Abstract
Background
Evaluation of indeterminate biliary strictures typically involves collection and analysis of tissue or cells. Brush cytology and intraductal biopsies that are routinely performed during ERCP to assess malignant-appearing biliary strictures are limited by relatively low sensitivity.
Objective
To study the comparative effectiveness of brushings for cytology and intraductal biopsies in the etiology of biliary strictures.
Design
Meta-analysis.
Setting
Referral center.
Patients
PUBMED and Embase databases were reviewed for studies published to April 2014 where diagnostic correlation of histology was available.
Intervention
Database and review of study findings.
Main Outcome Measurements
Sensitivity and specificity.
Results
The pooled sensitivity and specificity of brushings for the diagnosis of malignant biliary strictures was 45% (95% confidence interval [CI], 40%–50%) and 99% (95% CI, 98%–100%), respectively. The pooled diagnostic odds ratio to detect malignant biliary strictures was 33.43 (95% CI, 14.29–78.24). For intraductal biopsies, the pooled sensitivity and specificity were 48.1% (95% CI, 42.8%–53.4%) and 99.2% (95% CI, 97.6%–99.8%), respectively. The pooled diagnostic odds ratio to detect malignant biliary strictures was 43.18 (95% CI, 19.39–95.83). A combination of both modalities only modestly increased the sensitivity (59.4%; 95% CI, 53.7%–64.8%) with a specificity of 100% (95% CI, 98.8%–100.0%). The Begg-Mazumdar and Egger tests indicated a low potential for publication bias.
Limitations
Inclusion of low-quality studies.
Conclusion
Our study suggests that both brushings and biopsy are comparable and have limited sensitivity for the diagnosis of malignant biliary strictures. A combination of both only modestly increases the sensitivity.
Biliary strictures are challenging to diagnose and manage.1 Malignant strictures of the bile duct are commonly caused by periampullary cancer, cholangiocarcinoma (CCA), or pancreatic cancer. These strictures commonly manifest in their advanced stages and are often unresectable.1–3 When early diagnosis of malignancy is possible, the prognosis might improve with aggressive management.4 Surgery is often associated with high postoperative morbidities.5–7 Approximately 7% to 10% of patients undergoing surgery for suspected malignancy are found to have benign pathology.8,9 Confirming malignancy through cytological/tissue diagnosis is essential before considering aggressive surgical management.
ERCP is commonly performed to rule out malignancies in suspicious strictures, and cytological/tissue diagnosis of malignancies can be made with brush cytology or intraductal biopsies. Brush cytology is routinely done to diagnose malignant biliary strictures because it is easier to perform, associated with fewer adverse events, but is limited by its low sensitivity.10,11 Endobiliary forceps biopsies often require biliary sphincterotomy, and their advantage over brush cytology is not clearly established. Also, use of intraductal forceps would be difficult in narrow bile ducts. An ERCP-based diagnosis of indeterminate biliary strictures is important, particularly at centers where there is limited access to EUS. With the increasing costs of health care, if a second procedure (EUS) could be avoided through appropriate use of resources at the time of the ERCP for biliary drainage, it could result in a huge impact. We wanted to study the utility of the commonly used techniques when encountering a biliary stricture during ERCP to determine the optimal diagnostic approach. To our knowledge, no formal quantitative review of the available evidence has been published that comprehensively examined the diagnostic performance of biliary brushings for cytology and intraductal biopsies in the diagnosis of biliary strictures. The aim of this study was to perform a structured meta-analysis of all eligible studies to evaluate the comparative effectiveness of biliary brush cytology and intraductal biopsies in diagnosing malignant biliary strictures.
METHODS
Medical literature search
A comprehensive search of the medical literature was performed to identify peer-reviewed articles that examined the diagnostic accuracy of endoscopic retrograde biliary brush cytology and intraductal biopsies to detect malignancy as the etiology of biliary strictures. We systematically searched the PUBMED and Embase databases for studies published from January 1980 to April 2014 by using the following search terms: “ERCP brush cytology and forceps biopsy,” “bile duct brush cytology,” and “endobiliary forceps biopsy.” We searched for additional references by cross checking bibliographies of retrieved full-text papers. Two reviewers (V.L. and B.N.) independently screened the titles and abstracts of all the articles according to pre-defined inclusion and exclusion criteria. Any differences were resolved by mutual agreement and in consultation with the third reviewer (U.N.).
Selection criteria
Only studies involving both ERCP brushing and biopsies in the identification of biliary strictures with availability of data for the construction of 2 × 2 contingency tables were included. None of the studies included in our meta-analysis used cholangioscopy techniques for sampling the biliary stricture. Studies with insufficient data and a sample size of less than 10 were excluded from the analysis. The standard criterion for the confirmation of malignancy in the studies was the surgical pathology or autopsy and long-term clinical follow-up.
