Abstract
Evidence for an intracellular renin-angiotensin system (RAS) in various cell organelles now includes the endoplasmic reticulum, nucleus, and mitochondria (Mito). Indeed, angiotensin (ANG) AT1 and AT2 receptor subtypes were functionally linked to Mito respiration and nitric oxide production, respectively, in previous studies. We undertook a biochemical analysis of the Mito RAS from male and female sheep kidney cortex. Mito were isolated by differential centrifugation followed by a discontinuous Percoll gradient and were coenriched in Mito membrane markers VDAC and ATP synthase, but not β-actin or cathepsin B. Two distinct renin antibodies identified a 37-kDa protein band in Mito; angiotensinogen (Aogen) conversion was abolished by the inhibitor aliskiren. Mito Aogen was detected by an Aogen antibody to an internal sequence of the protein, but not with an antibody directed against the ANG I N terminus. ANG peptides were quantified by three direct RIAs; mitochondrial ANG II and ANG-(1–7) contents were higher compared with ANG I (23 ± 8 and 58 ± 17 vs. 2 ± 1 fmol/mg protein; P < 0.01, n = 3). 125I-ANG I metabolism primarily revealed the formation of 125I-ANG-(1–7) in Mito that reflects the endopeptidases neprilysin and thimet oligopeptidase. Last, immunoblot studies utilizing the ANG-(1–7)/Mas receptor antibody revealed the protein in isolated Mito from sheep renal cortex. Collectively, the current data demonstrate that Mito actively metabolize the RAS precursor protein Aogen, suggesting that ANG-(1–7) may be generated within Mito to establish an intramitochondrial RAS tone and contribute to renal mitochondrial function.
Keywords: ANG-(1–7), angiotensin-(1–7), kidney, renin-angiotensin system, mitochondria
the renin-angiotensin system (RAS) plays a pivotal role in regulating cardiovascular and renal function. Historically, the RAS is characterized as a circulating system that contributes to the regulation of blood pressure and fluid homeostasis. In a paracrine manner, the RAS influences local tissue systems including the brain, adrenal cortex, heart, and kidney. These organ systems were subsequently found to contain a functional RAS capable of responding in an autocrine fashion. Indeed, there is compelling evidence for functional actions of intracellular renin-angiotensin systems in various cell organelles, including the endoplasmic reticulum, nucleus, and the mitochondria within various tissues (1, 2, 4, 5, 25, 26, 42).
Mitochondria are the major energy-producing organelles within cells. These organelles are a primary source of reactive oxygen/nitrogen species and may regulate intracellular redox status and influence cellular signaling. Mitochondrial function and oxidant production are altered in cardiovascular pathologies that may reflect, in part, a role of the ANG II axis of the RAS. ANG II, a potent vasoconstrictor and inflammatory peptide hormone, stimulates the production of mitochondrial reactive oxygen species (ROS) (15, 29). In this regard, recent studies demonstrate an intramitochondrial RAS within a variety of tissue types (1, 5). Abadir et al. (1) report that ANG II type 2 (AT2) receptors are expressed on the inner mitochondrial membrane and are coupled to nitric oxide (NO) production in liver mitochondria. Moreover, an increased ratio of mitochondrial ANG II type 1 (AT1) to AT2 receptors was associated with aging; chronic treatment of aged mice with the AT1 receptor antagonist losartan abrogated the higher AT1 receptor expression (1). These results suggest that one target of chronic RAS blockade to blunt the effects of cellular aging is the mitochondria. Indeed, Ferder, Inversa, and colleagues (11–13) demonstrate that either angiotensin-converting enzyme (ACE) inhibition or angiotensin receptor blockers (ARBs) attenuated the decline in mitochondrial function. However, a more recent study has raised concerns over the biochemical evidence for an intramitochondrial RAS (5). Astin et al. (5) failed to detect RAS components in purified liver mitochondria and reported marginal inhibition of mitochondrial respiration by ANG II at a supraphysiological dose (1 μM), suggesting a nonspecific rather than a direct effect of ANG II.
Since the Abadir and Astin studies focused primarily on the ANG II-AT1/AT2 receptor axis in rodents, we undertook a biochemical analysis of the mitochondrial RAS that examined the ANG-(1–7) axis. The current study provides evidence for several RAS components, including angiotensinogen, active renin, ANG I, and the bioactive peptides ANG II and ANG-(1–7) in purified mitochondria from the sheep renal cortex. Moreover, we demonstrate that purified mitochondria process ANG I to ANG-(1–7) by the endopeptidases neprilysin and thimet oligopeptidase, suggesting the possibility for an intramitochondrial RAS pathway that forms ANG-(1–7).
METHODS
Animals
Mixed-breed sheep (obtained from a private local vendor) were delivered at term, farm-raised, and weaned at 3 mo of age. Adult male and female sheep (10–12 mo of age) were anesthetized with ketamine and isoflurane and euthanized by exsanguination. The kidneys were removed immediately, and the renal cortex was dissected out on ice for immediate isolation of mitochondria. Cortical tissue or isolated mitochondria were stored at −80°C. All procedures in the current study were approved by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee for animal care.
