Abstract
BACKGROUND
In November and December 2012, 6 patients at a hemodialysis clinic were given a diagnosis of new hepatitis C virus (HCV) infection.
OBJECTIVE
To investigate the outbreak to identify risk factors for transmission.
METHODS
A case patient was defined as a patient who was HCV-antibody negative on clinic admission but subsequently was found to be HCV-antibody positive from January 1, 2008, through April 30, 2013. Patient charts were reviewed to identify and describe case patients. The hypervariable region 1 of HCV from infected patients was tested to assess viral genetic relatedness. Infection control practices were evaluated via observations. A forensic chemiluminescent agent was used to identify blood contamination on environmental surfaces after cleaning.
RESULTS
Eighteen case patients were identified at the clinic from January 1, 2008, through April 30, 2013, resulting in an estimated 16.7% attack rate. Analysis of HCV quasispecies identified 4 separate clusters of transmission involving 11 case patients. The case patients and previously infected patients in each cluster were treated in neighboring dialysis stations during the same shift, or at the same dialysis station on 2 consecutive shifts. Lapses in infection control were identified. Visible and invisible blood was identified on multiple surfaces at the clinic.
CONCLUSIONS
Epidemiologic and laboratory data confirmed transmission of HCV among numerous patients at the dialysis clinic over 6 years. Infection control breaches were likely responsible. This outbreak highlights the importance of rigorous adherence to recommended infection control practices in dialysis settings.
Hepatitis C virus (HCV) infection is several times more prevalent among hemodialysis patients than the general US population.1–3 Outbreaks of new HCV infections have been reported in US dialysis centers, typically associated with lapses in infection control (IC), including improper parenteral medication handling and preparation, inadequate cleaning and disinfection of environmental surfaces between patient treatments, and poor hand hygiene and glove use.3–5
In November 2012, the Philadelphia Department of Public Health (PDPH) was notified of 2 patients at an outpatient hemodialysis clinic (Clinic A) who had documented seroconversion to HCV antibody positive status. Four additional seroconversions were identified in December 2012. Despite interventions by the clinic after PDPH assessed IC practices and provided recommendations, a new HCV infection was identified in April 2013. PDPH and the Centers for Disease Control and Prevention (CDC) performed an epidemiologic investigation to evaluate the extent of the outbreak and assess potential modes of transmission and risk factors for HCV acquisition.
METHODS
The clinic performed HCV antibody tests upon admission for all patients and then annually in January for susceptible patients. Monthly serum alanine aminotransferase (ALT) tests were performed on all patients. When ALT levels were elevated, HCV antibody testing was performed.
Definitions
We defined an incident case patient as a Clinic A patient who was HCV-antibody (anti-HCV) negative upon admission screening but subsequently found to be anti-HCV positive (ie, “newly infected”). A previously infected patient was one who was anti-HCV positive upon admission to Clinic A. Susceptible (ie, at-risk) patients were those not infected with HCV (ie, anti-HCV negative) upon admission.
Because most cases were asymptomatic, case patients’ estimated date of onset of HCV infection was defined as the first date the serum ALT level was above the upper limit of the normal range (ie, >45 IU/mL). The exposure period was defined as 3 months to 2 weeks prior to the estimated date of onset. For incident case patients whose seroconversion was not preceded by a documented serum ALT elevation, we defined the exposure period as the 6 months to 2 weeks before the first positive anti-HCV result.
Case Finding
We reviewed HCV test results of all patients who were treated at the clinic from January 1, 2008, through April 30, 2013 (including active patients and patients who transferred out or died), to identify incident case patients and previously infected patients.
Case Description
For all infected patients identified through case finding, medical records were abstracted and data were entered into Epi Info, version 7 (CDC). We obtained treatment schedule and station data—that is, the dialysis stations and shifts of patients’ treatment sessions—for all incident cases during their exposure periods. We compared station assignments and treatment schedules among infected patients identified as having closely related virus to evaluate potential links in space and time.
