Structured Summary
Objective
Low testosterone concentrations have been reported to be associated with increased risk of congestive heart failure, but the mechanisms are unclear. Our objectives was to examine the relationship between endogenous testosterone and measures of cardiac mass and function among men with type 1 diabetes.
Design
Secondary analysis of a prospective observational study.
Participants
Men (n=508) in the Epidemiology of Diabetes Interventions and Complications (EDIC) study, the observational follow-up of the Diabetes Control and Complications Trial.
Measurements
Testosterone assessed by liquid chromatography mass spectrometry at EDIC year 10 and cardiac magnetic resonance imaging (CMR) measures at EDIC years 14/15. Linear regression models were used to assess the relationship between testosterone, sex hormone binding globulin (SHBG) and left ventricular (LV) mass, volume, ejection fraction, and cardiac index before and after adjustment for age, randomization arm, alcohol and cigarette use, macroalbuminuria, hemoglobin A1c, insulin dose, body mass index, lipids, blood pressure, use of anti-hypertensive medications, and microvascular complications.
Results
In fully-adjusted models, total testosterone concentrations were significantly associated with LV mass (p=0.014), end-diastolic volume (p=0.002), end-systolic volume (p=0.012), and stroke volume (p=0.022) but not measures of LV function after adjustment for cardiac risk factors. Bioavailable testosterone was associated with LV mass but not volume or function, while SHBG was associated with volume but not mass or function.
Conclusions
Among men with type 1 diabetes, higher total testosterone was associated with higher LV mass and volume but not with function. The clinical significance of this association remains to be established.
Keywords: cardiac magnetic resonance imaging, testosterone, diabetes mellitus, sex hormones
Men with type 1 diabetes have a cardiovascular disease (CVD) mortality risk that is approximately 10 times that of men without diabetes.1 This may be due, in part, to abnormalities in ventricular mass, volume, or function.2 Although many studies have not noted strong correlations between hypertension and testosterone levels,3 at least one report has noted that low concentrations of endogenous testosterone correlate with higher blood pressure levels4 which would increase risk for left ventricular (LV) hypertrophy5 and heart failure.6 Through associations with CVD risk factors7 or apart from these risk factors,8 low testosterone concentrations have also been associated with increased prevalence of subclinical atherosclerosis and thus could increase risk of heart failure through ischemia.
However, few reports have actually examined the association between endogenous testosterone and measures of cardiac mass and function, and these reports conflict. In an examination of right ventricular mass and function, lower testosterone concentrations correlated with lower right ventricular mass and volume.9 In contrast, two other reports have noted that lower testosterone was associated with higher LV mass,10, 11 and lower testosterone was associated with higher, rather than lower, ejection fraction.11 A recent report noted no association between endogenous testosterone and incident congestive heart failure by death certificate or hospital discharge diagnosis.12 While exogenous testosterone may directly induce cardiac myocyte hypertrophy, apart from CVD risk factors,13 randomized trials in healthy younger men suggest that exogenous testosterone has little effect on cardiac mass and function as assessed on echocardiography.14 Of note, length of treatment and follow-up was limited, and therefore it is difficult to draw any conclusions about the effect of ongoing testosterone therapy on cardiac function. No reports examine the association between endogenous testosterone and measures of cardiac mass and function among men with type 1 diabetes.
Therefore, we examined the associations between endogenous testosterone concentrations and measures of cardiac mass and function in the Diabetes Control and Complications Trial (DCCT) follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study. This large, well-characterized cohort of adults with type 1 diabetes previously reported measurements of cardiac mass and function as assessed by cardiac magnetic resonance imaging (CMR).2 We hypothesized that lower concentrations of endogenous testosterone would be associated with poorer cardiac mass and function. We also investigated whether any associations persisted after adjustment for conventional CVD risk factors.
