Abstract
Chromobacterium violaceum is a bacterium associated with soil and water exposure in tropical regions and causes rare and serious clinical infections that are often fatal. We reviewed the demographic and clinical details of 28 patients with C. violaceum detected over 15 years from 2000 to 2015, from the Top End of the Northern Territory. Of these patients, 18 had infections attributable to C. violaceum. Patients with infections were more commonly male (55.6%), and in the 16- to 60-year (61.1%) age group. Skin and soft tissue infections (50%), predominantly involving the limbs, were the major clinical manifestation. Water, mud exposure, and trauma were all noted as precipitating circumstances and comorbidities were present in 61.1% of the patients with infections. Of the 28 patients, 10 (35.8%) had C. violaceum isolated as an incidental finding or as asymptomatic colonization; these 10 patients did not require or receive therapy for C. violaceum bacterial infections. There were no relapsing infections in this group. Chromobacterium violaceum remains a serious infection, with seven patients (25%) in our series requiring intensive care management. However, the mortality rate (7.1%) in our series was far lower than previously described. This case series of C. violaceum infections from a single geographic area provides additional information of the characteristics of infection with this pathogen.
Introduction
Chromobacterium violaceum is one of the four oxidase-positive gram-negative bacteria, Vibrio spp., Aeromonas spp., C. violaceum, and Shewanella spp. (known as VACS),1 associated with waterborne infections in tropical regions. Chromobacterium violaceum is a gram-negative bacillus characterized by the growth of smooth violet-black colonies on common laboratory media due to the pigment, violacein.2 A total of 154 cases of C. violaceum have been previously published. Location data available for 143 cases reveal a worldwide tropical distribution comprising western Pacific (Vietnam, Japan, Korea, Cambodia, Malaysia, China, Australia, Singapore, Laos, and Papua New Guinea) with 49 cases (34.3%),3–12 the Americas with 46 cases (30.0%),3,13,14 southeast Asia (Thailand, India, Sri Lanka, and Nepal) with 23 cases (16.0%),3,15–22 Africa with 22 cases (15.4%),23,24 Persian Gulf with two cases (1.4%),25 and Europe with one case (0.7%).26
Chromobacterium violaceum is associated with a spectrum of disease from localized skin and soft tissue infection (SSTI) to systemic or invasive infection including necrotizing fasciitis,13 visceral abscesses, osteomyelitis, and central nervous system disease.3 Published cases often describe severe sepsis and high case fatality rates (up to 60%)3 among patients, particularly in children with chronic granulomatous disease (CGD).10,22
In a recent analysis of VACS organisms from the Northern Territory of Australia, C. violaceum had significantly different epidemiology to the other organisms.1 The Top End of the Northern Territory is a geographical area of approximately 500,000 km2, with a 13,500 km coastline, tropical climate, and relatively low population density. The aim of this case series was to assess in detail the geographical distribution, demographic and clinical characteristics, and outcomes of C. violaceum infections in the Top End of the Northern Territory between January 2000 and March 2015 and assess factors associated with infection compared with colonization.
Methods
Cases of C. violaceum were identified between January 2000 and March 2015 using the common laboratory database of the Top End public hospitals (Darwin, Katherine, and Gove hospitals). As the public microbiology laboratory is the only microbiology laboratory in the Northern Territory, we believe our case detection method using the laboratory database identifies the majority of infections that are laboratory confirmed. Population prevalence was derived from Australian Bureau of Statistical Data for the Northern Territory (population data were urban population compared with remote population over the 15-year period of the study). Data on identified C. violaceum cases were linked to government electronic health records and patient files to collect information on demographics (age, sex, and residence), site of infection, potential exposure source, comorbidities (diabetes, cancer, immunosuppression, hazardous alcohol use, chronic lung disease/smoking, chronic liver disease, and chronic kidney disease), severity (hospitalization, intensive care unit [ICU] admission), antibiotic therapy, and clinical outcomes (cure or death at 30 days from sample isolation). Colonization or an incidental finding of bacteria was defined as the presence of the bacteria, without signs of illness or infection attributable to the bacteria, when a probable alternative diagnosis was present or when deemed clinically insignificant by the treating team and no directed therapy given.
