Abstract
Background
For unknown reasons a woman’s risk for developing the Metabolic Syndrome (MetS) increases dramatically with age and/or loss of ovarian function. The MetS is characterized by hepatic insulin resistance (IR), which is strongly associated with intrahepatic lipid (IHL) accumulation, mitochondrial dysfunction, and oxidative stress. Although circumstantial evidence suggests that the endocrine function of the ovary can directly impact hepatic mitochondrial function, this hypothesis remains untested. Thus, the purpose of this study was to assess the influence of age and secretory function of the ovary on mechanisms that regulate hepatic mitochondrial function.
Methods
Adult (10 week-old) and aged (88 week-old) female C57BL/6 mice were separated into two groups to undergo bilateral ovariectomy (OVX) or control surgery (SHAM). Eight weeks after surgery hepatic tissue was removed for measurements of total IHL and fatty acid species within hepatic triglycerides, mitochondrial function, and reactive oxygen species (ROS) production.
Results
Hepatic IHL content was not affected by OVX, but was increased by age. OVX had no effect on mitochondrial respiration, however, hepatic mitochondria from aged mice had lower O2 consumption, lower complex IV and higher complex I content. Mitochondrial H2O2 production was highest in OVX groups and exacerbated by age, while mitochondrial lipid peroxidation was highest in the aged mice and exacerbated by OVX. Regardless of age, OVX resulted in lower mitochondrial content of antioxidant glutathione peroxidase 1 (Gpx1). Isolated liver tissue from a sub-set of animals were acutely treated with conditioned ovarian media which increased Gpx1 mRNA expression compared to vehicle treated liver tissue.
Conclusion
Ovarian secretory function is necessary for the maintenance of hepatic ROS buffering capacity in the mitochondria, while age significantly influences mitochondrial respiration. These data suggest that when age is coupled with loss of ovarian function there is an increased risk for developing hepatic mitochondrial dysfunction, which may influence the onset of metabolic disease. Thus, in females there is critical organ cross-talk occurring between hepatic tissue and the ovary that impacts hepatic mitochondrial function.
Keywords: Ovariectomy, Mitochondrial function, Gpx1, Aging, Liver, Oxidative stress
1. INTRODUCTION
Current epidemiological dogma indicates that the ovary plays a critical role in regulating physiological and metabolic function in women. Thus, it should come as no surprise that disruptions of ovarian function in women can lead to increased risk of disease susceptibility. Indeed, recent evidence has shown that the prevalence of the metabolic syndrome (MetS) is higher in post-menopausal women or women who experience premature ovarian failure (Dorum et al., 2007; Coviello et al., 2006; Lindheim et al., 1994). With alterations in ovarian function and increased susceptibility to MetS, women are also at increased risk for cardiovascular disease and overall mortality (Lin et al., 2010). The increased susceptibility to disease highlights the importance of ovarian endocrine function in the metabolic health of women. Though epidemiological evidence points to the importance of the ovary, it is still unclear as to how the loss of ovarian hormonal function directly impairs metabolic and physiological function of various organs.
Previous results from our lab and others show that surgical removal of the ovaries (OVX) leads to alterations in metabolic function of hepatic tissue and skeletal muscle that affect whole body insulin sensitivity (Campbell et al., 2005; Jackson et al., 2013; Jackson et al., 2011; Wohlers et al., 2009). Although a substantial amount of work has shown the influence of ovarian hormones on skeletal muscle, our understanding of changes in hepatic function as a consequence of altered ovarian function is still incomplete. This is somewhat surprising given the importance of the liver in regulating whole body glucose and lipid dynamics. It is thought that the onset of menopause increases the susceptibility to accumulation of intrahepatic lipid (IHL), which is a risk factor for the development of insulin resistance (Petersen et al., 2007; Stefan et al., 2008). Recent evidence suggests that hepatic mitochondrial function is a strong predictor of susceptibility to hepatic steatosis in rodent and humans (Rector et al., 2010; Thyfault et al., 2009; Perez-Carreras, 2003). For example, mice with reduced flux in β-oxidation in the liver develop hepatic steatosis and insulin resistance (Zhang et al., 2006; Ibdah et al., 2005); moreover, enhancing hepatic mitochondrial function through exogenous delivery of carnitine palmitoyltransferase-1 (CPT-1) cDNA can prevent IHL in animal models susceptible to fatty liver disease (Stefanovic-Racic et al., 2008). Collectively, the results suggest that overall hepatic metabolic function is heavily dependent on the function of the mitochondria. Although insulin resistance is evident in humans and animals with impaired ovarian function, to the best of our knowledge no study has comprehensively assessed the impact of disruption of ovarian function on hepatic mitochondrial function.
We have previously shown that adult OVX mice develop characteristics of the MetS that were associated with alterations in fatty acid species within stored hepatic triglycerides (Jackson et al., 2011). Our results also demonstrate that the changes in hepatic fatty acid species were not due to changes in lipogenic function, which suggests that alterations in hepatic mitochondrial function could be the cause. Thus, in this study we sought to examine hepatic mitochondrial function in adult OVX animals. In addition, we sought to address the influence of age on hepatic mitochondrial function to determine if the effects of OVX were exacerbated in an aged cohort of mice. To the best of our knowledge, no studies have addressed the impact of age on hepatic tissue in the OVX model. We hypothesized that age will reduce mitochondrial function and promote oxidative stress, but when coupled with OVX, the changes in mitochondrial function will be exacerbated resulting in excessive IHL accumulation.
2. METHODS
2.1 Animal care
Eight week-old C57Bl/6 female mice obtained from Harlan (Frederick, MD) and 22 month-old C57Bl/6 female mice obtained from National Institute on Aging rodent colony (Bethesda, MD) were subdivided into two weight-matched groups. In both age groups, one group underwent bilateral ovariectomy (OVX), and the other underwent SHAM surgery as previously described (Jackson et al., 2011). Animals were housed in a temperature-controlled room on a twelve-hour light and dark cycle, and were provided with ad libitum access to water and standard rodent chow (Purina Laboratory Rodent Diet 5001: 23% protein, 4.5% fat, 6% fiber). Eight weeks post-surgery animals were euthanized following a 4–5 hr fast. The eight-week time point was chosen because we have previously shown that a number of significant metabolic differences manifest in the OVX group when compared to the SHAM group (Wohlers et al., 2009; Jackson, et al., 2011). The liver was surgically removed and a portion was used to isolate mitochondria as described by Frezza et al. (2007). Mitochondrial function and H2O2 measures were assessed immediately after isolation with the remaining mitochondrial material used for subsequent immunoblotting. The remaining portion of liver was snap frozen for biochemical assessments. All aspects of this study were approved by the University of Maryland Institutional Animal Care and Use Committee Review Board.
