Abstract
The intensification and concentration of animal production operations expose workers to high levels of organic dusts in the work environment. Exposure to organic dusts is a risk factor for the development of acute and chronic respiratory symptoms and diseases. Lung epithelium plays important roles in the control of immune and inflammatory responses to environmental agents to maintain lung health. To better understand the effects of organic dust on lung inflammatory responses, we characterized the gene expression profiles of A549 alveolar and Beas2B bronchial epithelial and THP-1 monocytic cells influenced by exposure to poultry dust extract by DNA microarray analysis using Illumina Human HT-12 v4 Expression BeadChip. We found that A549 alveolar and Beas2B bronchial epithelial and THP-1 cells responded with unique changes in the gene expression profiles with regulation of genes encoding inflammatory cytokines, chemokines, and other inflammatory proteins being common to all the three cells. Significantly induced genes included IL-8, IL-6, IL-1β, ICAM-1, CCL2, CCL5, TLR4, and PTGS2. Validation by real-time qRT-PCR, ELISA, Western immunoblotting, and immunohistochemical staining of lung sections from mice exposed to dust extract validated DNA microarray results. Pathway analysis indicated that dust extract induced changes in gene expression influenced functions related to cellular growth and proliferation, cell death and survival, and cellular development. These data show that a broad range of inflammatory mediators produced in response to poultry dust exposure can modulate lung immune and inflammatory responses. This is the first report on organic dust induced changes in expression profiles in lung epithelial and THP-1 monocytic cells.
Keywords: lung epithelium, cytokines, inflammation, occupational lung diseases, dust exposure
organic dusts are commonly found in confined agricultural environments and are derived from both animals and crops (1). They are complex in nature and contain inorganic and organic components and microbial bioactive agents such as lipopolysaccharide, peptidoglycan, β-glucans, and fungal antigens (1). Ammonia, hydrogen sulfide, and inorganic dust are also found in elevated levels in the agricultural environment (1). Agricultural workers are exposed to organic dusts in their work environment, and the prevalence of respiratory symptoms is directly correlated with dust exposure (10). Lower respiratory tract symptoms include fever, chills, cough, wheeze, shortness of breath, and chest tightness (1). Respiratory symptoms are associated with increased levels of inflammatory cytokines, neutrophils, and macrophages in the respiratory tract (13, 14). Prolonged exposure to agricultural environment is associated with chronic obstructive pulmonary disease and bronchitis in livestock farmers and a decline in mean forced expiratory volume in 1 s (4). Exposure to agents found in agricultural environments is correlated with declines in lung function and respiratory symptoms, although roles of individual agents could not be identified (4).
The poultry industry has grown rapidly in the United States and other countries in recent years and is a significant component of the United States agricultural economy (United States Department of Agriculture). In the United States alone, poultry industry employs >250,000 workers. The demand for low-cost and reliable supplies of meat and eggs has led to intensification and concentration of poultry production facilities. These changes have given rise to a number of environmental and worker health concerns. Because of the high density, the animal production poultry environment contains high levels of airborne dust. The poultry environment contains higher levels of dust, endotoxin, bacteria, and fungi, as well as ammonia and carbon dioxide, compared with the swine environment (18). Poultry workers have higher prevalence and severity of lower and upper respiratory symptoms and chronic bronchitis compared with other agricultural workers (11, 18, 22). Allergic and nonallergic rhinitis, hypersensitivity pneumonitis, and occupational asthma are also commonly found in poultry workers (11, 12, 23). Despite a higher prevalence of respiratory symptoms and respiratory diseases in agricultural workers, molecular mechanisms mediating development of respiratory symptoms and respiratory diseases are not well understood. In particular, information on mechanisms mediating the effects of poultry dust on lung inflammatory responses is lacking. Because of the vital role the poultry industry plays in the agricultural economy, it is important to maintain and improve the status of workers' health. A better understanding of molecular changes in the lung in response to poultry dust exposure may provide insights into the development of newer interventions and treatments.
Lung epithelium, apart from serving as a physical barrier against microbial pathogens, particulates, and other environmental agents, also serves important functions in host defense. Lung epithelial cells control immune and inflammatory responses through the production of a host of cytokines, chemokines, and other bioactive molecules (3, 16, 21). Alveolar macrophages through their phagocytic and microbicidal actions provide the first line of defense in the lower respiratory tract (7). In response to microbial pathogens and particulates, they produce cytokines and chemokines that control inflammatory responses (7). Therefore, characterization of gene expression changes elicited by organic dust exposure in lung epithelial cells and alveolar macrophages is key to understanding lung inflammatory responses to organic dust. Our recent studies showed that poultry dust extract is a strong inducer of interleukin-8 (IL-8) levels in A549 and Beas2B lung epithelial and THP-1 monocytic cells (5). Our studies also demonstrated that transcriptional and protein kinase signaling mechanisms mediate induction of IL-8 expression (5). In this investigation, we studied the effects of poultry dust extract on the gene expression profiles of A549 alveolar and Beas2B bronchiolar epithelial and THP-1 monocytic cells to understand molecular mechanisms mediating lung inflammatory responses. We found that lung epithelial and THP-1 cells responded with unique changes in the gene expression profiles with induction of a host of inflammatory cytokines, chemokines, and other inflammatory proteins being common to all the three cells. Changes in the expression of cytokines and chemokines were validated by real-time quantitative (q)RT-PCR, ELISA, and Western immunoblotting in cells and by a mouse model of dust extract exposure. Pathway analysis indicates that dust extract-induced changes in gene expression influence functions related to cellular growth and proliferation, cell death and survival, and cellular development. This is the first report on organic dust-induced changes in gene expression profiles in lung epithelial and THP-1 monocytic cells.
MATERIALS AND METHODS
Cell culture.
A549 (ATCC CCL-185) alveolar-like epithelial cells were maintained on plastic culture dishes in F12 K medium containing 10% fetal bovine serum. Beas2B bronchial epithelial cells (ATCC CRL-9609), normal human bronchial cells that have been virally transformed, were maintained on plastic culture dishes coated with fibronectin, bovine type I collagen, and bovine serum albumin in LHC 9 medium. THP-1 cells (ATCC TIB-202), a human acute monocytic leukemia cell line, were grown in suspension in plastic culture dishes in RPMI 1640 medium containing 10% fetal bovine serum and 0.05 mM β-mercaptoethanol. F12K and RPMI media contained 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. A549 and Beas2B cells were placed in serum-free F12K and RPMI 1640 medium containing penicillin, streptomycin, and amphotericin B, respectively, overnight prior to treatment with dust extract. THP-1 cells were placed in serum-free RPMI 1640 medium containing 0.05 mM β-mercaptoethanol and penicillin, streptomycin, and amphotericin B overnight prior to treatment with dust extract. Normal primary human small airway epithelial (SAE) cells (Lonza) were grown on plastic culture dishes in small airway epithelial growth media (Lonza) and placed in serum-free RPMI 1640 medium containing penicillin, streptomycin, and amphotericin B overnight prior to treatment with dust extract. A549, Beas2B, and SAEC cells were ∼80% confluent at the time of treatment. All cells were treated with dust extract in serum-free medium.
Preparation of dust extract.
