-α. BK, large-conductance Ca2+-activated K+ channels.
As the LNaHK diet becomes more acidic, IC-β are converted to IC-α (108, 126). In the CNT/CCD, IC-β and IC-α will coexist. In this state, secreted H+ from IC-α, which contain apical carbonic anhydrase IV (107), will react with HCO3− to form H2O and CO2. CO2 will be recycled back to IC-α. HCO3− recycling results in net Na+ reabsorption with Cl− rather than exchanging for K+. With a preponderance of Cl− in the lumen, some Cl− may be reabsorbed through the paracellular pathway (118). The consequence of decreased resistance to anion reabsorption via the paracellular pathway and H+ secretion will be less driving force for K+ secretion, thereby explaining the reduced transtubular K+ gradient and elevated P[K+] in the LNaHK-Cl− diet. The urine can be additionally acidified in medullary collecting ducts, where there is a predominance of IC-α (155).
In both models (Fig. 1, A and B), Cl− reabsorption with Na+ is dependent on pendrin, with luminal HCO3− activating ENaC (100). It is not understood at the molecular level how HCO3− activates ENaC from the luminal side. However, ENaC-mediated Na+ reabsorptive exchange for K+, along with HCO3− excretion, was enhanced in acid-loaded electrogenic Na+-HCO3− cotransporter (NBCe2) KO mice. Therefore, NBCe2, which resides in distal nephron cells, prevents ENaC-mediated Na+ for K+ exchange during acidosis (172–174). The role of NBCe2 and other transporters and signaling mechanisms linking ENaC and pendrin need to be determined.
Although it is clear that the LNaHK diet requires alkaline anions to prevent hyperkalemia, the results are mixed with respect to K+ homeostasis when mice or rats are given a normal-Na+, high-K+ diet with Cl−1 as the counteranion. Most studies have not reported the anion content associated with high K+. Some studies have shown that P[K+] is within the normal range when a 10% KCl (5% K+ and 5% Cl−) diet is given (40, 97); however, one of those studies administered the high-K+-Cl− for only 2 days (97). Other studies have shown that adapting mice (30) or rats (79, 156) with a high-K+-Cl− diet produces hyperkalemia with a P[K+] of 5.8–6.8 mM. Nevertheless, the degree of hyperkalemia in animals on a high K+-Cl− diet is not nearly as severe as animals on a LNaHK-Cl− diet. The reason is not clear, but increased Na+ availability to ENaC in the high K+-Cl− diet may reduce the dependency on pendrin-mediated HCO3− secretion to enhance ENaC activity.
The LNaHK Diet and Distinct Roles of P[Aldo], P[ANG II], and P[K+]
Aldo, ANG II, and K+ should all be considered critical regulators of K+ homeostasis in animals on a LNaHK diet. The independent importance of each for maintaining K+ balance will be discussed in this section. In Table 2, we compare data from three previously published studies (7, 31, 150) and some unpublished data from mice and rats on a variety of diets with differing Na+ and K+ contents to better understand the independent and interdependent roles of P[Aldo], P[K+], and P[ANG II] in the regulation of K+ excretion with the LNaHK diet. Values of P[Aldo] were measured by either radioimmunoassay or ELISA. Also shown are P[K+] and renal ANG II concentrations, when determined. Although the absolute values for P[Aldo] are different, probably because of the method of analysis, the relative changes in P[Aldo] are similar with the four diets. These values will be referenced below, which discuss Aldo versus high P[K+] and the ANG II regulation of K+ excretion.
Table 2.
Effects of varying dietary Na+ and K+ on plasma K+ concentration, plasma aldosterone concentration, and renal ANG II
| Na+, % | K+, % | Plasma K+ Concentration, mM | Plasma Aldosterone Concentration, pg/ml | Method | ANG II, fmol/g | |
|---|---|---|---|---|---|---|
| Rat (150) | ||||||
| Regular | 0.5 | 0.8 | 4.8 | 128 | RIA | 120 |
| Low Na+ | 0.001 | 0.8 | 4.7 | 428 | RIA | 230 |
| High K+* | 0.5 | 5 | 5.1 | 420 | RIA | 120 |
| LNaHK* | 0.001 | 5 | 5.9 | 1355 | RIA | 220 |
| Mouse (31) | ||||||
| Regular | 0.3 | 0.6 | 3.8 | 239 | ELISA | ND |
| Regular ADX | 0.3 | 0.6 | 4.5 | 56 | ELISA | ND |
| High K+ | 0.3 | 5 | 4.6 | 753 | ELISA | ND |
| LNaHK | 0.01 | 5 | 4.3 | 3630 | ELISA | ND |
| LNaHK-ADX-low Na+ | 0.01 | 5 | 7.4 | 607 | ELISA | ND |
| LNaHK-ADX-high Na+ | 0.01 | 5 | 4.2 | 4293 | ELISA | ND |
| Mouse (7) | ||||||
| Regular | 0.3 | 0.9 | 4.5 | 202 | RIA | ND |
| Low Na+ | 0.01 | 0.9 | ND | 440 | RIA | ND |
| Low K+ | 0.3 | 0.05 | 3 | 64 | RIA | ND |
| High K+* | 0.3 | 3 | 4.9 | 354 | RIA | ND |
| Mouse (unpublished observations) | ||||||
| Low Na+ | 0.01 | 0.6 | 4.1 | 700 | ELISA | ND |
| LNaHK-captopril | 0.01 | 5 | 9.2 | 9278 | ELISA | ND |
ADX, adrenalectomy; RIA, radioimmunoassay; ND, not determined. Unpublished observations were from the Sansom laboratory. High-K+ and LNaHK diets contained 5% citrate/carbonate/Cl−.
Anionic content unknown.
In response to high K+ intake, Aldo places K+ into extrarenal cells (182) as well as stimulating K+ excretion in the distal nephron. Aldo has two major roles in the regulation of Na+ and K+ transport in the distal nephron. With low volume or low Na+ intake, the renin-angiotensin system stimulates aldosterone production to retain Na+, Cl−, and volume. The major site of action of low volume-stimulated Aldo is NCC of the early distal tubule. The second responsibility of Aldo is to respond to elevated P[K+]. With elevating P[K+], Aldo eliminates K+ in the CNT/CCD via a process involving enhancement of the activity of ENaC, which reabsorbs Na+ in exchange for K+ secretion. The regulatory mechanisms involving the differential regulation of Na+ reabsorption via NCC versus ENaC are very complex and involve the WNK-SPAK system, as mentioned above in the Introduction. However, evidence is clear that high K+ intake, probably through an increase in P[K+], will inhibit Na+ reabsorption via NCC, resulting in increased delivery of Na+ to ENaC to enhance the elimination of K+.
As shown in Table 2, the P[K+] of 5.8 mM for rats on a LNaHK diet was greater compared with the value of 4.6 mM in mice. The best explanation for the hyperkalemia in rats on the LNaHK diet is that the diet is more acid producing, with Cl− as the counteranion of K+ (150). However, the anionic content of the diet was not revealed.
Aldo Versus High P[K+]
When a high-K diet is consumed, elevated plasma levels of K+ increase the production of Aldo in the adrenal glomerulosa. Aldo regulates K+ secretion by increasing the activity of ENaC, which enhances Na+ reabsorption and, consequently, the driving force for K+ secretion (14, 27, 39, 85, 145). A long-standing question has been whether an increase in K+ secretion can be stimulated by high P[K+], independent of an increase in P[Aldo].
The question of Aldo-independent K+ secretion was addressed for LNaHK diet (0.01% Na+ and 5% K+)-fed mice, which have a P[Aldo] of >3,500 pg/ml (31). When adrenalectomized (no corticosteroid replacement), LNaHK diet-fed mice do not survive more than 2 days without Aldo replacement (31). As shown in Table 2, when given low Aldo replacement to a reach P[Aldo] of 607 pg/ml, the mice had a P[K+] of 7.4 mM and survived only 5 days. The K+ balance was maintained only with a high replacement dose of Aldo, which yielded a P[Aldo] of 4,293 pg/ml. Therefore, a very high P[Aldo] was necessary to maintain K+ balance in mice on a LNaHK diet.
Unlike animals on a LNaHK diet, when animals are given a normal-Na+, high-K+ diet, the K+ balance is maintained fairly well in the absence of Aldo. Early studies showed that adrenalectomized animals on a high-K+ diet maintained K+ balance due to high P[K+]-stimulated K+ excretion (177, 181). However, Arrighi et al. (7) found that P[Aldo] increased by only 1.5-fold with a high-K+ diet of normal Na+ and 3% K+, indicating that K+ is secreted without the need of Aldo. However, when K+ intake increases, the demand for Aldo increases. For example, the K+ balance was maintained in aldosterone synthase KO (AS−/−) mice with normal Na+ and 2% dietary K+. When AS−/− mice were given a 5% high-K+ diet, the K+ balance was not maintained as P[K+] increased (146).
The mechanism for Aldo-independent K+ secretion is not clearly understood but may rely on high P[K+] directly stimulating Na+-K+-ATPase activity in the CNT/CCD (88) and inhibiting Na+ reabsorption by NCC (112), thereby enhancing Na+-reabsorptive exchange for secreted K+. ENaC activity decreases as luminal Na+ concentration increases (98). This feedback effect is the result of increased cell Na+ entry because ENaC activity returns with amiloride treatment (42). However, large increases in luminal Na+ concentration can enhance the intracellular Na+ concentration of PCs (102). Moreover, if Na+-K+-ATPase is stimulated with high P[K+], then the intracellular Na+ concentration is maintained at a low level (131).. Therefore, increased ENaC and Na+-K+-ATPase activity will stimulate K+ secretion, causing only a mild increase in P[K+] that only increases Aldo by 50%.