2.2 Hepatic TAG analysis
Using a portion of the liver, triacylglycerol (TAG) and diacylglycerol (DAG) were as extracted as previously described (Folch et al., 1957) and the fatty acid composition of the extracted TAG and DAG were measured as previously described (Jackson et al., 2011).
2.3 Mitochondrial Isolation
Liver tissue was collected and immediately placed in ice-cold isolation buffer (IBc) containing 10mM Tris-MOPS, 1mM EGTA/Tris, and 0.2M sucrose (pH 7.4). Methods were adapted from the ones described by Frezza et al. (2007). In brief, all procedures were performed in an ice bath; the liver was washed with IBc until the solution was clear, then it was minced with scissors and gently homogenized using a 30 mL glass/Teflon potter Elvehjem tissue grinder. The homogenate was transferred to a conical tube and centrifuged at 600g for 10 min at 4°C. The supernatant containing mitochondria was transferred to a glass centrifuge tube and spun at 7,000g for 10 min at 4°C. The remaining pellet was re-suspended in 5 mL IBc and centrifuged at 7,000g for 10 min at 4°C. The resulting supernatant was discarded and the pellet was loosened with an ice-cold glass rod and transferred to a microcentrifuge tube. Any excess buffer solution was removed. The protein concentration of the pellet was determined using the BCA protein assay (Pierce Protein Research, Rockford, Ill., USA). Only freshly isolated mitochondria were used to measure oxygen consumption and H2O2 production, and the remaining mitochondria were stored at −80°C.
2.4 Mitochondrial oxygen consumption
Oxygen consumption was measured polarographically using an Oxytherm (Hansatech, Norfolk, UK) as described by Garcia-Cazarin et al. (2011). Freshly isolated mitochondria (~150μg) from each group were placed in the calibrated electrode chamber containing 500 μL of respiration buffer (125mM KCl, 2mM K2PO4, 10mM HEPES, 1mM MgCl, 0.1mM EGTA, 1%BSA), 500μM glutamate, 25μM malate, 300μM ADP (Sigma-Aldrich, St. Louis, Mo., USA) as substrates to induce state 3 respiration. After 3 min, oligomycin (Calbiochem, Billerica, MA, USA) was added for a final concentration of 40μM to induce state 4 respiration. Eight replicates were measured from each animal’s mitochondrial sample, and oxygen consumption rates (nM O2/min) were normalized to mitochondrial protein content. To ensure mitochondrial integrity, values were only used when the respiratory control ratio (RCR= state 3 VO2/state 4 VO2) fell between 2.49 and 6.0 (Valle et al., 2007). All mitochondria measures with RCR values outside this range were discarded.
2.5 Mitochondrial H2O2
Accumulation of hydrogen peroxide (H2O2) produced by isolated mitochondria was measured fluorimetrically with the oxidation of nonfluorescent Amplex Red (Invitrogen, Carsbad, CA, USA) into fluorescent resorufin (Schonfeld, et al., 2009) using a Synergy H1 plate reader (BioTek, Winooski, VT, USA). All of these assays were measured at 37°C in a black-bottom 96-well plate. Mitochondria (~0.2mg protein) were added to respiration buffer with horseradish peroxidase 0.1 U/mL and 50μM Amplex Red reagent. Mitochondrial H2O2 accumulation was assessed in the presence of state 4 conditions and normalized to the total mitochondrial protein in each well. Four to five replicates were measured from each animal’s mitochondrial sample from all groups.
2.6 Immunoblot analysis of isolated mitochondria
Western blotting was used to assess protein content of electron transport chain (ETC) (MitoSciences, Eugene, OR, USA), superoxide dismutase (MnSOD) (Santa Cruz Biotechnology, Dallas, TX, USA), glutathione peroxidase-1 (Gpx1) (Abcam, Cambridge, MA), 4-hydroxy-nonenal (4-HNE) (Santa Cruz), long chain acyl-CoA dehydrogenases (kind gift of Dr. Gerald Vockley, University of Pittsburgh). The protocols for western blots have been previously described (Jackson et al., 2011). In brief, equal amounts of protein (30–35μg) were run in a Mini-PROTEAN®TGX™ Precast Gel (BioRad) and transferred onto a PDVF membrane. Blots were blocked for one hour using 3% nonfat dry milk dissolved in Tris-buffered saline with 0.05% or 0.01% Tween (TBS-T). Following, the membrane was incubated in the appropriate primary antibody diluted in TBS-T and 5% BSA overnight in 4°C. The membrane was then washed with TBS-T and incubated for one hour with horseradish peroxidase (HRP)-conjugated secondary antibody (Cell Signaling, Danvers, MA, USA) in a buffer of 3% nonfat dry milk and TBS-T. Membranes were incubated for one minute in enhanced chemiluminescence reagent (PierceProtein Research). Membranes were visualized with a chemiluminescence imager (Biorad, Hercules, CA) and quantified with the Biorad ImageLab software.
2.7 Ovary extract preparation and treatments
Ovaries were surgically removed from young adult female mice and placed in 250μL of ice-cold PBS. Ovaries were squeezed gently with smooth forceps four times and were kept rotating for one hr at 4°C. Ovaries were then removed from the PBS and both the ovary and PBS were snap frozen in liquid nitrogen, and stored at −80°C. The PBS exposed to the ovaries is now termed ovary extract (OE). Due to the hormone fluctuation change throughout the 4-day murine estrus cycle (Caligioni 2009), OE was prepared from ovaries extracted from four mice housed in the same cage for four consecutive days. After preparation all four of OE were mixed together and used in the experiments.
Protein concentration of the OE was quantified using the Pierce BCA protein assay to determine the volume of OE to use for treatment. The liver was surgically removed from six 4-month old mice and placed in Krebs-Ringer buffer (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 2.15 mM Na2HPO4, and 0.85 mM NaH2PO4, pH: 7.2). Each liver was cut into 100mg slices, to be individually treated with 4 mL of Krebs-Ringer buffer as control, or with the addition of 200μg of total protein from the OE, a concentration that yielded the most consistent effect on Gpx1 mRNA (data not shown). In addition, to determine if any OE effect was mediated through ERα/β activation or G-protein coupled estrogen receptor 1 (Gper-1) activation, conditions also included pretreatment with either 50 μM ICI 182,780 (Sigma) or 100 nM G1 (Calbiochem). All doses optimized in preliminary experiments. Hepatic samples were then snap-frozen in liquid nitrogen and stored at −80°C for subsequent RNA isolation which has been previously described (Jackson et al., 2011).
