Abstract
Given the fundamental role of β-catenin signaling in intestinal epithelial cell proliferation and the growth-promoting function of protein kinase D1 (PKD1) in these cells, we hypothesized that PKDs mediate cross talk with β-catenin signaling. The results presented here provide several lines of evidence supporting this hypothesis. We found that stimulation of intestinal epithelial IEC-18 cells with the G protein-coupled receptor (GPCR) agonist angiotensin II (ANG II), a potent inducer of PKD activation, promoted endogenous β-catenin nuclear localization in a time-dependent manner. A significant increase was evident within 1 h of ANG II stimulation (P < 0.01), peaked at 4 h (P < 0.001), and declined afterwards. GPCR stimulation also induced a marked increase in β-catenin-regulated genes and phosphorylation at Ser552 in intestinal epithelial cells. Exposure to preferential inhibitors of the PKD family (CRT006610 or kb NB 142-70) or knockdown of the isoforms of the PKD family prevented the increase in β-catenin nuclear localization and phosphorylation at Ser552 in response to ANG II. GPCR stimulation also induced the formation of a complex between PKD1 and β-catenin, as shown by coimmunoprecipitation that depended on PKD1 catalytic activation, as it was abrogated by cell treatment with PKD family inhibitors. Using transgenic mice that express elevated PKD1 protein in the intestinal epithelium, we detected a marked increase in the localization of β-catenin in the nucleus of crypt epithelial cells in the ileum of PKD1 transgenic mice, compared with nontransgenic littermates. Collectively, our results identify a novel cross talk between PKD and β-catenin in intestinal epithelial cells, both in vitro and in vivo.
Keywords: G protein-coupled receptors, angiotensin II, IEC-18 cells, PKA, PKD family inhibitors, CRT006610, kb NB 142-70, PKD1 transgenic mice
the mammalian intestine is covered by a single layer of epithelial cells that is renewed every 4–6 days throughout adult life. This high rate of turnover plays an essential role in the organization, maintenance, barrier function, and restoration of intestinal integrity in both normal and abnormal conditions (14). The sequential proliferation, lineage-specific differentiation, migration from the basal layer to the apex of the crypt, and death of the epithelial cells of the intestinal mucosa is a tightly regulated process modulated by a broad range of regulatory peptides, neurotransmitters, bioactive lipids, and differentiation signals. Many of these stimuli are known to initiate their biological effects through G protein-coupled receptors (GPCRs), but the intracellular signal transduction pathways involved, however, remain incompletely understood.
Protein kinase D (PKD), a protein kinase family within the CAMK group (49), has emerged as prominent downstream signal induced by GPCRs that function through Gq, G12, Gi, and Rho (3, 38, 48, 49, 65, 80–83). PKD1, the founding and most studied member of the family (23, 64), is rapidly activated through protein kinase C (PKC)-mediated phosphorylation of Ser744 and Ser748 in the PKD1 activation loop (19, 42, 66, 68). PKD1 catalytic activation within cells leads to its auto-phosphorylation at Ser916 and Ser748 (34, 52, 54, 55). Rapid PKC-dependent PKD1 activation is followed by a late, PKC-independent phase of activation induced by GPCR agonists (21, 52, 54). Accumulating evidence demonstrates that the PKD family plays an important role in a variety of cellular processes and activities, including gene expression, cell migration, and proliferation (48). In intestinal epithelial cells, PKD1 activation mediates migration and proliferation both in vitro and in vivo (54, 79). Accordingly, multiple growth-promoting stimuli rapidly activate PKD1 catalytic activity in intestinal epithelial cells (3, 6, 44, 54, 79) through activation loop phosphorylation (21, 52, 54, 66). Furthermore, transgenic (Tg) mice that express elevated PKD1 protein in intestinal epithelial cells display a marked increase in DNA-synthesizing cells in their intestinal crypts and a significant increase in the length and total number of cells per crypt (54). Collectively, these results support the notion that PKD1 signaling is a novel element in the pathway leading to proliferation of intestinal epithelial cells in vitro and in vivo. The mechanisms downstream of PKD1, however, remain to be identified.
β-Catenin is a multifunctional protein that plays pivotal roles in intercellular adhesion, motility, intestinal cell proliferation (7, 75), and in the pathogenesis of >90% of colorectal carcinomas (25, 45, 62, 72). Its different functions are coordinated by changes in its concentration, conformation, multisite phosphorylation, and the extent of binding to proteins, including E-cadherin and transcription factors (18, 63). In the absence of Wnt ligands, a multiprotein complex (referred as the destruction complex) comprised by axin/adenomatous polyposis coli (APC)/glycogen synthase kinase-3β (GSK-3β)/casein kinase I phosphorylates β-catenin at NH2-terminal sites and directs it to proteasome degradation (29, 33). The binding of Wnt ligands to their receptor Frizzled and coreceptor LRP5/6 inactivates the β-catenin destruction complex (13), thereby promoting β-catenin cellular accumulation and nuclear import, association with T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors, and activation of target genes (13, 33, 63). It is increasingly recognized that β-catenin nuclear localization is the initiating and driving element in β-catenin signaling, and that this step is regulated independently of its stabilization (35). While NH2-terminal phosphorylation of β-catenin targets it to degradation, phosphorylation at Ser552 and Ser675 promotes its dissociation from cell-cell contacts, induces its nuclear localization, and stimulates its transcriptional activity via Wnt-independent pathways (11, 16, 17, 27, 30, 41, 60, 61). β-Catenin phosphorylation at Ser552 has been implicated in inflammation-induced dysplastic transformation in the colon (27) and in intestinal polyposis (16). β-Catenin phosphorylation at Ser552 is regulated via phosphatidylinositol 3-kinase (PI3K)/Akt (11, 27) and/or cAMP/PKA (17, 60, 61, 84) through poorly understood cross-talk mechanisms with other signaling pathways (72).
Given the key role of β-catenin signaling in normal and abnormal intestinal epithelial cell proliferation (7, 45) and the growth-promoting functions of PKD1 in these cells, we hypothesized that PKD-mediated signaling interacts with β-catenin functions in intestinal cells. The results presented here provide several lines of evidence supporting positive cross talk between the PKD and the β-catenin signaling pathways: 1) PKD activity plays a critical role in mediating β-catenin nuclear localization and signaling in response to GPCR activation; 2) a novel PKD/PKA kinase cascade was identified that linked GPCR activation to β-catenin phosphorylation on Ser552; 3) GPCR stimulation induced the formation of a complex between PKD1 and β-catenin that was averted by cell treatment with inhibitors of PKD family catalytic activity; and 4) Tg PKD1 mice exhibited enhanced β-catenin nuclear localization and Ser552 phosphorylation in their intestinal epithelial cells. Taken together, our results identify a novel cross talk between PKD and β-catenin signaling pathways in intestinal epithelial cells, both in vitro and in vivo.
MATERIALS AND METHODS
Cell Culture
The nontransformed rat intestinal epithelial IEC-18 and IEC-6 cells (39, 40), originated from intestinal crypt cells, were purchased from ATCC. These cells express Gq-coupled receptors for angiotensin II (ANG II) and vasopressin (3–6, 44, 73, 78) and have been extensively used as a model system to examine signal transduction pathways in response to GPCR activation (3–6, 54, 56, 73, 79). Stock cultures of IEC-18 cells were maintained as described previously (36, 54). Briefly, cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum and penicillin-streptomycin and kept at 37°C in a humidified atmosphere containing 10% CO2 and 90% air. Human embryonic kidney (HEK)-293 cells were maintained in culture in DMEM supplemented with 10% fetal bovine serum, as described previously (77). Stock cultures were subcultured every 3–4 days. For experimental purposes, IEC-18 cells were seeded in 35-mm dishes at a density of 2 × 105 cells/dish.
Immunofluorescence
Immunofluorescence of IEC-18 cells was performed by fixing the cultures with 4% paraformaldehyde, followed by permeabilization with 0.4% Triton X-100. After extensive phosphate-buffered saline (PBS) washing, fixed cells were incubated for 2 h at 25°C in blocking buffer, consisting of PBS supplemented with 5% bovine serum albumin and then stained at 4°C overnight with a β-catenin (L54E2) mouse monoclonal antibody conjugated to Alexa Fluor 488 (1:200) diluted in blocking buffer. Subsequently, the cells were washed with PBS, and the nuclei were stained using Hoechst 33342 (1:10,000). The samples were imaged with an epifluorescence Zeiss Axioskop and a Zeiss water objective (Achroplan 40/0.75W Carl Zeiss). Images were captured as uncompressed 24-bit TIFF files with a cooled (−12°C) single charge-coupled device color digital camera (Pursuit, Diagnostic Instruments) driven by SPOT version 4.7 software. Alexa Fluor 488 signals were observed with a HI Q filter set 41001 (Chroma Technology). The selected cells displayed in the appropriate figures were representative of 90% of the population.
Image Analysis
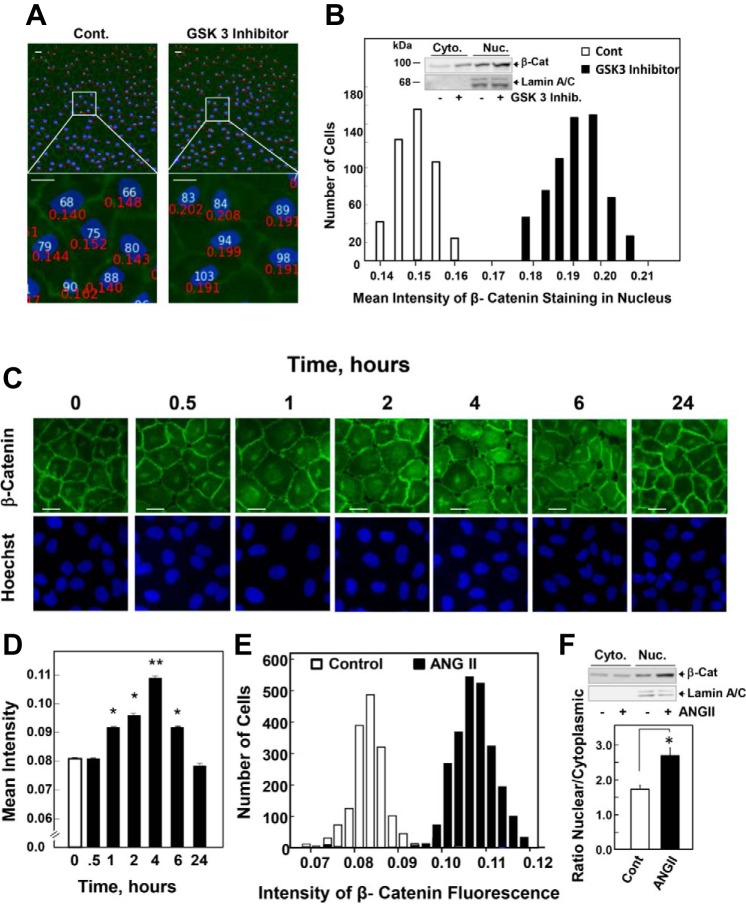
Average nuclear immunofluorescence intensities were determined using the Cell Profiler Software (www.cellprofiler.org). The CellProfiler software was programmed as follows: sequentially, the images of Hoechst 33342 and β-catenin Alexa Fluor 488 were loaded. The nuclear area was identified automatically using the Hoechst 33342 signal. These areas were used to measure the mean fluorescent intensity of β-catenin Alexa Fluor 488. An example of the assay used here is illustrated in Fig. 1, A and B. Representative images with identified cells were marked in white. Magnified portion displays quantification of fluorescent intensity in the nucleus, shown in red. In all cases, the data were exported to Excel, and the mean ± SE intensities from 8–10 fields (180–200 cells/field) were calculated from each experiment. Representative images were one of at least eight images taken for each treatment in each of at least three independent experiments.
Fig. 1.
