Abstract
Molecular chaperones that support protein quality control, including heat shock protein 70 (Hsp70), participate in diverse aspects of cellular and physiological function. Recent studies have reported roles for specific chaperone activities in blood platelets in maintaining hemostasis; however, the functions of Hsp70 in platelet physiology remain uninvestigated. Here we characterize roles for Hsp70 activity in platelet activation and function. In vitro biochemical, microscopy, flow cytometry, and aggregometry assays of platelet function, as well as ex vivo analyses of platelet aggregate formation in whole blood under shear, were carried out under Hsp70-inhibited conditions. Inhibition of platelet Hsp70 blocked platelet aggregation and granule secretion in response to collagen-related peptide (CRP), which engages the immunoreceptor tyrosine-based activation motif-bearing collagen receptor glycoprotein VI (GPVI)-Fc receptor-γ chain complex. Hsp70 inhibition also reduced platelet integrin-αIIbβ3 activation downstream of GPVI, as Hsp70-inhibited platelets showed reduced PAC-1 and fibrinogen binding. Ex vivo, pharmacological inhibition of Hsp70 in human whole blood prevented the formation of platelet aggregates on collagen under shear. Biochemical studies supported a role for Hsp70 in maintaining the assembly of the linker for activation of T cells signalosome, which couples GPVI-initiated signaling to integrin activation, secretion, and platelet function. Together, our results suggest that Hsp70 regulates platelet activation and function by supporting linker for activation of T cells-associated signaling events downstream of platelet GPVI engagement, suggesting a role for Hsp70 in the intracellular organization of signaling systems that mediate platelet secretion, “inside-out” activation of platelet integrin-αIIbβ3, platelet-platelet aggregation, and, ultimately, hemostatic plug and thrombus formation.
Keywords: chaperones, hemostasis, integrin, platelets, thrombosis
heat shock protein (Hsp) 70 is an ATP-powered, 70-kDa molecular chaperone that regulates a myriad of protein quality-control processes, including folding of nascent polypeptides, trafficking of proteins across membranes, prevention of protein aggregation, and protein complex assembly and disassembly (15, 34, 46). Hsp70 is an abundantly expressed and highly conserved protein with 50% sequence homology between mammals and prokaryotes, highlighting the importance of Hsp family members in protein homeostasis throughout evolution (10). In human cells, the significance and complexity of Hsp70 function are evident in the functions of the 13 members of the human Hsp70 family, including the cytosolic, stress-inducible Hsp70 (or Hsp72) and its constitutively expressed heat shock cognate 71-kDa protein (Hsc70), the endoplasmic reticulum (ER)-localized 78-kDa glucose-regulated protein (Grp78, or binding immunoglobulin protein), and the mitochondrial form mtHsp70 (or Grp75/mortalin) (39). These Hsp70 family members regulate diverse cellular processes through an association with >50 co-chaperones, and the arrangement of co-chaperones with Hsp70 varies across different contexts, allowing for a precise combinatorial control over the activity and specificity of Hsp70 (26, 39).
Platelets serve as the cellular guardians of vascular integrity. Upon exposure to extracellular matrix proteins such as collagen, platelets undergo a carefully orchestrated activation program, resulting in platelet shape changes, secretion, filopodia formation, and, ultimately, platelet-platelet aggregation (6, 8). Platelet-platelet aggregation is largely mediated by the activation of integrin-αIIbβ3, which undergoes conformational changes in response to platelet stimulation to bind soluble fibrinogen, bridging platelets to one another to form hemostatic plugs (32). Chaperone proteins such as protein disulfide isomerase (PDI), ER oxidoreductin 1α (Ero1α), ER protein 57 (ERp57), and ER protein 5 (ERp5) have emerging roles in regulating the activities of platelets and other hematopoietic cells (20, 21, 38, 55). However, while both Hsp70 and Hsp90 family members have been identified in platelets (31, 36, 42, 48), specific roles for Hsp70 activity in platelet function and aggregate formation have not been addressed.
Here we investigate the role of Hsp70 in platelet function. We find that platelets express abundant Hsp70 protein and that Hsp70 localizes throughout platelets, while partially colocalizing with other chaperones such as Hsp90 and PDI. In vitro and ex vivo assays of platelet physiological function point to a role for Hsp70 upstream of “inside-out” activation of integrin-αIIbβ3, granule secretion, and platelet aggregation, as well as aggregate formation under shear. Analyses of the intracellular signaling events of platelet activation suggest that Hsp70 has a minimal role in the activation of signaling events that mediate platelet activation but support a role for Hsp70 in regulating the assembly of linker for activation of T cells (LAT) signalosome to ensure a proper coordination of platelet signaling, activation, and function. Together, our results support roles for Hsp70 chaperone activity in platelet function and aggregation through regulation of the organization of the molecular events of platelet activation upstream of platelet integrin-αIIbβ3 activation and secretion.
MATERIALS AND METHODS
Reagents.
All reagents were obtained from Sigma except as noted. VER 155008 (VER) and thrombin receptor-activating peptide 6 (TRAP-6) were obtained from Tocris Bioscience, MKT-077 (MKT) from Sigma-Aldrich, collagen from Chrono-Log (Havertown, PA), collagen-related peptide (CRP) from R. Farndale (Cambridge University, UK), and human fibrinogen from Enzyme Research. For flow cytometry experiments, CD62E/CD62P-FITC antibody was obtained from Acris Antibodies, PAC-1-FITC antibody from Becton Dickinson, Oregon Green (OG488)-labeled human fibrinogen (OG488-FG) from Invitrogen, and CD61-phycoerythrin (PE) antibody from R & D Systems. Phosphorylated (Tyr416) Src (catalog no. 2101), phosphorylated (Tyr171) LAT (catalog no. 3581), LAT (catalog no. 9166), and phosphorylated (Ser473) Akt (catalog no. 9271) antibodies were obtained from Cell Signaling and Hsp70 (sc-24), Hsp90 (sc-101494), and PDI (sc-20132) antibodies were obtained from Santa Cruz Biotechnology. Tubulin (catalog no. T6199) antibody was obtained from Sigma and 4G10 antibody from EMD Millipore.
In vitro platelet studies.