Laboratory isolates were identified by biochemical and phenotypic methods, including API (BioMerieux, Marcy l'Etoile, France) and VITEK 2 (BioMerieux). As Clinical and Laboratory Standards Institute (Wayne, PA) and EUCAST breakpoints are not available for C. violaceum, antimicrobial susceptibilities were interpreted using Pseudomonas aeruginosa susceptibility criteria as indicative susceptibilities, for the following antibiotics: piperacillin–tazobactam, meropenem, ceftazidime, cefepime, ciprofloxacin, and the aminoglycosides.1 Statistical analysis was performed using χ2 or Fisher's exact tests where appropriate (Microsoft Excel 2010, Redmond, WA).
The study was registered with the Human Research Ethics Committee (HREC) of the Northern Territory Department of Health and Menzies School of Health Research (HREC reference number 2015-2359).
Results
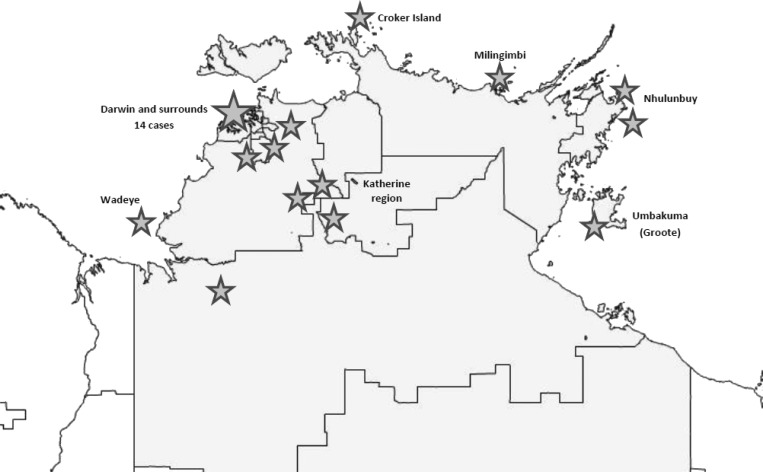
During the 15-year study period, 28 patients with C. violaceum were identified with 12 cases in the last 5 years compared with 16 cases in the 10 years from 2000. Patients with C. violaceum isolated resided more commonly in coastal regions, and though there were 14 isolations from the major population center of Darwin (Figure 1 ), the prevalence was 1.03/10,000 population in urban centers, over the 15-year period compared with 3.25/10,000 population in remote areas of the Top End. Isolation of C. violaceum did not occur in the inland centers of the Northern Territory (Alice Springs or Tennant Creek hospitals) in arid Central Australia, where year-round rainfall is low, and saltwater is absent. There was no obvious seasonal pattern of infection apparent, as infections occurred year-round.
Figure 1.
Residential locations of patients with Chromobacterium violaceum detected in the Top End of the Northern Territory, 2000–2015. Stars indicate the location of individual cases from that location.
Of the 28 patients with C. violaceum isolated, 10 met the criteria for colonization. Table 1 displays the demographic details and risk factors for both colonized and infected patients. There were no significant differences between the two groups according to demographics and clinical characteristics. The median age of all patients was 40 years (range = 0–82 years). Colonized patients were younger (mean age = 29.4 years), compared with infected patients (mean age = 42.5 years) and more commonly female (70%), but this was not statistically significant. Infected patients were commonly male (55.6%) between the ages of 16 and 60 (61.1%) years.
Table 1.
Demographic details of patients with Chromobacterium violaceum detected in the Northern Territory 2000–2015
| Demographic details of patients with C. violaceum isolated | Number of patients (%) | P value | Total (%) | |
|---|---|---|---|---|
| Incidental | Infected | |||
| Sex | ||||
| Female | 7 (70) | 8 (44.4) | 0.19 | 15 (53.6) |
| Male | 3 (30) | 10 (55.6) | 13 (46.4) | |
| Age (years) | ||||
| 0–15 | 5 (50) | 3 (16.7) | 0.11 | 8 (28.6) |
| 16–60 | 3 (30) | 11 (61.1) | 14 (50) | |
| > 60 | 2 (20) | 4 (22.2) | 6 (21.4) | |
| Specimen site (of initial isolate) | ||||
| Skin and soft tissue | 3 (30) | 9 (50) | 0.3 | 12 (66.7) |
| Lower limb | 3 (100) | 4 (44.4) | 7 (58.3) | |
| Upper limb | 0 | 3 (33.3) | 3 (25) | |