Glycogen synthase kinase (GSK)-3β inhibitor and angiotensin (ANG) II induce β-catenin (β-Cat) nuclear localization in intestinal epithelial IEC-18 cells. A: confluent cultures of IEC-18 cells were treated without [control (Cont)] or with 5 μM GSK-3 inhibitor XVI for 4 h. The cultures were then washed, fixed with 4% paraformaldehyde, and stained with an antibody that detects β-Cat and with Hoechst 33342 to visualize the cell nuclei. Quantification of β-Cat nuclear localization shown in A was determined with the CellProfiler software, as described in materials and methods. Top: representative images with identified cells marked with white numbers. Bottom: magnified portion quantification of fluorescent intensity in the nucleus is shown in red numbers. B: the histogram represents the distribution of Cont (open bars) and GSK-3β inhibitor treated cells (solid bars) as a function of their nuclear fluorescence intensity based on the analysis of 1,000 cells from 1 experiment. Similar results were obtained in 3 independent experiments. Inset: subcellular fractionation. Confluent cultures of IEC-18 cells were treated with 5 μM GSK-3 inhibitor XVI for 4 h, and cytosolic and nuclear fractions were prepared using the REAP (rapid, efficient, and practical) method, as described in materials and methods. Immunoblots are shown from a single representative experiment. Similar results were obtained from 2 further experiments. C: confluent cultures of IEC-18 cells were stimulated with 50 nM ANG II for the indicated times. The cultures were then washed, fixed with 4% paraformaldehyde, and stained with an antibody that detects β-Cat and with Hoechst 33342 to visualize the cell nuclei. D: quantification of β-Cat nuclear localization shown in A was determined with the CellProfiler software, as described in materials and methods and above. The bars shown are the mean nuclear intensities ± SE (n = 1,500), and they were compared with the Cont (time 0) by unpaired Student's t-test (*P < 0.01; **P < 0.001). E: the histogram represents the distribution of Cont and ANG II-stimulated cells (at 4 h) as a function of their nuclear fluorescence intensity based on the analysis of 1,000 cells from 1 experiment. Similar results were obtained in 8 independent experiments. F: subcellular fractionation. Confluent cultures of IEC-18 cells were stimulated with 50 nM ANG II for 4 h, and cytosolic and nuclear fractions were prepared using the REAP method, as described in materials and methods. Immunoblots of β-Cat are shown from a single representative experiment. Similar results were obtained from 4 further experiments using the REAP method and 1 experiment using the hypotonic buffer method, as described in materials and methods. The bars represent the mean from both methods expressed as the nuclear-to-cytoplasmic ratio ± SE (n = 6). *P < 0.02. Scale bars = 30 μm.
Immunoblotting and Detection of β-Catenin and PKD1 Phosphorylation
Serum-starved, confluent intestinal epithelial IEC-18 cells were lysed in 2 × SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer (20 mM Tris·HCl, pH 6.8, 6% SDS, 2 mM EDTA, 4% 2-mercaptoethanol, 10% glycerol) and boiled for 10 min. After SDS-PAGE (Bio-Rad Criterion 4–15% gels), proteins were transferred to Immobilon-P membranes. The transfer was carried out at 100 V, 0.4 A, at 4°C, for 4 h, using a Bio-Rad transfer apparatus. The transfer buffer consisted of 200 mM glycine, 25 mM Tris, 0.01% SDS, and 20% CH3OH. For detection of proteins, membranes were blocked using 5% nonfat dried milk in PBS (pH 7.2) and then incubated for at least 2 h with the desired antibodies diluted in PBS containing 0.1% Tween. Primary antibodies bound to immunoreactive bands were visualized by enhanced chemiluminescence detection with horseradish peroxidase-conjugated anti-mouse, anti-rabbit antibody and a FUJI LAS-4000 Mini Luminescent Image Analyzer. Quantification of Westerns was performed by using FUJI Multi Gauge V3.0 software.
Knockdown of PKD Family via siRNA Transfection
Silencer select small interfering RNA (siRNA) nontargeted and targeted duplexes were all purchased from Ambion, Life Technologies. The siRNAs were designed to target the mRNA of mouse/rat PKD1, PKD2, and PKD3 [GenBank mRNA sequences: Z34524.1 (PKD1), BC083592.1 (PKD2), BC092663.1 (PKD3)]. The sequences of the siRNAs were as follows: PKD1 sense, CGAUGACAAUGACAGCGAAtt, anti-sense, UUCGCUGUCAUUGUCAUCGct; PKD2 sense, GUUCUAUCGUGGACCAGAAtt, anti-sense, UUCUGGUCCACGAUAGAACag; and PKD3 sense, GCAUUUCACAAGGCAGUAAtt, anti-sense, UUACUGCCUUGUGAAAUGCtg. The non-targeted siRNA was Silencer Select Negative Control No. 1 (no. 4390844). For siRNA transfection, the reverse transfection method was used. The siRNA pool (either 20 nM of each of the PKD siRNAs or the equivalent concentration of nontargeted siRNA) was mixed with Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol and added to 35-mm dishes. IEC-18 cells were then plated on top of the siRNA/Lipofectamine RNAiMAX complex at a density of 2 × 105 cells/35-mm dish. Control transfections were carried out with Stealth siRNA negative control (Invitrogen, Carlsbad, CA). Four days after transfection, cells were used for experiments and subsequent Western blot analysis.
Reverse Transcription-Quantitative PCR
Relative transcript expression levels of c-myc were determined by reverse transcription-quantitative PCR using a SYBR Green-based method. Briefly, total RNA was extracted from cells by using TRIzol Reagent (Ambion, Life Technologies, Grand Island, NY). Reverse transcription was performed with the iScript reverse transcription supermix (Bio-Rad Laboratories, Hercules, CA), using 1 μg of total input RNA. The synthesized cDNA samples were used as templates for the real-time PCR analysis. All reactions were performed using the Roche LightCycler480 system, and the amplifications were done using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA). Gene-specific rat oligonucleotide primers for c-myc (unique assay ID: qRnoCID0007760) and GAPDH (unique assay ID: qRnoCID0057018) were purchased from Bio-Rad (Hercules, CA).
TCF/LEF Reporter Assay
HEK-293 cells were transfected with a mixture of β-catenin-responsive luciferase construct and a constitutively expressing Renilla luciferase reporter gene, or a noninducible firefly luciferase construct and constitutively expressing Renilla luciferase construct, all under the control of a CMV promoter (TCF/LEF Reporter Assay Kit no. CCS-018L, Qiagen, Valencia, CA) with either pcDNA3 or pcDNA3 expressing PKD1 using Lipofectamine 3000 (Invitrogen, Carlsbad, CA), as suggested by the manufacturer. Transiently transfected cells were analyzed 72 h posttransfection. Luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega, no. E910, Madison, WI), as described by the manufacturer.
Coimmunoprecipitation of PKD and β-Catenin
Confluent 100-mm dishes of IEC-18 cells (6 × 106 cells) were lysed in buffer containing 20 mM Tris·HCl (pH 7.5), 1% Triton, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (Pefabloc). PKD was immunoprecipitated from the cell extracts with a PKD C-20 antibody (1:100 dilution) from Santa Cruz Technologies. The immune complexes were recovered using protein-A coupled to agarose (30 μl of 50% suspension) and washed × with the lysis buffer.
Cell Fractionation
REAP method.
Cell fractionation was performed using the REAP (rapid, efficient, and practical) method (58). Briefly, confluent cultures of IEC-18 cells grown in 10-cm-diameter dishes and treated with or without ANG II were washed in ice-cold PBS, pH 7.4, scraped from the culture dishes on ice, and collected in 1.5-ml microcentrifuge tubes in 1 ml of ice-cold PBS. After centrifugation for 10 s in a table-top microfuge, supernatants were removed from each sample, and cell pellets were resuspended in 900 μl of ice-cold 0.1% NP-40 (Fisher Scientific, Pittsburgh, PA) in PBS and triturated five times using a p1000 micropipette. The lysates were centrifuged for 10 s in 1.5-ml microcentrifuge tubes, and 300 μl of the supernatants were removed as the “cytosolic fraction,” and 100 μl of 4 × SDS-PAGE sample buffer were added. After the remaining supernatant was removed, the pellet was suspended in 1 ml of ice-cold 0.1% NP-40 in PBS and centrifuged for 10 s, and the supernatant discarded. The pellet was designated as “nuclear fraction” and solubilized with 2 × SDS-PAGE sample buffer. In all experiments, lamin A/C was detected in nuclear but not in cytoplasmic fractions.
Hypotonic lysis method.
In addition, nuclear pellets were prepared using a hypotonic lysis buffer. Cells were first washed and then incubated for 15 min on ice with 3 ml of ice-cold hypotonic lysis buffer [10 mm HEPES-NaOH, pH 7.3, supplemented with 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride-HCl, and 10 μg/ml aprotinin], and subsequently harvested by gentle scraping, transferred to a 7-ml Dounce homogenizer, and homogenized by 50 strokes with a tight-fitting (type A) pestle. Nuclear pellets were obtained by spinning in a refrigerated microcentrifuge at 600 rpm for 6 min. Proteins were extracted from the nuclei using 2× SDS-PAGE sample buffer.
Assays of DNA Synthesis and β-Catenin Immunohistochemistry in Mice
To assess the effect of PKD1 on the localization of β-catenin in vivo, we used Tg mice that express elevated PKD1 protein in the intestinal epithelium and control non-Tg littermates. The generation of PKD1 Tg mice was described elsewhere (54). Briefly, we used the rat liver fatty acid-binding protein (FABP) promoter (kindly provided by Dr. J. Gordon), which has been well characterized to target transgene expression to these regions of the intestine (46, 51). The FABP promoter (−596 to +21) was fused to the cDNA for PKD (+40 to +2,910) and the bovine growth hormone polyadenylation signal sequence using conventional cloning methods. Tg mice were identified by PCR using genomic tail DNA and specific primers (sense, 5′-TATAACCGCTCTCTGGAC, corresponding to the PKD1 portion of the transgene, and antisense, 5′-ACTCAGACAATGCG, corresponding to the bovine growth hormone polyadenylation sequence) to detect the integrated transgene sequence as a product of 600 bp.
To perform anatomical dissection and tissue collection, mice were euthanized in a CO2 chamber. The ileum was selected for analysis because, in our initial studies, we noted that PKD1 expression driven by the FABP promoter was prominent in the distal region of the small intestine of the Tg mice. Overexpression of PKD1 in the ileum was verified using epithelial cells isolated sequentially along the crypt-villus axis by timed incubations in EDTA-PBS solutions. To measure the proliferation of intestinal epithelial cells in vivo, sex- and age-matched mice (8 PKD1 Tg mice and 8 non-Tg littermates) were injected intraperitoneally with the thymidine analog 5-bromo-2′-deoxyuridine (BrdU) at 100 μg/g body wt. After 3 h, the mice were anesthetized and cardio-perfused with PBS, followed by 4% paraformaldehyde in PBS. Sections (4 μm) of paraffin-embedded ileal tissue were deparaffinized and stained for BrdU incorporation using a BrdU staining kit (BrdU In-Situ Detection Kit II 551321, BD Pharmingen), according to the manufacturer's instructions. At least 20 full-length, longitudinally cut crypts were used from each animal to determine the percentage of labeled cells. Sections were also subjected to immunohistochemistry, with an antibody that detects β-catenin to determine the localization of β-catenin in the nucleus of crypt epithelial cells in the ileum of PKD1 Tg mice and non-Tg littermates. To measure PKD1 protein expression and signaling, lysates of intestinal cells isolated from sex- and age-matched mice were subjected to immunoblotting, as described in the corresponding figure legend. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Research Committee of the University of California, Los Angeles (protocol nos. 2001-142 and 2014-070). All mice were housed in specific pathogen-free barrier facilities, maintained on a 12:12-h light-dark cycle, and fed a standard autoclaved-stable rodent diet.
Repetition of Experiments, Number of Cells Analyzed, and Statistical Analysis
For each of our studies, the number of independent experiments ranged from two to eight, and the total number of cell analyzed ranged from 1,500 to 3,500, with the lower number of cells occurring in experiments where transfected cells with siRNA were analyzed. Values are means ± SE. Differences between groups were analyzed with the unpaired Student's t-test.
