Abstract
While pathological and clinical data suggest that small airways are involved in early cystic fibrosis (CF) lung disease development, little is known about how the lack of cystic fibrosis transmembrane conductance regulator (CFTR) function contributes to disease pathogenesis in these small airways. Large and small airway epithelia are exposed to different airflow velocities, temperatures, humidity, and CO2 concentrations. The cellular composition of these two regions is different, and small airways lack submucosal glands. To better understand the ion transport properties and impacts of lack of CFTR function on host defense function in small airways, we adapted a novel protocol to isolate small airway epithelial cells from CF and non-CF pigs and established an organotypic culture model. Compared with non-CF large airways, non-CF small airway epithelia cultures had higher Cl− and bicarbonate (HCO3−) short-circuit currents and higher airway surface liquid (ASL) pH under 5% CO2 conditions. CF small airway epithelia were characterized by minimal Cl− and HCO3− transport and decreased ASL pH, and had impaired bacterial killing compared with non-CF small airways. In addition, CF small airway epithelia had a higher ASL viscosity than non-CF small airways. Thus, the activity of CFTR is higher in the small airways, where it plays a role in alkalinization of ASL, enhancement of antimicrobial activity, and lowering of mucus viscosity. These data provide insight to explain why the small airways are a susceptible site for the bacterial colonization.
Keywords: cystic fibrosis transmembrane conductance regulator
cystic fibrosis (CF) is caused by defects in the membrane-bound channel cystic fibrosis transmembrane conductance regulator (CFTR), an anion channel that is permeable to both Cl− and bicarbonate (HCO3−) (52, 61). CF lung disease is the main cause of morbidity and mortality in people with CF. The main features of CF lung disease are bacterial colonization, chronic infection, and mucus plugging in both large and small conducting airways (1, 14, 43, 61). Genetically modified pigs with either deletion or expression of ΔF508 CFTR mutant have provided significant insight into the mechanism by which loss of CFTR function results in CF lung disease (42, 60). In large proximal airways of CF pigs, lack of CFTR results in host defense defects, including reduced mucociliary transport (MCT) and impaired bacterial killing, due in part to decreased airway surface liquid (ASL) pH (30, 43, 61). However, few studies have explored the role of the small airway in CF etiology, and the effect of loss of CFTR function on ASL pH, mucus viscosity, and antimicrobial activity in the distal small airways is not known.
Histopathology data show small airway abnormalities in early stages of CF lung disease (9, 41, 58). In addition, pulmonary function tests in people with CF demonstrate a progressive decline in 25–75% forced expiratory volume (FEV), which reflects small airway function, while FEV1, which more reflects central airway function, remains relatively stable in early stages of disease (6). Despite the fact that the distal small airways contribute to over 85–90% of the total surface area of airway epithelia (11, 25, 67) and the clear evidence that people with CF have some disease manifestation in the small airways, the field of small airway functional studies in CF has not significantly progressed due in part to the lack of experimental models.
Compared with extensive studies on CF large airways, previous studies on CF small airways are limited because it is difficult to access the small airways for detailed mechanistic studies (4, 7, 8, 13, 50, 51). We adapted a method to isolate, expand, and culture small airway epithelial cells from newborn CF and non-CF pigs before the onset of lung inflammation and infection (47). This method allowed us to characterize the bioelectric properties of both large and small airways and to investigate how CFTR deficiency affects ASL pH and host defense properties.
MATERIALS AND METHODS
Isolation, expansion, and culture of airway epithelia.
All animal studies were reviewed and approved by the University of Iowa Animal Care and Use Committee. Generation of CFTR−/− pigs has been previously reported (48). Animals were produced by mating CFTR+/− male and female pigs. Newborn CFTR+/+ or CFTR+/− (non-CF) and CFTR−/− (CF) piglets were obtained from Exemplar Genetics (Exemplar Genetics, Sioux Center, IA). We studied newborn pigs within 12 h after birth because their lungs have not manifested changes secondary to chronic infection and inflammation (36). Also in contrast to gut-corrected CFTR−/− pigs (62), the CFTR−/− pigs in this study develop severe meconium ileus, and it is difficult to keep them alive beyond 48 h after birth. Newborn piglet lungs were excised, and the whole airway tree was microdissected by carefully combing off the parenchymal tissue. Subsequently, the vascular tissue was separated from the airway tree by blunted dissection. Proximal large airways, including trachea and main stem bronchi, and distal small airways (diameter ∼200 μm) were dissected out separately from the airway tree. Next, primary porcine airway epithelia were isolated according to an adapted procedure originally developed for tracheal airway cells. Isolated large and small airway cells were expanded using a method developed to conditionally reprogram airway epithelial cells (39, 63). Briefly, large and small airway cells were separately cultured in F media in the presence of 10 μM Y-27632, a Rho kinase (ROCK) inhibitor, and low passages of irradiated fibroblast feeder cells NIH-3T3-J2 (obtained from Dr. H. Green's laboratory at Harvard University) (46) and maintained at 37°C with 5% CO2. After two passages of amplification, expanded epithelial cells were separated from feeder cells and seeded on collagen-coated semipermeable membranes (Corning no. 3470) at a density of 106 cells/cm2 and cultured at the air-liquid interface at 37°C in a 5% CO2 atmosphere as previously described (70) for 2–3 wk in the absence of feeder cells and ROCK inhibitor. In the 1st wk of seeding at the air-liquid interface, cells were maintained in Small Airway Growth Media (Lonza, Basel, Switzerland) supplemented with 10 ng/ml keratinocyte growth factor for 1 wk, after which cells were maintained in Ultroser G supplement media as described. All experiments were performed on matched large and small airway epithelia isolated from the same animal and cultured under identical conditions. We used the expanded epithelial cells between passage 2 and passage 5 to obtain consistent results.
Histology.
To stain cartilage, dissected airway trees were stained with Alcian blue using a method modified from Jegalian and De Robertis (34). Briefly, dissected airway trees were rinsed in water and incubated overnight in 70% ethanol, followed by staining with 0.03% Alcian blue in 80% ethanol/20% acetic acid, pH 1.0 for 3 days at 37°C with gentle shaking. Next, samples were dehydrated with sequential 80, 95, and 100% ethanol, cleared with xylene, and stored in methyl salicylate.
For histological analysis of native large and small airways and expanded cells cultured at an air-liquid interface, samples were fixed in 10% neutral buffered formalin for 48–96 h. Tissues were then processed, embedded, sectioned (∼4 μm), and stained with hematoxylin and eosin.
