Abstract
Interferon-gamma (IFN-γ) release assays (IGRAs) are used to detect cellular immune recognition of Mycobacterium tuberculosis. The chemokine IFN-γ-inducible protein 10 (IP-10) is an alternative diagnostic biomarker to IFN-γ. Several conditions interfere with IGRA test performance. We aimed to assess the possible influence of Plasmodium falciparum infection on the IGRA test QuantiFERON-TB GOLD® In-Tube (QFT) test and an in-house IP-10 release assay. In total, 241 Tanzanian adults were included; 184 patients with uncomplicated malaria (88 human immunodeficiency virus [HIV] coinfected) and 57 HIV-infected patients without malaria infection. Malaria was treated with artemether–lumefantrine (Coartem®). QFT testing was performed before initiation of malaria treatment and at days 7 and 42. In total, 172 patients completed follow-up. IFN-γ and IP-10 was measured in QFT supernatants. We found that during malaria infection IFN-γ and IP-10 levels in the unstimulated samples were elevated, mitogen responsiveness was impaired, and CD4 cell counts were decreased. These alterations reverted after malaria treatment. Concurrent malaria infection did not affect QFT test results, whereas there were more indeterminate IP-10 results during acute malaria infection. We suggest that IGRA and IP-10 release assay results of malaria patients should be interpreted with caution and that testing preferably should be postponed until after malaria treatment.
Introduction
Interferon-gamma (IFN-γ) release assays (IGRAs) are used to detect cellular immune recognition of Mycobacterium tuberculosis (Mtb) by measuring IFN-γ released by T cells on stimulation with peptide antigens specific for Mtb. The IGRAs are used to diagnose latent tuberculosis infections (LTBI). They have a high specificity, but are imperfect as they cannot differentiate between active and LTBI and have a sensitivity around 80%.1 A series of immunosuppressive conditions are known to compromise the IGRA test performance including human immunodeficiency virus (HIV) infection and low CD4 cell count,2–5 glucocorticoid treatment,6 cigarette smoking,7 severe disease in children,8 and helminth infection.9,10 So far, no systematic evaluation of the possible effect of malaria infection on IGRA test performance has been done.
IFN-γ-inducible protein 10 (IP-10) release assay is an alternative to IGRA for immunodiagnostic testing of Mtb infection.11 IP-10 is secreted by antigen-presenting cells interacting with T cells recognizing Mtb-specific peptides presented on its surface.12 IP-10 release assays perform on par with the commercially available IGRA QuantiFERON®-TB (Cellestis Ltd., Carnegie, Victoria, Australia) Gold In-Tube (QFT) test,11,12 but contrary to the QFT, IP-10 release assays appear less affected by immunosuppression such as a low CD4-cell count in HIV-infected individuals4,5 and in young-aged children.13,14
Malaria and tuberculosis (TB) are co-endemic in most of sub-Saharan Africa. Malaria is known to have several immunomodulating effects,15,16 including lymphopenia and decreased levels of CD4 cells during acute infection.17–20 Infection with malaria is therefore likely to interfere with the performance of QFT and IP-10 release assays.
With the aim to assess the impact of concurrent malaria infection on QFT and IP-10 release assay performance, we measured IFN-γ and IP-10 levels and evaluated results of QFT and IP-10 release assays before and after malaria treatment in adult HIV-infected and HIV-uninfected malaria patients in Tanzania.
Materials and Methods
Method.
From May 2010 to October 2011, a group of adult (> 15 years of age) patients were consecutively included from the ongoing InterACT study, a clinical trial evaluating clinical and pharmacological interactions between artemisinin-based combination treatment of uncomplicated falciparum malaria and first-line antiretroviral combination treatment of HIV/acquired immune deficiency syndrome (AIDS) in coinfected patients, conducted in Muheza in northeastern Tanzania (L. Vestergaard, unpublished data). Patients were recruited from the HIV/AIDS Care and Treatment Clinic or from the Outpatient Department of Muheza District Hospital. Three categories of patients were recruited for this sub-study: 1) HIV-infected patients with malaria, 2) HIV-uninfected patients with malaria, and 3) HIV-infected patients without malaria. We aimed at including a minimum of 50 patients in each group.
On the day of recruitment, patients had a clinical history collected, including history of possible past TB as well as Mtb exposure. Patients reporting active TB were all on standard anti-TB treatment.
Malaria patients were treated with the standard six-dose regimen of artemether–lumefantrine (Coartem® [Novartis, Cambridge, MA]). Non-malaria patients were treated according to alternative illness identified. Patients in all three study groups were re-examined clinically and tested again for malaria by microscopy after 7 and 42 days.
Laboratory assay.
A malaria rapid test, Paracheck Pf® (Orchid Biomedical Systems, Bambolim, Goa, India), was used to screen for malaria and if positive the diagnosis was confirmed and quantified by standard malaria microscopy. CD4 T-cell counts were determined using CD-4 Cell Counter (Coulter [Beckman Coulter, Brea, CA]). QFT and IP-10 tests were performed as previously described.21–23 In brief, whole blood was drawn into QFT tubes on days 0, 7, and 42 and stimulated with saline (negative control, unstimulated, and nil), Mtb-specific antigens, and mitogen phytohemagglutinin (PHA) (positive control) for 18–24 hours. Samples were centrifuged, and plasma supernatants were stored in cryo tubes at −80°C and shipped to Denmark on dry ice, where cytokine levels were measured. IFN-γ levels were measured using the QuantiFERON-TB GOLD® ELISA (Cellestis Ltd., Carnegie, Victoria, Australia) according to the manufacturer instructions.21 IP-10 levels were analyzed in duplicates using an in-house enzyme-linked immunosorbent assay (ELISA) as described previously.22,23 All samples from the same patient were analyzed on the same ELISA plate to eliminate between-run variation. Test results were classified as positive, negative, or indeterminate according to current diagnostic algorithms for QFT and IP-10 release assays.11,21 For determining the QFT or IP-10 test results, the cytokine level from the unstimulated sample is subtracted from the cytokine level in the antigen and mitogen samples.