Case patients were interviewed by PDPH to identify hepatitis C risk factors during exposure periods, symptoms of acute hepatitis C, and whether patients were notified of their diagnosis and referred for HCV treatment evaluation.
Laboratory Testing
Serum samples collected from HCV-infected patients (ie, incident case patients and previously infected patients) at the clinic in April 2013 were sent to CDC’s Division of Viral Hepatitis Laboratory. HCV RNA was amplified by real-time polymerase chain reaction from anti-HCV positive samples. HCV RNA-positive samples underwent sequencing of the 300-nucleotide NS5b coding region to determine genotype as described.6 In addition, the HCV hypervariable region 1 was sequenced to determine HCV quasispecies using the Roche/ 454 pyrosequencing technology.6 A phylogenetic tree was developed to illustrate and compare the hypervariable region 1 quasispecies distribution of incident cases and previously infected patients.
IC Observation
We observed patient flow and IC practices at the clinic. We focused on (1) staff hand hygiene and glove use, (2) vascular access care, (3) parenteral medication preparation and administration, and (4) environmental and machine surface cleaning and disinfection between shifts and at the end of day. A standard set of tools was used to record our observations (http://www.cdc.gov/dialysis/prevention-tools/index.html).
Environmental Assessment
An environmental assessment was performed with 3 objectives: (1) to identify blood contamination on surfaces, (2) to confirm the presence of human hemoglobin in potential blood contamination identified, and (3) to assess cleaning practices.
To identify potential invisible blood contamination on surfaces, we used Bluestar Forensic latent blood reagent (Bluestar), a forensic chemiluminescent agent that reacts with hemoglobin. Bluestar Forensic was used to identify the possible presence of blood on dialysis machines and patient chairs after terminal cleaning and on chairs in the clinic’s waiting area and in the patients' bathroom. An immune assay (Hexagon OBTI; Bluestar) was used to confirm the presence of human hemoglobin. We were able to perform only 1 assessment when no patients were being treated and all the machines and chairs had been cleaned and disinfected the evening before.
To evaluate the thoroughness of cleaning, we applied Glo Germ oil (Glo Germ) to a dialysis machine’s high-touch surfaces before completion of the last dialysis shift on the day of the assessment. After cleaning was performed, we exposed surfaces to UV light; fluorescence upon exposure to UV light indicates persistence of Glo Germ because of lack of adequate cleaning. This exercise was used to demonstrate to staff the importance of thorough cleaning and disinfection.
RESULTS
Description of Clinic A
Clinic A was a stand-alone dialysis center with 66 patients being dialyzed at the time of the investigation. The clinic had a total of 24 stations in 3 pods. Each pod had a total of 8 stations in 2 rows (4 stations in each row). Dialysis machines were close to each other, with no barrier between them. The clinic operated 3 shifts on a Monday-Wednesday-Friday schedule. Shifts 1 and 2 were very busy, and the transition time between those shifts was short, approximately 15 to 20 minutes.
There were 6 technicians (usually 2 assigned to each pod), 2 registered nurses, and 1 licensed practical nurse working per shift. The clinic maintained a log of technician and nurse schedules, along with the stations where specific patients were dialyzed each day, which were not assigned and could change daily.
