Abstract
The 2014–2015 Ebola epidemic has been the most protracted and devastating in the history of the disease. To prevent future outbreaks on this scale, it is imperative to understand the reasons that led to eventual disease control. Here, we evaluated the shifts of Ebola dynamics at national and local scales during the epidemic in Liberia. We used a transmission model calibrated to epidemiological data between June 9 and December 31, 2014, to estimate the extent of community and hospital transmission. We found that despite varied local epidemic patterns, community transmission was reduced by 40–80% in all the counties analyzed. Our model suggests that the tapering of the epidemic was achieved through reductions in community transmission, rather than accumulation of immune individuals through asymptomatic infection and unreported cases. Although the times at which this transmission reduction occurred in the majority of the Liberian counties started before any large expansion in hospital capacity and the distribution of home protection kits, it remains difficult to associate the presence of interventions with reductions in Ebola incidence.
Introduction
Since the first reported cases of Ebola Virus Disease (EVD) on March 30, 2014, a total of 10,670 cases and 4,807 deaths have been reported to the World Health Organization (WHO) from the Republic of Liberia.1,2 Though Liberia was the most affected country at the peak of the epidemic in west Africa, data from the end of 2014 suggest that Liberia was the first to experience a sharp decline in the number of new EVD cases, indicative of effective infection control measures.3
A variety of integrated interventions have been applied at different points over the course of the epidemic (Supplemental Tables 1–3). These interventions include classic infection control protocol such as “cordon sanitaire” procedures, isolation, quarantine, disease surveillance infrastructure, and active contact tracing of symptomatic and exposed individuals (Supplemental Tables 1–3). Other interventions such as hygienic burial of Ebola victims and distribution of household protective kits for family caregivers are specific to the transmission pathology of EVD. The continual implementation of non-pharmaceutical public health interventions such as isolation, quarantine, and personal protective equipment are needed to maintain the downward epidemic trend and to prevent a resurgence as occurred recently in Gueckedou, Guinea.3,4 Currently clinical trials of an experimental Ebola vaccine is underway in Sierra Leone and Guinea led respectively by a Sierra Leone and a Guinea WHO–Doctors Without Borders clinical research partnership.5 Despite the promise of these vaccine candidates to complement non-pharmaceutical interventions, disease countermeasures must remain vigilant during the interim study period and until the epidemic has concluded.
Herein, we conducted a retrospective analysis of the Ebola outbreak in Liberia to examine the main epidemiological factors that have contributed to decelerating the trend of disease transmission in Liberia. We used a stochastic model for Ebola transmission dynamics fitted to national and county-level epidemiological data to quantify the transmission rates of Ebola and hospital admission in Liberia. Through model calibration, we identified the pivotal turning points of the six county-level epidemiological trajectories. By evaluating the role and timing of intervention strategies that led to ultimate control over the largest Ebola epidemic in history can inform future disease management strategies.
Materials and Methods
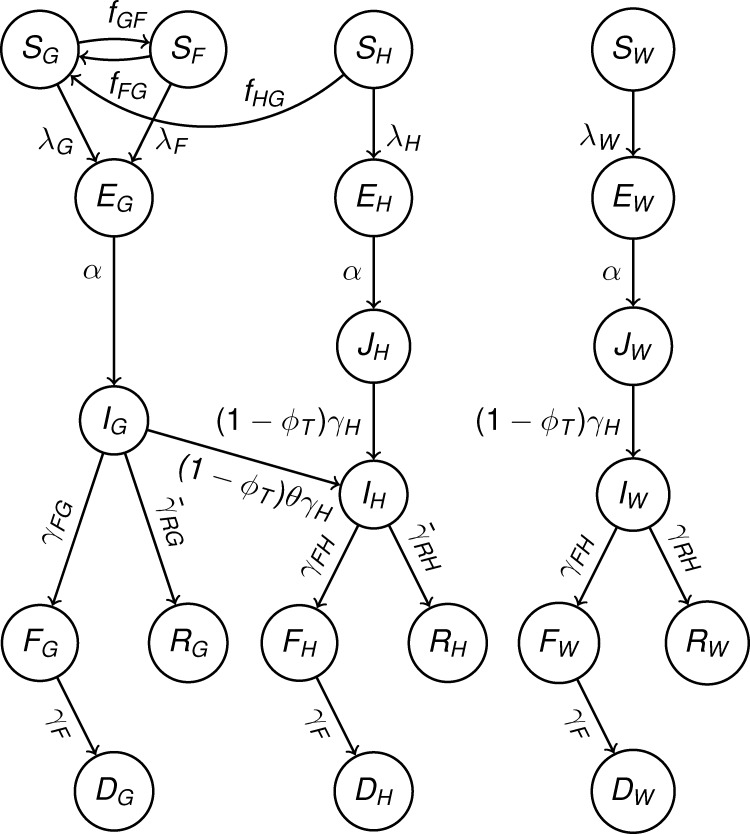
We extended a previously developed stochastic model of Ebola disease transmission within and between communities, hospitals, and funerals6 (Figure 1 and Supplemental Information). Our model tracks the density of individuals in the following epidemiological classes: uninfected and naive to Ebola infection (S), latently infected (E), infectious (I), deceased but infectious (F), recovered and immune (R), and buried (D). To account for the heterogeneity of contact and transmission between individuals in different locations, we stratified each of the epidemiological classes by location types fundamental to Ebola transmission. Our model tracks over time the density of people who are attending a funeral (subscript F), of hospitalized Ebola patients (subscript H), of health-care workers in a hospital (subscript W), and of people in the general community (subscript G).