Statistics.
Differences in cytokine levels and CD4 counts between study groups at enrollment were analyzed using Mann–Whitney U and Kruskal–Wallis test where malaria patients were stratified by parasite level. Changes in CD4 counts and cytokine levels during follow-up were analyzed using Wilcoxon signed-rank test. Test results for malaria patients according to parasite levels were analyzed using Cochran–Armitage trend test and changes in test results from enrollment to the last follow-up visit using McNemar's test. Agreement between QFT and IP-10 test results were assessed with Cohen's kappa. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC) and Graph Pad Prism 5 (GraphPad Software Inc., La Jolla, CA). As the IGRA reading frame has a defined upper limit of 10 IU/mL, it is not possible to transform the data to a normal distribution and therefore multivariate analysis was not conducted on the cytokine levels. Neither was multivariate analysis conducted on test results as we lacked power for these analyses.
Ethical considerations.
The study protocol was approved by the Medical Research Committee (MRCC) of the National Institute of Medical Research, Ministry of Health, Dar es Salaam, Tanzania, and the InterAct study; NCT00885287 (www.clinicaltrials.gov). All study participants gave their written consent after receiving detail information about the study and all involved procedures.
Results
A total of 241 patients were included in the study: 88 patients with both malaria and HIV, 96 patients with malaria only, and 57 patients with HIV only. Of these patients, 172 completed the full 42 days of follow-up (68, 55, and 49, respectively), and 167 of 172 were parasite free on both days 7 and 42 (67, 51, and 49, respectively). Seven study participants reported active TB (four malaria-HIV coinfected, two malaria-infected, and one non-malaria patient). In general, HIV-infected patients were older and more frequent female sex, reported more history of TB, and had lower median CD4 counts compared with HIV-uninfected patients (P < 0.001 for both HIV-infected groups). CD4 counts of the two HIV-infected groups were comparable (P = 0.48). Most HIV-infected patients were on combination antiretroviral treatment (cART). Two HIV-infected patients (both non-malaria) had initiated cART within 90 days of this study, and none initiated cART during the study period (Table 1). The characteristics of the patients that completed follow-up did not differ significantly from the study participants at enrollment (data not shown).
Table 1.
Baseline demographic characteristics and test results
| Group | Malaria | No malaria | |
|---|---|---|---|
| HIV infected | HIV uninfected | HIV infected | |
| N | 88 | 96 | 57 |
| Male, n (%) | 32 (36) | 54 (56) | 8 (14) |
| Age median (IQR) | 41 (36–47) | 28 (19–42) | 41 (37–46) |
| BMI median (IQR) | 20 (18–23) | 21 (18–23) | 21 (20–24) |
| Malaria | |||
| Plasmodium falciparum median parasite count/μL (IQR) | 3,920 (600–13,640) | 5,120 (680–20,840) | – |
| Hemoglobin (g/dL) | 11.2 (10.4–12.6) | 12.5 (11.0–13.6) | 11.9 (11.1–12.8) |
| HIV | |||
| cART, n/total (%) | 55/86 (64) | – | 57/57 (100) |
| Duration of ART median days (IQR) | 812 (499–1,288) | – | 1,026 (589–1,444) |
| Recently started on cART (< 90 days) | 0 | – | 2/57 |
| CD4 count* (cells/μL) | 382 (242–499) (N = 87) | 571 (488–834) (N = 76) | 382 (267–538) (N = 57) |
| CD4 ≤ 200/μL | 16/87 (18%) | 1/76 (1%) | 11/57 (19%) |
| TB history | |||
| Current TB, receiving anti-TB treatment, n/total (%) | 4/87 (5%) | 2/96 (2%) | 1/57 (2%) |
| Previous TB | 24/87 (28%) | 2/96 (2%) | 15/57 (26%) |
| In contact with person with TB | 23/87 (26%) | 10/95 (11%) | 11/56 (20%) |
| QFT test results | |||
| Positive | 20 (23%) | 17 (18%) | 14 (25%) |
| Negative | 66 (75%) | 72 (76%) | 42 (74%) |
| Indeterminate | 2 (2%) | 6 (6%) | 1 (2%) |
| IP-10 test results | |||
| Positive | 20 (23%) | 15 (16%) | 17 (30%) |
| Negative | 62 (71%) | 67 (70%) | 40 (70%) |
| Indeterminate | 6 (7%) | 14 (15%) | 0 (0%) |
BMI = body mass index; cART= combined antiretroviral therapy; HIV = human immunodeficiency virus; IP-10 = IFN-γ-inducible protein 10; IQR = interquartile range; QFT = QuantiFERON TB GOLD® In-Tube Test; TB = tuberculosis.
CD4 counts were unavailable in a period of the study, resulting in a lack of CD4 count for some patients.