Case Patient Characteristics
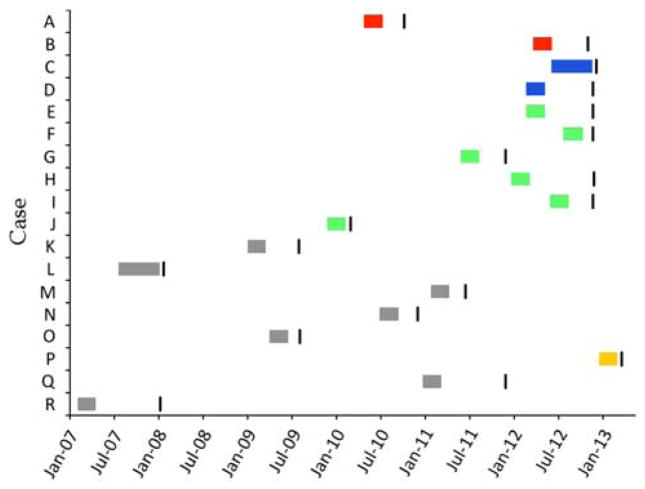
Of Clinic A’s 66 patients in April 2013, 26 (39%) were infected with HCV. Case finding identified a total of 18 patients who seroconverted since 2008 (Figure 1). Eight case patients seroconverted in 2012 (n = 7) or 2013 (n = 1) on the basis of their first anti-HCV positive tests. Forty-eight Clinic A patients were at risk during this time, thus the attack rate for 2012–2013 was 16.7% (8/48). Figure 1 describes the exposure periods and dates of the first positive HCV test for all 18 case patients. Only 3 case patients had documented symptoms consistent with acute hepatitis: 1 reported vomiting, 2 reported diarrhea. Two case patients had no ALT elevation (ie, all documented ALT values prior to seroconversion were normal); neither of them showed any symptoms suggestive of acute hepatitis. Table 1 describes the characteristics of case patients. Although 14 case patients (78%) had hospitalizations and 10 (56%) had invasive procedures during their exposure period, no common exposures (eg, overlap in hospitalizations or same surgeries) were identified. Three case patients had died and the cause of death was not reported in the charts.
Figure 1.
Exposure period and first hepatitis C virus (HCV)–positive test of 18 case patients, Clinic A, 2008–2013. Anti-HCV, antibody to HCV.
 , Positive HCV test (anti-HCV or HCV RNA);
, Positive HCV test (anti-HCV or HCV RNA);
 , Case patients’ exposure period (cluster 1);
, Case patients’ exposure period (cluster 1);
 , Case patients’ exposure period (cluster 2);
, Case patients’ exposure period (cluster 2);
 , Case patients’ exposure period (cluster 3);
, Case patients’ exposure period (cluster 3);
 , Case patient P’s exposure period;
, Case patient P’s exposure period;
 , Other case patients’ exposure period.
, Other case patients’ exposure period.
Table 1.
Characteristics of 18 Case Patients, Clinic A
| Characteristic | Cases |
|---|---|
| Male sex, n (%) | 9 (50) |
| Age, median (range), y | 63.5 (37–86) |
| Black race, n (%) | 18 (100) |
| History of diabetes, n (%)a | 9 (50) |
| History of hypertension, n (%) | 14 (78) |
| Time from first hemodialysis to seroconversion, median (range), months | 37.8 (5.6–220.1) |
| Symptoms of acute HCV infection, n (%) | 3 (17) |
| Highest documented ALT, median (range), IU/mLb | 183 (51–535) |
| During exposure period | |
| Most common treatment pod | |
| Pod 1, n (%) | 18 (100) |
| Most common treatment shift | |
| Shift 3, n (%) | 10 (56) |
| Heparin injection, n (%) | 11 (61) |
| Iron sucrose injection, n (%) | 8 (44) |
| Paricalcitol injection, n (%) | 14 (78) |
| Erythropoietin injection, n (%) | 14 (78) |
| CVC used for hemodialysis vascular access, n (%) | 3 (17) |
| History of intravenous use, n (%) | 0 (0) |
| Hospitalized or had hospital visit, n (%) | 14 (78) |
| Had invasive procedure, n (%) | 10 (56) |
| Other risk factors identified through interview (n =12) | |
| Close contact of confirmed or presumptive case, n (%) | 1 (8) |
| Received blood products, organs, or tissues, n (%) | 3 (25) |
| Exposure to another person’s blood, n (%) | 0 (0) |
| Sexual contact, n (%) | 5 (42) |
| Sexual contact without condoms, n (%) | 3 (25) |
| Injecting drugs, n (%) | 0 (0) |
| Tattoo, piercing, n (%) | 0 (0) |
NOTE. ALT, alanine aminotransferase; CVC, central venous catheter; HCV, hepatitis C virus.