Figure 1.
Model diagram of compartmental epidemiological classes (circles) with rates of movements between each class (arrows). Further details are presented in the Methods section, and corresponding model equations are presented in Supplemental Information.
We first calibrated the deterministic version of our model to the number of reported community Ebola cases, health-care worker cases, and Ebola hospitalizations in Liberia reported between June 9 and December 31, 2014. In addition, we calibrated the model to the proportion of cases attributable to transmission from funeral ceremonies for Ebola victims.7,8 We used national situation reports to fit eight epidemiological and behavioral parameters before and after a “transition point” to account for changes in control measures over the course of the epidemic. We estimated the transmission rate in the general community (βIβI, both before and after the transition point), the nosocomial transmission rate (βWβW, both before and after the transition point), the proportion of cases that present to a hospital (θθ, both before and after the transition point), the initial number of cases in the general community at the start of the outbreak (IG(0)IG(0)), and the relative risk of funeral transmission compared with transmission in the general community (ωω) before the transition points. We calibrated the model trajectories for Liberia using a range of weekly transition points that allowed us to determine the best-fit transition point week. We used the estimated value of relative risk for funeral transmission during the post-transition point period. Next, for county-level data, we estimated the first seven parameters, keeping the pre-transition point estimate of relative risk at a funeral constant and equal to that estimated from national data. We recalibrated the model for different transition points to estimate the best-fit transition point week for each county independently. We calibrated our model to data beginning from the first day of an Ebola case on or after June 9, 2014.
To evaluate the condition that the model parameterization changes piecewise by inclusion of a transition point as described above, we also fit a null model that uses constant parameters through the whole trajectory of the epidemic for comparison. Model fits were performed using a weighted least squares algorithm implemented using the fminunc routine in MATLAB,9 with bounded optimization where appropriate. Standard errors were calculated using the square root diagonal elements of the Hessian inverse from the derivative-based optimization algorithm. To ensure robustness of model fits, multiple initial conditions were considered, and the respective estimates for each optimization were then used as initial conditions for the next optimization routine until convergence of all implementations was achieved. To ensure that we were able to estimate the model transition point, we estimated all other parameters for a range of fixed transition point values and calculated the least squares metric in all cases to check the precision of transition point optimality. Further details are provided in our previous study that used the same fitting process.6 We conducted scenario analysis to account for potential asymptomatic and underreporting cases suggested by previous studies.10–12
A systematic review was conducted to determine the timing of key intervention events since the beginning of the Ebola outbreak in Liberia. All archived situation reports from the Liberian Ministry of Health and Social Welfare, the United Nations Children's Fund (UNICEF), and the WHO Ebola Response Roadmap were reviewed for documentation of intervention events. The events, the approximate dates of their implementation, the counties impacted, and a general classification (i.e., behavior, awareness, or health care) were recorded. If the details about a particular intervention event could not be inferred from the situation reports, additional information was sought from popular media articles, press releases from aid organizations, or the Centers for Disease Control and Prevention Morbidity and Mortality Weekly Reports.
