Abstract
Like any foreign object, orthopaedic implants are susceptible to infection when introduced into the human body. Without additional preventative measures, the absolute number of annual prosthetic joint infections will continue to rise, and may exceed the capacity of health care systems in the near future. Bacteria are difficult to eradicate from synovial joints due to their exceptionally diverse taxonomy, complex mechanistic attachment capabilities, and tendency to evolve antibiotic resistance. When a primary orthopaedic implant fails from prosthetic joint infection, surgeons are generally challenged by limited options for intervention. In this review, we highlight the etiology and taxonomic groupings of bacteria known to cause prosthetic joint infections, and examine their key mechanisms of attachment. We propose that antimicrobial strategies should focus on the most harmful bacteria taxa within the context of occurrence, taxonomic diversity, adhesion mechanisms, and implant design. Patient-specific identification of organisms that cause prosthetic joint infections will permit assessment of their biological vulnerabilities. The latter can be targeted using a range of antimicrobial techniques that exploit different colonization mechanisms including implant surface attachment, biofilm formation, and/or hematogenous recruitment. We anticipate that customized strategies for each patient, joint, and prosthetic component will be most effective at reducing prosthetic joint infections, including those caused by antibiotic-resistant and polymicrobial bacteria.
Keywords: infection, joint, arthroplasty, implant, individualized medicine
Currently, prosthetic joint infections (PJI) account for at least 16% of total joint revisions in the hip and knee,1,2 yet roughly 30% of joint revisions are thought to be caused by aseptic loosening.3–5 Due to the likelihood of undiagnosed PJIs,2,6–8 the exact number of PJI-related revisions is unclear. Importantly, however, known PJI-related revisions are more costly and time consuming than others, and increase the probability of poor prognosis (e.g., limb salvage operations or PJI recurrence).9–11 Therefore, recognizing the unique biological characteristics of relevant microbial organisms and their infection-causing mechanisms are of utmost importance for both preventing and treating PJIs.
A promising strategy for counteracting the negative effects of bacterial invasion and PJI is a multifaceted approach involving physical, chemical, and biological counter measures tested at multiple spatial scales both ex vivo and in vivo. The increasing occurrence of antibiotic resistance has raised awareness that each bacterial taxon may need a specifically catered formula for diagnosis, treatment, and long-term eradication. Further complicating this issue is the potential for concerted attacks by multiple types of bacteria (i.e., polymicrobial infections). More patients and the continued over-prescription of antibiotics can accelerate the rapid evolution of antibiotic-resistant bacteria and increase the potential for PJI complications with limited treatment solutions. In this review, we examine the taxa most responsible for orthopaedic PJIs, explore key mechanisms of implant PJI, and propose novel strategies to reduce the risk of PJI.
Etiology of Prosthetic Joint Infections
The etiologies of PJI in the hip and knee are similar: Staphylococcus is the most prevalent causative agent, accounting for more than half of all cases (Table 1). Surprisingly, S. aureus is widely known to be very pathogenic,12 but only causes about half of Staphylococcus-related PJIs. Others are caused by coagulase-negative Staphylococcus (CoNS), which includes several species: S. haemolyticus, S. capitis, and S. hominis, and most commonly S. epidermidis.13 A key mechanism of action for Staphylococcus-related PJIs is the formation of biofilms.
Table 1.
