Abstract
Dengue fever, caused by dengue virus (DENV), is endemic in more than 100 countries. The lack of effective treatment of patients and the suboptimal efficacies of the tetravalent vaccine in trials highlight the urgent need to develop alternative strategies to lessen the burden of dengue fever. Wolbachia pipientis, an obligate intracellular bacterium, is being developed as a biocontrol strategy against dengue because it limits the replication of the DENV in the mosquito vector, Aedes aegypti. However, several recent studies have demonstrated the sensitivity of pathogens, vectors, and their symbionts to temperature. To understand how the tripartite interactions between the mosquito, DENV, and Wolbachia may change under different temperature regimes, we assessed the vector competence and transmission potential of DENV-infected mosquitoes reared at a common laboratory setting of a constant 25°C and at two diurnal temperature settings with mean of 25°C and 28°C and a fluctuating range of 8°C (±4°C). Temperature significantly affected DENV infection rate in the mosquitoes. Furthermore, temperature significantly influenced the proportion of mosquitoes that achieved transmission potential as measured by the presence of virus in the saliva. Regardless of the temperature regimes, Wolbachia significantly and efficiently reduced the proportion of mosquitoes achieving infection and transmission potential across all the temperature regimes studied. This work reinforces the robustness of the Wolbachia biocontrol strategy to field conditions in Cairns, Australia, and suggests that similar studies are required for local mosquito genotypes and field relevant temperatures for emerging field release sites globally.
Introduction
An estimated 390 million people throughout tropical and subtropical regions of the globe contract dengue fever each year.1 The symptoms of clinically apparent cases of human infection range from mild to severe fever with some cases leading to fatal dengue shock syndrome.2–5 The ribonucleic acid (RNA) virus that causes the disease is vectored between humans mainly by the mosquito, Aedes aegypti and Aedes albopictus. Viruses such as dengue, West Nile, and yellow fever and other members of the Flavivirus genus have collectively become the most widespread arthropod-borne viruses affecting humans today.6 The lack of specific medical treatment of patients, the suboptimal performance of dengue vaccines in recent phase IIb and phase III trials, and rising rates of insecticide resistance in mosquito populations has highlighted the pressing need for alternative strategies to lessen the dengue burden.7–9 One of the most promising strategies in development is the use of the obligate endosymbiont Wolbachia pipientis to render Ae. aegypti, which does not naturally carry Wolbachia, incapable or less efficient at carrying the virus.8
Wolbachia is a maternally inherited intracellular bacterium that infects upwards of ~40% of insect species.10 Wolbachia manipulates its host reproduction for its own benefit and this serves as a driver for invading host populations.11 Additionally, the presence of Wolbachia reduces the replication of viruses in the host12,13 by competing for host resources critical for viruses14 and manipulating the host viral defense pathways such as the microRNA pathway.15 This antiviral property is the cornerstone of an initiative to use Wolbachia as a biocontrol agent against dengue virus (DENV) in the mosquito, Ae. aegypti.16–19 In controlled field releases in Cairns, Australia, the wMel strain of Wolbachia has successfully invaded natural populations of Ae. aegypti and infection frequencies have reached near-fixation a few months after the cessation of releases.20 More than 2 years later, the Wolbachia infection rate in the mosquito population remains high21 and Wolbachia-infected mosquitoes from these field populations continue to demonstrate reduced susceptibility to DENV under laboratory conditions.22 To assess the capacity of wMel-infected (wMel.F) mosquitoes to lower the transmission of the virus in a dengue endemic region, field-release trials are currently been carried out in Vietnam and Indonesia.8
Vectorial capacity is a measure of a vector-borne disease's transmission potential among humans. It is determined by a range of parameters including the contact rate between vector and human, the vector biting rate, daily survival of the vector, vector competence (VC), and the extrinsic incubation period (EIP).23,24 VC or the ability of the mosquito to carry a parasite is known to be influenced by mosquito genetics,25 virus genotype,26 mosquito by virus interactions,27 and mosquito gut microbiota.28 Furthermore, additional factors such as larval competition,29 nutrition,30 and temperature31 can influence the outcome of infection. Temperature in particular can influence how efficiently the host becomes infected with a parasite and how readily the parasite is transmitted by the host.32 For example, changes in temperature affect the length of the EIP33 or the time lag between when a mosquito consumes an infectious blood meal to when it is capable of secreting DENV in its saliva.34 EIP, relative to the other components of vectorial capacity, has the largest impact on rates of virus transmission as the earlier virus arrives in the saliva, the more humans the mosquito can potentially infect.24 This is particularly the case for Ae. aegypti that may seek blood meals every few days.35 Lastly, the diurnal temperature range (DTR), which emulates the degree or amplitude of daily cyclic temperature, was found to significantly influence the outcome of infection and survival of the mosquitoes, but not the EIP of DENV in Ae. aegypti as compared with a constant temperature.36,37 This relationship may be broadly applicable for other vector–virus interactions, with evidence of a similar effect on Plasmodium in Anopheles.38
The effects of changing temperature are not restricted to the vector. Wolbachia densities can also be affected by temperature,39 and DENV undergoes conformational changes under different temperatures.40 Such sensitivity to environmental temperature raises the possibility that the Wolbachia-mediated DENV-blocking phenotype is likely to be influenced by temperature.41 Indeed, various constant temperature regimes have been shown to affect the strength of Plasmodium inhibition in somatically Wolbachia transinfected Anopheles mosquitoes.42 However, previous knowledge gained on Wolbachia-mediated DENV blocking at constant temperatures, especially the most commonly used laboratory setting of 25°C, reveals very little on how the DENV blocking phenotype may vary in a natural environment. For example, mean daily temperature in Cairns, where dengue outbreaks occur in Australia,43 can range from 21°C to 28°C throughout the year with a DTR reaching 8°C (±4°C).44
To understand how the interactions between mosquitoes, DENV and Wolbachia may change in terms of different temperature regimes, we assessed the effects of both constant and diurnal temperature around a mean of 25°C on Wolbachia-mediated DENV blocking in wMel.F mosquitoes recaptured from the Wolbachia field release sites in Cairns. To simulate the warmer weather during the dengue outbreak seasons in this region, we further studied the effects of a DTR with a mean of 28°C. We demonstrated that temperature not only changes VC of the mosquitoes and DENV blocking, it also alters Wolbachia density in mosquitoes. Wolbachia-infected mosquitoes continued to strongly limit the replication of DENV, however, significantly reducing the proportion of mosquitoes achieving infection and transmission potential. This reinforces the likely robustness of Wolbachia-based biocontrol strategy to field condition variability temperatures in Cairns and affirms that experimental work carried out at 25°C may be taken as representative.