Isolation of Sheep Renal Cortex Mitochondria
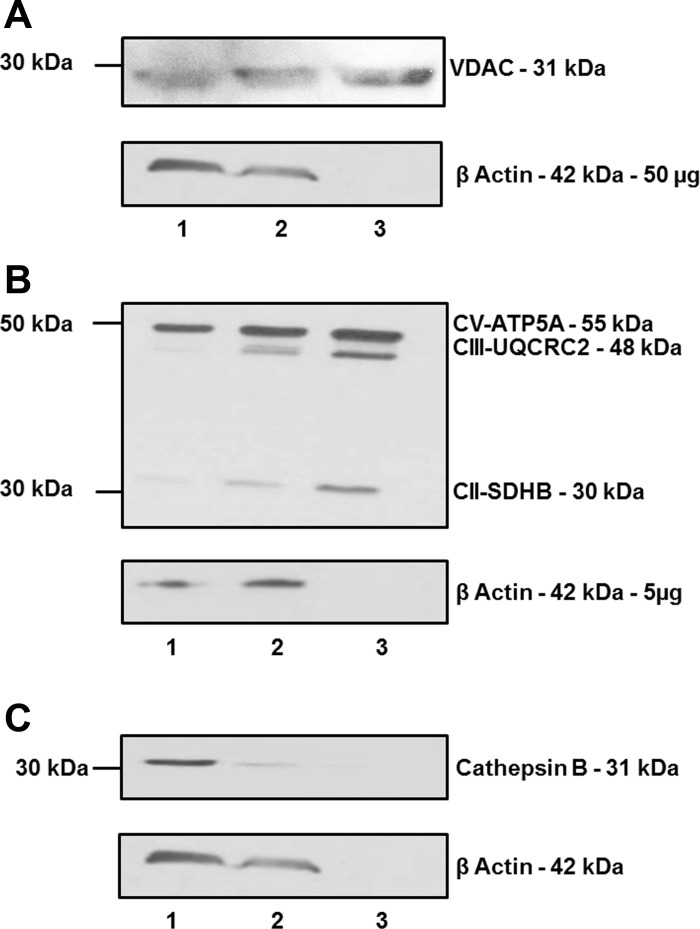
Mitochondria were isolated from fresh sheep renal cortex by a discontinuous Percoll gradient, as described previously (20, 39, 46). The tissue was homogenized in mitochondrial homogenization buffer [75 mM sucrose, 225 mM mannitol, 10 mM Na+-free HEPES, and 0.1 mM EDTA, pH 7.4 (KOH only)] using a Polytron Ultra-Turrax T25 Basic (setting 5), followed by a 50-ml all-glass Dounce homogenizer (Kontes Glass, Vineland, NJ). The homogenate was centrifuged for 5 min at 1,000 g, and then the supernatant was collected and filtered through a 70-μm metal strainer and centrifuged for 10 min at 10,000 g. The resulting pellet, representing the crude mitochondrial fraction, was resuspended in equal amounts of 12% Percoll (GE Healthcare, Buckinghamshire, UK) in Percoll buffer [250 mM sucrose, 0.4 mM EDTA, and 10 mM Na+-free HEPES]. This suspension was layered onto a discontinuous Percoll gradient (24 and 40% in Percoll buffer). The gradient was centrifuged for 5 min at 15,000 g, and the layer between the 24 and 40% Percoll suspensions containing the purified mitochondria was collected. The preparation was washed in Percoll buffer and centrifuged for 10 min at 15,000 g. The purity of the isolated mitochondria was determined by immunoblot analysis (Fig. 1, A–C). Antibodies were obtained from the following sources: voltage-dependent anion channel (VDAC; 1:1,000, Cell Signaling), an outer mitochondrial membrane marker, and several inner mitochondrial markers of the electron transport chain: ATP synthase complex V (CV-ATP5A); ubiquinol-cytochrome c reductase core protein II complex III (CIII-UCRC2); and succinate dehydrogenase complex subunit B complex II (CII-SDHB) obtained from Mito Sciences (1:1,000, Total OXPHOS Antibody Cocktail). A cathepsin B antibody was utilized as a marker for lysosomes and renin granules (50) (1:400, ab58802, Abcam). Membranes were probed with a mouse monoclonal anti-β-actin (1:5,000, Sigma, St. Louis, MO) antibody to assess the degree of purity.
Fig. 1.
Purification and enrichment of mitochondria from sheep renal cortex. Whole renal cortex homogenate (lane 1), crude mitochondrial fraction (lane 2), and Percoll-purified mitochondrial fraction (lane 3) were subjected to 10% SDS gel and immunoblotting with anti-VDAC (50 μg total protein; A) as well as ATP5A, UQCRC2, SDHB (5 μg total protein; B), and cathepsin B (50 μg total protein; C), as well as β-actin. Purified mitochondria exhibited greater expression of outer mitochondria marker (VDAC) and inner mitochondrial markers (ATP5A, UQCRC2, and SDHB), while cathepsin B and β-actin expression were diminished.
Immunoblot Analysis of RAS Components
Purified mitochondrial homogenates (∼100 μg) were boiled in PBS (pH 7.4), diluted in Laemmli buffer with β-mercaptoethanol, separated on 10% SDS polyacrylamide gels for 1 h at 120 V in Tris-glycine SDS, and transferred to a polyvinylidene difluoride (PVDF) membrane. Blots were blocked with 5% Bio-Rad Dry Milk (Bio-Rad, Hercules, CA) and Tris-buffered saline (TBS) with Tween (0.05%) and probed overnight at 4°C with primary antibodies against the following: rat angiotensinogen (internal sequence of angiotensinogen, Total angiotensinogen, residues 42–57, 1:1,000); ANG I sequence of angiotensinogen (ANG I-angiotensinogen, residues 25–34, 1:1,000); renin (1:3,000, Inagami antibody no. 826); renin (1:100, Aviva); prorenin receptor (ATP6IP2; 1:500, Abcam); neprilysin (NEP; 1:2,000, Cell Signaling); thimet oligopeptidase (TOP; 1:800, Epitomics); and the ANG-(1–7)/Mas receptor protein (MAS; 1:250, Alomone AAR-013). Membranes were treated with horseradish peroxidase (HRP)-labeled polyclonal anti-rabbit secondary antibodies (1:5,000) for 1 h and detected with ECL chemiluminescent substrates (Advansta, Menlo Park CA).
Renin Assay
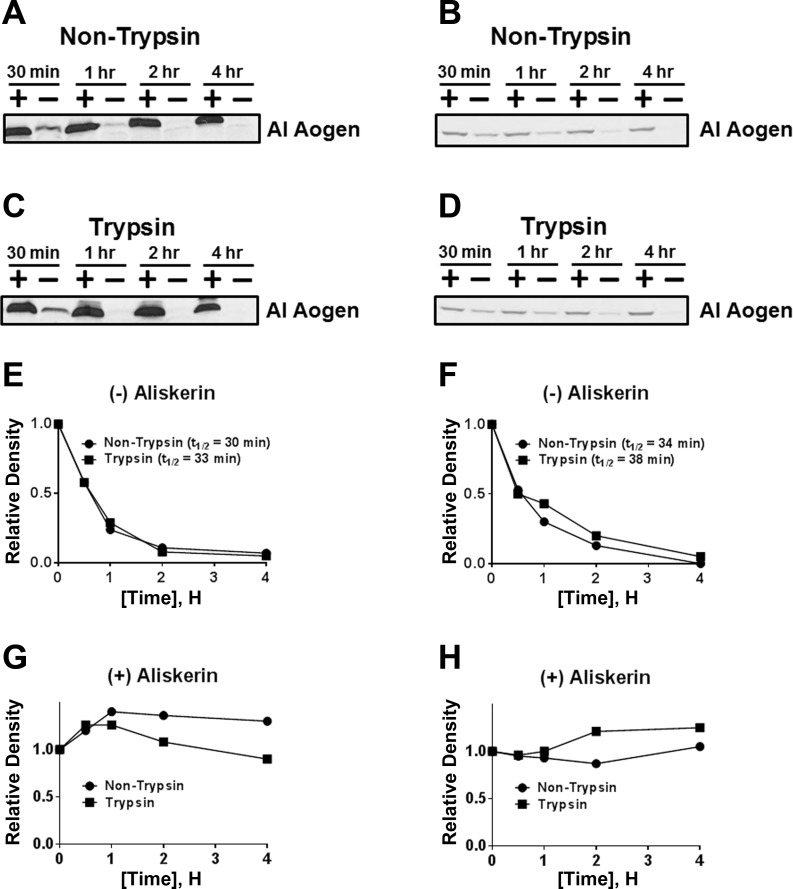
Purified mitochondria were isolated as described above and stored at −80°C. The mitochondrial pellet was reconstituted in 1 ml of metabolism buffer (25 mM HEPES, 125 mM NaCl, 10 μM ZnCl2) at pH 7.4, sonicated and left for 60 min on ice. A sample from the homogenate was taken for protein content. For a basal renin activity assay, 400 μg of mitochondrial homogenate was added to 10 μl of nephrectomized sheep plasma as the source of the exogenous angiotensinogen substrate. The assay was performed at 37°C for 4 h in the presence or absence of the renin inhibitor aliskiren (1 μM, final concentration). Aliquots were removed at 30 min, 1, 2, and 4 h and analyzed by Western blotting using the ANG I-angiotensinogen antibody. Activation of prorenin was performed by addition of 5 mg/ml trypsin (Sigma) to the mitochondrial sample for 60 min on ice. Trypsin was inactivated by incubation with an excess of soybean trypsin inhibitor (SBTI; 1 mg/ml, Sigma) for 15 min at room temperature. SBTI is a potent serine protease inhibitor that does not attenuate renin, an aspartyl protease. Following SBTI, the renin assay was performed as described above.