Results
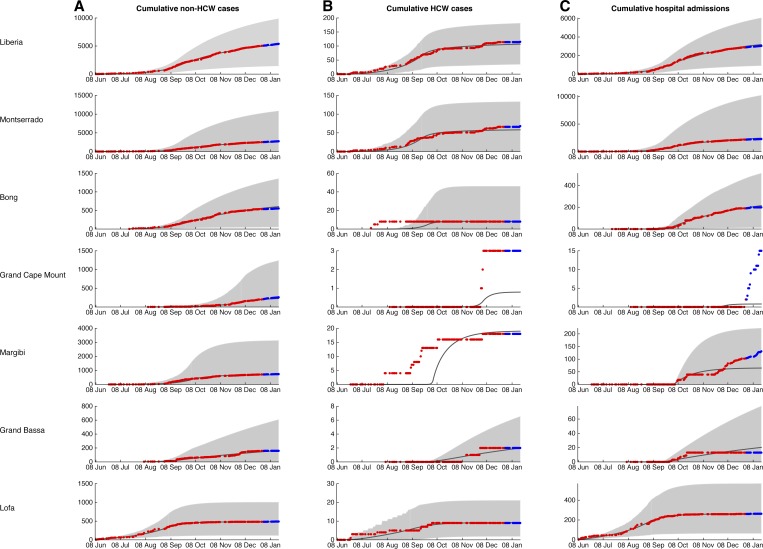
The piecewise model captures the initial exponential trajectory and the deceleration of the rate of new cases before eventual disease transmission decline (Figure 2 ). We found that all regional epidemics could be quantified by the inclusion of a natural transition point in transmission dynamics that signifies a transition from a period of high transmission to one of low transmission.
Figure 2.
Model fits. Deterministic trajectories of the model fitted to data from June 9, 2014 to December 30, 2014 (black line) with confidence intervals generated from over 1,000 runs of the stochastic version of our model (gray fill). Parameter values are displayed in Table 1. Data points for each county used to fit the model (red) and for validation (blue) are overlaid. Note that for Bong and Margibi counties, transmission to health-care workers increased before any Ebola patients being admitted to hospitals in those counties.
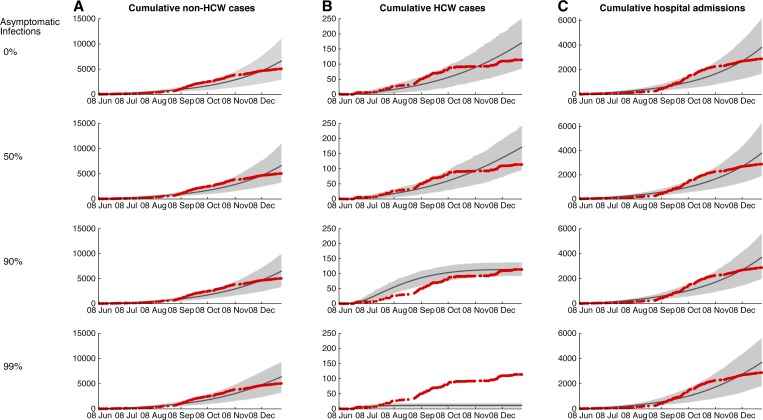
When transmission rates remain constant through the epidemic, the null model trajectory does not achieve the plateauing observed in the raw case data, even accounting for a substantial contribution of asymptomatic infections or underreporting (Figure 3 ). The discrepancy between the null model and the actual trajectories indicates that transmission rates were not constant through the epidemic, corroborating our alternate “transition point” model. Furthermore, the parameter estimation results of our alternate model suggest that the change in epidemic trajectory was caused primarily by reductions in community transmission, βIβI (Table 1).
Figure 3.
Model fits when only one community transmission rate (βIβI), health-care worker transmission rate (βWβW), and hospitalized proportion (θθ) are fit throughout the entire epidemic period. Scenario analysis of the model fits for varying proportions of asymptomatic infection.