Methods
Population and Setting
The DCCT has been described in detail.15 Briefly, the DCCT was a multicenter, randomized clinical trial designed to compare the impact of intensive and conventional diabetes treatment on the development and progression of early microvascular complications of type 1 diabetes.15 The DCCT included a primary prevention cohort and a secondary intervention cohort. The primary prevention cohort consisted of 726 subjects with no retinopathy, urinary albumin excretion rate < 40 mg/24 hours, and diabetes duration of 1–5 years at DCCT baseline. The secondary intervention cohort consisted of 715 subjects who had non-proliferative retinopathy, urinary albumin excretion rate ≤ 200 mg/24 hours, and diabetes duration of 1–15 years. Individuals were excluded if they had hypertension, significant dyslipidemia, were taking any blood pressure or lipid-lowering medications, or had a history of symptomatic ischemic heart disease or symptomatic peripheral neuropathy. Participants were followed for 3 to 9 years (mean 6.5 years), and at the end of the trial, intensive therapy was recommended for all participants. Annual observational follow-up of the DCCT cohort, the EDIC study, began in 1994 with 96% retention of the original cohort.16 By EDIC year 10, 97% of intensive and 94% of conventional treatment arm participants were implementing intensive therapy and similar levels of HbA1c had been attained.17 DCCT/EDIC procedures were approved by institutional review boards of all participating centers. Written informed consent was provided by all participants.
Data Collection
Clinical and biochemical endpoints were obtained annually by history, physical exam, and laboratory testing.16 Body mass index (BMI), insulin dosage, blood pressure, and HbA1c were assessed at randomization and quarterly during DCCT and annually in EDIC.18 Lipid profiles and urinary albumin excretion rates (AER) were obtained on alternate years. Medication use was assessed at each exam by EDIC staff. Alcohol use was self-reported and defined as consumption of an average of at least 1 alcoholic beverage (12 ounces of beer, 4 ounces of wine, or 1.5 ounces of hard liquor) per week in the past year. Cigarette use was also self-reported and smoking was defined as reporting any cigarette use currently.
All men enrolled in EDIC (n=641) were invited to participate in the UroEDIC study, an ancillary study designed to examine urologic complications. Of these participants, 628 had available stored serum and during the 10th year of EDIC follow-up and underwent measurements of total testosterone and sex hormone binding globulin (SHBG).17 Samples were obtained in the morning when participants were fasting. A rapid liquid chromatography-tandem mass spectrophotometry assay with a lower limit of detection of 0.25 nmol/l was used; the assay was certified by the Centers for Disease Control Hormone Standardization Program (http://www.cdc.gov/labstandards/hs.html) and passed intra-assay performance criterion of 6.4%. Intra-assay mean bias was 4.6% at 6.9 nmol and 1.8% at 34.9 nmol. Serum sex hormone binding globulin (SHBG) was measured on a Roche Elecsys 2010 analyzer using a sandwich immunoassay method, standardized against the first International Standard for SHBG from the National Institute for Biological Standards and Control code 95/560. The laboratory coefficient of variation was 3.0% at a concentration of 25.38 nmol/l. Calculated bioavailable testosterone was determined using the Vermeulen formula i.e. assuming a binding constant to albumin of 3.6 × 104 and a binding constant to SHBG of 10.19 At year 10, only 25 men reported using hormones that were not thyroid hormones or glucocorticoids.
CMR imaging in EDIC has been previously described.2 Briefly, CMR was performed during EDIC years 14/15 (2007-2009) at 27 centers with 1.5-T magnets, except for 1 center that had a 3-T magnet (Espree or Avanto, Siemens Medical Systems, Erlangen, Germany; Intera, Philips Medical Systems, Best, the Netherlands; Signa, GE Medical Systems, Waukesha, WI). CMR studies were centrally evaluated by readers blinded to all other study data. Briefly, a stack of short axis images covering the entire left ventricle was acquired to determine measures of mass, volume, and function.2 Endocardial and epicardial myocardial borders were contoured using a semiautomated method (QMASS software, version 6, Medis, Leiden, Netherlands), and LV mass was calculated as the difference between epicardial and endocardial areas for all slices multiplied by slice thickness and slice gap, then multiplied by the specific gravity of the myocardium (1.04 g/ml). LV end-diastolic volume and end-systolic volume were calculated using the summation of areas on each separate slide multiplied by the sum of slice thickness and image gap, and stroke volume was calculated as the difference between end-diastolic and end-systolic volume. Ejection fraction was calculated using systolic volume divided by diastolic volume and multiplied by 100 (percent). Cardiac output was calculated as systolic volume multiplied by the heart rate. Previous analyses of the CMR measures in the EDIC cohort did not note significant impact of indexing CMR measures to body surface area among men,2 and therefore we use the CMR measures that are not indexed to body surface area. Less than 5% of participants had myocardial scarring with gadolinium imaging, so separate analyses were not performed for this subgroup of participants. Fifty-nine of the 508 men with total testosterone measurements as well as CMR measures had limited serum sample volume and did not undergo measurement of SHBG (Figure 1), and thus bioavailable testosterone; these men were similar in age and microvascular complications to men who did undergo SHBG measurements (results not shown).
