Abstract
Triatoma dimidiata (Hemiptera: Reduviidae) is a secondary vector of Trypanosoma cruzi in Colombia and represents an important epidemiological risk mainly in the central and oriental regions of the country where it occupies sylvatic, peridomestic, and intradomestic ecotopes, and because of this complex distribution, its distribution and abundance could be conditioned by environmental factors. In this work, we explored the relationship between T. dimidiata distribution and environmental factors in the northwest, northeast, and central zones of Colombia and developed predictive models of infestation in the country. The associations between the presence of T. dimidiata and environmental variables were studied using logistic regression models and ecological niche modeling for a sample of villages in Colombia. The analysis was based on the information collected in field about the presence of T. dimidiata and the environmental data for each village extracted from remote sensing images. The presence of Triatoma dimidiata (Latreille, 1811) was found to be significantly associated with the maximum vegetation index, minimum land surface temperature (LST), and the digital elevation for the statistical model. Temperature seasonality, annual precipitation, and vegetation index were the variables that most influenced the ecological niche model of T. dimidiata distribution. The logistic regression model showed a good fit and predicted suitable habitats in the Andean and Caribbean regions, which agrees with the known distribution of the species, but predicted suitable habitats in the Pacific and Orinoco regions proposing new areas of research. Improved models to predict suitable habitats for T. dimidiata hold promise for spatial targeting of integrated vector management.
Introduction
In Colombia, there are 26 recorded triatomine species, of which Rhodnius prolixus and T. dimidiata are considered the most important vectors of Trypanosoma cruzi because of their infestation indexes and vectorial capacity.1–3 Trypanosoma cruzi is the causative agent of Chagas disease, a condition that according to the last estimation made by WHO in Colombia affected nearly 437,960 habitants by 2010, and there were at risk nearly 4,813,543 people, 131,388 persons with Chagasic cardiopathy, 5,274 annual cases due to vectorial transmission, 1,046 new cases due to congenital transmission each year, and 116,221 women with ages ranging between 15 and 44 years were infected.4
Triatoma dimidiata is a highly variable species with a high genetic diversity and is in fact a species complex; this diversity may indeed explain the ecological diversity of habitats and behavior encountered by this complex.5 This triatominae species have a wide geographical range from Mexico through Central America into Venezuela, Colombia, Ecuador, and northern Peru.6 It is currently the main vector of Chagas disease in Guatemala, El Salvador, Nicaragua, and Costa Rica, and the second most important vector in Honduras and Colombia.7–9
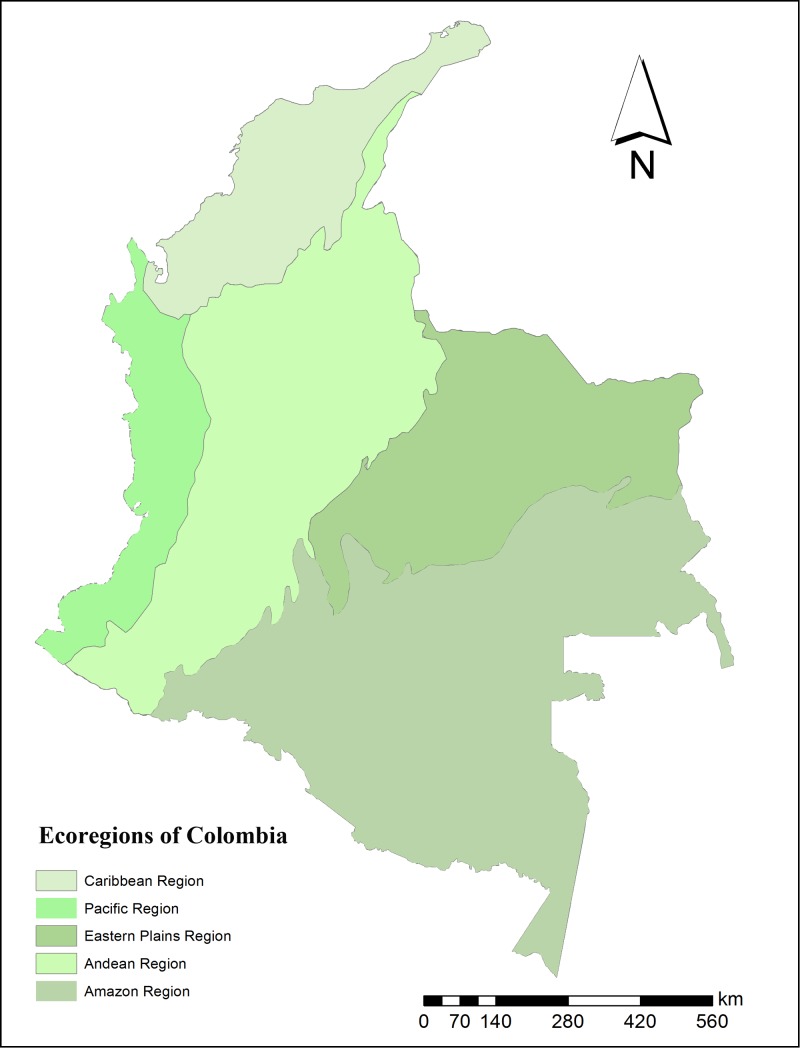
According to the Colombian Chagas Disease Control Program (CDCP), T. dimidiata is widespread in 13 departments, 79 municipalities, and 205 villages located in the northwest (Caribbean region), northeast, and central (Andean region) parts of the country (Figure 1 ). Through its distribution, T. dimidiata is not a strictly domiciliated species, presenting a complex epidemiological distribution including sylvatic, peridomestic, and domestic ecotopes. Non-domiciliated populations can act as reinfestation sources and may be engaged in the transmission of the parasite to humans.1
Figure 1.
Ecoregions of Colombia. Eastern Plains region also called Orinoco region.
