Abstract
Objective
To evaluate complications after post-mastectomy breast reconstruction, particularly in the setting of adjuvant radiotherapy.
Summary-Background Data
Most studies of complications after breast reconstruction have been conducted at centers of excellence; relatively little is known about complication rates in radiated patients treated in the broader community. This information is relevant for breast cancer patients' decision-making.
Methods
Using the claims-based MarketScan database, we described complications in 14,894 women undergoing mastectomy for breast cancer from 1998-2007 who received immediate autologous reconstruction (n=2637), immediate implant-based reconstruction (n=3007), or no reconstruction within the first two postoperative years (n=9250). We used a generalized estimating equation to evaluate associations between complications and radiotherapy over time.
Results
Wound complications were diagnosed within the first two postoperative years in 2.3% of patients without reconstruction, 4.4% with implants, and 9.5% with autologous reconstruction (p<0.001). Infection was diagnosed within the first two postoperative years in 12.7% of patients without reconstruction, 20.5% with implants, and 20.7% with autologous reconstruction (p<0.001). 5219 (35%) women received radiation. Radiation was not associated with infection in any surgical group within the first six months but was associated with an increased risk of infection in months 7-24 in all three groups (each p<0.001). In months 7-24, radiation was associated with higher odds of implant removal in patients with implant reconstruction (OR 1.48, p<0.001) and fat necrosis in those with autologous reconstruction (OR=1.55; P=0.01).
Conclusions
Complication risks after immediate breast reconstruction differ by approach. Radiation therapy appears to modestly increase certain risks, including infection and implant removal.
Introduction
An increasing proportion of American breast cancer patients receive breast reconstruction after mastectomy.1-6 Reconstruction can be accomplished using a variety of techniques that can involve the use of autologous tissues, implants, or a combination of the two. Patient factors (such as body habitus, comorbidities, and prior surgical procedures) affect which techniques are actually offered in any particular case, but many patients have a choice with respect to approach. In order to make decisions in this context, patients and their physicians must consider evidence regarding pertinent outcomes such as cosmetic satisfaction and complications with each approach.
Existing evidence suggests that satisfaction may vary considerably depending on technique,7 and complication rates are substantial.8 Patients who require post-mastectomy radiation therapy may be particularly vulnerable to post-reconstruction complications.9 Previous studies have suggested that radiation increases the risk of complications, both in patients receiving breast implants 10-15 and in those receiving autologous reconstruction, 16-18 although many patients appear to successfully undergo both radiation and breast reconstruction when treated with a systematic and carefully considered approach. 19-23 Unfortunately, most of these studies have come from centers of excellence, such as academic institutions, high-volume centers, or specialty practices; relatively little is known about complication rates in radiated patients treated in the broader community.
Therefore, additional research is necessary to investigate the rates of complications that occur in patients receiving breast reconstruction with different approaches, both with and without radiotherapy, given growing evidence of the importance of radiation treatment in improving not only locoregional control but also overall survival of appropriately selected patients.24-27 Therefore, we sought to evaluate surgical complications occurring within the first two postoperative years in a working-age, commercially insured sample of breast cancer patients who received mastectomy and immediate breast reconstruction. We specifically sought to document complication rates over time in patients who did and did not receive radiation treatment.
Methods
Dataset
We utilized the proprietary MarketScan® Commercial Claims & Encounters database, licensed by Truven Health Analytics. This large, nationwide, employment-based database includes medical claims data of employees and dependents from approximately 45 large employers covered by more than 100 payers. Initially, the database included only clients whose coverage was provided through large, self-insured companies; in 2002, the dataset was expanded to include health plan clients--employees and dependents receiving insurance coverage through small and medium-sized firms.
For the current analysis, we used claims collected from 1998 through 2009 derived from individuals identified as having a cancer diagnosis.
Cohort selection and definitions
As previously described 1 and detailed further in Supplementary Table 1, a validated, claims-based algorithm identified incident cases of female breast cancer treated with mastectomy between 1998 and 2007 (n=44,735).28 Given our intention to study complications within the first two years of mastectomy, we limited our cohort to individuals with continuous enrollment from 3 months before through 23 months after mastectomy (n=24,141). To enhance specificity, the cohort was then limited to patients without distant metastasis (as reconstruction is rarely performed in patients with metastatic disease), without radiation within 3 months prior to mastectomy (as this is uncommon and indicates extremely advanced disease), and at least two or more diagnosis codes for invasive or in situ breast cancer (which limits the impact of miscoding), leaving a sample size of 20,560. Of note, only patients with breast cancer were included; those receiving prophylactic mastectomy for genetic risk or other reasons were not included.
To allow for comparative analyses of complications within the first two postoperative years, we limited our analytic sample to patients receiving mastectomy and falling into one of three groups: those without claims for reconstruction within two years of mastectomy; those with claims for implant-based reconstruction on the same date as mastectomy (including placement of either a permanent breast implant or a temporary tissue expander followed later by placement of a permanent implant); and those with claims for autologous tissue-based reconstruction on the same date as mastectomy. Patients who fell outside of these criteria—including those patients who had claims for reconstruction on a date subsequent to their mastectomy but not on the date of mastectomy, as well as those who had claims for both autologous and implant-based procedures—were omitted in order to ensure adequate numbers and homogeneity within each group, as well as the same duration of follow-up in each group, to allow for meaningful comparisons. Receipt of breast reconstruction was determined using Common Procedural Terminology (CPT) and International Classification of Diseases version 9 (ICD-9) procedure codes indicating breast reconstruction (Supplementary Table 2) performed on the same date as mastectomy. The final analytic cohort included 14,894 patients.
Outcomes
We evaluated three general outcomes of interest in all patients: 1) 30-day rehospitalization, 2) wound complications within the first two postoperative years, and 3) skin or soft tissue infections within the first two postoperative years. In addition, among patients receiving implant-based reconstruction, we considered the outcome of implant removal within the first two postoperative years; of note, this included removal of the implant for any reason, including exchange of the implant (not necessarily permanent loss). Among those receiving autologous tissue-based reconstruction, we considered the outcome of fat necrosis. The claims codes used to define these outcomes were derived after consideration of previous algorithms used to measure these outcomes 29,30,31 are listed in Supplementary Table 3. These outcomes were selected because they are outcomes which can be measured using administrative claims and for which detailed risk information would be expected to be useful for surgical decision-making and decision satisfaction. Length of hospitalization for the index mastectomy with or without reconstruction was also determined, calculated as the number of days from admission to discharge, counting the admission date as day one. Some patients were admitted on the day following mastectomy. Since this could reflect patients initially held in 24-hour observation who were subsequently converted to inpatient hospitalization, these events were considered hospitalization for the index mastectomy and length of stay was calculated from the date of mastectomy to the date of discharge. To be consistent with this definition, the outcome rehospitalization within 30 days was defined as any hospitalization that occurred between the second and the thirtieth day after index mastectomy.