Washed human platelets were prepared from venous blood drawn from a rotating pool of 18 healthy volunteers by venipuncture into sodium citrate [1:9 (vol/vol)] in accordance with a protocol approved by the Oregon Health & Science University Institutional Review Board as previously described and with written informed consent from the volunteers (6). Blood was centrifuged at 200 g for 20 min to obtain platelet-rich plasma, and platelets were isolated from platelet-rich plasma by centrifugation at 1,000 g for 10 min in the presence of prostacyclin (0.1 μg/ml). Platelets were resuspended in modified HEPES-Tyrode buffer and washed once via centrifugation at 1,000 g for 10 min. Washed platelets were resuspended in modified HEPES-Tyrode buffer to the indicated concentration.
Platelet aggregation.
Aggregation studies were performed using 300 μl of platelets (2 × 108/ml) pretreated with inhibitors for 10 min. Platelet aggregation was initiated by CRP (1 or 3 μg/ml) and monitored under continuous stirring at 1,200 rpm at 37°C by measuring changes in light transmission using a PAP-4 aggregometer, as previously described (9).
Flow cytometry analysis.
Washed human platelets (2 × 107/ml) were pretreated with inhibitors for 10 min before stimulation with CRP (10 μg/ml) or thrombin (1 U/ml) for 20 min in the presence of CD62E/CD62P-FITC, PAC-1-FITC, OG488-FG, or CD61-PE. Samples were diluted in HEPES-Tyrode buffer and analyzed by flow cytometry (BD FACSCanto II, Becton Dickinson). Platelets were identified by logarithmic signal amplification for forward and side scatter, and the geometric mean fluorescence of each sample was recorded.
Platelet aggregate formation under flow.
Sodium citrate-anticoagulated blood was pretreated with inhibitors or antibodies for 10 min and perfused at 2,200 s−1 and 37°C through glass capillary tubes coated with collagen (100 μg/ml) and surface-blocked with denatured BSA to form platelet aggregates, as previously described (7). Imaging of aggregates was performed using Köhler-illuminated Nomarski differential interference contrast optics with a Zeiss ×40/0.75 NE EC Plan-Neofluar lens on a Zeiss Axiocam MRm camera and Slidebook 5.0 software (Intelligent Imaging Innovations). Aggregate surface area was computed by manual outlining and quantification of platelet aggregates, as previously described (7).
Hsp70 signaling and interaction studies.
For Hsp70 protein association studies, Hsp70-glutathione S-transferase (GST) and GST proteins were expressed in vitro from plasmid encoding GST-tagged human HSPA1A (Genecopoeia) or pGEX, as previously described (5). LAT immunoprecipitation and Western blotting were carried out as previously described (9).
Statistical analysis.
Data were analyzed by two-way ANOVA (date and treatment as factors), and post hoc analysis was performed via Tukey's honest significant difference test. P < 0.05 was considered statistically significant for all tests. Statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Hsp70 expression and localization in platelets.
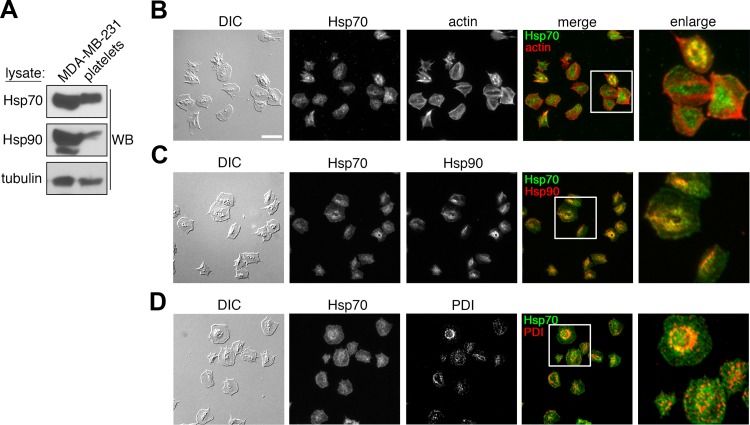
To investigate a role for Hsp70 in platelet physiological function, we first examined the relative expression of Hsp70 and Hsp90 proteins in human platelets. Human platelet lysates were separated by gel electrophoresis, transferred to nitrocellulose, and analyzed for Hsp70 and Hsp90 expression by Western blotting. As seen in Fig. 1A, human platelets contain abundant Hsp70 protein at levels similar to MDA-MB-231 breast cancer cells. Platelets also express Hsp90 protein; however, the ratio of Hsp90 to Hsp70 proteins was less in platelets than nucleated MDA-MB-231 cells. In eukaryotic cells, Hsp proteins regulate a multitude of functions associated with the maintenance of protein quality control in the ER and cytoplasm, as well as at specific intracellular membrane systems (59). Accordingly, we next examined the intracellular localization of Hsp70 and Hsp90 proteins in human platelets adherent to a surface of fibrinogen by fluorescence microscopy. As seen in Fig. 1B, Hsp70 localized in a punctate pattern throughout the platelet cytoplasm, as revealed by costaining of platelets for Hsp70 and actin. Costaining of platelet Hsp70 together with Hsp90 revealed partial colocalization of these Hsp chaperone proteins in the organelle- and vesicle-rich platelet granulomere, while Hsp70 showed a more diffuse localization throughout the platelet cytoplasm, as well as the platelet plasma membrane, than Hsp90 (Fig. 1C). Costaining of Hsp70 together with PDI, an ER-localized protein chaperone that is enriched in the platelet-dense tubular system (52), demonstrated minimal colocalization with Hsp70, suggesting that chaperone functions for Hsp70 and PDI are differentially compartmentalized in platelets (Fig. 1D).
Fig. 1.
Heat shock protein (Hsp) 70 expression and localization in human platelets. A: purified human platelets (1 × 109/ml) and MDA-MB-231 breast cancer cells were lysed directly into Laemmli sample buffer, separated by SDS-PAGE, and analyzed for Hsp70 and Hsp90 protein expression by Western blotting (WB). Samples were normalized to total α-tubulin content to control for protein loading. B–D: replicate samples of washed human platelets (2 × 107/ml) were spread on a surface of fibrinogen before fixation, staining, and visualization by differential interference contrast (DIC) and fluorescence microscopy for expression and colocalization of Hsp70 (green) and actin (red), Hsp70 (green) and Hsp90 (red), and Hsp70 (green) and protein disulfide isomerase (PDI; red). Scale bar = 10 μm.