It is not understood why P[Aldo] (and Aldo dependence) is more than fivefold in LNaHK diet-fed mice compared with high-K+ diet-fed mice. Based on transtubular Na+ gradients, estimated from urine osmolalities, the luminal Na+ concentration at the terminal CCD is between 30 and 40 mM in high-K+ diet-fed mice and only 1 mM in LNaHK diet-fed mice (169). Palmer et al. (98) found that ENaC activity was much greater in rats on a low-Na+ diet. Therefore, Aldo may be required for the increase in ENaC when Na+ intake is low. However, the Aldo demand to enhance ENaC activity may be reduced when the distal Na+ concentration is high.
In conclusion, high levels of P[Aldo] are required to maintain K+ homeostasis in LNaHK diet-fed mice. However, the Aldo-independent, P[K+]-stimulated K+ excretion of the high-K+ diet may be the result of an abundance of distal Na+ that is inhibited from reabsorbing via NCC and delivered to ENaC to stimulate K+ secretion. At the same time, Na+-K+-ATPase activity is stimulated by the high P[K+] from the basolateral side.
ANG II
Although very high Aldo is the most striking feature of the LNaHK diet, ANG II also has an important role to maintain K+ homeostasis. ANG II is increased in the plasma and locally in the kidney with the LNaHK diet in rats (150), probably because of low Na+ delivery to the macula densa. The relevance of ANG II was indicated when captopril (100 mg·kg−1·day−1 in drinking water) was given for the duration of the LNaHK diet (unpublished observation). Captopril treatment (n = 4) caused P[Aldo] to increase to very high levels of 9,278 ± 360 pg/ml, and P[K+] reached 9.23 ± 0.62 mM. Takaya et al. (141) showed that losartan, an ANG II type 1 receptor blocker given to low-Na+ diet-fed mice, resulted in increased P[K+] and a twofold increase in P[Aldo] (141). Therefore, ANG II may be necessary to increase P[Aldo] to 3,500 pg/ml; however, a very high P[Aldo] cannot compensate for the lack of ANG II, which is also required to maintain a normal P[K+].
The mechanism by which ANG II stimulates K+ secretion in LNaHK diet-fed mice can be complicated by the fact that inhibition of ANG II by captopril and losartan will decrease blood pressure with systemic effects. However, the mechanism may be connected to its direct effects on Na+ and K+ transport properties of the CNT/CCD. ANG II increases ENaC or Na+ reabsorption in isolated collecting ducts, independent of Aldo or any systemic effects (102). Mamenko et al. (82) showed that ANG II increased the open probability and membrane insertion of ENaC, as an independent and additive effect to that of Aldo. In isolated CCDs, ANG II application to either the luminal or basolateral side increased benzamil-sensitive Na+ uptake (102). In Aldo-sensitive distal nephron segments of mice, immunohistochemical staining revealed an increase in ENaC subunits (17), and patch-clamp analysis indicated an Aldo-independent increase in ENaC activity due to both low dietary Na+ and chronic ANG II infusion (83).
The role of ANG II in LNaHK diet-fed animals may be to insure a substantial ENaC-mediated Na+ reabsorptive driving force for K+ secretion with the minimally available luminal Na+ in the terminal CCD (102). A role for ANG II to maintain K+ homeostasis seemingly contradicts studies that have indicated that low volume-stimulated ANG II enhances NCC-mediated Na+ reabsorption. Since a high-K+ diet does not increase ANG II, its effect on NCC could explain the Aldo paradox (114, 127, 149, 151). Although ANG II may enhance NCC with low or normal P[K+], recent studies have shown that the high P[K+] of animals on a LNaHK diet should override the effects of ANG II and maximally inhibit NCC (150).
ANG II regulation of pendrin is also important for maintaining K+ homeostasis in animals on a LNaHK diet because it is necessary to secrete high HCO3− with K+. Pech et al. (99) showed with isolated perfused tubules that ANG II increased Cl− absorption via pendrin. Therefore, ANG II may be another common activator that links ENaC with pendrin activity (50).
Aldo and ANG II Regulation of K+ Secretory Channels
Aldo enhances not only the driving force for K+ secretion by increasing Na+ reabsorption (137) but also the K+ conductive pathways for K+ secretion (65, 116) in distal nephron segments. Initial studies have revealed that rabbits treated with mineralocorticoids or Aldo exhibited enhanced K+ conductance of the apical membrane of isolated rabbit CCDs (65, 116). These early studies measured the apical membrane conductance for K+. Subsequent patch-clamp studies made it possible to understand how the individual channel types might be regulated.
ROMK and BK channels have distinct and redundant roles to secrete K+ in the CCD (119). Although Aldo can increase the activity of both ROMK and BK channels, there may be separate signaling mechanisms for these channels. With a high-K+ diet, Aldo signaling increases insertion of ROMK channels in the apical membrane (156, 163, 180) and ANG II increases ROMK activity (164). A high-K+ diet increases ROMK mRNA (156) and protein expression (153) in the CNT/CCD. However, mice with KO of the Aldo-induced serum and glucokinase pathway (SGK−/− mice) exhibit elevated P[K+], a sixfold increase in P[Aldo], and downregulation of ENaC and Na+-K+-ATPase, as expected, but ROMK expression is enhanced (58). This finding indicates that ROMK channels may be regulated by elevated P[K+] rather than the Aldo-induced SGK pathway. In support of this notion, Todkar et al. (146) described an increase in ROMK expression in AS−/− mice, a model of elevated P[K+] in the absence of Aldo.
Patch-clamp analysis revealed that ANG II inhibited ROMK activity via ANG II type 1 receptors in mice on a low-K+ diet (165) but increased ROMK activity via ANG II type 2 receptors in mice on a high-K+ diet (164). Considering that ANG II is not elevated in rats on a high-K+ diet (150), the direct effects of ANG II on ROMK channels may be more relevant when animals are placed on a LNaHK diet. Van der Lubbe et al. (150) did not find an increase in ROMK (protein) expression in mice on a LNaHK diet. However, the open probability of ROMK channels or amount of ROMK channels in the membrane versus cytoplasm might be increased by ANG II (164). The ANG II-induced increase in ROMK activity, along with the increased ENaC activity, supports the notion that ANG II has a role to maintain K+ homeostasis in mice on a LNaHK diet.
Many studies found that BK channels in a variety of epithelial cells are regulated by Aldo (52, 60, 77, 133, 171). BK channels are contained in PCs as BK-α–BK-β1 (104) and in ICs as BK-α–BK-β4 (96). For the kidney, considerable evidence supports the notion that Aldo regulates BK-α–BK-β4 in ICs. A high-K+ diet enhanced the renal mRNA expression of BK-α and BK-β4 (90) and expression of BK-α protein in the cytoplasm of ICs (150, 171). However, BK-α remains in the cytoplasm if the high-K+ diet is acidic (171). BK-α does not express in the apical membrane of ICs of β4-KO mice on a LNaHK diet (171), which explains the considerably attenuated K+ excretion of β4-KO mice.
The role of BK-β4 in ICs is to prevent the lysosomal breakdown and promote the incorporation of BK-α into the plasma membrane (171). This trafficking function of BK-β4 is not supported by past studies, which have shown that BK-β4, along with BK-β1, prevents BK-α incorporation in the plasma membrane of cultured hair (8) and neuronal (130) cells. It is uncertain whether BK-α is associated with more than one subunit (159). The β2-subunit has been described in the CCD (90) and may also associate with BK-α in ICs. Moreover, it is now understood that palmitoylation of the β4-subunit controls the insertion of BK-α to the membrane by masking a trafficking motif on the COOH-terminus of BK-α (26). Thus, it remains to be determined how palmitoylation of BK-β4 is regulated in ICs. The roles and properties of β-subunits and their interactions with BK-α appear much more complex in different native cell systems than indicated in the original studies with cultured cells.
CNT cells and PCs of the CCD have been considered the primary targets of Aldo. Aldo-induced SGK (152) enhances ENaC (78) and Na+-K+-ATPase (138). However, IC-β also contains mineralocorticoid receptors as well as 11-β-steroid dehydrogenase (92), an enzyme that renders an Aldo-selective effect by inactivating glucorticoids (1, 134). These findings support the notion that Aldo regulates pendrin and BK channels in IC-β.
Although Aldo regulates IC-β of mice on a LNaHK diet, a recent study by Shibata et al. (128) reported that mineralocorticoids do not regulate IC transporters when mice are given a high-K+ (normal Na+) diet (128). Both IC-α and IC-β contain mineralocorticoid receptors with a specific site (MRS843) that prevents Aldo receptor binding when phosphorylated. High K+ enhances the phosphorylation of MRS843 via WNK4, thereby preventing an increase in Cl− reabsorption via pendrin in IC-β. Low extracellular volume, which increases ANG II in addition to Aldo, decreases the phosphorylation of MRS843, rendering mineralocorticoid receptor binding with enhanced H+-ATPase activity in IC-α and enhanced pendrin activity in IC-β. The model of Shibata et al. requires that IC-α secrete H+ that reacts with HCO3−, which is secreted from nearby IC-β (128, 158). Alternatively, H+-ATPase secretes H+ and pendrin secretes HCO3− from the same non-A-non-B ICs (63). Therefore, the study of Shibata et al. reports another explanation for the low volume or high K+ Aldo paradox.
