Summary
Dengue viruses cause more human morbidity and mortality than any other arthropod-borne virus. Dengue prevention relies primarily on vector control but the failure of traditional methods has promoted the development of novel entomological approaches. Although use of the intracellular bacterium Wolbachia to control mosquito populations was proposed half a century ago, it has only gained significant interest as a potential agent of dengue control in the last decade. Here, we review the evidence that supports a practical approach for dengue reduction through field release of Wolbachia-infected mosquitoes and discuss the additional studies that must be conducted before the strategy can be validated and operationally implemented. A critical next step is to assess the efficacy of Wolbachia deployment in reducing dengue virus transmission. We argue that a cluster-randomized trial is currently premature because Wolbachia strain choice for release as well as deployment strategies are still being optimized. We therefore present a pragmatic approach to acquiring preliminary evidence of efficacy via a suite of complementary methodologies: prospective cohort study, geographical cluster investigation, virus phylogenetic analysis, virus surveillance in mosquitoes, and vector competence assays. This multi-pronged approach could provide valuable intermediate evidence of efficacy to justify a future cluster-randomized trial.
Dengue is a major public health problem in tropical and sub-tropical regions, where almost 400 million infections are estimated to occur each year.1 The etiological agents are four dengue virus serotypes (DENV-1 to -4) in the genus Flavivirus that are transmitted among humans by mosquitoes. These viruses cause a systemic, debilitating though mostly self-limiting illness, which without careful management can lead to hypovolemic shock and death.2 In the absence of a licensed vaccine or therapeutic drug, dengue prevention efforts are currently limited to the control of its main mosquito vector, Aedes aegypti. With a few exceptions, the implementation of vector control methods has been largely unsuccessful due to the lack of sustained commitment of resources3 and inability to effectively scale-up and successfully apply interventions over large geographic areas and modern mega-cities. Novel entomological approaches to dengue control have been developed4 and some are now advancing to field testing.5
Wolbachia-based strategies for dengue control
One of the most promising entomological strategies being developed for dengue control relies on introduction of the intracellular bacterium Wolbachia into Ae. aegypti.6 Wolbachia pipientis is an bacterial endosymbiont that was originally identified in ovaries of Culex mosquitoes in the 1920s7 and is thought to infect two-thirds of all living insect species.8 The extraordinary evolutionary success of Wolbachia is attributed to their ability to manipulate the biology of their hosts in diverse ways.9 For example, Wolbachia can induce reproductive abnormalities such as feminization and sperm-egg cytoplasmic incompatibility (CI). Because Wolbachia is transmitted vertically via the egg, these female-biased reproductive manipulations can drive Wolbachia infections to high frequencies in wild populations. CI, the most common manipulation in insects, occurs when Wolbachia-infected males mating with Wolbachia-free females lead to the production of non-viable offspring. Wolbachia-infected females, in contrast, produce successful offspring regardless of the Wolbachia infection status of their mate.
The potential of Wolbachia to control pest insect populations was realized as early as half a century ago (Figure 1). Wolbachia-induced CI was then proposed to eliminate Culex mosquitoes10 or to introduce desirable genes into wild vector populations.11 To date, however, Wolbachia have never been operationally implemented as a vector control measure. A significant hurdle was the fact that several major vectors of human pathogens are not naturally infected by Wolbachia, including the main DENV vector Ae. aegypti. The mosquito vectors (Anopheles spp.) of human malaria parasites were also thought to be Wolbachia-free until a recent study reported evidence for Wolbachia in field populations of An. gambiae.12
Figure 1. Key dates in the development of Wolbachia-based dengue control strategies.