Covariates
The MarketScan enrollment file provided data on age, geographic region (Northeast, North Central, South, or West), relationship to employer (employee vs spouse), and insurance type (Health Maintenance Organization [HMO] or capitated Preferred Provider Organization [PPO] vs non-capitated plan types). Year of mastectomy, laterality of mastectomy (bilateral vs. unilateral), lymph node surgery (yes/no), chemotherapy (no, before mastectomy, or after mastectomy), and radiation (yes/no) were determined from claims (Supplementary Table 2).
Statistical analysis
For our initial analyses, all outcomes were classified as binary variables, with patients considered to have experienced the outcome of interest if any claims code indicating the outcome of interest was present within 2 years of index mastectomy. For these binary outcomes, unadjusted associations between covariates and outcomes were tested using Pearson's chi-square. Multivariable logistic models for each binary outcome were created, with type of reconstructive surgery (none, implant, autologous), receipt of radiation, receipt of chemotherapy, and age included a priori due to clinical relevance. Additional covariables were included if significant in unadjusted analysis at P<0.20. Models were iteratively refined to minimize colinearity. Goodness of fit was evaluated using the test of Hosmer and Lemeshow. For each model, the interaction term for type of reconstructive surgery with radiation was tested to determine whether radiation exerted a differential effect limited to certain reconstructive strategies.
To account for the distribution of events over time, and their temporal relationship relative to initiation of radiation therapy, we further divided outcomes on a month-by-month basis, with the outcome for month j equal to 1 if any claim for the outcome was present in month j, otherwise the outcome for month j was set to 0. Further, we a priori grouped outcomes into those that occurred in months 1 to 6 after mastectomy and those that occurred in months 7 to 24. These intervals were chosen based on median time from mastectomy to initiation of radiation, such that outcomes in the early interval generally reflected events that occurred prior to the completion of radiation, and outcomes in the late interval generally reflected events that occurred during and after radiation, if radiation was administered. For each outcome, we used generalized estimating equations, adjusted for age, receipt of chemotherapy, bilaterality, and year of diagnosis, to estimate the association between receipt of radiation and the outcome of interest for both the time intervals. In certain cases, models had to be iteratively refined to ensure convergence.
Patients with missing covariate values were excluded from all multivariable analyses. All analyses were conducted using SAS v9.2 (Cary, NC) using two-sided statistical tests. This project was granted exempt status by the University of Texas MD Anderson Institutional Review Board.
Results
Among the 14,894 patients in the analytic sample, median age was 52 years (IQR 46 to 57). As described in Table 1, surgery type varied significantly by age. For example, 40.4% of women under age 40 received mastectomy without reconstruction, 36.1% received mastectomy with immediate implant-based reconstruction, and 23.5% received mastectomy with autologous reconstruction; in contrast, 78.9% of women age 60 and older received mastectomy without reconstruction, 11.8% received mastectomy with immediate implant-based reconstruction, and 9.3% received mastectomy with immediate autologous reconstruction. In this sample, 19.0% (n=2831) of patients were in HMOs or capitated PPOs and nearly half were from the South. Overall, 11.6% underwent bilateral mastectomy, 52.4% received chemotherapy, and 35.0% received radiation therapy (of whom 22.2% received immediate reconstruction, as compared with 46.3% of those who did not receive radiation therapy).
Table 1. Baseline characteristics and type of surgery.
| Entire cohort (n=14,894) | Mastectomy and no reconstruction (n=9,250) | Mastectomy and immediate implant-based reconstruction (n=3,007) | Mastectomy and immediate autologous reconstruction (n=2,637) | P* | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Column % | N | Row % | N | Row % | N | Row % | ||
| Age at diagnosis (years) | <0.001 | ||||||||
| < 40 | 1122 | 7.5 | 453 | 40.4 | 405 | 36.1 | 264 | 23.5 | |
| 40-49 | 4378 | 29.4 | 2209 | 50.5 | 1203 | 27.5 | 966 | 22.1 | |
| 50-59 | 7378 | 49.5 | 4997 | 67.7 | 1162 | 15.8 | 1219 | 16.5 | |
| 60 and older | 2016 | 13.5 | 1591 | 78.9 | 237 | 11.8 | 188 | 9.3 | |
| Year of mastectomy | <0.001 | ||||||||
| 1998 | 381 | 2.6 | 258 | 67.7 | 30 | 7.9 | 93 | 24.4 | |
| 1999 | 592 | 4.0 | 394 | 66.6 | 59 | 10.0 | 139 | 23.5 | |
| 2000 | 608 | 4.1 | 410 | 67.4 | 58 | 9.5 | 140 | 23.0 | |
| 2001 | 874 | 5.9 | 566 | 64.8 | 118 | 13.5 | 190 | 21.7 | |