Materials
DMEM was obtained from Invitrogen (Carlsbad, CA). ANG II, forskolin (FSK), and 3-isobutyl-1-methylxanthine (IBMX) were obtained from Sigma Chemical (St. Louis, MO). The kinase inhibitors kb NB 142-70, CRT0066101, H89, AG1478, GSK690693, and LY29004 were obtained from R&D Systems (Minneapolis, MN). PKD C-20 antibody was obtained from Santa Cruz Biotechnology (Dallas, TX). The GSK-3 inhibitor XVI (no. 361559) was purchased from EMD Millipore (Billerica, MA).
Primary antibodies used were as follows: PKD C-20 (no. sc-639; final dilutions 1:400 for Western blotting and 1:100 for immunoprecipitation) and GAPDH (sc-365062; final dilution 1:400) from Santa Cruz Biotechnology (Dallas, TX), β-catenin (6B3) for immunohistochemistry (no. 9582; final dilution 1:100), β-catenin (6B3, no. 9582) or β-catenin (D10A8, no. 8480) for Western blotting (final dilutions 1:1,000), β-catenin (L54E2) conjugated to Alexa Fluor 488 for immunofluorescence (no. 2849; final dilution 1:200), phospho-β-catenin Ser552 (no. 9566; final dilution 1:1,000), PKD3 (no. 5655; final dilutions 1:1,000), phospho-PKD1 Ser916 (no. 2051; final dilution 1:1,000), phospho-ERK-1/2 Thr202/Tyr204 (E10, no. 9106; final dilution 1:1,000), β-tubulin (no. 2146; final dilution 1:1,000), and lamin A/C (no. 4777; final dilution 1:1,000) all from Cell Signaling Technology (Danvers, MA). The Cignal TCF/LEF Reporter Assay Kit (no. CCS-018L) was purchased from Qiagen (Valencia, CA), and the Dual-Luciferase Reporter Assay System was from Promega (no. E910) (Madison, WI). All other reagents were of the highest grade available. RT-quantitative PCR reagents were purchased from Bio-Rad (Hercules, CA).
RESULTS
GPCR Activation Induces β-Catenin Translocation in Intestinal Epithelial Cells
To determine whether nontransformed intestinal epithelial IEC-18 cells (39, 40) respond to Wnt signaling, confluent cultures of IEC-18 cells were treated with or without a selective GSK-3 inhibitor for 4 h (a surrogate of Wnt signaling) and then washed, fixed, and stained with an antibody that detects β-catenin and with Hoechst 33342 to visualize the cell nuclei. As shown in Fig. 1A, chemical inhibition of GSK-3 to inhibit the function of the degradation complex induced a striking increase in nuclear β-catenin. Quantification of β-catenin nuclear localization was determined with the CellProfiler software (Fig. 1A). The histogram representing the distribution of control (open bars) and GSK-3β inhibitor treated cells (solid bars) as a function of their nuclear fluorescence intensity indicates that chemical inhibition of GSK-3 induced β-catenin nuclear localization in most cells of the population (Fig. 1B). Furthermore, fractionation of IEC-18 cells corroborated that chemical inhibition of GSK-3 induced β-catenin accumulation, as shown by increased cytosolic and nuclear levels of β-catenin (Fig. 1B, inset). These results substantiated that nontransformed intestinal epithelial IEC-18 cells endogenously express a signal-responsive β-catenin pathway, as scored by image analysis and subcellular fractionation.
To identify cross talk between PKD1 and β-catenin in intestinal epithelial cells, we examined whether receptor-mediated activation of PKDs regulates β-catenin in IEC-18 cells (39, 40). The GPCR agonist ANG II acts as a potent growth factor for these cells (3–6, 56, 73) through PKD1 activation (54). Consequently, we determined whether stimulation with ANG II increases nuclear β-catenin localization in IEC-18 cells. As shown by representative images in Fig. 1C, β-catenin was predominantly located at the plasma membrane in unstimulated cells, most likely bound to E-cadherin (63). Some of the cells also exhibit a minor pool of β-catenin located at a perinuclear region. Stimulation with ANG II induced β-catenin intracellular redistribution in a time-dependent manner, characterized by cell retraction, loss of cell-cell adhesions, and increase in its nuclear levels (Fig. 1C). There was a concomitant decline of β-catenin expression at the surface in areas of diminished cell-cell adhesion. Using the CellProfiler software for image analysis, we quantified nuclear β-catenin fluorescence in thousands of individual IEC-18 after various times of GPCR stimulation. As shown in Fig. 1D, a significant increase in nuclear levels of β-catenin was evident within 1 h of ANG II stimulation (P < 0.01), reaching a peak after 4 h of stimulation (P < 0.001). After 24 h of ANG II stimulation, β-catenin was predominantly located at the plasma membrane, similar to that observed in the unstimulated cells. The histogram representing the distribution of control and ANG II-stimulated cells (at 4 h) as a function of their nuclear fluorescence intensity indicate that ANG II induced β-catenin nuclear localization in most cells of the population (Fig. 1E). Comparable results were obtained using nontransformed intestinal epithelial IEC-6 cells instead of IEC-18 (results not shown).
The increase in nuclear levels of β-catenin in response to ANG II in IEC-18 cells was corroborated using cytosol/nuclear fractionation of cells via the REAP method (58). Western blot analysis of cytoplasmic and nuclear fractions isolated from IEC-18 cells substantiated that stimulation with ANG II induced a marked increase (1.5-fold) in nuclear β-catenin levels without causing a concomitant increase in its cytoplasmic level (Fig. 1F). Furthermore, similar results were obtained when cell fractionation was performed with a different method using hypotonic buffers. These results show that GPCR stimulation of intestinal epithelial cells induces rapid and transient endogenous β-catenin redistribution, leading to nuclear localization, whereas chemical inhibition of GSK-3, a surrogate of Wnt signaling, produced β-catenin accumulation in the cytoplasm and nucleus.
GPCR Activation Induces β-Catenin Translocation Through PKD in Intestinal Epithelial Cells
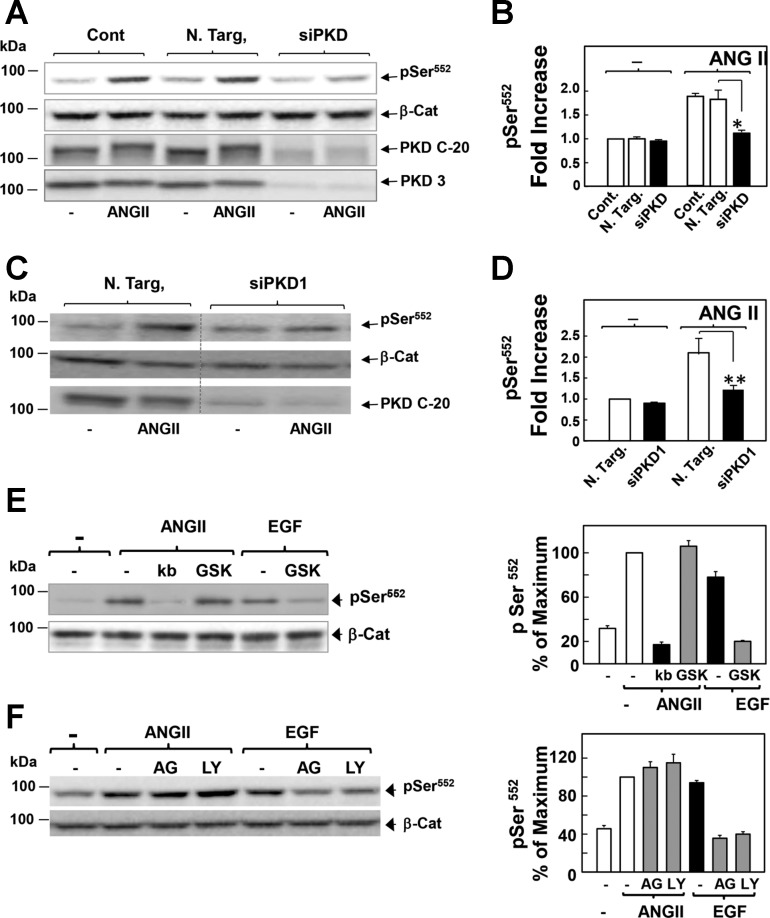
Since PKD has emerged as one of the prominent downstream elements in GPCR signaling (48, 49), we next determined whether PKDs play a role in mediating GPCR-induced nuclear β-catenin localization in intestinal epithelial cells. Cultures of IEC-18 cells were treated with the preferential PKD family inhibitors CRT0066101 (15) or kb NB 142-70 for 1 h and then stimulated with ANG II for 4 h. Exposure to 2.5 μM CRT006610 prevented the increase in nuclear localization of β-catenin in response to ANG II (Fig. 2A). This conclusion was substantiated by quantification of nuclear β-catenin immunofluorescence (Fig. 2B). Similarly, treatment of the cells with the PKD family inhibitor kb NB 142-70 (instead of CRT006610) also averted the increase in nuclear localization of β-catenin in response to ANG II (Fig. 2, A and B). In dose-response studies, we verified that CRT006610 and kb NB 142-70 prevented ANG II-induced nuclear β-catenin localization at concentrations that inhibit PKD1 activation within IEC-18 cells, as scored by PKD autophosphorylation at Ser916 (shown in Fig. 4, E and F). These results suggested that PKD signaling is required for GPCR-induced β-catenin nuclear localization in intestinal epithelial cells.
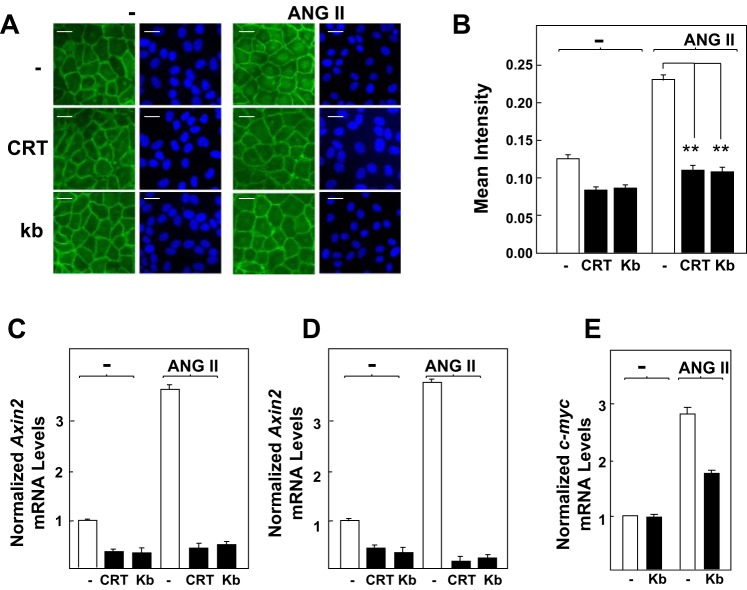
Fig. 2.
Protein kinase D (PKD) family inhibitors prevent β-Cat nuclear translocation and expression of Axin2 and c-myc in response to ANG II in intestinal epithelial cells. A: confluent cultures of IEC-18 cells were incubated in the absence (−) or presence of 2.5 μM CRT006610 (CRT) or 3.5 μM kb NB 142-70 (kb) for 1 h before stimulation of the cells without (−) or with 50 nM ANG II for 4 h. Then the cultures were fixed with 4% paraformaldehyde and stained with an antibody that detects β-Cat conjugated to Alexa Fluor 488 and with Hoechst 33342 to visualize the cell nuclei. B: quantification of β-Cat nuclear localization was determined with the CellProfiler software, as described in materials and methods and Fig. 1. Results shown here are the mean nuclear intensities ± SE (n = 1,500 cells) from 1 experiment, **P < 0.001. Similar results were obtained in 3 separate biological replicates. C and D: confluent cultures of IEC-18 cells were incubated in the absence or presence of CRT or kb for 1 h before stimulation of the cells without (−) or with 50 nM ANG II for either 1 h (C) or 2 h (D), as indicated. RNA was isolated, and the relative levels (n = 3) of Axin2 mRNA compared with GAPDH mRNA were measured by quantitative RT-PCR. E: confluent IEC-18 cells were stimulated with ANG II (50 nM) for 2 h. RNA was isolated, and the relative levels (n = 3) of c-myc mRNA compared with GAPDH mRNA were measured by quantitative RT-PCR. Similar results were obtained in separate experiment. Scale bars = 30 μm.