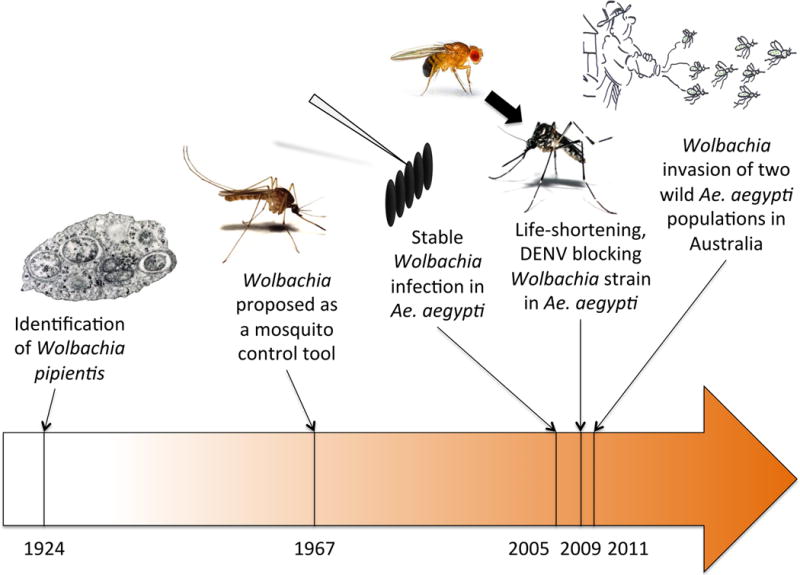
The timeline shows major achievements over the last century that have supported the development of Wolbachia as a dengue control tool.
A resurgence of interest for Wolbachia-based strategies to control vector-borne diseases occurred about a decade ago with the advent of transinfection techniques (Figure 1). Stable Wolbachia infections in naïve hosts can now be established by embryonic microinjections into the developing embryo germline. In general, Wolbachia transinfection is more likely to be successful between closely related donor and recipient hosts, and the expression of Wolbachia-induced phenotypes is conserved across hosts. In 2005, a stable infection by a Wolbachia strain from the mosquito Aedes albopictus was established in Ae. aegypti, which caused high rates of CI and rapidly spread to high frequencies in experimental populations.13 This was quickly followed by double transinfections of Ae. aegypti with two different Wolbachia strains from Ae. albopictus.14
A second wave of breakthroughs occurred a few years later with the discovery of Wolbachia-induced phenotypes in mosquitoes that had a direct effect on pathogen transmission (Figure 1). Until then Wolbachia was primarily considered a gene drive system, but the possibility to transinfect Wolbachia strains from more distant hosts by cell culture adaptation prior to microinjection,15 combined with the wide diversity of available Wolbachia strains and properties, resulted in new mosquito-Wolbachia associations. Stable introduction of a life-shortening strain of Wolbachia from Drosophila into Ae. aegypti halved the adult mosquito life-span under laboratory conditions, making mosquitoes unlikely to live long enough to transmit DENV.16 More importantly, this life-shortening Wolbachia strain directly inhibited the ability of a range of pathogens, including DENV, to infect and replicate in Ae. aegypti.17 Finally, semi-field and field trials in Australia demonstrated that Wolbachia can be persistently established in wild Ae. aegypti populations.18,19 Together, these properties form the basis of a practical approach for suppression of DENV transmission through field release of Wolbachia-infected mosquitoes.
Current status of Wolbachia deployment for dengue control
The critical next step is to assess the efficacy of medium-scale Wolbachia deployment in reducing human DENV infection. The gold standard, a cluster-randomized trial (CRT) of Wolbachia, has been considered in detail previously.20 CRT is a type of randomized controlled trial in which groups of subjects, instead of individual subjects, are randomly assigned to the alternative treatments under study. The CRT design is particularly useful when the intervention cannot be directed toward selected individual subjects, such as the release of Wolbachia-infected mosquitoes. In the classical two-armed CRT, clusters without intervention provide contemporaneous controls. In a stepped wedge CRT, the intervention is rolled-out sequentially to all the clusters so that the clusters are their own controls over time.
We believe that at this time a CRT is premature for the Wolbachia-based approach for several reasons. First, there are multiple strains of Wolbachia available for deployment, each with its own characteristic effects on DENV blocking and mosquito fitness. A process of selection through field-testing is still required before one or more final strain(s) can be chosen for a particular release area. In addition, while deployment in North Queensland has provided a basic template for release, this environment differs substantially from the large urban centers in Southeast Asia and Latin America where a CRT would likely be carried out. It is crucial to retain the capacity to learn during deployment about the effectiveness of release strategies and community engagement and to adjust practice accordingly. Past examples of adaptive changes made during deployment include releasing larger numbers of mosquitoes, changing the intensity of trap grids to monitor Wolbachia spread, supplementing releases with different mosquito developmental stages, and altering locations of deployment based on community concerns.18,21 In contrast, the standard CRT approach would lock-in all aspects of the release, preventing ‘on the fly’ improvements in design. Finally, a classical two-armed CRT would have to be large, with >40 clusters that each include approximately 100 study subjects who are monitored for infection to detect a 50% reduction in dengue with 90% power.20 Rough estimates of cost for such a design suggest it would exceed 5–10 million USD.