In Colombia, T. dimidiata occupies different ecological zones in a wide range of altitude between 0 and 2,400 m above sea level (masl).10 The domestic and peridomestic populations are found in the northeast and central zones of Colombia (Boyaca, Casanare, Cundinamarca, Huila, and Santander departments); the sylvatic populations are found in the northwest of Colombia (Antioquia, Guajira, Magdalena, and Sucre departments) where these insects are mainly found on palm trees (Attalea butyracea).11,12
Habitat preferences influenced by climatic factors determine the distribution of triatomines. These insects are able to move between microclimates within their habitats while seeking the most favorable conditions. The local environmental determinants for the presence of triatomines include altitude, climate, vegetation type, and land use.13 Analytical tools such as geographic information systems (GIS), remote sensing, and niche modeling have greatly enhanced our ability to evaluate the relationships between environmental factors and vector ecology or disease transmission.14
Remote sensing data are increasingly being used to measure environmental and topographic variables on the ground, and GIS are being used to model these data both spatially and temporally.15 Remote sensing has the advantages that numerous sensors has a wide range of spectral, spatial, and temporal resolutions16,17 and global coverage is at low or no cost. These properties allows GIS functions to be used to investigate environmental relationships and generate predictive maps throughout wide areas and thus focus control measures.18,19
Further evidence for the feasibility of mapping triatomine bug distributions comes from published maps for Triatoma infestans20 based on discriminant analysis using Fourier-processed satellite-derived descriptors of air temperature (AT), medium infrared radiation, and vegetation index. Potential distribution maps generated by ecological niche modeling (ENM) has also been applied broadly to understanding triatomine distribution in the last decade.21–29
Targeting and surveillance of T. dimidiata populations could potentially be improved by the generation of a distribution map, that is, by developing a quantitative prediction of infestation in each village on the basis of a GIS incorporating comprehensively available environmental variables such as altitude,30 vegetation and climate,14 or socioeconomic status.31 For example, T. dimidiata abundance in the Yucatan peninsula of Mexico was positively associated with agriculture and pasture, as opposed to less disturbed habitats such as forest or mangrove,14 and was also influenced by wind speed, rainfall, relative humidity (RH), and maximum temperature.
In fact, several studies have shown that bioclimatic factors can greatly influence triatomine geographic distribution.9,29,30 As with other vector-borne diseases, the knowledge of vector geographic distribution, vector competence, and overall areas of high risk of disease transmission can allow the development of sensitive tools for disease prediction and the optimization of planning and implementation of effective control strategies. Detailed risk maps for T. dimidiata have been developed in Santander Department (M. Florez, unpublished data), but not yet for the whole country.
In this work, we explored the relationship between T. dimidiata distribution and environmental factors in the northwest, northeast, and central zones of Colombia and developed predictive models of T. dimidiata infestation in the country.
Materials and Methods
Study area.
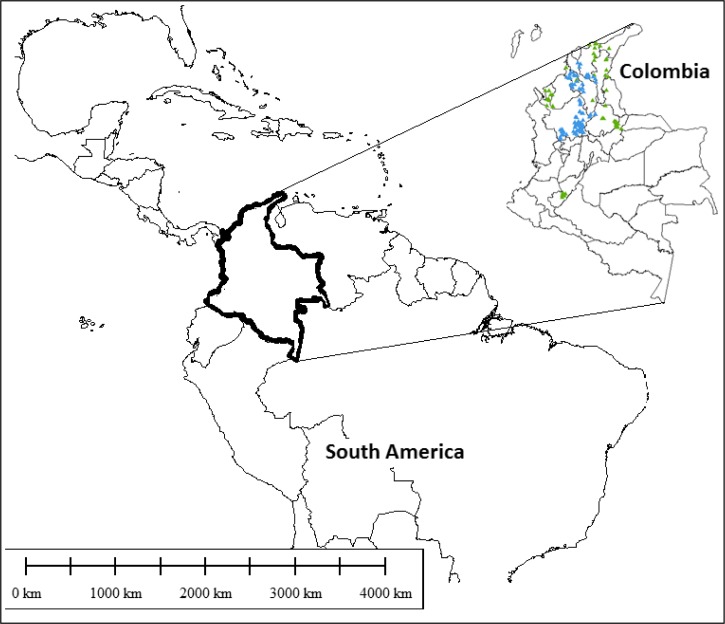
The study area was located at latitudes 5–11°N and longitudes 72–76°W and included nine departments. Following the CDCP protocols, entomologic field data from 340 houses belonging to 30 villages were obtained by direct inspection between 2006 and 2009. This included T. dimidiata infestation and infection rates by T. cruzi for each village (Figure 2 ).
Figure 2.
Collection sites of Triatoma dimidiata in Colombia. Green = presence; blue = absence.
Imaging.
Images of the LST, middle infrared (MIR), normalized difference vegetation index (NDVI), and digital elevation model (DEM) were obtained from the Advanced Very High Resolution Radiometer (AVHRR) sensor onboard the meteorological satellites of the National Oceanic and Atmospheric Administration. The images were obtained for the years 2006–2009. Averages for each variable was obtained and imported into a raster–vector based GIS (ArcGIS 9.3®; Esri, Redlands, CA, USA) to build a comprehensive database of entomologic and environmental factors for the study area.
Statistical model.
Data matching each 340 T. dimidiata collection sites were then extracted from the database for statistical analysis and modeling. The data set was divided randomly in two subsets, one for modeling (70%) and another for testing the models (30%). One subset was used to build a logistic regression model of the probability of presence of T. dimidiata through a forward stepwise procedure to select significantly associated variables with the response variable, and to reduce potential colinearity problems, retention of factors was relied in Wald test and the percentage of variance explained was valued taking into account the R-square of Nagelkerke. Goodness of fit of the models was assessed with Hosmer–Lemeshow test. The response variable was defined as presence of T. dimidiata, and the independent variables were a set of 12 environmental variables that include NDVI, LST, MIR (these three having account their maximum, minimum, and mean), DEM, RH, and AT. The coefficients from this model were then applied to the values of the predictor variables to generate a predicted probability of occurrence between 0 and 1; the threshold value used to determine between presence and absence was 0.5. Statistical analysis was performed in Stata 10.0 software (Stata Corp., College Station, TX). Maps of predicted probabilities of presence were created using the regression model equations and the map calculator of the “Spatial Analyst” extensions of ArcGIS, version 9.3.
Predictions of presence or absence were reached by comparing predictions and observations to measure sensitivity (ability to correctly predict “true” positives), specificity (ability to predict true negatives), and κ statistics (the proportion of observations that we would have expected to be incorrectly predicted on the basis of chance, but which were correctly predicted, i.e., a measure of the additional “skill” of the model over chance).32
As described by King and others,33 we used a procedure previously used in ecologic and veterinary mapping studies,34,35 and more recently applied in human disease mapping,36 of plotting sensitivity against specificity for all thresholds between 0 and 1 to generate a receiver–operator curve (ROC). The area under the ROC (AUC) gives a single comparable measure of overall model performance. AUC values from 0.5 to 0.7 indicate a poor discriminative capacity, 0.7–0.9 reasonable capacity, and > 0.9 a very good capacity; K values < 0.4 can be considered to show poor agreement, 0.4–0.75 good agreement, and > 0.75 excellent agreement.37
Ecological niche model.