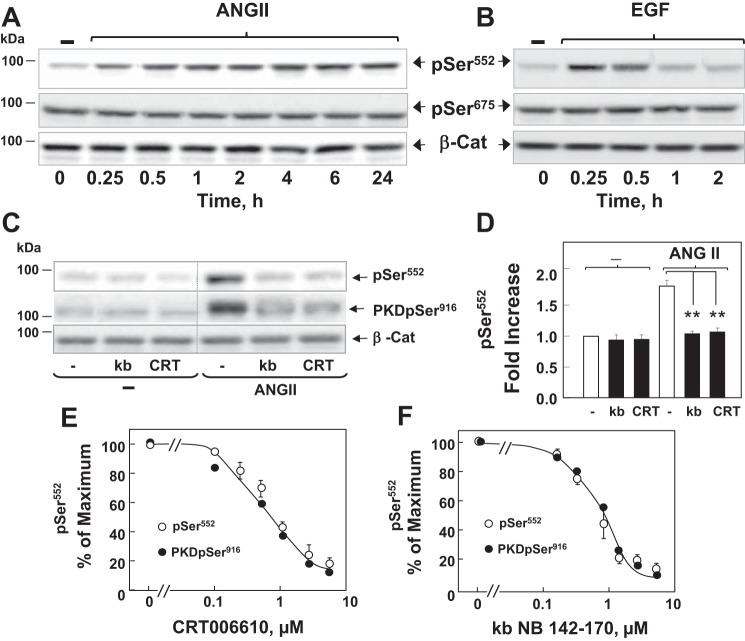
Fig. 4.
ANG II induces persistent PKD-dependent β-Cat phosphorylation at Ser552 in intestinal epithelial IEC-18 cells. A and B: confluent cultures of IEC-18 cells were incubated without (−) or with either 50 nM ANG II (A) or 50 ng/ml EGF (B) for the indicated times. Cultures were then lysed with 2 × SDS-PAGE sample buffer and analyzed by immunoblotting antibodies that detect β-Cat phosphorylated at Ser552 (pSer552) and Ser675 (pSer675) and total β-Cat to verify equal loading. C: confluent cultures of IEC-18 cells were incubated in the absence (−) or presence of 3.5 μM kb or 2.5 μM CRT for 1 h before stimulation of the cells without (−) or with 50 nM ANG II for 4 h. Cells were then lysed with 2 × SDS-PAGE sample buffer and analyzed by immunoblotting antibodies that detect β-Cat pSer552, PKD1 pSer916, and total β-Cat. D: quantification of β-Cat pSer552 was performed using Multi Gauge V3.0. The bars represent the fold increase in β-Cat pSer552. Values are means ± SE (n = 9). **P < 0.001. E and F: confluent cultures of IEC-18 cells were incubated in the absence (−) or presence of increasing concentrations of E or kb (F) for 1 h before stimulation of the cells with 50 nM ANG II for 4 h. Cells were then lysed with 2 × SDS-PAGE sample buffer and analyzed by immunoblotting and quantification for β-Cat pSer552 (○) and for PKD1 pSer916 (●). Values are means ± SE (n = 3) and are expressed as percentage of the maximum level of either β-Cat pSer552 or PKD1 pSer916, respectively. Image editing: irrelevant lanes were removed (indicated by a thin, vertical dotted line) from the acquired digital images, and flanking lanes were juxtaposed using Adobe Photoshop.
Axin2 gene expression is a well-established direct target of β-catenin signaling in the nucleus (28, 32). To determine the functional relevance of increased β-catenin nuclear localization in response to the GPCR/PKD, we determined Axin2 mRNA levels in IEC-18 cells stimulated with ANG II. Cultures of these cells were treated with or without the PKD inhibitors CRT006610 and kb NB 142-70 for 1 h and then challenged with ANG II for 1 or 2 h. As shown in Fig. 2, C and D, ANG II induced significant increase of Axin2 mRNA levels in IEC-18 cells (∼3.5-fold). This response was markedly attenuated by prior cell exposure to either CRT006610 or kb NB 142-70. Similar results were obtained when we examined the mRNA levels of c-myc, another direct target of β-catenin in the nucleus. Stimulation of IEC-18 cells with ANG II elicited significant increase of c-myc mRNA levels, an effect blunted by prior cell exposure to kb NB 142-70 (Fig. 2E).
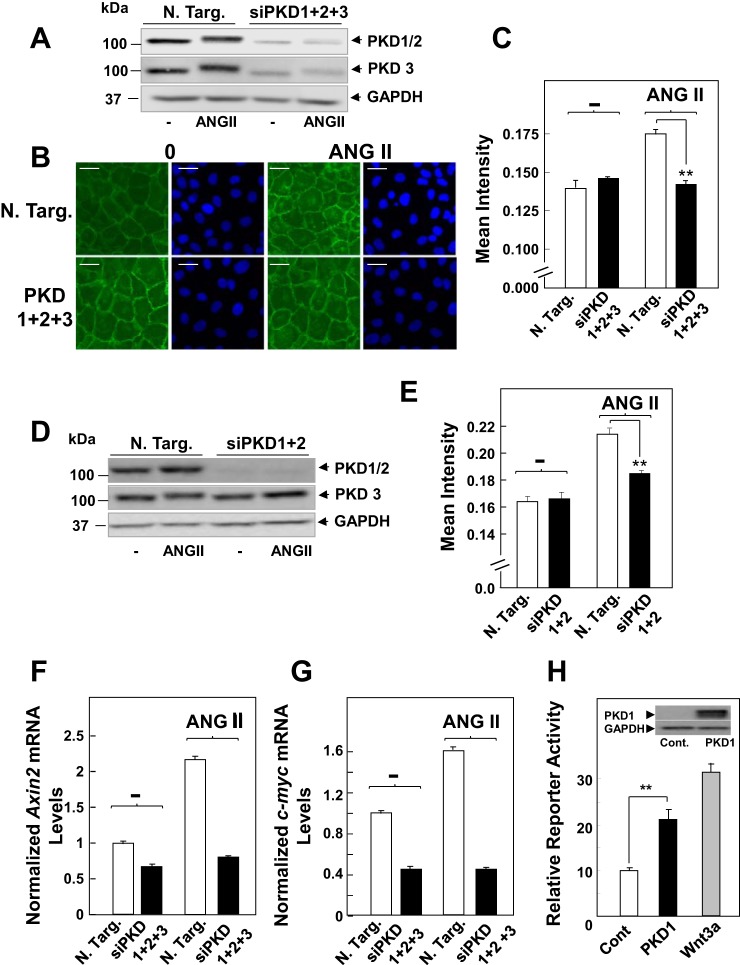
To corroborate the results obtained with pharmacological inhibitors, we used siRNA-mediated knockdown of PKD family expression. The protein level of PKD1, PKD2, and PKD3 in IEC-18 cells transfected with siRNA targeting these PKD isoforms was dramatically reduced compared with cells transfected with nontargeted negative control (Fig. 3A). Knockdown of PKD1, PKD2, and PKD3 completely blocked ANG II-induced β-catenin nuclear localization (Fig. 3B; quantification in Fig. 3C), similar to the inhibitory effects elicited by either CRT006610 or kb NB 142-70. Knockdown of PKD1 and PKD2 (Fig. 3D) was sufficient to greatly reduce nuclear β-catenin localization in response to ANG II (Fig. 3E). In addition, knockdown of PKDs also prevented the increase in Axin2 and c-myc mRNA levels induced by ANG II (Fig. 3, F and G). Further support for a role of PKD1 in promoting β-catenin nuclear signaling was obtained using HEK-293 cells, an extensively used cell model with intact Wnt/β-catenin signaling (29). Transient overexpression of PKD1 in these cells enhanced β-catenin reporter activity to a level comparable to that induced by stimulation with recombinant Wnt3a in these Wnt-responsive cells (Fig. 3H). Thus our results using pharmacological or genetic approaches indicate that PKD family activation mediates β-catenin nuclear localization and signaling induced by GPCR stimulation in intestinal epithelial cells.
Fig. 3.
Knockdown of PKD family expression prevents β-Cat nuclear translocation in response to ANG II in intestinal epithelial cells. A: cultures of IEC-18 cells were transfected with nontargeting small interfering RNA (siRNA) (N Targ) or with siRNAs targeting PKD1, 2, and 3 (siPKD1+2+3). Then the cultures were stimulated with 50 nM ANG II for 4 h and lysed with 2 × SDS-PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect total PKD1/2 or PKD3 to verify knockdown of all PKD isoforms and GAPDH to verify equal loading. B: parallel cultures transfected with N Targ or with siPKD1+2+3 were stimulated with 50 nM ANG II for 4 h, fixed with 4% paraformaldehyde, and stained with an antibody that detects β-Cat conjugated to Alexa Fluor 488 and with Hoechst 33342 to visualize the cell nuclei. C: quantification of β-Cat nuclear localization was determined with the CellProfiler software, as described in materials and methods and in Fig. 1. Results shown here are the mean nuclear intensities ± SE (n = 1,500 from 1 experiment), **P < 0.001. Similar results were obtained in 3 separate biological replicates. Scale bars = 30 μm. D and E: the experimental details were identical to those described above in A–C, except that IEC-18 cells were transfected with siRNAs targeting PKD1 and PKD2 (siPKD1+2) but not PKD3, **P < 0.001. F and G: cultures of IEC-18 cells were transfected with N Targ or with siPKD1+2+3, as above. Then the cells were stimulated with ANG II (50 nM) for 1 h. RNA was isolated, and the relative levels (n = 3) of Axin2 (F) and c-myc (G) mRNAs compared with GAPDH mRNA were measured by quantitative RT-PCR. Similar results were obtained in a separate experiment. H: PKD1 overexpression stimulates β-Cat transcriptional activity regulation. Human embryonic kidney-293 cells were transfected with a mixture of β-Cat-responsive luciferase construct and a constitutively expressing Renilla luciferase reporter gene, or a noninducible firefly luciferase construct and constitutively expressing Renilla luciferase construct, all under the control of a CMV promoter with either pcDNA3 (Cont) or pcDNA3 expressing PKD1. pcDNA3 (Cont) were then challenged without or with 100 ng/ml Wnt3a for 6 h, and luciferase activity was determined, as described under materials and methods. The results represent the mean relative reporter activity (n = 6), **P < 0.001.
GPCR Activation Induces β-Catenin Phosphorylation at Ser552 Through PKD in Intestinal Epithelial IEC-18 Cells
Phosphorylation of β-catenin plays a fundamental role in the regulation of localization, stability, and transcriptional activity. While the sequential phosphorylation of β-catenin in its NH2-terminal domain leads to its degradation, phosphorylation at Ser552 and Ser675 has been proposed to promote nuclear localization and stimulate its transcriptional activity via Wnt-independent pathways (11, 16, 17, 27, 60, 61). We next determined whether PKDs regulate β-catenin phosphorylation at Ser552 and Ser675 in these cells. Cultures of IEC-18 cells were challenged with ANG II and lysed at various times. The lysates were analyzed by Western blotting using antibodies that specifically detect the phosphorylated state of β-catenin at Ser552 and Ser675 (41). Stimulation with ANG II induced a marked increase in the phosphorylation of β-catenin at Ser552 in IEC-18 cells, which persisted for at least 24 h (Fig. 4A). Conversely, stimulation of these cells with EGF, used for comparison, induced transient Ser552 phosphorylation that declined to near baseline levels within 2 h (Fig. 4B). In contrast to Ser552, basal β-catenin phosphorylation at Ser675 was high, and it was not further enhanced by either ANG II or EGF (Fig. 4, A and B). Thus GPCR activation induced sustained increase in β-catenin phosphorylation at Ser552 in intestinal epithelial cells.