Materials and Methods
Ethical considerations.
Blood feeding of mosquitoes with human volunteers was performed in accordance to Human Research Ethics Committee of Monash University (permit CF11/0766-2011000387). The volunteers gave written informed consent prior to taking part in the study.
Temperature regimes.
Three temperature regimes were used in this study: a constant temperature of 25°C (25°C ± 0°C), a fluctuating range with a mean of 25°C and a DTR of 8°C (25°C ± 4°C), and a fluctuating range with a mean of 28°C with a DTR of 8°C (28°C ± 4°C). Fluctuating temperature regimes followed a sinusoidal progression during the day and a negative exponential decrease at night. The maximum and minimum temperatures were reached at 14:00 and 2:00, respectively. After oral infection of DENV, experimental mosquitoes were housed in MLR-352H-PE incubators (Panasonic Australia, New South Wales, Australia) that maintained the respective temperature regimes at ∼70% relative humidity, with 12:12 hours light : dark cycle. One fluorescent light was scheduled to turn on at 08:00 and off at 20:00. Temperature and humidity data loggers (EL-USB-2; Lascar Electronics, Salisbury, United Kingdom) recorded temperatures on half an hourly basis in the incubators. Actual air temperatures within the incubators matched the programmed temperature profile.
Mosquitoes.
The Wildtype (WT) mosquito colony (not infected with Wolbachia) was established from eggs routinely collected from ovitraps from neighboring suburbs outside of the Wolbachia field-release populations in Cairns, Australia. The wMel.F population was founded by mosquito eggs collected once from within the Wolbachia release areas in Cairns, Australia. Ae. aegypti species identification and Wolbachia screening at the point of collection was as previously described.20 All experimental work was carried out within four generations of field collection for the WT colony to limit the effects of inbreeding on genetic diversity. To prevent genetic drift between the two lines while maintaining Wolbachia infection, 20% of the males in the wMel.F line were replaced with WT males each generation. After hatching, mosquito larvae were reared at a constant 25°C, ∼70% relative humidity, with 12:12 hours light : dark cycle, at a density of 150 individuals/3 L of distilled water in plastic trays (30 × 40 × 8 cm) and fed fish food (Tetramin Tropical Tablets; Tetra, Melle, Germany) until pupation. Pupae were transferred to 30 × 30 × 30 cm cages to allow adult emergence at a density of approximately 400 individuals per cage. Adults were fed a 10% sucrose diet ad libitum.45
Virus.
A dengue virus serotype 3 (DENV-3) strain was used that was originally isolated from a patient diagnosed with dengue in the 2008/2009 outbreak in Cairns. This strain caused one of the largest outbreaks in recorded history (>900 cases) in far north Queensland, Australia.46 Virus was passaged, grown, and collected fresh from cell culture as previously described.47 The freezing and thawing of viruses reduces infectivity48 and therefore we used freshly propagated virus to achieve the greatest possible infection rate in the mosquitoes.
DENV infection.
Five- to 8-day-old female Ae. aegypti were starved for 24 hours and then provided defibrinated sheep blood containing live DENV to a final concentration of 3 × 106 plaque-forming units/mL as determined by plaque assay.45 The virus blood meal was held over a water-jacketed membrane-feeding apparatus by a piece of desalted pig intestine. The feeding apparatus was maintained at 37°C, and mosquitoes were allowed to feed for 3 hours. Previous research by our group demonstrated the stability of virus over this period.45 Unfed mosquitoes were removed from populations 24 hours later under carbon dioxide (CO2). Fed mosquitoes were then used for VC, transmission, and survival assays under their respective temperature regimes.
VC and Wolbachia density.