A pragmatic approach to optimize Wolbachia deployment
Here, we argue that well-designed observational studies could provide a suite of valuable indirect evidence that supports Wolbachia as a dengue intervention and, hence, justifies continued development, ultimately leading to a definitive efficacy trial. Ideally, several observational studies would be conducted in different settings and their outcomes combined in a meta-analytic framework to assess the impact on disease and infection incidence. Below we describe five possible approaches that could be used separately or in combination for acquiring such evidence.
Pediatric cohort study
A prospective longitudinal cohort study that tracks seroconversion rates in children could measure both the true incidence of DENV infections and the relative risk of infection between Wolbachia-treated and untreated areas.20 Because the overall DENV seroconversion rate is generally 5–10% per annum in endemic countries,22 a cohort would need to include at least several thousand individuals to be compatible with the statistical requirements of a CRT with sufficient power to detect a moderate intervention effect.20 A smaller cohort of 1,000–1,500 children, although underpowered in the context of a CRT, could be significantly enhanced by the concurrent approaches described below. Fine-scale entomological surveillance (e.g., a grid of traps) would allow monitoring the spatiotemporal dynamics of Wolbachia prevalence to distinguish, in real time, Wolbachia-free areas from areas where Wolbachia had established. The raw entomological data could be interpolated over time and space using standard methodology and serve as a covariate for DENV seroconversion. As in other epidemiological investigations, participants residing in the study area, but acquiring infections outside of the intervention area, represent a complication to this approach.23,24 However, geographical cluster studies of dengue cases and fine-scale spatiotemporal phylogenetic analyses of genomic DENV strain sequences (see below) would help to address this concern.
Geographical cluster investigation
DENV infections are acute, often mild, inapparent or with non-specific signs and symptoms, and thus are difficult to detect across populations in real time. Active surveillance of human infections can be efficiently achieved using geographical cluster sampling around dengue index cases.25,26 Here, ‘index case’ refers to the laboratory-diagnosed clinical dengue case that initiates a cluster investigation within a geographically restricted area around the home of a person with a documented DENV infection. Geographical cluster investigations could be used to compare the fine-scale spatial signature of DENV transmission in areas with and without Wolbachia (Figure 2). This methodology would test the hypothesis that concurrent and/or subsequent infections around an index case are reduced in areas where Wolbachia-infected mosquitoes are established. Inward migration of dengue infections acquired outside the treatment area would also be a confounding factor,23,24 although again potentially resolvable through detailed phylogenetic analysis of virus sequences and/or monitoring movement patterns of study participants. Nonetheless, if a Wolbachia intervention reduces local transmission at a micro-scale, it should be detectable by a cluster investigation methodology. An efficacious intervention would result in a lower overall number of index cases in the Wolbachia-treated areas and/or reduction in concurrent infections measured by a lower frequency of cases that are spatiotemporally linked to the index case.
Figure 2. Geographical cluster methodology.
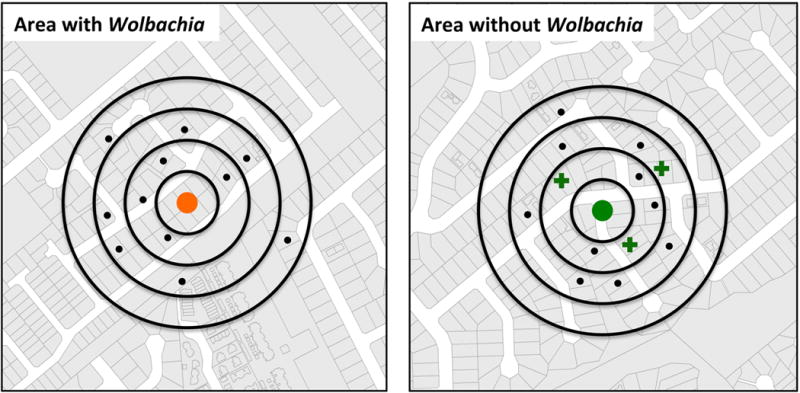
The central dot represents the home of a confirmed dengue case (orange: area with Wolbachia; green: area without Wolbachia). People living within a 100-m radius (black dots) are screened for concomitant and/or secondary DENV infection (crosses denote homes of additional DENV-infected individuals).