| Torso | 0 | 2 (22.2) | 2 (16.7) | |
| Respiratory | 3 (30) | 3 (16.7) | 6 (21.4) | |
| Feces | 4 (40) | 0 | 4 (14.3) | |
| Urine | 0 | 4 (22.2) | 4 (14.3) | |
| Blood | 0 | 2 (11.1) | 2 (7.1) | |
| Precipitating circumstances | ||||
| Water exposure | 2 (20) | 3 (16.7) | 0.23 | 5 (17.9) |
| Trauma | 2 (20) | 3 (16.7) | 5 (17.9) | |
| Remote location | 1 (10) | 3 (16.7) | 4 (14.3) | |
| Mud exposure | 0 | 3 (16.7) | 3 (10.7) | |
| Insect bite | 0 | 1 (5.6) | 1 (3.6) | |
| Nil recorded | 5 (50) | 5 (27.8) | 10 (35.7) | |
| Comorbidities (one or more) | ||||
| Alcohol excess | 1 (10) | 5 (22.2) | 0.28 | 6 (21.4) |
| Malignancy | 2 (20) | 3 (16.7) | 5 (17.9) | |
| Chronic lung disease/smoker | 2 (20) | 2 (11.1) | 4 (14.3) | |
| Diabetes | 0 | 3 (16.7) | 3 (10.7) | |
| Chronic liver disease | 1 (10) | 1 (5.6) | 2 (7.1) | |
| Severe chronic renal disease | 0 | 2 (11.1) | 2 (7.1) | |
| Nil | 6 (60) | 7 (38.9) | 13 (46.4) | |
In patients with infection, SSTI was the most frequent infection (50%), with limb infections (77.8%) more common. Two patients (11.1%) presented with septicemia. In the colonization group, fecal (40%) and respiratory (30%) sites were the most common.
Thirteen patients with infections (62.2%) had exposure to water, mud, or a traumatic episode, compared with 50% of the colonized patients.
Comorbidities were common, including malignancy, diabetes, and excessive alcohol use in 61.1% of infected patients, compared with comorbidities being present in 40% of patients who had C. violaceum as an incidental finding.
Co-isolation of other bacteria (Table 2) was present in 17 patients (60.7%) and more commonly in patients with infection (72.2%) compared with patients with colonization (40%). Co-pathogens included Enterobacteriaceae (28.6%), environmental gram negatives (21.4%), and Staphylococcus aureus (21.4%).
Table 2.
Co-pathogenic bacteria isolated in conjunction with Chromobacterium violaceum
| Presence of coexisting bacterial isolates | Number of patients (%) | Total (%) | |
|---|---|---|---|
| Incidental | Infected | ||
| Co-pathogen (one or more) | 4 (40) | 13 (72.2) | 17 (60.7) |
| Enterobacteriaceae | 1 (10) | 7 (38.9) | 8 (28.6) |
| Non-fermentative gram negative* | 2 (20) | 3 (16.7) | 6 (21.4) |
| Staphylococcus aureus | 0 | 4 (22.2) | 6 (21.4) |
| Yeast | 2 (20) | 1 (5.6) | 3 (10.7) |
| β-hemolytic streptococci | 1 (10) | 2 (11.1) | 3 (10.7) |
| Other VACS organisms | 0 | 2 (11.1) | 2 (7.1) |
| Anaerobes | 0 | 1 (5.6) | 1 (3.6) |
| Acinetobacter sp. | 0 | 1 (5.6) | 1 (3.6) |
| Fecal specimen† | 4 (40) | 0 | 4 (14.3) |
| No other bacterial isolates | 2 (20) | 5 (27.8) | 7 (25) |
VACS = Vibrio spp., Aeromonas spp., C. violaceum, and Shewanella spp.
Stenotrophomonas maltophilia, Chryseobacterium indologenes, Pseudomonas aeruginosa, and Burkholderia cepacia.
Presence of the other bacteria in four patients with fecal detection of C. violaceum not listed.
Table 3 lists the characteristics of the 18 individuals with C. violaceum infection and Table 4 lists the 10 patients with likely colonization or incidental isolation. Of the 28 patients, two (7.1%) with C. violaceum died as a direct result of the infection during the 15-year study period. Seven patients (25%) had severe disease defined by ICU admission during the clinical episode.
Table 3.