The head and carcass (body) of the mosquito 7 days postinfection (DPI) were used to measure the rate of DENV dissemination and infection, respectively. Head and carcass samples were homogenized using 2-mm-diameter glass beads (Merck KGaA, Darmstadt, Germany) in a mini-Beadbeater (BioSpec Products, Bartlesville, OK). Multiplex quantitative polymerase chain reaction assay was performed on a LightCycler 480 II machine using 4×TaqMan Fast Virus 1-Step Master Mix (Life Technologies Australia, New South Wales, Australia) according to the manufacturer's instructions. DENV concentrations in the samples were extrapolated from a standard curve of known DENV copy number. The data were expressed as concentration of virus per tissue by back calculating to the initial concentration of RNA.47,49 Quantification of Wolbachia density used Ae. aegypti ribosomal protein S17 as the reference gene. Wolbachia strain wMel was detected with primers/probes specific to the WD0513 gene as previously published.50
Transmission potential.
To estimate the transmission potential of the mosquitoes, saliva was collected from individual mosquitoes as modified from a previously established method.45 In brief, individual mosquitoes were lightly anesthetized with CO2 and placed inside a 70-mL polypropylene cup (Sarstedt, Nümbrecht, Germany) capped by a piece of 100% polyester mesh (Spotlight Pty Ltd., Victoria, Australia). The lid of a 2-mL polypropylene screw-cap tube (Sarstedt) was affixed to the bottom of the inside of the cup using adhesive plasticine (Bostik, Thomastown, Vic, Australia). The lid was then filled with 200 μL of 10% sucrose. The sucrose solution provided was the only source of food and fluid for the mosquito. The sucrose solution was collected every 2 days from 4 to 20 DPI to test for the presence of virus expectorated during feeding. Any mosquito death was recorded before each saliva collection. During each collection, the mosquito was lightly anesthetized using CO2 so that the bottom half of the 2-mL tube could be screwed onto the lid containing the sucrose solution and removed. A new lid was then provided containing fresh sucrose solution. DENV was detected in the collection tube as previously described.45
Data analysis.
Binary phenotypes (infection, dissemination, and infectivity) were analyzed using nominal logistic regression fitted with a binomial error distribution.
For the continuous phenotype (DENV titer in head and carcass), the dependent variable was log-transformed and analyzed using a linear regression model fitted with a normal error distribution. EIP and Wolbachia density data were analyzed using a general linear model fitted with an identity link function and normally distributed errors. The number of days DENV was detectable in mosquito saliva was analyzed using Mann–Whitney U tests due to its deviation from normality. Survival data were analyzed using log-rank statistics. Statistical analyses were performed using the software Statistica 8.0 (StatSoft, Inc., Tulsa, OK).
Results
Temperature and Wolbachia influence DENV infection rate in mosquitoes.
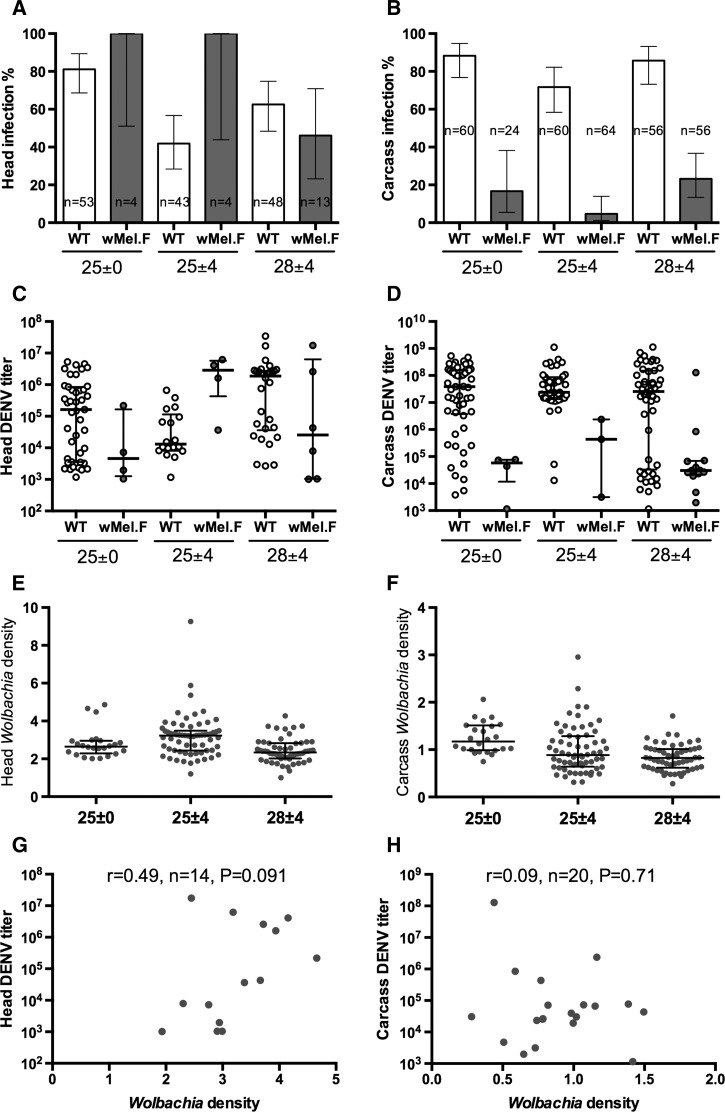
We used DENV infection of the carcass (body) as a measure of infection and presence of DENV in the head as an indication of dissemination. Only mosquitoes with carcass infection were used to calculate rate of dissemination. Temperature had a significant effect on DENV infection rate in the mosquito carcass (F = 6.8, degrees of freedom [df] = 2, P < 0.01; Figure 1B and Table 1). Mosquitoes reared at a constant temperature of 25°C (25°C ± 0°C) had a higher dissemination rate (F = 7.5, df = 1, P < 0.01) as compared with those reared at a diurnal temperature around a mean temperature of 25°C (25°C ± 4°C). Raising the diurnal temperature mean from 25°C to 28°C significantly (F = 11.4, df = 1, P < 0.01) increased infection rate from 72% to 86% and from 5% to 23% in WT and wMel.F, respectively. Of the mosquitoes that had a carcass infection, temperature had a significant effect on DENV dissemination rate to the mosquito head (F = 4.3, df = 2, P < 0.05; Figure 1 and Table 1). However, we did not find a significant change (F = 0.35, df = 1, P = 0.56) in dissemination rate when mosquitoes were reared at a constant temperature of 25°C (25°C ± 0°C) as compared with those reared at a diurnal temperature around a mean temperature of 25°C (25°C ± 4°C). Increasing the diurnal temperature mean from 25°C to 28°C also did not significantly (F = 3.3, df = 1, P = 0.21) change the dissemination rate of the mosquitoes.