Table 1.
Parameter estimates for the best-fit model before (top) and after (bottom) the best-fit transition point weeks
| Location | Week of parameter change (since June 9) | βI | βW | θ | ω | IG(0) |
|---|---|---|---|---|---|---|
| Liberia | 15 (September 20) | 0.235 (0.221, 0.256) | 0.042 (0.026, 0.085) | 0.377 (0.199, 0.499) | 0.090 | 17 |
| 0.128 (0.072, 0.156) | 0.004 (0.000, 0.009) | 0.450 (0.363, 0.522) | – | – | ||
| Montserrado | 15 (September 20) | 0.331 (0.307, 0.702) | 0.044 (0.026, 0.588) | 0.836 (0.053, 1.000) | – | 1 |
| 0.157 (0.072, 0.189) | 0.003 (0.000, 0.008) | 0.914 (0.500, 1.000) | – | – | ||
| Bong | 16 (September 27) | 0.205 (0.184, 0.241) | – | 0.003 (0.000, 0.325) | – | 7 |
| 0.118 (0.081, 0.147) | 0.000 | 0.321 (0.158, 1.000) | – | – | ||
| Grand Cape Mount | 26 (December 4) | 0.202 (0.189, 0.335) | – | 0.000 | – | 1 |
| 0.057 (0.000, 0.176) | – | 0.000 | – | – | ||
| Margibi | 17 (October 4) | 0.241 (0.212, 1.021) | – | 0.000 | – | 1 |
| 0.048 (0.008, 0.103) | 0.030 (0.000,0.182) | 0.065 (0.007, 0.088) | – | – | ||
| Grand Bassa | 15 (September 20) | 0.272 (0.180, 0.646) | – | 0.000 | – | 2 |
| 0.111 (0.106, 0.136) | 0.030 (0.030, 0.036) | 0.083 (0.059, 0.085) | – | – | ||
| Lofa | 13 (September 6) | 0.206 (0.166, 0.219) | 0.015 (0.015, 0.017) | 0.504 (0.481, 0.520) | – | 16 |
| 0.043 (0.000, 0.071) | 0.018 (0.000, 0.021) | 0.234 (0.000, 0.292) | – | – |
The shift in the dynamics occurred at different points in the different counties. For example, the counties that reported the first cases—Lofa and Montserrado—were also the counties where the earliest reductions in transmission occurred. Specifically, community transmission in Lofa decreased by 80% after September 6 and in both Montserrado and Grand Bassa by 50–60% after September 20. The turning points in other counties were later. In Bong County, transmission was reduced by 40% after September 27. A week later, on October 4, transmission in Margibi decreased by 80%, whereas in Grand Cape Mount a 75% reduction in community transmission occurred during the week of December 4. On a national level, there was a 45% decrease in community transmission the week of September 20, consistent with the shifts in Montserrado, where the majority of the cases occurred.
Model projections were validated using epidemiological data from January 1 to 20, 2015 (Figure 2). Model trajectories were consistent with empirical data of incidence within the community and among health-care workers in every county. Reported hospital admissions were consistent with projections for most counties, except Grand Cape Mount and Margibi where admissions increased relative to model projections during the first month of 2015.