Clinical features of patients with Chromobacterium violaceum infections
| Case number | Sex | Diagnosis year | Age at diagnosis | Site of isolate | Clinical disease | Mixed culture | Treatment | Outcome | Notes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 2000 | 23 | Lower limb | SSTI | Coliform sp. | Co-trimoxazole. Prior amoxycillin had failed | Resolved | Jeep bogged in mud, fell onto branch; leg wound. 8 months later, re-explored – chronic infection with granulation tissue. |
| 2 | M | 2000 | 12 | Upper limb | SSTI | Mixed coliforms | Cephazolin + gentamicin initially, 7 days co-trimoxazole | Resolved | Right elbow laceration, fell off a motorbike |
| 3 | F | 2001 | 42 | Torso | SSTI | Mixed coliforms, yeast on separate tissue | Piperacillin–tazobactam 3 days, then meropenem gentamicin 3/7 | Deceased | Polymicrobial gram-negative sepsis from ischemic bowel, abdominal wall abscess, and fat necrosis |
| 4 | F | 2001 | 53 | Lower limb | SSTI | Staphylococcus aureus | Gentamicin 7 days. Co-trimoxazole 2 weeks | Resolved | Diabetic foot infection—deep plantar collection |
| 5 | F | 2004 | 78 | Urine | Urinary tract infection | Pure growth | Co-trimoxazole 1 month | Resolved | Co-morbidity – ovarian malignancy with ascites |
| 6 | M | 2004 | 52 | Torso | SSTI | – | Piperacillin–tazobactam 10 days | Resolved | Sternal wound sepsis post cardiac graft surgery |
| 7 | M | 2006 | 19 | Sputum | Ventilator pneumonia | Burkholderia cepacia | Meropenem 4 days | Resolved | Trauma—hit by fiancé's ex with a bat. Subdural, ventilator pneumonia. No other exposure history |
| 8 | M | 2006 | 32 | Lower limb | SSTI | – | Co-trimoxazole 3 weeks | Resolved | Laceration third, fourth toe while in swamp. Pus drained |
| 9 | F | 2006 | 61 | Urine | Severe sepsis, urosepsis | Escherichia coli | Meropenem, then piperacillin–tazobactam | Resolved | Patient was septic from urosepsis, coinfection with E. coli and C. violaceum |
| 10 | M | 2008 | 57 | Torso | SSTI | Aeromonas sp., Acinetobacter sp., mixed anaerobes, enteric flora | Meropenem 14 days | Resolved | Found in creek outside hospital, chest wall infection |
| 11 | F | 2008 | 38 | Blood | Septicemia, pneumonia | – | Meropenem 1 week | Deceased | Homeless patient presented with severe sepsis |
| 12 | F | 2009 | 9 | Urine | Urinary tract infection | – | Co-trimoxazole 2 weeks | Resolved | Recurrent urosepsis, patient with vesicoureteric reflux |
| 13 | M | 2010 | 45 | Upper limb | SSTI | Staphylococcus aureus, Vibrio spp., group G Streptococcus | Doxycycline | Resolved | Stab injury to right hand |
| 14 | M | 2012 | 50 | Lower limb | SSTI | Enteric flora, cutaneous flora | Cephazolin, then doxycycline | Resolved | Toe abscess was debrided |
| 15 | M | 2013 | 76 | Sputum | Exacerbation chronic pulmonary disease | Stenotrophomonas maltophilia, Chryseobacterium indologenes | Co-trimoxazole | Resolved | Chromobacterium violaceum present with other environmental gram-negative bacteria |
| 16 | M | 2014 | 82 | Urine | Urinary tract infection | Pseudomonas sp. | Ceftazidime 2 days, then norfloxacin | Resolved | Symptomatic urinary tract infection, with C. violaceum and Pseudomonas aeruginosa both present |
| 17 | M | 2015 | 21 | Upper limb | SSTI | S. aureus, coliforms | Debridement, flucloxacillin, then amoxycillin-clavulanic acid | Resolved | Spider bite, abscess debrided, C. violaceum also present with S. aureus |
| 18 | F | 2015 | 15 | Blood | Skin wound, bacteremia, liver abscess | Group B Streptococcus, S. aureus | Meropenem 2 weeks, ciprofloxacin, doxycycline 4 weeks | Resolved | Liver abscesses and septicemia, required prolonged intensive care unit admission |
F = female; M = male; SSTI = skin and soft tissue infection.
Table 4.