Figure 1.
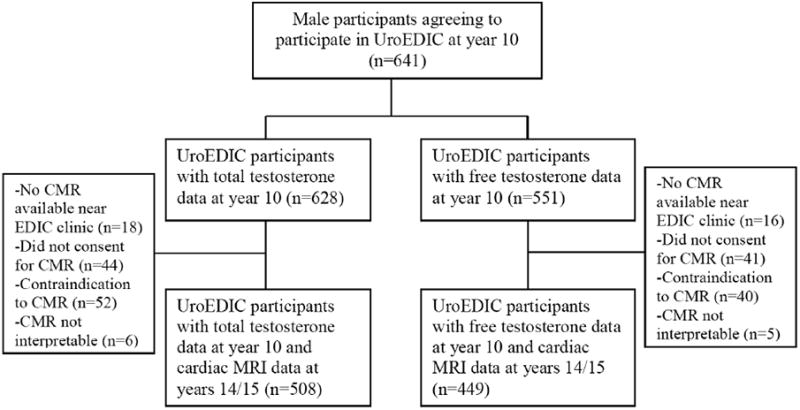
Flow diagram of Uro Epidemiology of Diabetes Interventions and Complication participants who underwent cardiac magnetic resonance imaging.
Statistical Analysis
Testosterone concentrations by sociodemographic, clinical, and diabetes-related characteristics were described at EDIC year 10. Separate multivariable linear regression models were built to estimate the associations between testosterone concentrations (independent variable) at EDIC year 10 and continuous CMR measures (dependent variable) at EDIC year 14/15. Due to previous reports modeling a non-linear relationship between testosterone and CVD events,20 we used quantile regression to determine if the effect of testosterone was homogeneous among quantiles of CMR;21 the effects were similar in the top and bottom quantile for each CMR measure. We did not assess whether testosterone concentrations consistent with hypogonadism were associated with CMR measures as only 26 participants had total testosterone concentrations < 10.4 nmol/l, a cutpoint commonly used to define hypogonadism.22 Therefore, testosterone was modeled as a continuous exposure.
Covariates included in the models were chosen based upon previous analyses of CMR in EDIC and values were selected that were concurrent with testosterone2 and potentially important risk factors including age (years), primary vs. secondary cohort, DCCT treatment arm (conventional vs. intensive therapy), smoking status at year 10 (yes/no), alcohol use at year 10 (at least one alcoholic beverage in the last week vs. none), any history of macroalbuminuria (albumin excretion ratio ≥ 300 mg/24 hours), glycemic control (HbA1c), insulin dose (units/kg/day), BMI (kg/m2), lipids (low-density lipoprotein cholesterol [LDL] and high-density lipoprotein cholesterol [HDL]), systolic blood pressure (SBP), and use of hypertensive medication. Previous EDIC reports have noted that SBP was highly correlated with DBP and had a stronger association with LV measures, so only SBP was included in these models.2 For levels of glycemic control, insulin dosage, blood pressure, and lipid levels, time-weighted values were used, which represent the running arithmetic means up to and including each study visit in the DCCT and EDIC. The quarterly DCCT and annual EDIC values were weighted by 3 and 12 months, respectively. We used these time-weighted values to determine whether the average value of the covariate from the time of randomization had greater influence on CMR than a single value of testosterone at year 10. Similar models were constructed using bioavailable testosterone as the primary independent variable. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant characteristics are shown in Table 1. At the time of testosterone measurement at year 10, participants had a mean age of 45 years. Approximately half reported drinking at least one alcoholic beverage per week, and only 12% reported current cigarette use. The majority of participants were overweight but had systolic blood pressure levels less than 130 mm Hg. At the time of the CMR measurement, LV mass, volumes, and ejection fraction, and output were consistent with normal cardiac size and function.
Table 1.