Glucose meter use was observed and found to be adequate.
2 case patients did not have documented elevation of ALT.
Quasispecies Analyses of Infected Patients
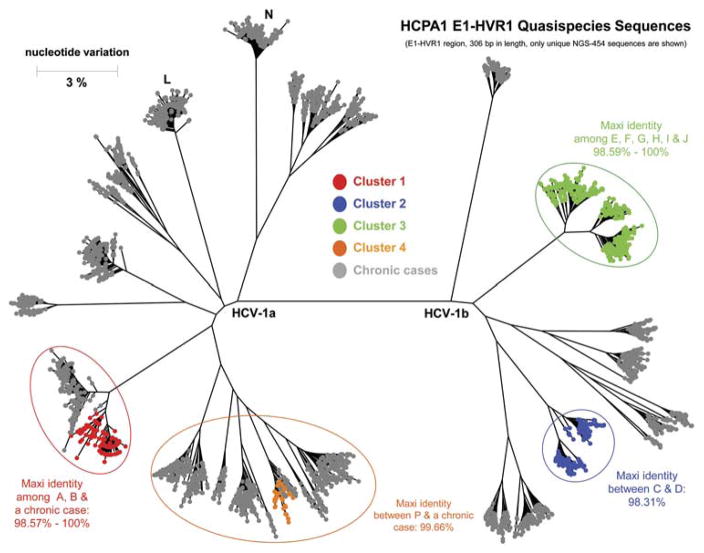
Twenty-three serum samples from 13 case patients and 10 previously infected patients at the clinic in April 2013 were available for additional laboratory testing. Of these 23 patients, 11 case patients and 2 previously infected patients belonged to 4 clusters (Figure 2).
Figure 2.
Results of hepatitis C virus (HCV) quasispecies analysis of 13 case patients and 10 previously infected patients at Clinic A. HVR, hypervariable region.
Given the high homology (99.66% nucleotide identity) and close clustering of HCV quasispecies between case patient P and 1 previously infected patient, HCV transmission could have occurred from this patient to case patient P. For the other clusters, there was a link between patients in each cluster; however, the transmission direction is more difficult to speculate. Secondary transmission could have occurred in any of the clusters.
We reviewed the treatment time and dialysis station assignment data for case patients and previously infected patients in each cluster to assess commonalities. For each cluster, the treatment station data for the exposure period of patients who seroconverted later was compared with the data for the same period of patients who were infected earlier. In each cluster, there was evidence that the case patients and previously infected patients were treated in nearby stations during the same shift, or at the same station on 2 consecutive shifts (with the case patients following the patients who were infected earlier). Most of these stations where transmission occurred were located in 1 particular pod.
IC Observations
Before our investigation, when PDPH visited the clinic in December 2012 and when the clinic’s manager performed audits of IC, lapses in IC practices were identified (eg, staff moved between machines without changing gloves or used ungloved hands without hand hygiene after touching machines, the clinic did not clearly label clean sinks and dirty sinks, a stationary medication cart in the nursing station within the treatment area was used as the location for medication preparation). The clinic then moved medication preparation to a separate room away from the patient care area and had only nurses draw the parenteral medications instead of technicians. During our investigation, generally good hand hygiene and aseptic technique during parenteral medication preparation and administration were observed. However, multiple lapses in IC were identified (Table 2). Most lapses were during vascular access (eg, lack of appropriate glove change and hand hygiene during vascular access procedures, inadequate application of antiseptic) and cleaning and disinfection of environmental surfaces (eg, insufficient application of disinfectant, wiping machine surfaces with patient still in station, not disinfecting all surfaces).
Table 2.