Examples of Organisms Commonly Detected in Prosthetic Joint Infections of the Hip and Knee (by Percentage of Isolated Samples Within Each Study)
| Reference | 66 | 112 | 113 | 114 | 15 | 22 |
|---|---|---|---|---|---|---|
| Cohort timeframe | 2000–03 | 1999–06 | 2001–06 | 10 studies | 2004–10 | 2013–14 |
| Number of patients | 699 | 147 | 63 | — | 109 | — |
| Staphylococcus sp. | 76% | 53% | 65% | 50% | 60% | 66% |
| Pseudomonas sp. | 7% | 5% | 2% | — | 11% | 2% |
| Enterococcus sp. | 5% | 6% | 5% | 3% | 11% | 6% |
| Streptococcus sp. | 2% | 7% | 11% | 8% | — | 4% |
| Enterobacteriaceae | 8% | 18% | 11% gram (−) | 10% gram (−) | 36% gram (−) | 17% |
| Polymicrobial | — | — | 7% | 16% | 17% | — |
In addition to Staphylococcus, many PJI-causing bacteria can form biofilms, which are particularly resistant to treatments14 because they often require genus-specific modes of eradication.15 Although biofilm structures vary, multiple species can coexist within one biofilm, which facilitates conjugation and could confer antibiotic resistance.16,17 Adding to this complexity, the extracellular polymeric substance of biofilms is largely composed of polysaccharides that encapsulate bacterial colonies,18 filter antimicrobial chemicals, prevent antibiotic perfusion, and limit pharmaceutical efficacy.19 Further, within a biofilm, bacteria can communicate via quorum sensing, which has been shown to regulate the expression of genes involved in virulence and dispersal.19 Quorum sensing, which controls gene expression in response to fluctuations in the density of microbial populations, has also been observed to promote DNA transformation.20 The latter not only elevates the potential for antibiotic resistance, but also provides a mechanism for the rapid evolution of harmful polymicrobial communities.
Among PJIs, polymicrobial infections occur in approximately, 7–17% of cases overall (Table 1) and are often most difficult to treat. Strikingly, though, Benito et al.15 reported a fivefold increase in the yearly occurrence of polymicrobial infections from 7.1 to 41.7% over a period of 6 years from 2004 to 2010, and an equally alarming increase in the yearly proportion of infections caused by gram-negative bacteria (21.4–66.7% over the same period). Of these, Enterobacteriaceae are challenging because they resist a wide range of antibiotics.21,22 making staged eradications less effective (Table 1). PJIs can occur at different times throughout the lifetime of an implant, and therefore, mitigation strategies need to consider temporality: Early (<3 m), delayed (3m-2y), and late (>3y).23 Early PJIs occur as a result of direct perioperative inoculation (either at the time of surgery or within 2–4 days of surgery), and according to the results presented by Benito et al.15 includes all polymicrobial infections. Delayed PJIs can be caused by perioperative inoculation of a less virulent bacterium, or a blood-borne (hematogenous) source. Late onset PJIs are more commonly caused by a remote infection (possibly due to an unrelated injury) that leads to hematogenous seeding of the implant surface or joint space by harmful bacteria.23 Remarkably, the species or strain(s) involved in a PJI may vary by time since surgery. For example, early and late PJIs are typically caused by virulent species (e.g., S. aureus), while delayed onset PJIs are caused by less virulent species that take longer to manifest (e.g., CoNS).23 Regardless of the timing of a PJI, treatment options generally follow the same algorithm, which results in the removal of an otherwise functional prosthesis.14
Some revisions labeled as “aseptic loosening” may instead be caused by undiagnosed, low-grade PJIs.2 Limited diagnostic techniques contribute to widely varying estimates of aseptic loosening rates;6 some reports state PJI as the cause of failure in 4–13% of revisions originally diagnosed as aseptic loosening,7,24 and patterns as high as 72% have been presented for this data mislabeling scenario.25 PCR methods can detect DNA from CoNS, Streptococcus, Salmonella, Propionibacterium, and Enterococcus species in “aseptic loosening” implants.7 Nevertheless, PCR can be prone to detecting false positives and is unlikely to accurately characterize polymicrobial PJIs.7 Other factors that could obfuscate a PJI include: biofilms, intracellular infections of peri-implant tissue, or phenotypic reductions of bacterial colony size in situ.2 Whether undetected PJIs of an implant are primarily responsible for loosening remains the subject of considerable debate,2 yet the need for preventing bacterial adhesion to orthopaedic implants is crucial for reducing all PJI-related complications.
Bacterial Adhesion to Orthopaedic Implants
According to some researchers, nearly 60% of PJIs occur during implantation procedures by known sources of pathogenic bacteria such as the patient’s skin or a contaminated surgical suite.26 Suboptimal surgical attire can also have generalizable effects on the prevalence of surgical wound infections.27 PJIs begin with bacterial adherence to the implant surface, making necessary an accurate understanding of the specific adhesion mechanisms employed by PJI-causing bacteria to prevent their establishment. Hip and knee implant surfaces are heterogeneous, with each modular component specifically designed to suit a particular function within a joint. For example, the femoral stem and acetabular cup of a hip implant are designed to promote osseointegration, and have therefore been subject to modifications in surface topography and chemistry. In contrast, the necks, liners and femoral heads of implants have a smooth composition designed to reduce friction between intercalating components.