We subsequently determined whether the PKDs play a role in linking GPCR activation to β-catenin phosphorylation at Ser552. As shown in Fig. 4C, ANG II-induced phosphorylation of endogenous β-catenin at Ser552 in IEC-18 cells was abrogated by treatment with either kb NB 142-70 or CRT006610. The quantification of nine independent experiments is illustrated in Fig. 4D. The PKD inhibitors kb NB 142-70 and CRT006610 prevented β-catenin phosphorylation at Ser552 and PKD1 autophosphorylation at Ser916 (a surrogate of PKD1 activation) in a dose-dependent manner (Fig. 4, E and F). The dose-response curves were superimposable, reinforcing the existence of a cause-effect relationship (Fig. 4, E and F). Furthermore, knockdown of the PKDs (i.e., PKD1, PKD2, and PKD3) completely blocked ANG II-induced β-catenin phosphorylation at Ser552 (Fig. 5A; quantification in Fig. 5B). Moreover, knockdown of only PKD1 greatly attenuated (by ∼80%) the increase in β-catenin phosphorylation at Ser552 induced by ANG II (Fig. 5, C and D). Collectively, these results indicate that GPCR stimulation activates a PKD-dependent signaling pathway that increases β-catenin phosphorylation at Ser552 in intestinal epithelial cells.
Fig. 5.
β-Cat phosphorylation at Ser552 in response to ANG II is mediated through a PKD-dependent but Akt-independent pathway in intestinal epithelial IEC-18 cells. A: cultures of IEC-18 cells were transfected with N Targ or with siPKD1+2+3. Other cultures were not subjected to transfection (Cont). Then the cultures were stimulated with 50 nM ANG II for 4 h. All cultures were then lysed with 2 × SDS-PAGE sample buffer and analyzed by immunoblotting with antibodies to detect β-Cat pSer552, total β-Cat, total PKD1/2 (PKD C-20), and total PKD3. B: quantification of β-Cat pSer552 was performed using Multi Gauge V3.0. The bars represent the fold increase in phosphorylated β-Cat at Ser552. Values are means ± SE (n = 3). *P < 0.05. C and D: the experimental details were identical to those described above in A and B, except that IEC-18 cells were transfected with siPKD1+2, but not PKD3. The bars represent the fold increase in phosphorylated β-Cat at Ser552. Values are means ± SE (n = 4). **P < 0.001. Image editing: irrelevant lanes were removed (indicated by a thin, vertical dotted line) from the acquired digital images, and flanking lanes were juxtaposed using Adobe Photoshop. E: confluent cultures of IEC-18 cells were incubated in the absence (−) or presence of 3.5 μM kb or 5 μM GSK690693 (GSK) for 1 h before stimulation of the cells without (−) or with 50 nM ANG II for 4 h or EGF for 30 min. The times of stimulation with the agonists were selected from the time course experiment shown in Fig. 4A. F: IEC-18 cells were incubated in the absence (−) or presence of 1 μM AG1478 (AG) or 10 μM LY294002 (LY) for 1 h before stimulation of the cells without (−) or with either 50 nM ANG II for 4 h or EGF for 30 min. The cultures in E and F were then lysed with 2 × SDS-PAGE sample buffer and analyzed by immunoblotting with the indicated antibodies to detect β-Cat pSer552 or total β-Cat. Quantification of β-Cat pSer552 was performed using Multi Gauge V3.0. The bars represent the fold increase in phosphorylated β-Cat at Ser552. Values are means ± SE (n = 4).
GPCR/PKD1 Activation Induces β-Catenin Phosphorylation at Ser552 Through an Akt-Independent But PKA-Dependent Pathway in Intestinal Epithelial Cells
The PKD family phosphorylates serine residues residing in a well-defined consensus motif (LXXRXS), in which a Leu (or Val) residue at position −5 is necessary (48). Given that Ser552 does not reside within a PKD family consensus sequence (TQRRTS), we hypothesized that PKDs promote β-catenin phosphorylation at this residue through other kinases implicated in Ser552 phosphorylation, including Akt and PKA (11, 16, 17, 27, 60, 61).
ANG II induces transient Akt activation in IEC-18 cells via transactivation of EGF receptor and PI3K activation (5), but stimulates sustained β-catenin phosphorylation at Ser552 in these cells (Fig. 4), suggesting that these pathways are not coupled. In keeping with this interpretation, inhibition of Akt (5 μM GSK690693), EGF receptor (1 μM AG1478), or PI3K (10 μM Ly29004) did not prevent Ser552 phosphorylation induced by ANG II in IEC-18 cells (Fig. 5, E and F). In contrast, each of these inhibitors suppressed β-catenin phosphorylation at Ser552 induced by EGF (Fig. 5, E and F), confirming their pharmacological activity in IEC-18 cells and demonstrating that ANG II and EGF stimulate β-catenin phosphorylation at Ser552 through different pathways in these cells. In addition, inhibition of PKD by kb NB 142-70 or CRT006610 prevented β-catenin phosphorylation at Ser552, but enhanced Akt activation, as shown by the increase in the phosphorylation of the Akt substrate PRAS40 at Ser246 (data not shown). These results are consistent with the notion that PKD mediates negative feedback on PI3K/Akt activation in GPCR-stimulated intestinal epithelial cells (36). Accordingly, treatment with the Akt inhibitor GSK690693 suppressed ANG II-induced phosphorylation of PRAS40 at Ser246 (data not shown), but did not prevent β-catenin Ser552 phosphorylation in response to ANG II (Fig. 5E). These results indicate that GPCR/PKD1 activation induces phosphorylation of β-catenin at Ser552 through an Akt-independent pathway.
In contrast to the results with PI3K/Akt inhibitors, treatment of IEC-18 cells with H89, an inhibitor of cAMP-dependent PKA, blunted β-catenin phosphorylation at Ser552 in response to ANG II as effectively as exposure to kb NB 142-70, but without impairing PKD1 autophosphorylation at Ser916 (Fig. 6A; quantification in Fig. 6B). Furthermore, cell stimulation with the adenylyl cyclase activator FSK and the cyclic nucleotide phosphodiesterase inhibitor IBMX to elevate the intracellular levels of cAMP potently induced β-catenin phosphorylation at Ser552 but did not induce PKD1 activation (Fig. 6, A and B). The phosphorylation of β-catenin at Ser552 in response to FSK + IBMX was abolished by exposure to H89, but it was unaffected by kb NB 142-70, suggesting that PKA mediates β-catenin phosphorylation at Ser552 downstream of PKD1. A role of PKA in mediating GPCR/PKD1 β-catenin phosphorylation at Ser552 was further substantiated by the finding that cell exposure to IBMX enhanced ANG II-induced Ser552 phosphorylation, an effect suppressed by treatment with H89 (Fig. 6A; quantification in Fig. 6B).
Fig. 6.
PKD functions upstream of PKA in mediating β-Cat phosphorylation at Ser552 in response to ANG II in intestinal epithelial IEC-18 cells. A: confluent cultures of IEC-18 cells were incubated in the absence (−) or presence of 3.5 μM kb or 5 μM H89 for 1 h before incubation of the cells without (−) or with 25 μM 3-isobutyl-1-methylxanthine (IBMX), 50 nM ANG II, 50 nM ANG II + 25 μM IBMX (ANGII+IBMX), or 10 μM forskolin + 25 μM IBMX (Forskolin+IBMX) for 4 h. Cultures were then lysed with 2 × SDS-PAGE sample buffer and analyzed by immunoblotting with the indicated antibodies. B: quantification of β-Cat pSer552 was performed using Multi Gauge V3.0. The bars corresponding to cultures incubated with 50 nM ANG II (ANG), 50 nM ANG II + 25 μM IBMX (ANG+IB), or 10 μM forskolin + 25 μM IBMX (FK+IB) or 25 μM IBMX (IB) represent the fold increase in phosphorylated β-Cat at Ser552. Values are means ± SE (n = 4). C: IEC-18 cells were incubated in the absence (−) or presence of increasing concentrations of H89, as indicated for 1 h before stimulation of the cells without (−) or with either 50 nM ANG II or 10 μM forskolin + 25 μM IBMX for 4 h. D: IEC-18 cells were incubated in the absence (−) or presence of 25 μM or 50 μM Rp-adenosine 3′,5′-cyclic monophosphorothioate (Rp-Ad), as indicated for 1 h before stimulation of the cells without (−) or with either 50 nM ANG II or 10 μM forskolin + 25 μM IBMX for 4 h. E: IEC-18 cells were incubated in the absence (−) or presence of 0.1 or 1 μM indomethacin (Indo) for 1 h before stimulation of the cells with either 50 nM ANG II or 10 μM forskolin + 25 μM IBMX (Fors+IBMX) for 4 h. F: quantification of β-Cat pSer552 was performed using Multi Gauge V3.0. The bars represent the fold increase in phosphorylated β-Cat at Ser552. Values are means ± SE (n = 5). All cultures were lysed with 2 × SDS-PAGE sample buffer and analyzed by immunoblotting with the indicated antibodies.
We verified that H89 prevented β-catenin phosphorylation at Ser552 in response to ANG II or FSK + IBMX in a dose-dependent manner (Fig. 6C). A significant inhibition was detected at 5 μM, and complete inhibition was achieved at 10 μM. Since H89 also inhibits other protein kinases (9), we examined the effect of a different PKA inhibitor. In line with the results obtained with H89, cell treatment with Rp diastereomer of adenosine 3′,5′-cyclic monophosphorothioate, an analog of cAMP that selectively inhibits PKA, also markedly attenuated β-catenin Ser552 phosphorylation in response to ANG II or FSK + IBMX in IEC-18 cells (Fig. 6D).
GPCR agonists or growth factors that do not couple directly to cAMP synthesis can elevate this second messenger via autocrine stimulation mediated by production of PGE2 (47). In support of this possibility, treatment of IEC-18 cells with the cyclooxygenase-1/2 inhibitor indomethacin greatly attenuated β-catenin Ser552 phosphorylation by ANG II but not by FSK + IBMX (Fig. 6, E and F). Similarly, the dual cyclooxygenase-1/2 inhibitors lornoxicam and meclofenamate prevented β-catenin Ser552 phosphorylation in response to ANG II (data not shown). Collectively, these results imply that PKD1 functions upstream of PKA in a novel pathway that links GPCR to β-catenin phosphorylation at Ser552 in IEC-18 cells.
Although β-catenin phosphorylation at Ser552 is implicated in the regulation of its function (17, 60, 61, 84), we found that treatment of IEC-18 cells with FSK + IBMX (i.e., bypassing the PKDs) was not sufficient to induce β-catenin nuclear localization in IEC-18 cells. Consequently, we anticipated that PKDs stimulate β-catenin nuclear localization, not only via PKD/PKA-mediated phosphorylation at Ser552, but also through an additional PKD-dependent mechanism(s).