Broiler poultry dust settled on vertical surfaces was collected at 8 wk of growth of chickens from the poultry facility at Stephen F. Austin State University, Nacogdoches, TX. Aqueous extract of settled poultry dust was prepared as described previously (5) by extracting dust with serum-free F12K medium containing penicillin (100 u/ml), streptomycin (100 μg/ml), and amphotericin (0.25 μg/ml) or saline at a ratio of 1:10 (wt/vol).
RNA isolation and real-time qRT-PCR.
RNA from cell cultures was isolated with TRI-Reagent (Molecular Research Center). We removed genomic DNA in RNA by treatment with DNase (Turbo DNA-free kit, Ambion) and quantified RNA by measuring absorbance at 260 nm. RNA was reverse transcribed with random hexamers to synthesize cDNA (Applied Biosystems). Typically, 200 ng of total RNA was reverse transcribed in 10 μl reaction by incubation first at 25°C for 10 min followed by incubation at 48°C for 30 min, and finally the reaction mixture was heated at 95°C for 5 min to inactivate reverse transcriptase. Levels of mRNAs and 18S rRNA were determined by TaqMan probe based assay (Applied Biosystems). Reaction conditions for PCR were 40 cycles of 95°C for 30 s, 95°C for 5 s, and 60°C for 30 s. Levels of mRNAs were normalized to 18S rRNA levels. TaqMan gene expression IDs for target mRNAs are listed in Table 1.
Table 1.
TaqMan gene expression IDs for target mRNAs
| Gene Symbol | Gene Name | Human Assay ID |
|---|---|---|
| IL-1β | interleukin-1beta | Hs01555410_m1 |
| IL-6 | interleukin-6 | Hs00985639_m1 |
| IL-8 | interleukin-8 | Hs00174103_m1 |
| ICAM-1 | intercellular adhesion molecule-1 | Hs00164932_m1 |
| CCL2 | chemokine (C-C motif) ligand 2 | Hs00234140_m1 |
| CCL5 | Hs00982282_m1 | |
| Cyr61 | Hs00998500_g1 | |
| TLR4 | toll-like receptor-4 | Hs00152939_m1 |
| SOD2 | Hs00167309_m1 | |
| PTGS2 | prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclo-oxygenase) | Hs00153133_m1 |
| 18S | 18S ribosomal RNA | Hs99999901_s1 |
DNA microarray analysis.
A549 and Beas2B cells (n = 2) were treated with medium alone or medium containing 0.25% dust extract for 1 and 3 or 6 h, and THP-1 cells (n = 2) were treated with medium alone or medium containing 0.1% dust extract for 1 and 3 h. Total RNA was isolated with Tri-Reagent, and the RNA integrity and quality were verified using an Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA). Gene expression profiles were performed using Illumina HumanHT-12 v4 bead array chips (Illumina, San Diego, CA). The HumanHT-12 v4 Expression Beadchip is a genome-wide gene expression array that targets >31,000 annotated transcripts with >47,000 probes derived from the National Center for Biotechnology Information Reference Sequence (NCBI). Synthesis of double-stranded cDNA, preparation and labeling of cRNA, and hybridization to HumanHT-12 v4 BeadChip (Illumina, San Diego, CA), washing, and scanning were performed according to Illumina standard protocols. Data were preprocessed with BeadStudio v3 (Illumina) using quantile normalization with background subtracted, and expressed genes were identified using a detection threshold of P < 0.01. The P values were determined by two-tailed t-test. Differential expression was assessed with Bonferroni correction and a false discovery rate of 0.05. Each of the two experiments was analyzed separately. Differentially expressed transcripts (P < 0.01) were subsequently analyzed using Ingenuity Pathway Analysis (IPA) software. Microarray analysis and analysis of microarray data were performed at the Quantitative Genomics Laboratory, the University of Texas Medical School at Houston. The microarray data sets can be accessed with the accession number GSE73063 from the NCBI Gene Expression Omnibus (GEO) repository. The complete manifest file for the arrays used in this study can be found at http://support.illumina.com/content/dam/illumina-support/documents/downloads/productfiles/humanht-12/humanht-12_v4_0_r2_15002873_b.txt.zip
ELISA.
Interleukin-6 (IL-6) and IL-8 levels in cell medium were determined by ELISA (R & D Systems) according to the manufacturer's protocol.
Western immunoblotting.
Cells were lysed in lysis buffer (50 mM Tris·HCl, pH 7.4, containing 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM sodium vanadate, 2.5 mM sodium pyrophosphate, 15% glycerol, and protease inhibitors), and protein levels determined by Bio-Rad Protein assay. Equal amounts of protein were separated by SDS-PAGE on 10% Bis-Tris gels using MOPS as the running buffer and transferred to PVDF membranes by electroblotting. Membranes were reacted with primary antibodies overnight at 4°C and subsequently with secondary antibodies conjugated with alkaline phosphatase for 1 h at room temperature. Monoclonal antibodies against ICAM-1 (Santa Cruz Biotechnology) and tubulin-α (Thermo Scientific) at 1:500 and 1:200 dilution were used. Protein bands were visualized according to enhanced chemifluorescence detection method by reacting the membrane with the substrate followed by fluorescence scanning. Membranes were reprobed with actin or tubulin antibodies for the determination of actin levels that served as loading and transfer controls.
Exposure of mice to dust extract.
The animal protocol had been approved by the Institutional Animal Use and Care Committee, University of Texas Health Science Center at Tyler. Female C57BL6 mice (18–20 g) (The Jackson Laboratory) (n = 4 for saline and n = 7 for dust extract) of 8–10 wk of age were first anesthetized with xylazine and ketamine and administered 50 μl of poultry dust extract (20% in saline) or saline via intranasal instillation. After 2 h, mice were euthanized and their lungs instilled with Excell Plus fixative (American MasterTech Scientific, Lodi, CA) under a constant hydrostatic pressure of 20 cm and stored in the fixative for at least 24 h. Lungs were embedded in paraffin, and 5 μm sections cut for histology and immunohistochemical analysis.
Immunohistochemical staining.
We first subjected tissue sections to antigen retrieval by heating them in 10 mM sodium citrate, pH 6.0, containing 0.05% Tween 20 at 95°C for 5 min. Sections were immunostained using Ultravision Detection System kit (Thermo Scientific) according to the kit instructions. Polyclonal antibodies against keratinocyte chemoattractant (KC) (BioVision, Milpitas, CA), IL-6 (Santa Cruz Biotechnology, Santa Cruz, CA) and tumor necrosis factor-α (TNF-α) (Pierce Biotechnology, Rockford, IL) at dilutions ranging from 1:100 were used in immunostaining experiments. Briefly, sections were incubated successively with primary antibody, biotinylated goat antipolyvalent secondary antibody, and streptavidin-peroxidase conjugate. Reaction was visualized by incubation with 3-amino-9-ethylcarbazole, and sections counterstained with hematoxylin. Images were captured with a Olympus BX41 microscope.
Statistical analyses.
Data are shown as means ± SD or SE. In experiments in which levels in control or untreated cells were arbitrarily set as 1, statistical significance was determined by one-sample t-test. In others, paired t-test was used to analyze statistical significance. One-tailed P values < 0.05 were considered significant.
RESULTS
DNA microarray analysis of the effects of poultry dust extract.