| 2002 | 1362 | 9.1 | 935 | 68.7 | 190 | 14.0 | 237 | 17.4 | |
| 2003 | 1749 | 11.7 | 1166 | 66.7 | 328 | 18.8 | 255 | 14.6 | |
| 2004 | 1779 | 11.9 | 1088 | 61.2 | 321 | 18.0 | 370 | 20.8 | |
| 2005 | 1829 | 12.3 | 1109 | 60.6 | 385 | 21.1 | 335 | 18.3 | |
| 2006 | 2537 | 17.0 | 1547 | 61.0 | 592 | 23.3 | 398 | 15.7 | |
| 2007 | 3183 | 21.4 | 1777 | 55.8 | 926 | 29.1 | 480 | 15.1 | |
| Relationship with employer | 0.26 | ||||||||
| Employee | 8698 | 58.4 | 5372 | 61.8 | 1765 | 20.3 | 1561 | 18.0 | |
| Spouse | 6177 | 41.5 | 3863 | 62.5 | 1238 | 20.0 | 1076 | 17.4 | |
| Unknown | 19 | 0.1 | 15 | 79.0 | 4 | 21.1 | 0 | 0.0 | |
| HMO or PPO with capitation | <0.001 | ||||||||
| No | 12063 | 81.0 | 7506 | 62.2 | 2366 | 19.6 | 2191 | 18.2 | |
| Yes | 2831 | 19.0 | 1744 | 61.6 | 641 | 22.6 | 446 | 15.8 | |
| Region of country | <0.001 | ||||||||
| Northeast | 1180 | 7.9 | 624 | 52.9 | 251 | 21.3 | 305 | 25.9 | |
| North Central | 3802 | 25.5 | 2360 | 62.1 | 863 | 22.7 | 579 | 15.2 | |
| South | 7234 | 48.6 | 4433 | 61.3 | 1321 | 18.3 | 1480 | 20.5 | |
| West | 2456 | 16.5 | 1683 | 68.5 | 544 | 22.2 | 229 | 9.3 | |
| Bilateral mastectomy | <0.001 | ||||||||
| No | 13160 | 88.4 | 8625 | 65.5 | 2229 | 16.9 | 2306 | 17.5 | |
| Yes | 1734 | 11.6 | 625 | 36.0 | 778 | 44.9 | 331 | 19.1 | |
| Lymph node surgery | <0.001 | ||||||||
| No | 2592 | 17.4 | 1277 | 49.3 | 721 | 27.8 | 594 | 22.9 | |
| Yes | 12302 | 82.6 | 7973 | 64.8 | 2286 | 18.6 | 2043 | 16.6 | |
| Chemotherapy | <0.001 | ||||||||
| No | 7084 | 47.6 | 3846 | 54.3 | 1754 | 24.8 | 1484 | 21.0 | |
| Before surgery | 1372 | 9.2 | 1026 | 74.8 | 164 | 12.0 | 182 | 13.3 | |
| After surgery | 6438 | 43.2 | 4378 | 68.0 | 1089 | 16.9 | 971 | 15.1 | |
| Radiation | <0.001 | ||||||||
| No | 9675 | 65.0 | 5191 | 53.7 | 2459 | 25.4 | 2025 | 20.9 | |
| Yes | 5219 | 35.0 | 4059 | 77.8 | 548 | 10.5 | 612 | 11.7 | |
- chi-square test
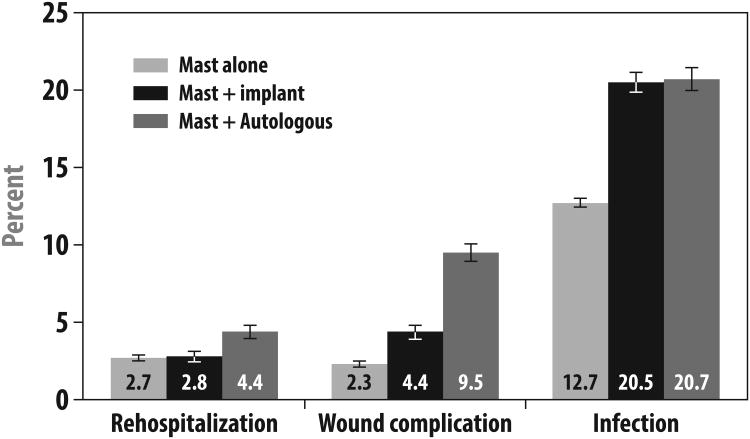
Overall, slightly more than half (52.2%) of women receiving mastectomy without reconstruction were hospitalized for their index surgery; most (74.6%) of those receiving immediate implant-based reconstruction and almost all (96.0%) of those receiving immediate autologous reconstruction were hospitalized. Among those who were hospitalized, the median length of stay was 2 days for those receiving mastectomy without reconstruction and those receiving immediate implant reconstruction, and it was 5 days for those receiving immediate autologous tissue reconstruction. As shown in Figure 1, the 30-day rehospitalization rate was 2.7% among patients receiving mastectomy without reconstruction, 2.8% among those receiving immediate implant reconstruction, and 4.4% among those receiving immediate autologous reconstruction (p<0.001).
Figure 1. Adverse Outcomes by Surgery Type.
This figure presents the proportion of patients who developed the following complications by type of initial breast surgery: (1) rehospitalization within 30 days of initial mastectomy, (2) a diagnosis code indicating wound complications within 2 years of initial mastectomy, and (3) a diagnosis code indicating infection within 2 years of initial mastectomy. P-values for all three outcomes by type of initial surgery were statistically significant at P<0.001. Abbreviations: Mast (mastectomy), Implant (implant-based reconstruction).
As also detailed in Figure 1, claims codes for wound complications within the first two postoperative years were observed in 2.3% of those not receiving reconstruction, 4.4% of those receiving implant reconstruction, and 9.5% of those receiving autologous reconstruction (p<0.001). Codes suggesting diagnoses of infections within the first two postoperative years were observed in 12.7% of those undergoing mastectomy without reconstruction, 20.5% of those undergoing immediate implant reconstruction, and 20.7% of those undergoing immediate autologous reconstruction (p<0.001). Implant removal was performed in 24.7% of those who received immediate implant reconstruction. Fat necrosis was diagnosed in 15.7% of those who received immediate autologous reconstruction.
In multivariable models, as shown in Table 2, patients who received immediate autologous reconstruction had increased odds of 30-day rehospitalization (OR 1.75), wound complication diagnosis within two years (OR 5.17), and infection diagnosis within two years (OR 1.94), compared to patients not receiving reconstruction. Patients who received immediate implant reconstruction had increased odds of diagnoses of wound complications (OR 2.10) and infections (OR 1.84) within two years, compared to those not receiving reconstruction. Radiation therapy was not associated with the risk of 30-day rehospitalization or wound complications but was significantly associated with the risk of infection (OR 1.29); there were no statistically significant interactions observed between radiation receipt and surgery type.