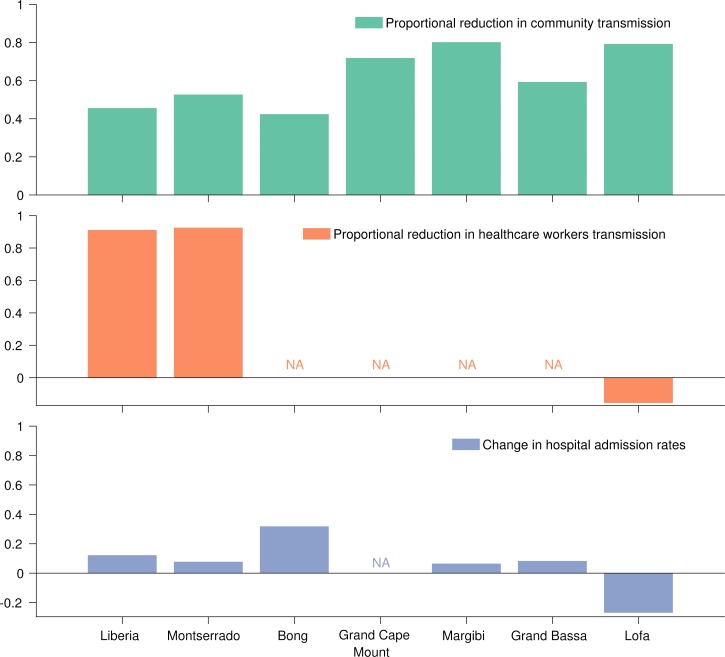
With the exception of Lofa County, the proportion of Ebola patients that were hospitalized increased after each county's best-fit transition point in transmission. This observation could be as a result of the combination of reduced community transmission rates and increased hospital capacity, consistent with the Ebola treatment unit (ETU) admission data (Figure 4 and Table 1). Although health-care worker transmission rate was estimated to increase by 15% after September 6 in Lofa, the lower hospital admittance resulted in an overall reduction in Ebola among health-care workers during this later period. In Montserrado, where the majority of ETUs are located, we observed a 90% reduction in transmission to health-care workers after the week of September 20.
Figure 4.
Changes in epidemiological parameters before and after the best-fit transition points.
Key intervention events implemented before mid-September were largely behavioral and awareness based (Supplemental Tables 1–3). After this time and leading up to October, there was increasing focus on awareness and health-care-related interventions, such as health promotion trainings for general community health volunteers, designation of airtime for Ebola-specific radio shows, distribution of home kits, and slight increases in hospital capacity. Although there were small increases in the bed capacity, after the opening of larger ETUs and the nation's first community care center occurred in early December, the majority of transmission rates started to decrease.
Accounting for 17% underreporting of symptomatic, infectious cases, as has been evaluated for the current Ebola outbreak in west Africa,10,13 our parameter estimates remain robust compared with an assumption of no underreporting (Table 2). If half of the infections are asymptomatic, consistent with a serological study from a previous Ebola outbreak,14 both community and health-care transmissions increased to about 2-fold. Nonetheless, the proportion of patients hospitalized, the relative reduction in community and health-care worker transmissions, and the approximate time when transmission rate reduction began remained consistent when compared with the assumption of no asymptomatic infection.
Table 2.
Effect of underreporting and asymptomatic infection on best-fit parameter estimates for Liberia before (top) and after (bottom) the best-fit transition point weeks
| Scenario | Week of parameter change (since June 9) | βI | βW | θ | ω | IG(0) |
|---|---|---|---|---|---|---|
| 100% reporting | 15 (September 20) | 0.235 | 0.042 | 0.377 | 0.090 | 17 |
| 0% asymptomatic | 0.128 | 0.004 | 0.500 | – | – | |
| 83% reporting | 15 (September 20) | 0.240 | 0.042 | 0.382 | 0.074 | 19 |
| 0% asymptomatic | 0.128 | 0.004 | 0.497 | – | – | |
| 50% reporting | 15 (September 20) | 0.251 | 0.045 | 0.398 | 0.044 | 27 |
| 0% asymptomatic | 0.128 | 0.006 | 0.489 | – | – | |
| 100% reporting | 15 (September 20) | 0.487 | 0.087 | 0.377 | 0.044 | 16 |
| 50% asymptomatic | 0.261 | 0.009 | 0.500 | – | – |
Discussion
We used a model of Ebola transmission calibrated to Liberian county-level situation report data to identify the transition points at which temporal shifts in Ebola transmission occurred. We found that both community and health-care worker transmissions were reduced over the course of the epidemic in all Liberian counties at different stages of the national epidemic. The drivers of these reductions in transmission are most likely multifactorial given that probabilities of infection depend on both the number and type of infectious contact. Behavioral response to an epidemic, particularly one of such a deadly disease as Ebola, can significantly impact transmission.15–17 In the general community, heightened awareness about Ebola may have spurred behavioral changes that reduce an individual's exposure risk, for example, limiting direct contact (e.g., handshaking) and improved sanitation (e.g., handwashing).16 Widespread public awareness may be the result of personal observations of Ebola victims, word-of-mouth and targeted public health campaigns. The success of any mandated control program requires the willingness of the public to adhere to the protocols. For example, the effectiveness of some control programs, such as the cordon sanitaire implemented in Monrovia, Montserrado County, have been questioned, due to lack of public support, infeasibility, and little evidence of incidence reduction.6,18