Any prosthetic component is susceptible to microbial colonization, which can lead to full-onset PJI. One study, for example, found no significant difference in the preference of bacteria between knee and hip implant components,28 possibly due to the heterogeneous adhesion abilities of different species of bacteria. Others found that acetabular cups and polyethylene liners were most commonly infected.29,30 Although these studies demonstrated that all components of knee and hip implants can become infected, they did not address the divergent behaviors among multiple species of bacteria. This is due, at least in part, to the fact that multiple components can become infected simultaneously or asynchronously, which can be difficult to measure in vivo. Further complicating this issue is the observation that different species of bacteria may better infect specific implant components creating a heterogeneous surface mosaic of infected sites. This is evidenced by an apparent strong preference of S. epidermidis for polyethylene liners,30 which is likely due to an adhesion mechanism that increases substrate suitability.
Causative agents of PJI have a diverse arsenal of adherence mechanisms. For adherence to an inert surface, non-specific adhesion is governed primarily by molecular chemistry (e.g., van der Waals, Lewis acid/base, electrostatic, and hydrophobic forces).31–34 Some researchers postulate that non-specific adhesion between a microbe and its substrate can only be explained by the combined interaction of both weak (van der Waals) and stronger (electrostatic) forces.32,34,35 Lewis acid/base forces, caused by the coupling between electron-accepting and -donating molecules, drive the potential for adherence. Therefore, on a Lewis acidic surface that has electron-accepting potential, bacteria can more readily donate electron-pairs. This was observed in three species of rod-shaped bacteria, whereby the species with highest electron-donating capacity had greatest attachment success.32 Similarly, the charge differential (electrostatic force) between a bacterium and surface can heavily influence adhesion potential.33 Thewes et al.31 suggested that hydrophobic interactions are the most important predictor of unspecific Staphylococcus adhesion. Using single cell force spectroscopy, an attraction between hydrophobic surface and bacteria was measurable to a distance of 50 nm, while hydrophilic surfaces resulted in only weak bacterial adhesion. These results suggest that the cell wall-bound proteins of Staphylococcus have high affinities for hydrophobic surfaces,31 but the responsible proteins have yet to be identified within this context. Variables such as surface proteins and overall cell wall charge vary greatly by bacterial taxon.36 A greater understanding of such surface proteins (and other key structural constituents) are important to consider when examining specific binding profiles, and identifying strategies for preventing bacterial adhesion to orthopaedic implants.
Pili (aka. fimbriae) are cell-bound elongated structures with many functions, such as an adhesin for gram-negative bacteria.19,37 Type I and Type IV pili are each critically important for the abiotic surface adherence of Escherichia coli38,39 and Pseudomonas aeruginosa,40 respectively. Most essential though is the role of curli fibers in E. coli adherence.41,42 Of note, certain laboratory strains of E. coli could not adhere to abiotic surfaces, but continuous culture led to mutants that could adhere.43 In this way, curli become a requirement for E. coli adhesion to abiotic surfaces.44 More recently, Mauclaire et al.45 proposed curli-mediated adhesion as the reason E. coli formed more extensive biofilms than S. aureus. The presence of curli may allow E. coli to overcome repulsive charge differentials between cell and surface to facilitate adhesion.46 Anatomical structures of bacteria, such as pili and curli, which have evolved to improve their survival and reproduction, are not only important for understanding cell behavior, but also for focused attempts at preventing their adhesion to prosthetic biomaterials in clinical orthopaedic settings.
Curiously, the main causative agents of PJI: S. aureus and S. epidermidis do not have anatomic protrusions for mechanistic attachment. Instead, the cell wall enzyme AltE increases hydrophobicity of S. epidermidis, while S. aureus has teichoic acids that influence cell charge and accommodate adhesion.47,48 Staphylococcus cell wall-anchored proteins also serve an important role in abiotic adherence,49 which commonly feature positively charged amino acid residues, hydrophobic extracellular domains, and functional adhesins that result from exposed ligand-binding domains.12 Chemical interactions (outlined above) are the primary forces modulating key proteins under abiotic conditions, making necessary ex vivo experimentation and implant optimization within this context to understand virulence factors that should be manipulated to attenuate PJIs.