We previously found that, in A549 and Beas2B cells treated with 0.25% dust extract, IL-8 mRNA levels increased after as short a time as 1 h of treatment to reach peak levels at 3 h and thereafter declined at 6 h (5). Although IL-8 levels at 6 h of treatment were lower than at 3 h, they were still significantly higher than in control cells. In THP-1 cells, 0.1% dust extract increased IL-8 mRNA levels after 1 h of treatment, and IL-8 mRNA levels continued to increase after 3 h with no appreciable decrease at 6 h (5). Our preliminary studies of the analysis of mRNA levels using Real-Time PCR Assay panel (Immune System Diseases Tier 1 H96, Bio-Rad) showed that exposure of A549 cells to 0.25% dust extract for 3 h significantly increased the mRNA levels of IL-6, IL-8, IL-1β, CCL2, CCL5, ICAM-1, PTGS2, SOD2, and TLR4 (data not shown).
To determine changes in gene expression profiles, A549, Beas2B, and THP-1 cells (n = 2) were exposed to medium alone or poultry dust extract (0.1% for THP-1 or 0.25% for A549 and Beas2B) for 1–3 or 6 h, and total RNA isolated and gene expression profiles determined by DNA microarray analysis using Illumina HumanHT-12 v4 Expression BeadChip. Exposure to dust extract (0.25%) for 1–6 h did not adversely affect the viabilities of A549 and Beas2B cells as assessed by MTS assay (data not shown). Exposure of THP-1 cells to 0.1% dust extract had no effect on the viability of cells after 1 h exposure but reduced viability by ∼40% after 3 h exposure. Treatment with dust extract caused significant changes in the expression of genes in A549, Beas2B, and THP-1 cells (Table 2). In A549 cells, exposure for as little as 1 h caused twofold or more increase in the expression of 38 genes in A549 cells but a decrease in the expression of only one gene. In Beas2B and THP-1 cells, exposure for 1 h increased the expression of 173 and 92 genes, respectively, while numbers of genes whose expression was decreased were much lower, 10 in case of Beas2B and 11 in case of THP-1. Exposure of A549 cells for 6 h reduced the number of induced genes; however, the number of downregulated genes increased marginally to three. In contrast, exposure of Beas2B cells to dust extract for 3 h increased the expression of 85 genes and decreased the expression of 78 genes, while in THP-1 cells, exposure for 3 h increased the expression of 798 genes and decreased the expression of 1,127 genes. The much larger changes in the expression of genes in THP-1 cells treated with dust extract for 3 h could be related to the reduced viability of cells. A comparison of induced transcripts (P < 0.01) by Venn diagram analysis was performed to identify unique and common transcripts in cells treated with dust extract for different periods of time. In A549 cells 33 or 17 genes were exclusively induced after treatment for 1 or 6 h with five genes in common for both time points, while in Beas2B cells 164 or 76 genes were exclusively induced after treatment for 1 or 3 h with nine genes in common for both time points, and in THP-1 cells 16 or 722 genes were exclusively induced after 1 or 3 h treatment with 76 genes in common for both (data not shown). A comparison of genes induced over twofold (P ≤ 0.01) in A549, Beas2B, and THP-1 cell at 1 h of treatment with dust extract by Venn diagram analysis (15) shows that although dust extract treatment elicited unique changes in gene expression in each cell line, several genes were common between and among the cell lines (Fig. 1).
Table 2.
Changes in the numbers of transcripts whose expression levels were altered after treatment with poultry dust extract for 1–6 h
| Treatment | A549 | Beas2B | THP-1 | |||
|---|---|---|---|---|---|---|
| ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | |
| 1 h | 38 | 1 | 173 | 10 | 92 | 11 |
| 3 h | 85 | 78 | 798 | 1127 | ||
| 6 h | 22 | 3 | ||||
Gene expression levels were determined by DNA microarray analysis using Illumina HumanHT-12 v4 Expression BeadChip. Upward or downward arrow indicates >2-fold increase or decrease in expression levels (P < 0.01) compared with untreated cells.
Fig. 1.
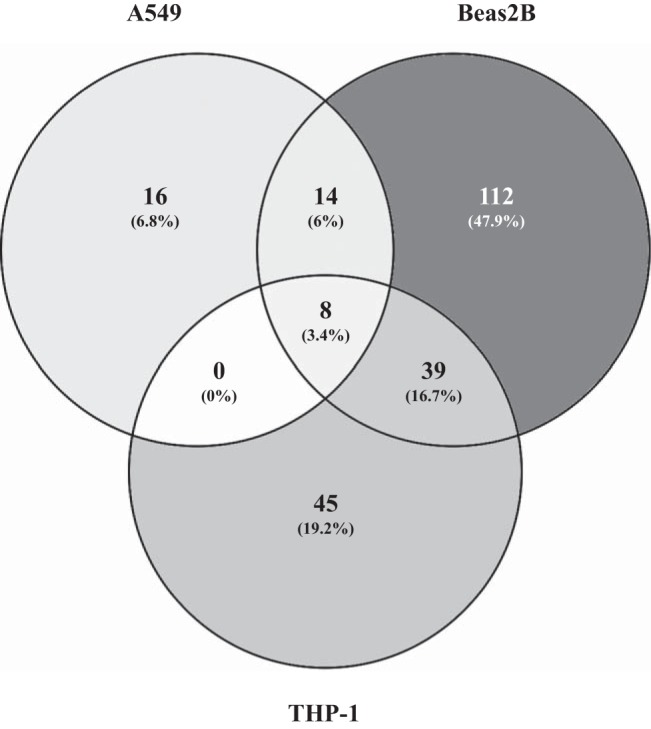
Venn diagram comparison of genes induced by poultry dust extract (DE) treatment in A549 and Beas2B lung epithelial and THP-1 cells. Genes induced (>2-fold, P < 0.01) by treatment with DE (0.25% for A549 and Beas2B cells and 0.1% for THP-1 cells) for 1 h relative to control cells were compared by Venn analysis diagram (15).
DNA microarray experiments indicate that exposure to poultry dust extract increased the expression of various members of cytokine (IL-6, IL-8, IL-11, IL-32) and chemokine (CCL2, CCL20, CXCL1, CXCL2, CXCL5) families, transcription factors (NF-κB, Jun, ATF3, FosB, EGR1), and other proteins (SOD, PTGS2, CYP4B1, Cyr61) implicated in cellular stress in A549, Beas2B, and THP-1 cells. Lists of some of the significantly induced genes in A549, Beas2B, and THP-1 cells are shown in Tables 3, 4, and 5, respectively. Data also indicate that several of these induced transcripts were differentially regulated in a time-dependent manner.
Table 3.