Angiotensin Peptides
Sheep renal cortex mitochondria were isolated as described above and stored at −80°C. The mitochondrial pellet was reconstituted in Milli-Q water and placed in a boiling water bath for 15 min. The mitochondrial fraction was acidified with heptafluorobutyric acid (HFBA) to a final concentration of 0.1%, sonicated, and centrifuged at 20,000 g for 20 min at 4°C. The resultant supernatant was applied to an activated Sep-Pak C18 extraction column, washed with 0.1% HFBA, and the peptide fraction was eluted with 3 ml of 100% methanol in 0.1% HFBA. Measurement of immunoreactive ANG I, ANG II, and ANG-(1–7) in the extracted mitochondria was performed using three distinct (RIAs) (4). The ANG-(1–7) RIA fully recognizes ANG-(1–7) and ANG-(2–7), but cross-reacts <0.01% with ANG-(3–7), ANG II, ANG I, and their fragments. The ANG II RIA equally recognizes ANG III, ANG-(3–8), and ANG-(4–8), but cross-reacts <0.01% with ANG I and ANG-(1–7). The limit of detection was 1 fmol/tube for ANG I, 0.5 fmol/tube for ANG II, and 4 fmol/tube for ANG-(1–7) (4, 35, 36).
125I-ANG I Metabolism
125I-ANG I processing was measured at 37°C in the metabolism buffer using the mitochondrial lysate (50 μg) in a final volume of 250 μl. Each reaction contained a final concentration of 0.5 nM 125I-ANG I and 100 nM ANG I with or without the inhibitors N-[N-[1-(S)-carboxyl-3-phenylpropyl]-(S)-phenyl-alanyl]-(S)-isoserine (SCH; 10 μM) and SCH (10 μM)/N-[1-(R,S)-carboxy-3-phenylpropyl]-Ala-Ala-Phe-p-aminobenzoate (CPP; 10 μM). The reactions were stopped after 60 min by addition of ice-cold 1.0% phosphoric acid and centrifugation at 16,000 g. The supernatants were immediately filtered, and the products were detected by HPLC-γ detection as previously described (34, 53). Preliminary studies also assessed 125I-ANG II metabolism using an identical approach as that for the mitochondrial metabolism of ANG I.
Statistical Analysis
All measurements are expressed as means ± SE. Differences between the groups were analyzed by one-way ANOVA and Newman-Keuls multiple comparison analysis. Statistical analyses were performed and figures constructed with GraphPad Prism V (GraphPad Software, San Diego, CA). A probability value of <0.05 was required for statistical significance.
RESULTS
Angiotensinogen and Renin
We verified mitochondrial enrichment and purity utilizing immunoreactive probes against proteins to outer mitochondrial (VDAC) and inner mitochondrial (ATP5A, UCRC2, and SDHB) membranes. Immunoreactivity for VDAC (Fig. 1A), ATP5A, UCRC2, and SDHB (Fig. 1B) was enriched as mitochondria were purified through differential centrifugation and a discontinuous Percoll gradient. In contrast, expression of the lysosomal and renin granule marker cathepsin B (Fig. 1C) in addition to the cellular protein β-actin was absent in the final Percoll fraction (Fig. 1, A–C). Based on the mitochondrial purity obtained by the Percoll gradient, this preparation was utilized in subsequent experiments to characterize the RAS components.
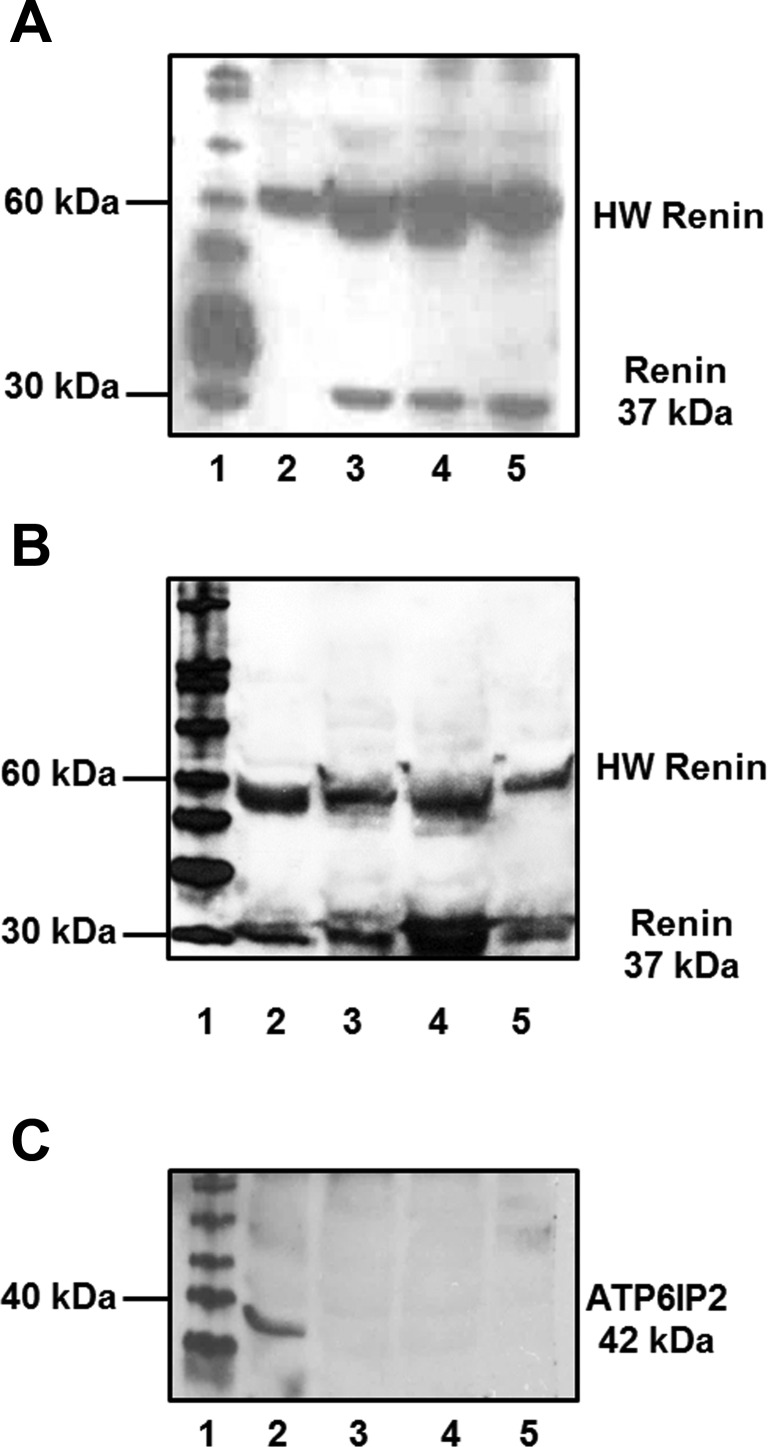
We initially assessed renin and the prorenin receptor expression in three mitochondrial homogenates and a renal cortex homogenate. Two distinct protein bands of 37 and 55 kDa were identified utilizing both the AVIVA renin antibody (Fig. 2A) and the Inagami renin antibody (Fig. 2B). Despite the presence of renin, there was no immunological evidence of the prorenin receptor (ATP6IP2) using the Abcam antibody in three separate mitochondrial preparations, although we noted a protein band of 35 kDa in the cortical homogenate (Fig. 2C). We then assessed renin activity in isolated mitochondria from male (Fig. 3, A, C, E, and G) and female (Fig. 3, B, D, F, and H) sheep by measuring the disappearance of intact angiotensinogen from nephrectomized sheep plasma (NSP) in the absence or presence of the renin inhibitor aliskiren (Fig. 3). The renin assay was facilitated by the specificity of the ANG I-angiotensinogen antibody to recognize the ANG I sequence of angiotensinogen. As shown in the immunoblot in Fig. 3 (A–D), renin activity in isolated mitochondria was evident in the time-dependent disappearance of intact