Index test
The index test was the use of brushings for cytology and intraductal biopsies with studies reporting positive for malignancy included in our analysis.
Quality of studies
Currently there are no consensus or criteria to evaluate the quality of studies without a control arm.12 The quality of studies was interpreted based on Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 criteria.13
Statistical analysis
Meta-analysis for the accuracy of biliary brush cytology and intraductal biliary biopsy to diagnose malignancy was performed by calculating the pooled estimates of sensitivity, specificity, likelihood ratios (LRs), and diagnostic odds ratio (DOR). Pooling was performed by using the Der Simonian-Laird method (random-effects model). Forest plots were constructed to show the point estimates in each study in relation to the summary pooled estimate. The width of the point estimates in the Forest plots corresponded to the assigned weight of the study. Heterogeneity was assessed by using χ2 statistics, I2 measure of inconsistency, and Cochran’s Q test.
A summary receiver-operating characteristic was constructed based on the Moses-Shapiro-Littenberg method as a way to summarize the true-positive and false-positive rates from different studies. The proximity of the area under the receiver-operating characteristic curve to 1 is a well-validated overall representation of the diagnostic accuracy of a test.
The robustness of the meta-analysis to publication bias was assessed by funnel plots and bias indicators, including the Begg-Mazumdar test and the Harbord–Egger test.14,15
Sensitivity analysis
A sensitivity analysis was conducted for every study to determine whether any single study was incurring undue weight in the analysis. We systematically removed 1 set of study data and checked the pooled results for the remaining studies to see whether there was any significant change in test performance.
Combined weighted sensitivity, specificity, positive LR, negative LR, summary receiver-operating characteristic curve, and meta-regression were determined by use of Meta-Disc version 1.4 (Unit of Clinical Biostatistics, Ramon y Cajal Hospital, Madrid, Spain).
RESULTS
Eligible studies and quality assessment
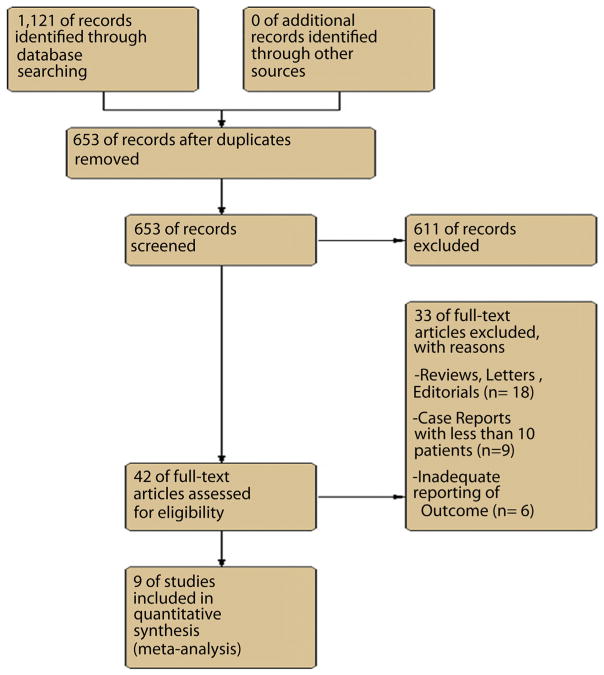
An initial medical literature search yielded 1121 articles. After excluding irrelevant studies, 42 potential studies were reviewed in detail. Of these, 9 studies (n = 730 patients) met the inclusion criteria and were included in the analysis.16–24 The reported pooled estimates were calculated by the random-effects model. Figure 1 shows the flow diagram for studies identified for the systematic review. Table 1 lists the various studies included in the meta-analysis. There were 2 studies by the same group.20,21 However, there was no overlap between the 2 studies, and hence both were included in our analysis.
Figure 1.
Flow chart of selected studies.
TABLE 1.
Characteristics of studies evaluating brushings and intraductal biopsies
| Study | Year | Sample size (pts) | Technique | Sensitivity, % |
|---|---|---|---|---|
| Pugliese et al21 | 1987 | 22 | Brush | 66 |
| Biopsy | 100 | |||
| Pugliese et al20 | 1995 | 94 | Brush | 54 |
| Biopsy | 53 | |||
| Ponchon et al19 | 1995 | 233 | Brush | 35 |
| Biopsy | 43 | |||
| Howell et al16 | 1996 | 28 | Brush | 42 |
| Biopsy | 15 | |||
| Sugiyama et al23 | 1996 | 52 | Brush | 48 |
| Biopsy | 81 | |||
| Jailwala et al17 | 2000 | 133 | Brush | 26 |
| Biopsy | 37 | |||
| Rösch et al22 | 2004 | 50 | Brush | 46 |
| Biopsy | 36 | |||
| Kitajima et al18,* | 2007 | 60 | Brush | 72 |
| Biopsy | 65 | |||
| Weber et al24,† | 2008 | 58 | Brush | 41 |
| Biopsy | 53 |
High-grade dysplasia was considered malignant to calculate the sensitivity.