Immunocytochemistry.
Native and expanded and cultured large and small airway epithelia were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 (Pierce, Rockford, IL). Apical surfaces were incubated with the following primary antibodies: 1) mouse anti-acetylated α-tubulin, 1:1,000 (Sigma, St. Louis, MO); 2) rabbit anti-zonula occludens-1 (anti-ZO-1), 1:100 (Zymed Laboratories, Carlsbad, CA); 3) mouse anti-Muc5AC, 1:50 (Thermo Fisher Scientific, Waltham, MA); 4) mouse anti-surfactant protein-D (SP-D, a gift from Dr. P. B. McCray Jr.), 1:50; and 5) phalloidin conjugated with Alexa 568, 1:50 (Thermo Fisher Scientific). Appropriate secondary antibody (goat anti-mouse-Alexa-Fluor-488, goat anti-rabbit-Alexa-Fluor-568, 1:500; Invitrogen, Carlsbad, CA) was used as described (38). Nuclei were counterstained with DAPI.
Bioelectrical properties.
Ussing chamber studies were performed as previously described (19, 54, 55). Apical and basolateral chambers contained the same bathing solution with symmetrical Cl− concentrations. CFTR-mediated Cl− current and amiloride-sensitive Na+ current were measured using a previously described protocol (19). First, Na+ current was inhibited with apical amiloride (100 μM), an inhibitor of the epithelial sodium channel (ENaC), which hyperpolarizes the apical membrane and increases the driving force for Cl− secretion through CFTR. Second, 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS, 100 μM) was added apically to block the calcium-activated Cl− channels to specifically examine the CFTR-mediated Cl− current. Next, CFTR activity was increased by raising cellular levels of cAMP with forskolin (10 μM) and 3-isobutyl-1-methylxanthine (IBMX, 100 μM). GlyH-101 (GlyH, 100 μM), a CFTR inhibitor, was added apically to block the CFTR-mediated Cl− current (19). Finally, the transepithelial Cl− current was reduced by inhibiting the Na+-K+-Cl− cotransporter with basolateral bumetanide (100 μM). The following parameters were calculated as previously described (38): decrease in current after apical addition of amiloride, decrease in current after apical addition of DIDS, cAMP-stimulated current after apical addition of forskolin and IBMX to epithelia already in the presence of apical amiloride and DIDS, and decrease in current after addition of GlyH-101 to the previous solutions. To study HCO3− transport, we used Cl−-free Krebs solution containing (in mM): 118.9 sodium gluconate, 25 NaHCO3, 2.4 K2HPO4, 0.6 KH2PO4, 5 calcium gluconate, 1 magnesium gluconate, and 5 dextrose and gassed with 5% CO2 as previously descried. The sequence of drug administration was the same as above.
Gene expression profiles.
Total RNA was isolated from non-CF expanded large and small airway epithelial cells at passage 4 with TRIzol Reagent (Invitrogen). After confirming RNA integrity, 5 μg of total RNA was used to generate biotinylated cRNA using the Affymetrix Gene Chip one-cycle target labeling kit (Affymetrix, Santa Clara, CA) and then hybridized to the Affymetrix Porcine GeneChip (23,937 probe sets that interrogate ∼23,256 transcripts from 20,201 Sus scrofa genes). All samples were hybridized on the same day. The arrays were scanned using the Affymetrix Model 450 Fluidics Station and Affymetrix Model 3000 scanner, and data were collected using the GeneChip Operating Software, version 1.4, and analyzed with Partek Genomics Suite Software (Partek, St. Louis, MO).
Quantitative real-time PCR.
Quantitative RT-PCR with SYBR Green chemistry and an ABI 7500 Fast Real-time PCR System were used to measure SP-D mRNA levels in tissue excised from native large and small airways. Briefly, tissues were collected in RNAlater (Ambion), and total RNA was isolated using the Purelink RNA kit (Invitrogen) and RNAeasy Mini Kit (Qiagen). First-strand cDNA was synthesized with random hexamers (SuperScript III; Invitrogen). Sequence-specific primers and probes were from IDT (Coralville, IA). SP-D mRNA levels were normalized to ZO-1 mRNA. The primer sequences of SP-D and ZO-1 genes were as follows: pig SP-D forward, 5′-AGC GGA GCA GAG AAC TGT GT-3′; pig SP-D reverse, 5′-CTC AGA ACT CGC AGA TCA CG-3′ (53); pig ZO-1 forward, 5′-AGA CCC GGC CAA GGT GTA TAG GA-3′; and pig ZO-1 reverse, 5′-ACG GGG GCT GGG GCT CAT AG-3′.
ASL pH measurement.
ASL pH was measured using the ratiometric fluorescent pH indicator SNARF conjugated to dextran (Molecular Probes, Eugene, OR) as previously reported (43). Briefly, the pH indicator was applied to the apical surface, and forskolin (10 μM) and IBMX (100 μM) were applied to basolateral medium. Two hours later epithelia were placed in a humidified chamber with 5% CO2 atmosphere at 37°C and examined by confocal microscopy (Zeiss 510 Meta NLO). pH was calculated as previously described (43).
ASL viscosity measurement.
ASL viscosity was assayed using fluorescence recovery after photobleaching as previously described (18) and our recent study (64).
Antimicrobial activity measurement.
Antimicrobial activity was examined using two separate assays as follows: 1) a luminescence-based antibacterial assay (2) modified for use on cells and 2) a grid-based assay (43). For the luminescence antibacterial assay, Staphylococcus aureus Xen-29 (Caliper LifeSciences Bioware), which contain a stable copy of the modified Photorhabdus luminescens luxABCDE operon, were grown to the exponential phase. Bacteria were harvested by centrifugation and suspended in a 1% tryptic soy broth buffer (2). Epithelial cells grown in a 24 Transwell plate (insert area 0.33 cm2) were exposed to bacteria (5 × 104 colony-forming units in 10 μl) for 2 min, followed by measurement of luminescence with a luminometer (Spectra Max L; Molecular Devices, Sunnyvale, CA). Data are reported as relative light units as a percentage of control (dead epithelial cells with no bacterial killing ability). For the grid assay, bacteria-coated grids were prepared as previously described (43) using S. aureus isolate 43SA (isolated from a CF pig with pulmonary infection) (60). Grids were place on the apical surface of epithelia in a humidified chamber with 5% CO2 at 37°C for 2 min, followed by assessment of bacterial killing using the Live/Dead BacLight Bacterial Viability kit (Invitrogen) per the manufacturer's instructions. All live and dead bacteria were counted in four to five individual fields (60×), and the percentage of live bacteria in each field was averaged.