Summary of Key Infection Control Observations
| Infection control area | Tools used | Positive practices | Selected suboptimal practices |
|---|---|---|---|
| Hand hygiene (HH)14,18 | http://www.cdc.gov/dialysis/prevention-tools/ |
|
|
| Medication preparation and administration14,19,20 |
http://www.cdc.gov/injectionsafety/IP07_standardPrecaution.html http://www.cdc.gov/injectionsafety/providers/provider_faqs.html |
|
|
| Vascular access care14,21–23 | http://www.cdc.gov/dialysis/prevention-tools/ |
|
|
| Cleaning and disinfection14,24,25 | http://www.cdc.gov/dialysis/prevention-tools/ |
|
|
Environmental Assessment
Table 3 lists the objects selected for assessment and results. The dialysis chair with reaction to Bluestar Forensic solution and positive Hexagon OBTI result was in the implicated pod mentioned above. This prompted us to inspect all the chairs in the clinic and we identified visible bloodstains on the inside of the armrests of 4 of the chairs, indicating that the chairs were not thoroughly cleaned.
Table 3.
Results of Environmental Assessments
| Tested object | Tested area | Bluestar Forensic test | Hexagon OBTI test | Interpretation |
|---|---|---|---|---|
| Dialysis machine 1 | ||||
| Screen | No reaction | … | … | |
| Keyboard | Reaction | Negative | Cross-reaction with remnant bleach or extremely low concentration of blood | |
| Acid and base administration port | No reaction | … | … | |
| Machine’s right side | Not tested | … | Visible bloodstains near blood pressure holder | |
| Artery blood pressure cuff | Reaction | Negative | Cross-reaction with remnant bleach or extremely low concentration of blood | |
| Prime bucket | Reaction | Negative | Cross-reaction with remnant bleach or extremely low concentration of blood | |
| Dialysis machine 2 | ||||
| Screen | No reaction | … | … | |
| Keyboard | No reaction | … | … | |
| Acid and base administration port | Reaction | Negative | Cross-reaction with remnant bleach or extremely low concentration of blood | |
| Machine’s right side | Reaction | Negative | Cross-reaction with remnant bleach or extremely low concentration of blood | |
| Blood pressure cuff | No reaction | … | … | |
| Prime bucket | No reaction | … | … | |
| Dialysis chair | ||||
| Headrest | No reaction | … | … | |
| Armrest | No reaction | … | … | |
| Footrest | No reaction | … | … | |
| Chair side | No reaction | … | … | |
| Side table | Reaction | Positive | Invisible bloodstain | |
| Two chairs in waiting area | ||||
| Seat surface | Reaction (1 chair) | Positive | Invisible bloodstain | |
| Bathroom | ||||
| Sink | Reaction | Negative | Cross-reaction with remnant bleach or extremely low concentration of blood | |
| Door handle | No reaction | … | … | |
| Light switch | No reaction | … | … | |
| Toilet handle | No reaction | … | … | |
| 4 television sets within dialysis stations | ||||
| 4 sets with visible stains (of 24 sets) | Not tested | Positive (1 stain tested) | Visible bloodstain | |
| Biohazard trash can with visible blood stain | ||||
| Opening area of can | Strong reaction | Positive | Served as positive control | |
NOTE. Bluestar Forensic is a forensic chemiluminescent agent that reacts with hemoglobin; Hexagon OBTI is an immune assay used to confirm the presence of human hemoglobin.
In summary, bloodstains were seen on 1 dialysis machine, on dialysis station televisions, and on patient chairs. Three samples produced a positive Hexagon OBTI result: 1 from the dialysis chair side table, 1 from the chair in the waiting area, and 1 from a dialysis station television. Multiple surfaces tested positive by Bluestar Forensic but negative by Hexagon OBTI. We attributed these results to either an extremely low concentration of blood in the samples or a false-positive reaction to dried bleach on the surfaces.
The use of Glo Germ on a machine identified numerous areas that were not cleaned thoroughly. We used this test as an educational tool to show technicians, facility managers, and the medical director where cleaning was suboptimal.