Suspicious cells were considered malignant to calculate the sensitivity.
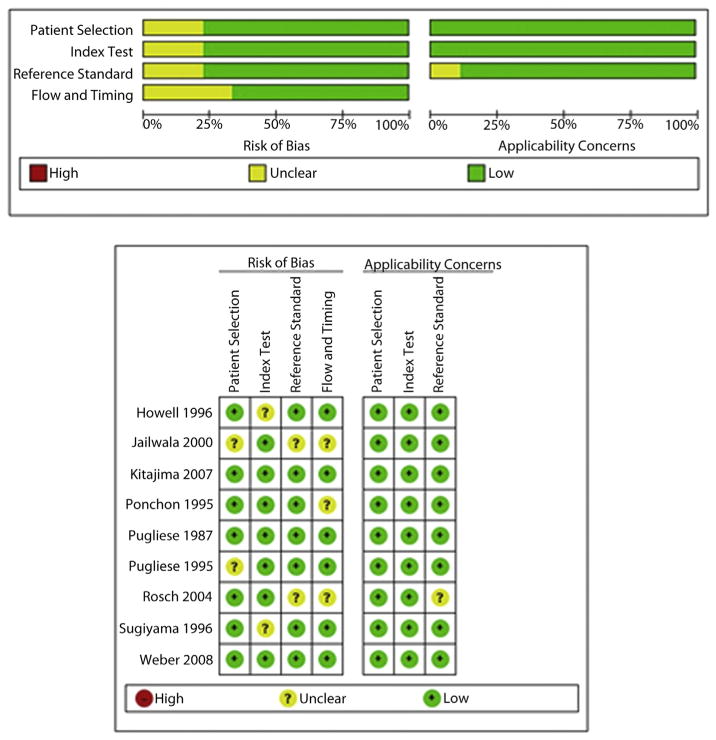
The quality of the eligible studies as assessed by Quality Assessment of Diagnostic Accuracy Studies 2 criteria is reported in Figure 2. In most studies, there was a low risk of bias regarding the selection of patients. There were no bias issues or concerns regarding applicability of the selection of patients. There was no risk of bias issues of the index test in any of the studies. In most studies, there was a low risk of bias to determine whether an appropriate reference standard was used or its applicability. Ultimately, 9 studies with sufficient data that met our inclusion criteria were included in the final meta-analysis.16–24
Figure 2.
The quality of the eligible studies as assessed by Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 criteria.
Of the 730 patients, 270 (37%) were found to have benign etiologies and 460 (63%) had malignant biliary strictures. Data regarding the etiologies of the malignant strictures were available in all the studies. Pancreatic cancer accounted for 35% (n = 160) and CCA for 46% (n = 211) of the malignancies. The remaining 19% (n = 88) of the malignancies were ampullary adenocarcinoma, gallbladder carcinoma, hepatocellular cancer, and metastasis presenting as biliary strictures.
Cytological samples were obtained by using various wire-guided brush catheters (standard DLB-35 brushes; Boston Scientific, Natick, Mass, or Geenen brushes, Wilson-Cook, Winston Salem, NC). Different kinds of forceps were used such as 31010 (Biomed, Paris, France), Olympus prototype FB-39Q (Olympus, Tokyo, Japan), HBIF-1.5-22/HBIF-1.5-220-S (Wilson-Cook Medical), and standard colonoscopy pinch forceps. Information on the number of brush passages required for the diagnosis of cancer was reported in 5 studies. The brush was advanced through the stricture, and samples were obtained with multiple passes (at least 5) across the strictures in 4 studies,17,18,21,24 and 2 passes in 1 study.22 The number of bites/biopsies was reported to be 1 to 3 in 2 studies17,21 and up to 5 bites in 2 studies.18,23 Six biopsy specimens were taken routinely for the patients in the study by Rösch et al.22 Table 2 lists the various study characteristics.
TABLE 2.
Characteristics of brushings, intraductal biopsies, and cytological interpretations
| Study | No. of brush passes, tissue bites | Cytological interpretations indicative of a positive FNA test result included in the analysis |
|---|---|---|
| Pugliese et al, 198720 | NR, NR | NR |
| Pugliese et al, 199521 | Multiple, 2–3 | Only positive |
| Ponchon et al, 199519 | NR, NR | Only positive |
| Howell et al, 199616 | NR, NR | Only positive (both positive or suspicious data reported by authors) |
| Sugiyama et al, 199623 | NR, 1–5 | NR |
| Jailwala et al, 200017 | 10–15, 1–2 | Only positive (both positive or suspicious data reported by authors) |
| Rösch et al, 200422 | 2, 6 | Only positive |
| Kitajima et al, 200718 | ≥5, 2–5 | High-grade dysplasia included as malignant |
| Weber et al, 200824 | Multiple, NR | Only positive |
NR, Not reported.