angiotensinogen at 37°C and that ANG I-angiotensinogen processing was abolished by aliskiren (Fig. 3, G and H). We did not attempt to quantify renin activity between the male and female mitochondria isolated from the renal cortex. To determine prorenin levels, mitochondria were pretreated with trypsin to process prorenin to active renin, and angiotensinogen processing was determined in the presence of the trypsin inhibitor SBTI. Trypsin activation of mitochondrial prorenin did not appear to markedly increase renin activity as the disappearance rate of ANG I-angiotensinogen was comparable for the trypsin- and non-trypsin-treated mitochondria samples in males and females (Figs. 3, C and D and E and F).
Fig. 2.
Renin and prorenin receptor expression in isolated mitochondria from sheep renal cortex. A: immunoblot of rat renal proximal tubular (NRK-52E) cells (lane 2) and sheep renal mitochondria preparations (lanes 3–5) with the Inagami renin antibody revealed 2 distinct protein bands (37 and 55 kDa). Lanes 3–4 represent female mitochondrial preparations, while lane 5 represents a male preparation. B: immunoblot of renal cortex homogenate (lane 2) and sheep renal mitochondria preparations from 3 male sheep (lanes 3–5) with the Aviva renin antibody revealed 2 distinct protein bands (37 and 55 kDa). C: immunoblot of prorenin receptor (ATP6IP2) revealed a predominant protein band at 35 kDa in renal cortex homogenate (lane 2), but not in the mitochondrial preparations from 3 male sheep (lanes 3–5). Lane 1 (A–C) is the protein molecular weight marker.
Fig. 3.
Renin activity in the mitochondria. Renin activity in the mitochondria of a male (A, C, E, and G) and female (B, D, F, and H) sheep. Non-trypsin (A and B)- or trypsin (C and D)-treated mitochondrial homogenates with (+) or without (−) the renin inhibitor aliskiren was detected by immunoblot analysis using an ANG I (AI)-angiotensinogen (Aogen)-directed antibody. Relative quantification of the ANG I-Aogen band without (E and F) or containing aliskiren (G and H) was performed. The disappearance or half-life (t½) of AI-Aogen was determined from each experiment.
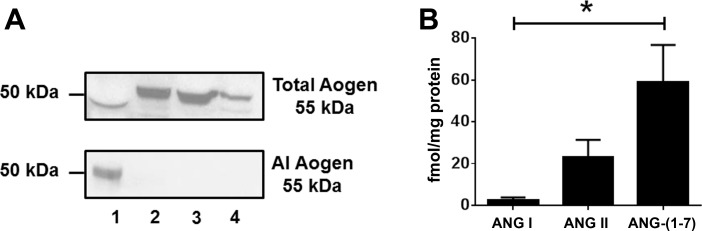
The protein precursor angiotensinogen was assessed by immunoblotting using two distinct antibodies: a “total” angiotensinogen antibody that targeted an internal amino acid sequence distal to ANG I and the ANG I-angiotensinogen antibody directed against the ANG I sequence (4). Thus the ANG I-angiotensinogen antibody detects only intact ANG I-containing angiotensinogen, and the total angiotensinogen antibody detects both intact and des-ANG I angiotensinogen (i.e., renin-processed angiotensinogen). The total angiotensinogen antibody recognized a 55-kDa protein band in three different male mitochondrial preparations and in NSP; however, the ANG I-angiotensinogen antibody failed to detect a protein band in the mitochondrial homogenates (Fig. 4A). The absence of ANG I-angiotensinogen may suggest active processing of the precursor; therefore, we determined the expression of angiotensin peptides. Mitochondrial ANG I, ANG II, and ANG-(1–7) content was then quantified using three direct RIAs targeted to each peptide in isolated mitochondria from three separate preparations (Fig. 4B). The mitochondrial content of ANG II and ANG-(1–7) were both significantly higher than ANG I [23 ± 8 and 58 ± 17 vs. 2 ± 1 fmol/mg protein; P < 0.01, n = 3].
Fig. 4.
Angiotensinogen (Aogen) and angiotensin peptide expression in isolated mitochondria from sheep renal cortex. A: immunoblot of Aogen in nephrectomized sheep plasma (NSP; lane 1) and isolated mitochondria from the renal cortex of 3 male sheep (lanes 2–4). A protein band of 55 kDa was detected for total Aogen in all lanes (top), but AI-Aogen was not detected in the mitochondria preparations (bottom). B: angiotensin peptide expression was quantified in isolated mitochondria from sheep renal cortex using distinct radioimmunoassays. ANG I, ANG II, and ANG-(1–7) were detected; however, there was a significant difference between the levels of ANG I compared with ANG II and ANG-(1–7). Values are means ± SE; n = 3. *P < 0.01 ANG I vs. ANG II, ANG I vs. ANG-(1–7).