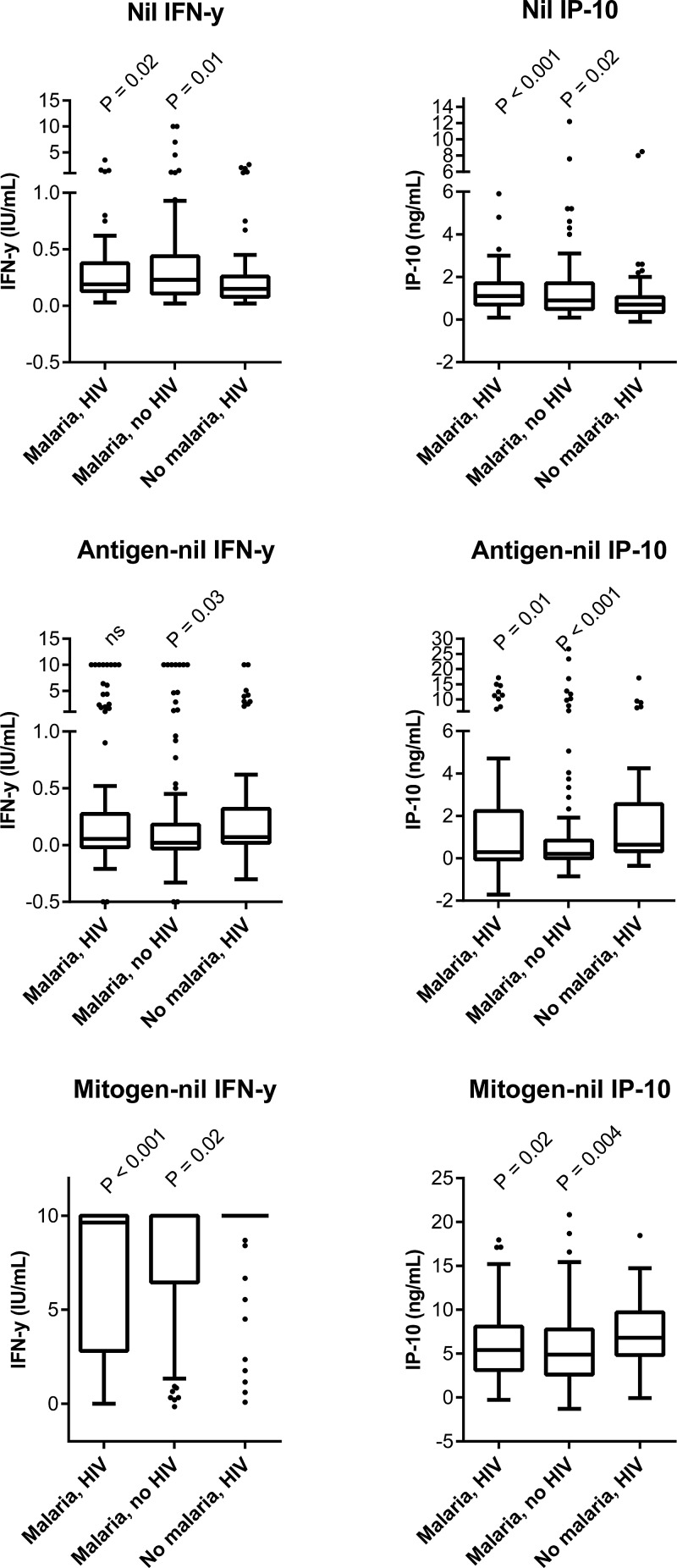
Before initiation of malaria treatment, the malaria patients had significantly higher IFN-γ and IP-10 levels in the unstimulated samples and lower IFN-γ and IP-10 responses to mitogen stimulation than the non-malaria group (Figure 1 ).
Figure 1.
Box-and-whiskers plot (Tukey) showing cytokine levels at enrollment (day 0). P values reflect comparison of the malaria groups to the non-malaria-HIV-infected group (Mann–Whitney U). The antigen levels depend on the Mycobacterium tuberculosis exposure of the group, whereas the nil and mitogen response reflect the patients' immune status. HIV = human immunodeficiency virus; ns = not significant. The Quantiferon test has an upper detection limit at 10 IU/mL. In general, we found higher nil levels and lower response to mitogen stimulation in the malaria-infected groups compared with the non-malaria-HIV-infected group.
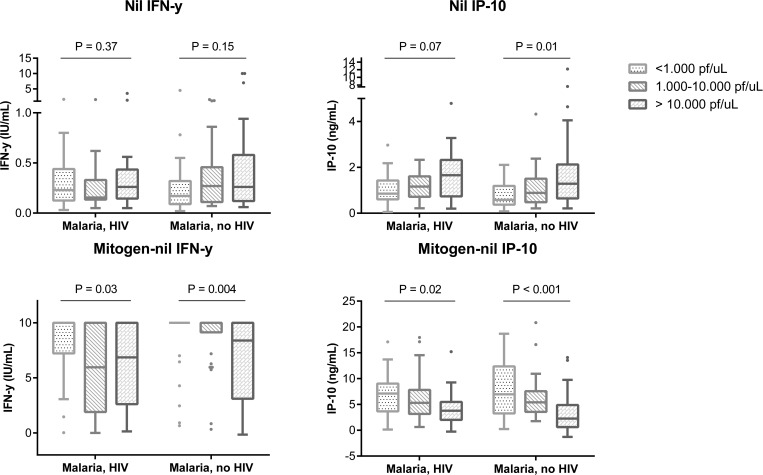
To assess the association between parasite density on day 0 and cytokine responses, we stratified the malaria patients according to the parasite levels: low parasite levels: < 1,000 Pf/μL, N = 58 (29 HIV infected); intermediate parasite levels: 1,000–10,000 Pf/μL, N = 60 (30 HIV infected); and high parasite levels: > 10,000 Pf/μL, N = 61 (35 HIV infected), where Pf = Plasmodium falciparum. We excluded data from six malaria patients who had missing parasite counts. Patients with high parasite levels had higher levels of IP-10 in the unstimulated samples, although only significant for the HIV-uninfected malaria patients, and impaired IFN-γ and IP-10 responses to mitogen stimulation compared with patients with lower parasite levels (Kruskal–Wallis test) (Figure 2 ). We found a significant lower CD4 cell count in patients with increasing parasite density; median CD4 counts of 538 cells/μL (interquartile range [IQR] = 820–382), 456 cells/μL (IQR = 325–559), and 430 cells/μL (IQR = 253–574) in patients with low, intermediate, and high parasite levels, respectively, P = 0.03, Kruskal–Wallis test. We observed significantly more indeterminate test results in patients with high parasite levels versus low and intermediate parasite levels. For the QFT test, we found 10% (N = 6) indeterminate results in the high parasite density group compared with 2% (N = 1) in both the low and intermediate parasite density groups (P = 0.04). For the IP-10 test, we found 25% (N = 15) indeterminate results in the high parasite density group compared with 5% (N = 3) and 3% (N = 2) in the low and intermediate parasite level groups, respectively (P < 0.001, Cochran–Armitage test for trend).