Endoscopic brush cytology
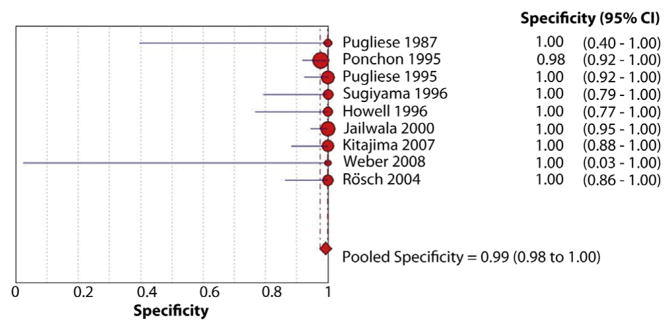
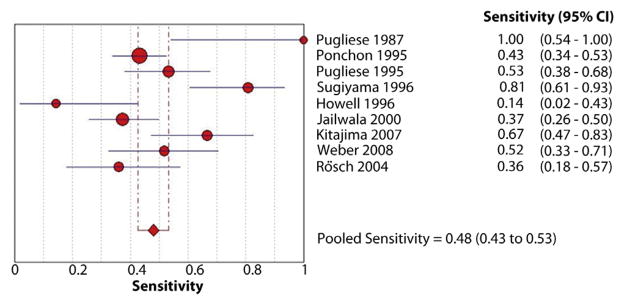
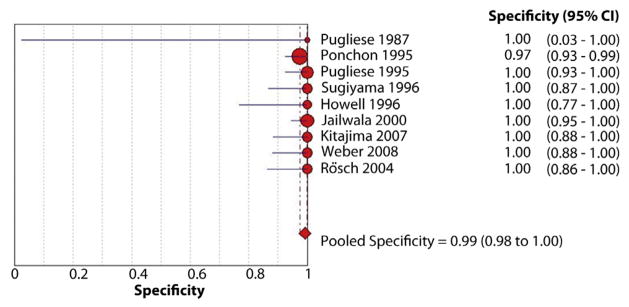
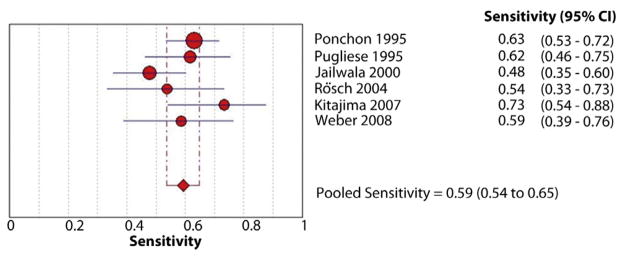
The pooled sensitivity and specificity of endoscopic brush cytology for the diagnosis of malignant biliary strictures was 45% (95% confidence interval [CI], 40%–50%) and 99% (95% CI, 98%–100%), respectively (Figs. 3 and 4). The pooled positive LR (LR+) was 15.73 (95% CI, 7.16–34.57) and negative LR (LR−) was 0.54 (95% CI, 0.45–0.66). The pooled DOR to detect malignant biliary strictures was 33.43 (95% CI, 14.29–78.24).
Figure 3.
Forest plot of studies reporting the diagnostic role of endoscopic brush cytology; the pooled sensitivity of brushings for diagnosis of malignant biliary strictures was 45%.
Figure 4.
Forest plot of studies reporting the diagnostic role of endoscopic brush cytology; the pooled specificity of brushings for diagnosis of malignant biliary strictures was 99%.
Intraductal biopsies
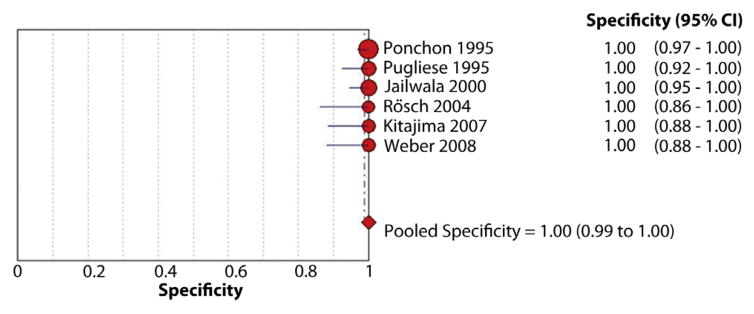
The pooled sensitivity and specificity of intraductal biopsies for the diagnosis of malignant biliary strictures was 48.1% (95% CI, 42.8%–53.4%) and 99.2% (95% CI, 97.6%–99.8%), respectively (Figs. 5 and 6). The pooled LR+ was 18.94 (95% CI, 9.07–39.55) and LR− was 0.54 (95% CI, 0.44–0.66). The pooled DOR to detect malignant biliary strictures was 43.18 (95% CI, 19.39–95.83).