Statistics.
Data are expressed as means ± SE. For microarray studies, a one-way ANOVA was used. P values are presented in legends for Figs. 1–6. For analyses that compared large and small airways from the same animal, we used a nonparametric Wilcoxon signed-rank test. For comparisons of different genotypes, we used the Wilcoxon rank-sum test. All analyses were done using Prism software version 6.0.
Fig. 1.
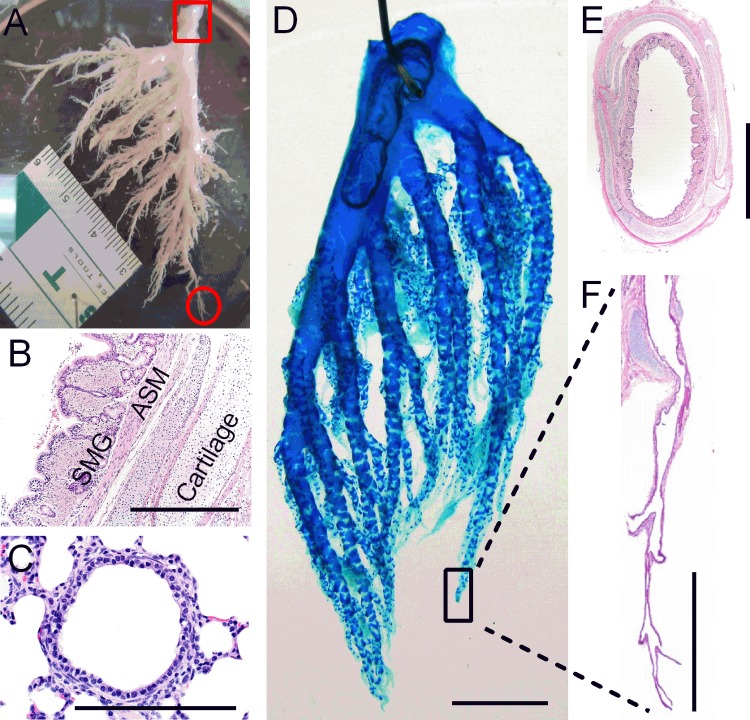
Large and small airways have different cellular compositions. A: image of intact airway tree dissected from the left lung of a newborn non-cystic fibrosis (CF) pig. The red square indicates where large airway epithelia was isolated, and the red circle indicates where small airway epithelia was isolated and used for expansion. B and C: hematoxylin and eosin (H&E) staining of large (B) and distal small (C) airways. In B, large airways consist of airway epithelial cells, submucosal glands (SMG), airway smooth muscle (ASM), and cartilage. Scale bar = 500 μm. In C, small airways lack complete cartilage ring support and SMG. Scale bar = 100 μm. D: Alcian blue staining of an intact right lung airway tree dissected from a newborn piglet. Scale bar = 1 cm. E: H&E staining of large cartilaginous airway. Scale bar = 2 mm. F: H&E staining demonstrates that only isolated cartilage plate is present in small airways. Scale bar = 1 mm.
Fig. 6.
CF small airway epithelia have higher ASL viscosity and lower bacterial killing ability compared with non-CF small airway epithelia. A: ASL viscosity as determined by fluorescence recovery after photobleaching of FITC-dextran in expanded small airway epithelia from non-CF and CF pigs. N = 6 non-CF piglets and 6 CF piglets. One Transwell insert of expanded epithelia from each pig was used. *P = 0.0043 vs. non-CF large airway epithelia, Wilcoxon rank-sum test. B: antimicrobial activity of expanded small airway epithelia from non-CF and CF pigs. Data are presented as the relative luminescence (RLU) of Staphylococcus aureus (Xen-29) as a percentage of control. N = 6 non-CF piglets and 6 CF piglets. One Transwell insert of expanded epithelia from each pig was used. *P = 0.0043 vs. non-CF large airway epithelia, Wilcoxon rank-sum test. C: antimicrobial activity of expanded small airway epithelia from non-CF and CF pigs as evaluated by S. aureus-coated grid assay. Data are presented as the percentage of live bacteria. N = 5 non-CF piglets and 5 CF piglets. One Transwell insert of expanded epithelia from each pig was used. *P = 0.0159 vs. non-CF small airway epithelia, Wilcoxon rank-sum test.
RESULTS
Isolation, expansion, and culture of distal small airway cells.
In human adults, “small airways” are defined as airways with an internal diameter of <2 mm (31), usually distal to the sixth generation of the bronchial tree. Because the size of the small airways is species and age specific, we defined small airways in newborn pigs based on histology. Previous work defined the airway tree nomenclature in newborn pigs (3). In newborn pigs, we define the small airways as distal airways, bronchioles without submucosal glands and complete cartilage ring support, with an internal diameter of <200 μm (Fig. 1). Newborn pigs were chosen because CF newborn pigs show no infection or inflammation. Figure 1A is an image of the intact airway tree dissected from the left lung of a non-CF newborn pig. The red square denotes the region where large airways were taken from while the red circle denotes the region where small airways were taken from for epithelial cell expansion. Large airways revealed a cartilaginous wall lined by pseudostratified epithelium with submucosal glands and smooth muscle (Fig. 1, B and E). In contrast, the small airways lacked submucosal glands and complete cartilage ring support and displayed thin bands of smooth muscle and low columnar to cuboidal lining epithelium (Fig. 1C). Figure 1D shows staining of an airway tree dissected from the right lung from a newborn pig; it was stained with Alcian blue, which mainly stains cartilages. The small airways contained only isolated cartilage plates and were devoid of complete cartilage ring support (Fig. 1F).
Whereas sufficient numbers of large airway cells can be obtained from newborn pig trachea for direct in vitro studies, substantially fewer cells can be recovered from the small airways. To perform a comparative analysis of large and small airway epithelia, we applied a recently developed method (39, 63) to expand and culture both large and small airway cells at the air-liquid interface. This method includes coculture with low-passage irradiated NIH 3T3-J2 fibroblast “feeder cells” and treatment with a ROCK inhibitor (39, 46).