Virus sequence analysis
Increasing access to viral genome sequence data has promoted the development of new methodologies to infer dengue epidemiological dynamics based on analyses of changing patterns in viral genetic diversity in time and space.27,28 Assuming that multiple lineages of various DENV serotypes co-circulate prior to an intervention, a reduction in local DENV transmission is expected to result in a decrease in viral genetic diversity across serotypes in the intervention area due to a major viral demographic bottleneck, and in an increase in the average dispersion distances travelled by DENV into the intervention area (Figure 3). Phylogenetic analysis provides a simple means to identify importation of ‘foreign’ viral lineages into the study area, provided that genetic diversity accumulates at a sufficiently high rate. Previous studies on DENV microevolution in Southeast Asia suggested that spatial patterns of genetic diversity are shaped by frequent virus immigration and highly focal transmission.28–30 Although the level of phylogenetic resolution to be obtained is uncertain, deep sequencing methods have recently undergone dramatic improvement, increasing the power of this approach. We expect that if local DENV transmission is reduced in Wolbachia-treated areas, some viruses will continue to be imported by human-mediated dispersal but will not persist locally, reducing the strong spatial clustering that is typically observed in DENV phylogenies.
Figure 3. Schematic representation of how Wolbachia intervention might change patterns of virus genetic diversity.
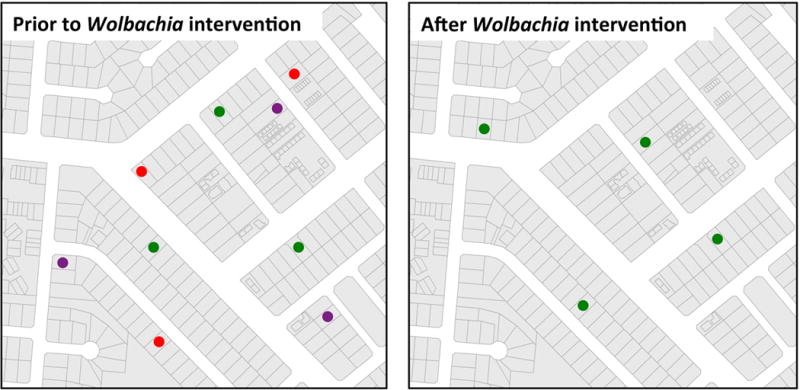
Assuming that multiple lineages of various DENV serotypes (colored dots) co-circulate prior to the intervention, a reduction in local DENV transmission is expected to result in a decrease in viral genetic diversity in the intervention area and a relative increase in the average dispersion distances.
Virus detection in mosquitoes
The release of Wolbachia-infected mosquitoes will require monitoring of the local Ae. aegypti population for changes in Wolbachia frequency and possibly in mosquito density. Recently, several sampling methods that effectively capture female Ae. aegypti have been developed.31–34 Virus detection could be combined with routine molecular tests for Wolbachia presence. Detecting DENV-infected Ae. aegypti mosquitoes is challenging because of the low infection rates (typically ~0.1%) in the adult females across the population, although infection rates can be higher in locations of geographical cluster investigations.25 Because mosquitoes that test positive for virus are not necessarily infectious, the proportion of DENV-infected mosquitoes does not directly translate into an estimate of virus transmission unless virus disseminated from the mosquito midgut or in saliva is also assayed, and even this approach is limited by the sensitivity of assays and variation of in vitro saliva collections. Nonetheless, a successful intervention is expected to reduce the incidence of viremic and infectious humans and, therefore, similarly reduce the incidence of DENV infection in mosquitoes in areas where Wolbachia infection predominates.
Vector competence assays
Following the release of Wolbachia-infected mosquitoes, it will be necessary to verify that the phenotype of reduced vector competence is maintained over time in field-collected mosquitoes.35 Vector competence assays consist of experimentally exposing laboratory-reared mosquitoes to either an artificial infectious blood meal or the blood of a viremic person.36 The proportion of infectious mosquitoes (i.e., with virus detected in saliva) is then measured over time. Wolbachia-infected mosquitoes have a strongly reduced ability to deliver DENV in their saliva compared to Wolbachia-free mosquitoes.19 Ideally, vector competence experiments would be extended to human-to-mosquito-to-human transmission experiments in a human challenge model.37 Vector competence assays will provide additional indirect evidence on the impact of the intervention, especially if the virus interference effect is strong.