Figure 5.
Forest plot of studies reporting the diagnostic role of intraductal biopsies; the pooled sensitivity of biopsies for diagnosis of malignant biliary strictures was 48%.
Figure 6.
Forest plot of studies reporting the diagnostic role of intraductal biopsies; the pooled specificity of biopsies for diagnosis of malignant biliary strictures was 99%.
Brushings and intraductal biopsies
Only 6 studies reported the combined sensitivity of diagnosing the strictures with both brush and forceps biopsy (Table 3). The pooled sensitivity and specificity of intraductal biopsies and brushings for cytology together for the diagnosis of malignant biliary strictures was 59.4% (95% CI, 53.7%–64.8%) and 100% (95% CI, 98.8%–100.0%), respectively (Figs. 7 and 8). The pooled LR+ was 53.83 (95% CI, 17.40–166.54) and LR− was 0.42 (95% CI, 0.36–0.49). The pooled DOR to detect malignant biliary strictures was 135.31 (95% CI, 42.10–434.86).
TABLE 3.
Characteristics of studies evaluating combined sensitivity of brushings and intraductal biopsies
| Study | Year | Sample size (pts) | Technique | Sensitivity, % |
|---|---|---|---|---|
| Pugliese et al21 | 1995 | 94 | Brush + biopsy | 61 |
| Ponchon et al19 | 1995 | 233 | Brush + biopsy | 63 |
| Jailwala et al17 | 2000 | 133 | Brush + biopsy | 48 |
| Rösch et al22 | 2004 | 50 | Brush + biopsy | 54 |
| Kitajima et al18,* | 2007 | 60 | Brush + biopsy | 74 |
| Weber et al24 | 2008 | 58 | Brush + biopsy | 60 |
High-grade dysplasia was considered malignant to calculate the sensitivity.
Figure 7.
Forest plot of studies reporting the diagnostic role of a combination of endoscopic brush cytology and biopsies; the pooled sensitivity for diagnosis of malignant biliary strictures was 59%.
Figure 8.
Forest plot of studies reporting the diagnostic role of a combination of endoscopic brush cytology and biopsies; the pooled specificity for diagnosis of malignant biliary strictures was 100%.
Pooled sensitivities could not be calculated separately for proximal and distal strictures and for specific etiologies of malignancies (pancreatic cancer vs CCA) because of the lack of data.
Sensitivity analysis
We systematically removed 1 dataset at a time and recalculated the DOR and area under the curve values for the remaining studies. The largest change occurred when removing the dataset from Jailwala et al,17 which changed the pooled sensitivity from 59.4% to 62.5% (+5.2%), and the corresponding change in LR− value was from 0.41 to 0.38. These results indicated that no single dataset carried enough weight to significantly influence the pooled test performance reported for the combination of brushing for cytology and intraductal biopsies for the diagnosis of malignant biliary strictures.
Publication bias
The Begg-Mazumdar indicator for bias gave a Kendall’s tau b of 0.16 (P = .09) and Egger’s test, another indicator of publication bias, was −0.12 (95% CI, −0.63 to 0.11; P = .31). Both of these values indicated no significant publication bias.
DISCUSSION
Pancreatic cancer and CCA usually manifest as biliary strictures that are difficult to differentiate from strictures caused by bile duct stones, chronic pancreatitis, or other benign conditions. Cytological or histological diagnosis of the strictures alters the management plan significantly in terms of aggressive treatment of confirmed malignancies and avoiding unnecessary surgery in benign conditions. ERCP-based tissue diagnosis of suspicious biliary strictures can be achieved with brush cytology or intraductal forceps biopsy.
From the 9 studies included in our meta-analysis, the pooled sensitivity of brush cytology and intraductal biopsy in diagnosing malignant biliary strictures were 45% and 48.1% respectively. The pooled negative LR− of 0.54 for both brush cytology and intraductal biopsy suggests that neither of these tests can be used as a stand-alone diagnostic test to exclude cancer in a stricture. This again challenges the diagnostic accuracy of both of these modalities in determining the nature of biliary strictures. A combination of both only modestly increased the sensitivity to 59.4%. Based on this meta-analysis, the best diagnostic approach in the absence of other resources is to perform both brushings and intraductal biopsies at the time of initial ERCP to maximize the diagnostic yield because both of these techniques clearly complement each other. Also, if the diagnosis of malignancy is obtained at the time of the initial ERCP, it could avoid a second procedure such as EUS and FNA and the risks and the costs associated with the procedure. Although both tests appear to complement each other, still better diagnostic tests are required for the evaluation of biliary strictures. In clinical practice, where pancreatic cancer is much more frequently seen than CCA, the low yield of brushings or biopsies may be because both techniques sample the bile duct and may not detect cancers unless there is infiltration of pancreatic cancer into the bile duct.