Table 2. Multivariable models to predict risk of adverse outcomes by type of initial surgery.
| 30-day rehospitalization | Wound complication diagnosis within 2 years | Infection diagnosis within 2 years | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Type of surgery | ||||||
| Mastectomy and no reconstruction | 1 | . | 1 | . | 1 | . |
| Mastectomy + implant | 1.116 | 0.86-1.45 | 2.10 | 1.67-2.66 | 1.84 | 1.64-2.07 |
| Mastectomy + autologous | 1.75 | 1.39-2.21 | 5.17 | 4.24-6.30 | 1.94 | 1.73-2.18 |
| Radiation | ||||||
| No | 1 | . | 1 | . | 1 | . |
| Yes | 1.038 | 0.83-1.30 | 1.154 | 0.94-1.42 | 1.29 | 1.15-1.44 |
| Chemotherapy | ||||||
| No | 1 | . | 1 | . | 1 | . |
| Yes | 1.186 | 0.96-1.47 | 1.16 | 0.96-1.40 | 1.082 | 0.98-1.20 |
| Age | ||||||
| < 40 | 1 | . | 1 | . | 1 | . |
| 40-49 | 0.949 | 0.65-1.38 | 1.07 | 0.76-1.51 | 1.063 | 0.89-1.27 |
| 50-59 | 1.016 | 0.71-1.46 | 1.34 | 0.97-1.86 | 1.158 | 0.97-1.38 |
| 60 and older | 1.21 | 0.79-1.85 | 1.97 | 1.35-2.87 | 1.20 | 0.97-1.47 |
| Year of mastectomy | ||||||
| 1998 | 1 | . | 1 | . | ||
| 1999 | 0.722 | 0.33-1.59 | 1.00 | 0.68-1.49 | ||
| 2000 | 1.196 | 0.58-2.45 | 1.088 | 0.74-1.60 | ||
| 2001 | 0.884 | 0.44-1.79 | 1.145 | 0.80-1.64 | ||
| 2002 | 1.078 | 0.56-2.08 | 1.117 | 0.79-1.58 | ||
| 2003 | 1.097 | 0.58-2.09 | 1.045 | 0.75-1.47 | ||
| 2004 | 1.111 | 0.59-2.09 | 1.394 | 1.00-1.94 | ||
| 2005 | 1.691 | 0.91-3.14 | 1.365 | 0.98-1.90 | ||
| 2006 | 1.785 | 0.97-3.28 | 1.29 | 0.93-1.79 | ||
| 2007 | 1.639 | 0.89-3.00 | 1.449 | 1.05-2.00 | ||
| Bilateral mastectomy | ||||||
| No | 1 | . | ||||
| Yes | 1.20 | 1.05-1.37 | ||||
| Region of country | ||||||
| Northeast | ||||||
| North Central | ||||||
| South | ||||||
| West | ||||||
| HMO or PPO with capitation | ||||||
| No | ||||||
| Yes | ||||||
|
| ||||||
| Goodness of fit (P-value) | 0.84 | 0.61 | 0.43 | |||
|
| ||||||
| Interaction of radiation by type of surgery (P-value) | 0.70 | 0.59 | 0.14 | |||
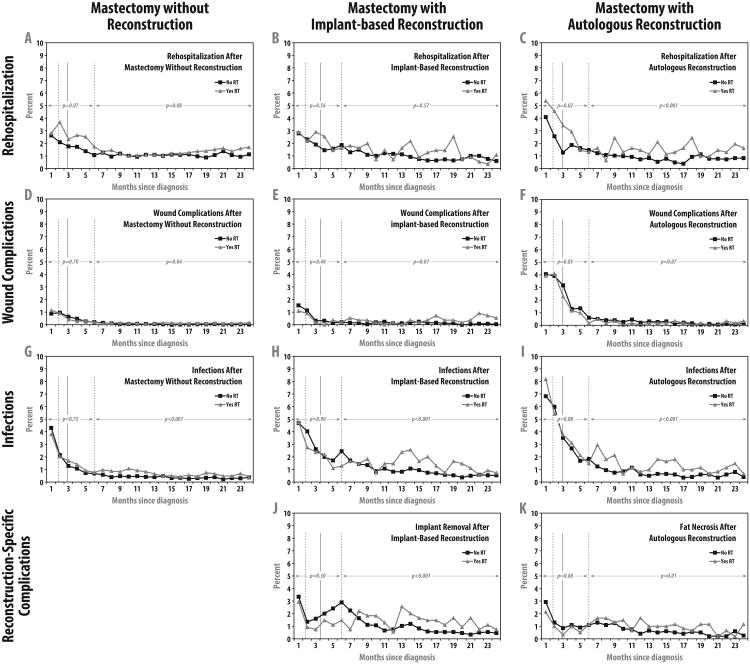
Figure 2 demonstrates the incidence of each adverse outcome over time in patients who did and did not receive radiotherapy, within subgroups by reconstruction type. The first two rows show similar outcomes in all groups with respect to hospitalization and wound complications, regardless of radiation receipt. Specifically, as shown in the first row, hospitalization rates were not significantly increased in patients receiving radiation in conjunction with mastectomy alone or implant reconstruction in either the first six months or months 7-24; hospitalization rates were increased in patients receiving radiation in conjunction with autologous reconstruction both in the first six months (P=0.02) and in months 7-24 (P<0.001). As shown in the second row, radiation receipt was not associated with wound complications in either group of reconstructed patients within months 1-6 or months 7-24. In the group receiving mastectomy alone, radiation receipt was associated with an increase in risk in months 7-24 in patients who did not receive reconstruction (OR=1.84, P=0.04), but the absolute magnitude was very small, as shown in Panel D.
Figure 2. Adverse Outcomes with and without Radiotherapy Among Reconstructed Patients Over Time.