The Shibata et al. model explains the paradox of either high K+ or low volume (high ANG II)-induced Aldo secretion by a mechanism that uses either high K+-induced PCs or low volume-induced IC transport. However, LNaHK diet-fed mice have increased plasma levels of Aldo, P[K+], and ANG II. Mice on a LNaHK diet require an effect of Aldo on both PCs and ICs, which are cooperating to maximize the ratio of K+ secreted to Na+ reabsorbed (169). Aldo enhances HCO3− secretion from a predominant population of IC-β and enhances ENaC-mediated Na+ reabsorption, which drives K+ secretion from both PCs and ICs (see Fig. 2). A modification of the Shibata et al. model has been proposed in which the high ANG II and Aldo of the LNaHK condition causes the dephosphorylation of MRS843 in only the predominating population of IC-β, allowing Aldo to enhance both pendrin and BK-α–BK-β4 activity as well as enhance the ENaC-mediated Na+ reabsorptive exchange for secreted K+ in PCs. This effect will enhance the exchange rate of secreted K+ per reabsorbed Na+ in animals on a LNaHK diet. This model explains why mice that consume the acid-loading LNaHK-Cl− diet are unable to maintain K+ balance. With a predominant population of IC-α with few IC-β (or non-A-non-B-cells), there will be increased Cl− reabsorption with Na+ rather than complete exchange of K+ for Na+, as observed with a predominant population of IC-β.
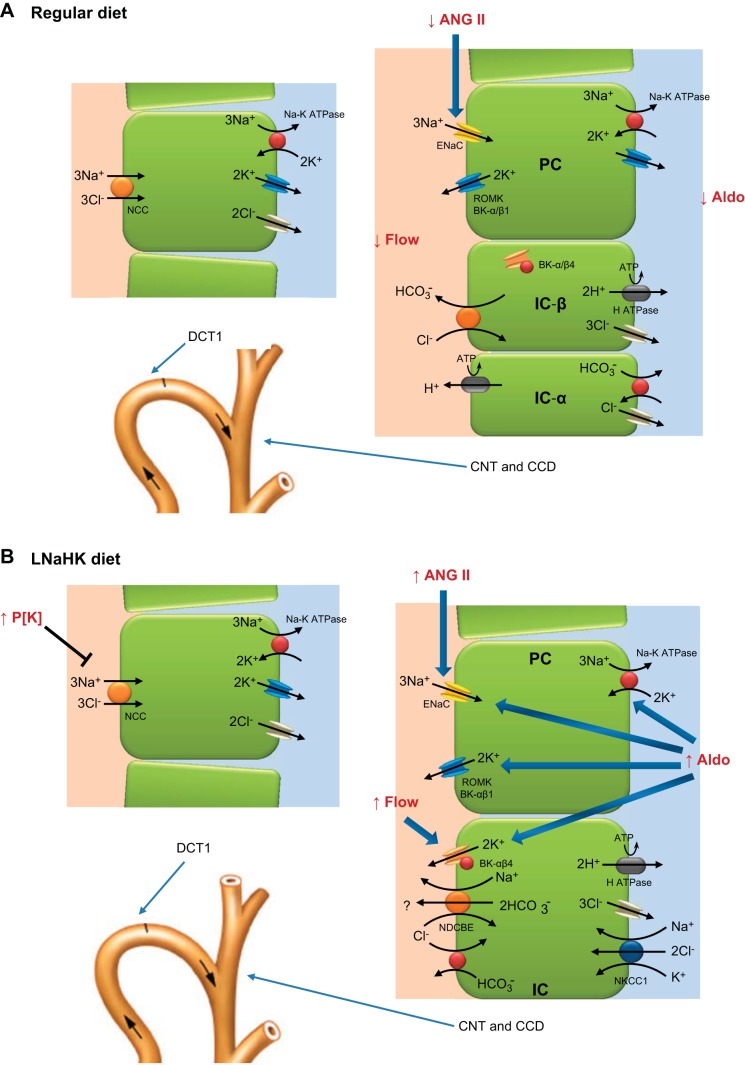
Fig. 2.
Cell models illustrating the effects of the LNaHK diet on Na+ and K+ transport properties of the distal nephron. A and B: illustrations of the Na+ and K+ transport pathways of the early distal convoluted tubule (DCT1) and CNT/CCD of animals on a regular diet (A) or LNaHK diet (B). A: for mice on a regular diet, ENaC reabsorbs 50% more Na+ than the Na+-Cl− cotransporter (NCC) in the distal nephron. Approximately 75% of the K+ transported on Na+-K+-ATPase is secreted into the lumen, and 25% is recycled across the basolateral membrane to yield a Na+ reabsorbed-to-K+ secreted ratio of 0.5. Cl− is reabsorbed via pendrin to account for the charge balance with Na+ reabsorption. B: the high K+ intake or elevating P[K+] turns off NCC of the DCT1, increasing the delivery of Na+ to ENaC. Renal and plasma ANG II levels elevate in response to the low Na+ intake with an increase in ENaC open probability and activation of renal outer medullary K+ (ROMK) channels. Aldosterone (Aldo) has several effects to enhance K+ secretion, including increasing BK channels in ICs and Na+-K+-ATPase, ENaC, and ROMK channels in PCs. The elevated flow stimulates BK channels in PCs and ICs. To enhance the secreted K+-to-Na+ reabsorbed ratio greater than 2, it is hypothesized that Na+ is recycled via the Na+-dependent Cl−/HCO3− exchanger.
Although a modification of the Shibata et al. model, with a predominance of IC-β, can explain the Aldo effects on both PCs and IC-β, it cannot explain the results of studies that showed that a high-K+ (normal Na+) diet causes Aldo-dependent BK-α–BK-β4-mediated K+ secretion in IC-β (171). In the high K+ condition, P[Aldo], but not ANG II, is elevated, and only PCs would be influenced by Aldo. However, the effects of Aldo on BK-α–BK-β4 were only observed with the alkaline-producing high-K+ diet (171). It remains to be determined whether an alkaline load causes dephosphorylation of MRS843 specifically in IC-β.
LNaHK and High Flow
Young et al. (183) originally reported that high K+ intake caused a profound natriuresis with hypotension. Although natriuresis is mild with low Na+ intake, high urinary flow is one of the most remarkable events associated with animals on a LNaHK diet (18, 30, 40, 146, 150, 169). In mice, flow increases from 1–1.5 ml/day with a regular diet to 4–6 ml/day when treated with a LNaHK diet (31). However, the mechanism underlying the high flow is uncertain.
High distal flow is necessary to eliminate a high K+ load. When a 12-fold normal K+ load is consumed, the rate of K+ excretion matches the input by increasing the rate of K+ secretion in the CNT and CCD. The rate of K+ secretion is composed of two components: the elevation of luminal K+ concentration and the rate of flow; however, the capacity to elevate luminal K+ concentration, which is ∼40 mM in mice on a regular diet, is limited, with a maximal transtubular K+ gradient of 20–25 (169). With a P[K+] near 4.5 mM, the luminal K+ concentration saturates at a value of 100–120 mM in mice on a LNaHK deit. Therefore, with an increase in luminal K+ concentration of only threefold (169), distal volume needs to increase by at least threefold to match the increase in K+ intake. High flow continually dilutes the volume and maintains the luminal K+ concentration near 110 mM.
While the high flow is necessary to prevent high K+ concentration in the CNT/CCD, conversely, the rate of K+ secretion must increase to match the rate of flow to maintain the high luminal K+ concentration. It has been shown that the continued reabsorption of Na+ is necessary to maintain a constant high transepithelial voltage driving force for K+ secretion during high flow (79). To increase Na+ reabsorption and transepithelial voltage with flow, it is necessary to continually activate ENaC. Isolated perfused tubule studies have shown that ENaC-mediated Na+ reabsorption is enhanced by flows from 0.5–3 (120) or 5 nl/min, even when ENaC membrane insertion is inhibited (87). Expression studies have shown that shear stress can increase the open probability of ENaC (3, 20, 120). Therefore, mechanical, flow-induced activation of ENaC may account for the increased Na+ reabsorption at a distal flow of 0.5–5 nl/min, which is considered a normal physiological range in rat distal tubules (56).
Malnic et al. (87) showed that the flow rate of the perfused distal tubule of high K+-treated rats stimulated K+ secretion to a steep level from 2 to10 nl/min, consistent with the flow range for mechanically activating ENaC and Na+ reabsorption. However, the rates of Na+ reabsorption and K+ secretion saturated and the luminal K+ concentration decreased with perfusion rates from 10 to 30 nl/min (79). The case was different with free flow micropuncture, in which high distal flow was increased by saline volume expansion. In these conditions, both Na+ reabsorption and K+ secretion continued with flows from 6 to 30 nl/min (61, 62, 68). These data indicate that the flow-induced shear stress of microperfusion increased the open probability of ENaC from 2 to 10 nl/min, as shown by the isolated tubule perfusion study by Morimoto et al. (87). However, as revealed by the free-flow experiments of Khuri et al. (61), another mechanism with more ENaC may be required for flows of >10 nl/min to increase Na+ reabsorption.