2.8 Glutathione peroxidase-1 mRNA expression
One microgram of isolated hepatic RNA was reverse transcribed as previously described (Jackson et al., 2011). PCR was performed using Qiagen’s HotStarTaq Master Mix to assess Gpx1 mRNA content in hepatic tissue samples. The primers utilized had the following sequence (5′ to 3′); CAGCCGGAAAGAAAGCGATG (Gpx1-forward) and CCATTCTCCTGGTGTCCGAA (Gpx1-reverse); TCCAGAGGAGGTACTACAAGCC (Scd1-forward); GCATCATTAACACCCCGATAGC (Scd1-reverse); GATCCATTGGAGGGGAAGTCT (18S-forward) and CCAAGATCCAACTACGAGCTTTTT (18S-reverse). All amplifications were done on a T100 thermocycler (Biorad) using the following protocols: Gpx-1 primers: 95°C for 5min, 95°C for 30sec, 51.8°C for 30sec, 72°C for 30 sec, 72°C for 5min, and stored at 4°C; Scd1 primers: 95°C for 5min, 95°C for 30sec, 52°C for 30sec, 72°C for 30 sec, 72°C for 5min, and stored at 4°C; 18S primers: 95°C for 5min, 95°C for 30sec, 53°C for 30sec, 72°C for 30 sec, 72°C for 5min, and stored at 4°C. The Gpx1 and Scd1 signal obtained were normalized to 18S.
2.9 Statistical Analysis
Assumptions of homoscedasticity and normality were verified for all outcome measures. Data were analyzed using multivariate ANOVA with pairwise comparisons or t-tests where appropriate. Statistical significance was accepted at P ≤ 0.05. Values are expressed as mean ± standard error of the mean.
3. RESULTS
3.1 OVX results in higher body weight and fat mass in young and aged mice
The OVX groups had significantly greater body mass compared to the SHAM groups (P < 0.05) (Table 1). Visceral fat mass (VCF) was significantly higher in the OVX groups compared to their SHAM counterparts even when normalized to total body weight (P < 0.01). Liver weight was significantly higher in the aged OVX group compared to young OVX and young SHAM groups but not when compared to the aged SHAM (P < 0.05). The values were also higher in the aged OVX group compared to young OVX group after normalizing liver mass to total body weight (P < 0.05). Uterine mass was significantly lower in the young OVX group compared to the young SHAM group (P < 0.05), while both aged groups (SHAM and OVX) exhibited significantly lower uterine mass compared to the young SHAM. Loss of uterine mass is the most accepted way to confirm that endogenous circulating estrogen levels are reduced (Holinka et al., 1977) as most commercial kits to measure circulating estradiol levels lack the necessary precision and scientific stringency (Stanczyk et al, 2003).
Table 1.
Anatomical characteristics of young and aged mice after eight weeks of SHAM or OVX surgery.
| YOUNG | AGED | |||
|---|---|---|---|---|
| SHAM | OVX | SHAM | OVX | |
| Body Weight (g) | 22.7±0.5 | 25.8±0.8* | 25.8±0.7* | 28.1±0.9#,* |
| Visceral Fat (mg) | 260±24 | 842±69#,* | 321±78 | 727±184#,* |
| VCF/BW (mg/g) | 11±1.0 | 32±3.0#,* | 12±2.0 | 25±6.0#,* |
| Liver (g) | 1.08±0.05 | 1.08±0.08 | 1.21±0.07 | 1.36±0.07€,* |
| Liver/BW (mg/g) | 47±1.0 | 42±3.0 | 47±3.0 | 48±2.0€ |
| Uterus (mg) | 160±47 | 55±40* | 81±19* | 82±27* |
Values presented as means ± SEM.
Indicates significantly different to young SHAM (P < 0.05),
indicates significantly different to aged SHAM (P < 0.05), and
indicates significantly different to young OVX (P < 0.05).
3.2 OVX alters stored hepatic fatty acid species and age was associated with elevated TAG content
The total content of stored hepatic DAG, TAG, and their fatty acid (FA) species were assessed in livers from each group (Table 2). Total DAG and TAG content was higher in the aged groups compared to both young groups (P < 0.05), but unaffected by OVX (Table 2). Aged animals had an increase in the content of hepatic lipid species 16:0, 18:0 and 18:1 in the TAG fraction. However minimal effects were seen as a result of OVX, with the exception of the desaturase index of 18:1/18:0 in both TAG and DAG within the young group only.
Table 2.
Hepatic fatty acid composition (ng/mg) of young and aged mice after eight weeks of SHAM or OVX surgery.
| YOUNG | AGED | |||
|---|---|---|---|---|
| SHAM | OVX | SHAM | OVX | |
| Total TAG | 7.78±2.62 | 10.46±1.76 | 18.07±4.13* | 18.38±2.86*,€ |
| 16:0 | 2.03±0.31 | 2.52±0.44 | 3.42±0.75 | 3.50±0.56* |
| 16:1 | 0.37±0.10 | 0.53±0.12 | 0.73±0.25 | 0.76±0.14* |
| 18:0 | 0.26±0.04 | 0.28±0.06 | 0.48±0.05* | 0.48±0.07*,#,€ |
| 18:1 | 2.92±0.45 | 3.91±0.64 | 6.50±1.67* | 6.98±1.25*, € |
| 16:1/16:0 | 0.17±0.03 | 0.201±0.01 | 0.19±0.03 | 0.21±0.01 |
| 18:1/18:0 | 11.56±1.30 | 14.7±0.91* | 12.90±2.03 | 14.45±1.13 |
| Total DAG | 0.66±0.07 | 0.77±0.07 | 1.11±0.20* | 1.09±0.16* |
| 16:0 | 0.16±0.02 | 0.19±0.02 | 0.26±0.03* | 0.27±0.03*,€,# |
| 16:1 | 0.02±0.00 | 0.03±0.00 | 0.03±0.01 | 0.03±0.01 |
| 18:0 | 0.07±0.01 | 0.06±0.01 | 0.09±0.01* | 0.08±0.02# |
| 18:1 | 0.21±0.02 | 0.25±0.02 | 0.30±0.08 | 0.31±0.05* |
| 16:1/16:0 | 0.12±0.02 | 0.14±0.02 | 0.11±0.03 | 0.11±0.01 |
| 18:1/18:0 | 3.20±0.17 | 4.27±0.43* | 3.08±0.59 | 4.11±0.60 |
Values presented as means ± SEM.