The top row of panels depicts rehospitalization rates; the second row wound complications; the third row wound complications; and the fourth row reconstruction-specific complications in the cohort of 14,894 women undergoing mastectomy for breast cancer from 1998-2007 who received no reconstruction within the first two postoperative years (n=9250), immediate implant-based reconstruction (n=3007), or immediate autologous reconstruction (n=2637), respectively. In each panel, the solid vertical line shows the median interval from mastectomy to start of radiotherapy, and the two dotted vertical lines show the interquartile range. Panel A shows that radiation was not associated with rehospitalization in patients not receiving reconstruction, either within the first 6 months (P=0.07) or in months 7 to 24 (P=0.08). Panel B shows that radiation was not associated with rehospitalization in patients receiving implant-based reconstruction, either within the first 6 months (P=0.56) or in months 7 to 24 (P=0.57). Panel C shows that radiation was associated with increased risk of rehospitalization in patients receiving autologous reconstruction, both within the first 6 months (OR=1.40; P=0.02) and in months 7 to 24 (OR=1.67; P<0.001). Panel D shows that within the first 6 months of mastectomy without reconstruction, wound complications were not associated with radiation (P=0.70); in months 7-24, radiation was associated with a modest increase in wound complications (OR=1.84, P=0.04), but absolute risks were extremely low. Panel E shows that radiation was not associated with wound complication risk within the first six months (P=0.40) or in months 7 to 24 (P=0.07) after mastectomy with immediate implant-based reconstruction. Panel F shows that radiation was not associated with wound complications either within the first 6 months (P=0.85) or in months 7 to 24 (P=0.87) after mastectomy with immediate autologous reconstruction. Panel G shows that within the first 6 months of mastectomy without reconstruction, infection was not associated with radiation (P=0.75), but in months 7 to 24, infection was more likely in radiated patients (OR=1.79; P<0.001). Panel H shows that within the first 6 months of mastectomy with immediate implant-based reconstruction, infection was not associated with radiation (P=0.90), but in months 7 to 24, infection was more likely in radiated patients (OR=1.66; P<0.001). Panel I shows that within the first 6 months of mastectomy with autologous reconstruction, infection was not associated with radiation (P=0.08), but in months 7 to 24, infection was more likely in radiated patients (OR=2.07; P<0.001). Panel J shows that after implant reconstruction, implant removal was not associated with radiation receipt (P=0.30), but in months 7 to 24, implant removal was more likely in radiated patients (OR=1.48; P<0.001). Panel K shows that radiation was not associated with increased risk of fat necrosis among patients receiving mastectomy with immediate autologous reconstruction within the first 6 months of surgery (P=0.88) but was associated with high risk of fat necrosis in months 7 to 24 (OR=1.55; P=0.01).
By contrast, the third and fourth rows demonstrate significant differences in rates of infections and procedure-specific complications by radiation receipt, all occurring within the time period following the expected time of radiation receipt and not preceding it. Specifically, as shown in the third row, regardless of surgery type, infection was not associated with radiation receipt within months 1-6 but was associated with radiation receipt in months 7-24 (P<0.001 for all comparisons). Specifically, during months 7-24, infections were observed in 5.0% of women who did not receive radiation vs 8.1% of women who had radiation in the mastectomy alone group; 10.9% (no radiation) vs 15.0% (with radiation) in the immediate implant reconstruction group; and 7.7% (no radiation) vs 14.5% (with radiation) in the immediate autologous reconstruction group. Finally, as shown in the fourth row of the figure, procedure-specific complications were more likely in radiated patients only in months 7-24. During months 7-24, 13.1% of women who underwent implant-based reconstruction and did not receive radiation had implant removal, compared with 21.9% of women in that group who received radiation; in the same time period, 8.7% of women who underwent autologous reconstruction and did not receive radiation had fat necrosis compared with 14.7% of women in that group who received radiation.
Discussion
In this large, claims-based analysis of women receiving mastectomy for breast cancer in recent years, we observed complications of immediate breast reconstruction to differ somewhat by approach. Autologous reconstruction is a more intensive surgical procedure that requires longer initial hospitalization and more frequent rehospitalization. However, both patients receiving immediate implant and autologous tissue reconstructions appear to face increased risks of infections and wound complications compared to those receiving mastectomy without reconstruction. In addition, we observed that radiation therapy appears to modestly increase certain risks that primarily develop over time after the initiation of radiation, such as infectious complications in all patients, implant removal in patients undergoing implant reconstruction, and fat necrosis in patients undergoing autologous reconstruction.
We believe these to be the first claims-based data on length-of-stay, rehospitalization, and incidence of various surgical complications in a cohort of women treated in diverse settings across the United States. Previous studies have shown that rates of post-operative complications after breast reconstruction are substantial. For example, in the Michigan Breast Reconstruction Outcomes Survey, investigators evaluated outcomes of 326 patients treated at 12 centers from 1994 to 1998, finding that 45% had experienced a complication within two years of surgery. Major complications—defined as those requiring reoperation, rehospitalization, or nonperioperative intravenous antibiotics—occurred in 32% of the sample.8 Unfortunately, however, the study included too few radiated patients to detect an impact of radiation on complication rates.
The effects of radiation therapy on the irradiated chest wall and reconstructed breast are widely feared but poorly understood. Radiation effects include acute toxicities that are generally inflammatory in nature, including soft tissue edema and skin erythema, sometimes accompanied by dry or moist desquamation of the treated skin. Late effects can include persistent skin changes, vascular compromise, and soft tissue fibrosis. In the setting of breast reconstruction, these toxicities may lead to an increased incidence of complications of the sort evaluated in this study, which may require repeated surgical interventions for correction.
Existing evidence regarding complications of breast reconstruction in the setting of radiation therapy comes largely from retrospective, single-institution series. Based on unique differences between reconstructive techniques, the specific complications observed postoperatively can be expected to vary. This variation in the complication profile of procedures makes head-to-head comparison of specific complications after autologous or implant reconstruction difficult. Information gained from an understanding of expected complications over time, however, can help guide decisions regarding reconstruction in the setting of radiation.
Autologous reconstruction techniques involve both a flap donor site and the reconstructed breast surgical site. Both sites are at risk for complications. As such, patients undergoing autologous breast reconstruction are theoretically at greater risk for wound complications and related readmissions, when compared to patients who are reconstructed with implants. This is consistent with our observation of the highest rates of wound complications in this subgroup, as compared to patients reconstructed with implants or those not receiving immediate reconstruction. However, we did not observe increased rates of wound complications in patients who received radiotherapy in the current study. Patients undergoing autologous reconstruction also face potentially increased risks of fat necrosis, fibrosis, atrophy, and flap contracture in the setting of radiotherapy.16-18 These flap-specific complications are difficult to quantify, and a lack of standardized reporting of these complications makes it difficult to assess their relative clinical significance. For instance, most flap contractures after radiation result in a firmer, less ptotic reconstructed breast which in many cases can be addressed with a mastopexy or reduction of the contralateral breast.23 On the other hand some flap contractures can be severe enough to warrant discarding the radiated flap with a revision of the reconstruction with alternative techniques. Along similar lines, the presence of a small area of fat necrosis may not require intervention or might be resolved with simple liposuction, but fat necrosis can also sometimes lead to atrophy and contracture of the reconstructed breast. 17, 18, 32-34 Although not all fat necrosis identified in this study may have been clinically consequential, we do believe that the increased rate of this complication in radiated patients merits consideration, particularly because it can necessitate additional imaging, cause anxiety, and even require surgical intervention.