We first determined that expanded large and small airway cells cultured at the air-liquid interface formed tight junctions and had the capacity to differentiate into ciliated (acetylated α-tubulin positive) (Fig. 2, A and B) and goblet (Muc5AC positive) (Fig. 2, C and D) cells. This is consistent with the cellular composition of the native large and small airways, which were mostly covered by ciliated cells, with fewer goblet cells in the small airway compared with large airway (Fig. 2, E–H).
Fig. 2.
Expanded small airway cells form differentiated epithelia when cultured at the air-liquid interface. A and B: expanded large and small airway cells cultured at the air-liquid interface form tight junctions [zonula occludens (ZO)-1, red] and develop cilia (acetylated α-tubulin, green). Nuclei (DAPI, blue), Scale bar = 20 μm. C and D: goblet cells are present in expanded large and small airway cells cultured at the air-liquid interface. Goblet cells (Muc5AC, green), tight junctions (ZO-1, red), and nuclei (DAPI, blue) are shown. Scale bar = 20 μm. E–H: cellular composition of the native large and small airways, which are mostly covered by ciliated cells (acetylated α-tubulin, green in E and F) with fewer goblet cells in small vs. large airway tissue (Muc5AC, green in G and H). Phalloidin was used to stain F-actin in red. DAPI staining for nuclei is in blue. Area surrounded by dotted box is enlarged in insets. Scale bar = 100 μm in large image and 20 μm in insets.
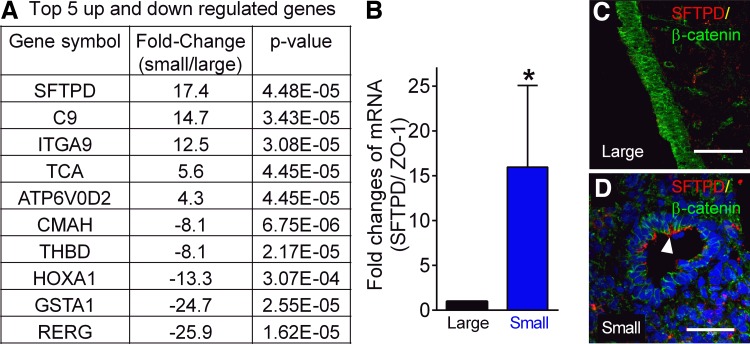
To further characterize the expanded small airway cells, we compared the gene expression profiles of non-CF small and large airways using a porcine gene microarray. We found that the expression of several genes was significantly different between expanded large and small airway epithelial cells (Fig. 3A). For example, SP-D was robustly expressed (17.4-fold higher) in expanded small airway epithelial cells (Fig. 3A), which is consistent with previous studies of SP-D distribution in pig, rat, and human small airways (21, 29, 40). Using real-time PCR and immunocytochemistry, we validated that SP-D expression was higher in excised native small airway tissue, with no SP-D-positive staining detected in large airway surface epithelia (Fig. 3, B–D). Other genes, such as complement component 9 and integrin-α9, had at least 10 times higher expression in the expanded small airway epithelia relative to the large airway cells (Fig. 3A). Conversely, there are some genes, such as glutathione-S-transferase α1 (GSTA1) (Fig. 3A), that have lower gene expression levels in small airways. The complete list of gene array data is included in the Supplemental Table S1 (Supplemental Material for this article is available online at the Journal website.). These data demonstrate that expanded epithelial cells retain some characteristics of the native tissue and small airway epithelia have a distinct gene expression profile compared with large airway epithelia.
Fig. 3.
Gene expression profile between expanded large and small airway epithelia is different. A: top five significantly upregulated and downregulated genes in expanded small vs. large airway epithelia from non-CF pigs. N = 3 non-CF piglets. Three Transwell inserts of expanded epithelia from each pig were pooled to isolate RNA. ANOVA was used, and the P value was listed. B: validation of surfactant protein D [SFTPD (SP-D)] mRNA changes in native large and small airway tissue by qRT-PCR. Data were normalized to ZO-1. N = 6 non-CF piglets. *P = 0.0498; Ratio-paired t-test. C and D: immunostaining of SP-D in native large and small airway tissue. The white arrowhead in D indicates the SP-D-positive surface epithelia in small airway but not in large airways. Scale bar = 60 μm in C and 40 μm in D.
Differential electrolyte transport properties in expanded large and small airway epithelia.
The physical and chemical properties of ASL, which play important roles in host defense and MCT, are dependent on transepithelial ion transport. Thus, we investigated the electrolyte transport properties in expanded large and small airway epithelia from both non-CF and CF pigs under short-circuit conditions. Expanded large and small airway epithelia from CF and non-CF pigs were used for Ussing chamber studies as previously described (54). The baseline transepithelial resistances were not different among groups of expanded cells studied: non-CF large airway epithelia (792.8 ± 146.7 Ω·cm2); non-CF small airway epithelia (907.8 ± 204.3 Ω·cm2); CF large airway epithelia (726.0 ± 128.0 Ω·cm2); and CF small airway epithelia (1014 ± 125.6 Ω·cm2). Summarized short-circuit current (Isc) data in non-CF and CF large and small airway epithelia are presented in Fig. 4, A and B. Data for large and small airways from the same animal were paired for each pig, allowing direct comparison within each genotype. Amiloride-sensitive ENaC current was similar in expanded large and small airway epithelia from CF and non-CF pigs (Fig. 4C). We found no difference in the DIDS-sensitive Ca+-activated Cl− channel activity in large and small CF and non-CF airway epithelia (Fig. 4D). However, we detected greater cAMP-stimulated Cl− current in non-CF small airway epithelia compared with large airway (Fig. 4E). Minimal cAMP-stimulated current was observed in either large or small CF airway epithelia (Fig. 4E). In line with these findings, the CFTR blocker GlyH produced a greater decrease in current in non-CF small airway cells relative to large airway cells, and application of GlyH had no effect in CF epithelia (Fig. 4F).
Fig. 4.