Figure 1.
Infection rate ± 95% confidence intervals for (A) head and (B) carcass of WT (white) and wMel-infected (wMel.F) mosquito (gray) reared on three temperature regimes orally infected with dengue virus serotype 3 (DENV-3). To measure dissemination rate of virus to the head, only mosquitoes with carcass infection was used as denominator. Medians and interquartile ranges of viral titer for (C) head and (D) carcass of WT and wMel.F mosquito reared on three temperature regimes. Wolbachia density of (E) head and (F) carcass of WT and wMel.F mosquito reared on three temperature regimes following orally infected with DENV-3. Mean ± standard error was plotted. Each data point represents 24–64 females (mean = 53). Wolbachia density vs. (G) head and (H) carcass viral titer of wMel.F mosquitoes across all three temperature regimes. Spearman's nonparametric r test is used to test for a correlation.
Table 1.
Summary of ANOVA on the effect of temperature regimes on DENV-3 infection (carcass) and dissemination (head) in Aedes aegypti and Wolbachia density
| All temperature regimes | 25°C ± 0°C vs. 25°C ± 4°C | 25°C ± 4°C vs. 28°C ± 4°C | |
|---|---|---|---|
| P value | Adjusted P value | Adjusted P value | |
| Head dissemination rate | |||
| Temperature | * | 0.56 | 0.21 |
| Wolbachia | 0.27 | 0.23 | 0.47 |
| Temperature × Wolbachia | 0.11 | 0.56 | 0.14 |
| Carcass infection rate | |||
| Temperature | ** | ** | ** |
| Wolbachia | **** | **** | **** |
| Temperature × Wolbachia | 0.71 | 0.65 | 0.64 |
| Head DENV titer | |||
| Temperature | 0.11 | 0.86 | 0.11 |
| Wolbachia | 0.73 | 0.56 | 0.60 |
| Temperature × Wolbachia | ** | ** | ** |
| Carcass DENV titer | |||
| Temperature | 0.45 | 0.34 | 0.34 |
| Wolbachia | **** | **** | **** |
| Temperature × Wolbachia | 0.65 | 0.74 | 0.74 |
| Head Wolbachia density | **** | 0.15 | *** |
| Carcass Wolbachia density | *** | * | * |
ANOVA = analysis of variance; DENV = dengue virus; DENV-3 = dengue virus serotype 3. Only mosquitoes with carcass infection were used to calculated dissemination rate. P values for post hoc pairwise comparison statistics (between two temperature regimes) were corrected using the Benjamini and Hochberg method.51 *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Regardless of the temperature regimes, Wolbachia was able to significantly reduce the rate of DENV infection in the mosquitoes (F = 242, df = 1, P < 0.0001; Table 1) but did not significantly reduce the dissemination rate of mosquitoes that already had carcass infection (F = 1.2, df = 1, P = 0.27). Temperature did not have a significant effect on Wolbachia-mediated reduction in infection rate (temperature × Wolbachia effect, F = 0.35, df = 1, P = 0.71; Table 1) or dissemination rate (F = 2.2, df = 1, P = 0.11).
Of the mosquitoes that achieved dissemination, temperature had little effect on the titer of DENV in the head (F = 2.2, df = 2, P = 0.11; Figure 1C) or the carcass (F = 0.79, df = 2, P = 0.45; Figure 1D) of the mosquitoes. Wolbachia significantly lowered the DENV titer in the carcass (F = 35, df = 1, P < 0.0001) but not in the head (F = 0.12, df = 1, P = 0.11). Head DENV titer was significant influenced by temperature by Wolbachia interactions (F = 6.1, df = 1, P < 0.01; Table 1).
Temperature influences Wolbachia density.
Temperature regimes significantly altered the Wolbachia density of the mosquitoes in both the head (F = 10.1, df = 2, P < 0.0001; Figure 1E) and the carcass (F = 9.4, df = 2, P < 0.001; Figure 1F). It was noted that Wolbachia density was highest when mosquitoes were reared on a diurnal temperature around the mean of 25°C, which coincided with the lowest DENV dissemination rate in wMel.F mosquitoes. However, we observed no clear trend between the changes in Wolbachia density with DENV infection rate in the carcass.