We used 340 georeferenced records of T. dimidiata in Colombia to produce ecological niche models. Distributional data of T. dimidiata were separated randomly into two sets: one for model calibration (75% of points) and the other for model evaluation (25% of points). To characterize environmental variation across Colombia, we used nine climatic variables: annual mean temperature, diurnal temperature range, temperature seasonality, maximum temperature in the warmest month, maximum temperature in the warmest quarter, minimum temperature in the coldest month, annual precipitation, precipitation in the wettest month, and precipitation in the driest month. We obtained these variables from the WorldClim project (worldclim.org), which were developed via the interpolation of mean monthly climatic data from meteorological stations over 30–50 (1950–2000) years, depending on their availability at the stations.38 To summarize aspects of vegetation and land cover, we used the multitemporal (monthly) NDVI (a “greenness” index) drawn from the AVHRR satellite (http://daac.gsfc.nasa.gov/avhrr/). The environmental databases used in our analysis covered the areas at a spatial resolution of 2.5″ (5 × 5 km per pixel).
Models were produced using Maxent version 3.2.1 (Florham Park, NJ). Maxent assumes a priori uniform distribution and performs a series of iterations in which weights are adjusted to maximize the average probability of the point localities, expressed as the training gain.39 Within the processing of the Maxent, these weights are used to compute the maximum entropy probability distribution over the entire geographic space, with values expressing the environmental suitability of each grid cell as a function of the environmental conditions presented there. A high value of the function in a particular grid cell indicates suitable conditions.
We assessed model accuracy by examining omission rates associated with test points.40 To test model significance, we compared predictive success of models against null expectations using a cumulative binomial test. In particular, we assessed whether each test point fell in areas identified by the model as suitable and compared this success rate with overall proportions of pixels identified as suitable or unsuitable for that species. Statistical significance was assessed via a cumulative binomial probability calculation in Excel (Microsoft Corp., Redmond, WA). We also used Maxent's jackknife test to identify variables that most influence model predictions; this approach drop out each variable in turn. Variables are considered important if they cause low training when removed from the model.41 The quality of the predictions generated by the models was also evaluated using the ROC. In Table 1, the entomological indices of T. dimidiata in the biogeographic regions of Colombia are shown.
Table 1.
Entomological indices of Triatoma dimidiata in the biogeographic regions of Colombia
| Biogeographic zone | Department | Municipality | Village | Sample size (%) | DII (%) | CI (%) | NII (%) | DI (%) |
|---|---|---|---|---|---|---|---|---|
| Santander mountains | Santander | Capitanejo | Chorreras | 15 (2.9) | 68.7 | 12.5 | 20.6 | 100 |
| Macaravita | Buraga | 4 (0.8) | 9.8 | 9.8 | 50 | 100 | ||
| Boyacá mountains | Boyacá | Soatá | El Espinal | 5 (1.0) | 60 | 20 | 10 | 75 |
| El Hatillo | 4 (0.8) | 22 | 22 | 0 | ||||
| La Costa | 8 (1.5) | 12.5 | 12.5 | 15.4 | ||||
| Jabonera | 6 (1.2) | 0 | 0 | 0 | ||||
| Tipacoque | El Palmar | 9 (1.7) | 0 | 0 | 0 | 75 | ||
| Nogal–Carrera | 2 (0.4) | 33 | 0 | 0 | ||||
| Bavatá | 3 (0.6) | 25 | 25 | 20 | ||||
| Ovachía | 3 (0.6) | 33 | 100 | 0 | ||||
| Upper Magdalena | Huila | El Agrado | Remolinos | 1 (0.2) | 10 | 0 | 0 | 100 |
| Pital | San Joaquín | 12 (2.3) | 9.09 | 0 | 0 | 100 | ||
| Arrayán | 6 (1.2) | 54.5 | 0 | 0 | ||||
| Gigante | Veracruz | 8 (1.5) | 0 | 0 | 0 | 50 | ||
| Río Loro | 14 (2.7) | 40 | 0 | 0 | ||||
| Depresión Momposina | Bolívar | Mompox | San Fernando | 1 (0.2) | 0 | 0 | 0 | 0 |
| Sucre | San Onofre | Las Brisas | 1 (0.2) | 0 | 0 | 0 | 0 | |
| Galeras | Baraya | 1 (0.2) | 0 | 0 | 0 | 0 | ||
| Sierra Nevada of Santa Marta | Magdalena | Santa Marta | Guachaca | 18 (3.4) | 0 | 0 | 0 | 0 |
| Santa Marta | Cacahualito | 16 (3.0) | 0 | 0 | 0 | 0 | ||
| Santa Marta | Palomino–Gumake | 3 (0.6) | 0 | 0 | 0 | 0 | ||
| Cesar | Valledupar | Seynimen | 104 (19.8) | 11.3 | 7.5 | 4 | 100 | |
| Gulf of Uraba | Antioquia | Apartadó | La Victoria | 8 (1.5) | 0 | 0 | 0 | 0 |
| Chigorodó | Barranquillita | 11 (2.1) | 0 | 0 | 0 | 0 | ||
| Necoclí | Caña Flechal | 16 (3.0) | 0 | 0 | 0 | 0 | ||
| Turbo | Aguas Frías | 6 (1.1) | 0 | 0 | 0 | 0 | ||
| Los Enamorados | 8 (1.5) | 0 | 0 | 0 | 0 | |||
| Middle Magdalena | Santander | San Vicente | El Peltrecho | 47 (9.0) | 4.4 | 0 | 0 | 75 |
| Granada | 35 (6.7) | 13.5 | 0 | 25 | ||||
| Santa Rosa | 49 (9.3) | 0 | 0 | – | ||||
| Pradera | 2 (0.4) | 33 | 0 | 0 | ||||
| El Carmen | Cirales | 21 (4.0) | 7.7 | 0 | 100 | 16 | ||
| El Diviso | 16 (3.0) | 0 | 0 | – | ||||
| Honduras | 24 (4.8) | 0 | 0 | – | ||||
| Delia–Victoria–Belleza | 11 (2.1) | 0 | 0 | – |
CI = colonization index (proportion of houses with T. dimidiata nymph or eggs); DI = Dispersion Index (proportion of villages within municipality with triatomines); DII = Domestic Infestation Index (proportion of infested houses with T. dimidiata; NII = Natural Infection Index (proportion of Trypanosoma cruzi-infected triatomines).
Ethical considerations.
The ethical committee of the Colombian Institute of Tropical Medicine approved the research protocol through the resolution 31. Informed consent was obtained from each head of household.
Results
Statistical model.
The variables that did not have a significant association with the presence of T. dimidiata were excluded from the logistic regression model by a forward stepwise procedure. Table 2 summarizes the performance of logistic regression model in predicting the geographic distribution of T. dimidiata. P value of Hosmer–Lemeshow test was 0.58.