Characteristics of male participants at EDIC year 10 (n=508), means (SD) or n (%).
| Total testosterone (nmol/l) | 20.9 (7.1) |
| Bioavailable testosterone (nmol/l) | 0.34 (0.08) |
| Sex hormone binding globulin (nmol/l) | 55.3 (23.6) |
| Age (years) | 45 (6) |
| Randomization arm (n,%) | |
| Conventional | 259 (51%) |
| Intensive | 249 (49%) |
| Primary cohort (n,%) | 257 (51%) |
| Current smoking (n,%) | 59 (12%) |
| Current alcohol use (n,%) | 260 (51%) |
| Body mass index (n,%) | |
| < 25 kg/m2 | 173 (34%) |
| 25-29.9 kg/m2 | 288 (57%) |
| ≥30 kg/m2 | 47 (9%) |
| Time-weighted body mass index (kg/m2) | 26.1 (2.8) |
| Time-weighted insulin dose (units/kg/day) | 0.7 (0.2) |
| Time-weighted DCCT/EDIC HbA1c (mmol/mol) | 64 (11) |
| Anti-hypertensive medication use (%) | 279 (55%) |
| Time-weighted systolic blood pressure (mm Hg) | 120 (8) |
| Time-weighted low-density lipoprotein cholesterol (mg/dl) | 114 (23) |
| Time-weighted high-density lipoprotein cholesterol (mg/dl) | 49 (11) |
| History of albumin excretion ratio ≥ 300 mg/24 hours (n, %) | 49 (10%) |
| Left ventricular mass index (g/m2) | 76.5 (12.3) |
| End-diastolic volume (ml/m2) | 73.3 (12.6) |
| End-systolic volume (ml/m2) | 29.2 (7.8) |
| Stroke volume index (ml/m2) | 44.1 (8.0) |
| Ejection fraction (%) | 60.3 (6.3) |
| Cardiac output (L• min-1•m-2) | 3.1 (0.6) |
| Left ventricular mass/end-diastolic volume (mg/ml) | 1.1 (0.2) |
Table 2 shows the associations between total testosterone and CMR measures. Patterns were similar in partially and fully-adjusted models. In fully-adjusted models, higher testosterone levels were associated with greater LV mass and end-diastolic volume, systolic volume, and stroke volume (Table 2). Of note, the strength of the associations between testosterone and LV mass (as indicated by the β-coefficient relative to the standard errors) were less pronounced than the strongest risk factor, time-weighted SBP, and LV mass in fully-adjusted models (p<0.0001). However, the magnitude of the effect size between testosterone and LV mass (as indicated by the ratio of the β-coefficient to standard error) was comparable to that of age (p=0.04), time-weighted hemoglobin A1c (p=0.01) and greater than that of current alcohol use (p=0.76) or smoking (p=0.35). Despite the relationships between testosterone level with LV mass and volume, testosterone was not associated with ejection fraction, cardiac output, or LV mass:end-diastolic volume after adjustment for covariates (Table 2).
Table 2.
Associations between testosterone and SHBG and CMR measures, β-coefficients (standard errors) and p-values.*
| Left-ventricular mass (g/m2) |
End-diastolic volume (ml/m2) |
End-systolic volume (ml/m2) |
Stroke volume (ml/m2) |
Ejection fraction (%) |
Cardiac output (L• min-1•m-2) |
Left ventricular mass/end- diastolic volume (mg/ml) |
|
|---|---|---|---|---|---|---|---|
| Total testosterone (ng/dl) | 0.0070 (0.0028) | 0.0093 (0.0032) | 0.0048 (0.0019) | 0.0045 (0.0019) | -0.0015 (0.0015) | 0 (0.0001) | 0.0001 (0) |
| p=0.014 | P=0.003 | p=0.012 | p=0.023 | p=0.339 | p=0.879 | p=0.568 | |
| Bioavailable testosterone (ng/dl) | 0.578 (0.253) | 0.063 (0.274) | 0.164 (0.169) | -0.10 (0.173) | -0.145 (0.134) | -0.0094 (0.013) | 0.0075 (0.0034) |
| p=0.023 | p=0.818 | p=0.332 | p=0.563 | p=0.279 | p=0.47 | p=0.026 | |
| Sex hormone binding globulin (nmol/l) | 0.0196 (0.0252) | 0.076 (0.027) | 0.037 (0.017) | 0.039 (0.017) | -0.014 (0.013) | 0.0004 (0.0013) | 0.0008 (0.0003) |
| p=0.437 | p=0.005 | p=0.03 | p=0.022 | p=0.30 | p=0.758 | p=0.025 |
Table 2 also shows the associations between bioavailable testosterone and CMR measures. Higher bioavailable testosterone was associated with higher LV mass and LV mass:end-diastolic volume in fully-adjusted models. In contrast to the models with total testosterone as the primary independent predictor, the associations between bioavailable testosterone and other CMR measures were largely insignificant.