Hsp70 inhibition reduces platelet aggregation and P-selectin exposure.
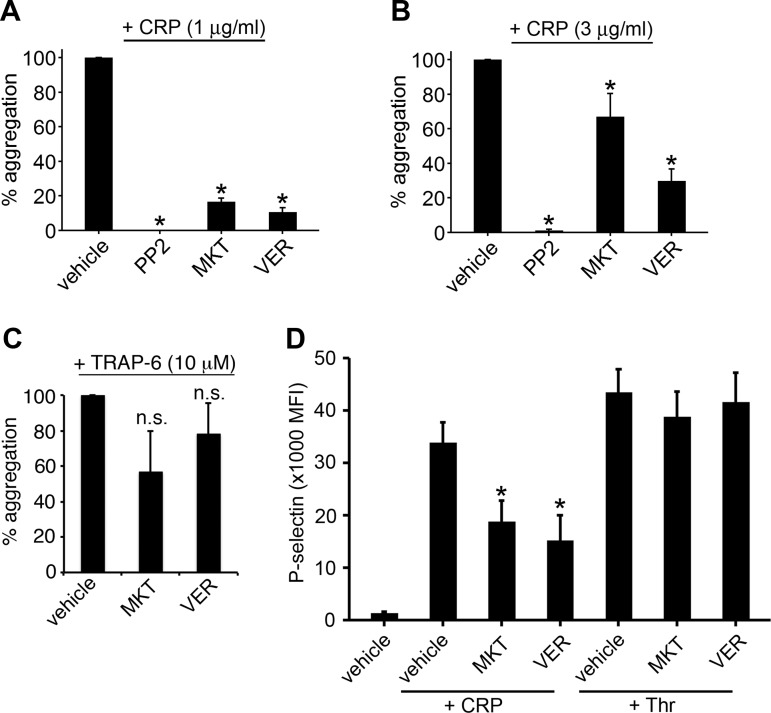
Upon stimulation of platelets with CRP, a cross-linked peptide that mimics fibrillar collagen and specifically engages and activates the platelet glycoprotein VI in the absence of integrin-α2β1 activation, leads to platelet Src kinase activation and phosphorylation of the glycoprotein receptor VI (GPVI)-associated-Fc receptor-γ chain complex (56). These receptor-mediated complex assembly and phosphorylation events then promote the assembly of the LAT signalosome at the platelet plasma membrane to support intracellular calcium signaling events, as well as inside-out-mediated conformational changes in integrin-αIIbβ3 to, in turn, drive fibrinogen binding, granule secretion, and platelet-platelet aggregation (56). To test the hypothesis that Hsp70 activity has roles in the platelet activation program, we first examined the ability of platelets to aggregate in response to CRP under Hsp70-inhibited conditions. To inhibit Hsp70 activities, we took advantage of two separate pharmacological inhibitors of Hsp70, VER and MKT. VER is an adenosine derivative that interacts directly with the ATP binding pocket of Hsp70, Hsc70, and Grp78 (33, 47, 57). MKT is a rhodacyanine dye that binds mitochondrial Hsp70 (mtHsp70 or mortalin), Hsc70, and Hsp70 at an allosteric site near the ATP binding site, preventing allosteric communication between the Hsp70 ATP binding site and substrate binding sites (45, 54). As seen in Fig. 2A, aggregation of washed human platelets in response to stimulation with 1 μg/ml CRP was strongly inhibited by 20 μM MKT or VER (16.6 ± 2.2% and 10.7 ± 2.4% of control, respectively); aggregation in response to 3 μg/ml CRP was also inhibited by 20 μM MKT or VER. Conversely, aggregation in response to thrombin receptor-activating peptide 6, which promotes platelet activation through protease-activated receptor (PAR)-coupled Gq and G13 pathways independent of immunoreceptor tyrosine-based activation motif (ITAM) receptor and LAT signalosome-mediated signaling, was not significantly inhibited by 20 μM MKT or VER (56.5 ± 23.1% and 78.2 ± 17.3% of control aggregation, respectively; Fig. 2C).
Fig. 2.
Hsp70 inhibitors block platelet aggregation and P-selectin exposure. A–C: replicate samples of washed human platelets (2 × 108/ml, n = 3–5) were pretreated with the Src kinase inhibitor PP2 (20 μM), the Hsp70 inhibitor MKT-077 (MKT, 20 μM), the Hsp70 inhibitor VER-155008 (VER, 20 μM), or vehicle alone (0.1% DMSO) prior to stimulation with 1 μg/ml collagen-related peptide (CRP), 3 μg/ml CRP, or 10 μM thrombin receptor-activating peptide 6 (TRAP-6) and analysis for platelet aggregation by Born aggregometry. Changes in optical density were recorded as a vertical drop and lag times to quantify the extent of platelet aggregation. *P < 0.05. ns, Not significant (P > 0.05). D: replicate samples of washed human platelets (2 × 108/ml, n = 3–7) were pretreated with PP2 (20 μM), MKT (20 μM), VER (20 μM), or vehicle alone (0.1% DMSO) prior to stimulation with 10 μg/ml CRP or 1 U/ml thrombin (Thr) and analysis for P-selectin (CD62P) surface exposure by flow cytometry. MFI, mean fluorescence intensity. *P < 0.05.
Platelet activation by CRP initiates the secretion of P-selectin from platelet α-granules to support platelet aggregate growth and stability through interactions with integrin-αIIbβ3 that also allow for fibrinogen binding and platelet-platelet aggregation (35). To examine the role of Hsp70 in platelet granule secretion and P-selectin exposure by flow cytometry, washed human platelets were treated with Hsp70 inhibitors before stimulation with CRP or thrombin and labeling with antibodies against P-selectin (CD62P). As seen in Fig. 2D, MKT or VER (20 μM) significantly decreased P-selectin exposure upon stimulation by CRP (55.7 ± 1.8% and 45.1 ± 4.2% of control, respectively) but not by thrombin (89.1 ± 11.2% and 95.4 ± 13.0% of control, respectively).
Hsp70 inhibition blocks platelet integrin-αIIbβ3 activation.