ANG I Processing
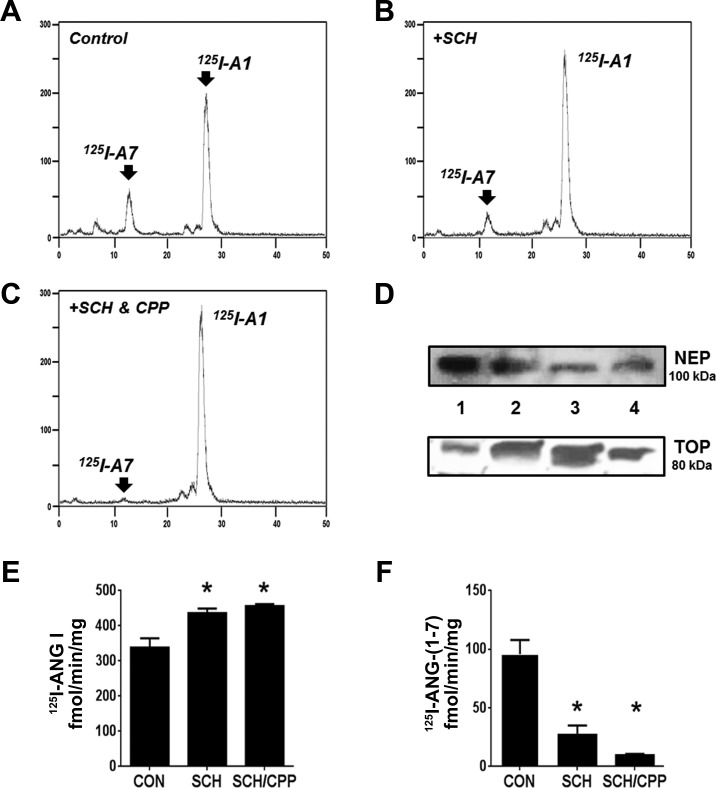
We examined the potential processing pathway of ANG I in isolated mitochondrial homogenates from sheep renal cortex using the 125I-labeled peptide as a substrate. As shown in the chromatographs for Fig. 5, 125I-ANG I was metabolized primarily to 125I-ANG-(1–7). Addition of the neprilysin inhibitor SCH reduced the 125I-ANG-(1–7) peak (Fig. 5B). The coaddition of SCH and the thimet oligopeptidase inhibitor CPP further reduced the 125I-ANG-(1–7) peak and preserved the 125I-ANG I peak (Fig. 5C). Immunoblot analysis of the 100,000-g mitochondrial membranes revealed NEP expression while the corresponding 100,000-g soluble fraction expressed thimet oligopeptidase (Fig. 5D). Quantification of ANG I metabolism revealed that the neprilysin inhibitor significantly increased ANG I and reduced ANG-(1–7) (Fig. 5, E and F, respectively). The addition of the thimet oligopeptidase inhibitor further reduced ANG-(1–7), but this did not attain significance compared with the effect of the neprilysin inhibitor alone. We did not observe ANG I-to-ANG II processing in the mitochondrial preparation with or without the endopeptidase inhibitors (Fig. 5, A–C).
Fig. 5.
Mitochondrial processing of 125I-ANG I. Chromatograph reveals 125I-ANG I (125I-AI) was metabolized to 125I-ANG-(1–7) (125I-A7) in renal mitochondrial homogenate fractions (A). 125I-AI metabolism to 125I-A7 was reduced by 10 μM SCH (B), and 125I-AI metabolism to 125I-A7 was reduced by 10 μM SCH in combination with 10 μM CPP (C). Immunoblots for neprilysin (NEP) and thimet oligopeptidase (TOP) in mitochondrial homogenates (lanes 2–4, n = 3) along with renal cortex homogenate as a positive control (lane 1; D). 125I-labeled products were separated by HPLC under gradient conditions. Statistical quantification of 125I-AI metabolism demonstrated SCH (10 μM) and SCH in combination with CPP (10 μM) preserved 125I-ANG I, while SCH (10 μM) and CPP (10 μM) reduced 125I-ANG-(1–7) formation (E and F). The addition of SCH and CPP did not significantly further influence 125I-ANG-(1–7) formation or preservation of 125I-ANG I (E and F). Values are means ± SE; n = 3. *P < 0.05 vs. control (CON).
Expression of the Mas Receptor
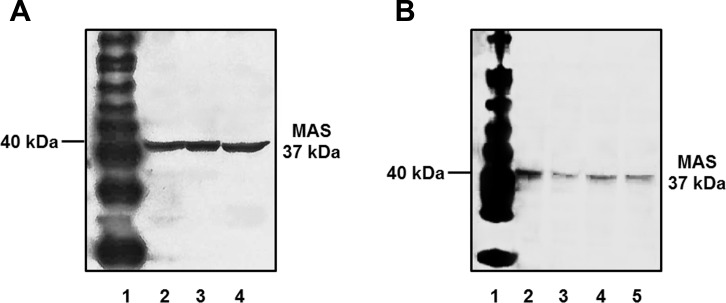
In lieu of the mitochondrial content of ANG-(1–7), we further assessed whether these organelles express the protein for the ANG-(1–7)/Mas receptor. For these studies, we utilized the Alomone antibody against the Mas receptor. We previously demonstrated that the antibody identified the receptor protein within the cortical tubules of the sheep kidney by immunofluorescent staining (26). Using a new batch of the antibody, the immunoblot revealed a single band of 40 kDa in the cortical homogenate, crude mitochondrial pellet, and the purified Percoll fraction from the sheep renal cortex (Fig. 6A). A single immunoreactive band for the Mas protein was also evident in the Percoll mitochondrial fraction from three male sheep (Fig. 6B).
Fig. 6.
Immunodetection of receptor expression in sheep renal mitochondria. A: whole renal cortex homogenate (lane 2), crude mitochondrial fraction (lane 3), and Percoll-purified mitochondrial fraction (lane 4) were subjected to 10% SDS gel and immunoblotting with an antibody against the MAS receptor protein. B: whole renal cortex homogenate (lane 2) and purified mitochondria (lane 3–5) from 3 distinct male sheep exhibited protein expression of the MAS receptor. Lane 1 (A and B) is the protein molecular weight marker.