One explanation for the difference in the response of ENaC to the high flows of tubule perfusion and free flow is the presence of an unknown paracrine regulator of Na+ reabsorption in the endogenous tubular fluid. The precedence for this notion is the finding that flow-induced fluid reabsorption in the proximal tubule is exhibited with free-flow micropuncture but inconsistently, or at all, with microperfusion (49). It has been shown that if the proximal tubule is microperfused for 10 min with a solution containing the plasma ultrafiltrate, as opposed to an artificial solution, then flow-induced fluid reabsorption can be demonstrated (11). However, we have not found reports of similar studies that determined the relation between flow and ENaC-mediated Na+ reabsorption using plasma ultrafiltrate. A paracrine effect would be more evident with high flow in vivo as it is observed to act over the course of several minutes to increase the number of ENaC in the membrane. In contrast, the shear stress effect to increase ENaC open probability with flows from 1 to 5 nl/min could be regulated in the order of seconds.
High flow not only maintains a chemical driving force for K+ secretion, but also the luminal K+ conductive pathways are enhanced. This is accomplished by flow stimulation of the activity of BK channels in the distal nephron (9, 73, 142, 178, 178). However, very few in vivo studies have supplied evidence showing that BK channels are activated by flow. Mice with KO of the BK β1-subunit, which is localized in CNT cells, exhibit a deficient kaliuretic response to high flow induced by NaCl volume expansion (104). In ROMK KO mice, micropuncture revealed that K+ secretion in the distal nephron was inhibited by the BK channel blocker iberiotoxin (9). It is not known, however, whether the BK channels were responding to high flow from reduced Na+ reabsorption in the TAL or compensating for the lack of ROMK-mediated K+ secretion in the CNT. Another study revealed that BK-α–BK-β4 in the medullary collecting ducts were activated to reduce the volume of ICs and increase the luminal diameter of the tubules to accommodate the flow (52). However, with a LNaHK diet, BK-α–BK-β4 secretes K+ as a resident of the CNT, where there is a preponderance of HCO3− secreting IC-β (89), especially under alkaline conditions. Urinary flow is decreased only slightly, but K+ excretion is considerably reduced in β4-KO mice on a high-K+ or LNaHK diet (52). Therefore, BK-α–BK-β4 in the medullary collecting ducts activate to reduce cell volume in response to high flow, and BK channels of the CNT/CCD mediate net K+ secretion with high flow.
That flow-induced K+ secretion is blocked by iberiotoxin, a BK channel blocker, is seemingly inconsistent with the notion that BK-α–BK-β4 of ICs respond to flow. A previous expression study (86) with Xenopus oocytes indicated that the β4-subunit renders BK-α insensitive to iberiotoxin. However, other more recent reports have indicated that the BK-α/BK-β4 resistance to Iberiotoxin is cell specific (159) and that BK-α–BK-β4 is sensitive to iberiotoxin in native cells (179). Patch-clamp experiments are needed to determine whether BK-α–BK-β4 in IC-β is resistant to iberiotoxin.
Flow may activate BK channels by stimulating Ca2+ cell entry (73, 76) or releasing ATP, which increases cell Ca2+ through purinergic receptors (46, 55). Ca2+ enters the cell via flow-stimulated transient receptor potential vanilloid (TRPV)4 channels (15, 16) or ATP-activated TRPV3 channels (46). Mice with KO of TRPV4 do not exhibit flow-mediated K+ secretion (81, 143).
We do not completely understand how high flow is generated in mice on a LNaHK diet. High flow can be initiated in the kidney by either inhibiting Na+ reabsorbing transporters or increasing the osmolality of the distal tubule. Most of these Na+ reabsorbing transporters can be inhibited by diuretic pharmaceutical agents. Accordingly, these diuretics can be used to diagnose the origin of the site of Na+ inhibition, with the notion that a diuretic will be relatively ineffective if the Na+ transporter is already inhibited in animals on a LNaHK diet.
NKCC2 and NCC Pathways
High K+ consumption can enhance flow by acting as a loop diuretic inhibiting NKCC2 or inhibit the NCC like a thiazide diuretic. Previous studies have suggested that K+ recycling/reabsorption from the medullary region inhibits Na+ and Cl− absorption in the TAL (12, 25, 136), thereby acting as a loop diuretic. Furosemide was given in vivo to determine whether the TAL was inhibited in mice on a LNaHK diet. Furosemide was found to inhibit the TAL in mice on a LNaHK diet, with the osmolality decreasing from 2,555 ± 522 to 717 ± 15 mosmol/L and the Na+ output increasing from 9.0 ± 1.4 to 98.8 ± 20.4 (160). This magnitude of change of Na+ and osmolality was the same as found for furosemide-treated mice on a regular diet (160). It is therefore unlikely that the transport of NKCC2 in the TAL is affected by the LNaHK diet.
NCC localized in the early distal convoluted tubule (DCT) normally reabsorbs 5% of filtered Na+. With a LNaHK diet, the high P[K+] inhibits NCC of the early DCT, acting as a thiazide diuretic (41, 112, 112, 132, 148, 150). A recent study (59) indicated that acute inhibition of NCC does not result in enhanced Na+ reabsorption via ENaC. Rather, long-term inhibition of NCC causes morphological adaptations of the distal nephron with enhanced ENaC-mediated reabsorption for secreted K+. That high K+-induced natriuresis was prevented in NCC KO mice is evidence that high P[K+] inhibits NCC (132). High P[K+] probably causes a slight DCT depolarization that increases intracellular Cl−, resulting in the dephosphorylation of NCC (144).
The question is whether the natriuresis due to inhibition of NCC can result in fivefold diuresis. An early study (80) has shown that substantial Na+ and Cl− are delivered to NCC with a Na+ concentration of ∼50 mM, even with a low-Na+ diet. Therefore, ∼100 mosM will be delivered to the CNT/CCD. On a LNaHK diet, Na+ is reabsorbed by ENaC in the CNT/CCD, as indicated by <5 mM Na+ concentration in the urine of LNaHK diet-fed mice (169). Before water is extracted in the medullary regions, the Na+ concentration at the end of the CCD is <2 mM. Moreover, hydrochlorothiazide (HCTZ), an inhibitor of NCC, only decreased tubular fluid/plasma inulin from 3.79 to 2.78 in the late distal nephron of dogs (34), indicating a mild increase in flow of only 1.4-fold in the late DCT. HCTZ caused a natriuresis in normal diet-fded mice, but the urinary flow was not significantly increased (169). Thus, the natriuresis from inhibition of NCC might carry enough volume to stimulate BK channels but would probably not account for a four- to fivefold increase in flow through the CNT/CCD.
Kaliuretic Osmotic Diuresis and the IC-β Pathway
Luminal Na+ in the CNT/CCD of mice on a LNaHK diet is avidly reabsorbed via ENaC; however, the luminal Na+ and Cl− are replaced by K+ and HCO3−, such that there is no net loss of solutes. It was hypothesized that the ENaC-mediated Na+-dependent K+ secretion could be greater than the ENaC-dependent Na+ reabsorption. This hypothesis was tested with amiloride, an inhibitor of ENaC. It was important to establish with HPLC that the amiloride concentration of the tubular fluid totally inhibited ENaC. At a luminal concentration of >22 μM (169) and a Ki of ∼100 nM (123), ENaC activity was inhibited by >99%. When mice were placed on a normal diet and then given amiloride for 12 h, the amiloride-induced natriuresis was more than double the amount of amiloride-sensitive K+ excretion, consistent with a ratio for K+ secreted to Na+ reabsorbed of 0.5. However, when mice on a LNaHK diet for 7 days were given amiloride for 12 h, the amiloride-sensitive Na+ diuresis was ∼30% of the amount of amiloride-sensitive kaliuresis (169). This experiment indicated that more ENaC-dependent K+ is secreted than ENaC-dependent Na+ is reabsorbed in the CNT/CCD.
With the alkaline-producing LNaHK diet, the secreted K+ is essentially a nonreabsorbable solute because the major reabsorbing pathway in the CCD and medullary collecting ducts, H+-K+-ATPase, is inhibited (176). Thus, there is no net loss of solutes in the distal lumen when inhibiting NCC and Na+ is delivered to ENaC. The net addition of solute in the CNT/CCD could account for a large osmotic diuresis.
A kaliuretic osmotic diuresis could also result from Na+-independent K+ secretion, with the addition of luminal K+ without reabsorbing Na+, in the CNT/CCD. Rats exhibited Na+-independent K+ excretion when fed a high-K+-Cl− diet (5% K+ and 5% Cl−) for 7–9 days, and amiloride treatment significantly inhibited kaliuresis (40). This diet with Cl− as the counteranion produced an acid load, as indicated by the high urine Cl− output. However, amiloride treatment only increased P[K+] to 5.5 mM, which is considered only slightly hyperkalemic. In contrast, when mice were given the high-K+-Cl− diet for 7–10 days, they exhibited hyperkalemia (P[K+] = 6.85 mM), and P[K+] increased to lethal levels when mice were given amiloride (169). These results suggest that rats, unlike mice, may have a very efficient way to adapt to the high-K+-Cl− diet.
The question arises regarding how more K+ secretion is inhibited than Na+ reabsorbed when inhibiting ENaC when the Na+-K+-ATPase ratio of PCs is only 0.67 (2 K+ for 3 Na+). It has been suggested that K+ is secreted from a parallel pathway from ICs when mice are on a LNaHK diet (169). IC-β normally secrete HCO3− via pendrin, do not contain ENaC, and contain minimal, if any, Na+-K+-ATPase. It was therefore very surprising when amiloride caused a much greater reduction in K+ secretion in WT mice compared with β4-KO mice. These data supported the notion that BK-α–BK-β4 of ICs mediate ENaC-dependent K+ secretion in mice on a LNaHK diet.