Conclusions and perspectives
The current challenge is to convert a promising strategy into a validated public health intervention through rigorous assessment of its epidemiological impact. The suite of approaches described above is not a substitute for a CRT. Nonetheless, this strategy has at least two major strengths that can lay the foundations for a future CRT. First, the proposed investigations are not dependent on the uniform application of the intervention, which by nature will vary through time and space. Instead, an association between Wolbachia presence and proxies of DENV transmission (e.g., DENV seroconversion or occurrence of secondary cases around index cases) can be inferred dynamically from the spatiotemporal correlation between these factors. Second, comprehensive observation and detection of correlations between multiple environmental and biological factors will likely improve fundamental understanding of dengue epidemiology that will inform and underpin future trial designs. A multi-pronged approach would also help to evaluate potential impacts on other Ae. aegypti-borne arboviruses (e.g., chikungunya virus), and the likelihood of unexpected outcomes such as viral evolution to escape the inhibitory effects of Wolbachia, or other unanticipated, adverse events.
Measuring the epidemiological impact of a Wolbachia deployment to reduce DENV transmission is challenging. The intervention is not based on individuals, as a vaccine trial would be, but on populations defined by spatial areas. The fundamental test of the impact of the intervention is a comparison between areas where Wolbachia-infected mosquitoes are present versus areas where they are not (Figures 2, 3). Although limited dispersal of Ae. aegypti38 and, therefore, spread of Wolbachia, is expected to maintain spatial delineation of the intervention, a buffer zone will be necessary to avoid unanticipated overlap between treatment and control areas. The intervention needs to be deployed over a large enough geographic area to ensure that a sufficient number of dengue cases (or absence of cases if the intervention is effective) is captured. Prior knowledge of the study area will help to assign intervention and control areas with similar baseline transmission trends. Virus importation into the intervention area (through human-mediated dispersal23,24), which is likely to occur and may reduce the signal-to-noise ratio, can be explored with geographic cluster studies and by accounting for movement of study subjects.
One advantage of our proposed approach is that interpretation of seroconversion data from a small-scale pediatric cohort can be enhanced by data from geographical cluster investigations, viral sequencing and virus detection in mosquitoes, collectively resulting in a body of evidence that could support continued development of Wolbachia as public health tool. In any case, virus importation by study participants exposed to infected mosquitoes outside of the treatment area would result in false positive cases in the Wolbachia-treated area and conservatively lead to an underestimation of efficacy. A true placebo treatment (i.e., release of Wolbachia-free mosquitoes) is not ethically possible. The human and mosquito samples can, however, be blinded prior to laboratory testing.
We have described a pragmatic approach for evaluation of novel entomological interventions for dengue control through a coordinated, cross-disciplinary, ecological study that combines several proxies of efficacy at the epidemiological, entomological and virological levels. It relies on a combination of methodologies that have been successfully used to monitor dengue epidemiological dynamics, as well as novel methodologies. Although this approach has no precedent for dengue, it has the potential to provide valuable intermediate evidence of efficacy that supports the Wolbachia methodology and justifies funding for a CRT or deployment.
Acknowledgments
We thank Stephanie James and Fil M. Randazzo for their insights, and two anonymous reviewers for helpful comments. This work was supported by a grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative of the Bill and Melinda Gates Foundation.
Footnotes
Contributors
LL wrote the first draft of the manuscript. All other authors contributed equally to edit the manuscript.