By contrast, implant patients who receive radiation therapy may be at increased risk for capsular contracture, infection, pain, skin necrosis, fibrosis, and impaired wound healing.10-15, 35 Specific complications related to radiation such as infection and impaired wound healing would also be expected to present within the 7-24 month observation period. As with autologous reconstructions, revision procedures including tissue expander exchanges are many times performed months after completion of chemotherapy and radiation. Therefore, we also find it noteworthy that an increased risk of infection was observed in radiated patients with implant reconstruction only within months 7-24 and not within the first 6 months of surgery. These infections were likely related to revision procedures performed after the completion of radiation. Revision procedures in this setting would include procedures performed during the exchange of tissue expanders for implants or subsequent to an expander-implant exchange. As the radiated breast soft tissue tends to be more resistant to the stretch required for breast ptosis, revision procedures are typically necessary to improve on the breast appearance and achieve symmetry with the non-radiated contralateral breast. These procedures include capsulotomies, capsulectomies and capsulorrhaphies, in addition to techniques utilized to lower the inframammary fold or re-establish the fold when needed. Most of these procedures require re-entry into the implant pocket, potentially increasing the risk for postoperative infection. Radiation changes to the soft tissue are also a likely cause of the observed increased risk of infection as was shown in a recent study from Memorial Sloan-Kettering Cancer Center, by Cordeiro et al.36 Evaluating 319 implants exposed to radiation therapy after expander-implant exchange in comparison to 1814 non-radiated implants, they found a significantly higher infection rate (10.3% vs 2.7%, p<0.01) in radiated patients; implant infections were the most common reason (41.4%) for implant removal in these radiated patients.
Indeed, our findings strongly suggest that radiation is a risk factor for infection regardless of type of surgery or receipt of reconstruction, illustrating that the normal tissue effects of radiation, either through immune-mediated effects or damage to the skin and soft tissues or congestion of the lymphatic channels, result in a physiologic state characterized by increased susceptibility to infection. Still, although a rise in infections was also noted in autologous reconstruction patients in the later time period, a critical difference exists in the ultimate clinical implication of this complication. Autologous reconstruction infections typically are managed and resolve with oral antibiotic therapy. Implant infections tend to require an extended course of intravenous antibiotics with some of these infections ultimately resulting in removal of the infected implant. In this study population, 21.9% of the radiated patients reconstructed with implants had their implants taken out during the 7-24 month observation period in comparison to 13.1% of non-radiated patients; we believe that infections are a potential reason for a substantial number of the implant removals. Thus, the implications of infections in the radiated patient reconstructed with implants may be of greater clinical significance than they are in patients reconstructed with flaps. We do find it reassuring that the rates of implant removal observed in this sample of patients treated in the community were generally similar to rates reported from centers of excellence.21, 37
The few multi-center studies investigating outcomes of the integration of radiation and reconstruction have had significant limitations, including failure to include patients treated with different techniques for comparison, failure to measure covariates, and/or limitation to centers of excellence or populations substantially different from those treated in the United States.38,39,40 Given the wide variation in complication rates reported in different institutional series, the general lack of comparison to patients not receiving reconstruction, and the lack of inclusion of sufficient numbers of irradiated patients in studies such as the multicenter Michigan Breast Reconstruction Outcomes Survey discussed above, the evidence assembled in the current study fills a void in the existing literature.
This study has a number of strengths, including its access to information on a large sample of patients treated in diverse settings across the United States. It also has limitations. First, as in any observational study, associations may not reflect causation and may instead reflect imbalances in other features. We did adjust our models for those potentially confounding factors to which we had access, but this does not ensure the absence of confounding, particularly by factors that could not be measured from the claims data, including smoking history and body mass index. Second, the sample was restricted to patients with commercial insurance and mostly those of working age; if complications differ in settings in which patients with other insurance types are treated or, perhaps more plausibly, among older patients, our findings should not be generalized to those populations. The dataset we used also includes a disproportionate share of patients from the Southern United States, so if outcomes differ by region, this may also affect generalizability.
Third, the measures were derived from claims, which can be vulnerable to underascertainment or misclassification. Certain outcomes are more likely to be accurately measured through claims (such as rehospitalizations and implant removal procedures) than others, including complications assessed on the basis of diagnosis codes (such as wound complications, infections, and fat necrosis). Moreover, our algorithms identified complications that would be expected to be heterogeneous in terms of severity. However, even for those complications assessed using diagnosis codes, one would not expect misclassification, underascertainment, or heterogeneity to vary systematically by surgery group for the complications we assessed across the different surgery types, so this is unlikely to undermine the validity of the differences observed between the surgery groups. Similarly, we do not expect systematic differences in the accuracy of claims-based measures of complications in radiated versus uninrradiated patients, so this is unlikely to have biased our estimates of the impact of radiation therapy. Nevertheless, it should be noted our results suggest that a small proportion (4%) of patients undergoing autologous reconstruction were not hospitalized at the time of their reconstructive surgery. To our knowledge, this is not a common practice. While our finding may be reflective of actual practice, we cannot rule out the possibility of a low frequency of coding errors, for example errors in the date of hospital admission, which could account for this finding.
Fourth, we were unable to differentiate what type of axillary surgery was received by patients in this cohort. There was not a specific CPT or ICD-9 procedure code for sentinel lymph node biopsy with blue dye mapping during the span of years included in this study. Such patients may have been coded with more generic CPT or ICD-9 procedure codes, such as axillary lymph node excision, not otherwise specified. In our prior work, approximately one-third of patients could not be accurately classified as receiving either sentinel lymph node biopsy or axillary lymph node dissection when using only insurance billing claims.41 Studies seeking to differentiate outcomes in patients receiving sentinel node biopsy versus axillary lymph node dissection must, therefore, await maturation and release of contemporary datasets, in which the recently introduced CPT code for SLNB with blue dye mapping will facilitate this distinction in the future.