GPCR Activation Induces Complex Formation Between Endogenous β-Catenin and PKD1 in Intestinal Epithelial IEC-18 Cells
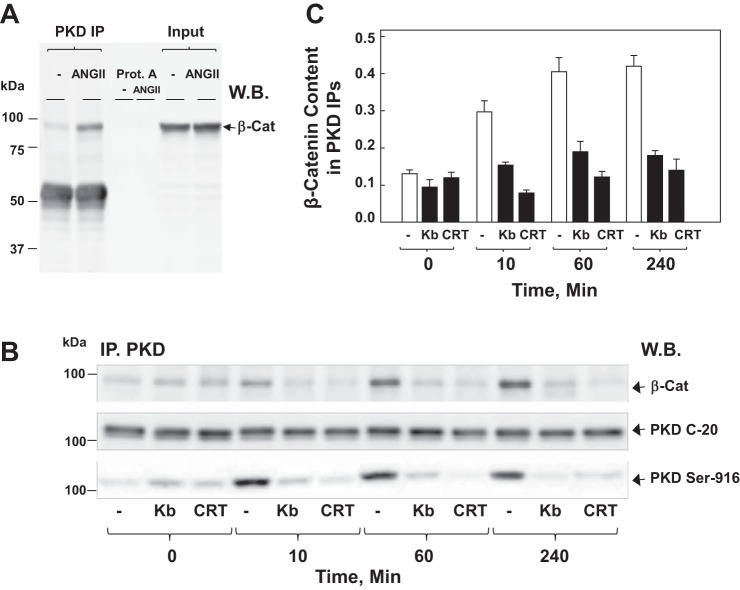
As the function and regulation of β-catenin depends on its binding to other proteins, we hypothesized that cross talk between PKD and β-catenin could be mediated, at least in part, by a physical complex between these proteins. To examine this hypothesis, we determined whether GPCR activation induces association between endogenous PKD1 and β-catenin in intestinal epithelial IEC-18 cells. PKD1 was immunoprecipitated from lysates of IEC-18 cells treated with ANG II for 1 h. The immune complexes were recovered using protein-A coupled to agarose. Western blotting of the resulting PKD1 immunoprecipitates with β-catenin antibodies revealed that cell stimulation with ANG II induced the formation of a complex between PKD1 and β-catenin (Fig. 7A). We verified that omission of the PKD1 antibody prevented the coimmunoprecipitation of β-catenin (Fig. 7A). In time course experiments, the coimmunoprecipitation of PKD1 with β-catenin was detected as early as 10 min after cell stimulation with ANG II, reached a maximum within 1 h, and remained nearly at the maximal level for at least 4 h (Fig. 7B). We verified that similar amounts of total PKD1 were recovered in the immunoprecipitates (Fig. 7B, PKD-C20).
Fig. 7.
ANG II induces complex formation between β-Cat and PKD1 in IEC-18 cells. A: confluent cultures (100 mm dish/treatment, 6 × 106 cells) of IEC-18 cells were stimulated with 50 nM ANG II for 60 min. Then the cells were lysed, and PKD C-20 immunoprecipitates (PKD IP) and protein A agarose negative controls (omitting the PKD C-20 antibody) were analyzed by Western blotting (W.B.) with antibodies that detect β-Cat. The input represents 1/30 of the total lysate used for IP. B: confluent cultures of IEC-18 cells were incubated in the absence (−) or presence (kb) of 3.5 μM kb or 2.5 μM CRT for 1 h before stimulation of the cells with 50 nM ANG II for either 10, 60, or 240 min, as indicated. Cells were lysed, and PKD IP were analyzed by W.B. with antibodies that detect β-Cat, PKD1 (PKD C-20), or PKD1 pSer916 (PKD-Ser916). C: bars represent the means ± SE obtained in 3 independent experiments. Individual values are the level of β-Cat band intensity normalized to the corresponding PKD band intensity in each experiment.
Given that our preceding results indicated that β-catenin nuclear translocation depended on PKD activation, we determined whether exposure of IEC-18 cells to either kb NB 142-70 or CRT006610 before ANG II impairs association between PKD1 and β-catenin. Treatment with the PKD family inhibitors before ANG II stimulation prevented the physical interaction between PKD1 and β-catenin, as well as PKD1 autophosphorylation at Ser916, implying that PKD1/β-catenin complex formation depends on PKD1 activation/phosphorylation (Fig. 7B; quantification in Fig. 7C). Collectively, these results indicate that GPCR stimulation rapidly induces the formation of a complex between PKD1 and β-catenin that depends on PKD1 catalytic activation in intestinal epithelial cells.
PKD1 Signaling Induces β-Catenin Translocation in Intestinal Crypt Epithelial Cells
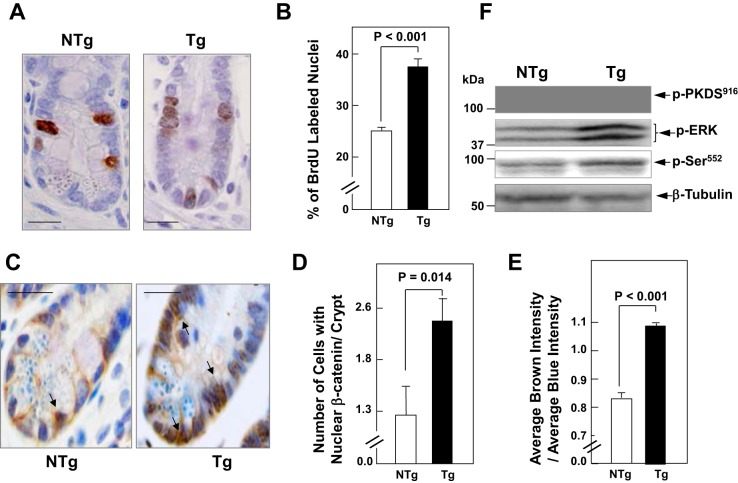
To examine whether PKD1 signaling enhances β-catenin localization in the nuclei of crypt cells in vivo, we used Tg mice that express elevated PKD1 protein in intestinal epithelial cells (PKD1 Tg mice), as described previously (54). As PKD1 is prominently overexpressed in the ileum of the Tg mice (Ref. 54 and unpublished results), we focused our analysis on the distal region of the small intestine. In agreement with our laboratory's previous results (54), BrdU immunostaining showed a marked increase (P < 0.001) in the proportion of DNA synthesizing cells in the crypts of the ileum of PKD1 Tg mice compared with non-Tg littermates (examples of labeled crypts of PKD1 Tg and non-Tg littermates in Fig. 8A; quantification in Fig. 8B). Using immunohistochemistry, we detected a marked increase in the localization of β-catenin in the nucleus of crypt epithelial cells in the ileum of PKD1 Tg mice, compared with non-Tg littermates. Representative histological sections showed that Tg overexpression of PKD1 induced β-catenin nuclear localization that extended beyond the transit-amplifying region in the midcrypt toward the lower region of the crypt (Fig. 8C). The number of cells with nuclear β-catenin in well-oriented crypts of PKD1 Tg mice (2.4 ± 0.3; mean ± SE; n = 8) was 88% higher than in control animals (1.29 ± 0.25; mean ± SE; n = 8), an increase that was statistically significant (P = 0.014) (Fig. 8D). Furthermore, an independent quantitative analysis of the nuclear content of β-catenin of at least 600 crypts of PKD1 Tg mice (n = 8) and 536 of non-Tg littermates (n = 8) showed a significant increase (P < 0.001) in the ratio of β-catenin to nuclear staining in crypt cells of PKD1 Tg mice compared with non-Tg littermates (Fig. 8E).
Fig. 8.
PKD1 signaling induces β-Cat nuclear translocation in intestinal crypt epithelial cells. A: the proportion of crypt cell DNA synthesis was determined by immunohistochemical detection of 5-bromo-2′-deoxyuridine (BrdU) incorporated into the nuclei of crypt cells of the ileum. Transgenic (Tg) mice and nontransgenic (NTg) littermates were injected intraperitoneally with BrdU (100 μg/g body wt) and killed 3 h after the injection. Tissue fixation, histological sections, and routine staining were performed as described in materials and methods. Scale bars = 20 μm. B: the bars represent the percentage of BrdU-labeled crypt cells (means ± SE) in the PKD1 Tg mice (solid bar) and in the NTg littermates in Tg mice (open bar). C: immunohistochemistry of histological sections from PKD1 Tg mice and NTg littermates with β-Cat (6B3) rabbit monoclonal antibody. Scale bars = 20 μm. D: the bars represent the number of cells with nuclear β-Cat in well-oriented crypts of PKD1 Tg mice and NTg littermates. Values are means ± SE (n = 8). E: the bars represent the ratio of brown intensity to blue intensity (β-Cat/nuclear) staining in crypt cells of PKD1 Tg compared with NTg. Values are means ± SE (n = 600 cells in Tg mice and n = 536 cells in NTg mice). F: epithelial cells from the ileum of Tg mice and NTg littermates mice were isolated sequentially by timed incubations in a EDTA-PBS solution. W.B. was used to analyze lysates of these cells for PKD1 autophosphorylated at Ser916 (pPKDS916), ERK at Thr202 and Tyr204 (pERK), and β-Cat at Ser552 (pSer552). Equivalent loading was verified by immunoblotting for tubulin.
Using lysates from epithelial cells isolated from ileum of PKD1 Tg and non-Tg mice, we verified that PKD1 overexpressed in the Tg mice is catalytic active, as judged by PKD1 autophosphorylation at Ser916, class IIa histone deacetylase phosphorylation at residues targeted by PKDs (53), and stimulation of downstream signaling, including ERK activation (Fig. 8F). In accord with results obtained in IEC-18 cells, PKD1 overexpression also promoted β-catenin phosphorylation at Ser552 in the intestinal epithelial lysates (Fig. 8F). The results indicate that PKD1 signaling promotes nuclear β-catenin localization and phosphorylation, thus providing evidence for the existence of cross talk between PKD1 and β-catenin in intestinal crypt epithelial cells in vivo.
DISCUSSION
It is increasingly recognized that β-catenin nuclear localization is the initiating and driving element in β-catenin signaling, and that this step is regulated independently of its stabilization (35). A number of previous studies demonstrated that GPCR activation regulates β-catenin function and localization, but the mechanism(s) involved remained incompletely defined (50). PKD1, a major downstream element in GPCR signaling, plays a critical role in mediating migration and proliferation of intestinal epithelial cells (54, 79). The importance and overlapping of the biological responses mediated by β-catenin and PKD1 in intestinal epithelial cell regulation prompted us to examine whether these signaling pathways are connected through positive cross talk.
In previous studies, our laboratory demonstrated that the GPCR agonists are a potent growth factor for IEC-18 cells (3–6, 56, 73) through PKD1 activation (54). In addition to its well-established role in the regulation of systemic blood pressure, ANG II is a regulatory peptide generated locally in the gastrointestinal tract that plays a role in gastrointestinal mucosal inflammation and carcinogenesis (20, 24, 76). Using ANG II to induce receptor-mediated PKD activation in IEC-18 cells (3, 4, 36, 53, 54), we produced several lines of evidence indicating that PKDs interact with endogenous β-catenin at multiple points in these cells. 1) Stimulation of intestinal epithelial cells with ANG II induced β-catenin nuclear localization and image analysis indicated that β-catenin redistribution occurred in a time-dependent manner in most cells of the population. The immunofluorescence analysis was corroborated by using cytosol/nuclear fractionation. 2) Treatment with structurally unrelated PKD family inhibitors (kb NB 142-70 or CRT006610), at concentrations that inhibited PKD1 activation within intact IEC-18 cells, prevented ANG II-induced β-catenin nuclear localization. 3) Knockdown of the isoforms of the PKD family (PKD1, PKD2, and PKD3) or of PKD1 and PKD2 also averted the increase in nuclear localization of β-catenin in response to ANG II. 4) Axin2 and c-myc gene expression, direct downstream targets of β-catenin signaling, were stimulated by ANG II in a PKD-dependent manner, reinforcing the functional relevance of increased β-catenin nuclear localization in response to the GPCR/PKD axis. Furthermore, overexpression of PKD1 in HEK-293 cells, a model for studies of Wnt/β-catenin signaling, enhanced β-catenin reporter activity. Collectively, these results demonstrate that PKDs mediate GPCR-induced β-catenin nuclear localization and signaling in intestinal epithelial cells.