Table 2.
Logistic regression model for Triatoma dimidiata in Colombia
| B | SE | Wald | df | Sig | Exp(B) | |
|---|---|---|---|---|---|---|
| DEM | −0.002 | 0.001 | 4.171 | 1 | 0.041 | 0.998 |
| MaxNDVI | −0.051 | 0.008 | 37.465 | 1 | 0.000 | 0.950 |
| MinLST | −0.052 | 0.018 | 8.439 | 1 | 0.004 | 0.949 |
| Constant | 242.499 | 64.143 | 14.293 | 1 | 0.000 | 2.07 |
B = regression coefficient; DEM = digital elevation model; df = degrees of freedom; Exp(B) = exponential of regression coefficient; MaxNDVI = maximum vegetation index; MinLST = minimum land surface temperature; SE = standard error; Wald = significance statistical Wald test; Sig = significance. Variables with statistical significance in final model: DEM, MaxNDVI, and MinLST.
The first variable included in the model, maximum vegetation index (MaxNDVI), explains 78.5% of the presence of T. dimidiata in the predicted area; when the second variable, minimum LST (MinLST), was added to the model the percentage of explain achieved 80.1%. The last variable added, elevation (DEM Digital Elevation Model), increased that explanation until 81.0%. So, the most important variable to explain the presence of T. dimidiata was MaxNDVI.
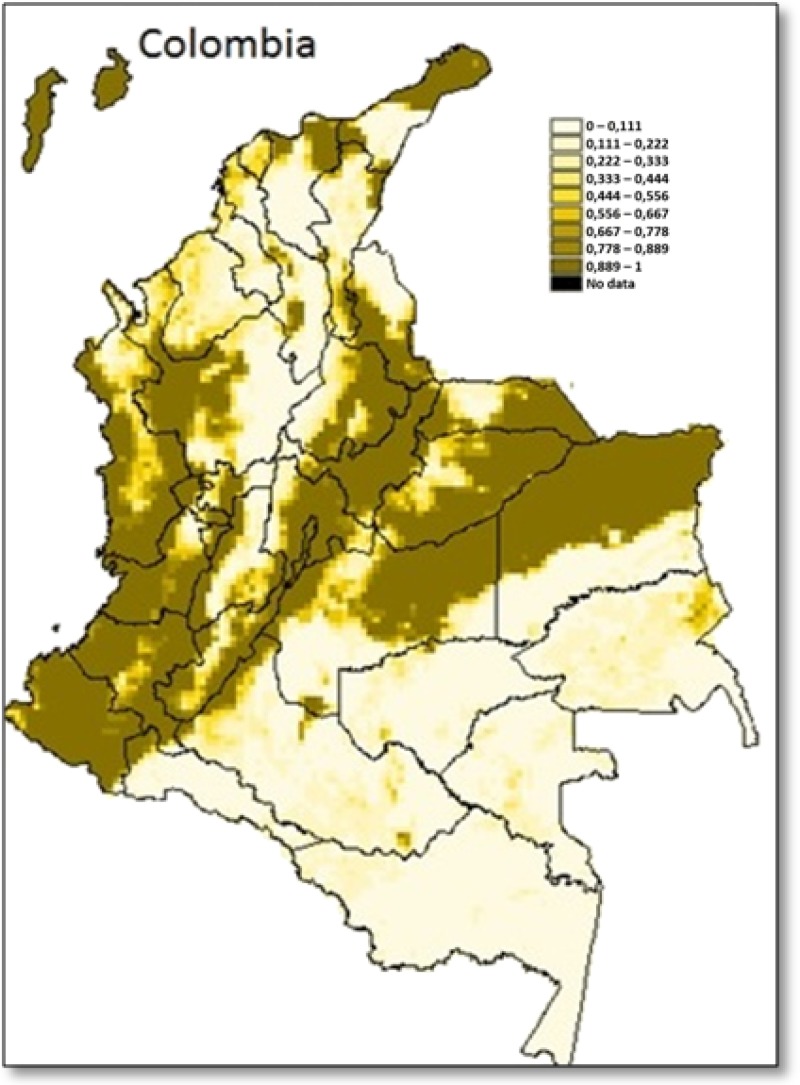
The variables obtained with the third model (DEM, MaxNDVI, and MinLST) were used to construct the equation for the predictive model. The equation was as follows: Exp ((−0.051MaxNDVI) + (−0.052MinLST) + (−0.002DEM) + 242.499)/1 + Exp ((−0.051MaxNDVI) + (−0.052MinLST) + (−0.002DEM) + 242.499). This equation was introduced in the map calculator, and after an iteration process developed by the spatial analyst extension of ArcGIS 9.3, we obtained the risk map for T. dimidiata (Figure 3 ). Odds ratio (OR) for each variable was set with the exponential of regression of their coefficient, DEM: OR = 0.99, MaxNDVI: OR = 0.95, and MinLST: OR = 0.95, showing these variables as characteristics that decrease the odds of T. dimidiata presence.
Figure 3.
Predictive model (logistic regression) for Triatoma dimidiata distribution in Colombia. Scale in probabilities from 0 = absence to 1 = presence.
The overall model performance is as follows: AUC = 0.87; κ value = 0.87; sensitivity = 93.6%; and specificity = 92.1%. Our model shows a reasonable discriminative capacity and an excellent agreement. The values for the predictive potential of the model on the rest of the data set were κ value = 0.91; sensitivity = 91.2%; and specificity = 91.9%.
Ecological niche model.