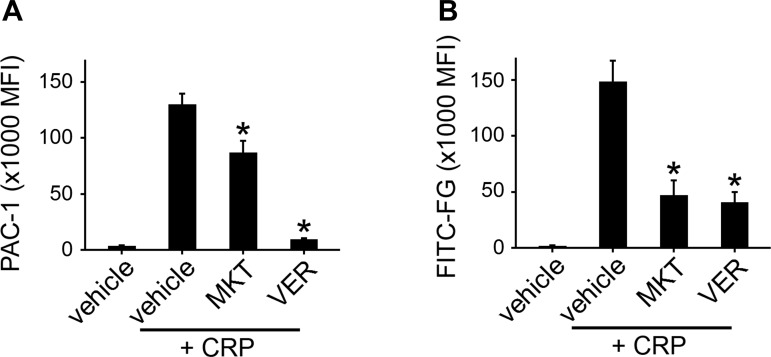
Next, to specifically examine inside-out integrin-αIIbβ3 activation following Hsp70 inhibition, Hsp70-inhibited platelets were stimulated with CRP before incubation with fluorescently labeled PAC-1 antibodies that bind specifically to activated integrin-αIIbβ3. As seen in Fig. 3A, MKT or VER (20 μM) decreased the activation of integrin-αIIbβ3 upon CRP stimulation as indicated by decreased detection of PAC-1 (67.1 ± 7.8% and 7.2 ± 0.7% of control, respectively). Hsp70 inhibition and loss of αIIbβ3-integrin activation were associated with a reduced capacity of stimulated platelets to bind to soluble fibrinogen, as measured by flow cytometry analysis of the capacity of Hsp70-inhibited platelets to bind OG488-FG (31.7 ± 8.7% and 27.5 ± 6.1% of control, respectively; Fig. 3B). The reduction in fibrinogen binding following Hsp70 inhibition was not likely the result of a reduced presence of integrin-αIIbβ3 on the surface of the platelet plasma membrane, as resting integrin-β3 (CD61) surface expression was not significantly changed by treatment with 20 μM MKT or VER (107.1 ± 7.1% and 104.5 ± 8.0% of control, respectively, n = 4) as determined by flow cytometry analysis.
Fig. 3.
Inhibition of Hsp70 prevents platelet integrin-αIIbβ3 activation and fibrinogen binding. A and B: replicate samples of washed human platelets (2 × 108/ml, n = 5) were pretreated with PP2 (20 μM), MKT (20 μM), VER (20 μM), or vehicle alone (0.1% DMSO) prior to stimulation with 10 μg/ml CRP and flow cytometry analysis for integrin-αIIbβ3 activation as determined by PAC-1 binding or fluorescent fibrinogen (FITC-FG) binding. *P < 0.05.
Inhibition of Hsp70 dampens platelet aggregate formation under shear.
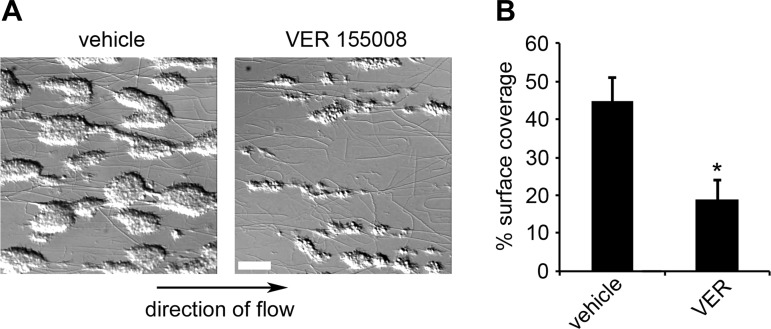
In addition to supporting platelet aggregation in solution, GPVI signaling, integrin-αIIbβ3 inside-out activation, and fibrinogen binding serve roles in platelet aggregate growth on surfaces of extracellular matrix proteins under conditions of physiological shear (22). Accordingly, using control and Hsp70-inhibited conditions, we examined platelet aggregate formation on collagen under physiological shear. Whole, citrated human blood was pretreated with Hsp70 inhibitor before perfusion over a surface of collagen at an arterial shear rate of 2,200 s−1 for 5 min. Adherent cells were fixed, visualized, and quantified for surface area. As seen in Fig. 4, VER (40 μM), which is bioavailable in vivo (33), reduced aggregate surface area to 42.7 ± 11.4% of control (0.1% DMSO). This ex vivo assay of platelet function supports a role for Hsp70 in regulating integrin-αIIbβ3-mediated platelet aggregate formation in whole blood under shear.
Fig. 4.
Ex vivo inhibition of Hsp70 prevents platelet aggregate formation under physiological shear. A: whole blood was treated with vehicle (DMSO, 0.1%) or VER (40 μM) and perfused at 2,200 s−1 at 37°C through capillary tubes coated with collagen (100 μg/ml) to form platelet aggregates. B: percent surface area covered by aggregates was computed by outlining and quantifying platelet aggregates. *P < 0.05 vs. DMSO control (vehicle). Values are means ± SE; n = 3. Scale bar = 100 μm.
Hsp70 interacts with and regulates components of the LAT signalosome.