We then tested for a correlation between Wolbachia density and DENV titer in mosquitoes. Due to the small number of Wolbachia-infected mosquitoes that were simultaneously infected with DENV, we plotted and analyzed these mosquitoes across all three temperature regimes. We observed no correlation between Wolbachia density and DENV titer in the head (Spearman's r = 0.49, N = 14, P = 0.091; Figure 1G) or the carcass (Spearman's r = 0.09, N = 20, P = 0.71; Figure 1H).
Both temperature and Wolbachia influence the proportion of mosquitoes achieving transmission potential.
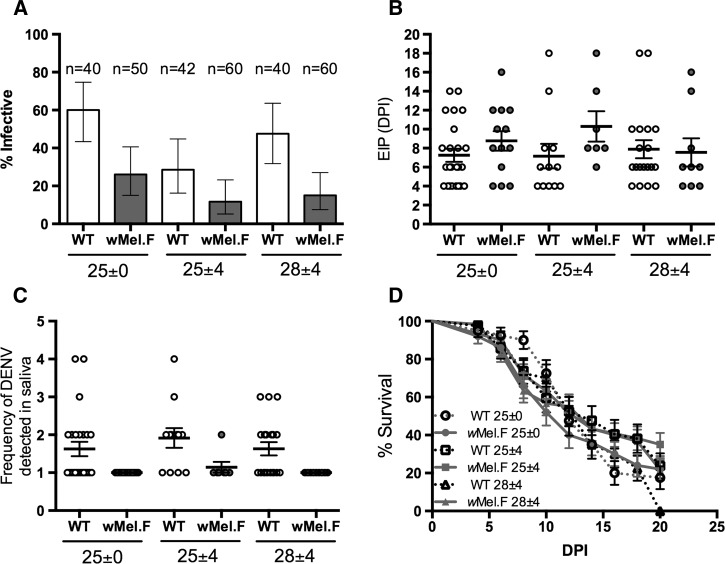
To determine the effect of temperature and Wolbachia on the transmission potential of the mosquitoes for DENV, we measured transmission traits including proportion of mosquitoes with detectable DENV in saliva at any collection time point from 4 to 20 DPI (infectivity), the earliest time point DENV can be detected in the saliva of mosquitoes (EIP), and the frequency (number of time points) that DENV can be detected in the saliva. Temperature significantly altered the proportion of mosquitoes that became infective (F = 6.8, df = 2, P < 0.01; Figure 2A ). Consistent with the trend in dissemination rate (Figure 1A), mosquitoes reared at a constant temperature of 25°C are more likely to be infective (F = 13.6, df = 1, P < 0.001) compared with those reared at a diurnal regimen around a mean of 25°C (25°C ± 4°C). There was no significant temperature × Wolbachia interaction affecting whether a mosquito becomes infective (F = 1.2, df = 1, P = 0.3).
Figure 2.
(A) Proportion of infective ± 95% confidence intervals, (B) extrinsic incubation period (EIP), (C) frequency of dengue virus (DENV) detected in saliva, (D) and survival of WT (white) and wMel-infected (wMel.F) mosquito (gray) reared on three temperature regimes orally infected with dengue virus serotype 3. Infective rate (A) of WT and wMel.F mosquito. Each data point represents 40–60 females (mean = 49).
Neither temperature (F = 0.3, df = 2, P = 0.71) nor Wolbachia (F = 2.3, df = 1, P = 0.13; Figure 2B) significantly change the EIP of the mosquitoes. Wolbachia significantly reduced the frequency that DENV is detectable in the saliva (F = 15.9, df = 1, P < 0.001; Figure 2C). However, this frequency was not influenced by temperature (F = 0.62, df = 2, P = 0.54), nor was a temperature × Wolbachia interaction evident (F = 0.07, df = 1, P = 0.93). Lastly, we measured the survival of DENV-3 fed WT and wMel.F mosquitoes from the point of DENV feed to 20 DPI. We found that change in temperature regimes had little effect on the mosquito survival of either WT or wMel.F mosquitoes (Figure 2D and Table 2).
Table 2.