DISCUSSION
We identified multiple clusters of transmission of HCV at Clinic A with a total of 18 newly infected patients from January 1, 2008, through April 30, 2013. Genetic links between infected patients’ viruses and analysis of epidemiologic data (treatment station assignment, healthcare exposures) supported our hypothesis that transmission occurred to case patients at Clinic A, most likely owing to lapses in IC.
We observed multiple lapses in IC practices, including vascular access care, hand hygiene, as well as cleaning and disinfection of dialysis stations. The presence of bloodstains on multiple surfaces at the clinic pointed to poor environmental cleaning and disinfection. Although not observed at the time of our investigation, poor medication handling practices, including the preparation of medications on a cart in the treatment area, might also have been a risk factor. In addition, Clinic A presumably restricted technicians from performing medication administration because they might not have adhered to recommended aseptic technique. We believe that these suboptimal IC practices likely contributed to the repeated transmission of HCV among patients in Clinic A. These factors are well-known causes of HCV transmission in this setting.3–5,7–10 The transmission of HCV among patients who were in proximity during the same shift or shared a station between consecutive shifts has also been reported.11–13
In this investigation, 15 case patients lacked documented symptoms and 2 lacked ALT elevations. These 2 case patients were identified by reviewing serial anti-HCV results; the new acute HCV infection case definition used by the National Notifiable Diseases Surveillance System does not require clinical symptoms if a documented seroconversion occurs (http://wwwn.cdc.gov/nndss/conditions/hepatitis-c-acute/case-definition/2012/). This highlights the importance of routine hepatitis C serology testing, which is a recommended practice.14,15 The clinic identified the seroconversions in 2012 and 2013 as a result of screening and reviewing tests; however, previous case patients had not been promptly identified. Therefore, when routine ALT and HCV screening is performed, providers should review the results and take appropriate action in a timely manner15; early intervention at the clinic before 2012 could have prevented many subsequent HCV infections. Unfortunately, in this clinic, and as with another investigation,4 we found that not all case patients were informed of their HCV diagnosis.
Our environmental testing allowed us to confirm human blood contamination on surfaces at the clinic. The use of forensic chemiluminescent agents and hemoglobin detection kits to identify blood contamination in dialysis settings has been reported.7,16 In a previous outbreak, Bluestar Forensic was used with limited success.12 The agent requires a completely dark environment that can be difficult to achieve in healthcare facilities. In addition, the agent also reacts with bleach, which is a widely used disinfectant in dialysis clinics. In this investigation, we were able to arrange a completely dark environment in a laboratory room for the testing. To address the potential for false-positive results from bleach, we used Hexagon OBTI to confirm the presence of human hemoglobin. We believe this provided more convincing evidence of blood contamination of surfaces at the clinic.
This investigation raised several issues regarding clinic management and layout. First, we believe that the space available for each station at the clinic was not adequate—for example, we observed 2 dialysis machines in use within 1 foot of each other. The Facility Guidelines Institute’s Guidelines for Design and Construction of Hospitals and Outpatient Facilities recommends a space of 80 square feet for each treatment station and a minimum of 4 feet (1.22 meters) between dialysis chairs, which was not followed at Clinic A.17 Clinics not meeting these criteria should consider expanding the space for each station and between stations, or install walls between stations to reduce crowding between patients and equipment that could facilitate cross-contamination. Second, the clinic operated only 3 days per week. We believe this condensed schedule contributed to the crowding in the facility; quick turnaround time between shifts may have also increased the workload burden on staff, all of which could lead to suboptimal IC practices. A 6-day-per-week schedule is more typical in facilities of similar size and patient volume. This was suggested to the clinic as one way to reduce the number of patients dialyzed per day and spread out treatments in space and time. Third, poor IC practices have been highlighted many times as contributors to HCV outbreaks in dialysis settings5; therefore, having strong IC support at the clinic is important. The support may include (1) providing regular infection prevention training to staff and performing staff competency assessments, (2) having a sufficient number of staff to ensure all IC procedures are performed adequately throughout the day, and (3) having a staff member on site with infection prevention expertise to serve as an IC manager, perform practice audits, and address breaches in a timely manner. The necessary support for IC should be made as a policy at both the clinic level and the corporate level. Following the investigation, PDPH worked with Clinic A to provide multiple training sessions to clinic staff. The clinic increased turnaround time between patient treatment shifts to allow adequate environmental cleaning and disinfection but maintained the 3-day-per-week operations schedule. The clinic also used audit tools to routinely audit IC practices. Follow-up visits by PDPH confirmed improved IC practices at the clinic.