The order of performing brush cytology and forceps biopsy varied among the studies. There is always the concern for inadequate sampling with both brush cytology and intraductal biopsy. Disrupting the strictures with a dilating balloon or scraping brush before sampling has been suggested in some studies to increase the cellular yield of brush/bile cytology,25–27 whereas in other studies,28 this intervention did not affect the yield. Performing forceps biopsy before brushing could possibly disrupt the stricture, especially with previous dilation, and might increase the cellular yield of brushing. In the studies included in our meta-analysis, when biopsies were followed with brushings,16,21,24 the sensitivity of brushing did not change considerably from the overall pooled sensitivity (44.5%). Brushing followed by biopsy seems to be more practical and was the order of performing tissue sampling in most of the included studies.
There might be concerns that dilating the strictures could make the walls thinner, increasing the risk of perforation, especially where multiple biopsies are performed in close proximity to each other. In the study by Pugliese et al,21 both cytology and forceps biopsy were performed in the first 52 consecutive patients, but the occurrence of perforation in patient 52 led to the adoption of brush cytology alone in the remaining 42 patients. Intraductal biopsy was cited as the cause of the perforation. In the studies included in this meta-analysis, ductal perforations were few. Kitajima et al18 reported a perforation in a patient with infiltrating carcinoma of the bile duct undergoing ERCP biopsy. In another study, a retroperitoneal perforation occurred in a patient with benign stricture undergoing brush cytology.19 Duct infiltration by the tumor, multiple large biopsy specimens, and excessive biliary dilation are independent risk factors for perforation.18,20,21
Performing forceps biopsy may require biliary sphincterotomy more often than brushing.16,17,21 Performing sphincterotomy did not lead to a significant increase in adverse events except minimal bleeding. Biopsies provide information about the tissue structure and can also provide details on tissue invasion when the biopsy has adequate depth. Such information cannot be obtained by brush cytology. In our meta-analysis, the sensitivity of endobiliary biopsy for the detection of malignancy did not differ much from that of brush cytology. The success of both of the techniques in sample acquisition and diagnosis is largely dependent on the quality of the cytopathologists available, as suggested in other studies of EUS-guided FNA.29
The limitations of both modalities for the detection of cancer can be attributed to many factors. In almost all included studies, the “suspicious for malignancy” category or “atypical cells” category was not included in calculating the sensitivity of these modalities. Often there is concern for inadequate cellularity of the sample, especially in brush cytology specimens, and the desmoplastic nature of CCA can further decrease the cellular yield.30 Unlike other instances in which biopsies can be targeted to the tumor mass, the biliary or pancreatic malignancies present as strictures difficult to be targeted on ERCP. Advanced imaging options such as peroral cholangioscopy can provide better visualization of tissues for targeted biopsy if malignancy is suspected, but the costs and technical expertise make its routine use difficult.31 The question of whether the use of on-site cytopathology improves the yield of biopsies and/or brushings of the bile duct remains unresolved. There is an ongoing clinical trial to address this (http://clinicaltrials.gov/ct2/show/NCT01815619). The yield may be better in strictures where good brushing specimens can be obtained such as in ragged strictures rather than in smooth strictures. However, there is no evidence to support this. The role of whether sending a recently removed stent removed during ERCP for cytology increases the diagnostic yield also remains unknown. All of these factors could increase the diagnostic yield of brushings and/or biopsies and need to be explored. Pancreatic head malignancies often present with extrinsic compression of bile ducts, and hence ERCP might not be representative of the malignant cells unless there is bile duct invasion.32 The use of EUS is well proven to be safe in the setting of pancreatic cancer and distal CCA.33–35 In proximal/hilar CCA, there is controversy. A Mayo Clinic study suggested that EUS with FNA may increase the risk of peritoneal seeding,36 whereas a recent study suggested that FNA did not increase the risk of adverse events.37 However, in centers where Mayo Clinic transplantation protocol is used for CCA, the patients are excluded if FNA is performed. However, EUS still plays a major role in lymph node staging of hilar CCA. Also, most patients with seeding in the setting of the Mayo Clinic study had a biopsy performed by interventional radiology.