DISCUSSION
Compelling evidence strongly supports an intracellularly based RAS in the kidney that contributes to renal function, as well as the regulation of oxidative stress (3, 25, 26, 29, 42). Mitochondria are now considered a key organelle regarding their role in chronic cardiovascular diseases and the mechanisms that underlie hypertension and renal dysfunction (10, 14, 16, 38, 45). Mitochondria are one of several intracellular organelles that contain elements of a RAS, and ANG II may directly influence oxidative stress and mitochondrial respiration (1, 5, 21). The present study provides further support for an intramitochondrial RAS pathway that is distinct from the peripheral RAS. Although previous reports have indicated associations between RAS components and mitochondrial function (1, 5, 21, 45, 47), the biochemical approach in this study allowed for a more complete characterization of a mitochondrial RAS regarding the alternative ANG-(1–7) axis. Our studies revealed that mitochondrial renin was active and cleaved exogenous angiotensinogen. Mitochondria expressed the des-ANG I form of angiotensinogen, but not intact angiotensinogen, suggesting that renin may process endogenous angiotensinogen within the mitochondria. We also show by direct RIA the presence of the angiotensin peptides ANG I, ANG II, and ANG-(1–7) within mitochondria. Moreover, the mitochondrial preparation processed ANG I to ANG-(1–7) by the endopeptidases neprilysin and thimet oligopeptidase. Finally, the current study revealed that purified mitochondria expressed a single protein band for the AT7/Mas receptor.
Peters and colleagues (44) originally identified an alternative transcript of renin that lacked exon 1 which precluded entry of the transcribed protein into the secretory pathway; this isoform was predicted to reside within the cell. These investigators subsequently showed that the truncated form of renin was catalytically active and preferentially localized to the mitochondria (9). Overexpression of this renin isoform in a human cardiomyocyte cell line was recently shown to protect the cells from necrosis in response to reduced glucose conditions; this protective effect was not reversed by a renin inhibitor, suggesting a direct effect of renin rather than renin-dependent processing of angiotensinogen (52). Clausmeyer et al. (9) reported that truncated renin was actively taken up and internalized by isolated mitochondria, but that the full-length prorenin form did not undergo internalization. Ishigami et al. (27) have recently demonstrated an additional renin transcript that lacks a portion of the pre-pro segment such that the enzyme is constitutively active; the isoform constitutes ∼10% to total renin and was expressed predominantly along the tubular elements of the mouse kidney. The forced overexpression of this renin isoform was associated with higher blood pressure in the transgenic mice (27). Although the Ishigami study found no change in circulating renin suggesting that the renin isoform is not secreted, the intracellular localization of the renin isoform within the tubules was not established (27). It is not currently known whether different renin transcripts are expressed within the sheep kidney or the extent to which renin isoforms traffic to the mitochondria.
The renal cortex consists of multiple cell populations, and although tubules constitute the major cell type, we cannot definitively exclude juxtaglomerular or other cell contamination. Juxtaglomerular cells are the major source of renin and contain both prorenin and active forms of renin (28, 37). Renin-containing granules sediment at a higher density than purified mitochondria; however, the possibility of renin contamination from granules is plausible, and isolated mitochondria were probed with a cathepsin B antibody. Renin and cathepsin B coexist within renin granules, and cathepsin B is a major component of lysosomes (50). Both cathepsin B and β-actin protein expression were diminished as mitochondria were purified from sheep renal cortex, suggesting a negligible contamination of renin granules or lysosomes in the mitochondrial preparation. Moreover, trypsin treatment did not result in a marked increase in renin activity. The inability of trypsin to enhance renin activity further argues for the lack of renin granule contamination in the isolated mitochondria. We found no immunological evidence for the prorenin receptor in mitochondria, which is consistent with predominant intracellular localization of this receptor on the endoplasmic reticulum, as well as the plasma membrane (4, 26, 27, 52).
Evidence of catalytically active renin in the mitochondria would support the predominant des-ANG I form of angiotensinogen in this organelle. Peters et al. (44) originally identified angiotensinogen by electron microscopy (EM) in mitochondria from the adrenal cortex along with other RAS components. Moreover, our preliminary data suggest that angiotensinogen is internalized by isolated mitochondria from both the sheep renal cortex and human HK-2 proximal tubule cells (54). Evidence for the angiotensin peptides ANG I and ANG-(1–7) within the mitochondria may also reflect the renin-dependent processing of angiotensinogen. ANG I metabolism studies revealed the generation of ANG-(1–7) that was attributed to the endopeptidases neprilysin (EC 3.4.24.11) and thimet oligopeptidase (EC 3.4.24.15). Neprilysin contributes to the generation of circulating ANG-(1–7), particularly following ACE inhibition; however, to our knowledge, these are the first studies to indicate neprilysin activity within the renal mitochondria (4, 19, 40, 53). Although the current understanding of a mitochondrial role for neprilysin is limited, there is evidence for the peptidase in mitochondrial-mediated amyloid β degradation (33). Transgenic mice engineered to produce amyloid β that contained the human mitochondria-targeted antioxidant catalase were found to upregulate neprilysin expression that was associated with reduced amyloid β and oxidative DNA damage (33). A role for thimet oligopeptidase in the intracellular processing of ANG I to ANG-(1–7) was evident in rat vascular smooth muscle cells, the NRK-52E renal epithelial cell line, and the rat brain (4, 7, 43). The enzyme is primarily an intracellular metallopeptidase of the M3 family of metallopeptidases and hydrolyzes peptides with an optimal length of 9–17 amino acids. Thimet oligopeptidase activity was originally localized to the mitochondria from the rat liver; however, Barrett and colleagues (30, 31) subsequently identified the mitochondrial peptidase activity to be neurolysin (EC 3.4.24.16), a metallopeptidase that shares 60% homology with thimet oligopeptidase and is sensitive to higher concentrations of the CPP inhibitor. Although our immunoblot studies identified a single protein band for thimet oligopeptidase in purified mitochondria, additional studies may be required to definitively confirm that the sheep renal mitochondrial activity is indeed thimet oligopeptidase or its homolog neurolysin. We note that both neprilysin and thimet oligopeptidase are ubiquitous peptidases that are found in the kidney of various species (8). Finally, ANG II metabolism studies did not reveal conversion to ANG-(1–7) in the isolated mitochondria, and this would further support an ACE2/ANG II-independent pathway for ANG-(1–7) formation (Wilson BA and Chappell MC, unpublished observations).