Some of the significantly induced transcripts in A549 cells by treatment with poultry dust extract
| Gene Alias | Gene Name | Fold change (1 h) | P Value | Fold Change (6 h) | P Value | Fold Change by qRT-PCR (6 h) |
|---|---|---|---|---|---|---|
| IL-6 | interleukin-6 | 62.9 | 1.85E-11 | 19.32 | 2.52E-07 | 59.8 ± 18.2 (P < 0.01) |
| IL-8 | inteleukin-8 | 35.75 | 4.06E-07 | 14.37 | 3.03E-11 | 42 ± 5.9 (P < 0.0001) |
| IL-11 | interleukin-11 | 2.92 | 0.00017 | 2.14 | 0.00095 | |
| IL-1β | interleukin-1β | ND | ND | 15.6 ± 3.98 (P < 0.01) | ||
| CCL2 | chemokine (C-C motif) ligand 2 | 6.3 | 6.10E-08 | 6.3 | 4.96E-09 | 9.2 ± 2.5 (P < 0.01) |
| CCL5 | 5.9 | 2.94E-05 | 17.4 ± 6.4 (P < 0.05) | |||
| CCL20 | chemokine (C-C motif) ligand 20 | 154.83 | 5.36E-13 | 80.42 | 1.92E-09 | |
| CXCL1 | chemokine (C-X-C motif) ligand 1 | 9.45 | 6.16E-09 | 8 | 9.44E-07 | |
| CXCL2 | chemokine (C-X-C motif) ligand 2 | 27.84 | 1.48E-08 | 7.64 | 3.03E-10 | |
| CXCL5 | chemokine (C-X-C motif) ligand 5 | 2.03 | 0.0028 | 5.23 | 3.73E-11 | |
| Cyr61 | cysteine rich angiogenic inducer 61 | 7.66 | 9.89E-09 | ND | 2.1 ± 0.3 (P < 0.05) | |
| ICAM1 | intercellular adhesion molecule 1 | ND | ND | 34.2 ± 10.3 (P < 0.01) | ||
| TLR4 | toll-like receptor 4 | ND | ND | 4.1 ± 0.66 (P < 0.001) | ||
| SOD2 | superoxide dismutase 2 | ND | 4.59 | 0.00016 | 2 ± 0.6 (P < 0.05) | |
| PTGS2 | prostaglandin-endoperoxidase synthase 2 | 5.14 | 3.94E-05 | 1.89 | 0.0069 | 4.5 ± 0.78 (P < 0.001) |
| EGR1 | early growth response protein 1 | 25.92 | 7.21E-14 | ND | ||
| JUN | v-Jun avian sarcoma virus 17 oncogene homolog | 4.64 | 1.24E-06 | 0.47 | 0.023 | |
| ETS1 | v-Ets avian erythroblastosis virus E26 oncogene homolog 1 | 4.86 | 0.00060 | ND | ||
| FOSB | FBJ murine osteosarcoma viral oncogene homolog B | 30852 | 7.34E-13 | ND | ||
| ATF3 | activating transcription factor 3 | 29.67 | 1.73E-12 | ND |
Changes (means ± SE, n = 4–7) in the levels of transcripts induced by dust extract, relative to untreated cells, determined by qRT-PCR are also shown. Genes listed include cytokines, chemokines, and transcription factors and proteins implicated in inflammatory responses. ND, not determined.
Table 4.
Some of the significantly induced transcripts in Beas2B cells by treatment with poultry dust extract
| Gene Alias | Gene Name | Fold Change (1 h) | P Value | Fold Change (3 h) | P Value | Fold Change by qRT-PCR (3 h) |
|---|---|---|---|---|---|---|
| IL-6 | interleukin-6 | 47.5 | 3.67E-38 | 71.68 | 3.67E-38 | 85.29 ± 19.47 (P < 0.01) |
| IL-8 | inteleukin-8 | 111.53 | 3.67E-38 | 124 | 8.88E-16 | 222 ± 57.8 (P < 0.01) |
| IL-11 | interleukin-11 | 3.46 | 6.27E-06 | 5.81 | 2.10E-10 | |
| IL-1β | interleukin-1β | 21.58 | 7.31E-12 | 85.1 | 3.68E-38 | 100.7 ± 23.8 (P < 0.01) |
| CCL2 | chemokine (C-C motif) ligand 2 | 16 | 6.38E-13 | 41.99 | 1.91E-10 | 30.5 ± 9.1 (P < 0.01) |
| CCL5 | chemokine (C-C motif) ligand 5 | 32.2 ± 9.14 | ||||
| CCL20 | chemokine (C-C motif) ligand 20 | 1110.93 | 3.55E-15 | 2220 | 3.77E-15 | |
| CXCL1 | chemokine (C-X-C motif) ligand 1 | 35 | 1.22E-11 | 49.74 | 1.45E-12 | |
| CXCL2 | chemokine (C-X-C motif) ligand 2 | 245 | 3.67E-38 | 85.75 | 3.67E-38 | |
| CXCL5 | chemokine (C-X-C motif) ligand 5 | 26.38 | 5.60E-05 | 100.73 | 4.85E-10 | |
| Cyr61 | cysteine rich angiogenic inducer 61 | 2.9 | 7.25E-08 | ND | 1.75 ± 0.25 | |
| ICAM1 | intercellular adhesion molecule 1 | 7.28 | 2,2E-08 | 15.3 | 3.65E-08 | 29.5 ± 9.95 (P < 0.05) |
| TLR4 | toll-like receptor 4 | ND | ND | 7.37 ± 2.77 (P < 0.05) | ||
| SOD2 | superoxide dismutase 2 | 1.69 | 0.012 | 7.36 | 2.04E-14 | 1.32 ± 0.22 |
| PTGS2 | prostaglandin-endoperoxidase synthase 2 | 84 | 3.67E-38 | 27.32 | 9.73E-08 | 43 ± 12.1 (P < 0.05) |
| EGR1 | early growth response protein 1 | 4.62 | 2.03E-10 | ND | ||
| JUN | v-Jun avian sarcoma virus 17 oncogene homolog | 3.12 | 1.33E-07 | ND | ||
| ETS1 | v-Ets avian erythroblastosis virus E26 oncogene homolog 1 | 2.09 | 0.00027 | 2.97 | 2.13E-06 | |
| FOSB | FBJ murine osteosarcoma viral oncogene homolog B | 55.46 | 3.67E-38 | 3.12 | 0.0018 | |
| ATF3 | activating transcription factor 3 | 9.67 | 1.11E-15 | 2.38 | 5.57E-05 |
Changes (means ± SE/SD, n = 2–5) in the levels of transcripts induced by dust extract, relative to untreated cells, determined by qRT-PCR are also shown. Genes listed include cytokines, chemokines, and transcription factors and proteins implicated in inflammatory responses.
Table 5.