A hypothetical mechanism, as shown in Fig. 2, was previously presented that describes a parallel path that is dependent on ENaC-mediated Na+ reabsorption yet results in a ratio of K+ secretion per Na+ reabsorbed of much greater than 2:3 and could be as much as 3:1 (169). The model was partly based on recent findings by Chambrey et al. (22) showing that transepithelial transport is powered by H+-ATPase, which helps establish an electronegative cell potential and sets up the driving force for HCO3− secretion via pendrin or one of the recently discovered members of the solute carrier (SLC)4 family across the apical cell membrane (35, 72) and the exit of Na+ via Na+-HCO3− cotransporter 1 (NBCn1) in the basolateral cell membrane. This model was established with chemical gradients that supported reabsorption of Na+ and Cl−. However, the transporters would reverse when faced with the chemical driving forces for animals on a LNaHK diet.
With a Na+-deficient diet, the urine Na+ concentration is reduced to <5 mM in rats (129) and mice (169). Accounting for water extraction, the Na+ concentration in the terminal CCD is <2 mM. The low luminal Na+ concentration establishes a chemical gradient for Na+ to passively recycle back through a paracellular pathway and be pumped back through PCs. Na+ can enter IC-β via NBCn1 in the basolateral membrane (22) or NKCC1. Although NBCn1 has been shown to extrude Na+ across the basolateral membrane of ICs, NBCn1 carries Na+ into the TAL (70). NKCC1 has been described functionally with the isolated perfused tubule (74) and was localized with immunohistochemistry in the basolateral membrane of ICs (45). That NKCC1 is involved in K+ excretion was indicated by Wall et al. (157), who showed that NKCC1 KO mice, compared with WT mice, on a regular K+ diet exhibited decreased K+ excretion and a significant increase in P[K+] (157). However, the increased P[K+] (to 4.6–4.8 mM) was still in the normal range. It would be interesting to determine the K+ handling of NKCC1 KO mice when treated with a high-K+ diet. A role for basolateral NKCC1 in K+ secretion has also been demonstrated for the intestine (48) and a variety of secretory epithelial cells (37).
K+ and Cl− can enter ICs via NKCC1 and exit via apical K+ and basolateral Cl− channels, respectively. However, it is not understood how Na+ exits ICs with levels of Na+-K+-ATPase too low to support transport. The best candidate for Na+ exit is the luminal Na+-dependent Cl−/HCO3− exchanger (45, 74), with chemical forces driving the HCO3− and Na+ back to the lumen. In the intestine, Na+ is also recycled from the blood to the lumen compartment (175). Moreover, a substantial Na+ permeability for Na+ backflux has been shown with isolated perfused CCDs (124, 125, 135). With Na+ secreting/recycling, more net K+ is secreted than net Na+ reabsorbed, thereby causing an addition of K+ solute that is nonreabsorbable. However, this mechanism is only hypothetically based on previously identified transporters and needs to be verified with in vivo and in vitro studies.
It is difficult to compare recent in vivo studies using LNaHK diets with earlier isolated perfused tubule experiments because most of these early studies focused on direct effects of Aldo on transport properties of the CCD by treating animals with Aldo or the synthetic mineralocorticoid DOCA (65, 94, 115, 116, 124, 124, 135). Using electrophysiological analysis with microelectrodes, DOCA treatment elicited a threefold increase in the amounts of secreted K+ and reabsorbed Na+ in the isolated perfused rabbit CCD (117). The ratio of K+ secreted to Na+ reabsorbed was ∼0.5 in both control and mineralocorticoid-treated rabbits. A ratio of K+ secreted to Na+ reabsorbed of 0.5 is much less than the ratio of >1 found for LNaHK diet-fed mice. However, with exogenous mineralocorticoid treatment, the plasma K+ concentration is reduced and the volume expansion reduces ANG II. It remains to be determined with isolated perfused tubules whether the high ratio of K+ secreted to Na+ reabsorbed indicated in vivo with the LNaHK diet can be demonstrated with the isolated CNT or CCD.
In summary, three different mechanisms, including natriuresis from inhibition of Na+ reabsorption in the TAL, natriuresis from inhibition of NCC in the DCT, and a kaliuretic osmotic kaliuresis, can contribute to the high flows associated with a high-K+ diet. It is possible that the natriuretic effects increase flow to reach a threshold value that is enough to activate BK channels, which contribute to the kaliuresis.
LNaHK Diet and Diuretic Therapy
With the LNaHK diet, Na+ reabsorption along the nephron is altered to maximize the use of the available Na+ that is necessary for exchange with secreted K+. It should not be surprising, therefore, that diuretics will have a different effect on electrolyte homeostasis in patients on LNaHK than in patients on a Western diet. Diet may explain hyperkalemia, instead of the expected hypokalemia, that often results from antihypertensive diuretics meant to reduce extracellular fluid volume (111, 139). When disregarding the patient's diet, administration of loop, thiazide, and K+-sparing diuretics could give unexpected changes in P[K+] and can lead to increased morbidity, expense, and time wasted in an attempt to obtain the correct response. We found several differences in the electrolyte response to three diuretics (furosemide, HCTZ, and amiloride) in mice on a LNaHK diet that were not observed in mice on a regular diet.
The loop diuretic furosemide, which inhibits NKCC2 of the TAL, caused a natriuresis and small reduction in P[K+] in mice on a regular diet. Decreased P[K+] is a prevalent complication of loop diuretics as high flow presumably causes BK channel-mediated K+ secretion. In mice on a LNaHK diet, furosemide still caused a small natriuresis, despite low Na+ intake, and decreased urine osmolality. Unexpectedly, however, furosemide inhibited K+ excretion in mice on a LNaHK diet (160). The mechanism for the inhibition of K+ excretion by furosemide is not understood and is under investigation. We conclude, however, that furosemide, normally considered a K+-wasting diuretic, may actually prevent K+ excretion in subjects on an ancient diet.
A striking feature of the LNaHK diet is the total ineffectiveness of thiazide diuretics, such as HCTZ. HCTZ normally inhibits NCC-mediated Na+ and Cl− reabsorption in the DCT (169). In the normal diet condition, NCC is phosphorylated and traffics to the apical membrane so that Na+ and Cl− are reabsorbed. It is now well documented that high K+ intake results in a dephosphorylation and reduced abundance of NCC (112, 132, 150) and low external K+ increases the phosphorylation of NCC (144, 154). Therefore, the high K+ intake and elevated P[K+] have already effectively turned off the NCC, rendering HCTZ ineffective as a diuretic.
The ENaC inhibitor amiloride also has a different effect in mice on a LNaHK diet. Amiloride is a more effective diuretic than HCTZ and reduces K+ excretion and causes a natriuresis in mice on a regular diet (170). However, in mice on a LNaHK diet, amiloride decreases K+ excretion more than it increases Na+ excretion. Because ENaC-dependent K+ secretion per Na+ reabsorption is close to 3, creating an osmotic diuresis, amiloride treatment tended to reduce urinary volume of mice on a LNaHK diet (169). Even with a very large increase in Na+ excretion, amiloride decreased flow and urine osmolality due to the large decrease in K+ excretion.
Conclusions
The LNaHK diet is an exaggerated form of present day “ancient” diets, but in vivo studies have revealed underlying physiological mechanisms for the regulation of K+ balance that have not been considered previously. Maintenance of K+ balance with a LNaHK diet requires that the diet is alkaline because HCO3− excretion plays a major role to assist in BK-α–BK-β4-mediated K+ secretion from IC-β. In contrast to animals on a high-K+ diet, the LNaHK diet requires a very high P[Aldo] of >10-fold normal to maintain K+ balance. However, with normal, high K+, the demand for Aldo is minimal as the elevating P[K+] stimulates Na+-K+-ATPase in the CCD and inhibits NCC, causing an increased delivery of Na+ to ENaC. Both ANG II and Aldo are required to maximize the ENaC-mediated Na+ reabsorptive driving force to maintain K+ balance in mice on the LNaHK diet. While ANG II may increase Cl− reabsorption from IC-β and IC-α in animals on a LNaHK-Cl− diet, ANG II may dephosphorylate mineralocorticoid receptors and enhance Aldo effects on K+ secretion from IC-β in animals on a LNaHK diet. The high urinary flow of mice on a LNaHK diet may be attributed to a combination of inhibiting NCC-mediated Na+ reabsorption and the high excretion rate of K+ in the CNT/CCD, causing an osmotic diuretic flow that activates BK channels.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.J.C. and S.C.S. conception and design of research; R.J.C. performed experiments; R.J.C., B.W., and J.W.-F. analyzed data; R.J.C., B.W., and J.W.-F. interpreted results of experiments; R.J.C., B.W., and S.C.S. prepared figures; R.J.C. drafted manuscript; R.J.C., B.W., J.W.-F., and S.C.S. edited and revised manuscript; R.J.C., B.W., J.W.-F., and S.C.S. approved final version of manuscript.