Indicates significantly different to young SHAM (P < 0.05),
indicates significantly different to aged SHAM (P < 0.05), and
indicates significantly different to young OVX (P < 0.05).
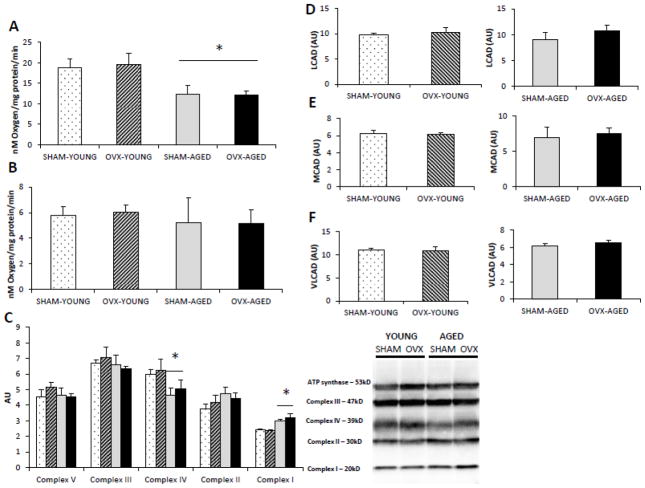
3.3 Aged groups had lower mitochondrial respiration capacity and altered ETC protein content
Regardless of group, isolated mitochondria from hepatic tissue of aged mice had lower oxygen consumption under state 3 conditions (Fig 1A) while no differences were evident in state 4 conditions (Fig 1B). Since the capacity of mitochondria to consume oxygen highly depends on the electron transport chain (ETC) of the inner mitochondrial membrane, we assessed specific protein content of each of the five complexes in the isolated mitochondria (Fig 1C). Complex content was not affected by OVX, but the aged group had lower complex IV and higher complex I content compared to young group. Protein content of long acyl-CoA dehydrogenase (LCAD), medium acyl-CoA dehydrogenase (MCAD), and very long acyl-CoA dehydrogenase (VLCAD) did not change as a result of OVX (Fig. D–F).
Figure 1.
(A–F). Age, but not OVX reduced the respiratory capacity of isolated hepatic mitochondria (A-B) and altered the protein content of ETC (C). Hepatic mitochondria from both age groups exhibited lower respiration rates when provided with 0.5mM glutamate, 125μM malate, and 300μM ADP state 3 respiration (A), but not with state 4 respiration (40μM oligomycin) (B). Respiration rates were measured in isolated mitochondria from hepatic tissue, and are expressed as nM of oxygen consumed per minute per milligram of mitochondrial protein. Ovariectomy (OVX) did not affect the protein content of β-oxidation proteins LCAD (D), MCAD (E), or VLCAD (F) in hepatic tissue of young or aged groups. *Significant statistical difference from young groups (P < 0.05). n=6–7 animals/group.
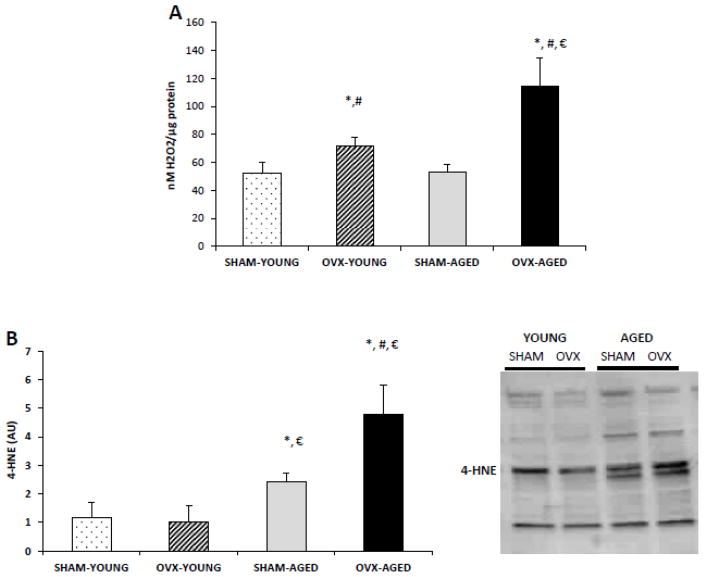
3.4 OVX leads to increased mitochondrial H2O2 production, whereas age leads to increased mitochondrial lipid peroxidation
H2O2 measured in isolated mitochondria was significantly higher in the OVX groups compared to the SHAM groups (Fig 2A). Surprisingly, age had no effect on hepatic mitochondrial H2O2 production in the SHAM groups, but age exacerbated H2O2 production in the OVX groups (Fig 2A). Mitochondrial 4-HNE content was higher in hepatic tissue from the aged mice compared to the young mice; however, the highest 4-HNE content detected was evident in the aged OVX group (P<0.05) (Fig 2B).
Figure 2.
(A–B): OVX groups exhibited higher oxidative stress in isolated hepatic mitochondria, which was exacerbated in the aged group (A). H2O2 production was measured under state 4 conditions, expressed relative to mitochondrial protein. (B) Lipid peroxidation was determined by 4-HNE content of the mitochondrial fraction. *Significant statistical difference from young SHAM; #significantly different from aged SHAM; significantly different from young OVX (P < 0.05). n=6–7 animals/group.
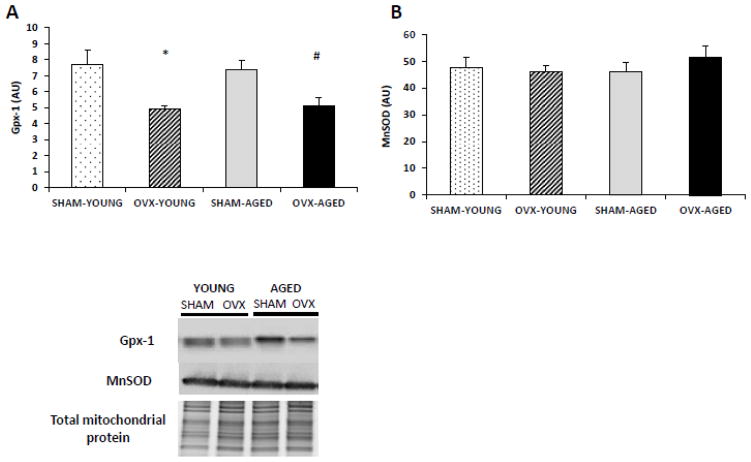
3.5 OVX resulted in decreased hepatic Gpx1 protein content, but no differences in MnSOD
The protein contents of two known mitochondrial antioxidant proteins involved in H2O2 dynamics, MnSOD and Gpx1, were assessed through immunoblotting of the mitochondrial fraction (Fig. 3A–B). Gpx1 protein content was lower in both OVX groups compared to the SHAM groups, while no differences were detected in MnSOD content across any of the groups.