Some of the significantly induced transcripts in THP-1 cells by treatment with poultry dust extract
| Gene Alias | Gene Name | Fold Change (1 h) | P Value | Fold Change by qRT-PCR (1 h) |
|---|---|---|---|---|
| IL-6 | interleukin-6 | ND | 138 ± 115 (P < 0.05) | |
| IL-8 | interleukin-8 | 106.79 | 3.67E-38 | 434 ± 323 |
| IL-11 | interleukin-11 | |||
| IL-1β | interleukin-1β | 332 | 3.68E-38 | 1350 ± 1137 |
| CCL2 | chemokine (C-C motif) ligand 2 | 17.04 | 3.67E-38 | 38.94 ± 35 |
| CCL5 | chemokine (C-C motif) ligand 5 | |||
| CCL20 | chemokine (C-C motif) ligand 20 | 325.64 | 3.67E-38 | |
| CXCL1 | chemokine (C-X-C motif) ligand 1 | 128.04 | 3.67E-38 | |
| CXCL2 | chemokine (C-X-C motif) ligand 2 | |||
| CXCL5 | chemokine (C-X-C motif) ligand 5 | |||
| Cyr61 | cysteine rich angiogenic inducer 61 | |||
| ICAM1 | intercellular adhesion molecule 1 | 14.3 | 8.27E-10 | 22.67 ± 11.1 |
| TLR4 | toll-like receptor 4 | ND | 2.67 ± 1.18 | |
| SOD2 | superoxide dismutase 2 | 6.65 | 3.67E-38 | |
| PTGS2 | prostaglandin-endoperoxidase synthase 2 | 10.47 | 3.67E-38 | 165.9 ± 143 |
| EGR1 | early growth response protein 1 | 28.85 | 3.67E-38 | |
| JUN | v-Jun avian sarcoma virus 17 oncogene homolog | |||
| ETS1 | v-Ets avian erythroblastosis virus E26 oncogene homolog 1 | ND | ||
| FOSB | FBJ murine osteosarcoma viral oncogene homolog B | 188.77 | 7.51E-11 | |
| ATF3 | activating transcription factor 3 | 8.1 | 3.67E-38 |
Changes (means ± SE, n = 3) in the levels of transcripts induced by dust extract, relative to untreated cells, determined by qRT-PCR are also shown. Genes listed include cytokines, chemokines, and transcription factors and proteins implicated in inflammatory responses.
Validation of DNA microarray data.
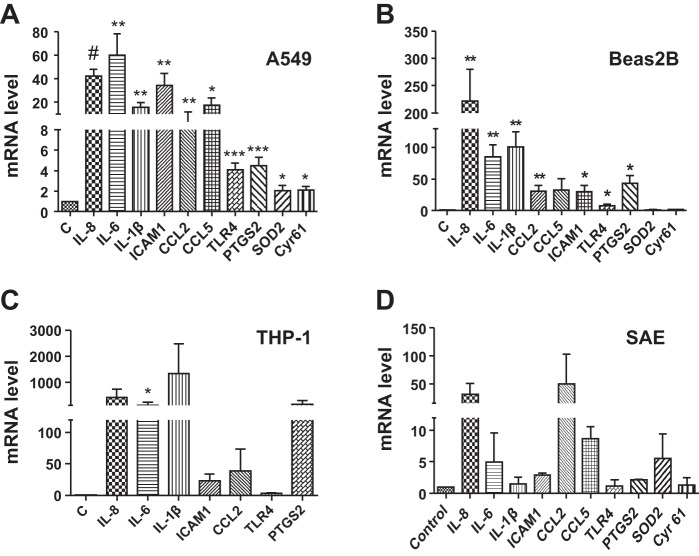
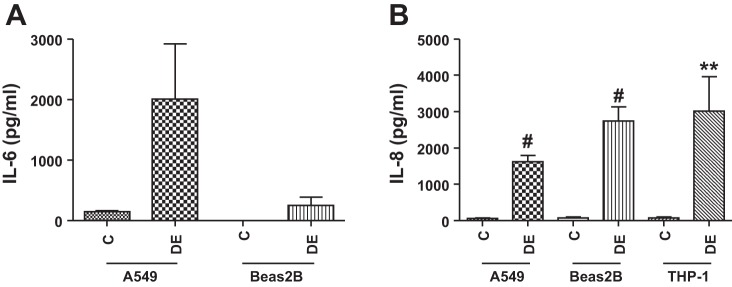
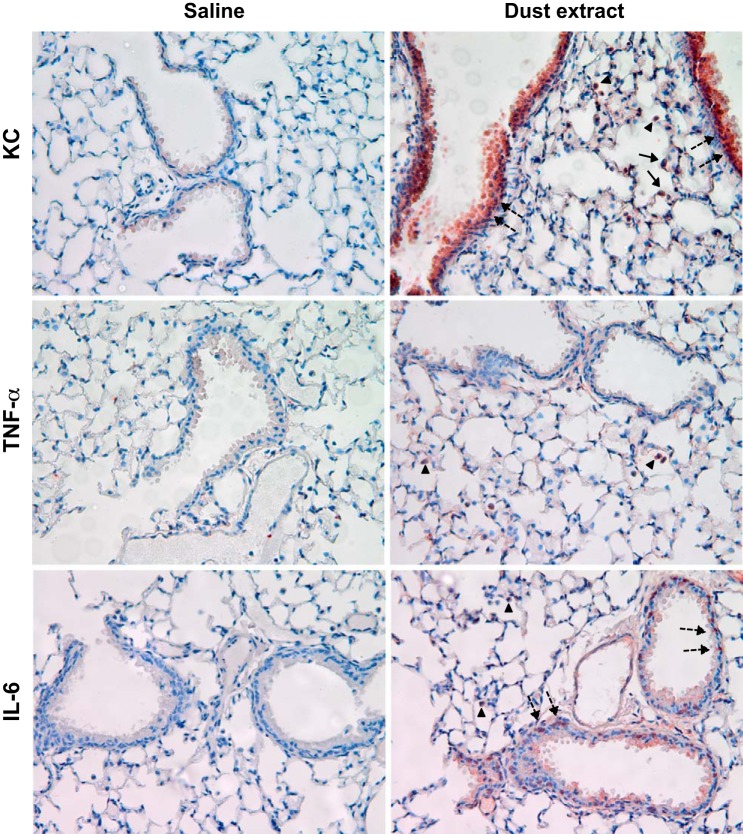
In agreement with DNA microarray data, preliminary experiments of the analysis of mRNA levels using a Real-Time PCR Assay panel (Immune System Diseases Tier 1 H96, Bio-Rad) showed that exposure of A549 cells to 0.25% dust extract for 3 h significantly increased the mRNA levels of IL-6, IL-8, IL-1β, CCL2, CCL5, ICAM-1, PTGS2, SOD2, and TLR4 (data not shown). We analyzed the levels of these mRNAs in A549, Beas2B, normal human SAE, and THP-1 cells treated with dust extract by qRT-PCR. In agreement with the microarray data, treatment of A549, Beas2B, and SAE cells with 0.25% dust extract or THP-1 cells with 0.1% dust extract induced the mRNA levels of several chemokine/cytokines as well as PTGS2, SOD2, ICAM-1, Cyr61, and TLR4 (Fig. 2). Increases in mRNA levels for chemokines/cytokines and other genes relative to levels in untreated cells varied between different cells. The degree of induction of each mRNA by dust extract varied between experiments likely due to extremely low levels of mRNAs in untreated cells; however, in each experiment treatment with dust extract was found to increase mRNA levels. ELISA (Fig. 3) and Western immunoblotting experiments (Fig. 4) show that dust extract increased IL-6 and IL-8 levels in cell medium and cellular ICAM-1 expression, further validating the inductive effects of poultry dust extract. Our preliminary studies indicated that administration saline (50 μl) or dust extract (50 μl, 20%) to mice via intranasal instillation increased lung KC, a mouse ortholog of IL-8, IL-6, and TNF-α, to peak levels at 2 h postexposure, which subsided to control levels by 5 h (Mitchell C and Boggaram V, unpublished observations, 2015). Immunohistochemical staining of lung sections from control mice and mice exposed to dust extract for 2 h showed increased expression of KC and IL-6 in alveolar and bronchiolar epithelial cells and inflammatory cells, while increased TNF-α expression was found only in inflammatory cells (Fig. 5).