The biochemical detection of ANG II corroborates several studies that have localized ANG II to the mitochondrial by immunogold EM (1, 18, 44). We did not detect ACE activity in isolated mitochondria from the renal cortex, consistent with the findings of Astin et al. (5) in liver mitochondria. Moreover, we found no evidence of other ANG II-forming activities (cathepsin G, elastase-2, chymase) from ANG I in the absence or presence of the combined neprilysin and thimet oligopeptidase inhibitors that abolished ANG-(1–7) formation, and these data do not support the conversion of ANG I to ANG II within mitochondria (8). However, Peters and colleagues (44) detected immunoreactive ACE in mitochondria isolated from the rat adrenal cortex, and it is possible that species or tissue differences may underlie apparent discrepancies in the expression of RAS components in this organelle. Alternatively, both the Abadir (1) and Astin (5) studies reported AT1 receptors in isolated mitochondria and mitochondrial ANG II may derive from trafficking of the ANG II-AT1 receptor complex from the cell surface. Early studies by Sirett et al. (48) described the highest density of ANG II binding sites on the purified mitochondrial fraction from the rat brain. Moreover, Goodfriend and colleagues (21) originally reported direct effects of ANG II on mitochondrial phosphorylation in isolated preparations. Danser and colleagues (51) found that uptake of circulating 125I-ANG II by the kidney was also associated with the mitochondrial fraction, and Li et al. (30) recently demonstrated the trafficking of the ANG II-AT1 receptor complex to the mitochondria in proximal tubule cells. We also identified the AT7/Mas receptor on purified mitochondria based on Western blot analysis using the Alomone antibody and had previously demonstrated Mas expression primarily on the tubular elements of the sheep kidney with the same antibody (26). AT1, AT2, and Mas receptors do not express a canonical N-terminal mitochondrial targeting sequence, nor are these receptors predicted to localize to the mitochondria based on subcellular localization prediction software (TargetP 1.1 Server) (17). Moreover, to our knowledge, there is no evidence that mammalian mitochondrial DNA genes code for these receptors based on the MitoCarta inventory (6). However, AT1, AT2, and Mas receptor subtypes are evident on other intracellular organelles, including the nucleus and endoplasmic reticulum; thus there may exist alternative mechanisms for the intracellular expression of angiotensin receptors (1, 4, 22–24, 26, 31, 32, 41, 42, 47, 49).
The present study isolated mitochondria from the renal cortex, and although the tubular epithelial cells are the most predominant cell type in this tissue distinguished by a high density of mitochondria, we cannot definitely localize all the RAS elements to one particular cell type at this time. However, it should be noted that Peters et al. (44) localized multiple components of the RAS, including immunoreactive renin, angiotensinogen, ACE, and ANG II, to the mitochondria of the rat adrenal cortex. Moreover, we did not evaluate any functional properties of ANG-(1–7) or ANG II within the sheep mitochondria. The ANG-(1–7)-AT7/Mas receptor axis may act as a physiological antagonist of ANG II that counterbalances the deleterious effects of ANG II-mediated intracellular signaling (7, 38). In this regard, ANG-(1–7) attenuated mitochondrial ROS production and cellular apoptosis induced by ANG II in renal NRK-52E epithelial cells (29). Since the ANG-(1–7)-AT7/Mas receptor axis is typically linked to NO generation in the kidney and other tissues, investigation of this pathway to influence mitochondrial function under normal and pathological conditions may be warranted, particularly as ANG-(1–7) was recently shown to be more effective than the AT1 receptor antagonist to attenuate renal injury in a model of diabetic nephropathy (55).
Perspectives and Significance
The RAS may influence kidney dysfunction and oxidative stress maintenance within a variety of intracellular organelles (2). In general, the ANG-(1–7)-AT7/Mas axis opposes or functionally antagonizes a stimulated ACE-ANG II-AT1R pathway. The renal actions of an intramitochondrial ANG-(1–7) system may encompass the release of NO, activation of antiapoptotic pathways, and/or the reduction of oxidative stress. Moreover, the loss of ANG-(1–7) tone within the kidney may accelerate deleterious mitochondrial pathways that increase oxidative stress and enhance apoptosis under pathological conditions. Therefore, the presence of a mitochondrial ANG-(1–7)-AT7/Mas axis within the kidney could constitute a novel therapeutic target to influence intracellular levels of oxidative stress and respiration; elucidation of the peptide's functional role may identify specific intracellular targets to attenuate renal disease.
GRANTS
B. A. Wilson is supported by an American Heart Association (AHA) predoctoral fellowship grant (15PRE25120007). Additional support for this study was provided by National Institutes of Health Grants HD-047584, HD-017644, HD-084227, 0HL-51952, T32 grant (HL091797); the Groskert Heart Fund, the Wake Forest Venture Fund, and the Farley-Hudson Foundation (Jacksonville, NC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.A.W. performed experiments; B.A.W. analyzed data; B.A.W. and M.C.C. interpreted results of experiments; B.A.W. prepared figures; B.A.W. and M.C.C. drafted manuscript; B.A.W., M.N., T.M.G., J.C.R., and M.C.C. edited and revised manuscript; B.A.W., M.N., T.M.G., J.C.R., and M.C.C. approved final version of manuscript; M.C.C. provided conception and design of research.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Eric LeSaine and Nancy Pirro for technical and surgical support.
Portions of this research were originally presented at the American Heart Association's Council on Hypertension 2015 Scientific Sessions meeting in Washington, DC.
This work represents partial fulfillment of the requirements for the degree of Doctorate of Philosophy in the Department of Molecular Medicine and Translational Sciences at Wake Forest University School of Medicine for B. A. Wilson.
REFERENCES
- 1.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abadir PM, Walston JD, Carey RM. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides 38: 437–445, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzayadneh EM, Chappell MC. Angiotensin-(1–7) abolishes AGE-induced cellular hypertrophy and myofibroblast transformation via inhibition of ERK1/2. Cell Signal 26: 3027–3035, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzayadneh EM, Chappell MC. Nuclear expression of renin-angiotensin system components in NRK-52E renal epithelial cells. J Renin Angiotensin Aldosterone Syst 16: 1135–1148, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astin R, Bentham R, Djafarzadeh S, Horscroft JA, Kuc RE, Leung PS, Skipworth JR, Vicencio JM, Davenport AP, Murray AJ. No evidence for a local renin-angiotensin system in liver mitochondria. Sci Rep 3: 2467–2473, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res 44: D1251–D1257, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the ACE2-Ang-(1–7)-Mas receptor axis. Front Endocrinol 4: 201–216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chappell MC. Biochemical evaluation of the renin-angiotensin system—the good, bad, and absolute? Am J Physiol Heart Circ Physiol 310: H137–H152, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clausmeyer S, Stürzebecher R, Peters J. An alternative transcript of the rat renin gene can result in a truncated prorenin that is transported into adrenal mitochondria. Circ Res 84: 337–344, 1999. [DOI] [PubMed] [Google Scholar]
- 10.De Cavanagh E, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol 27: 545–553, 2007. [DOI] [PubMed] [Google Scholar]
- 11.De Cavanagh E, Toblli J, Ferder L, Piotrkowski B, Stella I, Fraga C, Inserra F. Angiotensin II blockade improves mitochondrial function in spontaneously hypertensive rats. Cell Mol Biol (Noisy-le-grand) 51: 573–578, 2005. [PubMed] [Google Scholar]
- 12.De Cavanagh EM, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, Fraga CG. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J 17: 1096–1098, 2003. [DOI] [PubMed] [Google Scholar]