Figure 3.
(A–B). OVX groups had lower mitochondrial protein content of antioxidant Gpx-1 but not of MnSOD. Protein content was measured using immunoblotting of protein from the isolated hepatic mitochondrial fraction and normalized to total mitochondrial protein. *Significant statistical difference from young SHAM (P < 0.05); #significantly different from aged SHAM (P < 0.01). n=6–7 animals/group.
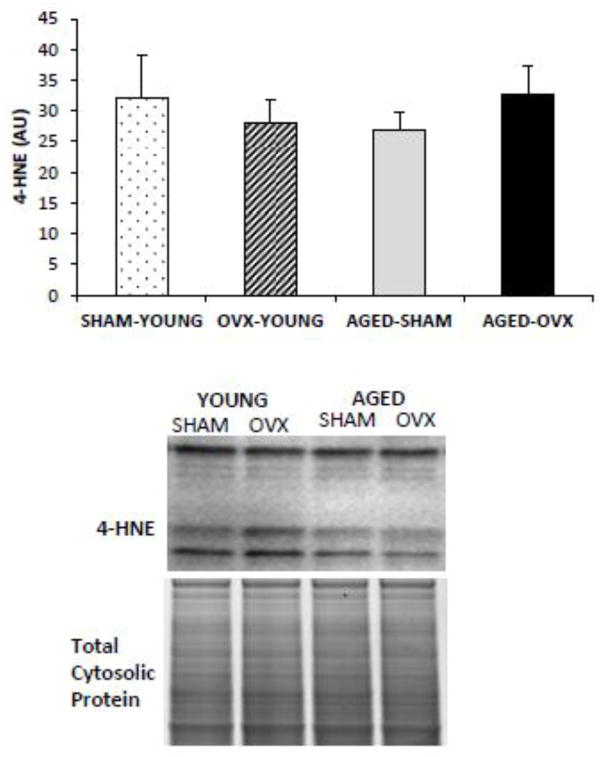
3.5 Increased lipid peroxidation is specific to the mitochondria with age or OVX
To address whether the changes we detected were specific to the mitochondria, we measured 4-HNE content in the cytosolic fraction of hepatic tissue. No significant differences were detected between groups (Fig. 4).
Figure 4.
4-HNE content in the cytosolic fraction of hepatic tissue was normalized to total cytosolic protein. There were no differences in 4-HNE content between groups.
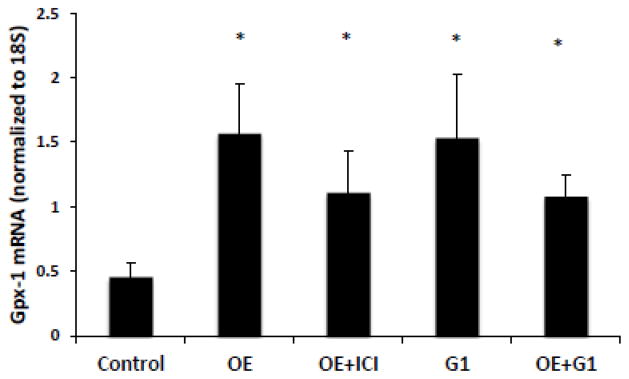
3.6 Ovarian secretions increase Gpx1 mRNA expression in hepatic tissue ex-vivo
Isolated hepatic tissue was treated with OE to determine whether ovary secretions exert a direct effect on hepatic Gpx-1 mRNA expression (Fig. 5). There was a two-fold increase in Gpx1 mRNA expression after OE treatment when compared to vehicle (P<0.05). In contrast, we found no effect of OE treatment on SCD-1 mRNA which is thought to be an estrogen sensitive gene in hepatic tissue (data not shown). The OE effect on Gpx-1 was not affected by pre-treatment of the hepatic tissue with ERα/β-antagonist ICI 182,780. In contrast, exposure of the liver tissue to the Gper-1 agonist, G-1, resulted in a similar effect to OE exposure. Further, OE and G-1 co-treatment did not result in an additive increase in Gpx1 mRNA expression.
Figure 5.
Gpx1 mRNA expression in hepatic tissue increased after acute ovary extract (OE) exposure. Inclusion of the ER-antagonist ICI 182,870 (50μM) failed to block the effect, while treatment with Gper-1 agonist G1 (100nM) increased Gpx1 mRNA, with no additive effect when co-treated with OE.*Significant statistical difference from control (P < 0.05).
4. DISCUSSION
Our findings demonstrate the potential for tissue-to-tissue cross-talk between the ovaries and hepatic tissue. Specifically, surgical removal of the ovaries results in increased H2O2 production and lowered Gpx1 protein content in isolated hepatic mitochondria. Mitochondrial H2O2 is produced from the reduction of superoxide (O2−) by the enzyme MnSOD, and H2O2 is then reduced into water by Gpx1 (Chance et al., 1979). Since we did not find any differences in MnSOD content across groups, the accumulation of H2O2 in OVX groups is likely the result of reduced Gpx1 content in hepatic mitochondria. Our results suggest that endocrine function of the ovary can enhance the expression of Gpx1 in hepatic tissue through activation of Gper-1 (also known as GPR30). Thus, our data indicate that maintenance of ovary function irrespective of age is necessary for protection from oxidative stress in the mitochondria of hepatic tissue.
The results partially confirmed our initial hypothesis that age would exacerbate effects of OVX. Specifically, we demonstrated a greater accumulation of H2O2 in isolated hepatic mitochondria from aged OVX mice compared to age-matched SHAM mice and young OVX mice. Mitochondrial H2O2 accumulation can lead to lipid peroxidation of polyunsaturated fatty acids in lipid membranes resulting in 4-HNE accumulation. 4-HNE is a reactive lipid aldehyde that induces protein carbonylation promoting further oxidative stress and insulin resistance (Pillon et al., 2012). Thus, our data suggest OVX in aged animal results in enhanced oxidative stress even though the mice were anestrous prior to the OVX, suggesting that presence of the ovary in the female induces a protective effect on peripheral tissue independent of the estrous cycle. Interestingly, this effect is specific to the mitochondria, as we found no difference in 4-HNE content in the cytosolic fraction of hepatic tissue. These data suggest that the combination of age coupled with loss of ovarian function results in enhanced mitochondrial oxidative stress in the form of lipid peroxidation. Therefore, the biological changes that occur as the result of reduced ovarian function and age are likely to impact each other, and in some cases increasing the resulting effect.