Because the phosphorylation of β-catenin on Ser552 has been implicated in stimulating β-catenin nuclear localization (17, 60, 61, 84), we determined whether PKDs promote the phosphorylation of this site. Our results demonstrated, for the first time, that ANG II stimulates a persistent increase in β-catenin phosphorylation on Ser552 in intestinal epithelial cells. Pharmacological and genetic approaches showed that PKD1 positively regulates β-catenin phosphorylation on Ser552 in ANG II-stimulated intestinal cells. Surprisingly, inhibition of PKA blunted β-catenin phosphorylation at Ser552 in response to ANG II without impairing PKD1 activation, suggesting that PKA lies downstream of PKD1. In line with this interpretation, cAMP-elevating agents potently induced sustained β-catenin phosphorylation at Ser552 without eliciting PKD1 activation. The results indicate that PKD1 functions upstream of PKA in a novel PKD/PKA kinase cascade that links GPCR activation to β-catenin phosphorylation at Ser552 in intestinal epithelial cells. Given that β-catenin phosphorylation at Ser552 has been implicated in inflammation-induced dysplastic transformation in the colon (27) and in intestinal polyposis (16), as well as results obtained in other systems (17, 60, 61, 84), it is likely that β-catenin phosphorylation on Ser552 facilitates GPCR/PKD-induced β-catenin nuclear localization. However, our results indicated that PKA-mediated β-catenin phosphorylation at Ser552 was not sufficient to induce nuclear localization of β-catenin in response to GPCR activation in IEC-18 cells, implying that other PKD-dependent mechanism(s) are also required.
As the function and regulation of β-catenin depends on its binding to other proteins, we hypothesized that cross talk between PKD and β-catenin could be also mediated, at least in part, through a physical complex between these proteins. Accordingly, our results revealed that GPCR stimulation induced rapid association between endogenous PKD1 and β-catenin in intestinal epithelial cells, thus providing further evidence for PKD/β-catenin cross talk. Previous studies showed dynamic nuclear-cytoplasmic shuttling of PKD family members in response to cell stimulation (43, 54, 67). Specifically, the time course of nuclear translocation of PKD1 in response to GPCR agonists is similar to that of β-catenin nuclear localization shown in this study. Interestingly, GPCR-induced nuclear localization of both β-catenin and PKD1, as well as their complex formation, were abrogated by inhibitors of PKD family catalytic activity. These results raise the attractive possibility that activated PKD kinases facilitate β-catenin translocation to the nucleus, a location of critical importance for regulating the output of this pathway.
While the importance of nuclear import of β-catenin for the function of the pathway in normal and cancer cells is well recognized (22), the mechanism(s) involved is incompletely understood. However, there are examples of interacting molecules promoting β-catenin translocation to the nucleus in the absence of Wnt signaling or mutations in APC or CTNNB. For example, the Forkhead-box transcription factor M1 and the proto-oncogene BCL9 have been implicated in β-catenin nuclear import and/or its retention in the nucleus (1, 85). We propose that PKD family activation promotes β-catenin localization to the nucleus of intestinal epithelial cells through direct complex formation that protects it from degradation by the destruction complex and indirectly, via PKA-mediated β-catenin phosphorylation at Ser552.
Since our results with IEC-18 cells revealed positive cross talk between PKD and β-catenin localization and phosphorylation, we hypothesized that PKD1 regulates β-catenin activity of intestinal epithelial cells in vivo. To test this hypothesis, we used Tg mice that express elevated PKD1 protein in the intestinal epithelium (54). In agreement with our laboratory's previous results, the proportion of DNA synthesizing cells in the crypts of the ileum of PKD1 Tg mice was markedly higher, compared with non-Tg littermates. We also found that overexpression of PKD1 was associated with a significant increase in the localization of β-catenin in the nucleus of crypt intestinal epithelial cells and with augmented β-catenin phosphorylation on Ser552 in lysates of crypt cells. Collectively, the results indicate that PKD1 mediates positive cross talk between GPCR and β-catenin signaling pathways within intestinal epithelial cells in vitro and in vivo.
In contrast to our results obtained with untransformed intestinal epithelial cells, PKD1 has been implicated in the negative regulation of β-catenin function via phosphorylation of Thr112 and Thr120 in prostate cancer cells (10). Intriguingly, these residues reside in sequences of β-catenin that are sharply different from the consensus sequence phosphorylated by PKDs in most of its targets, characterized by Arg at position −3 and Leu (or Val) at position −5. Furthermore, PKDs preferentially phosphorylate Ser rather than Thr residues (48). The sequence of β-catenin surrounding Thr120 possesses Ala at position −3, and Asp at position −5. Although these discrepancies remain unexplained, it is intriguing that a recent study identified mutations in protein kinases that alter their specificity for phosphorylation sites (8). In the case of PKD1, a single amino acid mutation (D665N), found in prostate cancer samples, removed the requirement of Arg at position −3 (8). More experimental work is necessary to determine whether the D665N mutation of PKD1 explains β-catenin phosphorylation of Thr120 in prostate cancer cells. It should also be noted that other reports concluded that PKDs are pro-oncogenic in prostate cancer (2, 26, 59).
The identification of positive PKD/β-catenin cross talk assumes an added significance in view of increasing evidence implicating PKDs in human proliferative diseases. For example, PRKD1 gene expression is increased in subpopulations of cancers of the colon, liver, and upper aero-digestive system, as revealed by searching COSMIC (catalog of somatic mutations in cancer). Recently, high PKD1 mRNA levels have been linked to lower cancer-specific survival in heavy smokers with esophageal squamous cell cancers (74) and predicts recurrence in laryngeal cancer (12). Indeed, the level of PRKD1 expression has been proposed as a laryngeal cancer prognostic marker for routine clinical applications. Notably, activating PRKD1 somatic mutations (e.g., E710D) have been detected in >70% of a salivary gland cancer (70). New recurrent PRKD1 gene rearrangements and variant fusions have been found in cribriform adenocarcinoma of the oropharynx (71). PKD1 has been proposed as a therapeutic target in pancreatic (15, 31, 37) and colon (69) cancers, although a different conclusion has also been presented (57). It is conceivable that the PKD/β-catenin cross talk identified here contributes to the mechanism(s) by which PKDs promote malignant transformation of epithelial cells, but further systematic studies are needed to prove this hypothesis.
In conclusion, our results show that GPCR activation promotes β-catenin nuclear localization, phosphorylation at Ser552, and signaling through a PKD-dependent pathway. In the course of these studies, a novel PKD/PKA kinase cascade that links GPCR activation to β-catenin phosphorylation at Ser552 was also discovered. GPCR signaling induced formation of a physical complex between PKD1 and β-catenin that was abrogated by inhibitors of PKD family catalytic activity. Using a PKD1 Tg mouse model, we corroborated that PKD1 overexpression promotes β-catenin nuclear localization and phosphorylation at Ser552 in cryptal epithelial cells. Collectively, the results identify a novel positive cross talk between PKD and β-catenin in intestinal epithelial cells, both in vitro and in vivo.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK-100405, P30-DK-41301, and P01-CA-163200 and by Department of Veterans Affair Grant 1I01BX001473 (to E. Rozengurt). L. Han and L.-L. Han were both supported by a scholarship from the Chinese Scholarship Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.W., L.H., J.S.-S., L.-L.H., J.V.S., N.R., and S.H.Y. performed experiments; J.W., J.S.-S., N.R., S.H.Y., and E.R. analyzed data; J.S.-S. and E.R. conception and design of research; J.S.-S. and E.R. interpreted results of experiments; J.S.-S. prepared figures; J.S.-S. and E.R. edited and revised manuscript; E.R. drafted manuscript; E.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the Imaging and Stem Cell Biology Core of the CURE: Digestive Diseases Research Center (supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-41301) for help. E. Rozengurt is the holder of the Ronald S. Hirshberg Chair in Pancreatic Cancer Research.
Present addresses: L. Han is affiliated with the Department of Hepatobiliary Surgery, First Affiliated Hospital of Medical College, Xi'an Jiaotong University, Xi'an 710061, China; L.-L. Han is affiliated with the Department of Oncology, First Affiliated Hospital, College of Medical of Xi'an Jiaotong University, Xi'an, 710061, China.
REFERENCES
- 1.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between β-catenin's adhesive and transcriptional functions. Genes Dev 18: 2225–2230, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Deng F, Singh SV, Wang QJ. Protein kinase D3 (PKD3) contributes to prostate cancer cell growth and survival through a PKCepsilon/PKD3 pathway downstream of Akt and ERK 1/2. Cancer Res 68: 3844–3853, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Chiu T, Rozengurt E. PKD in intestinal epithelial cells: rapid activation by phorbol esters, LPA, and angiotensin through PKC. Am J Physiol Cell Physiol 280: C929–C942, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Chiu T, Santiskulvong C, Rozengurt E. ANG II stimulates PKC-dependent ERK activation, DNA synthesis, and cell division in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 285: G1–G11, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chiu T, Santiskulvong C, Rozengurt E. EGF receptor transactivation mediates ANG II-stimulated mitogenesis in intestinal epithelial cells through the PI3-kinase/Akt/mTOR/p70S6K1 signaling pathway. Am J Physiol Gastrointest Liver Physiol 288: G182–G194, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Chiu T, Wu SS, Santiskulvong C, Tangkijvanich P, Yee HF Jr, Rozengurt E. Vasopressin-mediated mitogenic signaling in intestinal epithelial cells. Am J Physiol Cell Physiol 282: C434–C450, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Clevers H. Wnt/β-catenin signaling in development and disease. Cell 127: 469–480, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Creixell P, Schoof Erwin M, Simpson Craig D, Longden J, Miller Chad J, Lou Hua J, Perryman L, Cox Thomas R, Zivanovic N, Palmeri A, Wesolowska-Andersen A, Helmer-Citterich M, Ferkinghoff-Borg J, Itamochi H, Bodenmiller B, Erler Janine T, Turk Benjamin E, Linding R. Kinome-wide decoding of network-attacking mutations rewiring cancer signaling. Cell 163: 202–217, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du C, Zhang C, Li Z, Biswas MHU, Balaji KC. Beta-catenin phosphorylated at threonine 120 antagonizes generation of active beta-catenin by spatial localization in trans-Golgi network. PLoS One 7: e33830, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 282: 11221–11229, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fountzilas E, Kotoula V, Angouridakis N, Karasmanis I, Wirtz RM, Eleftheraki AG, Veltrup E, Markou K, Nikolaou A, Pectasides D, Fountzilas G. Identification and validation of a multigene predictor of recurrence in primary laryngeal cancer. PLoS One 8: e70429, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal 22: 717–727, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Gracz AD, Magness ST. Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways. Am J Physiol Gastrointest Liver Physiol 307: G260–G273, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harikumar KB, Kunnumakkara AB, Ochi N, Tong Z, Deorukhkar A, Sung B, Kelland L, Jamieson S, Sutherland R, Raynham T, Charles M, Bagherazadeh A, Foxton C, Boakes A, Farooq M, Maru D, Diagaradjane P, Matsuo Y, Sinnett-Smith J, Gelovani J, Krishnan S, Aggarwal BB, Rozengurt E, Ireson CR, Guha S. A novel small-molecule inhibitor of protein kinase D blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther 9: 1136–1146, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 39: 189–198, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol 25: 9063–9072, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/β-catenin signalling during epithelial-mesenchymal transition. PLoS One 6: e23899, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iglesias T, Waldron RT, Rozengurt E. Identification of in vivo phosphorylation sites required for protein kinase D activation. J Biol Chem 273: 27662–27667, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Inokuchi Y, Morohashi T, Kawana I, Nagashima Y, Kihara M, Umemura S. Amelioration of 2,4,6-trinitrobenzene sulphonic acid induced colitis in angiotensinogen gene knockout mice. Gut 54: 349–356, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacamo R, Sinnett-Smith J, Rey O, Waldron RT, Rozengurt E. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser(744) and Ser(748) phosphorylation. J Biol Chem 283: 12877–12887, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamieson C, Sharma M, Henderson BR. Targeting the β-catenin nuclear transport pathway in cancer. Semin Cancer Biol 27: 20–29, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem 269: 6140–6148, 1994. [PubMed] [Google Scholar]