By partitioning the epidemic into a primary and a secondary phase, each with its own reproductive number, our model predicts that the transition points—synonymous with decreases in transmission intensity—occurred around September 6 for the earliest epidemic in Lofa County and around December 4 for the latest epidemic in Grand Cape Mount County. For Liberia overall, the transition point of around September 20 aligned with that of the hardest hit area, Montserrado County. The county-level transition points calculated in this study are generally consistent with a previous country-level phenomenological analysis in Liberia that found an initial reduction in the reproductive number around the week of September 6 and a secondary drop around the week of October 1.19
In the initial phase of the epidemic, the ETUs (JFK Medical Center in Montserrado, ELWA Hospital in Montserrado, and Foya Hospital in Lofa County) were filled to capacity and remained insufficient to accommodate the rising incidence throughout September. Notably, the delayed but substantial expansion in hospital capacity, such as the opening of a 200-bed ETU at the former Ministry of Defense compound, and the distribution of 50,000 home protection kits were not implemented until late October 2014, after all but one of the calculated county-level transition points in the epidemic trajectory. Throughout October, increasing involvement of the U.S. Africa Command (AFRICOM) as part of Operation United Assistance resulted in two additional mobile medical laboratories for improved Ebola diagnosis in Liberia. Each laboratory had the capacity to process up to 80 samples daily with two trained staff members. As the first of the AFRICOM-led ETUs opened in November 2014 (Supplemental Table 4), the impact of patient isolation had a delayed effect on overall disease transmission, consistent with results from a previous study.20 By contrast, before mid-September transition points, significant behavioral and awareness interventions were being introduced, including the “Wash Away Ebola” strategy and UNICEF's multi-county hygiene promotion efforts.21,22 These interventions mobilized community members through training workshops that provided instruction regarding the dissemination of Ebola education with an emphasis on behavior change. Taken together, our model results and the timeline of intervention implementation support the explanation that behavioral changes had an important impact in initial decline of Ebola incidence toward the end of September, and before expansion of isolation and treatment facilities. Although evidence suggests that the expansion of ETU capacity, the setup of community care centers (CCCs) and distribution of home kits will likely reduce Ebola incidence in Liberia,23,24 it is difficult to disentangle the effects of social awareness necessary for patients to present early at treatment centers from the availability of ETU and CCC beds.25
It is theoretically possible that a downturn in an epidemiological trajectory may be attributable to the depletion of susceptible individuals as the system approaches herd immunity. Although the number of recovered Ebola cases that have been reported in Liberia is far from the herd immunity threshold, a high proportion of asymptomatic infections that result in seroconversion without case detection reduces the pool of susceptibles that could conceivably achieve herd immunity.10 Our results indicate that asymptomatic infections can lead to some tapering of the epidemic over the current time scale, although accumulation of asymptomatic infections may have a greater impact on transmission at a more localized scale than county-level captured in our model. Nevertheless, we found that even if 99% of Ebola infections are asymptomatic, this factor would not have been sufficient to mediate the dramatic reduction of transmission that occurred.
Understanding the drivers of the dramatic, albeit delayed, halting of transmission will be vital to curbing future outbreaks before they escalate and disseminate as far as occurred in the 2014–2015 Ebola epidemic. Our model results and the timeline of intervention implementation support the importance of behavioral change on Ebola incidence.
Supplementary Material
Disclaimer: The views expressed are those of the authors and not necessarily those of the NIH, NSF, NHS, NIHR, Department of Health, or Public Health England.
Footnotes
Financial support: The work was supported by the National Institutes of Health (NIH U01 GM087719 and U01 GM105627), and the National Science Foundation (NSF RAPID 1514673). Katherine E. Atkins was also funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Immunisation at the London School of Hygiene and Tropical Medicine in partnership with Public Health England (PHE).