REFERENCES
- 1.Agarwal AK, Monder C, Eckstein B, White PC. Cloning and expression of rat cDNA encoding corticosteroid 11β-dehydrogenase. J Biol Chem 264: 18939–18943, 1989. [PubMed] [Google Scholar]
- 2.Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, Kahle KT. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal 7: re3, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J 21: 2389–2399, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 47: 296–308, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 336: 1117–1124, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol 22: 1981–1989, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrighi I, Bloch-Faure M, Grahammer F, Bleich M, Warth R, Mengual R, Drici MD, Barhanin J, Meneton P. Altered potassium balance and aldosterone secretion in a mouse model of human congenital long QT syndrome. Proc Natl Acad Sci USA 98: 8792–8797, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai JP, Surguchev A, Navaratnam D. β4-Subunit increases Slo responsiveness to physiological Ca2+ concentrations and together with β1 reduces surface expression of Slo in hair cells. Am J Physiol Cell Physiol 300: C435–C446, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Barfod ET, Moore AL, Lidofsky SD. Cloning and functional expression of a liver isoform of the small-conductance Ca2+-activated K+ channel SK3. Am J Physiol Cell Physiol 280: C836–C842, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Bartoli E, Earley LE. Importance of ultrafilterable plasma factors in maintaining tubular reabsorption. Kidney Int 3: 142–150, 1973. [DOI] [PubMed] [Google Scholar]
- 12.Battilana CA, Dobyan DC, Lacy FB, Bhattacharya J, Johnston PA, Jamison RL. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest 62: 1093–1103, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Dietary potassium intake and risk of stroke in US men and women: National Health and Nutrition Examination Survey I epidemiologic follow-up study. Stroke 32: 1473–1480, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci USA 95: 9424–9429, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berrout J, Jin M, Mamenko M, Zaika O, Pochynyuk O, O'Neil RG. Function of transient receptor potential cation channel subfamily V member 4 (TRPV4) as a mechanical transducer in flow-sensitive segments of renal collecting duct system. J Biol Chem 287: 8782–8791, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berrout J, Mamenko M, Zaika OL, Chen L, Zang W, Pochynyuk O, O'Neil RG. Emerging role of the calcium-activated, small conductance, SK3 K+ channel in distal tubule function: regulation by TRPV4. PLos One 9: e95149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41: 1143–1150, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Brooks DP, Crofton JT, Share L, Nasjletti A. High potassium intake increases the plasma concentration and urinary excretion of vasopressin in the rat. Experientia 42: 1012–1014, 1986. [DOI] [PubMed] [Google Scholar]
- 19.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem 279: 4120–4126, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Carrisoza-Gaytan R, Salvador C, Satlin LM, Liu W, Zavilowitz B, Bobadilla NA, Trujillo J, Escobar LI. Potassium secretion by voltage-gated potassium channel Kv1.3 in the rat kidney. Am J Physiol Renal Physiol 299: F255–F264, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hubner CA, Eladari D. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci USA 110: 7928–7933, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, Tsai SY, Pan WH. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr 83: 1289–1296, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Cheema-Dhadli S, Lin SH, Keong-Chong C, Kamel KS, Halperin ML. Requirements for a high rate of potassium excretion in rats consuming a low electrolyte diet. J Physiol 572: 493–501, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Bi D, Tian L, McClafferty H, Steeb F, Ruth P, Knaus HG, Shipston MJ. Palmitoylation of the beta4-subunit regulates surface expression of large conductance calcium-activated potassium channel splice variants. J Biol Chem 288: 13136–13144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, Malsure S, Ronzaud C, Stehle JC, Rossier BC, Hummler E. Sodium and potassium balance depends on αENaC expression in connecting tubule. J Am Soc Nephrol 21: 1942–1951, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapp JR, Rector FC Jr, Seldin DW. Effect of unreabsorbed anions on proximal and distal transtubular potentials in rats. Am J Physiol 202: 781–786, 1962. [DOI] [PubMed] [Google Scholar]
- 29.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 81: 341–354, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Cornelius RJ, Wen D, Hatcher LI, Sansom SC. Bicarbonate promotes BK-α/β4-mediated K excretion in the renal distal nephron. Am J Physiol Renal Physiol 303: F1563–F1571, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornelius RJ, Wen D, Li H, Yuan Y, Wang-France J, Warner PC, Sansom SC. Low Na, high K diet and the role of aldosterone in BK-mediated K excretion. PLos One 10: e0115515, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derst C, Konrad M, Kockerling A, Karolyi L, Deschenes G, Daut J, Karschin A, Seyberth HW. Mutations in the ROMK gene in antenatal Bartter syndrome are associated with impaired K+ channel function. Biochem Biophys Res Commun 230: 641–645, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Ecelbarger CA, Kim GH, Knepper MA, Liu J, Tate M, Welling PA, Wade JB. Regulation of potassium channel Kir 1.1 (ROMK) abundance in the thick ascending limb of Henle's loop. J Am Soc Nephrol 12: 10–18, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Edwards BR, Baer PG, Sutton RA, Dirks JH. Micropuncture study of diuretic effects on sodium and calcium reabsorption in the dog nephron. J Clin Invest 52: 2418–2427, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74: 325–349, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem 277: 37551–37558, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr 25: 247S–255S, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Frindt G, Ergonul Z, Palmer LG. Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol 131: 617–627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol 297: F389–F396, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frindt G, Silver RB, Windhager EE, Palmer LG. Feedback regulation of Na channels in rat CCT. II. Effects of inhibition of Na entry. Am J Physiol Renal Fluid Electrolyte Physiol 264: F565–F574, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Geleijnse JM, Kok FJ, Grobbee DE. Impact of dietary and lifestyle factors on the prevalence of hypertension in Western populations. Eur J Public Health 14: 235–239, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Giebisch G, Malnic G, Klose RM, Windhager EE. Effect of ionic substitutions on distal potential differences in rat kidney. Am J Physiol 211: 560–568, 1966. [DOI] [PubMed] [Google Scholar]
- 45.Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, Coleman RA, Wade JB. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol 7: 2533–2542, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Goel M, Schilling WP. Role of TRPC3 channels in ATP-induced Ca2+ signaling in principal cells of the inner medullary collecting duct. Am J Physiol Renal Physiol 299: F225–F233, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray DA, Frindt G, Palmer LG. Quantification of K+ secretion through apical low-conductance K channels in the CCD. Am J Physiol Renal Physiol 289: F117–F126, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Gustafsson JK, Linden SK, Alwan AH, Scholte BJ, Hansson GC, Sjovall H. Carbachol-induced colonic mucus formation requires transport via NKCC1, K+ channels and CFTR. Pflügers Arch 467: 1403–1415, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haberle DA, von BH. Characteristics of glomerulotubular balance. Am J Physiol Renal Fluid Electrolyte Physiol 244: F355–F366, 1983. [DOI] [PubMed] [Google Scholar]
- 50.Hadchouel J, Busst C, Procino G, Valenti G, Chambrey R, Eladari D. Regulation of extracellular fluid volume and blood pressure by pendrin. Cell Physiol Biochem 28: 505–512, 2011. [DOI] [PubMed] [Google Scholar]
- 51.He J, Tell GS, Tang YC, Mo PS, He GQ. Relation of electrolytes to blood pressure in men. The Yi people study. Hypertension 17: 378–385, 1991. [DOI] [PubMed] [Google Scholar]
- 52.Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-α/β4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol 21: 634–645, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoorn EJ, Nelson JH, McCormick JA, Ellison DH. The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol 22: 605–614, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoorn EJ, van der Lubbe N, Zietse R. The renal WNK kinase pathway: a new link to hypertension. Nephrol Dial Transplant 24: 1074–1077, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155–170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hropot M, Fowler N, Karlmark B, Giebisch G. Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney Int 28: 477–489, 1985. [DOI] [PubMed] [Google Scholar]
- 57.Huang CL, Cheng CJ. A unifying mechanism for WNK kinase regulation of sodium-chloride cotransporter. Pflügers Arch 467: 2235–2241, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang DY, Wulff P, Volkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15: 885–891, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Hunter RW, Craigie E, Homer NZ, Mullins JJ, Bailey MA. Acute inhibition of NCC does not activate distal electrogenic Na+ reabsorption or kaliuresis. Am J Physiol Renal Physiol 306: F457–F467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanthesh BM, Sandle GI, Rajendran VM. Enhanced K+ secretion in dextran sulfate-induced colitis reflects upregulation of large conductance apical K+ channels (BK; Kcnma1). Am J Physiol Cell Physiol 305: C972–C980, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khuri RN, Strieder N, Wiederholt M, Giebisch G. Effects of graded solute diuresis on renal tubular sodium transport in the rat. Am J Physiol 228: 1262–1268, 1975. [DOI] [PubMed] [Google Scholar]