Summary of ANOVA on the effect of temperature regimes on transmission potential of Aedes aegypti for DENV-3 and mosquito survival
| All temperature regimes | 25°C ± 0°C vs. 25°C ± 4°C | 25°C ± 4°C vs. 28°C ± 4°C | |
|---|---|---|---|
| P value | Adjusted P value | Adjusted P value | |
| Proportion of infective mosquitoes | |||
| Temperature | ** | *** | 0.08 |
| Wolbachia | **** | *** | *** |
| Temperature × Wolbachia | 0.3 | 0.18 | 0.18 |
| EIP | |||
| Temperature | 0.71 | 0.52 | 0.46 |
| Wolbachia | 0.13 | 0.26 | 0.52 |
| Temperature × Wolbachia | 0.37 | 0.52 | 0.52 |
| Frequency of DENV detected in saliva | |||
| Temperature | 0.54 | 0.49 | 0.49 |
| Wolbachia | *** | ** | ** |
| Temperature × Wolbachia | 0.93 | 0.74 | 0.74 |
| Survival | |||
| WT | NA | 0.57 | 0.24 |
| wMel.F | NA | 0.24 | 0.32 |
ANOVA = analysis of variance; DENV = dengue virus; DENV-3 = dengue virus serotype 3; EIP = extrinsic incubation period; NA = not applicable; wMel.F = wMel-infected. P values for post hoc pairwise comparison statistics (between two temperature regimes) were corrected using the Benjamini and Hochberg method.51 *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
Wolbachia replication and density in its host is known to be sensitive to temperature changes. In Ae. albopictus, an increase of constant temperature from 25°C to 37°C reduced Wolbachia density.52 Changes in temperature can also affect a wide range of Wolbachia-induced phenotypes in its host such as maternal transmission, cytoplasmic incompatibility, and host fitness including longevity.53,54 Effects of temperature on Wolbachia-mediated pathogen blocking have only been reported in somatically infected Anopheles.42 To our knowledge, this is the first report that compares constant and diurnal temperature regimes on Wolbachia-mediated DENV blocking. To simulate the daily cyclic temperature fluctuations, a DRT of 8°C (±4°C) was chosen to test if DENV blocking happens at the same efficiency in a diurnal temperature as compared with a constant temperature both with the same mean temperature. To further mimic the warmer months in summer in Cairns where dengue outbreaks occur,43 a diurnal temperature around a mean temperature of 28°C was selected. We showed here that the rearing of mosquitoes at a constant temperature overestimates the body infection and the proportion of mosquitoes achieving transmission capability as compared with a diurnal of temperature around the same mean temperature of 25°C. We also found that raising the diurnal temperature regimes around the mean by 3°C enhances VC parameters and the transmissibly of DENV. Wolbachia efficiently reduced carcass infection rate and the proportion of mosquitoes achieving transmission potential across all temperature regimes. Wolbachia infection did not significantly change the dissemination rate of those mosquitoes that had already achieved infection in the body. It should be noted, however, that the small number of wMel.F mosquitoes with carcass infection greatly limited the power to test the effect of temperature on dissemination rate.
Infection and transmission of DENV in Ae. aegypti is influenced by the amplitude and pattern of daily temperature variation. This amplitude of change, which is represented by the DTR, affects both Ae. aegypti infected with DENV36 and Anopheles carrying Plasmodium38 in a nonlinear manner. DTR and mean temperature in combination dictates how fast Ae. aegypti can transmit DENV. With field release of Wolbachia-infected mosquitoes in dengue endemic region such as Vietnam and Indonesia, each site with its unique mean temperature and DTR, it will be important to examine how these two variables also impact the efficiency of Wolbachia-mediated DENV blocking.
Further, temperature is known to have an effect on insect immunity.55 Though the fundamental mechanism involved in viral blocking, demonstrated in both Drosophila and Aedes, does not seem to be dependent on the upregulation of the immune effectors, it is likely that Wolbachia-associated immune priming in mosquitoes can enhance the virus blocking phenotype.56,57 Furthermore, microbial gut flora of mosquito interacts with host immune system to determine the outcome of host–pathogen interactions,28,58 and evidence is emerging on how temperature can affect the presence and quantity of symbionts in insect's gut to influence host fitness.59 How different temperature regimes can affect DENV blocking by possibly changing host immunity and its gut microbiota also needs to be investigated.
Finally, VC parameters such as the proportion of mosquitoes with a body infection, rate of dissemination of virus, and virus titer in mosquito tissue are dependent on the combination of mosquito genotype and virus genotype.27 To determine the role of environment on host and parasite interactions, a study of chikungunya viruses in Ae. albopictus populations extended the concept to include both interacting genotypes (virus and mosquito) and temperature. The study demonstrated that these sources of variation do not necessarily act independently and may interact with each other (mosquito genotype × virus genotype × environment) to determine the outcome of VC.60 To accurately assess the impact and plasticity of Wolbachia-mediated DENV blocking, it is also necessary to take this G × G × E interaction into consideration.
A limitation of this study is the use of a single DENV strain. We selected this DENV-3 isolate as it achieves a high infection rate in WT mosquitoes. The efficiency of Wolbachia-mediated DENV blocking greatly limited our choice of virus strains in this study. For example, wMel.F was found to completely limit some DENV strains from disseminating to the head of the mosquito.18,22 To examine the generality of DENV blocking and effects on transmission parameters, a diversity of viral genotypes, including representatives of the other three serotypes, need be used in the future.
We have previously reported a lengthening of EIP in mosquitoes infected with wMel.F as compared with uninfected ones. In that study, we used saliva pooled from a group of mosquitoes and at any one time point only ∼5% of the wMel.F cages/pools were DENV positive for saliva.45 Here we used live DENV in individual mosquitoes to examine their EIP and have observed no significant delay in EIP due to the presence of Wolbachia. With only ∼20% of the wMel.F mosquitoes producing a valid EIP this greatly limits the power to tease apart the effects of temperature and Wolbachia on EIP. Challenging Wolbachia-infected mosquitoes with viremic blood from dengue patients or concentrating DENV using ultracentrifugation methods may help to increase the proportion of wMel.F mosquitoes that achieve transmission potential to study the EIP trait at the individual mosquito level.