Figure 2.
Box-and-whiskers plot (Tukey) showing cytokine levels at enrollment (day 0) of unstimulated (nil) and mitogen-stimulated samples of the malaria patients stratified by parasite densities. Cytokine levels were compared using Kruskal–Wallis test. HIV = human immunodeficiency virus; Pf = Plasmodium falciparum. N = 29, 30, and 35 for the malaria-HIV-infected group, and N = 29, 30, and 25 for the malaria-non-HIV group. The Quantiferon test has an upper detection limit at 10 IU/mL. In general, we found higher nil levels and lower mitogen responses in patients with high parasite levels.
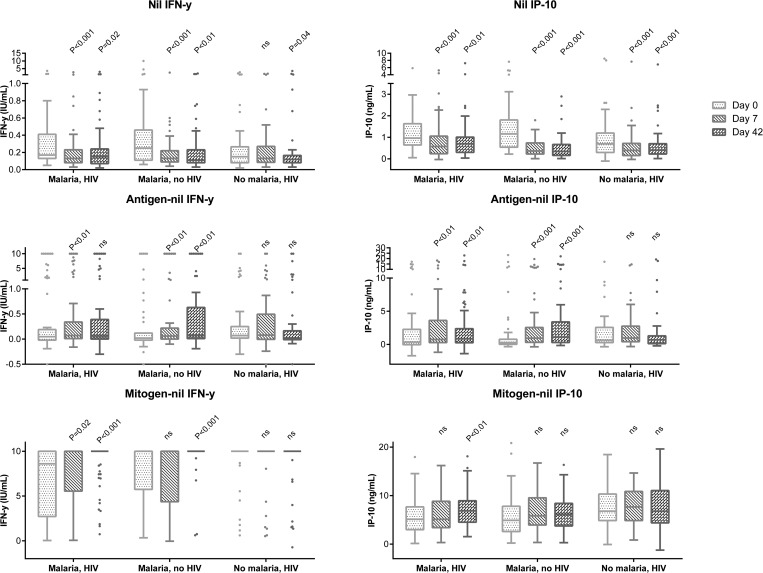
Changes in IFN-γ and IP-10 levels, as well as CD4-cell counts, during follow-up were assessed in 167 patients with complete follow-up and successful treatment. The IFN-γ and IP-10 levels in the unstimulated samples declined with effective treatment in all patient groups. IFN-γ responses to antigen stimulation increased for both groups of malaria patients, although only significantly for the HIV-uninfected malaria patients. IP-10 responses to antigen stimulation increased as well, significantly for both HIV-infected and HIV-uninfected malaria patients. IFN-γ responses to mitogen stimulation similarly improved after malaria treatment, and this was significant for both HIV-infected and HIV-uninfected malaria patients. IP-10 responses to mitogen stimulation also increased, although only significant for the HIV-infected malaria patients (Figure 3 ). There was a significant increase in CD4 counts already at day 7, and this increase in CD4-cell counts was greatest for the HIV-uninfected malaria patients (Table 2).
Figure 3.
Box-and-whiskers plot (Tukey) showing cytokine levels during follow-up at day 0, 7, and 42. Only patients with complete follow-up and no malaria reinfections were included for this analysis. Malaria-HIV-infected group N = 67, malaria-non-HIV-group N = 51, and non-malaria-HIV-group N = 49, P values reflect comparison to levels at inclusion (day 0), Wilcoxon signed-rank test. HIV = human immunodeficiency virus; ns = not significant. The Quantiferon test has an upper detection limit at 10 IU/mL. In general, we found a reduction in nil levels for all study groups and an increase in antigen and mitogen responses in the malaria groups during the follow-up.
Table 2.
CD4 counts (cells/μL) at inclusion and during follow-up for patients with complete follow-up and no malaria reinfection
| Group | Malaria | No malaria | ||||
|---|---|---|---|---|---|---|
| HIV infected (N = 67) | HIV uninfected (N = 51) | HIV infected (N = 59) | ||||
| CD4 counts | Median (IQR) | P | Median (IQR) | P | Median (IQR) | P |
| Day 0 | 364 (242–483) | – | 563 (450–903) | – | 382 (243–538) | – |
| N = 66 | N = 43 | N = 49 | ||||
| Day 7 | 453 (359–625) | < 0.001 | 875 (713–1,021) | < 0.001 | 399 (247–546) | NS |
| N = 57 | N = 26 | N = 46 | ||||
| Day 42 | 482 (302–636) | < 0.001 | 888 (727–1,069) | < 0.001 | 418 (254–540) | NS |
| N = 66 | N = 48 | N = 49 | ||||
HIV = human immunodeficiency virus; IQR = interquartile range; NS = not significant.
Changes from enrollment levels were assessed with Wilcoxon signed-rank test. CD4-counts were unavailable in a period of the study, resulting in a lack of CD4 count for some patients. At inclusion (day 0) CD4 counts were higher in the HIV-uninfected group compared with the HIV-infected groups (P < 0.001 for both HIV-infected groups). The CD4 counts were comparable for the HIV-infected groups at inclusion (P = 0.73).