Clinical features of patients with Chromobacterium violaceum considered incidental or minor in nature
| Case number | Sex | Diagnosis year | Age at diagnosis | Site of isolate | Mixed culture | Therapy for admission diagnosis | Outcome | Notes |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 2004 | 33 | Sputum | Oral flora, yeast, Pseudomonas | Piperacillin–tazobactam for 4 days | Resolved | Patient in hospital after a motor vehicle accident. Chromobacterium violaceum noted on sputum, thought to be incidental |
| 2 | M | 2006 | 53 | Sputum | Chryseobacterium sp., Stenotrophomonas sp., yeast | Ceftazidime 4 weeks. Co-trimoxazole 2–3 months for melioidosis | Resolved | Patient had disseminated melioidosis with abscesses in prostate and spleen. Incidental C. violaceum in feces |
| 3 | F | 2006 | 5 | Feces | – | Metronidazole 3 days | Resolved | Patient being investigated for chronic colitis. Chromobacterium violaceum in feces was felt to be incidental |
| 4 | F | 2009 | 15 | Skin | Group A Streptococcus | Flucloxacillin 4 weeks | Resolved | Septic laceration left leg, resolved with therapy for group A Streptococcus. No therapy for C. violaceum |
| 5 | M | 2011 | 54 | Lower limb | – | Dicloxacillin 10 days | Resolved | Admitted with knee cellulitis. This resolved with 48 hours antibiotics before the swab result became available |
| 6 | M | 2013 | 1 | Feces | – | Flucloxacillin and co-trimoxazole 12 days in total | Resolved | Incidental fecal finding, culture performed for inpatient diarrhea. Patient admitted with facial abscess |
| 7 | F | 2013 | 64 | Sputum | Not listed | Nil | Resolved | Exacerbation of severe chronic pulmonary disease. Chromobacterium violaceum in sputum, not treated, the exacerbation resolved |
| 8 | F | 2013 | 4 | Feces | – | Nil | Resolved | Self-limiting diarrhea in both patient and her sister |
| 9 | F | 2013 | 64 | Skin | Cutaneous flora, mixed coliforms | Nil | Resolved | Chronic calf wound for 2 months. Chromobacterium violaceum detected on a wound swab (not present on earlier swabs) and not treated |
| 10 | F | 2014 | 1 | Feces | – | One dose of ceftriaxone before detection of bacteria | Resolved | Admitted after a 9-day febrile illness with diarrhea and an irritable hip, spontaneous resolution of diarrhea |
F = female; M = male.
Susceptibilities of C. violaceum are presented in Table 5. All isolates had inferred susceptibility to ciprofloxacin, gentamicin, and meropenem. Minimum inhibitory concentration to ciprofloxacin was 0.012 mg/L for three isolates. Susceptibility to other antimicrobials, including ceftazidime (30%), amikacin (87.6%), tobramycin (75%), and co-trimoxazole (TMP-SMX) (88.9%) was found, though the overall numbers of isolates tested was small.
Table 5.
Chromobacterium violaceum antimicrobial susceptibility results
| Antibiotic | C. violaceum |
|---|---|
| Isolates tested (% susceptible) | |
| Amikacin | 8 (87.6) |
| Ampicillin | 6 (0) |
| Amoxycillin–clavulanate | 7 (0) |
| Ceftriaxone | 5 (20) |
| Ceftazidime | 10 (30) |
| Cephazolin | 4 (0) |
| Ciprofloxacin | 13 (100) |
| Cefepime | 4 (100) |
| Gentamicin | 13 (100) |
| Meropenem | 9 (100) |
| Piperacillin–tazobactam | 6 (84) |
| Ticarcillin–clavulanate | 9 (22) |
| Tobramycin | 8 (75) |
| Co-trimoxazole | 9 (88.9) |
Comparison data with the other VACS organisms is presented in Table 6. These organisms were isolated from patients from the same geographic location, in the same period and are all associated with tropical waterborne infections.
Table 6.
Comparison of demographic and clinical details Chromobacterium violaceum to other VACS organisms
| C. violaceum | Aeromonas spp.* | Vibrio spp.* | Shewanella spp.* | |
|---|---|---|---|---|
| No. of bacterial isolates | 28 | 312 | 71 | 61 |
| Sex | ||||
| Males (% of total) | 13 (46) | 203 (68) | 47 (77) | 51 (84) |
| Age | ||||
| 0–15 | 8 (28.6) | 27 (9) | 8 (13) | 2 (3) |
| 16–60 | 14 (50) | 223 (74) | 43 (70) | 51 (84) |
| > 60 | 6 (21.4) | 50 (17) | 10 (16) | 8 (13) |
| Site of isolation (%) | ||||
| Skin and soft tissue | 12 (42.9) | 260 (83) | 52 (73) | 50 (82) |
| Blood | 2 (7.1) | 11 (3.5) | 7 (9.9) | 6 (9.8) |
| Feces | 4 (14.3) | 16 (5.1) | 6 (8.5) | 0 (0) |
| Respiratory | 6 (21.4) | 4 (1.3) | 2 (2.8) | 4 (6.6) |
| Peritoneal | 0 (0) | 3 (1.0) | 1 (1.4) | 0 (0) |
| Bile | 0 (0) | 2 (0.6) | 0 (0) | 0 (0) |
| Bone | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) |
| Eye | 0 (0) | 6 (1.9) | 1 (1.4) | 1 (1.6) |
| Urine | 4 (14.3) | 5 (1.6) | 1 (1.4) | 0 (0) |
| Genital tract | 0 (0) | 3 (1.0) | 0 (0) | 0 (0) |
| Ear | 0 (0) | 1 (0.3) | 1 (1.4) | 0 (0) |
| ICU admission (% of total) | 7 (25) | 13 (4.2) | 3 (4.1) | 4 (6.6) |
ICU = intensive care unit; VACS = Vibrio spp., Aeromonas spp., C. violaceum, and Shewanella spp.