We also want to highlight the importance of HCV surveillance, prompt reporting of new infections to health departments, and referral of infected patients to medical care. Current guidelines recommend routine HCV antibody testing every 6 months for all susceptible patients, and at more frequent intervals following the identification of 1 or more seroconversions.14,15 Because infected patients often lack symptoms, timely testing and careful review of results are critical to identifying new HCV infections. In the wake of this outbreak, Clinic A performed monthly HCV antibody and ALT testing of all uninfected patients under the guidance of PDPH. As of December 2013, no new cases had been identified and the clinic has resumed semiannual anti-HCV screening. The effort to frequently screen, communicate results to patients, and refer infected patients to care requires coordination between clinic staff, its medical director, and specialists outside the facility.
The investigation had several limitations. First, we were unable to obtain blood samples from all cases to perform viral sequencing because some patients had died. Therefore, the relatedness of some cases was unknown. Second, we were not able to perform the environmental assessment using Bluestar on all the machines and chairs as well as the medication preparation area because of the facility lighting. We were also unable to interrupt the patient care schedule to assess adequacy of cleaning in between patient treatments (vs at the end of the day). The former would be a more relevant representation of surface contamination experienced by most patients. However, evidence of blood contamination on the randomly chosen machine and chair may reflect the generally suboptimal cleaning and disinfection practice at Clinic A. Finally, there were important changes in IC practices (eg, injectable medication preparation) that occurred before our investigation. Therefore, our observations might not reflect the breaches that contributed to transmission.
In conclusion, this was one of the largest outbreaks of HCV infection among dialysis patients that we have encountered in the past decade. Although it remains critically important for dialysis clinic staff to continue adherence to current recommended IC practices to prevent spread of HCV and other infections, more aggressive strategies may be needed to stop HCV outbreaks from occurring in outpatient hemodia-lysis centers.
Acknowledgments
We thank all staff at Clinic A and PDPH who helped us with the investigation.
Financial support. None reported.
Footnotes
Presented in part: 2014 Council of State and Territorial Epidemiologists Annual Conference; Nashville, TN; June 22-26, 2014 (Abstract 141).
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The use of trade names and commercial sources in this report is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention. The CDC Experience is a 1-year fellowship in applied epidemiology at CDC made possible by a public/ private partnership supported by a grant to the CDC Foundation from External Medical Affairs, Pfizer Inc.