We found that exposure of hepatic tissue to an ovarian extract solution was sufficient to increase Gpx1 mRNA expression. The induction of Gpx1 mRNA was not mediated by activation of the classic estrogen receptor signaling since pre-treatment of the hepatic tissue with known inhibitory concentrations of ERα/β antagonist (ICI 182,780) failed to inhibit the response. Interestingly, we found that acute exposure of the hepatic tissue to G1, a highly specific Gper-1 agonist (Bologa et al., 2006), was sufficient to increase Gpx-1 mRNA expression suggesting that ovary extract exposure was acting through the membrane bound Gper-1 protein. This was further confirmed since no additive effect was detected when the ovary extract and G1 was delivered simultaneously. Although Gper-1 is highly expressed in the liver (Hsieh et al., 2007), the role of Gper-1 in this tissue function is largely unknown. At this point, very few gene targets of Gper-1 signaling have been empirically identified, but these data indicate that Gpx1 is likely a downstream target of the Gper-1. Other Gpx proteins have been found to be estrogen sensitive (i.e. Gpx3), however of them only Gpx1 is enriched in the liver compared to other tissue with the majority of it found in the mitochondria. Although others have shown that exogenous estrogens can induce gene expression of various antioxidant systems in a variety of different tissues (Baltgalvis et al., 2010; Stirone et al., 2005; Borras et al., 2003), our data suggest that endogenous estrogens are critical for the regulation of mitochondrial ROS production and/or buffering of ROS. However, due to the nature of our ex vivo experiments it is not possible to determine if ovarian media or Gper-1 agonists are sufficient to affect downstream effects of antioxidant gene expression such as protein content or mitigating oxidative stress.
Contrary to previous studies (Pighon et al., 2011; Paquette et al., 2008), we found that OVX does not alter hepatic metabolic function by promoting increased hepatic lipid storage, though we did find an increase in intrahepatic lipid (IHL) content as a result of age, which agrees with findings in aged rats (Zhao et al. 2014). However, the effect of IHL accumulation was not exacerbated with OVX in the aged groups. Although IHL accumulation is often assumed to be a result of OVX, not all groups have equivocally demonstrated significant increases in IHL (Camporez et al, 2013). In addition, other studies have shown that hepatic estrogen receptor alpha knockout (LERKO) mice exposed to a high fat diet are not more susceptible to IHL accumulation compared to age-matched control mice exposed to the same diet (Matic et al., 2013). Previous data by Jackson et al. (2011) demonstrated a non-significant elevation in IHL, which we further confirm in this current study. We also have found that the expression of numerous genes in lipogenesis or triglyceride synthesis were unaffected by OVX (Jackson et al, 2011). In contrast to the mouse results, using the rat model for OVX surgery results in more predictable increases in IHL (Hao et al., 2010); however, the rat and mouse OVX models exhibit some fundamental differences in metabolic and behavioral function. For example, the OVX surgery in the rat results in hyperphagia and reduced physical activity leading to a positive energy balance (Nigro et al., 2014; Picard et al., 2004; Lavoie & Pighon, 2011), while the OVX mouse exhibits lower or similar food consumption in addition to lower physical activity levels (Jackson et al. 2013, Wohlers et al. 2010, Rogers et al., 2009; Gorzek et al., 2007). At this point, the data appear to suggest that OVX mice are not more susceptible to IHL accumulation and greater caution may be necessary when comparing the effects of OVX across species.
However, our data support previous findings that OVX results in a greater proportion of monounsaturated fatty acids being stored in hepatic triglycerides (Jackson et al, 2011). Additionally, in both the young and aged groups, we found an increase in the desaturase index of the stored TAG and DAG with OVX. Our previous results found a higher TAG desaturase index in hepatic tissue of OVX mice which correlated to an increased hepatic content of stearoyl-CoA desaturase (SCD-1), a metabolic gene that is thought to be estrogen sensitive (Gao et al., 2006). However, our data did not suggest that ovary secretions directly affected SCD-1 mRNA expression, thus it is possible that secondary effects (i.e. visceral adiposity or higher insulin concentrations) of the OVX are affecting hepatic SCD-1 content. Our previous data found a strong positive relationship between the hepatic desaturase index and visceral adiposity (Jackson et al., 2011), thus it is possible that the storage of particular fatty acid species in the hepatic tissue is influenced through secondary actions of ovary removal. Nonetheless, the data do indicate that the dynamics involved in fatty acid storage are altered upon interference with normal ovarian function.
To the best of our knowledge, only one study has assessed hepatic mitochondrial function (ie. O2 consumption) in the OVX mouse model. Camporez et al. (2013) demonstrated that isolated hepatocytes from OVX mice had lower O2 consumption compared to OVX mice treated with exogenous 17β-estradiol; however, because this study design did not include a control (i.e SHAM) group it is impossible to determine if OVX reduced O2 consumption or if exposure to exogenous estradiol stimulated increased mitochondrial respiration. Our data show that regardless of age, OVX did not affect hepatic mitochondrial O2 consumption, showing that loss of ovarian function alone is not sufficient to reduce mitochondrial respiratory function. Collectively, previous findings suggest that although estrogens appear to exert a powerful effect on mitochondrial function, our findings indicate that ovary loss does not impact respiratory function in the hepatic mitochondria of mice. This finding is somewhat surprising given the effects of estrogens on the mitochondria and when considering the work of Valle et al (2007) who found that hepatic mitochondria from female mice had greater complex I and complex III activities compared to male mice. Recent suggestions have argued that mitochondrial dysfunction in hepatic tissue is not a prerequisite for IHL accumulation (Sunny et al., 2011), furthermore, metabolic insults predicted to induce mitochondrial dysfunction often induce better coupling of the mitochondria (Franko et al., 2014). Thus, it possible that mitochondria adapt in the OVX model to ensure function is maintained. Conversely, hepatic mitochondrial respiratory function evidenced by O2 consumption was clearly lower in the aged groups compared to the young groups, which is consistent with the work of others (Lanza et al., 2008; Shigenaga et al.,1994). In contrast to findings from other groups, mitochondrial content of enzymes that contribute to β-oxidation or to ETC complexes were not affected by OVX in both young or aged mice (Campbell et al., 2003). Altogether, these findings suggest that OVX does not substantially alter mitochondrial oxidation enzymes or mitochondrial respiratory function.