We assessed the effect of malaria infection and malaria treatment on QFT and IP-10 release assay test results by comparing the change in proportion of positive and indeterminate results, from the time of inclusion and till the last follow-up visit on day 42 for patients with complete follow-up and no parasites on day 42, N = 167 (McNemar's test). In brief, we found no changes in QFT test results during follow-up, but we found that the indeterminate IP-10 test results in HIV-uninfected malaria patients declined from eight (16%) to two (4%), P = 0.03, and from five (7%) to zero (0%) in HIV-infected malaria patients (Table 3). Compared with patients with determinate IP-10 result at enrollment, the 13 malaria patients with indeterminate results constituted a subgroup of patients with very high parasite levels (36,120 Pf/μL (IQR = 4,040–79,040) versus 2,680 Pf/μL (IQR = 400–9,360), P = 0.003) and low CD4-cell counts (275 cells/μL (IQR = 241–405) versus 457 cells/μL (IQR = 290–674), P = 0.02).
Table 3.
QFT and IP-10 test results at inclusion and on follow-up at days 7 and 42 for patients with complete follow-up and no malaria reinfection (N = 167)
| Group | Malaria | No Malaria | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV infected (N = 67) | HIV uninfected (N = 51) | HIV infected (N = 49) | |||||||
| QFT, n (%) | |||||||||
| Positive | Negative | Ind. | Positive | Negative | Ind. | Positive | Negative | Ind. | |
| Day 0 | 15 (22) | 51 (76) | 1 (1) | 10 (20) | 39 (76) | 2 (4) | 11 (22) | 38 (78) | 0 (0) |
| Day 7 | 16 (24) | 50 (75) | 1 (1) | 11 (22) | 38 (75) | 2 (4) | 13 (27) | 36 (73) | 0 (0) |
| Day 42 | 17 (25) | 50 (75) | 0 (0) | 15 (29) | 36 (71) | 0 (0) | 9 (18) | 40 (82) | 0 (0) |
| IP-10, n (%) | |||||||||
| Positive | Negative | Ind. | Positive | Negative | Ind. | Positive | Negative | Ind. | |
| Day 0 | 16 (24) | 46 (69) | 5 (7) | 8 (16) | 35 (69) | 8 (16) | 15 (31) | 34 (70) | 0 (0) |
| Day 7 | 19 (28) | 43 (64) | 5 (7) | 13 (25) | 33 (65) | 5 (10) | 14 (29) | 34 (70) | 1 (2) |
| Day 42 | 18 (27) | 49 (73) | 0 (0) | 14 (27) | 35 (69) | 2 (4) | 7 (14) | 40 (82) | 2 (4) |
HIV = human immunodeficiency virus; Ind. = indeterminate; IP-10 = IFN-γ-inducible protein 10; QFT = QuantiFERON TB GOLD® In-Tube Test.
Concordance between QFT and IP-10 release assay was determined for samples collected 42 days after treatment. Patients with indeterminate results were excluded from this analysis. The tests agreed in 143 of 163 cases with a kappa value of 0.66.
Discussion
In this study, we aimed to explore the potential influence of malaria on the Mtb-specific immune responses in HIV-infected and HIV-uninfected Tanzanian adults. We found that during malaria infection and malaria-HIV coinfection, IP-10 and IFN-γ levels in the unstimulated samples were elevated, mitogen responsiveness was impaired, and CD4-cell counts were decreased, resulting in an increased rate of indeterminate IP-10 results. These alterations reverted rapidly after malaria treatment, and led to a decrease in indeterminate IP-10 test results, but did not influence the QFT test results.
The antigen responses is dependent on Mtb infections, and it is not possible to assess whether malaria contributes to the differences observed between groups at inclusion. However, we did find a small increase in the antigen response in malaria patients after treatment (Figure 3). With only 8–16 participants with positive results in each study group with complete follow-up, the possible increase for patients with true Mtb infection could theoretically be greater, but we do not have the power to investigate this aspect further, and it remains speculative whether malaria influences the immunologic response to Mtb-specific stimulation of Mtb-infected individuals in a matter that could be decisive for the test result.
Both IP-10 and IFN-γ levels measured in the unstimulated samples were elevated at time of acute malaria infection and decreased after malaria treatment. These pro-inflammatory markers can be upregulated during acute infections, and our results support previous findings of elevated IP-10 levels in malaria patients.24–26 High levels of IFN-γ have been associated with severe malaria infections, but the evidence is ambiguous as reviewed in a study.27 Petrone and others investigated IP-10 as a marker of TB in children and found raised levels of IP-10 in blood and urine but not significant higher than levels found in children with other respiratory diseases. This supports that IP-10 is an unspecific marker of inflammation rather than of any specific disease.28 Inflammation due to high parasite levels could explain the high nil IP-10 found in our study. The decrease during follow-up could reflect the effect of treatment.