Since the association between total testosterone, mass and volume was more pronounced than that between bioavailable testosterone, mass, and volume, we examined whether SHBG contributed to the associations observed between total testosterone and CMR measures. Of note, the correlation between total testosterone and SHBG was 0.60 (p<0.01). Table 2 shows the associations between SHBG and CMR measures after full adjustment (Table 2). SHBG was not associated with LV mass, although higher SHBG was associated with higher end-systolic and end-diastolic volume. We also created models that included both total testosterone and SHBG, as well as the other covariates; of note, in these models, the association between testosterone and LV mass (p=0.02) was more pronounced than that between SHBG and LV mass (p=0.52), while the association between SHBG and LV mass/end-diastolic volume was more pronounced for SHBG (p=0.02) than between testosterone and LV mass/end-diastolic volume (p=0.40). Neither SHBG nor testosterone was associated with measures of function, consistent with the high correlation between SHBG and testosterone resulting in reduction in associations between each hormone and CMR measure. Finally, we examined whether models including total testosterone without SHBG had better fit than models including SHBG without testosterone using F-values, with larger values corresponding to better fit. For LV mass, end-diastolic volume, and end-systolic volume, total testosterone had slightly higher F-values than SHBG values, suggesting again that the relationship between total testosterone and CMR measures was not better explained by SHBG, despite the slight difference in F-values (results not shown).
Discussion
In a well-characterized cohort of middle-aged men with well-controlled type 1diabetes, we found that lower endogenous total testosterone concentrations were associated with lower LV mass and volume using CMR data. Testosterone was not associated with cardiac function as represented by ejection fraction or cardiac output. These patterns persisted after adjustment for factors previously reported to be associated with LV mass, volume, and function, including age and CVD risk factors such as hypertension, as well as factors specific to our study population of men with type 1 diabetes including randomization to intensive insulin therapy and glycemic control. These associations suggest that lower testosterone concentrations may influence LV mass apart from standard CVD risk factors, and suggest a possible mechanism linking lower endogenous testosterone concentrations with higher CVD event rates that has been observed in some,23 but not all studies.24 However, the associations between bioavailable testosterone with CMR measures were largely not significant, suggesting that factors other than testosterone activity may account for the association. It is also possible that estimates of bioavailable testosterone do not accurately represent testosterone activity.
Only a few reports have examined the relationship between endogenous testosterone and measures of cardiac mass and volume. Ventetuolo and colleagues reported that higher bioavailable testosterone concentrations were associated with greater right ventricular mass and volume among men in the Multiethnic Study of Atherosclerosis, a result that parallels our findings between testosterone and LV mass.9 Of note, right ventricular mass and volume are subject to influences such as pulmonary pressures that may be of less relevance for the left ventricle. In contrast, an inverse relationship between testosterone and LV mass was noted in the Tromsø Study; however, associations were no longer significant after adjustment for obesity.10 Similarly, a Polish study reported that LV mass was not correlated with testosterone in approximately 100 middle-aged men.25 We may have found different relationships between testosterone and LV mass in the EDIC cohort due to the use of CMR, considered a more precise measure of cardiac function than echocardiography. Due to their lack of endogenous insulin, it is also possible that men with type 1 diabetes have different relationships between testosterone and cardiac mass and volume.