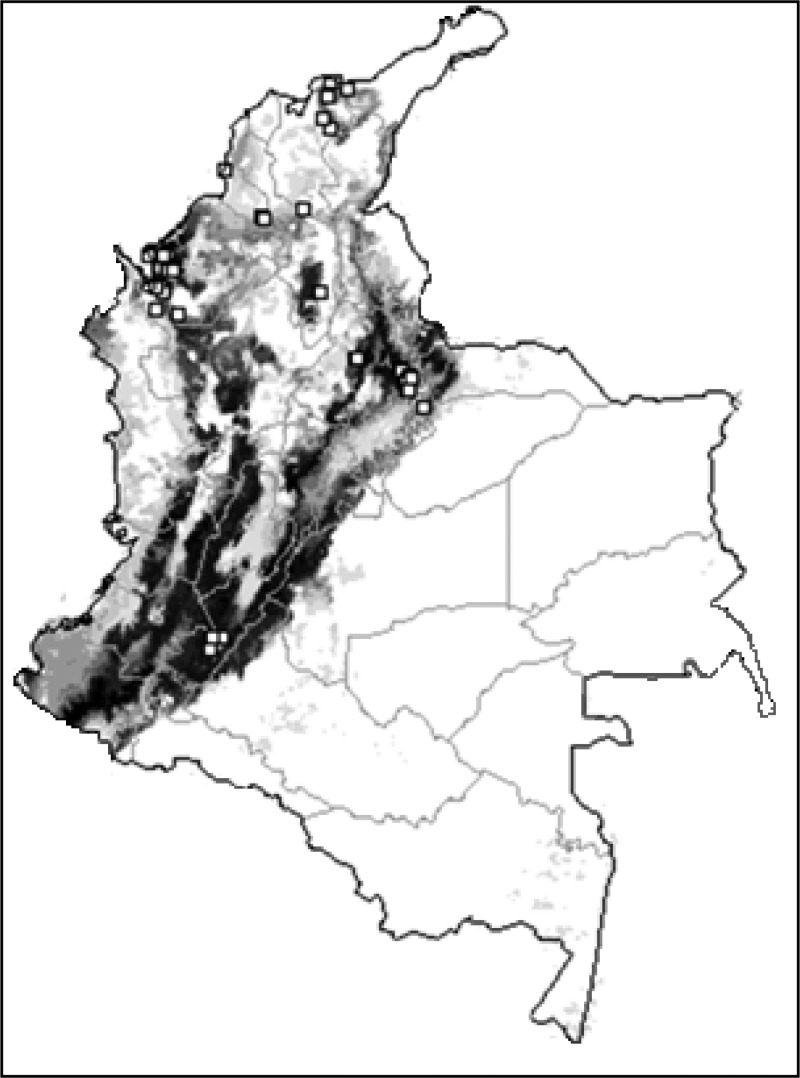
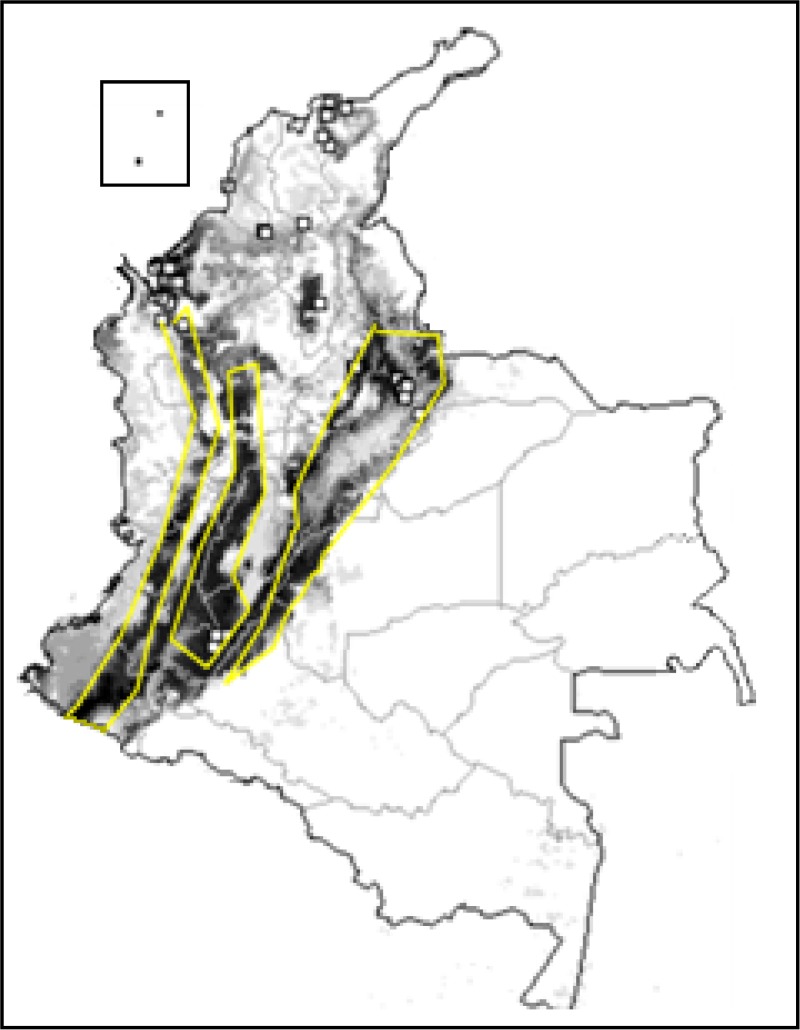
Triatoma dimidiata showed a wide potential distribution in the Andean region and northwest of Colombia. Temperature seasonality, annual precipitation, and NDVI were the variables that most influenced the model of T. dimidiata distribution (Figures 4 and 5 ) All test points were included in the predicted occurrence (0% omission), and AUC was 0.989. The binomial tests indicated high statistical significance (P < 0.01). These results indicated a good model performance.
Figure 4.
Ecological niche model for Triatoma dimidiata in Colombia. Blank areas represent triatomine absence predicted by the model. Areas identified as suitable based on environmental variables are shown in monochromatic scale: light gray (low suitability) to black (high suitability). Squares represent known occurrences of T. dimidiata. Lines represent political limits (gray = departments, black = country).
Figure 5.
Potential distribution of Triatoma dimidiata by ecological niche modeling and ecoregions of Colombia.
Discussion
Similar to other vector insects, triatomines are influenced by bioclimatic variables, and numerous studies have investigated the effect of climatic factors on several aspects of triatomine life cycle.14,15 During the past few years, a growing number of studies have attempted to establish integrated relationships between environmental variables and triatominae distribution.13,14,21,22,24–29,42
In this study, environmental variables that showed association with the presence of T. dimidiata were MaxNDVI, MinLST, and the DEM in the statistical model that we developed using variables extracted from remote sensors and NDVI and temperature seasonality and annual precipitation (both from WorldClim database) in ENM. Both models were in agreement with the importance of the vegetation index associated with T. dimidiata infestation. The models also shows the need to include data from different sources as annual precipitation, seasonal temperatures in ENM and MinLST and DEM in remote sensing associated with the presence of T. dimidiata. Those variables plus another variables have been used to model the distribution of other triatominae species.13,20,25,27,42,43 According to the values of the variables that better explain the presence of T. dimidiata in the predicted areas, we found variability in the values of MaxNDVI and MinLST and a high variability in the value of DEM.