Isolate data from 2000 to 2013 inclusive, as reported by McAuliffe and others.1
Discussion
Our series of C. violaceum cases from the Top End of the Northern Territory provides some novel clinical insights into the nature of infection caused by this tropical and water-associated bacterium. We present a relatively large case series of C. violaceum infection from a single center collected over 15 years. Consistent with previous reports in the literature documented, most C. violaceum infections manifested as SSTI with exposure of wounds to environmental water or soil.
In comparison with other waterborne tropical VACS organisms, C. violaceum epidemiology in the Northern Territory differs significantly with respect to sex, age, and site of isolation compared with the other organisms.1 Chromobacterium violaceum patients were more evenly matched for sex (M:F ratio 0.85:1 versus 2:1), infections occurred more commonly in children < 15 years of age (28.6% versus 8.3%) and isolates from feces (14.3% versus 5.0%), respiratory samples (21.4% versus 2.2%), and urine (14.3% versus 1.4%) was more frequent, compared with the pooled results of the other three VACS organisms, respectively.1
Chromobacterium violaceum was also more commonly isolated from patients in ICU (seven cases, 25%) than patients with Vibrio spp. (three cases, 4.1%), Aeromonas spp. (13 cases, 4.2%), or Shewanella spp. (four cases, 6.6%) (for all P < 0.05), supporting the potential seriousness and virulence of the organism.1
In contrast to previous case series, which have relied heavily on clinical reporting of notable cases, we reviewed all laboratory isolates of C. violaceum in one location. This resulted in the identification of 10 cases (35.7%) that were not associated with clinical disease, demonstrating a role of C. violaceum in asymptomatic colonization of humans. Previous reports of asymptomatic infection are rare.15
Severe illness due to C. violaceum was frequent (25%), but the overall mortality rate in this series was small (7.1%) compared with previous reviews that have reported mortality of 53%.3 Potential causes of the reduced mortality include 1) C. violaceum is a relatively rare infection with only 154 cases reported, and therefore published case reports tend to favor serious infection, over more mild disease, as series from one location are not common; 2) It is not clear what determines the clinical disease and virulence in C. violaceum. Host risk factors such as CGD and glucose-6-phosphate dehydrogenase (G6PD) deficiency exist, but the many cases have been reported in immunocompetent patients. Microbial factors may be more significant. The pigment violacein has been related to cytotoxic activity; however, non-pigmented strains have been isolated with similar clinical severity.27 It is possible that there are strain differences with respect to virulence factors,28 postulated to be related to elevated levels of superoxide dismutase and catalase. Potentially, strains from both hemispheres and different disease manifestations could be compared by whole-genome sequencing as a next step. There is a precedent with other tropical water-associated bacteria such as Burkholderia pseudomallei having geographic strain differences29; or 3) As the Top End of the Northern Territory is an endemic zone for B. pseudomallei, the bacteria that causes melioidosis, protocols for sepsis include the early empiric use of meropenem. These measures have improved outcomes in patients presenting with septic presentations of melioidosis,30 and our low C. violaceum mortality may be an unexpected side benefit. Meropenem usually has excellent activity against C. violaceum and may explain the lower mortality seen.
The limitations of this study, by its retrospective nature, were the inability to have formal G6PD and CGD testing performed on the patient group. In future, more formal immune function and G6PD screening should be considered, particularly in pediatric patients.
The findings of this case review confirm that C. violaceum is a rare human pathogen, with 28 clinical isolates identified over 15 years in a tropical setting. This pathogen has the potential to cause severe and fatal disease. However, the increased frequency of asymptomatic colonization, and less severe clinical spectrum of disease reported, suggest that in the endemic setting, C. violaceum may not be as pathogenic as previously thought. Early targeted antibiotic therapy and early sepsis recognition may be factors associated with improved outcomes.