Non-CF small airway cells have increased cystic fibrosis transmembrane conductance regulator (CFTR)-mediated cAMP-stimulated Cl− current. A and B: summarized short-circuit current (Isc) data of expanded large and small airway epithelial cells from non-CF (A) and CF (B) pigs. C: summary data of amiloride-sensitive ΔIsc (ΔIscAmil) in expanded large and small airway epithelia from non-CF and CF pigs. D: summary data of 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS)-sensitive ΔIsc (ΔIscDIDS) in expanded large and small airway epithelia from non-CF and CF pigs. E: summary data of forskolin (F) + 3-isobutyl-1-methylxanthine (I)-stimulated ΔIsc (ΔIscF&I) in expanded large and small airway epithelia from non-CF and CF pigs. N = 11 non-CF piglets and 11 CF piglets. One Transwell insert of expanded epithelia from each pig was used. *P = 0.002 vs. non-CF large airway epithelia, Wilcoxon signed-rank test. F: summary data of GlyH-101 (a CFTR inhibitor)-sensitive ΔIsc (ΔIscGlyH) in expanded large and small airway epithelia from non-CF and CF pigs. N = 11 non-CF piglets and 11 CF piglets. One Transwell insert of expanded epithelia from each pig was used. *P = 0.0029 vs. non-CF large airway epithelia, Wilcoxon signed-rank test.
To determine whether small airway epithelia have differences in HCO3− transport, we measured electrogenic HCO3− transport by Ussing chamber using Cl−-free Krebs solution gassed with 5% CO2. Representative Isc traces in non-CF and CF large and small airway epithelia are presented in Fig. 5, A and B. Compared with expanded non-CF large airway epithelia, addition of 10 μM forskolin and 100 μM IBMX and the addition of 100 μM GlyH to non-CF small airway epithelia resulted in a greater change in current (Fig. 5, A, C, and D). These data suggest that the greater CFTR activity in small airway epithelia is associated with greater HCO3− transport. The minimal response to treatments (forskolin and IBMX; GlyH) in CF large and small airway epithelia confirms the role of CFTR in HCO3− transport (Fig. 5, B, C, and D).
Fig. 5.
CFTR-mediated bicarbonate (HCO3−) transport and airway surface liquid (ASL) pH are higher in non-CF small vs. large airway cells. A and B: representative Isc traces of expanded large and small airway epithelial cells from non-CF (A) and CF (B) pigs. C: summary data of ΔIscF&I in expanded large and small airway epithelia from non-CF and CF pigs. N = 7 non-CF piglets and 7 CF piglets. One Transwell insert of expanded epithelia from each pig was used. *P = 0.0313 vs. non-CF large airway epithelia, Wilcoxon signed-rank test. D: summary data of ΔIscGlyH in expanded large and small airway epithelia from non-CF and CF pigs. N = 7 non-CF piglets and 7 CF piglets. One Transwell insert of expanded epithelia from each pig was used. *P = 0.0313 vs. non-CF large airway epithelia, Wilcoxon signed-rank test. E: calibration curve for ASL pH measurement. x-Axis indicates the ratio of fluorescence reading of SNARF at 580 vs. 640 nm at different pH values; n = 5, error bars are hidden by symbols. F: ASL pH in non-CF small airway epithelia is higher than that in non-CF large airway epithelia. ASL pH is similar in CF large and small airway epithelia. Epithelia were exposed to 5% CO2 under stimulated conditions with cAMP-elevating agents (F&I). N = 7 non-CF piglets and 7 CF piglets. One Transwell insert of expanded epithelia from each pig was used. *P = 0.0469 vs. non-CF large airway epithelia, Wilcoxon signed-rank test.
ASL pH is higher in non-CF small airway epithelia than in expanded large airway epithelia.
Changes in HCO3− transport will alter ASL pH given a constant Pco2 (43). Thus, we examined if the differences in HCO3− transport in large and small airway epithelia result in a change in ASL pH. We used the dextran-conjugated fluorescent ratiometric pH indicator SNARF to measure ASL pH, and the calibration cure is shown in Fig. 5E. We found that non-CF small airway epithelia had a higher ASL pH compared with non-CF large airway epithelia under 5% CO2 conditions (Fig. 5F). These data are consistent with the differences in CFTR activity (Fig. 4, E and F) and HCO3− transport (Fig. 5, C and D) in large vs. small airway epithelia from non-CF pigs.
CF small airway epithelia have lower ASL pH, higher viscosity, and impaired bacterial killing.
Previous studies in large airway epithelia of pig and human origin have established that loss of CFTR results in decreased HCO3− secretion and reduced ASL pH (19, 43, 56, 59). We therefore compared ASL pH in expanded CF and non-CF pig small airway epithelia and found that ASL pH was lower with CFTR deficiency (Fig. 5F), consistent with that CF ASL pH is more acidic (43).
Studies in newborn CF pigs identified at least two respiratory host defense defects in the large airway that were present at birth: 1) impaired MCT (30) and 2) impaired endogenous bacterial killing in ASL lining fluid due to lower pH (43, 61). Because we observed lower pH in CF vs. non-CF small airway epithelia, we next examined ASL viscosity and bacterial killing to assess if these same defects are present in the CF small airways. We found that ASL viscosity was higher in CF small airway epithelia compared with non-CF cells (Fig. 6A), consistent with a role for CFTR in MCT defects. In addition, we used two independent methods to evaluate bacterial killing: luminescence-based assessment of bacterial viability (2) and bacteria-coated grids placed on the apical surface of expanded airway epithelia (43). Compared with expanded non-CF small airway epithelia, both methods demonstrated a significant impairment of bacterial killing in CF small airway cells (Fig. 6, B and C). These data highlight the important implications for host defense of CFTR deficiency in small airway epithelia.
DISCUSSION
Recent work in the large airway has provided compelling data that ASL pH and MCT are altered by CFTR deficiency, resulting in impaired host defense against bacterial challenge (30, 43, 61). While clinical and pathology data suggest that the small airways are involved in the early development of CF lung disease (65), progress toward understanding the precise role of small airway epithelia has been significantly hampered by the lack of experimental models. Using a novel approach to isolate, expand, and culture small airway epithelia from CF and non-CF pigs, we were able to directly compare bioelectric properties of small vs. large airway epithelia. We found that small airway cells from non-CF pigs have greater CFTR activity, HCO3− transport, and ASL pH relative to large airway cells. Lack of CFTR function in small airway epithelia blunted HCO3− transport, decreased ASL pH, and significantly impaired ASL viscosity and bacterial killing. In summary, this method for expanding and culturing small airway epithelia allowed us to uncover differences in ASL pH, viscosity, and antimicrobial properties between CF and non-CF small airway epithelia.