Authors' addresses: Katherine E. Atkins, Department of Infectious Disease Epidemiology, London School of Hygiene and Public Health, London, United Kingdom, and Center for Infectious Disease Modeling and Analysis, Yale School of Public Health, New Haven, CT, E-mail: katherine.atkins@lshtm.ac.uk. Abhishek Pandey, Natasha S. Wenzel, Laura Skrip, Dan Yamin, Martial L. Ndeffo-Mbah, and Alison P. Galvani, Center for Infectious Disease Modeling and Analysis, Yale School of Public Health, New Haven, CT, E-mails: abhishek.pandey@yale.edu, natasha.wenzel@yale.edu, laura.skrip@yale.edu, dan.yamin@yale.edu, martial.ndeffo-mbah@yale.edu, and alison.galvani@yale.edu. Tolbert G. Nyenswah, Mosoka Fallah, and Luke Bawo, Ministry of Health and Social Welfare, Monrovia, Liberia, E-mails: tgnyenswah74@yahoo.com, mfallah1969@gmail.com, and lukebawo@gmail.com. Jan Medlock, Department of Biomedical Sciences, Oregon State University, Corvallis, OR, E-mail: jan.medlock@oregonstate.edu. Frederick L. Altice, Section of Infectious Diseases, Yale University School of Medicine, New Haven, CT, E-mail: frederick.altice@yale.edu. Jeffrey Townsend, Department of Biostatistics, Yale School of Public Health, New Haven, CT, E-mail: jeffrey.townsend@yale.edu.
References
- 1.World Health Organization Ebola Situation Report—28 January 2015. 2015. http://apps.who.int/ebola/en/ebola-situation-report/situation-reports/ebola-situation-report-28-january-2015 Available at. Accessed February 14, 2015.
- 2.World Health Organization Ebola Virus Disease in Liberia. 2014. http://www.who.int/csr/don/2014_03_30_ebola_lbr/en/ Available at. Accessed August 20, 2014.
- 3.Gulland A. WHO reports decline in number of new Ebola cases in Liberia. BMJ. 2014;349:g6542. doi: 10.1136/bmj.g6542. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Guinea: The Ebola Virus Shows its Tenacity. 2015. http://www.who.int/csr/disease/ebola/one-year-report/guinea/en/ Available at. Accessed February 14, 2015.
- 5.Bellan SE, Pulliam JR, Pearson CA, Champredon D, Fox SJ, Skrip L, Galvani AP, Gambhir M, Lopman BA, Porco TC, Meyers LA, Dushoff J. Statistical power and validity of Ebola vaccine trials in Sierra Leone: a simulation study of trial design and analysis. Lancet Infect Dis. 2015;2015:14. doi: 10.1016/S1473-3099(15)70139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey A, Atkins KE, Medlock J, Wenzel N, Townsend JP, Childs JE, Nyenswah TG, Ndeffo-Mbah ML, Galvani AP. Strategies for containing Ebola in west Africa. Science. 2014;346:991–995. doi: 10.1126/science.1260612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Ebola Response Team Ebola virus disease in west Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merler S, Ajelli M, Fumanelli L, Gomes MFC, Piontti AP, Rossi L, Chao DL, Longini IM, Halloran ME, Vespignani A. Spatiotemporal spread of the 2014 outbreak of Ebola virus disease in Liberia and the effectiveness of non-pharmaceutical interventions: a computational modelling analysis. Lancet Infect Dis. 2015;15:204–211. doi: 10.1016/S1473-3099(14)71074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The MathWorks Inc. MATLAB. 2014. http://www.mathworks.com/products/matlab/ Available at.