Different studies suggest that there is an association between malaria infection, reduced CD4-cell count, and impaired immune response.17–20 This study adds to these observations by showing that acute malaria had a negative influence on the CD4-cell count with a significantly lower CD4-cell count in patients with high parasitemia, and we found a significant increase in CD4-cell counts after treatment (Table 2). The latter finding was most pronounced in HIV-negative patients indicating the possible association to CD4 cells is not HIV associated. A reduction in of CD4-cell counts during malaria infections has been observed by others,18,19 and several studies have shown rapid increase of lymphocytes, including an increase in CD4 cells, when malaria drug therapy has been initiated.17,18 It has been proposed that the transient decrease of lymphocytes during acute malaria infection is caused by accumulation of CD4 cells to sites of inflammation and redistribution to the circulation after successful therapy.17,18,20
The impaired response to mitogen stimulation may not solely be explained by the lower number of CD4 cells in patients with high parasite levels, that is, the CD4-cell counts in the group of patients with the highest parasite level were comparable to the CD4-cell counts of the non-malaria HIV-infected group that responded stronger to mitogen stimulation (Figure 1). It is likely that malaria causes a functional immune suppression greater than what can be attributed to the quantitative fall in CD4 cells. Hviid and others29 found in a small study published in 1991 that patients who were previously responsive to antigens (soluble malaria antigens and purified protein derivate of tuberculin) lost their responsiveness to antigen stimulation during acute malaria infection and regained it during convalescence. In contrast to our findings, the study by Hviid and others found no impact on responses to the mitogen PHA stimulation during acute malaria. The reason could be manifold, that is, the use of peripheral blood mononuclear cell versus whole blood, the concentration of PHA, and the differences in study population.
An association between P. falciparum infections and increased rate of indeterminate QFT test results due to failed mitogen response has previously been reported.10 Although we found decreased IFN-γ responsiveness to mitogen stimulation during acute malaria infections, the majority of the malaria patients responded well above the cutoff for positive mitogen response, and we were unable to demonstrate a direct effect on the QFT test. However, we did find an increased IP-10 indeterminate rate. Both the QFT and the IP-10 release assay are developed as diagnostic tests for latent Mtb infection with defined cutoffs for both the antigen and the mitogen levels. The reason for the higher proportion of indeterminate test results using the IP-10 release assay compared with the QFT assay could be a matter of a relatively high cut-off for mitogen-response for the IP-10 release assay. The QFT test parameters have been extensively validated whereas the IP-10 release assay cut off was based on a small case control study.22 Multiple alternative cutoffs for IP-10 have been suggested (reviewed in ref. 11), and a larger study is currently ongoing to validate the cutoff (M. Ruhwald, unpublished data).
Surprisingly, we found a significant reduction in IP-10 positive test results during follow-up for non-malaria patients. We did not find any connection between the patients or samples in terms of collection dates, laboratory dates, or plate numbers. It is well documented that QFT test results within patients can fluctuate around cutoff upon serial testing.30 We cannot dismiss that the within test uncertainties could have contributed to some of the changes we observed during follow-up. Factors that contribute to within subject variability include incubation time, contamination, storage conditions, and so forth.1,30 We have eliminated the between-run variability by analyzing all samples for the same patient within the same plate. As the IP-10 based test is not commercially available, this test has not been as extensively studied as the QFT test.
Limitations.
We were not able to assess the influence on the Mtb-specific response because of low number of positive test results. The proper study to assess this question would include TB-malaria coinfected individuals or persons with known LTBI, two groups that are difficult to find. This study was designed to be conducted alongside the InterACT study with an aim to examine pharmacologic interactions between HIV and malaria medication. Thus, we did not include patients who were not infected with HIV and/or malaria and therefore not able to compare our results to healthy individuals, which would have been preferable. Further, we did not collect data on other diagnosis of our study participants, which eliminated our possibilities to investigate whether other illnesses could have contributed to our observations.
Clinical trials have shown that the sensitivity of Paracheck Pf varies with parasite density. In general, sensitivity of > 90% has been reported when parasite levels > 200 Pf/uL,31,32 leaving a possibility that malaria-infected study participants could have been misplaced in the non-malaria group.
Conclusion
We have showed that malaria affects the IFN-γ and IP-10 levels of the IGRA and IP-10 release assay. The changes in cytokine responses did not influence QFT results, whereas the IP-10 based test results were affected by concurrent malaria infection, with increased rate of indeterminate test results. We recommend interpreting especially IP-10 test results of malaria patients with caution. The changes observed in IFN-γ levels during follow-up suggest that the QFT in some degree is also influenced by concurrent malaria infection. It would be preferable to postpone testing until malaria infection has been treated. The role of the IGRAs is a topic of continued discussion, and the findings of this study add to the list of precautions the clinicians should take into consideration when using this diagnostic tool.
ACKNOWLEDGMENTS
We thank all study participants for their willingness to participate in the study. We also thank all staff at the Muheza District Hospital for their assistance and support. Finally, we thank the Director General of NIMR for accepting the publication of this study.
Footnotes
Financial support: The ACT Consortium was funded through a grant from the Bill & Melinda Gates Foundation to the London School of Hygiene and Tropical Medicine. This sub-study was partly funded by Danida. Camilla H. Drabe is a recipient of a research grant from Copenhagen University Hospital Nordsjaelland Hospital, Hillerød.
Disclosure: PR and MR hold a pending patent on the IP-10 release assay. MR is employed at Statens Serum Institut who holds and licenses IP on immunodiagnostic antigens included in QuantiFERON®. No other conflicts of interests declared.