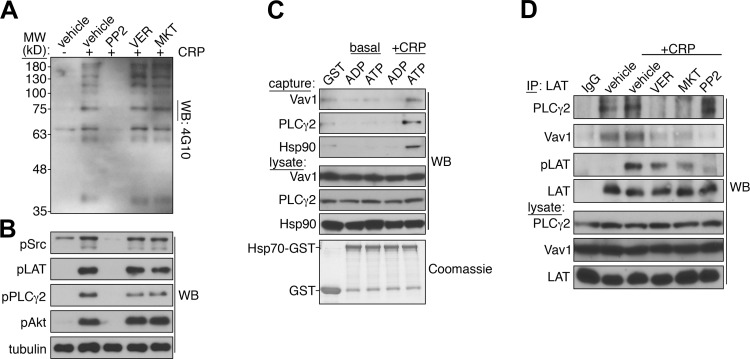
Inhibition of integrin-αIIbβ3 activation and platelet aggregation, as well as P-selectin exposure, in response to CRP, but not PAR, agonists under Hsp70-inhibited conditions suggested a role for Hsp70 in the intracellular signaling events that drive platelet activation downstream of GPVI engagement (56). Accordingly, we examined the activation of signaling pathways downstream of CRP stimulation in platelets under Hsp70-inhibited conditions. As seen in Fig. 5A, under control conditions, stimulation of platelets with CRP promoted the activation of Src-mediated tyrosine kinase signaling events, as determined by Western blotting for total phosphotyrosine proteins with 4G10 antiserum, phosphorylated Src, phosphorylated LAT, and phospholipase Cγ2 (PLCγ2), as well as Akt phosphorylation (Fig. 5B). As expected, the activation of kinase signaling was abolished by preincubation of platelets with the Src kinase inhibitor PP2, which blocks the earliest steps of platelet activation initiated by CRP stimulation (Fig. 5, A and B). However, despite a decrease in platelet integrin activation, aggregation, and P-selectin exposure following CRP stimulation (Figs. 2 and 3), inhibition of Hsp70 did not significantly affect CRP-stimulated protein tyrosine phosphorylation, Src kinase phosphorylation, and Akt activation, while it mildly inhibited PLCγ2 phosphorylation (Fig. 5, A and B).
Fig. 5.
Hsp70 interacts with and regulates organization of the linker for activation of T cells protein (LAT) signalosome. A: replicate samples of washed human platelets (5 × 108/ml) were pretreated with PP2 (20 μM), VER (20 μM), MKT (20 μM), or vehicle alone (0.1% DMSO) for 10 min prior to stimulation with CRP (10 μg/ml, 5 min). Protein tyrosine phosphorylation was analyzed by Western blotting using 4G10 antiserum. MW, protein molecular weight markers. B: phosphorylation of Src kinase pTyr416 (pSrc), LAT pTyr171 (pLAT), PLCγ2 pTyr759 (pPLCγ2), and Akt pSer473 (pAkt). α-Tubulin levels serve as a control for equal protein loading. C: recombinant glutathione S-transferase (GST) protein (1 μg) or Hsp70-GST protein (1 μg) preloaded with 10 μM ADP or 10 μM ATP was incubated with basal and CRP-stimulated platelet lysates prior to protein capture with glutathione-Sepharose and Western blot analysis for co-captured Vav1, PLCγ2, and Hsp90. Coomassie staining confirmed efficient pulldown of GST and Hsp70-GST proteins. D: platelets (5 × 108/ml) were pretreated with PP2 (20 μM), VER (20 μM), MKT (20 μM), or vehicle alone (0.1% DMSO) prior to stimulation with CRP (10 μg/ml, 5 min) and lysis into immunoprecipitation buffer. LAT immunoprecipitates were examined for capture of total (LAT) and phosphorylated LAT (pLAT) protein, as well as coprecipitating PLCγ2 and Vav1, by Western blotting. Representative signaling, protein capture, and LAT immunoprecipitation results are representative of 3 experiments.
Similar to inhibition of Hsp70, genetic deletion of LAT, a scaffolding protein that organizes functional interactions among key mediators of platelet activation downstream of ITAM receptor stimulation, including 76-kDa SH2 domain-containing leukocyte protein, Vav1/3, and PLCγ2, has limited effects on tyrosine kinase activation and protein phosphorylation downstream of GPVI engagement (37). LAT deletion also disrupts the organization of PLCγ2-mediated signaling requisite for platelet integrin activation, aggregation, and granule secretion (24, 58). Given the diverse roles for Hsp70 in protein complex assembly in a number of intracellular signaling processes (43), particularly at the intracellular surface of the plasma membrane (16), we examined the ability of ADP- and ATP-loaded Hsp70 to interact with components of the LAT signalosome from resting and activated platelets in vitro. Under ATP-loaded conditions, immobilized GST-Hsp70 supported the capture of LAT signalosome components, including Vav1 and PLCγ2, from CRP-stimulated platelet lysates (Fig. 5C). As a positive control, ATP-loaded Hsp70 also supported the capture of Hsp90 from platelet lysates (Fig. 5C).
Next, to examine roles for Hsp70 activity in LAT signalosome organization and platelet function, we immunoprecipitated endogenous LAT from resting and CRP-activated platelets under control and Hsp70-inhibited conditions and monitored the coprecipitation of LAT signalosome-associated proteins (Fig. 5D). Western blot analyses of LAT immunoprecipitates confirmed that LAT readily immunoprecipitated from platelet lysates (Fig. 5D). In agreement with signaling studies (Fig. 5A), stimulation of platelets with CRP prior to lysis and LAT immunoprecipitation upregulated the phosphorylation of immunocaptured LAT, which was inhibited in the presence of PP2, but not the Hsp70 inhibitors VER and MKT (Fig. 5D). Notably, PLCγ2, which coprecipitated with LAT under resting as well as CRP-stimulated conditions, did not associate with LAT in the presence of the Hsp70 inhibitors VER and MKT. Hsp70 inhibition similarly prevented the detectable interaction of LAT with Vav1, which associates with the LAT signalosome to regulate PLCγ2 activation and platelet aggregation (40). Together, these results suggest that, in platelets, Hsp70 activity has a role in organizing and maintaining the assembly of the LAT signalosome independent of the activation of tyrosine kinases that signal the initiation and progression of the platelet activation program in response to CRP.
DISCUSSION
Here we report a role for the molecular chaperone Hsp70 in platelet function. Physiological, biochemical, and flow cytometry experiments highlighted a role for Hsp70 in platelet integrin-αIIbβ3 activation, granule secretion, and aggregation, as two distinct Hsp70 inhibitors reduced CRP-induced PAC-1 or fluorescent fibrinogen binding as well as P-selectin surface exposure and platelet aggregation. Hsp70 inhibition also prevented platelet aggregate formation in an ex vivo model of blood flow under arterial shear, demonstrating an inhibitory effect under physiological shear in addition to static conditions. Together with intracellular signaling and protein complex studies of the LAT signalosome, our results suggest that, following the activation of ITAM-bearing receptor systems in platelets, Hsp70 helps organize intracellular molecular processes that orchestrate inside-out integrin activation as well as secretion events that drive platelet-platelet aggregation.