- 10.Bellan SE, Pulliam JRC, Dushoff J, Meyers LA. Ebola control: effect of asymptomatic infection and acquired immunity. Lancet. 2014;384:1499–1500. doi: 10.1016/S0140-6736(14)61839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkins KE, Wenzel NS, Ndeffo-Mbah ML, Altice FL, Townsend JP, Galvani AP. Under-reporting and case fatality estimates for emerging epidemics. BMJ. 2015;350:h1115. doi: 10.1136/bmj.h1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Why the Ebola Outbreak Has Been Underestimated. Geneva, Switzerland: World Health Organization; 2014. http://www.who.int/mediacentre/news/ebola/22-august-2014/en/ Available at. Accessed August 27, 2014. [Google Scholar]
- 13.Scarpino SV, Iamarino A, Wells C, Yamin D, Ndeffo-Mbah M, Wenzel NS, Fox SJ, Nyenswah T, Altice FL, Galvani AP, Meyers LA, Townsend JP. Epidemiological and viral genomic sequence analysis of the 2014 Ebola outbreak reveals clustered transmission. Clin Infect Dis. 2014;60:1079–1082. doi: 10.1093/cid/ciu1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leroy EM, Baize S, Volchkov VE, Fisher-Hoch SP, Georges-Courbot MC, Lansoud-Soukate J, Capron M, Debré P, McCormick JB, Georges AJ. Human asymptomatic Ebola infection and strong inflammatory response. Lancet. 2000;355:2210–2215. doi: 10.1016/s0140-6736(00)02405-3. [DOI] [PubMed] [Google Scholar]
- 15.Yamin D, Gertler S, Ndeffo-Mbah ML, Skrip LA, Fallah M, Nyenswah TG, Altice FL, Galvani AP. Effect of Ebola progression on transmission and control in Liberia. Ann Intern Med. 2014;162:11–17. doi: 10.7326/M14-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funk S, Knight GM, Jansen VA. Ebola: the power of behaviour change. Nature. 2014;515:492. doi: 10.1038/515492b. [DOI] [PubMed] [Google Scholar]
- 17.Bauch CT, Galvani AP. Epidemiology. Social factors in epidemiology. Science. 2013;342:47–49. doi: 10.1126/science.1244492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towers S, Patterson-Lomba O, Castillo-Chavez C. Temporal variations in the effective reproduction number of the 2014 west Africa Ebola outbreak. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.9e4c4294ec8ce1adad283172b16bc908. doi:10.1371/currents.outbreaks.9e4c4294ec8ce1adad283172b16bc908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowell G, Simonsen L, Viboud C, Kuang Y. Is west Africa approaching a catastrophic phase or is the 2014 Ebola epidemic slowing down? Different models yield different answers for Liberia. PLoS Curr. 2014;1 doi: 10.1371/currents.outbreaks.b4690859d91684da963dc40e00f3da81. doi:10.1371/currents.outbreaks.b4690859d91684da963dc40e00f3da81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucharski A, Camacho A, Flasche S, Glover RE, Edmunds WJ, Funk S. Measuring the impact of Ebola control measures in Sierra Leone. Proc Natl Acad Sci USA. 2015;112:14366–14371. doi: 10.1073/pnas.1508814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UNICEF Liberia Ebola Situation Report 51. 2014. pp. 1–3.http://www.unicef.org/appeals/files/UNICEF-Liberia_Sitrep_51_Ebola_Outbreak_12_September_2014.pdf Available at. Accessed February 14, 2015.
- 22.UNICEF UNICEF Liberia Ebola Virus Disease: SitRep #50. 2014. pp. 1–4.http://www.unicef.org/appeals/files/UNICEF_Liberia_Ebola_Virus_Disease_Epidemic__5_Sept_2014.pdf Available at. Accessed February 14, 2015.
- 23.WHO Ebola Response Team West African Ebola epidemic after one year–slowing but not yet under control. N Engl J Med. 2015;372:584–587. doi: 10.1056/NEJMc1414992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucharski AJ, Camacho A, Checchi F, Waldman R, Grais RF, Cabrol JC, Briand S, Baguelin M, Flasche S, Funk S, Edmunds WJ. Evaluation of the benefits and risks of introducing Ebola community care centers, Sierra Leone. Emerg Infect Dis. 2014;21:393–399. doi: 10.3201/eid2103.141892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camacho A, Kucharski A, Aki-Sawyerr Y, White MA, Flasche S, Baguelin M, Pollington T, Carney JR, Glover R, Smout E, Tiffany A, Edmunds WJ, Funk S. Temporal changes in Ebola transmission in Sierra Leone and implications for control requirements: a real-time modelling study. PLoS Curr. 2015;7 doi: 10.1371/currents.outbreaks.406ae55e83ec0b5193e30856b9235ed2. doi:10.1371/currents.outbreaks.406ae55e83ec0b5193e30856b9235ed2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.