Conflicts of interest
We declare that we have no conflict of interest
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 3.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5:e68. doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGraw EA, O’Neill SL. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol. 2013;11:181–93. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 5.James S, Simmons CP, James AA. Ecology. Mosquito trials. Science. 2011;334:771–2. doi: 10.1126/science.1213798. [DOI] [PubMed] [Google Scholar]
- 6.Iturbe-Ormaetxe I, Walker T, O’Neill SL. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011;12:508–18. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertig M, Wolbach SB. Studies on Rickettsia-like micro-organisms in insects. J Med Res. 1924;44:329–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?–A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–20. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–51. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 10.Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967;216:383–4. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- 11.Curtis CF. Population replacement in Culex fatigans by means of cytoplasmic incompatibility. 2. Field cage experiments with overlapping generations. Bull World Health Organ. 1976;53:107–19. [PMC free article] [PubMed] [Google Scholar]
- 12.Baldini F, Segata N, Pompon J, Marcenac P, Robert Shaw W, Dabire RK, et al. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nature Commun. 2014;5:3985. doi: 10.1038/ncomms4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi Z, Khoo CC, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–8. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 14.Ruang-Areerate T, Kittayapong P. Wolbachia transinfection in Aedes aegypti: a potential gene driver of dengue vectors. Proc Natl Acad Sci U S A. 2006;103:12534–9. doi: 10.1073/pnas.0508879103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMeniman CJ, Lane AM, Fong AW, Voronin DA, Iturbe-Ormaetxe I, Yamada R, et al. Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl Environ Microbiol. 2008;74:6963–9. doi: 10.1128/AEM.01038-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–4. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 17.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–78. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–7. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 19.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–3. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 20.Wolbers M, Kleinschmidt I, Simmons CP, Donnelly CA. Considerations in the design of clinical trials to test novel entomological approaches to dengue control. PLoS Negl Trop Dis. 2012;6:e1937. doi: 10.1371/journal.pntd.0001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris AF, McKemey AR, Nimmo D, Curtis Z, Black I, Morgan SA, et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nature Biotechnol. 2012;30:828–30. doi: 10.1038/nbt.2350. [DOI] [PubMed] [Google Scholar]
- 22.Endy TP, Yoon IK, Mammen MP. Prospective cohort studies of dengue viral transmission and severity of disease. Curr Top Microbiol Immunol. 2010;338:1–13. doi: 10.1007/978-3-642-02215-9_1. [DOI] [PubMed] [Google Scholar]
- 23.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A. 2013;110:994–9. doi: 10.1073/pnas.1213349110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoddard ST, Morrison AC, Vazquez-Prokopec GM, Paz Soldan V, Kochel TJ, Kitron U, et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis. 2009;3:e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJ, et al. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012;6:e1730. doi: 10.1371/journal.pntd.0001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes M, Mercado JC, Standish K, Matute JC, Ortega O, Moraga B, et al. Index cluster study of dengue virus infection in Nicaragua. Am J Trop Med Hyg. 2010;83:683–9. doi: 10.4269/ajtmh.2010.10-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabaa MA, Ty Hang VT, Wills B, Farrar J, Simmons CP, Holmes EC. Phylogeography of recently emerged DENV-2 in southern Viet Nam. PLoS Negl Trop Dis. 2010;4:e766. doi: 10.1371/journal.pntd.0000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghwani J, Rambaut A, Holmes EC, Hang VT, Hien TT, Farrar J, et al. Endemic dengue associated with the co-circulation of multiple viral lineages and localized density-dependent transmission. PLoS Pathog. 2011;7:e1002064. doi: 10.1371/journal.ppat.1002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarman RG, Holmes EC, Rodpradit P, Klungthong C, Gibbons RV, Nisalak A, et al. Microevolution of dengue viruses circulating among primary school children in Kamphaeng Phet, Thailand. J Virol. 2008;82:5494–500. doi: 10.1128/JVI.02728-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabaa MA, Klungthong C, Yoon IK, Holmes EC, Chinnawirotpisan P, Thaisomboonsuk B, et al. Frequent in-migration and highly focal transmission of dengue viruses among children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis. 2013;7:e1990. doi: 10.1371/journal.pntd.0001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrera R, Mackay AJ, Amador M. An improved trap to capture adult container-inhabiting mosquitoes. J Am Mosq Control Assoc. 2013;29:358–68. doi: 10.2987/13-6343.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krockel U, Rose A, Eiras AE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J Am Mosq Control Assoc. 2006;22:229–38. doi: 10.2987/8756-971X(2006)22[229:NTFSOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie SA, Buhagiar TS, Townsend M, Hoffmann A, Van Den Hurk AF, McMahon JL, et al. Field validation of the gravid Aedes trap (GAT) for collection of Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2014;51:210–9. doi: 10.1603/me13105. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J Med Entomol. 2009;46:1256–9. doi: 10.1603/033.046.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen N, Thi Hue Kien D, Tuan T, Quyen N, Tran C, Vo Thi L, et al. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2013;110:9072–7. doi: 10.1073/pnas.1303395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas SJ. Dengue human infection model: re-establishing a tool for understanding dengue immunology and advancing vaccine development. Hum Vaccin Immunother. 2013;9:1587–90. doi: 10.4161/hv.24188. [DOI] [PubMed] [Google Scholar]
- 38.Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–20. [PubMed] [Google Scholar]