- 62.Khuri RN, Strieder WN, Giebisch G. Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 228: 1249–1261, 1975. [DOI] [PubMed] [Google Scholar]
- 63.Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin W, Verlander JW, Sutliff RL, Wall SM. Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol Renal Physiol 293: F1314–F1324, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Koeppen BM, Giebisch GH. Mineralocorticoid regulation of sodium and potassium transport by the cortical collecting duct. Soc Gen Physiol Ser 39: 89–104, 1985. [PubMed] [Google Scholar]
- 66.Kohda Y, Ding W, Phan E, Housini I, Wang J, Star RA, Huang CL. Localization of the ROMK potassium channel to the apical membrane of distal nephron in rat kidney. Kidney Int 54: 1214–1223, 1998. [DOI] [PubMed] [Google Scholar]
- 67.Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol 10: 653–662, 2014. [DOI] [PubMed] [Google Scholar]
- 68.Kunau RT Jr, Webb HL, Borman SC. Characteristics of the relationship between the flow rate of tubular fluid and potassium transport in the distal tubule of the rat. J Clin Invest 54: 1488–1495, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee HL, Hering-Smith KS, Nakhoul NL. Acid-base and potassium homeostasis. Semin Nephrol 33: 257–264, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Lee S, Lee HJ, Yang HS, Thornell IM, Bevensee MO, Choi I. Sodium-bicarbonate cotransporter NBCn1 in the kidney medullary thick ascending limb cell line is upregulated under acidic conditions and enhances ammonium transport. Exp Physiol 95: 926–937, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lemann J., Jr Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: a review. Nephron 81, Suppl 1: 18–25, 1999. [DOI] [PubMed] [Google Scholar]
- 72.Leviel F, Hubner CA, Houillier P, Morla L, El MS, Brideau G, Hatim H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Liu W, Schreck C, Coleman RA, Wade JB, Hernandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu W, Wei Y, Sun P, Wang WH, Kleyman TR, Satlin LM. Mechanoregulation of BK channel activity in the mammalian cortical collecting duct: role of protein kinases A and C. Am J Physiol Renal Physiol 297: F904–F915, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, Song X, Shi Y, Shi Z, Niu W, Feng X, Gu D, Bao HF, Ma HP, Eaton DC, Zhuang J, Cai H. WNK1 activates large-conductance Ca2+-activated K+ channels through modulation of ERK1/2 signaling. J Am Soc Nephrol 26: 844–854, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280: F675–F682, 2001. [DOI] [PubMed] [Google Scholar]
- 79.Malnic G, Berliner RW, Giebisch G. Flow dependence of K+ secretion in cortical distal tubules of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 256: F932–F941, 1989. [DOI] [PubMed] [Google Scholar]
- 80.Malnic G, Klose RM, Giebisch G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol 211: 529–547, 1966. [DOI] [PubMed] [Google Scholar]
- 81.Mamenko M, Zaika O, Boukelmoune N, O'Neil RG, Pochynyuk O. Deciphering physiological role of the mechanosensitive TRPV4 channel in the distal nephron. Am J Physiol Renal Physiol 308: F275–F286, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension 62: 1111–1122, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mancilha-Carvalho JJ, Souza e Silva NA. The Yanomami Indians in the INTERSALT study. Arq Bras Cardiol 80: 289–300, 2003. [DOI] [PubMed] [Google Scholar]
- 85.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97: 5562–5567, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Muto S, Giebisch G, Sansom S. An acute increase of peritubular K stimulates K transport through cell pathways of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 255: F108–F114, 1988. [DOI] [PubMed] [Google Scholar]
- 89.Muto S, Yasoshima K, Yoshitomi K, Imai M, Asano Y. Electrophysiological identification of α- and β-intercalated cells and their distribution along the rabbit distal nephron segments. J Clin Invest 86: 1829–1839, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005. [DOI] [PubMed] [Google Scholar]
- 91.Nanami MM, Pech V, Lazo-Fernandez Y, Weinstein AM, Wall SM. ENaC inhibition stimulates HCl secretion in the mouse cortical collecting duct II: bafilomycin-sensitive H+ secretion. Am J Physiol Renal Physiol 309: F259–F268, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naray-Fejes-Toth A, Rusvai E, Fejes-Toth G. Minealocorticoid receptors and 11β-steroid dehydrogenase activity in renal principal and intercalated cells. Am J Physiol Renal Fluid Electrolyte Physiol 266: F76–F80, 1994. [DOI] [PubMed] [Google Scholar]
- 93.O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 371: 612–623, 2014. [DOI] [PubMed] [Google Scholar]
- 94.O'Neil RG, Helman SI. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol Renal Fluid Electrolyte Physiol 233: F544–F558, 1977. [DOI] [PubMed] [Google Scholar]
- 95.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation 52: 146–151, 1975. [DOI] [PubMed] [Google Scholar]
- 96.Pacha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991. [DOI] [PubMed] [Google Scholar]
- 97.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999. [DOI] [PubMed] [Google Scholar]
- 98.Patel AB, Frindt G, Palmer LG. Feedback inhibition of ENaC during acute sodium loading in vivo. Am J Physiol Renal Physiol 304: F222–F232, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007. [DOI] [PubMed] [Google Scholar]
- 100.Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM. Pendrin modulates ENaC function by changing luminal HCO3−. J Am Soc Nephrol 21: 1928–1941, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pech V, Wall SM, Nanami MM, Bao HF, Kim YH, Lazo-Fernandez Y, Yue Q, Pham TD, Eaton DC, Verlander JW. Pendrin gene ablation alters ENaC subcellular distribution and open probability. Am J Physiol Renal Physiol 309: F154–F163, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J Am Soc Nephrol 13: 1131–1135, 2002. [DOI] [PubMed] [Google Scholar]
- 103.Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-β1−/− mice. Am J Physiol Renal Physiol 284: F1274–F1279, 2003. [DOI] [PubMed] [Google Scholar]
- 104.Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-β1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005. [DOI] [PubMed] [Google Scholar]
- 105.Procino G, Mastrofrancesco L, Sallustio F, Costantino V, Barbieri C, Pisani F, Schena FP, Svelto M, Valenti G. AQP5 is expressed in type-B intercalated cells in the collecting duct system of the rat, mouse and human kidney. Cell Physiol Biochem 28: 683–692, 2011. [DOI] [PubMed] [Google Scholar]
- 106.Procino G, Milano S, Tamma G, Dossena S, Barbieri C, Nicoletti MC, Ranieri M, Di MA, Nofziger C, Svelto M, Paulmichl M, Valenti G. Co-regulated pendrin and aquaporin 5 expression and trafficking in type-B intercalated cells under potassium depletion. Cell Physiol Biochem 32: 184–199, 2013. [DOI] [PubMed] [Google Scholar]
- 107.Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int 71: 103–115, 2007. [DOI] [PubMed] [Google Scholar]
- 108.Purkerson JM, Tsuruoka S, Suter DZ, Nakamori A, Schwartz GJ. Adaptation to metabolic acidosis and its recovery are associated with changes in anion exchanger distribution and expression in the cortical collecting duct. Kidney Int 78: 993–1005, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rau WS, Fromter E. Electrical properties of the medullary collecting ducts of the golden hamster kidney. I. The transepithelial potential difference. Pflügers Arch 351: 99–111, 1974. [DOI] [PubMed] [Google Scholar]
- 110.Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev 80: 277–313, 2000. [DOI] [PubMed] [Google Scholar]
- 111.Remme WJ, Swedberg K. Comprehensive guidelines for the diagnosis and treatment of chronic heart failure. Task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Eur J Heart Fail 4: 11–22, 2002. [DOI] [PubMed] [Google Scholar]
- 112.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA. Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA 104: 4025–4029, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA 106: 4384–4389, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sansom S, Muto S, Giebisch G. Na-dependent effects of DOCA on cellular transport properties of CCDs from ADX rabbits. Am J Physiol Renal Fluid Electrolyte Physiol 253: F753–F759, 1987. [DOI] [PubMed] [Google Scholar]
- 116.Sansom SC, O'Neil RG. Mineralocorticoid regulation of apical cell membrane Na+ and K+ transport of the cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 248: F858–F868, 1985. [DOI] [PubMed] [Google Scholar]
- 117.Sansom SC, O'Neil RG. Effects of mineralocorticoids on transport properties of cortical collecting duct basolateral membrane. Am J Physiol Renal Fluid Electrolyte Physiol 251: F743–F757, 1986. [DOI] [PubMed] [Google Scholar]
- 118.Sansom SC, Weinman EJ, O'Neil RG. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 247: F291–F302, 1984. [DOI] [PubMed] [Google Scholar]
- 119.Sansom SC, Welling PA. Two channels for one job. Kidney Int 72: 529–530, 2007. [DOI] [PubMed] [Google Scholar]
- 120.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001. [DOI] [PubMed] [Google Scholar]
- 121.Schild L. The epithelial sodium channel and the control of sodium balance. Biochim Biophys Acta 1802: 1159–1165, 2010. [DOI] [PubMed] [Google Scholar]
- 122.Schild L, Kellenberger S. Structure function relationships of ENaC and its role in sodium handling. Adv Exp Med Biol 502: 305–314, 2001. [DOI] [PubMed] [Google Scholar]
- 123.Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol 109: 15–26, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schwartz GJ, Burg MB. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 235: F576–F585, 1978. [DOI] [PubMed] [Google Scholar]
- 125.Schwartz GJ, Burg MB. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 235: F576–F585, 1978. [DOI] [PubMed] [Google Scholar]
- 126.Schwartz JH, Alexander EA. Adaptation of intercalated cells along the collecting duct to systemic acid/base changes. Kidney Int 78: 949–951, 2010. [DOI] [PubMed] [Google Scholar]
