The Ziehl–Neelsen (ZN) stain, also known as the acid-fast stain, has been reported to be helpful in detection and identification of Schistosoma eggs. 1, 2 In histological sections, Schistosoma mansoni egg shells appear as ZN positive and Schistosoma haematobium shells as ZN negative. 1, 2 The staining target of the responsible ZN component (carbolfuchsin) in the shell is unknown. Because carbolfuchsin is supposed to stain mycolic acids in the mycobacterial cell wall, 3 unidentified substances in the egg shell were proposed as target. 2 However, in histopathological examination, biopsies are exposed to concentrated ethanol and xylene during their embedding. This may alter the egg shell and allow some unusual staining reactions not seen in eggs from other sources. In fact, the ZN stain is not consistently positive in feces. 3 In addition, in some intact S. mansoni eggs, the shell was found to be only weakly ZN positive while the miracidium was found intensely positive. 3 Fuchsin is a known nucleic acid stain, 4 and it was already shown that mycobacteria with insufficiently retained carbolfuchsin may be invisible in bright-field microscopy. Yet, they can be easily detected because of a strong red fluorescence when excited with green light. 5
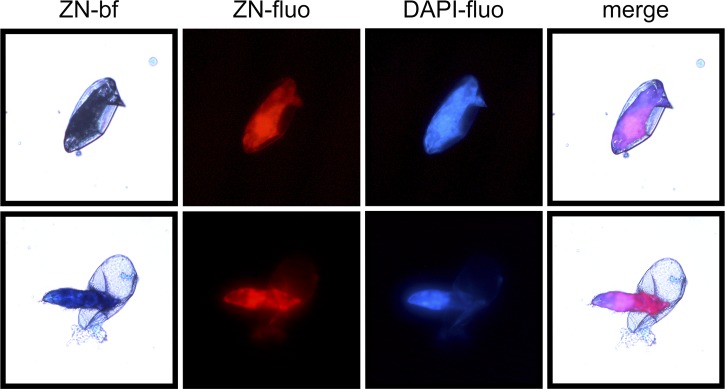
A smear of S. mansoni eggs was prestained with the nucleic acid stain 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) and then stained with the common ZN procedure. The smear was observed (40× objective) using bright-field and fluorescent microscopy where carbolfuchsin fluoresces red (ZN-fluo) 5 and DAPI fluoresces blue (DAPI-fluo) ( Figure 1 ). In bright-field microscopy, the shell appears to stain very little, whereas the miracidium within the intact egg and outside the egg appears acid-fast negative, apparently only retaining the counterstain methylene blue. Contrary to this, fluorescent microscopy shows strong staining of the miracidium with carbolfuchsin (ZN-fluo) and DAPI (DAPI-fluo). The co-localization (merged) reinforces the idea that carbolfuchsin is indeed a nucleic acid stain. 4 Because acid-fast stains and low-cost light-emitting diode fluorescent microscopy are now commonly used in many regions where schistosomiasis is endemic, it may be the time to revisit the staining mechanisms of acid-fast stains 4 and investigate the use of these stains for their capacity to improve the detection of Schistosoma eggs.
Figure 1.
Bright-field and fluorescent image of ZN-stained S. mansoni eggs.
Footnotes
Authors' addresses: Soraia Vieira and Thomas Hänscheid, Instituto de Microbiologia, Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal, E-mails: soraiavieira@medicina.ulisboa.pt and t.hanscheid@medicina.ulisboa.pt. Silvana Belo, Instituto de Higiene e Medicina Tropical, Universidade Nova de Lisboa, Medical Parasitology Unit, Global Health and Tropical Medicine, Lisbon, Portugal, E-mail: silvanabelo@ihmt.unl.pt.
References
- 1.Bustinduy AL, King CH. Schistosomiasis. In: Farrer J, Hoetz PJ, Junghanss T, Kang G, Lalloo D, White NJ, editors. Manson's Tropical Diseases. 23rd edition. Philadelphia, PA: Saunders Ltd.; 2014. pp. 698–725. [Google Scholar]
- 2.Lichtenberg F, Lindenberg M. An alcohol-acid-fast substance in eggs of Schistosoma mansoni. Am J Trop Med Hyg. 1954;3:1066–1076. [PubMed] [Google Scholar]
- 3.Richards OW. The staining of acid-fast tubercle bacteria. Science. 1941;93:190. doi: 10.1126/science.93.2408.190. [DOI] [PubMed] [Google Scholar]
- 4.Hänscheid T, Ribeiro CM, Shapiro HM, Perlmutter NG. Fluorescence microscopy for tuberculosis diagnosis. Lancet Infect Dis. 2007;7:236–237. doi: 10.1016/S1473-3099(07)70058-0. [DOI] [PubMed] [Google Scholar]
- 5.Hänscheid T, Badura R, Fernandes ML, Antunes F, Cristino JM. The case of the disappearing mycobacteria in Ziehl-Neelsen-stained smears. Int J Infect Dis. 2011;15:e291. doi: 10.1016/j.ijid.2011.01.002. [DOI] [PubMed] [Google Scholar]