With respect to MinLST, for the populations of the northwest region of the country, the high values of this variable can be explained because these populations mainly inhabit in palm trees where microclimate that favors the presence of the populations is present. In the eastern region of the country, the low values of MinLST are consistent with the values given in a study (M. Florez, unpublished data) for Santander Department where T. dimidiata appear more abundant in milder temperatures with low vegetation index. This seems to contrast with T. dimidiata populations from the northwest region of the country, which are more abundant in warmer and drier climate with high vegetation indexes. This may suggest important differences in the ecologic niches between these distinct populations and may confirm the ecological differences observed between them.44
According to the description of M. Florez (unpublished data), although the relation of the arthropod vectors with the temperature is recognized in Santander Department, T. dimidiata is found in zones with less temperature; our results for the east region of the country are according with these results, but contrast with the work of Bustamante and others,13 where they found correlation between the presence of T. dimidiata in Guatemala and the maximum absolute temperature, results that are more close to our results for the populations of the Caribbean plains of Colombia. The maximum and minimum temperatures might not be critical factors for the distribution of T. dimidiata but those variables reflect a pattern of habitat partitioning between the population of the east and northwest regions of Colombia.
In relation to the vegetation index, as discussed by other authors,20 although it is a synthetic variable, could reflect changes in temperature, water availability, and humidity that affect arthropod vectors. In the work of Dumonteil and Gourbiéri,14 vegetation type appears as an important predictor of abundance and infection of T. dimidiata. Our results show strong negative relation of high values of NDVI with the distribution of T. dimidiata in Colombia, this variable is a general proxy for environmental characterization because it integrates a wide number of climatic, geologic, zoologic, and anthropologic factors and is indeed one of the variables most often associated with vector geographic distribution.14,20,45,46 In the Yucatan peninsula, higher bug abundance appeared to be associated with perturbed vegetation (agriculture and pasture), suggesting that deforestation and habitat degradation are important factors contributing to the domiciliation of T. dimidiata, and conversely higher bug infection coincided with high forests, in agreement with the zoonotic origin of T. cruzi.14 These findings are according with the sites of collections because the search was made inside of the houses and its peridomicile, the latter which was mainly composed by chicken coops, ban, and bushes, the search was limited to these areas and no searches were realized in forest areas. Our results are in accordance with the results of Dumonteil and Gourbiéri for the Yucatan peninsula; we found higher T. dimidiata abundance and infestation of houses in the departments of Boyacá and Santander where deforestation and habitat degradation are conspicuous and conversely less abundance and in the Sierra Nevada of Santa Marta and Uraba regions where high forests are present with high numbers of palm trees. Although in the Caribbean plains (Bolivar and Sucre) agriculture- and pasture-driven ecological changes and perturbation of the vegetation occur, we found a high number of palm trees between the pastures, where bug population could survive.
The topographic variable (DEM) is also a synthetic variable that influences temperature, rainfall, and humidity and has been widely used in the mapping of infectious diseases,47 because of which the interpretation of this variable should be referenced in relation with any other mentioned. It would have been interesting to test the covariance between the topographic variable and others. Some works have demonstrated that mosquitoes and malaria transmission are sensitive to altitude.48 In relation to geographical factors linked to climatic conditions of triatominae vector species, several articles have been published in which latitude and altitude were correlated.49–53 Most of the species are found in tropical and subtropical areas and altitudes ranging from 100 to 1,800 masl. However, the most important vector species, T. infestans, is found at higher altitudes (4,100 masl in Bolivia).
In Colombia, Rhodnius pallescens were found in altitudes below 500 masl; R. prolixus in an altitudinal range from 0 to 2,800 masl; Panstrongylus geniculatus from 0 to 1,700 masl and T. dimidiata from 0 to 2,700 masl (V. Angulo, unpublished data).
The results suggest that northwest Andean montane forest, Cordillera Oriental montane forests, Magdalena valley montane forests, and Santa Marta montane forests are most favorable areas for the occurrence of T. dimidiata. The Choco-Darien moist forests (in the west) and the Oriental plains are less suitable for T. dimidiata presence. These results are different than that obtained by the statistical model, which predicted those areas, the Choco-Darien and the Oriental plains, as having high probability of T. dimidiata presence.
Triatoma dimidiata was predicted to be widely present in the pacific coast of Colombia (region without previous reports of this species), oriental plains/Orinoco region, Andean region, Guajira Department, and insular areas such as San Andres with high predicted probabilities of presence (Figure 1). Active surveillance for triatominae must be made in these regions, particularly Orinoco region, which shows the association of odds of T. dimidiata presence with the most recently reported oral Chagas outbreak in Paz de Ariporo (Casanare) where 31 people linked to mining activities were infected, although the most recent review of the species in that area does not report the presence of the species in Orinoco region.54 However, it would be useful to develop a more sensitive model that further stratifies the distribution of T. dimidiata to support control and surveillance activities.14 In this work, we explored the relationship between T. dimidiata and bioclimatic factors in Colombia and developed a distribution predictive model for this vector species in the country.
This is the first potential distribution model of T. dimidiata in Colombian territory and should be valuable for the design and implementation of effective targeting of vector control programs in the country.
Footnotes
Financial support: Departamento Administrativo Nacional de Ciencia y Tecnología de Colombia “Fondo Francisco José de Caldas–COLCIENCIAS, Colombia” and “Unión Temporal Programa Nacional de Investigación para la prevención, control y tratamiento integral de la enfermedad de Chagas en Colombia,” grant no. 380-2011, code 5014-537-30398380-2011.
Authors' addresses: Gabriel Parra-Henao, Red Chagas Colombia, Instituto Colombiano de Medicina Tropical, Medellín, Colombia, E-mail: gparrahenao@gmail.com. Oscar Quirós-Gómez and Ángela Segura Cardona, Grupo de Epidemiología y Bioestadística, Universidad CES, Medellín, Colombia, E-mails: quiromez@hotmail.com and asegura@ces.edu.co. Nicolas Jaramillo-O, Instituto de Biología, Universidad de Antioquia, Medellín, Colombia, E-mail: nicolas.jaramillo@udea.edu.co.