- 13.De Cavanagh EM, Toblli JE, Ferder L, Piotrkowski B, Stella I, Inserra F. Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol 290: R1616–R1625, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Dikalov SI, Ungvari Z. Role of mitochondrial oxidative stress in hypertension. Am J Physiol Heart Circ Physiol 305: H1417–H1427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension 60: 1242–1249, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2: 953–971, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Erdmann B, Fuxe K, Ganten D. Subcellular localization of angiotensin II immunoreactivity in the rat cerebellar cortex. Hypertension 28: 818–824, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Smith R D, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int 68: 2189–2196, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Gáspár T, Snipes JA, Busija AR, Kis B, Domoki F, Bari F, Busija DW. ROS-independent preconditioning in neurons via activation of mitoKATP channels by BMS-191095. J Cereb Blood Flow Metab 28: 1090–1103, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Goodfriend T, Fyhrquist F, Allmann D. Biochemical effects of angiotensin. In: Angiotensins, edited by Page I and Bumpus FM. Berlin: Springer, 1974, p. 511–517. [Google Scholar]
- 22.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R518–R530, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1–7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension 55: 166–171, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol 296: F1484–F1493, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwathmey TM, Shaltout HA, Rose JC, Diz DI, Chappell MC. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension 57: 620–626, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1–7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol 299: F983–F990, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishigami T, Kino T, Chen L, Minegishi S, Araki N, Umemura M, Abe K, Sasaki R, Yamana H, Umemura S. Identification of bona fide alternative renin transcripts expressed along cortical tubules and potential roles in promoting insulin resistance in vivo without significant plasma renin activity elevation. Hypertension 64: 125–133, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Kawamura M, McKenzie JC, Hoffman LH, Tanaka I, Parmentier M, Inagami T. The storage form of renin in renin granules from rat kidney cortex. Hypertension 8: 706–711, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW, Ihm CG, Moon JY. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One 7: e39739, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XC, Sandoval RM, Molitoris BA, Zhuo JL. In vivo evidence of AT1a receptor-mediated uptake of angiotensin II by the proximal tubule visualized by intravital multiphoton imaging. Hypertension 66: A077, 2015. [Google Scholar]
- 31.Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-β1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol 294: C1034–C1045, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licea H, Walters MR, Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung 89: 427–438, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Mao P, Manczak M, Calkins MJ, Truong Q, Reddy TP, Reddy AP, Shirendeb U, Lo HH, Rabinovitch PS, Reddy PH. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid β production and BACE1 in a mouse model of Alzheimer's disease: implications for neuroprotection and lifespan extension. Hum Mol Genet 21: 2973–2990, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall AC, Pirro NT, Rose JC, Diz DI, Chappell MC. Evidence for an angiotensin-(1–7) neuropeptidase expressed in the brain medulla and CSF of sheep. J Neurochem 130: 313–323, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall AC, Shaltout HA, Nautiyal M, Rose JC, Chappell MC, Diz DI. Fetal betamethasone exposure attenuates angiotensin-(1–7)-Mas receptor expression in the dorsal medulla of adult sheep. Peptides 44: 25–31, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall AC, Shaltout HA, Pirro NT, Rose JC, Diz DI, Chappell MC. Enhanced activity of an angiotensin-(1–7) neuropeptidase in glucocorticoid-induced fetal programming. Peptides 52: 74–81, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris BJ, Johnston CI. Isolation of renin granules from rat kidney cortex and evidence for an inactive form of renin (prorenin) in granules and plasma. Endocrinology 98: 1466–1474, 1976. [DOI] [PubMed] [Google Scholar]
- 38.Nautiyal M, Arnold AC, Chappell MC, Diz DI. The brain Renin-Angiotensin system and mitochondrial function: influence on blood pressure and baroreflex in transgenic rat strains. Int J Hypertens 2013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nautiyal M, Katakam PVG, Busija DW, Gallagher PE, Tallant EA, Chappell MC, Diz DI. Differences in oxidative stress status and expression of MKP-1 in dorsal medulla of transgenic rats with altered brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 303: R799–R806, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olszanecki R, Madej J, Suski M, Gebska A, Bujak-Gizycka B, Korbut R. Angiotensin metabolism in rat stomach wall: prevalence of angiotensin-(1–7) formation. J Physiol Pharmacol 60: 191–196, 2009. [PubMed] [Google Scholar]
- 41.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun 384: 149–154, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira MG, Souza LL, Becari C, Duarte DA, Camacho FR, Oliveira JAC, Gomes MD, Oliveira EB, Salgado MCO, Garcia-Cairasco N. Angiotensin II-independent angiotensin-(1–7) formation in rat hippocampus involvement of thimet oligopeptidase. Hypertension 62: 879–885, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Peters J, Kranzlin B, Schaeffer S, Zimmer J, Resch S, Bachmann S, Gretz N, Hackenthal E. Presence of renin within intramitochondrial dense bodies of the rat adrenal cortex. Am J Physiol Endocrinol Metab 271: E439–E450, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Piotrkowski B, Fraga CG, de Cavanagh EM. Mitochondrial function and nitric oxide metabolism are modified by enalapril treatment in rat kidney. Am J Physiol Regul Integr Comp Physiol 292: R1494–R1501, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Rajapakse N, Shimizu K, Payne M, Busija D. Isolation and characterization of intact mitochondria from neonatal rat brain. Brain Res Brain Res Protoc 8: 176–183, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Re RN, Cook JL. The mitochondrial component of intracrine action. Am J Physiol Heart Circ Physiol 299: H577–H583, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirett NE, McLean AS, Bray JJ, Hubbard JI. Distribution of angiotensin-II receptors in rat-brain. Brain Res 122: 299–312, 1977. [DOI] [PubMed] [Google Scholar]
- 49.Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem 285: 22338–22349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taugner R, Bührle C, Nobiling R, Kirschke H. Coexistence of renin and cathepsin B in epithelioid cell secretory granules. Histochemistry 83: 103–108, 1985. [DOI] [PubMed] [Google Scholar]
- 51.Van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MA, Danser AJ. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens 19: 583–589, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Wanka H, Keβler N, Ellmer J, Endlich N, Peters BS, Clausmeyer S, Peters J. Cytosolic renin is targeted to mitochondria and induces apoptosis in H9c2 rat cardiomyoblasts. J Cell Mol Med 13: 2926–2937, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson BA, Cruz-Diaz N, Marshall AC, Pirro NT, Su Y, Gwathmey TM, Rose JC, Chappell MC. Identification of an angiotensin-(1–7) endopeptidase in the kidney cortex, proximal tubules, and human HK-2 epithelial cells that is distinct from insulin-degrading enzyme. Am J Physiol Renal Physiol 308: F594–F601, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson BA, Pirro NT, Gwathmey TM, Rose JC, Chappell MC. A mitochondrial Renin-Angiotensin System: internalization of angiotensinogen. Hypertension 66: A020, 2015. [Google Scholar]
- 55.Zhang K, Meng X, Li D, Yang J, Kong J, Hao P, Guo T, Zhang M, Zhang Y, Zhang C. Angiotensin (1–7) attenuates the progression of streptozotocin-induced diabetic renal injury better than angiotensin receptor blockade. Kidney Int 87: 359–369, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]