Estradiol levels are reduced with the loss of ovarian function as it occurs with OVX surgery and when ovaries become senescent with age (Danilovich & Ram Sairam, 2006). Uterine weight, which is a common indicator of circulating estrogen levels, was clearly reduced in all groups compared to the young SHAM group. This was used to determine the efficacy of OVX surgery and that the aged-SHAM groups were anestrous. Uterine mass is used since commercially available kits to quantify circulating endogenous estrogen levels are not reliable or accurate (Haisenleder et al., 2011). In a subset of our mice, we further confirmed that aged-SHAM mice were anestrous based on vaginal smears (data not shown), which likely explains the reduced uterine mass and indicates that circulating levels of estrogens were reduced in the aged-SHAM group. Although speculative, this suggests that the ovary plays an important physiological role in the female that may be independent of the estrous cycle. Specifically, we only found reduced Gpx1 content in the OVX groups and not in the aged-SHAM group, which suggests that ovary plays a critical role in biological function of female rodents that extends beyond just the estrous cycle. Unfortunately, until more precise and reliable methods are developed to accurately quantify endogenous rodent estrogens, this is a challenging hypothesis to empirically confirm.
Overall, these data suggest that the endocrine secretions of the ovary protect hepatic mitochondria from oxidative stress by regulating mitochondrial Gpx1 content through the Gper-1 protein. Increased ROS production is known to contribute to IR in a variety of tissues, and thus, our data provide novel direction for our understanding of why women with loss of ovarian function can become more susceptible to metabolic conditions such as type 2 diabetes, cardiovascular disease, or the MetS. In conclusion, our results suggest that IHL and loss of mitochondrial function does not develop as a consequence of lost ovarian function but rather as a consequence of age. On the other hand, the ability to protect hepatic tissue from oxidative stress is directly influenced by ovarian function regardless of age suggesting that the ovary is important across the lifespan of females and may still play an important role even after the loss of the estrous cycle in mice, and possibly during menopause in women.
Highlights.
Ovary removal results in hepatic oxidative stress which is exacerbated in aged mice.
Ovary removal exacerbates mitochondrial lipid peroxidation in aged mice.
Ovary removal results in decreased Gpx1 protein content in hepatic mitochondria.
Ovary secretions directly enhance Gpx1 expression in liver through Gper-1.
Hepatic mitochondrial oxygen consumption is lower in aged mice compared to young mice.
Ovary removal results in more monounsaturated fatty acids incorporated into stored liver triacylglycerol.
Acknowledgments
This work was funded in part by Baltimore Diabetes Research Training Center (DRTC-P60DK079637) (E. E Spangenburg); A. P. Valencia was funded by National Institute on Aging Grant (AG-000268).
Abbreviations
- MetS
Metabolic syndrome
- IR
insulin resistance
- IHL
intrahepatic lipid
- OVX
ovariectomized
- Gpx1
glutathione peroxidase-1
- Gper-1
G-protein coupled estrogen receptor
- OE
ovary extract
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PlosOne. 2010;5(4):e10164. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Viña J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radical Bio Med. 2003;34(5):546–552. doi: 10.1016/S0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath J, Galuska D, Steiler T, Dahlman-Wright K. Evidence that oestrogen receptor alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49(3):588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Lundholm L, Portwood N, Gustafsson AK, Efendic S, Dahlman-Wright K. Mechanisms of antidibetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295(4):E904–E912. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SE, Febbraio MA. Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281(4):E803–E808. doi: 10.1152/ajpendo.2001.281.4.E803. [DOI] [PubMed] [Google Scholar]
- Campbell SE, Mehan KA, Tunstall RJ, Febbraio MA, Cameron-Smith D. 17β-estradiol upregulates the expression of peroxisome proliferator-activated receptor α and lipid oxidative genes in skeletal muscle. J Mol Endocrinol. 2003;31:37–45. doi: 10.1677/jme.0.0310037. [DOI] [PubMed] [Google Scholar]
- Camporez JPG, Jornayvaz FR, Lee H, Kanda S, Guigni BA, Kahn M, Samuel VT, Carvalho CR, Petersen KF, Jurczak MJ, Shulman GI. Cellular mechanisms by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinol. 2013;154(3):1021–1028. doi: 10.1210/en.2012-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91(2):492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- Danilovich N, Ram Sairam M. Recent female mouse models displaying advanced reproductive aging. Exp Gerontol. 2006;41(2):117–22. doi: 10.1016/j.exger.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Dorum A, Tonstad S, Liavaag AH, Michelsen TM, Hildrum B, Dahl AA. Bilateral oophorectomy before 50 years of age is significantly associated with the metabolic syndrome and Framingham risk score: a controlled, population-based study (HUNT-2) Gynecol Oncol. 2008;109(3):377–83. doi: 10.1016/j.ygyno.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Franko A, von Kleist-Retzow JC, Neschen S, Wu M, Schommers P, Bose M, Kunze A, Hartmann U, Weisner RJ. Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. J Hepatol. 2014;60(4):816–823. doi: 10.1016/j.jhep.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat Protoc. 2007;2(2):287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson J, Dahlman-Wright K. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and impoves insulin sensitivity in ob/ob mice: A possible mechanism is through direct regulation of signal transducer activator factor of transcription 3. Mol Endocrinol. 2008;20(6):1287–99. doi: 10.1210/me.2006-0012. [DOI] [PubMed] [Google Scholar]
- Garcia-Cazarin M, Snider N, Andrade F. Video Article Mitochondrial Isolation from Skeletal Muscle. J Vis Exp. 2011;49(e2452) doi: 10.3791/2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39(2):248–56. doi: 10.1249/01.mss.0000241649.15006.b8. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: Evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152(11):4443–4447. doi: 10.1210/en.2011-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han E, Muller FL, Perez V, Qi W, Liang H, Xi L, Fu C, Doyle E, Rickardson A. The in vivo gene expression signature of oxidative stress. Physiol Genomic. 2008;34(1):112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Wang Y, Duan Y, Bu S. Effects of treadmill exercise training on liver fat accumulation and estrogen receptor alpha expression in intact and ovariectomized rats with or without estrogen replacement treatment. Eur J Appl Physiol. 2010;109(5):879–86. doi: 10.1007/s00421-010-1426-6. [DOI] [PubMed] [Google Scholar]