ACKNOWLEDGMENTS
We thank Laboratory staff of the Top End public health laboratories.
Footnotes
Authors' addresses: Yi dan Lin, Department of Infectious Diseases, Royal Darwin Hospital, Darwin, Australia, E-mail: yidanl@gmail.com. Suman S. Majumdar, Centre for International Health, Burnet Institute, Victoria, Australia, E-mail: suman.majumdar@burnet.edu.au. Jann Hennessy and Robert W. Baird, Department of Microbiology, Royal Darwin Hospital, Darwin, Australia, E-mails: jann.hennessy@nt.gov.au and rob.baird@nt.gov.au.
References
- 1.McAuliffe GN, Hennessy J, Baird RW. Relative frequency, characteristics and antimicrobial susceptibility patterns of Vibrio spp., Aeromonas spp., Chromobacterium violaceum, and Shewanella spp. in the northern territory of Australia, 2000–2013. Am J Trop Med Hyg. 2014;92:605–610. doi: 10.4269/ajtmh.14-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitman WB, Goodfellow M, Kämpfer P, Busse H-J, Trujillo M, Ludwig W, K-i Suzuki, Parte A, editors. Bergey's Manual of Systematic Bacteriology. New York, NY: Springer-Verlag; 2012. [Google Scholar]
- 3.Yang CH, Li YH. Chromobacterium violaceum infection: a clinical review of an important but neglected infection. J Chin Med Assoc. 2011;74:435–441. doi: 10.1016/j.jcma.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Yang CH. Nonpigmented Chromobacterium violaceum bacteremic cellulitis after fish bite. J Microbiol Immunol Infect. 2011;44:401–405. doi: 10.1016/j.jmii.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JI, Lan NPH, Qui PT, Dung LT, Farrar JJ, Baker S. A successful antimicrobial regime for Chromobacterium violaceum induced bacteremia. BMJ Infectious Diseases. 2013;13:4. doi: 10.1186/1471-2334-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheong BM. A fatal case of pulmonary Chromobacterium violaceum infection in an adult. Med J Malaysia. 2010;65:148–149. [PubMed] [Google Scholar]
- 7.Hagiya H, Murase T, Suzuki M, Shibayama K, Kokumai Y, Watanabe N, Maki M, Otsuka F. Chromobacterium violaceum nosocomial pneumonia in two Japanese patients at an intensive care unit. J Infect Chemother. 2014;20:139–142. doi: 10.1016/j.jiac.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Ke L, An KP, Heng S, Riley M, Sona S, Moore C, Parry C, Stoesser N, Chanpheaktra N. Paediatric Chromobacterium violaceum in Cambodia: the first documented case. Trop Doct. 2012;42:178–179. doi: 10.1258/td.2012.120054. [DOI] [PubMed] [Google Scholar]
- 9.Lim IW, Stride PJ, Horvath RL, Hamilton-Craig CR, Chau PP. Chromobacterium violaceum endocarditis and hepatic abscesses successfully treated with meropenem and ciprofloxacin. Med J Aust. 2009;190:386–387. doi: 10.5694/j.1326-5377.2009.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 10.Huffam SE, Nowotny MJ, Currie BJ. Chromobacterium violaceum in tropical northern Australia. Med J Aust. 1998;168:335–337. doi: 10.5694/j.1326-5377.1998.tb138962.x. [DOI] [PubMed] [Google Scholar]
- 11.Baker S, Campbell JI, Stabler R, Nguyen HV, To DS, Nguyen DV, Farrer J. Fatal wound infection caused by Chromobacterium violaceum in Ho Chi Minh City, Vietnam. J Clin Microbiol. 2008;46:3853–3855. doi: 10.1128/JCM.01068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slesak G, Douangdala P, Inthalad S, Silisouk J, Vongsouvath M, Sengduangphachanh A, Moore CE, Mayxay M, Matsuoka H, Newton PN. Fatal Chromobacterium violaceum septicaemia in northern Laos, a modified oxidase test and post-mortem forensic family G6PD analysis. Ann Clin Microbiol Antimicrob. 2009;8:24. doi: 10.1186/1476-0711-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seigal JK, Stadler ME, Lombrano JL, Almony JS, Couch ME, Belhorn TH. Chromobacterium violaceum necrotizing fasciitis: a case report and review of the literature. Ear Nose Throat J. 2012;91:479–483. doi: 10.1177/014556131209101108. [DOI] [PubMed] [Google Scholar]