This meta-analysis has some limitations. Although all of the studies included details on the number of cases of pancreatic/ampullary adenocarcinoma and CCA, specific sensitivities of the modalities in detecting different malignancies were not reported in most of them. Hence, the results of the meta-analysis are for malignant biliary strictures in general and cannot be extrapolated to specific malignancies. Furthermore, this meta-analysis does not apply to patients with primary sclerosing cholangitis as it was not described in the included studies. The strength of this meta-analysis is that final confirmation was available in all of the studies, and surgical pathology was the confirmatory modality in nearly all cases. A recent review highlighted the yield of brushings of 45%, validating the results of this meta-analysis.10 In addition, in almost all of included studies, the pathologists were blinded to the clinical information, eliminating observer bias. The sensitivities reported in our study were exclusive of the atypical/suspicious cytologies in almost all of the studies. Thus, a potentially major source of data variability was avoided in our meta-analysis. Bias calculation by using the Egger bias indicator and the Begg-Mazumdar indicator showed no statistically significant publication bias.
To conclude, the diagnostic yield of brush cytology or forceps biopsy alone does not appear very convincing, and their combination moderately increases the sensitivity for diagnosing malignant biliary strictures. Both techniques are almost 100% specific. With the recent advances in biomarker identification,38–40 complementing these techniques with serum or biliary tumor markers can further improve the sensitivity and seems to be a reasonable option for enhancing the diagnostic yield.
Abbreviations
- CCA
cholangiocarcinoma
- CI
confidence interval
- DOR
diagnostic odds ratio
- LR
likelihood ratio
- LR+
positive likelihood ratio
- LR−
negative likelihood ratio
Footnotes
DISCLOSURE: Dr Vargo is a consultant for Olympus, Boston Scientific, Ethicon Endosurgery, and Cook Endoscopy. Dr Parsi is a consultant for Boston Scientific. All other authors disclosed no financial relationships relevant to this article.
References
- 1.Friman S. Cholangiocarcinoma–current treatment options. Scand J Surg. 2011;100:30–4. doi: 10.1177/145749691110000106. [DOI] [PubMed] [Google Scholar]
- 2.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–67. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 3.Saif MW. Pancreatic neoplasm in 2011: an update. JOP. 2011;12:316–21. [PubMed] [Google Scholar]
- 4.Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology. 2014;14:27–35. doi: 10.1016/j.pan.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown EG, Yang A, Canter RJ, et al. Outcomes of pancreaticoduodenectomy: where should we focus our efforts on improving outcomes? JAMA Surg. 2014;149:694–9. doi: 10.1001/jamasurg.2014.151. [DOI] [PubMed] [Google Scholar]
- 6.Chok AY, Koh YX, Ow MY, et al. A systematic review and meta-analysis comparing pancreaticoduodenectomy versus limited resection for duodenal gastrointestinal stromal tumors. Ann Surg Oncol. 2014;21:4329–38. doi: 10.1245/s10434-014-3788-1. [DOI] [PubMed] [Google Scholar]
- 7.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1. [DOI] [PubMed] [Google Scholar]
- 8.Gerritsen A, Molenaar IQ, Bollen TL, et al. Preoperative characteristics of patients with presumed pancreatic cancer but ultimately benign disease: a multicenter series of 344 pancreatoduodenectomies. Ann Surg Oncol. doi: 10.1245/s10434-014-3810-7. Epub 2014 May 29. [DOI] [PubMed] [Google Scholar]
- 9.Abraham SC, Wilentz RE, Yeo CJ, et al. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all ‘chronic pancreatitis’? Am J Surg Pathol. 2003;27:110–20. doi: 10.1097/00000478-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res. 2013;184:304–11. doi: 10.1016/j.jss.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Trikudanathan G, Navaneethan U, Njei B, et al. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis-a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:783–9. doi: 10.1016/j.gie.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Thompson SG, Higgins JT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 13.Whiting P, Rutjes AS, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 15.Harbord RM, Egger M, Sterne JC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 16.Howell DA, Parsons WG, Jones MA, et al. Complete tissue sampling of biliary strictures at ERCP using a new device. Gastrointest Endosc. 1996;43:498–502. doi: 10.1016/s0016-5107(96)70294-8. [DOI] [PubMed] [Google Scholar]
- 17.Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51:383–90. doi: 10.1016/s0016-5107(00)70435-4. [DOI] [PubMed] [Google Scholar]
- 18.Kitajima Y, Ohara H, Nakazawa T, et al. Usefulness of transpapillary bile duct brushing cytology and forceps biopsy for improved diagnosis in patients with biliary strictures. J Gastroenterol Hepatol. 2007;22:1615–20. doi: 10.1111/j.1440-1746.2007.05037.x. [DOI] [PubMed] [Google Scholar]