Authors' addresses: Camilla H. Drabe, Department of Infectious Diseases, Copenhagen University Hospital, Hvidovre, Kettegaard Alle 30, 2650 Hvidovre, Denmark, E-mail: camilla.h.drabe@gmail.com. Lasse S. Vestergaard, InterACT Project, Muheza District Hospital, Muheza, Tanzania, Department of Immunology and Microbiology, Centre for Medical Parasitology, University of Copenhagen, Copenhagen, Denmark, Department of Public Health, University of Copenhagen, Copenhagen, Denmark, Department of Infectious Diseases, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark, and Department of Clinical Microbiology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark, E-mail: lasse.vestergaard@dadlnet.dk. Marie Helleberg, Department of Infectious Diseases, Copenhagen University Hospital Rigshospitalet, University of Copenhagen, CHIP, Copenhagen, Denmark, E-mail: marie.helleberg@regionh.dk. Nyagonde Nyagonde, Tanzania National Institute for Medical Research, Tanga Center, Tanga, Tanzania, E-mail: drnyagonde@yahoo.com. Michala V. Rose, Department of Infectious Diseases, Copenhagen University Hospital, Hvidovre, Denmark, E-mail: michala.rose@gmail.com. Filbert Francis, National Institute for Medical Research, Tanga, Tanzania, E-mail: ffrancis8@gmail.com. Ola P. Theilgaard, Lægehuset i Hedehusene, Hedehusene, Denmark, E-mail: olaptheilgaard@gmail.com. Jens Asbjørn, Danish Health and Medicines Authority, Public Health Medical Officers of Southern Denmark, Kolding, Denmark, E-mail: asbjorn_1@hotmail.com. Ben Amos, InterACT Project, Muheza District Hospital, Muheza, Tanzania, E-mail: ben@teule.org.uk. Ib Christian Bygbjerg, Department of Public Health, University of Copenhagen, Copenhagen, Denmark, E-mail: iby@sund.ku.dk. Morten Ruhwald, Department of Infectious Disease Immunology, Statens Serum Institut, Copenhagen, Denmark, E-mail: moru@ssi.dk. Pernille Ravn, Department of Pulmonary and Infectious Diseases, Nordsjaelland Hospital, Hillerød, Denmark, E-mail: peravn@gmail.dk.
References
- 1.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santin M, Muñoz L, Rigau D. Interferon-γ release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLoS One. 2012;7:e32482. doi: 10.1371/journal.pone.0032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sester M, van Leth F, Bruchfeld J, Bumbacea D, Cirillo DM, Dilektasli AG, Domínguez J, Duarte R, Ernst M, Eyuboglu FO, Gerogianni I, Girardi E, Goletti D, Janssens J-P, Julander I, Lange B, Latorre I, Losi M, Markova R, Matteelli A, Milburn H, Ravn P, Scholman T, Soccal PM, Straub M, Wagner D, Wolf T, Yalcin A, Lange C. TBNET Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med. 2014;190:1168–1176. doi: 10.1164/rccm.201405-0967OC. [DOI] [PubMed] [Google Scholar]
- 4.Goletti D, Raja A, Syed Ahamed Kabeer B, Rodrigues C, Sodha A, Carrara S, Vernet G, Longuet C, Ippolito G, Thangaraj S, Leportier M, Girardi E, Lagrange PH. Is IP-10 an accurate marker for detecting M. tuberculosis-specific response in HIV-infected persons? PLoS One. 2010;5:e12577. doi: 10.1371/journal.pone.0012577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanini V, Petruccioli E, Gioia C, Cuzzi G, Orchi N, Rianda A, Alba L, Giancola ML, Conte A, Schininà V, Busi Rizzi E, Girardi E, Goletti D. IP-10 is an additional marker for tuberculosis (TB) detection in HIV-infected persons in a low-TB endemic country. J Infect. 2012;65:49–59. doi: 10.1016/j.jinf.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Belard E, Semb S, Ruhwald M, Werlinrud AM, Soborg B, Jensen FK, Thomsen H, Brylov A, Hetland ML, Nordgaard-Lassen I, Ravn P. Prednisolone treatment affects the performance of the QuantiFERON gold in-tube test and the tuberculin skin test in patients with autoimmune disorders screened for latent tuberculosis infection. Inflamm Bowel Dis. 2011;17:2340–2349. doi: 10.1002/ibd.21605. [DOI] [PubMed] [Google Scholar]
- 7.Aabye MG, Hermansen TS, Ruhwald M, Praygod G, Faurholt-Jepsen D, Jeremiah K, Faurholt-Jepsen M, Range N, Friis H, Changalucha J, Andersen AB, Ravn P. Negative effect of smoking on the performance of the QuantiFERON TB gold in tube test. BMC Infect Dis. 2012;12:379. doi: 10.1186/1471-2334-12-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose MV, Kimaro G, Nissen TN, Kroidl I, Hoelscher M, Bygbjerg IC, Mfinanga SG, Ravn P. QuantiFERON®-TB Gold in-tube performance for diagnosing active tuberculosis in children and adults in a high burden setting. PLoS One. 2012;7:e37851. doi: 10.1371/journal.pone.0037851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas M, Nicol P, McKinnon E, Whidborne R, Lucas A, Thambiran A, Burgner D, Waring J, French M. A prospective large-scale study of methods for the detection of latent Mycobacterium tuberculosis infection in refugee children. Thorax. 2010;65:442–448. doi: 10.1136/thx.2009.127555. [DOI] [PubMed] [Google Scholar]
- 10.Banfield S, Pascoe E, Thambiran A, Siafarikas A, Burgner D. Factors associated with the performance of a blood-based interferon-gamma release assay in diagnosing tuberculosis. PLoS One. 2012;7:e38556. doi: 10.1371/journal.pone.0038556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruhwald M, Aabye MG, Ravn P. IP-10 release assays in the diagnosis of tuberculosis infection: current status and future directions. Expert Rev Mol Diagn. 2012;12:175–187. doi: 10.1586/erm.11.97. [DOI] [PubMed] [Google Scholar]