- 127.Seva PB, van der Lubbe N, Verdonk K, Roks AJ, Hoorn EJ, Danser AH. Key developments in renin-angiotensin-aldosterone system inhibition. Nat Rev Nephrol 9: 26–36, 2013. [DOI] [PubMed] [Google Scholar]
- 128.Shibata S, Rinehart J, Zhang J, Moeckel G, Castaneda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 18: 660–671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shirley DG, Walter SJ, Unwin RJ. Mechanism of the impaired natriuretic response to frusemide during sodium depletion: a micropuncture study in rats. Clin Sci (Lond) 91: 299–305, 1996. [DOI] [PubMed] [Google Scholar]
- 130.Shruti S, Urban-Ciecko J, Fitzpatrick JA, Brenner R, Bruchez MP, Barth AL. The brain-specific Beta4 subunit downregulates BK channel cell surface expression. PLos One 7: e33429, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Silver RB, Frindt G, Windhager EE, Palmer LG. Feedback regulation of Na channels in rat CCT. I. Effects of inhibition of Na pump. Am J Physiol Renal Fluid Electrolyte Physiol 264: F557–F564, 1993. [DOI] [PubMed] [Google Scholar]
- 132.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. [DOI] [PubMed] [Google Scholar]
- 133.Sorensen MV, Matos JE, Sausbier M, Sausbier U, Ruth P, Praetorius HA, Leipziger J. Aldosterone increases KCa1.1 (BK) channel-mediated colonic K+ secretion. J Physiol 586: 4251–4264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CH, Edwards CR. Mineralocorticoid activity of liquorice: 11-β-hydroxysteroid dehydrogenase deficiency comes of age. Lancet 2: 821–824, 1987. [DOI] [PubMed] [Google Scholar]
- 135.Stokes JB. Potassium secretion by cortical collecting tubule: relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am J Physiol Renal Fluid Electrolyte Physiol 241: F395–F402, 1981. [DOI] [PubMed] [Google Scholar]
- 136.Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle's loop. J Clin Invest 70: 219–229, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stoner LC, Burg MB, Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol 227: 453–459, 1974. [DOI] [PubMed] [Google Scholar]
- 138.Summa V, Mordasini D, Roger F, Bens M, Martin PY, Vandewalle A, Verrey F, Feraille E. Short term effect of aldosterone on Na,K-ATPase cell surface expression in kidney collecting duct cells. J Biol Chem 276: 47087–47093, 2001. [DOI] [PubMed] [Google Scholar]
- 139.Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure? J Card Fail 10: 297–303, 2004. [DOI] [PubMed] [Google Scholar]
- 140.Takahashi D, Mori T, Nomura N, Khan MZ, Araki Y, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S. WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep 34: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takaya J, Matsusaka T, Katori H, Tamura M, Miyazaki Y, Homma T, Ichikawa I. In situ demonstration of angiotensin-dependent and independent pathways for hyperaldosteronism during chronic extracellular fluid volume depletion. Mol Endocrinol 15: 2229–2235, 2001. [DOI] [PubMed] [Google Scholar]
- 142.Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol 164: 35–45, 1998. [DOI] [PubMed] [Google Scholar]
- 143.Taniguchi J, Tsuruoka S, Mizuno A, Sato JI, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol 292: F667–F673, 2007. [DOI] [PubMed] [Google Scholar]
- 144.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thomas W, McEneaney V, Harvey BJ. Aldosterone-induced signalling and cation transport in the distal nephron. Steroids 73: 979–984, 2008. [DOI] [PubMed] [Google Scholar]
- 146.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J. Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26: 425–438, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tsuruoka S, Schwartz GJ. Mechanisms of HCO3− secretion in the rabbit connecting segment. Am J Physiol Renal Physiol 277: F567–F574, 1999. [DOI] [PubMed] [Google Scholar]
- 148.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79: 66–76, 2011. [DOI] [PubMed] [Google Scholar]
- 150.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013. [DOI] [PubMed] [Google Scholar]
- 151.van der Lubbe N, Zietse R, Hoorn EJ. Effects of angiotensin II on kinase-mediated sodium and potassium transport in the distal nephron. Curr Opin Nephrol Hypertens 22: 120–126, 2013. [DOI] [PubMed] [Google Scholar]
- 152.Verrey F. Sodium reabsorption in aldosterone-sensitive distal nephron: news and contributions from genetically engineered animals. Curr Opin Nephrol Hypertens 10: 39–47, 2001. [DOI] [PubMed] [Google Scholar]
- 153.Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300: F1385–F1393, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wade JB, Liu J, Coleman R, Grimm PR, Delpire E, Welling PA. SPAK-mediated NCC regulation in response to low-K+ diet. Am J Physiol Renal Physiol 308: F923–F931, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wagner CA, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid-base transport in the collecting duct. Pflügers Arch 458: 137–156, 2009. [DOI] [PubMed] [Google Scholar]
- 156.Wald H, Garty H, Palmer LG, Popovtzer MM. Differential regulation of ROMK expression in kidney cortex and medulla by aldosterone and potassium. Am J Physiol Renal Physiol 275: F239–F245, 1998. [DOI] [PubMed] [Google Scholar]
- 157.Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin W, Pham TD, Meyer JW, Lorenz JN, Beierwaltes WH, Dietz JR, Shull GE, Kim YH. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol 290: F409–F416, 2006. [DOI] [PubMed] [Google Scholar]
- 158.Wall SM, Pech V. The interaction of pendrin and the epithelial sodium channel in blood pressure regulation. Curr Opin Nephrol Hypertens 17: 18–24, 2008. [DOI] [PubMed] [Google Scholar]
- 159.Wang B, Jaffe DB, Brenner R. Current understanding of iberiotoxin-resistant BK channels in the nervous system. Front Physiol 5: 382, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang B, Wen D, Cornelius RJ, Yuan Y, Li H, Wang J, Sansom SC. Net K secretion in thick ascending limbin mice on low Na, high K diet. Experimental Biology 2015 2015. [Google Scholar]
- 161.Wang WH. Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects. Am J Physiol Renal Physiol 290: F14–F19, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang WH, Giebisch G. Regulation of potassium (K) handling in the renal collecting duct. Pflügers Arch 458: 157–168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990. [DOI] [PubMed] [Google Scholar]
- 164.Wei Y, Liao Y, Zavilowitz B, Ren J, Liu W, Chan P, Rohatgi R, Estilo G, Jackson EK, Wang WH, Satlin LM. Angiotensin II type 2 receptor regulates ROMK-like K+ channel activity in the renal cortical collecting duct during high dietary K+ adaptation. Am J Physiol Renal Physiol 307: F833–F843, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem 282: 6455–6462, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weinberger MH. Hypertension in African Americans: the role of sodium chloride and extracellular fluid volume. Semin Nephrol 16: 110–116, 1996. [PubMed] [Google Scholar]
- 167.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 27: 481–490, 1996. [DOI] [PubMed] [Google Scholar]
- 168.Weinstein AM. Potassium excretion during antinatriuresis: perspective from a distal nephron model. Am J Physiol Renal Physiol 302: F658–F673, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wen D, Cornelius RJ, Rivero-Hernandez D, Yuan Y, Li H, Weinstein AM, Sansom SC. Relation between BK-α/β4-mediated potassium secretion and ENaC-mediated sodium reabsorption. Kidney Int 86: 139–145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wen D, Cornelius RJ, Sansom SC. Interacting influence of diuretics and diet on BK channel-regulated K homeostasis. Curr Opin Pharmacol 15C: 28–32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wen D, Cornelius RJ, Yuan Y, Sansom SC. Regulation of BK-α expression in the distal nephron by aldosterone and urine pH. Am J Physiol Renal Physiol 305: F463–F476, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wen D, Sansom SC. Physiological role of NBCe2 in the regulation of electrolyte transport in the distal nephron. Am J Physiol Renal Physiol 309: F489–F491, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wen D, Yuan Y, Cornelius RJ, Li H, Warner PC, Wang B, Wang-France J, Boettger T, Sansom SC. Deficient acid handling with distal RTA in the NBCe2 knockout mouse. Am J Physiol Renal Physiol 309: F523–F530, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wen D, Yuan Y, Warner PC, Wang B, Cornelius RJ, Wang-France J, Li H, Boettger T, Sansom SC. increased epithelial sodium channel activity contributes to hypertension caused by Na+. Hypertension 66: 68–74, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.White JF, Imon MA. Intestinal HCO3− secretion in Amphiuma: stimulation by mucosal Cl− and serosal Na+. J Membr Biol 68: 207–214, 1982. [DOI] [PubMed] [Google Scholar]
- 176.Wingo CS, Cain BD. The renal H-K-ATPase: physiological significance and role in potassium homeostasis. Annu Rev Physiol 55: 323–347, 1993. [DOI] [PubMed] [Google Scholar]
- 177.Wingo CS, Seldin DW, Kokko JP, Jacobson HR. Dietary modulation of active potassium secretion in the cortical collecting tubule of adrenalectomized rabbits. J Clin Invest 70: 579–586, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. [DOI] [PubMed] [Google Scholar]
- 179.Xu WX, Ban T, Wang LQ, Zhao M, Yin L, Li G, Chen H, Schield J, Qiao GF, Yan JL, Li BY. KCa1.1 β4-subunits are not responsible for iberiotoxin-resistance in baroreceptor neurons in adult male rats. Int J Cardiol 178: 184–187, 2015. [DOI] [PubMed] [Google Scholar]
- 180.Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem 278: 23066–23075, 2003. [DOI] [PubMed] [Google Scholar]
- 181.Young DB. Relationship between plasma potassium concentration and renal potassium excretion. Am J Physiol Renal Fluid Electrolyte Physiol 242: F599–F603, 1982. [DOI] [PubMed] [Google Scholar]
- 182.Young DB. Quantitative analysis of aldosterone's role in potassium regulation. Am J Physiol Renal Fluid Electrolyte Physiol 255: F811–F822, 1988. [DOI] [PubMed] [Google Scholar]
- 183.Young DB, McCaa RE, Pan YJ, Guyton AC. The natriuretic and hypotensive effects of potassium. Circ Res 38: 84–89, 1976. [DOI] [PubMed] [Google Scholar]