- 19.Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995;42:565–72. doi: 10.1016/s0016-5107(95)70012-9. [DOI] [PubMed] [Google Scholar]
- 20.Pugliese V, Barone D, Saccomanno S, et al. Tissue sampling from the common bile duct through endoscopic retrograde cholangiopancreatography, endoscopic papillo(sphinctero)tomy and drainage in juxta-papillary malignancies. Surg Endosc. 1987;1:83–7. doi: 10.1007/BF00312690. [DOI] [PubMed] [Google Scholar]
- 21.Pugliese V, Conio M, Nicolò G, et al. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995;42:520–6. doi: 10.1016/s0016-5107(95)70004-8. [DOI] [PubMed] [Google Scholar]
- 22.Rösch T, Hofrichter K, Frimberger E, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390–6. doi: 10.1016/s0016-5107(04)01732-8. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama M, Atomi Y, Wada N, et al. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol. 1996;91:465–7. [PubMed] [Google Scholar]
- 24.Weber A, von Weyhern C, Fend F, et al. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J Gastroenterol. 2008;14:1097–101. doi: 10.3748/wjg.14.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohandas KM, Swaroop VS, Gullar SU, et al. Diagnosis of malignant obstructive jaundice by bile cytology: results improved by dilating the bile duct strictures. Gastrointest Endosc. 1994;40:150–4. doi: 10.1016/s0016-5107(94)70157-1. [DOI] [PubMed] [Google Scholar]
- 26.Farrell RJ, Jain AK, Brandwein SL, et al. The combination of stricture dilation, endoscopic needle aspiration, and biliary brushings significantly improves diagnostic yield from malignant bile duct strictures. Gastrointest Endosc. 2001;54:587–94. doi: 10.1067/mge.2001.118715. [DOI] [PubMed] [Google Scholar]
- 27.Parasher VK, Huibregtse K. Endoscopic retrograde wire-guided cytology of malignant biliary strictures using a novel scraping brush. Gastrointest Endosc. 1998;48:288–90. doi: 10.1016/s0016-5107(98)70193-2. [DOI] [PubMed] [Google Scholar]
- 28.de Bellis M, Fogel EL, Sherman S, et al. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest Endosc. 2003;58:176–82. doi: 10.1067/mge.2003.345. [DOI] [PubMed] [Google Scholar]
- 29.Harewood GC, Baron TH, Stadheim LM, et al. Prospective, blinded assessment of factors influencing the accuracy of biliary cytology interpretation. Am J Gastroenterol. 2004;99:1464–9. doi: 10.1111/j.1572-0241.2004.30845.x. [DOI] [PubMed] [Google Scholar]
- 30.Patel T. Cholangiocarcinoma–controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin MW, Byrne MF. Update of cholangioscopy and biliary strictures. World J Gastroenterol. 2011;17:3864–9. doi: 10.3748/wjg.v17.i34.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97–104. doi: 10.1016/j.gie.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Ngamruengphong S, Xu C, Woodward TA, et al. Risk of gastric or peritoneal recurrence, and long-term outcomes, following pancreatic cancer resection with preoperative endosonographically guided fine needle aspiration. Endoscopy. 2013;45:619–26. doi: 10.1055/s-0033-1344216. [DOI] [PubMed] [Google Scholar]
- 34.Khashab MA, Fockens P, Al-Haddad MA. Utility of EUS in patients with indeterminate biliary strictures and suspected extrahepaticcholangiocarcinoma (with videos) Gastrointest Endosc. 2012;76:1024–33. doi: 10.1016/j.gie.2012.04.451. [DOI] [PubMed] [Google Scholar]
- 35.Navaneethan U, Njei B, Venkatesh PG, et al. Endoscopic ultrasound in the diagnosis of cholangiocarcinoma as the etiology of biliary strictures: a systematic review and meta-analysis. Gastroenterol Rep (Oxf) doi: 10.1093/gastro/gou057. Epub 2014 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heimbach JK, Sanchez W, Rosen CB, et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–60. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Chafic AH, Dewitt J, Leblanc JK, et al. Impact of preoperative endoscopic ultrasound-guided fineneedle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy. 2013;45:883–9. doi: 10.1055/s-0033-1344760. [DOI] [PubMed] [Google Scholar]
- 38.Navaneethan U, Gutierrez NG, Venkatesh PG, et al. Lipidomic profiling of bile in distinguishing benign from malignant biliary strictures: a single-blinded pilot study. Am J Gastroenterol. 2014;109:895–902. doi: 10.1038/ajg.2014.60. [DOI] [PubMed] [Google Scholar]
- 39.Lankisch TO, Metzger J, Negm AA, et al. Bile proteomic profiles differentiate cholangiocarcinoma from primary sclerosing cholangitis and choledocholithiasis. Hepatology. 2011;53:875–84. doi: 10.1002/hep.24103. [DOI] [PubMed] [Google Scholar]
- 40.Navaneethan U, Parsi MA, Gutierrez NG, et al. Volatile organic compounds in bile can diagnose malignant biliary strictures in the setting of pancreatic cancer: a preliminary observation. Gastrointest Endosc. doi: 10.1016/j.gie.2014.04.016. Epub 2014 Jun 11. [DOI] [PubMed] [Google Scholar]