- 12.Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M. Beyond the IFN-γ horizon: biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur Respir J. 2014;43:1472–1486. doi: 10.1183/09031936.00151413. [DOI] [PubMed] [Google Scholar]
- 13.Kabeer BSA, Sikhamani R, Raja A. Comparison of interferon gamma and interferon gamma-inducible protein-10 secretion in HIV-tuberculosis patients. AIDS. 2010;24:323–325. doi: 10.1097/QAD.0b013e328334895e. [DOI] [PubMed] [Google Scholar]
- 14.Aabye MG, Ruhwald M, Praygod G, Jeremiah K, Faurholt-Jepsen M, Faurholt-Jepsen D, Range N, Friis H, Changalucha J, Andersen AB, Ravn P. Potential of interferon-γ-inducible protein 10 in improving tuberculosis diagnosis in HIV-infected patients. Eur Respir J. 2010;36:1488–1490. doi: 10.1183/09031936.00039010. [DOI] [PubMed] [Google Scholar]
- 15.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 16.Enwere GC, Ota MO, Obaro SK. The host response in malaria and depression of defence against tuberculosis. Ann Trop Med Parasitol. 1999;93:669–678. doi: 10.1080/00034989957907. [DOI] [PubMed] [Google Scholar]
- 17.Chougnet C, Tallet S, Ringwald P, Deloron P. Kinetics of lymphocyte subsets from peripheral blood during a Plasmodium falciparum malaria attack. Clin Exp Immunol. 1992;90:405–408. doi: 10.1111/j.1365-2249.1992.tb05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hviid L, Kurtzhals JA, Goka BQ, Oliver-Commey JO, Nkrumah FK, Theander TG. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect Immun. 1997;65:4090–4093. doi: 10.1128/iai.65.10.4090-4093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Geertruyden J-P, Mulenga M, Kasongo W, Polman K, Colebunders R, Kestens L, D'Alessandro U. CD4 T-cell count and HIV-1 infection in adults with uncomplicated malaria. J Acquir Immune Defic Syndr. 2006;43:363–367. doi: 10.1097/01.qai.0000243125.98024.da. [DOI] [PubMed] [Google Scholar]
- 20.Elhassan IM, Hviid L, Satti G, Akerstrom B, Jakobsen PH, Jensen JB, Theander TG. Evidence of endothelial inflammation, T cell activation, and T cell reallocation in uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 1994;51:372–379. doi: 10.4269/ajtmh.1994.51.372. [DOI] [PubMed] [Google Scholar]
- 21.Cellestis XXX. Manufacturer of QuantiFERON® Diagnostics and Research Products: Homepage. http://www.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf Available at. Accessed June 11, 2013.
- 22.Aabye MG, Eugen-Olsen J, Werlinrud AM, Holm LL, Tuuminen T, Ravn P, Ruhwald M. A simple method to quantitate IP-10 in dried blood and plasma spots. PLoS One. 2012;7:e39228. doi: 10.1371/journal.pone.0039228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruhwald M, Latorre I, Diaz J, Maldonado J, Mialdea I, Ravn P, Dominguez J, Aabye M. Specific diagnosis of tuberculosis infection sent via mail—IGRAs on paper. Eur Respir J. 2011;38((Suppl 55)):1897. [Google Scholar]
- 24.Lucchi NW, Jain V, Wilson NO, Singh N, Udhayakumar V, Stiles JK. Potential serological biomarkers of cerebral malaria. Markers. 2011;31:327–335. doi: 10.3233/DMA-2011-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armah HB, Wilson NO, Sarfo BY, Powell MD, Bond VC, Anderson W, Adjei AA, Gyasi RK, Tettey Y, Wiredu EK, Tongren JE, Udhayakumar V, Stiles JK. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson NO, Jain V, Roberts CE, Lucchi N, Joel PK, Singh MP, Nagpal AC, Dash AP, Udhayakumar V, Singh N, Stiles JK. CXCL4 and CXCL10 predict risk of fatal cerebral malaria. Markers. 2011;30:39–49. doi: 10.3233/DMA-2011-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCall MBB, Sauerwein RW. Interferon—central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol. 2010;88:1131–1143. doi: 10.1189/jlb.0310137. [DOI] [PubMed] [Google Scholar]
- 28.Petrone L, Cannas A, Aloi F, Nsubuga M, Sserumkuma J, Nazziwa RA, Jugheli L, Lukindo T, Girardi E, Reither K, Goletti D. Blood or urine IP-10 cannot discriminate between active tuberculosis and respiratory diseases different from tuberculosis in children. BioMed Res Int. 2015;2015:1–11. doi: 10.1155/2015/589471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hviid L, Theander TG, Abu-Zeid YA, Abdulhadi NH, Jakobsen PH, Saeed BO, Jepsen S, Bayoumi RA, Jensen JB. Loss of cellular immune reactivity during acute Plasmodium falciparum malaria. FEMS Microbiol Immunol. 1991;3:219–227. doi: 10.1111/j.1574-6968.1991.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 30.Tagmouti S, Slater M, Benedetti A, Kik SV, Banaei N, Cattamanchi A, Metcalfe J, Dowdy D, van Zyl Smit R, Dendukuri N, Pai M, Denkinger C. Reproducibility of interferon gamma (IFN-γ) release assays. A systematic review. Ann Am Thorac Soc. 2014;11:1267–1276. doi: 10.1513/AnnalsATS.201405-188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falade CO, Adesina-Adewole B, Dada-Adegbola HO, Ajayi IO, Akinyemi JO, Ademowo OG, Adewole IF, Kanki P. Evaluation of Paracheck-Pf™ rapid malaria diagnostic test for the diagnosis of malaria among HIV-positive patients in Ibadan, south-western Nigeria. Pathog Glob Health. 2013;107:69–77. doi: 10.1179/2047773213Y.0000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djallé D, Gody JC, Moyen JM, Tekpa G, Ipero J, Madji N, Breurec S, Manirakiza A. Performance of ParacheckTM-Pf, SD Bioline malaria Ag-Pf and SD Bioline malaria Ag-Pf/pan for diagnosis of falciparum malaria in the Central African Republic. BMC Infect Dis. 2014;14:109. doi: 10.1186/1471-2334-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]