Previous studies have alluded to roles for Hsp70, as well as Hsp90, family members in platelet function. Phosphorylated Hsc70 associates with Hsp90 and PP1 in resting platelets and dissociates from this complex upon platelet activation by collagen, but not thrombin (42). Inhibition of Hsp90 reduces the trafficking of the ATP-gated ion channel P2X1 to the platelet surface and also interferes with P2X1 calcium channel gating, reducing receptor responsiveness to ATP (29). Grp94, the ER paralog of Hsp90, assists in folding and assembly of the platelet glycoprotein Ib-IX-V complex, the receptor for von Willebrand factor-mediated platelet adhesion and activation (48). In this study we identified a role for Hsp70 in platelet activation and aggregation associated with the inside-out activation of integrin-αIIbβ3. While inhibition of Hsp70 did not limit the localization of integrin-αIIbβ3 on the platelet surface, Hsp70 inhibition abrogated platelet functional responses associated with inside-out integrin-αIIbβ3 activation, notably, platelet aggregation as well as granule secretion (Fig. 2) and platelet aggregate formation under physiological shear (Fig. 4).
Despite the inability of platelets to fully activate integrin-αIIbβ3 and aggregate in response to CRP stimulation under Hsp70-inhibited conditions (Figs. 2 and 3), the intracellular signaling systems that support these processes appear to activate normally (Fig. 5A), suggesting potential organizational defects in the molecular events of platelet activation when Hsp70 is inhibited. As the effects of Hsp70 inhibition phenocopy those reported for genetic deletion of LAT in platelets (37), we examined roles for Hsp70 in the assembly and organization of the LAT signalosome. After activation of ITAM-bearing receptors such as GPVI/Fc receptor-γ chain and C-type lectin-like receptor-2, tyrosine phosphorylation of LAT organizes a complex of key mediators of platelet activation, most notably PLCγ2, as well as Vav1, SLP-76, Btk/Tec, Grb2, and Gads (2), to support platelet granule secretion, integrin activation, and platelet aggregation (25, 37). We found that recombinant, ATP-loaded Hsp70 captured Vav1 and PLCγ2 from CRP-stimulated platelet lysates (Fig. 5C). Immunoprecipitation of endogenous LAT from resting and CRP-activated platelets revealed that Hsp70 inhibition blocked the association of PLCγ2, as well as Vav1, from LAT but had no effect on LAT phosphorylation (Fig. 5D). There have been no reports of chaperone activities in the regulation of LAT-associated signaling and cellular physiological function. However, Vav1, a component of the LAT signalosome, associates with and is regulated by Hsc70 in tumor cells (44). Also, the Hsp70 co-chaperone BCL2-associated athanogene (BAG)-3 helps regulate phospholipase protein signaling downstream of epidermal growth factor receptor activation (18, 19). While the role of BAG proteins in platelet physiology has not been examined, proteomics studies have reported expression of a number of BAG family members in platelets (11).
In addition to the Hsp family members, oxidoreductase chaperones have emerging roles in platelet activation and thrombotic function. PDI and two of its homologs (ERp5 and ERp57) have been found to be released from endothelial cells and platelets: endothelial PDI is essential for thrombus formation, and platelet PDI is essential for thrombus growth (20). Extracellular PDI, ERp5, ERp57, and the ER oxidoreductase Ero1α have been found to regulate the active conformation of integrin-αIIbβ3, and blocking these thiol isomerases inhibits activation of integrin-αIIbβ3 (14, 38, 49, 55). Similar to the oxidoreductase chaperones PDI, ERp5, ERp57, and Ero1α, our results show a role for Hsp70 chaperone activity in integrin activation, suggesting that chaperones, in addition to thiol isomerases, may regulate platelet integrin conformational changes and activation. While our data support a role for intracellular Hsp70 in the regulation of platelet secretion as well as integrin activation, we cannot exclude a role for extracellular Hsp70 in supporting platelet integrin activation, fibrinogen binding, and thrombus formation. Indeed, while Hsp70 is primarily present intracellularly, under activating conditions, Hsp70 can translocate to the cell surface and is also secreted from exosomes (30, 53). Extracellular Hsp70 binds Toll-like receptors and other surface receptors on neighboring macrophages, monocytes, neutrophils, or dendritic cells, which initiates an inflammatory response through activation of the NFκB and MAP kinase pathways (1, 3, 4, 13, 23, 50). A hypothetical role for extracellular Hsp70 activity is also supported by work in other cell models showing that Hsp70 associates with integrins (12), that the Hsp70 family member Grp78/binding immunoglobulin protein and PDI cooperate to carry out chaperone activities (17), and that the cytosolic Hsp70 member Hsc70 associates with PDI and ERp57 in endothelial cell lipid rafts to facilitate integrin activation (17, 42).
Apart from specific platelet-based functions, it has been hypothesized that Hsp70 has roles in cardiovascular health that may be linked to platelet physiology. Circulating Hsp70 has a role in vascular calcification in patients with atherosclerosis (28). Cardioprotective roles have also been shown for circulating Hsp70, suggesting that higher levels of Hsp70 in hypertensive individuals can mitigate the development of atherosclerosis, potentially through inflammatory functions mediated by Hsp70 (41). Furthermore, intracellular Hsp70 has been shown to exert a cardioprotective effect through association with integrin-linked kinase (ILK) in cardiomyocytes (51). While platelet ILK has a role in integrin-αIIbβ3 activation and thrombus growth (27), specific connections between ILK and Hsp70 in platelets have not been investigated. Surface Grp78 in platelets was also found to have an atheroprotective role through binding and inactivation of tissue factor (36). The contrasting effects of circulating and intracellular Hsp70 suggest that Hsp70 plays diverse roles in circulatory cell function in a manner related to the cardiovascular and inflammatory state of the patient or experimental subject in question.