Similarly, few reports have examined whether endogenous testosterone is associated with measures of cardiac function. We found no significant associations between testosterone and ejection fraction or cardiac output. We also found that testosterone did not correlate with LV mass:end-diastolic volume, a marker of concentric hypertrophy observed in hypertensive heart failure.26 In contrast, lower testosterone was associated with lower ejection fraction in one series of Iranian men,27 with higher ejection fraction in a cohort of Norwegian men,11 and with neither increased nor decreased risk for congestive heart failure hospitalizations or mortality in another cohort.12 We may not have found an association for several reasons. We were able to adjust for an extensive list of CVD risk factors, as well as levels of glycemic control that may be particularly important among men with type 1 diabetes. However, despite their long duration of type 1 diabetes, the men in EDIC are middle-aged, were not obese, have mild degrees of impairment in CMR measures,2 and have had few CVD events.28 Thus, longer follow-up may reveal stronger relationships between testosterone and CMR measures. In contrast, Davoodi and colleagues examined a cohort of men referred for angiographic studies who had greater levels of impairment.27
The lack of association between bioavailable testosterone and CMR measures suggest that the associations between total testosterone and CMR measures were at least in part attributable to SHBG concentrations. The relationship between total testosterone and CMR measures was largely attenuated when SHBG was added to models including total testosterone, although models with total testosterone had slightly better fit than models with SHBG. Individuals with type 1 diabetes lack endogenous insulin, which down-regulates SHBG production by the liver.29 Administration of exogenous insulin bypasses the portal circulation and thus has relatively reduced impact upon SHBG concentrations compared to endogenous insulin.29 SHBG, the primary binding of testosterone, is inversely associated with bioavailable testosterone. It is controversial whether current methods of adjusting for SHBG adequately reflect active testosterone concentrations and which method is optimal,30 and whether associations observed between sex steroids and metabolic outcomes might reflect the amount of circulating SHBG rather than sex steroid activity.31 To our knowledge, no studies have examined whether SHBG is associated with incident heart failure or subclinical measures of function assessed by CMR. SHBG is associated with fat volume and adipokines, and while speculative, it is possible that these mechanisms, rather than testosterone, account for the relationship between total testosterone and CMR measures.32
Strengths and unique aspects of this report include its examination of a large number of adults at high-risk for CVD, men with type 1 diabetes; the use of state of the art methods to assess cardiac structure, i.e. cardiac magnetic resonance imaging; and the use of mass spectrometry to assess testosterone. Our study has several limitations. Although single measures of testosterone are thought to be an adequate indicator of androgen status, only a single measure of testosterone was obtained before CMR. Furthermore the measurement of SHBG utilized an “antibody” which may identify epitopes on more than one type of molecule. Although testosterone declines slowly at a rate of 1-2% per year, the decline can be variable and the time lapse between the measurement of testosterone and the assessment of CMR may have led to an underestimate of associations.22 We did not have additional measures of sex steroids, particularly estradiol, that would have enabled an assessment of the androgen/estrogen balance as a predictor of cardiac structure and function. We could not examine change in CMR measures or incident congestive heart failure, as the number of these events was too few. Although men with type 1 diabetes are at increased risk for CVD events, the EDIC cohort has been characterized by excellent glycemic control and management of risk factors, and thus has had fewer events than general populations of type 1 diabetes.28 Finally, we conducted a large number of comparisons, and it is possible that several associations are false-positive findings.
We conclude that in a cohort of middle-aged men with well-controlled type 1 diabetes, lower total testosterone concentrations are associated with smaller LV mass and volume. SHBG or other confounders may have contributed to the association between total testosterone and CMR measures. It is possible that as the EDIC cohort ages and testosterone and cardiac function decline, the relationship between testosterone and function may alter. Replication of these results among populations with and without type 1 diabetes are needed to determine whether these relationships between testosterone and CMR are unique to men with type 1 diabetes and whether testosterone is an independent risk factor for changes in cardiac mass and size. Future analyses include examination of the relationship between testosterone and incident CVD events among men with type 1 diabetes.
Acknowledgments
A complete list of participants in the DCCT/EDIC research group can be found in New England Journal of Medicine, 2011;365:2366-2376.
Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants' adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi-Aventis (Bridgewater NJ).
Funding: Financial support for this work provided by the NIDDK Diabetic Complications Consortium (DiaComp, www.diacomp.org), grant DK076169. The DCCT/EDIC has been supported by cooperative agreement grants (1982-1993, 2012-2017), and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA. Additional funding was provided by the Diabetes Complications Consortium. A complete list of participants in the DCCT/EDIC research group can be found in New England Journal of Medicine, N Engl J Med 2015 372:1722-33. Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants' adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi-Aventis (Bridgewater NJ).
Trial Registration: clinicaltrials.gov NCT00360815 and NCT00360893.