Finally, this claims-based study was limited to the assessment of certain outcomes that were measurable through claims data and could not evaluate other meaningful endpoints such as pain and cosmetic satisfaction that cannot be measured using information present in claims data. Nevertheless, we believe that the outcomes we could measure, including wound complications, infections, rehospitalization, fat necrosis, and implant removals, are important to study because they are likely to have a meaningful impact on patients' quality of life, as they can compromise physical well-being, cause psychological distress, and impair social role functioning. To assess the absolute magnitude of all potentially relevant, patient-centered outcomes in this setting, future researchers should attempt to build plastic surgery registries with active, prospective data collection to this end.
In sum, thousands of women with breast cancer each year must decide whether to pursue breast reconstruction, and if so, with what approach. Information on hospitalization experiences and complication rates are relevant for decision-making in this context, especially if patients are also considering radiation therapy. The current study suggests that the complications of immediate breast reconstruction do differ depending on approach and that radiation therapy appears to modestly increase certain risks. Further research is necessary to determine whether the increasing use of acellular dermal matrices42 and autologous fat grafting 43 in implant-based reconstruction or the use of delayed reconstruction approaches (including a staged, “delayed-immediate” approach to autologous reconstruction with transfer of the flap after radiation) may alter these risks9,44,45,46.
Supplementary Material
Supplementary Table 1. Cohort creation
Supplementary Table 2. Diagnosis and procedure codes used to select cohort and define surgery, reconstruction, and covariates
Supplementary Table 3. Definition of outcomes derived from claims
Acknowledgments
Dr. Jagsi was supported by a grant from the American Cancer Society (MRSG-09-145-01-CPHPS). Drs. Smith and Giordano are supported by a grant from the Cancer Prevention & Research Institute of Texas (RP101207). This research was also supported by the Duncan Family Institute. MD Anderson investigators were supported by the NIH/NCI under award number P30CA016672, using the Biostatistics Resource Group. None of the funding organizations played any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Smith had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Presented in preliminary abstract form at the ASTRO Annual Meeting in Atlanta, Georgia, September 2013.
Author Contributions: This was a complex multidisciplinary study of plastic surgical complications in the setting of radiation therapy using claims based data. Therefore, the study required expertise from multiple disciplines, including radiation oncology (RJ, LJP, TAB, BDS), plastic surgery (AM, AA, SK), and health services methodology (SG, JJ). As a result, more than 8 (9) individuals contributed substantially to this work and merit authorship.
- Reshma Jagsi contributed substantially to conception and design of the study, analysis and interpretation of the data, wrote the manuscript, and gave final approval of the version to be published.
- Jing Jiang contributed substantially to the analytic design and performed all analyses; she critically reviewed, edited, and approved the final manuscript.
- Adeyiza O. Momoh contributed substantially to the design of the study, analysis and interpretation of data, and critically reviewed, edited, and approved the final manuscript.
- Amy Alderman contributed substantially to the design of the study, analysis and interpretation of data, and critically reviewed, edited, and approved the final manuscript.
- Sharon H. Giordano contributed substantially to data acquisition, the analysis and interpretation of data, and critically reviewed, edited, and approved the final manuscript.
- Thomas A. Buchholz contributed substantially to the analysis and interpretation of data; he critically reviewed, edited, and approved the final manuscript.
- Lori J. Pierce contributed substantially to the analysis and interpretation of data; she critically reviewed, edited, and approved the final manuscript.
- Steven J. Kronowitz contributed substantially to the analysis and interpretation of data; he critically reviewed, edited, and approved the final manuscript.
- Benjamin D. Smith contributed substantially to the conception and design of the study, analysis and interpretation of the data, and critically reviewed, edited, and approved the final manuscript
References
- 1.Jagsi R, Jiang J, Momoh AO, Alderman A, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32:919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderman AK, McMahon L, Jr, Wilkins EG. The national utilization of immediate and early delayed breast reconstruction and the effect of sociodemographic factors. Plast Reconstr Surg. 2003;111:695–703. doi: 10.1097/01.PRS.0000041438.50018.02. [DOI] [PubMed] [Google Scholar]
- 3.Christian CK, Niland J, Edge SB, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: a study of the National Comprehensive Cancer Network. Ann Surg. 2006;243:241–249. doi: 10.1097/01.sla.0000197738.63512.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderman AK, Hawley ST, Janz NK, et al. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population- based study. J Clin Oncol. 2009;27:5325–5330. doi: 10.1200/JCO.2009.22.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuben BC, Manwaring J, Neumayer LA. Recent trends and predictors in immediate breast reconstruction after mastectomy in the United States. Am J Surg. 2009;198:237–243. doi: 10.1016/j.amjsurg.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Polednak AP. How frequent is postmastectomy breast reconstructive surgery? A study linking two statewide databases. Plast Reconstr Surg. 2001;108:73–77. doi: 10.1097/00006534-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Alderman AK, Kuhn LE, Lowery JC, Wilkins EG. Does patient satisfaction with breast reconstruction change over time? Two-year results of the Michigan Breast Reconstruction Outcomes Study. J Am Coll Surg. 2007;204:7–12. doi: 10.1016/j.jamcollsurg.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in post-mastectomy breast reconstruction: two year results of the Michigan breast reconstruction outcome study. Plast Reconstr Surg. 2002;109:2265–2274. doi: 10.1097/00006534-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis in Breast. Breast Cancer Res Treat. 2011;127:15–22. doi: 10.1007/s10549-011-1401-x. [DOI] [PubMed] [Google Scholar]