Fig. 2.
Effects of poultry DE on inflammatory gene expression in A549, Beas2B, small airway epithelial (SAE), and THP-1 cells. A549 (A), Beas2B (B) and SAE (D) cells were treated with medium alone or medium containing 0.25% DE for 3 h. THP-1 cells (C) were treated with medium alone or medium containing 0.1% dust extract for 1 h. Levels of mRNAs and 18 S rRNA were determined by quantitative RT-PCR, and mRNA levels normalized to 18S rRNA level. The level in cells treated with control medium (C) was arbitrarily set as 1, and levels in dust extract treated cells are shown relative to control level. Data shown are means ± SE/SD (n = 4–7 for A549 cells, n = 4–5 for Beas2B cells, n = 3 for THP-1 cells, and n = 2 for SAEC). *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.0001 compared with cells treated with control medium.
Fig. 3.
Effects of poultry DE on IL-6 (A) and IL-8 (B) levels in cell culture medium from A549 and Beas2B cells. A549 and Beas2B cells were treated with medium alone (C) or medium containing 0.25% DE for 3 h. THP-1 cells were treated with medium alone (C) or medium containing 0.1% DE for 1 h. IL-6 and IL-8 levels in cell medium were quantified by ELISA. Data shown are means ± SE/SD (A549, n = 3 and Beas2B, n = 2 for IL-6 ELISA), (A549, n = 11; Beas2B, n = 13; THP-1, n = 11 for IL-8 ELISA). **P < 0.01, #P < 0.0001 compared with cells treated with control medium.
Fig. 4.
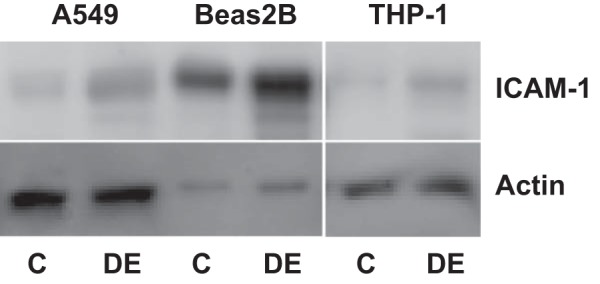
Effects of poultry DE on ICAM-1 levels in A549, Beas2B, and THP-1 cells. A549 and Beas2B cells were treated with medium alone or medium containing 0.25% DE for 3 h. THP-1 cells were treated with medium alone or medium containing 0.1% DE for 1 h. ICAM-1 levels in cell lysates were determined by Western immunoblotting. The same blot was probed for actin, which served as a loading and transfer control. Similar results were obtained in a 2nd independent experiment.
Fig. 5.
Effects of poultry DE exposure on keratinocyte chemoattractant (KC), TNF-α, and IL-6 immunostaining in mouse lung sections. Mice were exposed to saline or DE (20%) via intranasal instillation; 2 h later the mice were killed and their lungs processed for immunohistochemical detection of KC, TNF-α, or IL-6. Results shown are representative of data from 2 mice from each group. Solid arrows, alveolar type II epithelial cells; broken arrows, bronchiolar epithelial cells; arrowheads, inflammatory cells.
Our recently published data suggested that lipopolysaccharide (LPS) present in poultry dust extract may not be solely responsible for IL-8 induction (5). In agreement with these data, exposure of A549 cells to Escherichia coli LPS modestly increased IL-6, IL-8, IL-1β, CCL2, ICAM-1, PTGS2, and TLR4 mRNA levels in A549 cells (Fig. 6). We have used aqueous extracts of poultry dust in our studies, as inclusion of detergents and organic solvents for extraction would adversely affect cell viability. We compared the effects of dust extract (0.25%, 2 ml) directly with that of dust particles (1 or 2 mg/ml, 2 ml) on the induction of inflammatory gene expression in A549 cells to determine the efficacy of induction (Fig. 6). Results show that the dust extract at 0.25% (represents extract derived from 0.5 mg dust particles) was nearly as effective as dust particles at 0.5 and 1 mg/ml in inducing inflammatory gene expression, indicating that the extraction procedure has successfully extracted active components of dust particles.
Fig. 6.
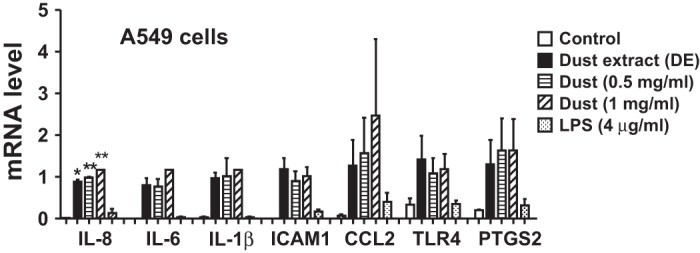
Effects of poultry DE, poultry dust particles, or lipopolysaccharide (LPS) on inflammatory gene expression in A549 cells. Cells were treated with control medium, poultry DE (0.25%), poultry dust particles (0.5 or 1 mg/ml), or Escherichia coli LPS (4 μg/ml) for 3 h. Effects on inflammatory gene expression was determined by quantitative RT-PCR. Data shown are means ± SD (n = 2).
Pathway analysis of differentially expressed genes.
The changes in gene expression profiles in A549, Beas2B, and THP-1 cells elicited by poultry dust extract treatment were analyzed with the IPA bioinformatics program to predict cellular pathways and cellular functions influenced by dust extract treatment. Genes that were differentially expressed at P < 0.01 were imported into IPA and pathways analyzed. Putative molecular and cellular functions and the top canonical pathways in A549, Beas2B, and THP-1 cells predicted by the IPA are shown (Table 6). Analysis by IPA reveals that dust extract-induced changes in gene expression influenced functions related to cellular growth and proliferation, cell death and survival, and cellular development in all the three cell lines. Signaling pathways influenced by dust extract exposure shared by the different cell lines included IL-6, IL-17, and TNFR signaling pathways. The other noteworthy signaling pathways affected include LPS-stimulated mitogen-activated protein kinases (MAPK) signaling, toll-like receptor signaling, and NRF2-mediated oxidative stress response signaling.
Table 6.