References
- 1.Ramírez CJ, Jaramillo CA, Delgado M, Pinto N, Aguilera G, Guhl F. Genetic structure of sylvatic, peridomestic and domestic populations of Triatoma dimidiata (Hemiptera: Reduviidae) from an endemic zone of Boyacá, Colombia. Acta Trop. 2005;93:23–29. doi: 10.1016/j.actatropica.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Control of Chagas Disease. Geneva, Switzerland: WHO; 2002. Technical report series 905. 1–07. [Google Scholar]
- 3.Parra-Henao G, Segura Á, Quirós-Gómez O, Angulo V, Alexander N. House-level risk factors for Triatoma dimidiata infestation in Colombia. Am J Trop Med Hyg. 2005;92:193–200. doi: 10.4269/ajtmh.14-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Epidemiol Rec Health Sect Secr Leag Nations. 2015;90:33–43. [PubMed] [Google Scholar]
- 5.Bargues MD, Klisiowicz DR, Gonzalez-Candelas F, Ramsey JM, Monroy C, Ponce C, Salazar-Schettino PM, Panzarea F, Abad-Franch F, Sousa OE, Schofield CJ, Dujardin JP, Guhl F, Mas-Coma S. Phylogeography and genetic variation of Triatoma dimidiata, the main Chagas disease vector in Central America, and its position within the genus Triatoma. PLoS Negl Trop Dis. 2008;2:e233. doi: 10.1371/journal.pntd.0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorn PL, Monroy C, Curtis A. Triatoma dimidiata (Latreille, 1811): a review of its diversity across its geographic range and the relationship among populations. Infect Genet Evol. 2007;7:343–352. doi: 10.1016/j.meegid.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Petana WB. American trypanosomiasis in British honduras. X. Natural habitats and ecology of Triatoma dimidiata (Hemiptera, Reduviidae) in the El Cayo and Toledo districts, and the prevalence of infection with Trypanosoma (Schizotrypanum) cruzi in the wild-caught bugs. Ann Trop Med Parasitol. 1971;65:169–178. [PubMed] [Google Scholar]
- 8.Zeledon R, Rabinovich JE. Chagas disease: an ecological appraisal with special emphasis on its insect vectors. Annu Rev Entomol. 1981;26:101–133. doi: 10.1146/annurev.en.26.010181.000533. [DOI] [PubMed] [Google Scholar]
- 9.Monroy MC, Bustamante DM, Rodas AG, Enriquez ME, Rosales RG. Habitats, dispersion and invasion of sylvatic Triatoma dimidiata (Hemiptera: Reduviidae: Triatominae) in Petén, Guatemala. J Med Entomol. 2003;40:800–806. doi: 10.1603/0022-2585-40.6.800. [DOI] [PubMed] [Google Scholar]
- 10.Parra-Henao G, Angulo V, Jaramillo N, Restrepo M. Factores de riesgo de infestación domiciliaria por Triatoma dimidiata. Biomedica. 2009;13((Suppl 1)):316. [Google Scholar]
- 11.Parra-Henao G, Angulo V, Jaramillo N, Restrepo M. Triatominos (Hemiptera: Reduviidae) de la Sierra Nevada de Santa Marta, Colombia. Aspectos epidemiológicos, entomológicos y de distribución. CES Med. 2010;23:17–26. [Google Scholar]
- 12.Parra-Henao G, Restrepo M, Restrepo B, Dominguez J. Estudio de tripanosomiasis americana en dos poblados indígenas de la Sierra Nevada de Santa Marta. CES Med. 2004;18:43–50. [Google Scholar]
- 13.Bustamante DM, Monroy MC, Rodas AG, Juarez JA, Malone JB. Environmental determinants of the distribution of Chagas disease vectors in south-eastern Guatemala. Geospat Health. 2007;1:199–211. doi: 10.4081/gh.2007.268. [DOI] [PubMed] [Google Scholar]
- 14.Dumonteil E, Gourbiére S. Predicting Triatoma dimidiata abundance and infection rate: a risk map for natural transmission of Chagas disease in the Yucatan peninsula of Mexico. Am J Trop Med Hyg. 2004;70:514–519. [PubMed] [Google Scholar]
- 15.Carcavallo RU. Climatic factors related to Chagas disease transmission. Mem Inst Oswaldo Cruz. 1999;94((Suppl 1)):367–369. doi: 10.1590/s0074-02761999000700071. [DOI] [PubMed] [Google Scholar]
- 16.Lillesand TM, Kiefer RW. Remote Sensing and Image Interpretation. 3rd edition. Toronto, Canada: John Wiley and Sons; 1994. p. 750. [Google Scholar]
- 17.Beck LR, Lobitz BM, Wood BL. Remote sensing and human health: new sensors and new opportunities. Emerg Infect Dis. 2000;6:217–227. doi: 10.3201/eid0603.000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitron U. Risk maps: transmission and burden of vector-borne diseases. Parasitol Today. 2000;16:324–325. doi: 10.1016/s0169-4758(00)01708-7. [DOI] [PubMed] [Google Scholar]
- 19.Thomson MC, Connor SJ. Environmental information systems for the control of arthropod vectors of disease. Med Vet Entomol. 2000;14:227–244. doi: 10.1046/j.1365-2915.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 20.Gorla DE. Remotely sensed environmental variables as indicators of Triatoma infestans (Heteroptera: Reduviidae) distribution. Ecol Austral. 2002;12:117–127. [Google Scholar]
- 21.Costa J, Peterson AT, Beard CB. Ecologic niche modeling and differentiation of populations of Triatoma brasiliensis neiva, 1911, the most important Chagas' disease vector in northeastern Brazil (hemiptera, reduviidae, triatominae) Am J Trop Med Hyg. 2002;67:516–520. doi: 10.4269/ajtmh.2002.67.516. [DOI] [PubMed] [Google Scholar]
- 22.Peterson AT, Sánchez-Cordero V, Beard CB, Ramsey JM. Ecologic niche modeling and potential reservoirs for Chagas disease, Mexico. Emerg Infect Dis. 2002;8:662–667. doi: 10.3201/eid0807.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beard CB, Pye G, Steurer F, Rodriguez R, Campman R, Peterson A, Ramsey J, Wirtz R, Robinson L. Chagas disease in a domestic transmission cycle in southern Texas, USA. Emerg Infect Dis. 2003;9:103–105. doi: 10.3201/eid0901.020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandoval-Ruiz CA, Zumaquero-Rios JL, Rojas-Soto OR. Predicting geographic and ecological distributions of triatomine species in the southern Mexican state of Puebla using ecological niche modeling. J Med Entomol. 2008;45:540–546. doi: 10.1603/0022-2585(2008)45[540:pgaedo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Gurgel-Goncalves R, Cuba CA. Predicting the potential geographical distribution of Rhodnius neglectus (Hemiptera, Reduviidae) based on ecological niche modeling. J Med Entomol. 2009;46:952–960. doi: 10.1603/033.046.0430. [DOI] [PubMed] [Google Scholar]
- 26.Gurgel-Gonçalves R, Silva R. Analysis of the geographical distribution of Psammolestes Bergroth (Heteroptera: Reduviidae) in South America with new records of Psammolestes tertius Lent & Jurberg. Zootaxa. 2009;2033:41–48. [Google Scholar]