The majority of published studies of the small airways rely on large animals, namely pigs and sheep. Murine models are not suitable because the size of the small airways precludes study. Moreover, CF mice do not develop the airway disease typically found in humans (28). Microperfusion or micro-Ussing chamber has been used to study non-CF pig small airway tissue and provided evidence that small airways have a large anion conductance (4, 7, 8, 13, 50, 51). Two studies have also used human primary cells isolated from the small airways (12, 13), which allows for examining both bioelectrical and functional properties of the small airway epithelia. However, the tissue is very limited and typically only available from diseased lungs, in particular from people with CF with terminal lung disease.
Using a recently reported approach for culturing primary cells in the presence of a ROCK inhibitor (39, 63), we isolated large and small airway cells from newborn pig lungs and expanded and cultured the expanded cells at the air-liquid interface. We confirmed that our model retains many features of the native tissue, including gene expression profile and distribution of SP-D in the small but not large airway epithelia (21, 29). We also observed that several other genes as listed in Fig. 3A are differentially expressed in large vs. small airway cells, although they do not have obvious roles in ion transport properties and host defense. They likely present an interesting area for further investigation. Moreover, we demonstrate that this approach can be applied to study small airway cells from CF pigs, which facilitates comparative studies of small airway function in CF and non-CF pigs.
A limitation of this study is that expanded airway epithelia may have altered properties compared with native tissue or primary cells. For example, published data from expanded human alveolar cells demonstrate loss of ion channel transport properties and expression of surfactant proteins at higher passage numbers (16). We observed a similar trend in our expanded airway cells. For this reason, we only used expanded cells with passage numbers under six. We also observed a non-negligible batch effect. To overcome this limitation, experiments were performed using matched cells derived from the large and small airways of the same pig, cultured under the same conditions, and at the same passage number.
Our data provide clear evidence that CFTR activity is greater in small airway epithelia compared with large airway epithelia. A previous in situ hybridization study in human lung tissue demonstrated that CFTR expression is higher in small vs. large airways (23). As expected, deficiency of CFTR abrogated Cl− transport in expanded small airway epithelia. Consistent with the greater CFTR activity in small airways, HCO3− transport was greater in the small compared with the large airway epithelia. Others have measured HCO3− secretion in native small airways using micro-Ussing chamber assays (50), but ours is the first study to directly compare HCO3− transport in large and small airway epithelia. We also confirmed that HCO3− transport in the small airways is CFTR dependent, which has been previously reported in large airway (20, 32, 33, 45, 57). While our data provide evidence that HCO3− transport is mainly mediated by CFTR in the small airways, it remains possible that the channel TMEM16A (17, 22, 49, 69) and the transporter SLC26A9 (10) may contribute to HCO3− transport, perhaps by working in concert with the Cl−/HCO3− exchanger SLC26A4 (26). In addition, ASL pH may be regulated by other mechanisms besides HCO3− secretion. It has been reported that acid secretion can also regulate ASL pH in airway epithelia (24). Our data suggest that higher HCO3− secretion through CFTR can at least partially explain why ASL pH is higher in non-CF small airways. Further studies are needed to elucidate the detailed ASL pH regulation mechanism, such as measurement of anion gaps and proton secretion in non-CF large and small airways (24, 35).
The lower ASL pH in the small airways of CF pigs was associated with increased viscosity and decreased bacterial killing compared with non-CF pigs. These data indicate that the small airways are susceptible to bacterial colonization, which may be caused by the same antibacterial host defense defects present in the large airways. In large CF airways, the submucosal glands secrete mucus strands that remain tethered to gland ducts (30), resulting in impaired MCT. Because the small airways lack submucosal glands, we speculate that acidic ASL pH in CF small airways alters goblet cell-derived mucus properties, providing an alternate mechanism for the MCT defect. The impaired bacterial killing in CF small airways may be explained by the impact of ASL pH on antimicrobial proteins, such as SP-D, which is exclusively expressed in the small airways. We have reported that acidic pH impairs the ability of two key airway antimicrobial peptides, β-defensin-3 and LL-37, to kill bacteria (2). When these peptides are combined, they exhibit synergistic bacterial killing, but an acidic pH reduced their synergistic effect. Future studies are warranted to understand how changing ASL pH affects SP-D antimicrobial activity and its synergy with other antimicrobial proteins/peptides. Even though we suggest that lower ASL pH in CF small airways could contribute to CF pathogenesis, there could be other factors. For example, several studies of cultured large airway epithelia reported that loss of the CFTR led to ASL height depletion (15). Considering that there could be some difference in water permeability between large and small airways (37), ASL height will be important to determine in expanded large and small airways in future studies. However, we did not detect any difference in aquaporin gene expression in our expanded large and small airway epithelia as described in a previous study (37). In addition, it is not clear what the physiological and pathophysiological significance of several other differentially expressed genes in large and small airways is. Interestingly, it has been reported that GSTA1 polymorphism is associated with asthma, which is another small airway disease (44). Similar investigations are needed to determine if these differences contribute to CF lung disease pathogenesis.
Our data leave the interesting question unanswered: why would the small airways have higher CFTR activity and a more alkaline ASL pH than the large airway? This observation is consistent with previous studies (13, 23, 66). We speculate several possibilities. First, because the small airways lack submucosal glands, and the submucosal glands in large airways secrete fluid and HCO3−, the epithelia of the small airways have to compensate by secreting more liquid and HCO3− through elevated CFTR activity (13, 68). Because our culture model lacks submucosal glands, this results in higher ASL pH in the small airways. Second, the small airways are exposed to a constant ∼5% CO2 concentration during the respiratory cycle. In contrast, the large airways are exposed to oscillations in CO2, with lower CO2 concentrations during inspiration (27). Thus the average CO2 concentration in small airways is higher than in the large airways during the respiratory cycle. A higher HCO3− concentration in the small airway would partially counteract the differences in CO2 concentration. Third, the optimal pH needed for host defense mechanisms, including MCT and antimicrobial capacity, may be different between large and small airways. Finally, because distal airways may have greater susceptibility to particles/bacterial accumulation due to slower MCT in the distal lung region (5), enhanced pH-mediated antimicrobial activity and MCT may be required to maintain sterile epithelia.
Detailed mechanistic studies are needed to test these possibilities and understand why ASL pH is higher in the small airways under normal physiological conditions. Moreover, these questions should be taken into account when developing therapeutic approaches to overcome the effect of CFTR deficiency that leads to CF lung disease.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL-091842 and HL-51670, by a Cystic Fibrosis Foundation Pilot & Feasibility Grant to X. Li, and Research Development Grant (M. J. Welsh).