Pathway analysis of gene expression changes induced by poultry dust extract
| Cell | Molecular and Cellular Functions | P Value | Molecules, n | Top Canonical Pathways | P Value | Enrichment Ratio |
|---|---|---|---|---|---|---|
| 1 h | ||||||
| A549 | cellular development | 2.08E-11–1.22E-02 | 41 | cholecysstokinin/gastrin-mediated signaling | 5.25E-05 | 5/106 (0.047) |
| gene expression | 4.12E-11–1.22E-02 | 32 | IL-6 signaling | 1.07E-04 | 5/124 (0.04) | |
| cell death and survival | 5.55E-11–1.22E-02 | 39 | LPS-stimulated MAPK signaling | 2.19E-04 | 4/82 (0.049) | |
| cellular growth and proliferation | 6.35E-11–1.22E-02 | 40 | ErbB signaling | 3.92E-04 | 4/87 (0.046) | |
| cell cycle | 4.18E-08–1.22E-02 | 22 | HMGB1 signaling | 5.52E-04 | 4/99 (0.04) | |
| Beas2B | cellular growth and proliferation | 3.36E-32–5.56E-08 | 242 | IL-17A signaling in fibroblasts | 1.05E-11 | 13/40 (0.325) |
| cell death and survival | 2.14E-31–7.02E-08 | 221 | role of IL-17F in allergic inflammatory diseases | 1.06E-10 | 13/47 (0.277) | |
| cellular development | 1.26E-28–5.56E-08 | 221 | IL-6 signaling | 1.49E-09 | 19/124 (0.153) | |
| cellular movement | 6.76E-27–6.89E-08 | 167 | HMGB1 signaling | 1.93E-09 | 17/99 (0.172) | |
| cellular function and maintenance | 4.62E-20–2.48E-10 | 132 | TNFR2 signaling | 4.22E-09 | 10/33 (0.303) | |
| THP-1 | cellular function and maintenance | 9.45E-20–4.56E-06 | 71 | IL-6 signaling | 2.92E-10 | 14/124 (0.113) |
| cell death and survival | 7.75E-19–8.34E-06 | 105 | IL-10 signaling | 1.03E-09 | 11/78 (0.141) | |
| cellular development | 2.98E-18–8.41E-06 | 102 | TNFR2 signaling | 1.71E-09 | 8/33 (0.242) | |
| cellular growth and proliferation | 2.98E-18–7.22E-06 | 105 | TNFR1 signaling | 7.57E-09 | 9/52 (0.173) | |
| gene expression | 2.45E-14–1.89E-06 | 105 | IL-17A signaling in fibroblasts | 1.2E-08 | 8/40 (0.2) | |
| 3 or 6 h | ||||||
| A549 | cell death and survival | 9.74E-11–1.42E-02 | 34 | TWEAK signaling | 2.14E04 | 3/38 (0.079) |
| cellular growth and proliferation | 8.73E-06–1.42E-02 | 29 | role of macrophages, fibroblasts and endothelial cells in rheumatoid arthritis | 4.92E-04 | 6/332 (0.018) | |
| cellular development | 1.07E-05–1.42E-02 | 26 | pancreatic adenocarcinoma signaling | 5.38E-04 | 4/120 (0.033) | |
| cellular movement | 1.18E05–1.42E-02 | 21 | toll-like receptor signaling | 9.75E-04 | 3/62 (0.048) | |
| cell-to-cell signaling and interaction | 3.71E-05–1.42E-02 | 20 | role of osteoblasts, osteoclasts and chindrocytes in rheumatoid arthritis | 9.84E-04 | 5/238 (0.021) | |
| Beas2B | cell cycle | 1.84E-15–2.22E-03 | 281 | hereditary breast cancer signaling | 7.62E-10 | 33/128 (0.258) |
| cell death and survival | 4.16E-12–2.19E-03 | 474 | cyclins and cell cycle regulation | 2.13E-07 | 23/89 (0.258) | |
| cellular growth and proliferation | 2.52E-08–2.02E-03 | 469 | small cell lung cancer signaling | 8.56E-07 | 21/89 (0.236) | |
| cellular assembly and organization | 1.53E-07–2.10E-03 | 210 | ErbB2-ErbB3 Signaling | 1.35E-06 | 18/60 (0.3) | |
| DNA replication, recombination, and repair | 1.53E-07–1.76E-03 | 173 | mitotic roles of polo-like kinase | 1.56E-06 | 19/70 (0.271) | |
| THP-1 | cellular function and maintenance | 2.39E-15–1.56E-03 | 506 | molecular mechanisms of cancer | 6.89E-07 | 107/378 (0.283) |
| cellular development | 1.53E-14–1.55E-03 | 706 | B cell receptor signaling | 2.62E-06 | 57/170 (0.335) | |
| cellular growth and proliferation | 1.53E-14–1.55E-03 | 970 | TNFR2 signaling | 4.52E-06 | 17/33 (0.515) | |
| cell death and survival | 3.51E-13–1.53E-03 | 998 | NRF2-mediated oxidative stress response | 1.31E-05 | 61/192 (0.318) | |
| gene expression | 2.43E-11–1.2E-03 | 730 | death receptor signaling | 2.96E-05 | 26/64 (0.406) | |
Potential molecular and cellular functions and top canonical pathways influenced by poultry dust extract in A549, Beas2B, and THP-1 cells were analyzed using Ingenuity Pathway Analysis (IPA). Mappable (identified and annotated) transcripts were imported into IPA for analysis. A549 cells, 53 at 1 h and 25 at 6 h; for Beas2B cells, 420 at 1 h and 1226 at 3 h; for THP-1 cells, 191 at 1 h and 4,187 at 6 h.
DISCUSSION
We have used the DNA microarray analysis as a first screening step in identifying genes whose expression is altered by treatment with poultry dust extract. Our subsequent validation by qPCR, ELISA, Western blotting, and immunohistochemical staining provided more definitive quantification. Our data show that lung epithelial cells and THP-1 monocytic cells exposed to poultry dust extract express a wide variety of genes including cytokines (IL-6, IL-11, IL-32), chemokines (IL-8, CCL2, CCL20, CXCL1, CXCL2, CXCL5), PTGS2, an enzyme responsible for the production of inflammatory prostaglandins, ICAM-1, an intracellular adhesion molecule associated with inflammatory response, TLR4, whose activation leads to release of inflammatory modulators, and Cyr61, a secreted protein that promotes adhesion of endothelial cells. The expression of elevated levels of cytokines, chemokines, and other inflammatory mediators indicates that lung epithelial cells play important roles in the modulation of inflammatory responses to poultry dust extract. We validated the expression of several of these genes by real-time qRT-PCR and ELISA to prove DNA microarray results. Furthermore, exposure of mice to dust extract increased immunostaining for KC, IL-6, and TNF-α in alveolar and bronchial epithelial cells and inflammatory cells in agreement with results obtained with lung epithelial and THP-1 cells in vitro. Marked induction of IL-8, CCL2, CCL5, and ICAM-1 by lung epithelial cells indicates that these cells can chemoattract and interact with a wide variety of immune cells to modulate inflammatory and innate immune responses. Induction of PTGS2 in lung epithelial and THP-1 cells treated with dust extract indicates the potential involvement of prostaglandins and their metabolites such as prostacyclins and thromboxanes in eliciting lung inflammatory responses. Induction of SOD2 is perhaps a reflection of oxidant stress in dust extract-treated cells. The involvement of oxidant stress in the modulation of gene expression by organic dust is not known. Our unpublished studies (Boggaram V, 2015) have shown that exposure of A549 and Beas2B lung epithelial and THP-1 cells to poultry dust extract increases intracellular reactive oxygen species levels. Our experiments also indicate that aqueous poultry dust extract was equally potent as poultry dust particles in its ability to induce inflammatory gene expression, indicating its suitability for studying the effects of dust. Furthermore, poultry dust extract was significantly more effective than E. coli LPS to induce inflammatory gene expression, in agreement with our previous observations of the lack of effect of polymixin B, an inhibitor of LPS, on dust extract induction of IL-8 expression (5).