Footnotes
Financial disclosure/conflicts of interest: nothing to declare
Disclosures: None
Contributor Information
Ionut Bebu, Email: ibebu@bsc.gwu.edu.
Barbara Braffett, Email: braffett@bsc.gwu.edu.
Patricia A. Cleary, Email: cleary@bsc.gwu.edu.
Valerie Arends, Email: aren0085@umn.edu.
Michael Steffes, Email: steff001@umn.edu.
Hunter Wessells, Email: wessells@uw.edu.
Trevor Orchard, Email: orchardt@edc.pitt.edu.
Aruna V. Sarma, Email: asarma@umich.edu.
References
- 1.Orchard T, Dorman J, Maser R, Becker D, Drash A, Ellis D, LaPorte R, Kuller L. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 2.Turkbey E, Backlund J, Genuth S, Jain A, Miao C, Cleary P, Lachin J, Nathan D, van der Geest R, Soliman E, Liu C, Lima J, Bluemke D, Group DER. Myocardial structure, function, and scar in patients with type 1 diabetes mellitus. Circulation. 2011;124:1737–1746. doi: 10.1161/CIRCULATIONAHA.111.022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad R, Kennedy C, Caples S, Tracz M, Bolona E, Sideras K, Uraga M, Erwin P, Montori V. Testosterone and cardiovascular risk in men: a systemic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;81:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 4.Guder G, Allolio B, Angermann C, Stork S. Androgen deficiency in heart failure. Curr Heart Fail Rep. 2011;8:131–139. doi: 10.1007/s11897-011-0053-9. [DOI] [PubMed] [Google Scholar]
- 5.Kannel W, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence, and mortality in the Framingham Study. Ann Intern Med. 1969;71:89–105. doi: 10.7326/0003-4819-71-1-89. [DOI] [PubMed] [Google Scholar]
- 6.Kannel W, Castelli W, McNamara P, McKee P, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham Study. N Engl J Med. 1972;287:781–787. doi: 10.1056/NEJM197210192871601. [DOI] [PubMed] [Google Scholar]
- 7.Makimura H, Stanley T, Sun N, Connelly J, Hemphill L, Grinspoon S. The relationship between reduced testoserone, stimulated growth hormone secretion and increased carotid intima-media thickness in obese men. Clin Endocrinol (Oxf) 2010;73:622–629. doi: 10.1111/j.1365-2265.2010.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller M, Van den Beld A, Bots M, Grobbee D, Lamberts S, van der Schouw Y. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109:2074–2079. doi: 10.1161/01.CIR.0000125854.51637.06. [DOI] [PubMed] [Google Scholar]
- 9.Ventetuolo C, Ouyang P, Bluemke D, Tandri H, Barr R, Bagiella E, Cappola A, Bristow M, Johnson C, Kronmal R, Kizer J, Lima J, Kawut S. Sex hormones are associated with right ventricular structure and function: the MESA-right ventricular study. Am J Respir Crit Care Med. 2011;183:659–667. doi: 10.1164/rccm.201007-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svartberg J, von Muhlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosteorne with blood pressure and left ventricular mass in men. The Tromso Study. Eur J Endocrinol. 2004;150:65–71. doi: 10.1530/eje.0.1500065. [DOI] [PubMed] [Google Scholar]
- 11.Ruige J, Rietzschel E, De Buyzere M, Bekaert S, Segers P, De Bacquer D, De Backer G, Gillebert T, Kaufman J AsklepiosInvestigators. Modest opposite associations of endogenous testosterone and oestradiol with left ventricular remodeling and function in healthy middle-aged men. Int J androl. 2011;34:e587–593. doi: 10.1111/j.1365-2605.2011.01191.x. [DOI] [PubMed] [Google Scholar]
- 12.Srinath R, Golden S, Carson K, Dobs A. Endogenous testosterone and its relationship to preclinical and clinical measures of cardiovascular disease in the Atherosclerosis Risk on Communites (ARIC) Study. J Am Coll Cardiol. 2015 doi: 10.1210/jc.2014-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell J, Bernasochi G, Varma U, Raaijmakers A, Delbridge L. Sex and sex hormones in cardiac stress-mechanistic insights. J Steroid Biochem Mol Biol. 2013;137:124–135. doi: 10.1016/j.jsbmb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Chung T, Kelleher S, Liu P, Conway A, Kritharides L, Handelsman D. Effects of testosterone and nandrolone on cardiac function: a randomized, placebo-controlled study. Clin Endocrinol (Oxf) 2007;66:235–245. doi: 10.1111/j.1365-2265.2006.02715.x. [DOI] [PubMed] [Google Scholar]