References
- 1.Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18:52–61. doi: 10.1111/j.1525-139X.2005.18108.x. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis. 2010;56:371–378. doi: 10.1053/j.ajkd.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Hepatitis C virus transmission at an outpatient hemodialysis unit–New York, 2001–2008. MMWR Morb Mortal Wkly Rep. 2009;58:189–194. [PubMed] [Google Scholar]
- 5.Thompson ND, Novak RT, Datta D, et al. Hepatitis C virus transmission in hemodialysis units: importance of infection control practices and aseptic technique. Infect Control Hosp Epidemiol. 2009;30:900–903. doi: 10.1086/605472. [DOI] [PubMed] [Google Scholar]
- 6.Forbi JC, Campo DS, Purdy MA, et al. Intra-host diversity and evolution of hepatitis C virus endemic to Cote d’Ivoire. J Med Virol. 2014;86:765–771. doi: 10.1002/jmv.23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girou E, Chevaliez S, Challine D, et al. Determinant roles of environmental contamination and noncompliance with standard precautions in the risk of hepatitis C virus transmission in a hemodialysis unit. Clin Infect Dis. 2008;47:627–633. doi: 10.1086/590564. [DOI] [PubMed] [Google Scholar]
- 8.Savey A, Simon F, Izopet J, Lepoutre A, Fabry J, Desenclos JC. A large nosocomial outbreak of hepatitis C virus infections at a hemodialysis center. Infect Control Hosp Epidemiol. 2005;26:752–760. doi: 10.1086/502613. [DOI] [PubMed] [Google Scholar]
- 9.Shimokura G, Chai F, Weber DJ, et al. Patient-care practices associated with an increased prevalence of hepatitis C virus infection among chronic hemodialysis patients. Infect Control Hosp Epidemiol. 2011;32:415–424. doi: 10.1086/659407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidney Disease Improving Global Outcomes (KDIGO) Guideline 3: preventing HCV transmission in hemodialysis units. Kidney Int. 2008;73:S46–S52. [Google Scholar]
- 11.Spada E, Abbate I, Sicurezza E, et al. Molecular epidemiology of a hepatitis C virus outbreak in a hemodialysis unit in Italy. J Med Virol. 2008;80:261–267. doi: 10.1002/jmv.21088. [DOI] [PubMed] [Google Scholar]
- 12.Rao AK, Luckman E, Wise ME, et al. Outbreak of hepatitis C virus infections at an outpatient hemodialysis facility: the importance of infection control competencies. Nephrol Nurs J. 2013;40:101–110. 164. [PubMed] [Google Scholar]
- 13.Thompson ND, Novak RT, White-Comstock MB, et al. Patient-to-patient hepatitis C virus transmissions associated with infection control breaches in a hemodialysis unit. J Nephrol Therapeutic. 2012;S10:002. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Recommendations for preventing transmission of infections among chronic hemo-dialysis patients. MMWR Recomm Rep. 2001;50:1–43. [PubMed] [Google Scholar]
- 15.Mbaeyi C, Thompson ND. Hepatitis C virus screening and management of seroconversions in hemodialysis facilities. Semin Dial. 2013;26:439–446. doi: 10.1111/sdi.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergervoet PW, van Riessen N, Sebens FW, van der Zwet WC. Application of the forensic Luminol for blood in infection control. J Hosp Infect. 2008;68:329–333. doi: 10.1016/j.jhin.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Facility Guidelines Institute. Guidelines for design and construction of healthcare facilities. [Accessed May 2015];Facility Guidelines Institute website. http://www.fgiguidelines.org/guidelines2010.php. Published 2010.
- 18.Boyce JM, Pittet D Healthcare Infection Control Practices Advisory Committee. HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/ Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51:1–45. [PubMed] [Google Scholar]
- 19.Siegel JD, Rhinehart E, Jackson M, Chiarello L HICPAC. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, Healthcare Infection Control Practices Advisory Committee, National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion. Guide to Infection Prevention for Outpatient Settings Minimum Expectations for Safe Care. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion; 2011. [Accessed May 2015]. http://www.cdc.gov/HAI/pdfs/guidelines/standatds-of-ambulatory-care-7-2011.pdf. Published 2011. [Google Scholar]
- 21.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39:S1–34. doi: 10.1016/j.ajic.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Levin A, Rocco M. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:S10–S179. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48:S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Rutala WA, Weber DJ Centers for Disease Control and Prevention, Healthcare Infection Control Practices Advisory Committee. Guideline for disinfection and sterilization in healthcare facilities, 2008. Atlanta, GA: Centers for Disease Control and Prevention; 2008. [Accessed May 2015]. http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf. Published 2008. [Google Scholar]
- 25.Sehulster L, Chinn RY CDC, HICPAC. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52:1–42. [PubMed] [Google Scholar]