Treatment with poultry dust extract induced early growth response 1 (EGR1), Jun, and FosB, members of the activator protein 1 (AP-1), and activation transcription factor 3 (ATF3), transcription factors. EGR1 is a zinc finger transcription factor that is involved in cellular stress responses (2). AP-1 is a heterodimeric protein composed of members of the Fos, Jun, and ATF family of transcription factors and controls gene expression in response to a wide variety of stimuli that include microbial infections, cellular stress, cytokines, and growth factors (8). ATF3 is a member of activation transcription factor/cAMP response element-binding (CREB) protein family of transcription factors and is involved in cellular stress response (6). We previously found that poultry dust extract increased AP-1 and NF-κB DNA binding activities in lung epithelial and THP-1 cells and induced IL-8 promoter activity by binding to cognate DNA elements in the IL-8 proximal promoter (5). Interestingly, EGR1 expression is controlled at the transcriptional level by AP-1, CREB, NF-κB, as well as ETS1 (2, 20), suggesting a complex transcriptional network controlling gene expression in response to poultry dust extract treatment. The activities of members of AP-1 and ATF3 family of transcription factors and EGR1 are controlled by MAPK (9, 24). Our studies have shown that poultry dust extract induction of IL-8 expression in lung epithelial and THP-1 cells is controlled by MAPK activation (5), pointing to the potential importance of AP-1, ATF3, and EGR1 in the control of lung inflammatory responses to poultry dust. Pathway analysis indicated that poultry dust extract-induced changes in gene expression influence cellular development, cell death and survival, cellular growth and proliferation in agreement with the functions of cytokines, chemokines, and other inflammatory proteins whose expression is altered. Some of the top canonical signaling pathways influenced by poultry dust extract treatment include IL-6, IL-10, IL-17A, MAPK, TNFR, ErbB, and HMGB1 signaling, indicating effects on immune and inflammatory responses.
Our experimental data of the inductive effects of poultry dust extract on the mRNA levels of cytokines, chemokines, ICAM-1, TLR4, and PTGS2 in A549, Beas2B, and THP-1 cells were quite similar to effects in primary normal human SAEC. This indicates that the effects of the dust extract to induce inflammatory gene expression in lung epithelial cells are independent of whether the cell was a tumor-derived or a transformed cell. Similar inductive effects on the protein levels of IL-6, IL-8, and ICAM-1 indicate that the effects of dust to increase mRNA levels resulted in similar increases in protein levels. Our group and others have reported that organic dust extracts induce IL-8 and IL-6 expression in lung epithelial cells via activation of protein kinase C and MAPK signaling pathways (5, 19). Exposures of mice to poultry dust extract increased immunostaining for KC, IL-6, and TNF-α in lung epithelial and inflammatory cells in agreement with the cell culture data, further validating the proinflammatory effects of poultry dust in an animal model. It was recently shown that exposure of mice to swine barn dust extract increased IL-6, KC, and TNF-α levels in the bronchoalveolar lavage fluid and lung inflammation (17). In summary, our studies have shown that lung epithelial and THP-1 cells respond to poultry dust extract treatment by expressing cytokines, chemokines, and transcription factors that serve important functions in immune and inflammatory responses. This study is the first genome-wide expression investigation into the effects of organic dust on lung epithelial and THP-1 cells.
GRANTS
This research was supported by Grant U54 OH007541 from the Centers for Disease Control and the National Institute of Occupational Safety and Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.B. conception and design of research; V.B., K.R.G., K.N., and C.T.M. performed experiments; V.B., D.S.L., and K.R.G. analyzed data; V.B., D.S.L., and K.N. interpreted results of experiments; V.B., K.R.G., K.N., and C.T.M. prepared figures; V.B. drafted manuscript; V.B. and D.S.L. edited and revised manuscript; V.B., D.S.L., K.R.G., K.N., and C.T.M. approved final version of manuscript.
REFERENCES
- 1.Respiratory health hazards in agriculture. Am J Respir Crit Care Med 158: S1–S76, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Aicher WK, Sakamoto KM, Hack A, Eibel H. Analysis of functional elements in the human Egr-1 gene promoter. Rheumatol Int 18: 207–214, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Chuquimia OD, Petursdottir DH, Periolo N, Fernandez C. Alveolar epithelial cells are critical in protection of the respiratory tract by secretion of factors able to modulate the activity of pulmonary macrophages and directly control bacterial growth. Infect Immun 81: 381–389, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest 136: 716–725, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Gottipati KR, Bandari SK, Nonnenmann MW, Levin JL, Dooley GP, Reynolds SJ, Boggaram V. Transcriptional mechanisms and protein kinase signaling mediate organic dust induction of IL-8 expression in lung epithelial and THP-1 cells. Am J Physiol Lung Cell Mol Physiol 308: L11–L21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 273: 1–11, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunology 14: 81–93, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Ke J, Gururajan M, Kumar A, Simmons A, Turcios L, Chelvarajan RL, Cohen DM, Wiest DL, Monroe JG, Bondada S. The role of MAPKs in B cell receptor-induced down-regulation of Egr-1 in immature B lymphoma cells. J Biol Chem 281: 39806–39818, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Kirkhorn SR, Garry VF. Agricultural lung diseases. Environ Health Perspect 108, Suppl 4: 705–712, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirychuk SP, Senthilselvan A, Dosman JA, Juorio V, Feddes JJ, Willson P, Classen H, Reynolds SJ, Guenter W, Hurst TS. Respiratory symptoms and lung function in poultry confinement workers in Western Canada. Can Respir J 10: 375–380, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Larsson BM, Larsson K, Malmberg P, Martensson L, Palmberg L. Airway responses in naive subjects to exposure in poultry houses: comparison between cage rearing system and alternative rearing system for laying hens. Am J Ind Med 35: 142–149, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Larsson BM, Palmberg L, Malmberg PO, Larsson K. Effect of exposure to swine dust on levels of IL-8 in airway lavage fluid. Thorax 52: 638–642, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson KA, Eklund AG, Hansson LO, Isaksson BM, Malmberg PO. Swine dust causes intense airways inflammation in healthy subjects. Am J Respir Crit Care Med 150: 973–977, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Oliveros JC. Venny. An interactive tool for comparing lists with Venn's diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html.
- 16.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 45: 189–201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 296: L1085–L1095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radon K, Weber C, Iversen M, Danuser B, Pedersen S, Nowak D. Exposure assessment and lung function in pig and poultry farmers. Occup Environ Med 58: 405–410, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 93: 289–296, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Schwachtgen JL, Campbell CJ, Braddock M. Full promoter sequence of human early growth response factor-1 (Egr-1): demonstration of a fifth functional serum response element. DNA Seq 10: 429–432, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Shaykhiev R, Bals R. Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J Leukoc Biol 82: 1–15, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Simpson JC, Niven RM, Pickering CA, Fletcher AM, Oldham LA, Francis HM. Prevalence and predictors of work related respiratory symptoms in workers exposed to organic dusts. Occup Environ Med 55: 668–672, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viegas S, Faisca VM, Dias H, Clerigo A, Carolino E, Viegas C. Occupational exposure to poultry dust and effects on the respiratory system in workers. J Toxicol Environ Health A 76: 230–239, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta 1773: 1285–1298, 2007. [DOI] [PubMed] [Google Scholar]