DISCLOSURES
M. J. Welsh is an Investigator of the Howard Hughes Medical Institute. No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
X.L., I.M.T., M.J.W., and J.Z. conception and design of research; X.L., X.X.T., L.G.V.B., P.H.K., P.J.T., and D.K.M. performed experiments; X.L., X.X.T., L.G.V.B., S.R., K.S., M.J.W., D.K.M., and J.Z. analyzed data; X.L., X.X.T., L.G.V.B., A.P.C., I.M.T., P.H.K., M.H.A.A., M.J.W., D.A.S., and J.Z. interpreted results of experiments; X.L. and X.X.T. prepared figures; X.L., X.X.T., L.G.V.B., M.J.W., and J.Z. drafted manuscript; X.L., X.X.T., L.G.V.B., A.P.C., I.M.T., S.R., P.H.K., P.J.T., K.S., M.H.A.A., M.J.W., D.K.M., D.A.S., and J.Z. edited and revised manuscript; X.L., X.X.T., L.G.V.B., A.P.C., I.M.T., S.R., P.H.K., P.J.T., K.S., M.H.A.A., M.J.W., D.K.M., D.A.S., and J.Z. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Lakshmi Durairaj, Dr. Lynda Ostedgaard, Sarah E. Ernst, Viral Shah, Ping Tan, and Drake Bouzek for technical assistance, suggestions, and advice. We thank Dr. Kristina W. Thiel for assistance in manuscript preparation. We thank the University of Iowa In Vitro Models and Cell Culture Core and DNA Core Facility.
Present address for S. Ramachandran: Children's Hospital of Philadelphia, Philadelphia, PA.
REFERENCES
- 1.Abou Alaiwa MH, Beer AM, Pezzulo AA, Launspach JL, Horan RA, Stoltz DA, Starner TD, Welsh MJ, Zabner J. Neonates with cystic fibrosis have a reduced nasal liquid pH; a small pilot study. J Cyst Fibros 13: 373–377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, Zabner J, Welsh MJ. pH modulates the activity and synergism of the airway surface liquid antimicrobials beta-defensin-3 and LL-37. Proc Natl Acad Sci USA 111: 18703–18708, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam RJ, Michalski AS, Bauer C, Abou Alaiwa MH, Gross TJ, Awadalla MS, Bouzek DC, Gansemer ND, Taft PJ, Hoegger MJ, Diwakar A, Ochs M, Reinhardt JM, Hoffman EA, Beichel RR, Meyerholz DK, Stoltz DA. Air trapping and airflow obstruction in newborn cystic fibrosis piglets. Am J Respir Crit Care Med 188: 1434–1441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Bazzaz FJ, Hafez N, Tyagi S, Gailey CA, Toofanfard M, Alrefai WA, Nazir TM, Ramaswamy K, Dudeja PK. Detection of Cl–HCO3− and Na+-H+ exchangers in human airways epithelium. JOP 2: 285–290, 2001. [PubMed] [Google Scholar]
- 5.Asmundsson T, Kilburn KH. Mucociliary clearance rates at various levels in dog lungs. Am Rev Respir Dis 102: 388–397, 1970. [DOI] [PubMed] [Google Scholar]
- 6.Bakker EM, Borsboom GJ, van der Wiel-Kooij EC, Caudri D, Rosenfeld M, Tiddens HA. Small airway involvement in cystic fibrosis lung disease: routine spirometry as an early and sensitive marker. Pediatr Pulmonol 48: 1081–1088 2013. [DOI] [PubMed] [Google Scholar]
- 7.Ballard ST, Fountain JD, Inglis SK, Corboz MR, Taylor AE. Chloride secretion across distal airway epithelium: relationship to submucosal gland distribution. Am J Physiol Lung Cell Mol Physiol 268: L526–L531, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Ballard ST, Schepens SM, Falcone JC, Meininger GA, Taylor AE. Regional bioelectric properties of porcine airway epithelium. J Appl Physiol 73: 2021–2027, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Bedrossian CW, Greenberg SD, Singer DB, Hansen JJ, Rosenberg HS. The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol 7: 195–204, 1976. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol 133: 421–438, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blouquit-Laye S, Chinet T. Ion and liquid transport across the bronchiolar epithelium. Respir Physiol Neurobiol 159: 278–282, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Blouquit-Laye S, Dannhoffer L, Braun C, Dinh-Xuan AT, Sage E, Chinet T. Effect of nitric oxide on epithelial ion transports in noncystic fibrosis and cystic fibrosis human proximal and distal airways. Am J Physiol Lung Cell Mol Physiol 303: L617–L625, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Blouquit S, Regnier A, Dannhoffer L, Fermanian C, Naline E, Boucher R, Chinet T. Ion and fluid transport properties of small airways in cystic fibrosis. Am J Respir Crit Care Med 174: 299–305, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med 13: 231–240, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med 261: 5–16, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Bove PF, Dang H, Cheluvaraju C, Jones LC, Liu X, O'Neal WK, Randell SH, Schlegel R, Boucher RC. Breaking the in vitro alveolar type II cell proliferation barrier while retaining ion transport properties. Am J Respir Cell Mol Biol 50: 767–776, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Chang EH, Tang XX, Shah VS, Launspach JL, Ernst SE, Hilkin B, Karp PH, Abou Alaiwa MH, Graham SM, Hornick DB, Welsh MJ, Stoltz DA, Zabner J. Medical reversal of chronic sinusitis in a cystic fibrosis patient with ivacaftor. Int Forum Allergy Rhinol 5: 178–181, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143: 911–923, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, O'Neal WK, Boucher RC. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA 100: 16083–16088, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crouch E, Parghi D, Kuan SF, Persson A. Surfactant protein D: Subcellular localization in nonciliated bronchiolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 263: L60–L66, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol 279: C461–C479, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Engelhardt JF, Zepeda M, Cohn JA, Yankaskas JR, Wilson JM. Expression of the cystic fibrosis gene in adult human lung. J Clin Invest 93: 737–749, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol 211: 139–150, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishman AP. Structure and function. In: Pulmonary Diseases and Disorders. New York, NY: McGraw-Hill, 1988. [Google Scholar]
- 26.Garnett JP, Hickman E, Burrows R, Hegyi P, Tiszlavicz L, Cuthbert AW, Fong P, Gray MA. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J Biol Chem 286: 41069–41082, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavriely N, Eckmann DM, Grotberg JB. Intra-airway gas transport during high-frequency chest vibration with tracheal insufflation in dogs. J Appl Physiol 79: 243–250, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev 79: S193–S214, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Herias MV, Hogenkamp A, van Asten AJ, Tersteeg MH, van Eijk M, Haagsman HP. Expression sites of the collectin SP-D suggest its importance in first line host defence: power of combining in situ hybridisation, RT-PCR and immunohistochemistry. Mol Immunol 44: 3324–3332, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, Stoltz DA, Welsh MJ. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345: 818–822, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 278: 1355–1360, 1968. [DOI] [PubMed] [Google Scholar]