- 24.Katada K, Yoshida N, Suzuki T, Okuda T, Mizushima K, Takagi T, Ichikawa H, Naito Y, Cepinskas G, Yoshikawa T. Dextran sulfate sodium-induced acute colonic inflammation in angiotensin II type 1a receptor deficient mice. Inflamm Res 57: 84–91, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal 26: 570–579, 2014. [DOI] [PubMed] [Google Scholar]
- 26.LaValle C, Bravo-Altamirano K, Giridhar K, Chen J, Sharlow E, Lazo J, Wipf P, Wang QJ. Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility. BMC Chem Biol 10: 5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM, Weber CR, Turner JR, He XC, Katzman RB, Li L, Barrett TA. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology 139: 869–881, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by β-catenin-T cell factor: a feedback repressor pathway regulating Wnt signaling. J Biol Chem 277: 21657–21665, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 149: 1245–1256, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Shao Y, Tong Y, Shen T, Zhang J, Li Y, Gu H, Li F. Nucleo-cytoplasmic shuttling of PAK4 modulates β-catenin intracellular translocation and signaling. Biochim Biophys Acta 1823: 465–475, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Liou GY, Döppler H, Braun UB, Panayiotou R, Scotti Buzhardt M, Radisky DC, Crawford HC, Fields AP, Murray NR, Wang QJ, Leitges M, Storz P. Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nat Commun 6: 6200, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/Axin2 in colorectal and liver tumors. Mol Cell Biol 22: 1184–1193, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maher MT, Mo R, Flozak AS, Peled ON, Gottardi CJ. Beta-catenin phosphorylated at serine 45 is spatially uncoupled from beta-catenin phosphorylated in the GSK3 domain: implications for signaling. PLoS One 5: e10184, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews SA, Rozengurt E, Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/protein kinase C mu. J Biol Chem 274: 26543–26549, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Morgan RG, Ridsdale J, Tonks A, Darley RL. Factors affecting the nuclear localization of β-catenin in normal and malignant tissue. J Cell Biochem 115: 1351–1361, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Ni Y, Sinnett-Smith J, Young SH, Rozengurt E. PKD1 mediates negative feedback of PI3K/Akt activation in response to G protein-coupled receptors. PLoS One 8: e73149, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochi N, Tanasanvimon S, Matsuo Y, Tong Z, Sung B, Aggarwal BB, Sinnett-Smith J, Rozengurt E, Guha S. Protein kinase D1 promotes anchorage-independent growth, invasion, and angiogenesis by human pancreatic cancer cells. J Cell Physiol 226: 1074–1081, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Paolucci L, Sinnett-Smith J, Rozengurt E. Lysophosphatidic acid rapidly induces protein kinase D activation through a pertussis toxin-sensitive pathway. Am J Physiol Cell Physiol 278: C33–C39, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Quaroni A, May RJ. Establishment and characterization of intestinal epithelial cell cultures. Methods Cell Biol 21B: 403–427, 1980. [PubMed] [Google Scholar]
- 40.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248–265, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rey O, Chang W, Bikle D, Rozengurt N, Young SH, Rozengurt E. Negative cross-talk between calcium-sensing receptor and beta-catenin signaling systems in colonic epithelium. J Biol Chem 287: 1158–1167, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rey O, Reeve JR Jr, Zhukova E, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor-mediated phosphorylation of the activation loop of protein kinase D: dependence on plasma membrane translocation and protein kinase C epsilon. J Biol Chem 279: 34361–34372, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Rey O, Sinnett-Smith J, Zhukova E, Rozengurt E. Regulated nucleocytoplasmic transport of protein kinase D in response to G protein-coupled receptor activation. J Biol Chem 276: 49228–49235, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Rey O, Zhukova E, Sinnett-Smith J, Rozengurt E. Vasopressin-induced intracellular redistribution of protein kinase D in intestinal epithelial cells. J Cell Physiol 196: 483–492, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 434: 843–850, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Roth KA, Hermiston ML, Gordon JI. Use of transgenic mice to infer the biological properties of small intestinal stem cells and to examine the lineage relationships of their descendants. Proc Natl Acad Sci U S A 88: 9407–9411, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213: 589–602, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Rozengurt E. Protein kinase D signaling: multiple biological functions in health and disease. Physiology 26: 23–33, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem 280: 13205–13208, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Shevtsov SP, Haq S, Force T. Activation of β-catenin signaling pathways by classical G-protein-coupled receptors: mechanisms and consequences in cycling and non-cycling cells. Cell Cycle 5: 2295–2300, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Simon TC, Roth KA, Gordon JI. Use of transgenic mice to map cis-acting elements in the liver fatty acid-binding protein gene (Fabpl) that regulate its cell lineage-specific, differentiation-dependent, and spatial patterns of expression in the gut epithelium and in the liver acinus. J Biol Chem 268: 18345–18358, 1993. [PubMed] [Google Scholar]
- 52.Sinnett-Smith J, Jacamo R, Kui R, Wang YM, Young SH, Rey O, Waldron RT, Rozengurt E. Protein kinase D mediates mitogenic signaling by Gq-coupled receptors through protein kinase C-independent regulation of activation loop Ser744 and Ser748 phosphorylation. J Biol Chem 284: 13434–13445, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinnett-Smith J, Ni Y, Wang J, Ming M, Young SH, Rozengurt E. Protein kinase D1 mediates class IIa histone deacetylase phosphorylation and nuclear extrusion in intestinal epithelial cells: role in mitogenic signaling. Am J Physiol Cell Physiol 306: C961–C971, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinnett-Smith J, Rozengurt N, Kui R, Huang C, Rozengurt E. Protein kinase D1 mediates stimulation of DNA synthesis and proliferation in intestinal epithelial IEC-18 cells and in mouse intestinal crypts. J Biol Chem 286: 511–520, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinnett-Smith J, Zhukova E, Hsieh N, Jiang X, Rozengurt E. Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in Swiss 3T3 cells. J Biol Chem 279: 16883–16893, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Slice LW, Chiu T, Rozengurt E. Angiotensin II and epidermal growth factor induce cyclooxygenase-2 expression in intestinal epithelial cells through small GTPases using distinct signaling pathways. J Biol Chem 280: 1582–1593, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Sundram V, Ganju A, Hughes JE, Khan S, Chauhan SC, Jaggi M. Protein kinase D1 attenuates tumorigenesis in colon cancer by modulating β-catenin/T cell factor activity. Oncotarget 5: 6867–6684, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki K, Bose P, Leong-Quong R, Fujita D, Riabowol K. REAP: a two minute cell fractionation method. BMC Res Notes 3: 294, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tandon M, Salamoun JM, Carder EJ, Farber E, Xu S, Deng F, Tang H, Wipf P, Wang QJ. SD-208, a novel protein kinase D inhibitor, blocks prostate cancer cell proliferation and tumor growth in vivo by inducing G2/M cell cycle arrest. PLoS One 10: e0119346, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem 281: 9971–9976, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Taurin S, Sandbo N, Yau DM, Sethakorn N, Dulin NO. Phosphorylation of beta-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells. Am J Physiol Cell Physiol 294: C1169–C1174, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thorstensen L, Lind GE, Løvig T, Diep CB, Meling GI, Rognum TO, Lothe RA. Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia 7: 99–108, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J 31: 2714–2736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci U S A 91: 8572–8576, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waldron RT, Innamorati G, Torres-Marquez ME, Sinnett-Smith J, Rozengurt E. Differential PKC-dependent and -independent PKD activation by G protein alpha subunits of the Gq family: selective stimulation of PKD Ser(7)(4)(8) autophosphorylation by Galphaq. Cell Signal 24: 914–921, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waldron RT, Rey O, Iglesias T, Tugal T, Cantrell D, Rozengurt E. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J Biol Chem 276: 32606–32615, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Waldron RT, Rey O, Zhukova E, Rozengurt E. Oxidative stress induces protein kinase C-mediated activation loop phosphorylation and nuclear redistribution of protein kinase D. J Biol Chem 279: 27482–27493, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Waldron RT, Rozengurt E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J Biol Chem 278: 154–163, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Wei N, Chu E, Wipf P, Schmitz JC. Protein kinase D as a potential chemotherapeutic target for colorectal cancer. Mol Cancer Ther 13: 1130–1141, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CKY, Perez-Ordonez B, Harding NJ, Alfaro J, Chu KC, Viale A, Fusco N, da Cruz Paula A, Marchio C, Sakr RA, Lim R, Thompson LDR, Chiosea SI, Seethala RR, Skalova A, Stelow EB, Fonseca I, Assaad A, How C, Wang J, de Borja R, Chan-Seng-Yue M, Howlett CJ, Nichols AC, Wen YH, Katabi N, Buchner N, Mullen L, Kislinger T, Wouters BG, Liu F-F, Norton L, McPherson JD, Rubin BP, Clarke BA, Weigelt B, Boutros PC, and Reis-Filho JS. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet 46: 1166–1169, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinreb I, Zhang L, Tirunagari LMS, Sung YS, Chen CL, Perez-Ordonez B, Clarke BA, Skalova A, Chiosea SI, Seethala RR, Waggott D, Boutros PC, How C, Liu FF, Irish JC, Goldstein DP, Gilbert R, ud Din N, Assaad A, Hornick JL, Thompson LDR, Antonescu CR. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosomes Cancer 53: 845–856, 2014. [DOI] [PubMed] [Google Scholar]
- 72.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology 142: 219–232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu SS, Chiu T, Rozengurt E. ANG II and LPA induce Pyk2 tyrosine phosphorylation in intestinal epithelial cells: role of Ca2+, PKC, and Rho kinase. Am J Physiol Cell Physiol 282: C1432–C1444, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Xie X, Zhang SS, Wen J, Yang H, Luo KJ, Yang F, Hu Y, Fu JH. Protein kinase D1 mRNA level may predict cancer-specific survival in heavy smokers with esophageal squamous cell cancers. Dis Esophagus 27: 188–195, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell 123: 889–901, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yasumaru M, Tsuji S, Tsujii M, Irie T, Komori M, Kimura A, Nishida T, Kakiuchi Y, Kawai N, Murata H, Horimoto M, Sasaki Y, Hayashi N, Kawano S, Hori M. Inhibition of angiotensin II activity enhanced the antitumor effect of cyclooxygenase-2 inhibitors via insulin-like growth factor I receptor pathway. Cancer Res 63: 6726–6734, 2003. [PubMed] [Google Scholar]
- 77.Young SH, Rozengurt E. Amino acids and Ca2+ stimulate different patterns of Ca2+ oscillations through the Ca2+-sensing receptor. Am J Physiol Cell Physiol 282: C1414–C1422, 2002. [DOI] [PubMed] [Google Scholar]
- 78.Young SH, Rozengurt E. Qdot nanocrystal conjugates conjugated to bombesin or ANG II label the cognate G protein-coupled receptor in living cells. Am J Physiol Cell Physiol 290: C728–C732, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Young SH, Rozengurt N, Sinnett-Smith J, Rozengurt E. Rapid protein kinase D1 signaling promotes migration of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 303: G356–G366, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan J, Rey O, Rozengurt E. Activation of protein kinase D3 by signaling through Rac and the alpha subunits of the heterotrimeric G proteins G12 and G13. Cell Signal 18: 1051–1062, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Yuan J, Slice LW, Gu J, Rozengurt E. Cooperation of Gq, Gi, and G12/13 in protein kinase D activation and phosphorylation induced by lysophosphatidic acid. J Biol Chem 278: 4882–4891, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Yuan J, Slice LW, Rozengurt E. Activation of protein kinase d by signaling through rho and the alpha subunit of the heterotrimeric G protein G13. J Biol Chem 276: 38619–38627, 2001. [DOI] [PubMed] [Google Scholar]
- 83.Yuan JZ, Slice L, Walsh JH, Rozengurt E. Activation of protein kinase D by signaling through the alpha subunit of the heterotrimeric G protein G(q). J Biol Chem 275: 2157–2164, 2000. [DOI] [PubMed] [Google Scholar]
- 84.Zhang M, Mahoney E, Zuo T, Manchanda PK, Davuluri RV, Kirschner LS. Protein kinase a activation enhances β-catenin transcriptional activity through nuclear localization to PML bodies. PLoS One 9: e109523, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, Huang H, Xue J, Liu M, Wang Y, Sawaya R, Xie K, Yung WKA, Medema René H, He X, Huang S. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell 20: 427–442, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]