Lastly, recent studies showed that virus dissemination in the mosquito was reduced when the immature stages of Ae. albopictus were spent in cooler conditions.61 The interaction between temperature and food experienced during larval and pupal stages of the mosquitoes was also found to influence DENV-1 infection of adult Ae. albopictus.62 Future experiments should extend to study the effect of rearing larvae and pupae under different temperature regimes on DENV infection and transmission.62
Conclusions
We showed that whether a mosquito becomes infected, reaches dissemination, and achieves transmission of dengue is heavily dependent on temperature. Wolbachia density is also sensitive to temperature changes, and temperature can interact with Wolbachia to determine the VC of the mosquito. However, we found that Wolbachia efficiently reduced the proportion of mosquitoes achieving infection and transmission potential across all temperature regimes tested. As field releases of Wolbachia-infected mosquitoes are currently underway in dengue-endemic regions each with its unique climate, it is important to take into consideration the mean temperature and extent of fluctuations in daily temperature to better access the impact of Wolbachia on DENV transmission. This work, however, shows a robustness of Wolbachia-based blocking to temperature variation that mimic the warmer months in summer in Cairns. Parallel studies should be carried out with mosquito genotypes and field temperatures relevant to emerging release sites globally.
ACKNOWLEDGMENTS
The authors thank Nichola Kenny for assistance with mosquito rearing.
Footnotes
Financial support: The research was supported by the National Health and Medical Research Council of Australia (No. APP1037003 and No. APP1020607).
Authors' addresses: Yixin H. Ye, Alison M. Carrasco, Yi Dong, Carla M. Sgrò, and Elizabeth A. McGraw, School of Biological Sciences, Monash University, Clayton, Victoria, Australia, E-mails: henry.ye@monash.edu, ali_clem@hotmail.com, yi.dong@monash.edu, carla.sgro@monash.edu, and beth.mcgraw@monash.edu.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 4.Ross TM. Dengue virus. Clin Lab Med. 2010;30:149–160. doi: 10.1016/j.cll.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead SB. Dengue virus-mosquito interactions. Annu Rev Entomol. 2008;53:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- 6.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 7.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 8.McGraw EA, O'Neill SL. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 9.Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, Pitisuttithum P, Thisyakorn U, Yoon IK, van der Vliet D, Langevin E, Laot T, Hutagalung Y, Frago C, Boaz M, Wartel TA, Tornieporth NG, Saville M, Bouckenooghe A, Group CYDS Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 10.Zug R, Koehncke A, Hammerstein P. Epidemiology in evolutionary time: the case of Wolbachia horizontal transmission between arthropod host species. J Evol Biol. 2012;25:2149–2160. doi: 10.1111/j.1420-9101.2012.02601.x. [DOI] [PubMed] [Google Scholar]
- 11.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 12.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caragata EP, Rances E, Hedges LM, Gofton AW, Johnson KN, O'Neill SL, McGraw EA. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013;9:e1003459. doi: 10.1371/journal.ppat.1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Hussain M, O'Neill SL, Asgari S. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc Natl Acad Sci USA. 2013;110:10276–10281. doi: 10.1073/pnas.1303603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xi Z, Khoo CC, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 17.McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, O'Neill SL. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 18.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O'Neill SL, Hoffmann AA. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 19.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O'Neill SL. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips B, Billington K, Axford JK, Montgomery B, Turley AP, O'Neill SL. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, McGraw EA, O'Neill SL. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Adv Virus Res. 2003;60:187–232. doi: 10.1016/s0065-3527(03)60006-0. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald G. The Epidemiology and Control of Malaria. London and New York: Oxford University Press; 1951. [Google Scholar]
- 25.Bosio CF, Beaty BJ, Black WCt. Quantitative genetics of vector competence for dengue-2 virus in Aedes aegypti. Am J Trop Med Hyg. 1998;59:965–970. doi: 10.4269/ajtmh.1998.59.965. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JR, Rico-Hesse R. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. Am J Trop Med Hyg. 2006;75:886–892. [PMC free article] [PubMed] [Google Scholar]
- 27.Lambrechts L, Chevillon C, Albright RG, Thaisomboonsuk B, Richardson JH, Jarman RG, Scott TW. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol Biol. 2009;9:160. doi: 10.1186/1471-2148-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez JL, Souza-Neto J, Cosme RT, Rovira J, Ortiz A, Pascale JM, Dimopoulos G. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Neglect Trop Dis. 2012;6:e1561. doi: 10.1371/journal.pntd.0001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects Arbovirus infection in Aedes mosquitoes. Ecology. 2005;86:3279–3288. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimstad PR, Walker ED. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. IV. Nutritional deprivation of larvae affects the adult barriers to infection and transmission. J Med Entomol. 1991;28:378–386. doi: 10.1093/jmedent/28.3.378. [DOI] [PubMed] [Google Scholar]
- 31.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue-2 virus. Am J Trop Med Hyg. 1987;36:143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 32.Thomas MB, Blanford S. Thermal biology in insect-parasite interactions. Trends Ecol Evol. 2003;18:344–350. [Google Scholar]
- 33.Turell MJ, Rossi CA, Bailey CL. Effect of extrinsic incubation temperature on the ability of Aedes taeniorhynchus and Culex pipiens to transmit Rift Valley fever virus. Am J Trop Med Hyg. 1985;34:1211–1218. doi: 10.4269/ajtmh.1985.34.1211. [DOI] [PubMed] [Google Scholar]
- 34.Black WC, Bennett KE, Gorrochotegui-Escalante N, Barillas-Mury CV, Fernandez-Salas I, Munoz MD, Farfan-Ale JA, Olson KE, Beaty BJ. Flavivirus susceptibility in Aedes aegypti. Arch Med Res. 2002;33:379–388. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- 35.Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- 36.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrington LB, Armijos MV, Lambrechts L, Scott TW. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl Trop Dis. 2013;7:e2735. doi: 10.1371/journal.pntd.0002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mouton L, Henri H, Charif D, Bouletreau M, Vavre F. Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol Lett. 2007;3:210–213. doi: 10.1098/rsbl.2006.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang XZ, Sheng J, Plevka P, Kuhn RJ, Diamond MS, Rossmann MG. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc Natl Acad Sci USA. 2013;110:6795–6799. doi: 10.1073/pnas.1304300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu P, Bian G, Pan X, Xi Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl Trop Dis. 2012;6:e1754. doi: 10.1371/journal.pntd.0001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murdock CC, Blanford S, Hughes GL, Rasgon JL, Thomas MB. Temperature alters Plasmodium blocking by Wolbachia. Sci Rep. 2014;4:3932. doi: 10.1038/srep03932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanna JN, Ritchie SA. Outbreaks of dengue in north Queensland, 1990–2008. Commun Dis Intell. 2009;33:32–33. doi: 10.33321/cdi.2009.33.5. [DOI] [PubMed] [Google Scholar]
- 44.weatherzone.comweatherzone.com Available at. Accessed January 2015.