- 32.Hug MJ, Tamada T, Bridges RJ. CFTR and bicarbonate secretion by [correction of to] epithelial cells. News Physiol Sci 18: 38–42, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Itani OA, Chen JH, Karp PH, Ernst S, Keshavjee S, Parekh K, Klesney-Tait J, Zabner J, Welsh MJ. Human cystic fibrosis airway epithelia have reduced Cl- conductance but not increased Na+ conductance. Proc Natl Acad Sci USA 108: 10260–10265, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jegalian BG, De Robertis EM. Homeotic transformations in the mouse induced by overexpression of a human Hox3.3 transgene. Cell 71: 901–910, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Joris L, Dab I, Quinton PM. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis 148: 1633–1637, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Keiser NW, Birket SE, Evans IA, Tyler SR, Crooke AK, Sun X, Zhou W, Nellis JR, Stroebele EK, Chu KK, Tearney GJ, Stevens MJ, Harris JK, Rowe SM, Engelhardt JF. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am J Respir Cell Mol Biol 52: 683–694, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol 24: 224–234, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Comellas AP, Karp PH, Ernst SE, Moninger TO, Gansemer ND, Taft PJ, Pezzulo AA, Rector MV, Rossen N, Stoltz DA, McCray PB Jr, Welsh MJ, Zabner J. CFTR is required for maximal transepithelial liquid transport in pig alveolar epithelia. Am J Physiol Lung Cell Mol Physiol 303: L152–L160, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol 180: 599–607, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol 164: 5866–5870, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Oppenheimer EH, Esterly JR. Pathology of cystic fibrosis review of the literature and comparison with 146 autopsied cases. Perspect Pediatr Pathol 2: 241–278, 1975. [PubMed] [Google Scholar]
- 42.Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, Rogan MP, Davis GJ, Dohrn CL, Wohlford-Lenane C, Taft PJ, Rector MV, Hornick E, Nassar BS, Samuel M, Zhang Y, Richter SS, Uc A, Shilyansky J, Prather RS, McCray PB Jr, Zabner J, Welsh MJ, Stoltz DA. The DeltaF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med 3: 24–74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Banfi B, Horswill AR, Stoltz DA, McCray PB Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piacentini S, Polimanti R, Iorio A, Cortesi M, Papa F, Rongioletti M, Liumbruno GM, Manfellotto D, Fuciarelli M. GSTA1*-69C/T and GSTO2*N142D as asthma- and allergy-related risk factors in Italian adult patients. Clin Exp Pharmacol Physiol 41: 180–184, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372: 415–417, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6: 331–343, 1975. [DOI] [PubMed] [Google Scholar]
- 47.Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB Jr, McLennan G, Meyerholz DK, Namati E, Ostedgaard LS, Prather RS, Sabater JR, Stoltz DA, Zabner J, Welsh MJ. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 295: L240–L263, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321: 1837–1841, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shamsuddin AK, Quinton PM. Native small airways secrete bicarbonate. Am J Respir Cell Mol Biol 50: 796–804, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shamsuddin AK, Quinton PM. Surface fluid absorption and secretion in small airways. J Physiol 590: 3561–3574, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev 79: S23–S45, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Skovgaard K, Mortensen S, Boye M, Hedegaard J, Heegaard PM. Hepatic gene expression changes in pigs experimentally infected with the lung pathogen Actinobacillus pleuropneumoniae as analysed with an innate immunity focused microarray. Innate Immun 16: 343–353, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Smith JJ, Karp PH, Welsh MJ. Defective fluid transport by cystic fibrosis airway epithelia. J Clin Invest 93: 1307–1311, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85: 229–236, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest 89: 1148–1153, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith JJ, Welsh MJ. Fluid and electrolyte transport by cultured human airway epithelia. J Clin Invest 91: 1590–1597, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sobonya RE, Taussig LM. Quantitative aspects of lung pathology in cystic fibrosis. Am Rev Respir Dis 134: 290–295, 1986. [DOI] [PubMed] [Google Scholar]
- 59.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol 290: C741–C749, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GAt Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB Jr, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2: 29–31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 372: 351–362, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoltz DA, Rokhlina T, Ernst SE, Pezzulo AA, Ostedgaard LS, Karp PH, Samuel MS, Reznikov LR, Rector MV, Gansemer ND, Bouzek DC, Alaiwa MH, Hoegger MJ, Ludwig PS, Taft PJ, Wallen TJ, Wohlford-Lenane C, McMenimen JD, Chen JH, Bogan KL, Adam RJ, Hornick EE, Nelson GAt Hoffman EA, Chang EH, Zabner J, McCray PB Jr, Prather RS, Meyerholz DK, Welsh MJ. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest 123: 2685–2693, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suprynowicz FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, Yuan H, Cheluvaraju C, Clapp PW, Boucher RC Jr, Kamonjoh CM, Randell SH, Schlegel R. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci USA 109: 20035–20040, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, Choudhury B, Varki A, Stoltz DA, Welsh MJ. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tiddens HA, Donaldson SH, Rosenfeld M, Pare PD. Cystic fibrosis lung disease starts in the small airways: can we treat it more effectively? Pediatr Pulmonol 45: 107–117, 2010. [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Lytle C, Quinton PM. Predominant constitutive CFTR conductance in small airways. Respir Res 6: 7, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weibel ER. Morphometry of the Human Lung. Berlin, Germany: Springer-Verlag, 1963. [Google Scholar]
- 68.Widdicombe JH, Wine JJ. Airway Gland Structure and Function. Physiol Rev 95: 1241–1319, 2015. [DOI] [PubMed] [Google Scholar]
- 69.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Zabner J, Wadsworth SC, Smith AE, Welsh MJ. Adenovirus-mediated generation of cAMP-stimulated Cl- transport in cystic fibrosis airway epithelia in vitro: effect of promoter and administration method. Gene Ther 3: 458–465, 1996. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.