- 10.Kronowitz SJ. Current status of implant-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130:513e–523e. doi: 10.1097/PRS.0b013e318262f059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitfield GA, Horan G, Irwin MS, et al. Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother Oncol. 2009;90:141–147. doi: 10.1016/j.radonc.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Jhaveri JD, Rush SC, Kostroff K, et al. Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. Int J Radiat Oncol Biol Phys. 2008;72:859–865. doi: 10.1016/j.ijrobp.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 13.Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:713–721. doi: 10.1016/s0360-3016(00)01402-4. [DOI] [PubMed] [Google Scholar]
- 14.Contant CM, van Geel AN, van der Holt B, et al. Morbidity of immediate breast reconstruction (IBR) after mastectomy by a subpectorally placed silicone prosthesis: the adverse effect of radiotherapy. Eur J Surg Oncol. 2000;26:344–350. doi: 10.1053/ejso.1999.0896. [DOI] [PubMed] [Google Scholar]
- 15.Tallet AV, Salem N, Moutardier V, et al. Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: complications and esthetic results. Int J Radiat Oncol Biol Phys. 2003;57:136–142. doi: 10.1016/s0360-3016(03)00526-1. [DOI] [PubMed] [Google Scholar]
- 16.Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130:282–292. doi: 10.1097/PRS.0b013e3182589be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams JK, Carlson GW, Bostwick J, 3rd, et al. The effects of radiation treatment after TRAM flap breast reconstruction. Plast Reconstr Surg. 1997;100:1153–1160. doi: 10.1097/00006534-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg. 2002;109:1919–1924. doi: 10.1097/00006534-200205000-00022. discussion 1925-1926. [DOI] [PubMed] [Google Scholar]
- 19.Crisera CA, Chang EI, Da Lio AL, et al. Immediate free flap reconstruction for advanced-stage breast cancer: is it safe? Plast Reconstr Surg. 2011;128:32–41. doi: 10.1097/PRS.0b013e3182174119. [DOI] [PubMed] [Google Scholar]
- 20.Chawla AK, Kachnic LA, Taghian Ag, et al. Radiotherapy and breast reconstruction: complications and cosmesis with TRAM versus tissue expander/implant. Int J Radiat Oncol Biol Phys. 2002;54:520–526. doi: 10.1016/s0360-3016(02)02951-6. [DOI] [PubMed] [Google Scholar]
- 21.Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer. 2012;118:2552–2559. doi: 10.1002/cncr.26521. [DOI] [PubMed] [Google Scholar]
- 22.Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg. 2011;127:2154–2166. doi: 10.1097/PRS.0b013e3182131b8e. [DOI] [PubMed] [Google Scholar]
- 23.Chang EI, Liu TS, Festekjian JH, et al. Effects of radiation therapy for breast cancer based on type of free flap reconstruction. Plast Reconstr Surg. 2013;131:1e–8e. doi: 10.1097/PRS.0b013e3182729d33. [DOI] [PubMed] [Google Scholar]
- 24.EBCTCG (Early Breast Cancer Trialists' Collaborative Group) McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 26.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 27.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 28.Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39(6 Pt 1):1733–1749. doi: 10.1111/j.1475-6773.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen MA, Fraser VJ. Use of diagnosis codes and/or wound culture results for surveillance of surgical site infection after mastectomy and breast reconstruction. Infect Control Hosp Epidemiol. 2010;31:544–547. doi: 10.1086/652155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CL, Shore AD, Johns R, et al. The impact of obesity on breast surgery complications. Plast Reconstr Surg. 2011;128:395e–402e. doi: 10.1097/PRS.0b013e3182284c05. [DOI] [PubMed] [Google Scholar]
- 31.Smith GL, Xu Y, Buchholz TA, Giordano SH, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307:1827–1837. doi: 10.1001/jama.2012.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khansa I, Momoh AO, Patel PP, et al. Fat necrosis in autologous abdomen-based breast reconstruction: a systematic review. Plast Reconstr Surg. 2013;131:443–452. doi: 10.1097/PRS.0b013e31827c6dc2. [DOI] [PubMed] [Google Scholar]
- 33.Gill PS, Hunt JP, Guerra AB, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg. 2004;113:1153–1160. doi: 10.1097/01.prs.0000110328.47206.50. [DOI] [PubMed] [Google Scholar]
- 34.Tran NV, Evans GR, Kroll SS, et al. Postoperative adjuvant irradiation: Effects on transverse rectus abdominis muscle flap breast reconstruction. Plast Reconstr Surg. 2000;106:313–317. doi: 10.1097/00006534-200008000-00011. discussion 318–320. [DOI] [PubMed] [Google Scholar]
- 35.Nahabedian MY, Tsangaris T, Momen B, Manson PN. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112:467–476. doi: 10.1097/01.PRS.0000070727.02992.54. [DOI] [PubMed] [Google Scholar]
- 36.Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K. The Impact of Postmastectomy Radiotherapy on Two-Stage Implant Breast Reconstruction: An Analysis of Long-Term Surgical Outcomes, Aesthetic Results, and Satisfaction over 13 Years. Plast Reconstr Surg. 2014;134:588–595. doi: 10.1097/PRS.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 37.Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21:118–124. doi: 10.1245/s10434-013-3284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy CM, Klassen AF, Cano SJ, et al. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer. 2010;116:5584–5591. doi: 10.1002/cncr.25552. [DOI] [PubMed] [Google Scholar]
- 39.Cowen D, Gross E, Rouannet P, et al. Immediate post-mastectomy breast reconstruction followed by radiotherapy: risk factors for complications. Breast Cancer Res Treat. 2010;121:627–634. doi: 10.1007/s10549-010-0791-5. [DOI] [PubMed] [Google Scholar]
- 40.Alderman AK, Jagsi R. Discussion: Immediate post-mastectomy breast reconstruction followed by radiotherapy: risk factors for complications. Breast Cancer Res Treat. 2010;121:635–637. doi: 10.1007/s10549-010-0878-z. [DOI] [PubMed] [Google Scholar]
- 41.Black DM, Jiang J, Kuerer HM, Buchholz TA, Smith BD. Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg. 2014;149(8):788–796. doi: 10.1001/jamasurg.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyer HR, Pinell-White X, Losken A. The effect of radiation on acellular dermal matrix and capsule formation in breast reconstruction: clinical outcomes and histologic analysis. Plast Reconstr Surg. 2014;133:214–221. doi: 10.1097/01.prs.0000437255.01199.42. [DOI] [PubMed] [Google Scholar]
- 43.Kakagia D, Pallua N. Autologous Fat Grafting: In Search of the Optimal Technique. Surg Innov. 2014;21:327–336. doi: 10.1177/1553350613518846. [DOI] [PubMed] [Google Scholar]
- 44.Albino FP, Patel KM, Smith JR, et al. Delayed versus Delayed-Immediate Autologous Breast Reconstruction: A Blinded Evaluation of Aesthetic Outcomes. Arch Plast Surg. 2014;41:264–270. doi: 10.5999/aps.2014.41.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113:1617–1628. doi: 10.1097/01.prs.0000117192.54945.88. [DOI] [PubMed] [Google Scholar]
- 46.Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124:395–408. doi: 10.1097/PRS.0b013e3181aee987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Cohort creation
Supplementary Table 2. Diagnosis and procedure codes used to select cohort and define surgery, reconstruction, and covariates
Supplementary Table 3. Definition of outcomes derived from claims