- 45.Ye YH, Carrasco AM, Frentiu FD, Chenoweth SF, Beebe NW, van den Hurk AF, Simmons CP, O'Neill SL, McGraw EA. Wolbachia reduces the transmission potential of Dengue-infected Aedes aegypti. PLoS Negl Trop Dis. 2015;9:e0003894. doi: 10.1371/journal.pntd.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, Christofferson RC, Gubler DJ, Bennett SN, van den Hurk AF. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One. 2013;8:e68137. doi: 10.1371/journal.pone.0068137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye YH, Ng TS, Frentiu FD, Walker T, van den Hurk AF, O'Neill SL, Beebe NW, McGraw EA. Comparative susceptibility of mosquito populations in North Queensland, Australia to oral infection with dengue virus. Am J Trop Med Hyg. 2014;90:422–430. doi: 10.4269/ajtmh.13-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards SL, Pesko K, Alto BW, Mores CN. Reduced infection in mosquitoes exposed to blood meals containing previously frozen flaviviruses. Virus Res. 2007;129:224–227. doi: 10.1016/j.virusres.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kien DTH, Tuan TV, Hanh TNT, Chau TNB, Huy HLA, Wills BA, Simmons CP. Validation of an internally controlled one-step real-time multiplex RT-PCR assay for the detection and quantitation of dengue virus RNA in plasma. J Virol Methods. 2011;177:168–173. doi: 10.1016/j.jviromet.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson NM, Kien DT, Clapham H, Aguas R, Trung VT, Chau TN, Popovici J, Ryan PA, O'Neill SL, McGraw EA, Long VT, Dui le T, Nguyen HL, Chau NV, Wills B, Simmons CP. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7:279ra37. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 52.Wiwatanaratanabutr I, Kittayapong P. Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J Invertebr Pathol. 2009;102:220–224. doi: 10.1016/j.jip.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Clancy DJ, Hoffmann AA. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia-infected Drosophila simulans. Entomol Exp Appl. 1998;86:13–24. [Google Scholar]
- 54.Reynolds KT, Thomson LJ, Hoffmann AA. The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics. 2003;164:1027–1034. doi: 10.1093/genetics/164.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murdock CC, Paaijmans KP, Cox-Foster D, Read AF, Thomas MB. Rethinking vector immunology: the role of environmental temperature in shaping resistance. Nat Rev Microbiol. 2012;10:869–876. doi: 10.1038/nrmicro2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rances E, Ye YH, Woolfit M, McGraw EA, O'Neill SL. The relative importance of innate immune priming in wolbachia-mediated dengue interference. PLoS Pathog. 2012;8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan XL, Zhou GL, Wu JH, Bian GW, Lu P, Raikhel AS, Xi ZY. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2012;109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramirez JL, Short SM, Bahia AC, Saraiva RG, Dong Y, Kang S, Tripathi A, Mlambo G, Dimopoulos G. Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog. 2014;10:e1004398. doi: 10.1371/journal.ppat.1004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prado SS, Hung KY, Daugherty MP, Almeida RP. Indirect effects of temperature on stink bug fitness, via maintenance of gut-associated symbionts. Appl Environ Microbiol. 2010;76:1261–1266. doi: 10.1128/AEM.02034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zouache K, Fontaine A, Vega-Rua A, Mousson L, Thiberge JM, Lourenco-De-Oliveira R, Caro V, Lambrechts L, Failloux AB. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc Biol Sci. 2014;281 doi: 10.1098/rspb.2014.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alto BW, Bettinardi D. Temperature and dengue virus infection in mosquitoes: independent effects on the immature and adult stages. Am J Trop Med Hyg. 2013;88:497–505. doi: 10.4269/ajtmh.12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buckner EA, Alto BW, Philip Lounibos L. Larval temperature-food effects on adult mosquito infection and vertical transmission of dengue-1 virus. J Med Entomol. 2015;53:91–98. doi: 10.1093/jme/tjv145. [DOI] [PMC free article] [PubMed] [Google Scholar]