- Hewitt K, Pratis K, Jones ME, Simpson E. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145(4):1842–48. doi: 10.1210/en.2003-1369. [DOI] [PubMed] [Google Scholar]
- Holinka CF, Hetland MD, Finch CE. The response to a single dose of estradiol in the uterus of ovariectomized C57BL/6J mice during aging. Biol Reprod. 1977;17(2):262–264. doi: 10.1095/biolreprod17.2.262. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Yu HP, Frink M, Suzuki T, Choudhry MW, Schwacha MG, Chaudry IH. G Protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage. Am J Pathol. 2007;170(4):1210–1218. doi: 10.2353/ajpath.2007.060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibdah JA, Perlegas P, Zhao Y, Anddisen J, Borgerink H, Shadoan MK, Cline JM. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenerology. 2005;128:1381–90. doi: 10.1053/j.gastro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Jackson KC, Wohlers LM, Lovering RM, Schuh RA, Maher AC, Bonen A, Koves TR, Ilkayeva O, Thomson DM, Muoio DM, Spangenburg EE. Ectopic lipid deposition and the metabolic profile of skeletal muscle in ovariectomized mice. Am J Physiol Regul Comp Physiol. 2013;304(3):R206–17. doi: 10.1152/ajpregu.00428.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KC, Wohlers LM, Valencia AP, Cilenti M, Borengasser SJ, Thyault JP, Spangenburg EE. Wheel running prevents the accumulation of monounsaturated fatty acids in liver of ovariectomized mice by attenuating changes in SCD-1 content. Appl Physiol Nutr Metab. 2011;36(6):798–810. doi: 10.1139/h11-099. [DOI] [PubMed] [Google Scholar]
- Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell Menopause and the metabolic syndrome: The study of women’s health across the nation. Arch Intern Med. 2008;168(14):1568–75. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57(11):2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JM, Pighon A. NAFLD, estrogens, and physical exercise: the animal model. J Nutr Metab. 2012:914938. doi: 10.1155/2012/914938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WY, Yang WS, Lee LT, Chen CY, Liu CS, Lin CC, Huang KC. Insulin resistance, obesity, and metabolic syndrome among non-diabetic pre- and post-menopausal women in North Taiwan. Int J Obes (Lond) 2006;30(6):912–917. doi: 10.1038/sj.ijo.0803240. [DOI] [PubMed] [Google Scholar]
- Lindheim SR, Buchanan TA, Duffy DM, Vijod MA, Kojima T, Stanczyk FZ, Lobo RA. Comparison of estimates of insulin sensitivity in pre- and postmenopausal women using the insulin tolerance test and the frequently sampled intravenous glucose tolerance test. J Soc Gynecol Investig. 1994;1(2):150–154. doi: 10.1177/107155769400100210. [DOI] [PubMed] [Google Scholar]
- Matic M, Bryzgalova G, Hui G, Antonson P, Humire P, Omoto Y, Portwood N, Pramfalk C, Efendic S, Berggren P, Gustafsson J, Dahlman-Wright K. Estrogen signaling and the metabolic syndrome: Targeting the hepatic estrogen receptor alpha action. Plos One. 2013;8(2):e57458. doi: 10.1371/journal.pone.0057458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol. 2012;393(8):G979–92. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro M, Santos AT, Barthem CS, Louzada RAN, Fortunato RS, Ketzer LA, Carvalho DP, de Meis L. A change in liver metabolism but not in brown adipose tissue thermogenesis is an early event in ovariectomy-induced obesity in rats. Endocrinology. 2014;155(8):2881–2891. doi: 10.1210/en.2013-1385. [DOI] [PubMed] [Google Scholar]
- Paquette A, Wang D, Jankowski M, Gutkowska J, Lavoie JM. Effects of ovariectomy on PPAR alpha, SREBP-1c, and SCD-1 gene expression in the rat liver. Menopause. 2008;15(6):1169–75. doi: 10.1097/gme.0b013e31817b8159. [DOI] [PubMed] [Google Scholar]
- Perez-Carreras M, Del Hoyo P, Martin MA, Rubio JC, Martin A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratiory chain in patients with nonalcoholic steatohepatitis. Hepatol. 2003;38:999–1007. doi: 10.1002/hep.1840380426. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Savage DB, Solomon G, Yonemitsu S, Cline GW, Belfroy D, Zemany L…, Shulman GI. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104(31):12587–94. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pighon A, Gutkowska J, Jankowski M, Rabasa-Lhorel R, Lavoie JM. Exercise training in ovariectomized rats stimulates estrogenic-like effects on expression of genes involved in lipid accumulation and subclinical inflammation in liver. Metabolism. 2011;60(5):629–39. doi: 10.1016/j.metabol.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Pillon NJ, Croze ML, Vella RE, Soulere L, Lagarde M, Soulage CO. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology. 2012;153(5):2099–111. doi: 10.1210/en.2011-1957. [DOI] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Uptergrove G, Morris NS, Borengasser S, Mikus C, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52(5):727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ. Steroids. 2003;68(14):1173–1178. doi: 10.1016/j.steroids.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–1616. doi: 10.1001/arcinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O’Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab. 2008;294(5):E969–77. doi: 10.1152/ajpendo.00497.2007. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 2005;68(4):959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- Sunny NE, Parks EJ, Browning JD, Burguess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholoc fatty liver disease. Cell Metab. 2011;14(6):804–10. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyfault JP, Rector SR, Uptergrove GM, Borengasser SJ, Morris E, Wei Y, Laye MJ, Burant CF, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and suceptibility to hepatic steatosis and injury. J Physiol. 2009;587(8):1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle A, Guevara R, Garcia-Palmer FJ, Roca P, Oliver J. Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. Am J Physiol: Cell Physiol. 2007;293:C1302–08. doi: 10.1152/ajpcell.00203.2007. [DOI] [PubMed] [Google Scholar]
- Wohlers LM, Sweenew SM, Ward CW, Lovering RM, Spangenburg EE. Changes in contraction-induced phosphorylation of AMP-activated protein kinase and mitogen-activates protein kinases in skeletal muscle after ovariectomy. J Cell Biochem. 2009;107(1):171–178. doi: 10.1002/jcb.22113. [DOI] [PubMed] [Google Scholar]
- Wohlers LM, Spangenburg EE. 17beta-estradiol supplementation attenuated ovariectomy-induced increases in ATGL signaling and reduced perilipin expression in visceral adipose tissue. J Cell Bioshem. 2010;110(2):420–427. doi: 10.1002/jcb.22553. [DOI] [PubMed] [Google Scholar]
- Zhang D, Liu Z, Chio CC, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI. Mitochodnrial dysfunction due to long-chain acyl-coa dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. PNAS. 2006;104(43):17075–17080. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zou X, Feng Z, Luo C, Liu J, Li H, Chang L, Wang H, Li Y, Long J, Gao F, Liu J. Evidence for association of mitochondrial metabolism alteration with lipid accumulation in aging rats. Exp Gerontol. 2014;56:3–12. doi: 10.1016/j.exger.2014.02.001. [DOI] [PubMed] [Google Scholar]