- 15.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 16.Epidemiology of Diabetes Interventions and Complications (EDIC) Group. Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt S, Lopushnyan N, Hotaling J, Sarma A, Dunn R, Cleary P, Braffett B, Gatcomb P, Martin C, Herman W, Wessells H Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Prevalence of low testosterone and predisposing risk factors in men with type 1 diabetes mellitus: findings from the DCCT/EDIC. J Clin Endocrinol Metab. 2014;99:E1655–1660. doi: 10.1210/jc.2014-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffes M, Cleary P, Goldstein D, Little R, Wiedmeyer HM, Rohlfing C, England J, Bucksa J, Nowicki M. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem. 2005;51:753–758. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeulen A, Verdonck L, Kaufman J. A critical calculation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;86:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 20.Kim C, Cushman M, Kleindorfer D, Safford M, Redberg R, Lisabeth L. A review of the relationships between endogenous sex steroids and incident ischemic stroke and coronary heart disease events. Curr Cardiol Rev. 2015 doi: 10.2174/1573403X1103150515110749. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koenker R. Quantile regression. Cambridge University Press; Cambridge, England: 2005. [Google Scholar]
- 22.Araujo A, Esche G, Kupelian V, O'Donnell A, Travison T, Williams R, Clark R, McKinlay J. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin G, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haring R, Teng Z, Xanthakis V, Coviello A, Sullivan L, Bhasin S, Murabito J, Wallaschofski H, Vasan R. Association of sex steroids, gonadotrophins, and their trajectories with clinical cardiovascular disease and all-cause mortality in elderly men from the Framingham Heart Study. Clinical Endocrinology. 2013;78:629–634. doi: 10.1111/cen.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barud W, Makaruk B, Myslinski W, Palusinski R, Hanzlik J. Hypertension, and not sex hormones or insulin resistance, affects left ventricular mass in aging men. Ann Univ Mariae Curie Sklodowska Med. 2004;59:232–236. [PubMed] [Google Scholar]
- 26.Gaasch W, Zile M. Left ventricular structural remodeling in health and disease. J Am Coll Cardiol. 2011;58:1733–1740. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Davoodi G, Amirzadegan A, Boroumand M, Dehkordi M, Saeid A, Sharif A, Rezvanfard M, Anvari M. Association between androgenic hormone levels and left ventricular ejection fraction. J Tehran Heart Cent. 2010;5:141–145. [PMC free article] [PubMed] [Google Scholar]
- 28.Lachin J, Orchard T, Nathan D DCCT/EDIC Research Group. Update on cardiovascular outcomes at 30 years of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care. 2014;37:39–43. doi: 10.2337/dc13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yki-Jarvinen H, Makimattila S, Utrainen T, Rutanen E. Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone binding globulin and insulin-like growth factor binding protein 1 in vivo. J Clin Endocrinol Metab. 1995;80:3227–3232. doi: 10.1210/jcem.80.11.7593430. [DOI] [PubMed] [Google Scholar]
- 30.Shea J, Wong P, Chen Y. Free testosterone: clinical utility and important analytical aspects of measurement. Adv Clin Chem. 2014;63:59–84. doi: 10.1016/b978-0-12-800094-6.00002-9. [DOI] [PubMed] [Google Scholar]
- 31.Hsu B, Cumming R, Naganathan V, Blyth F, Le Couteur D, Seibel M, Waite L, Handelsman D. Associations between circulating reproductive hormones and SHBG and prevalent and incident metabolic syndrome in community-dwelling older men: the Concord Health and Ageing in Men Project. J Clin Endocrinol Metab. 2014;99:12e2686–2691. doi: 10.1210/jc.2014-2464. [DOI] [PubMed] [Google Scholar]
- 32.Laughlin G, Barrett-Connor E, May S. Sex-specific association of the androgen to oestrogen ratio with adipocytokine levels in older adults: the Rancho Bernardo Study. Clin Endocrinol (Oxf) 2006;65:506–513. doi: 10.1111/j.1365-2265.2006.02624.x. [DOI] [PubMed] [Google Scholar]