- 27.Arboleda S, Gorla DE, Porcasi X, Saldaña A, Calzada J, Jaramillo ON. Development of a geographical distribution model of Rhodnius pallescens Barber, 1932 using environmental data recorded by remote sensing. Infect Genet Evol. 2009;9:441–448. doi: 10.1016/j.meegid.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Ibarra-Cerdeña CN, Sánchez-Cordero V, Townsend Peterson A, Ramsey JM. Ecology of North American triatominae. Acta Trop. 2009;110:178–186. doi: 10.1016/j.actatropica.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Gurgel-Gonçalves R, Galvão C, Costa J, Peterson AT. Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modeling. J Trop Med. 2012;2012:e705326. doi: 10.1155/2012/705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabaru Y, Monroy C, Rodas A, Mejia M, Rosales R. The geographical distribution of vectors of Chagas disease and population at risk of infestation in Guatemala. Med Entomol Zool. 1999;50:9–17. [Google Scholar]
- 31.Starr MD, Rojas JC, Zeledón R, Hird DW, Carpenter TE. Chagas' disease: risk factors for house infestation by Triatoma dimidiata, the major vector of Trypanosoma cruzi in Costa Rica. Am J Epidemiol. 1991;133:740–747. doi: 10.1093/oxfordjournals.aje.a115949. [DOI] [PubMed] [Google Scholar]
- 32.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 33.King RJ, Campbell-Lendrum DH, Davies CR. Predicting geographic variation in cutaneous leishmaniasis, Colombia. Emerg Infect Dis. 2004;10:598–607. doi: 10.3201/eid1004.030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv. 1997;24:38–49. [Google Scholar]
- 35.Pearce JL, Ferrier S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Modell. 2000;133:225–245. [Google Scholar]
- 36.Brooker S, Hay S, Tchuem Tchuenté L, Ratard R. Using NOAA-AVHRR data to model human helminth distributions in planning disease control in Cameroon, west Africa. Photogramm Eng Remote Sensing. 2002;68:175–179. [Google Scholar]
- 37.Rogers DJ. Models for vectors and vector-borne diseases. Adv Parasitol. 2006;62:1–35. doi: 10.1016/S0065-308X(05)62001-5. [DOI] [PubMed] [Google Scholar]
- 38.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 39.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190:231–259. [Google Scholar]
- 40.Anderson RP, Gómez-Laverde M, Peterson AT. Geographical distributions of spiny pocket mice in South America: insights from predictive models. Glob Ecol Biogeogr. 2002;11:131–141. [Google Scholar]
- 41.Pearson R, Raxworthy C, Nakamamura M, Peterson A. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr. 2006;34:102–117. [Google Scholar]
- 42.King RJ, Cordon-Rosales C, Cox J, Davies CR, Kitron UD. Triatoma dimidiata infestation in Chagas disease endemic regions of Guatemala: comparison of random and targeted cross-sectional surveys. PLoS Negl Trop Dis. 2011;5:e1035. doi: 10.1371/journal.pntd.0001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carbajal de la Fuente AL, Porcasi X, Noireau F, Diotaiuti L, Gorla DE. The association between the geographic distribution of Triatoma pseudomaculata and Triatoma wygodzinskyi (Hemiptera: Reduviidae) with environmental variables recorded by remote sensors. Infect Genet Evol. 2009;9:54–61. doi: 10.1016/j.meegid.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Grisales N, Triana O, Angulo V, Jaramillo N, Parra-Henao G, Panzaera F, Gómez-Palacio A. Genetic differentiation of three Colombian populations of Triatoma dimidiata (Heteroptera: Reduviidae) by ND4 mitochondrial gene molecular analysis. Biomedica. 2010;30:207–214. [PubMed] [Google Scholar]
- 45.Cross ER, Newcomb WW, Tucker CJ. Use of weather data and remote sensing to predict the geographic and seasonal distribution of Phlebotomus papatasi in southwest Asia. Am J Trop Med Hyg. 1996;54:530–536. doi: 10.4269/ajtmh.1996.54.530. [DOI] [PubMed] [Google Scholar]
- 46.Thomson MC, Connor SJ, D'Alessandro U, Rowlingson B, Diggle P, Creewell M, Greenwood B. Predicting malaria infection in Gambian children from satellite data and bed net use surveys: the importance of spatial correlation in the interpretation of results. Am J Trop Med Hyg. 1999;61:2–8. doi: 10.4269/ajtmh.1999.61.2. [DOI] [PubMed] [Google Scholar]
- 47.Guerra CA, Snow RW, Hay SI. Defining the global spatial limits of malaria transmission in 2005. Adv Parasitol. 2006;62:157–179. doi: 10.1016/S0065-308X(05)62005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox J, Craig M, Le Seur D, Sharp B. Mapping malaria risk in the highlands of Africa) 1999 (MARA/HIMA, 1999. [Google Scholar]
- 49.Carcavallo R, Curto de Casas S, Gálindez-Girón I, Jurberg J, Mena-Segura C. Geographical distribution and alti-latitudinal dispersion of genera and species of the tribes Alberproseniini, Bolboderini and Cavernicolini (Hemiptera, Reduviidae, Triatominae) Entomol Vectores. 1995;2:127–144. [Google Scholar]
- 50.Curto de Casas S, Carcavallo R, Gálindez-Girón I, Jurberg J, Mena-Segura C. Geographical distribution and alti-latitudinal dispersion of species of Panstrongylus (Hemiptera, Reduviidae, Triatominae, Triatomini) Entomol Vectores. 1996;3:43–58. doi: 10.1590/s0074-02761998000100007. [DOI] [PubMed] [Google Scholar]
- 51.Jurberg J, Galvao C, Galíndez-Girón I, Carcavallo RU, Mena Segura CA, Curto de Casas SI. Distribución geográfica y dispersión alti-latitudinal de las especies del género Triatoma Laporte, 1832 de Norte América, América Central y el Caribe. Entomol Vectores. 1996;3:87–117. [Google Scholar]
- 52.Galvão C, Jurberg J, Carcavallo RU, Segura CA, Galíndez-Girón I, Curto de Casas SI. Geographical distribution and alti-latitudinal dispersion of some genera and species of the tribe Triatomini Jeannel, 1919 (Hemiptera, Reduviidae, Triatominae) Mem Inst Oswaldo Cruz. 1998;93:33–37. doi: 10.1590/s0074-02761998000100007. [DOI] [PubMed] [Google Scholar]
- 53.Ramsey JM, Ordoñez R, Cruz-Celis A, Alvear AL, Chavez V, Lopez R, Pintor JR, Gama F, Carrillo S. Distribution of domestic triatominae and stratification of Chagas disease transmission in Oaxaca, Mexico. Med Vet Entomol. 2000;14:19–30. doi: 10.1046/j.1365-2915.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 54.Parra-Henao G, Florez Martinez M, Angulo Silva V. Vigilancia de Triatominae (Hemiptera: Reduviidae) en Colombia. Bogotá, Colombia: Sic Editorial Ltda; 2015. [Google Scholar]