In conclusion, this study finds a role for Hsp70 activity in platelet hemostatic function associated with the regulation of integrin activation and granule secretion following stimulation of platelet ITAM receptors. Our data suggest that Hsp70 activity ensures the proper molecular organization of intracellular mediators of the platelet activation program around the LAT signalosome, including Vav1 and PLCγ2, which support activation of integrin-αIIbβ3 and granule secretion to mediate platelet aggregation and aggregate formation under shear. Given the emerging roles of molecular chaperones in the regulation of cellular signaling pathways and cellular physiology, Hsp70-associated activities represent an interesting topic of investigation for future studies of physiological and pathological processes that impact platelet activation in normal physiology and disease states.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL-101972 and R01 GM-116184 (to O. J. T. McCarty) and T32 AI-007472 (to L. D. Healy) and American Heart Association Grants 13POST13730003 (to J. E. Aslan) and 13EIA12630000 (to O. J. T. McCarty). M. L. D. Thierheimer is an Oregon State University Johnson Scholar. R. A. Rigg is a Whitaker International Fellow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A.R., J.M., O.J.T.M., and J.E.A. developed the concept and designed the research; R.A.R., L.D.H., M.S.N., M.L.D.T., J.P., and J.E.A. performed the experiments; R.A.R., L.D.H., M.S.N., and J.E.A. analyzed the data; R.A.R., M.S.N., O.J.T.M., and J.E.A. interpreted the results of the experiments; R.A.R. and J.E.A. prepared the figures; R.A.R. and J.E.A. drafted the manuscript; R.A.R., J.M., O.J.T.M., and J.E.A. edited and revised the manuscript; R.A.R., L.D.H., M.S.N., J.M., M.L.D.T., J.P., O.J.T.M., and J.E.A. approved the final version of the manuscript.
REFERENCES
- 1.Anand PK. Exosomal membrane molecules are potent immune response modulators. Commun Integr Biol 3: 405–408, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asazuma N, Wilde JI, Berlanga O, Leduc M, Leo A, Schweighoffer E, Tybulewicz V, Bon C, Liu SK, McGlade CJ, Schraven B, Watson SP. Interaction of linker for activation of T cells with multiple adapter proteins in platelets activated by the glycoprotein VI-selective ligand, convulxin. J Biol Chem 275: 33427–33434, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6: 435–442, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Aslan JE, Baker SM, Loren CP, Haley KM, Itakura A, Pang J, Greenberg DL, David LL, Manser E, Chernoff J, McCarty OJ. The PAK system links Rho GTPase signaling to thrombin-mediated platelet activation. Am J Physiol Cell Physiol 305: C519–C528, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslan JE, Itakura A, Gertz JM, McCarty OJ. Platelet shape change and spreading. Methods Mol Biol 788: 91–100, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Aslan JE, Itakura A, Haley KM, Tormoen GW, Loren CP, Baker SM, Pang J, Chernoff J, McCarty OJ. p21 activated kinase signaling coordinates glycoprotein receptor VI-mediated platelet aggregation, lamellipodia formation, and aggregate stability under shear. Arterioscler Thromb Vasc Biol 33: 1544–1551, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslan JE, McCarty OJ. Rho GTPases in platelet function. J Thromb Haemost 11: 35–46, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslan JE, Tormoen GW, Loren CP, Pang J, McCarty OJ. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood 118: 3129–3136, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boorstein WR, Ziegelhoffer T, Craig EA. Molecular evolution of the HSP70 multigene family. J Mol Evol 38: 1–17, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, Geiger J, Sickmann A, Zahedi RP. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 120: e73–e82, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Chan YC, Greenwood DR, Yang Y, Leung E, Krissansen GW. Leukocyte integrin α4β7 associates with heat shock protein 70. Mol Cell Biochem 409: 263–269, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Cao X. Stress for maintaining memory: HSP70 as a mobile messenger for innate and adaptive immunity. Eur J Immunol 40: 1541–1544, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Cho J, Kennedy DR, Lin L, Huang M, Merrill-Skoloff G, Furie BC, Furie B. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of β3-integrins. Blood 120: 647–655, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerico EM, Tilitsky JM, Meng W, Gierasch LM. How Hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J Mol Biol 427: 1575–1588, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colvin TA, Gabai VL, Gong J, Calderwood SK, Li H, Gummuluru S, Matchuk ON, Smirnova SG, Orlova NV, Zamulaeva IA, Garcia-Marcos M, Li X, Young ZT, Rauch JN, Gestwicki JE, Takayama S, Sherman MY. Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer Res 74: 4731–4740, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delom F, Mallet B, Carayon P, Lejeune PJ. Role of extracellular molecular chaperones in the folding of oxidized proteins. Refolding of colloidal thyroglobulin by protein disulfide isomerase and immunoglobulin heavy chain-binding protein. J Biol Chem 276: 21337–21342, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Doong H, Price J, Kim YS, Gasbarre C, Probst J, Liotta LA, Blanchette J, Rizzo K, Kohn E. CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase Cγ and Hsp70/Hsc70. Oncogene 19: 4385–4395, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Doong H, Vrailas A, Kohn EC. What's in the “BAG”? A functional domain analysis of the BAG-family proteins. Cancer Lett 188: 25–32, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Furie B, Flaumenhaft R. Thiol isomerases in thrombus formation. Circ Res 114: 1162–1173, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbins JM. Platelets using proteins creatively. Blood 122: 3553–3554, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Goncalves I, Hughan SC, Schoenwaelder SM, Yap CL, Yuan Y, Jackson SP. Integrin-αIIbβ3-dependent calcium signals regulate platelet-fibrinogen interactions under flow. Involvement of phospholipase Cγ2. J Biol Chem 278: 34812–34822, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez T, Simmen T. Endoplasmic reticulum chaperones and oxidoreductases: critical regulators of tumor cell survival and immunorecognition. Front Oncol 4: 291, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes CE, Auger JM, McGlade J, Eble JA, Pearce AC, Watson SP. Differential roles for the adapters Gads and LAT in platelet activation by GPVI and CLEC-2. J Thromb Haemost 6: 2152–2159, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Judd BA, Myung PS, Obergfell A, Myers EE, Cheng AM, Watson SP, Pear WS, Allman D, Shattil SJ, Koretzky GA. Differential requirement for LAT and SLP-76 in GPVI versus T cell receptor signaling. J Exp Med 195: 705–717, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11: 