- 14.Richard K, Lovvorn J, Oliver S, Ross S, Benner K, Kong M. Chromobacterium violaceum sepsis: rethinking conventional therapy to improve outcome. Am J Case Rep. 2015;16:740–744. doi: 10.12659/AJCR.894509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pant ND, Sharma M, Khatiwada S. Asymptomatic bacteriuria caused by Chromobacterium violaceum in an immunocompetent adult. Case Rep Med. 2015;2015:652036. doi: 10.1155/2015/652036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pant ND, Sharma M. Urinary tract infection caused by Chromobacterium violaceum. Int J Gen Med. 2015;2015:293–295. doi: 10.2147/IJGM.S89886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swain B, Otta S, Sahu KK, Panda K, Rout S. Urinary tract infection by Chromobacterium violaceum. J Clin Diagn Res. 2014;8:DD01–DD02. doi: 10.7860/JCDR/2014/9230.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saboo AR, Vijaykumar R, Save SU, Bavdekar SB. A rare nonfatal presentation of disseminated Chromobacterium violaceum sepsis. J Microbiol Immunol Infect. 2015;48:574–577. doi: 10.1016/j.jmii.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Kumar MR. Chromobacterium violaceum: a rare bacterium isolated from a wound over the scalp. Int J Appl Basic Med Res. 2012;2:70–72. doi: 10.4103/2229-516X.96814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madi DR, Vidyalakshmi K, Ramapuram J, Shetty AK. Case report: successful treatment of Chromobacterium violaceum sepsis in a south Indian adult. Am J Trop Med Hyg. 2015;93:1066–1067. doi: 10.4269/ajtmh.15-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karthik R, Pancharatnam P, Balaji V. Fatal Chromobacterium violaceum septicemia in a south Indian adult. J Infect Dev Ctries. 2012;6:751–755. doi: 10.3855/jidc.1866. [DOI] [PubMed] [Google Scholar]
- 22.Ray P, Sharma J, Marak RS, Singhi S, Taneja N, Garg RK, Sharma M. Chromobacterium violaceum septicaemia from north India. Indian J Med Res. 2004;120:523–526. [PubMed] [Google Scholar]
- 23.Anah MU, Udo JJ, Ochigbo SO, Abia-Bassey LN. Neonatal septicaemia in Calabar, Nigeria. Trop Doct. 2008;38:126–128. doi: 10.1258/td.2006.006037. [DOI] [PubMed] [Google Scholar]
- 24.Bottieau E, Mukendi D, Kalo JR, Mpanya A, Lutumba P, Barbe B, Chappuis F, Lunguya O, Boelaert M, Jacobs J. Fatal Chromobacterium violaceum bacteraemia in rural Bandundu, Demographic Republic of Congo. New Microbes New Infect. 2015;3:21–23. doi: 10.1016/j.nmni.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Khalifa SM, Al Khaldi T, Algahtani MM, Al Ansari AM. Two siblings with fatal Chromobacterium violaceum sepsis linked to drinking water. BMJ Case Rep. 2015;2015:pii bcr2015210987. doi: 10.1136/bcr-2015-210987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arosio M, Raglio A, Ruggeri M, Serna Ortega P, Morali L, De Angelis C, Goglio A. Chromobacterium violaceum lymphadenitis successfully treated in a northern Italian hospital. New Microbiol. 2011;34:429–432. [PubMed] [Google Scholar]
- 27.Sivendra R, Tan SH. Pathogenicity of nonpigmented cultures of Chromobacterium violaceum. J Clin Microbiol. 1977;5:514–516. doi: 10.1128/jcm.5.5.514-516.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller DP, Blevins WT, Steele DB, Stowers MD. A comparative study of virulent and avirulent strains of Chromobacterium violaceum. Can J Microbiol. 1988;34:249–255. doi: 10.1139/m88-046. [DOI] [PubMed] [Google Scholar]
- 29.Podin Y. Burkholderia pseudomallei isolates from Sarawak, Malaysian Borneo, are predominantly susceptible to aminoglycosides and macrolides. Antimicrob Agents Chemother. 2014;58:162–166. doi: 10.1128/AAC.01842-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitman MC, Luck T, Marshall CS, Anstey NM, Ward L, Currie BJ. Intravenous therapy duration and outcomes in melioidosis: a new treatment paradigm. PLoS Negl Trop Dis. 2015;93:e0003586. doi: 10.1371/journal.pntd.0003586. [DOI] [PMC free article] [PubMed] [Google Scholar]