579–592, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keularts IM, van Gorp RM, Feijge MA, Vuist WM, Heemskerk JW. α2A-Adrenergic receptor stimulation potentiates calcium release in platelets by modulating cAMP levels. J Biol Chem 275: 1763–1772, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Krepuska M, Szeberin Z, Sotonyi P, Sarkadi H, Fehervari M, Apor A, Rimely E, Prohaszka Z, Acsady G. Serum level of soluble Hsp70 is associated with vascular calcification. Cell Stress Chaperones 16: 257–265, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalo U, Jones S, Roberts JA, Mahaut-Smith MP, Evans RJ. Heat shock protein 90 inhibitors reduce trafficking of ATP-gated P2X1 receptors and human platelet responsiveness. J Biol Chem 287: 32747–32754, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem 280: 23349–23355, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Ma C, Yao Y, Yue QX, Zhou XW, Yang PY, Wu WY, Guan SH, Jiang BH, Yang M, Liu X, Guo DA. Differential proteomic analysis of platelets suggested possible signal cascades network in platelets treated with salvianolic acid B. PLos One 6: e14692, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma YQ, Qin J, Plow EF. Platelet integrin-αIIbβ3: activation mechanisms. J Thromb Haemost 5: 1345–1352, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Massey AJ, Williamson DS, Browne H, Murray JB, Dokurno P, Shaw T, Macias AT, Daniels Z, Geoffroy S, Dopson M, Lavan P, Matassova N, Francis GL, Graham CJ, Parsons R, Wang Y, Padfield A, Comer M, Drysdale MJ, Wood M. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol 66: 535–545, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62: 670–684, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merten M, Thiagarajan P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation 102: 1931–1936, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Molins B, Pena E, Padro T, Casani L, Mendieta C, Badimon L. Glucose-regulated protein 78 and platelet deposition: effect of rosuvastatin. Arterioscler Thromb Vasc Biol 30: 1246–1252, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Pasquet JM, Gross B, Quek L, Asazuma N, Zhang W, Sommers CL, Schweighoffer E, Tybulewicz V, Judd B, Lee JR, Koretzky G, Love PE, Samelson LE, Watson SP. LAT is required for tyrosine phosphorylation of phospholipase Cγ2 and platelet activation by the collagen receptor GPVI. Mol Cell Biol 19: 8326–8334, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passam FH, Lin L, Gopal S, Stopa JD, Bellido-Martin L, Huang M, Furie BC, Furie B. Both platelet- and endothelial cell-derived ERp5 support thrombus formation in a laser-induced mouse model of thrombosis. Blood 125: 2276–2285, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patury S, Miyata Y, Gestwicki JE. Pharmacological targeting of the Hsp70 chaperone. Curr Topics Med Chem 9: 1337–1351, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearce AC, Senis YA, Billadeau DD, Turner M, Watson SP, Vigorito E. Vav1 and Vav3 have critical but redundant roles in mediating platelet activation by collagen. J Biol Chem 279: 53955–53962, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Pockley AG, Calderwood SK, Multhoff G. The atheroprotective properties of Hsp70: a role for Hsp70-endothelial interactions? Cell Stress Chaperones 14: 545–553, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polanowska-Grabowska R, Simon CG Jr, Falchetto R, Shabanowitz J, Hunt DF, Gear AR. Platelet adhesion to collagen under flow causes dissociation of a phosphoprotein complex of heat-shock proteins and protein phosphatase 1. Blood 90: 1516–1526, 1997. [PubMed] [Google Scholar]
- 43.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 228: 111–133, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Razidlo GL, Wang Y, Chen J, Krueger EW, Billadeau DD, McNiven MA. Dynamin 2 potentiates invasive migration of pancreatic tumor cells through stabilization of the Rac1 GEF Vav1. Dev Cell 24: 573–585, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rousaki A, Miyata Y, Jinwal UK, Dickey CA, Gestwicki JE, Zuiderweg ER. Allosteric drugs: the interaction of antitumor compound MKT-077 with human Hsp70 chaperones. J Mol Biol 411: 614–632, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol 14: 630–642, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlecht R, Scholz SR, Dahmen H, Wegener A, Sirrenberg C, Musil D, Bomke J, Eggenweiler HM, Mayer MP, Bukau B. Functional analysis of Hsp70 inhibitors. PLos One 8: e78443, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staron M, Wu S, Hong F, Stojanovic A, Du X, Bona R, Liu B, Li Z. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood 117: 7136–7144, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swiatkowska M, Padula G, Michalec L, Stasiak M, Skurzynski S, Cierniewski CS. Ero1α is expressed on blood platelets in association with protein-disulfide isomerase and contributes to redox-controlled remodeling of αIIbβ3. J Biol Chem 285: 29874–29883, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett 579: 1951–1960, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Traister A, Walsh M, Aafaqi S, Lu M, Dai X, Henkleman MR, Momen A, Zhou YQ, Husain M, Arab S, Piran S, Hannigan G, Coles JG. Mutation in integrin-linked kinase [ILK(R211A)] and heat-shock protein 70 comprise a broadly cardioprotective complex. PLos One 8: e77331, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Nispen Tot Pannerden HE, van Dijk SM, Du V, Heijnen HF. Platelet protein disulfide isomerase is localized in the dense tubular system and does not become surface expressed after activation. Blood 114: 4738–4740, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol 180: 4299–4307, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Wadhwa R, Sugihara T, Yoshida A, Nomura H, Reddel RR, Simpson R, Maruta H, Kaul SC. Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Res 60: 6818–6821, 2000. [PubMed] [Google Scholar]
- 55.Wang L, Wu Y, Zhou J, Ahmad SS, Mutus B, Garbi N, Hammerling G, Liu J, Essex DW. Platelet-derived ERp57 mediates platelet incorporation into a growing thrombus by regulation of the αIIbβ3-integrin. Blood 122: 3642–3650, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin-αIIbβ3 signaling in platelets. J Thromb Haemost 3: 1752–1762, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, Foloppe N, Francis GL, Graham CJ, Howes R, Macias AT, Murray JB, Parsons R, Shaw T, Surgenor AE, Terry L, Wang Y, Wood M, Massey AJ. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem 52: 1510–1513, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Wonerow P, Obergfell A, Wilde JI, Bobe R, Asazuma N, Brdicka T, Leo A, Schraven B, Horejsi V, Shattil SJ, Watson SP. Differential role of glycolipid-enriched membrane domains in glycoprotein VI- and integrin-mediated phospholipase Cγ2 regulation in platelets. Biochem J 364: 755–765, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci 28: 541–547, 2003. [DOI] [PubMed] [Google Scholar]