The immune response is activated in PJI, which does not clear the infection, but is deleterious in the joint environment. One reason for this pattern is that toxins secreted by S. aureus and other pathogens may diminish the immune response. It has been well-established that S. aureus biofilms can impair immune responses in recipients of implants, at least in part by preventing the destruction of microbes by macrophages. Phenotypic analysis of S. aureus isolated from musculoskeletal infections indicates differences in virulence50 and that S. aureus may already reside endogenously in many patients prior to PJI occurrence.51 Therefore, there is considerable interest in the development of vaccines that could specifically neutralize the virulence of common PJI-causing bacteria, such as S. aureus, to mitigate joint infection rates.52 Recent studies have elucidated potential mechanisms in S. aureus biofilms that alter macrophage activity through microbial toxins that disrupt immune responses.53 Accurate identification and characterization of PJI-causing bacteria, including additional insights into virulence factors, will ultimately improve patient-specific treatment strategies.
Among many virulence factors (e.g., evasion or suppression of the host immune response) employed by PJI-causing bacteria, adhesins allow for their survival and persistence within the joint by facilitating attachment to prosthetic materials.54 Once a prosthesis is implanted, autogenous host fluids are quickly adsorbed,12,36,49,55,56 which generates a conditioning film (e.g., albumin, fibronectin, fibrinogen, laminin).18 However, bacteria have other virulence factors that effectively regulate adhesion to conditioning films.36,49,55 For example, S. aureus and S. epidermidis adhesins specifically bind to microbial surface component recognizing adhesive matrix molecules (MSCRAMMs); a class of molecules with similar protein structures and ligand binding mechanisms.49,57 Although named for attachment to the host extracellular matrix, their functions also include binding to conditioning films49,57 via fibronectin binding proteins, collagen adhesins, clumping factors, and bone sialoprotein binding protein.58–63 Importantly, virulence factors can interfere with host cell activity, accelerate or delay PJI formation, and make treatment strategies susceptible to failure or misdiagnosis.54,64
Protein adsorption onto biomaterials may further facilitate bacterial binding in a positive feedback fashion, because denaturation exposes additional binding sites.36 The presence of specific MSCRAMMs varies among species and strains, as seen in a study involving more than 200 PJI-related isolates of S. aureus.55 Fibronectin binding proteins appear crucial for S. aureus implant adherence as one study reported that all isolates carried at least one form of fibronectin binding protein.65 This observation was supported by Arciola et al.,66 who showed an even higher prevalence of fibronectin binding proteins among S. aureus isolated from PJIs of the hip and knee.66 Fibronectin binding is not exclusive to Staphylococcus, because curli also have this ability.41,67–69 Notably, glycoproteins present in the bloodstream can also be used by mannose-binding fimbria (e.g., E. coli and P. aeruginosa possess these structures) can also bind to conditioning films.41,70 As outlined, virulent bacteria have multiple strategies for adhering to biomaterials, such as orthopaedic implants, and therefore require a multifaceted approach to preventing their attachment and progression into a PJI.
Current Methods for Preventing PJI
Recent efforts to reduce prosthetic joint infections include increased surgical suite sterility, and improved antibiotic prophylaxis.71,72 Prophylactic antibiotics used in hip and knee reconstructions target gram-positive PJIs,71 which may selectively favor gram-negative PJIs. Irrigating wounds with a broad-range antimicrobial, such as Betadine, are effective at reducing PJI rates,73,74 and widely used due to cost efficiency and availability.73 However, further strategies to diminish the evolutionary capacity of bacteria need to be investigated and delivered to the exact site of PJI establishment. In particular, the implant itself should counteract PJI induction. Biomaterial manufacturing techniques have promoted research into optimizing topographies for osseointegration,75 but such modifications could also promote bacterial colonization. By combining approaches that negate bacterial attachment and/or kill bacteria upon contact, antimicrobial orthopaedic implants have emerged (Table 2).
Table 2.
Strategies to Prevent Prosthetic Joint Infections by Improving the Antimicrobial Surface Properties of Titanium-Based Orthopaedic Implant Materials
| Method | Organism(s) | Antibacterial | System | Observation(s) | Ref |
|---|---|---|---|---|---|
| Oxidative nanopatterning of Ti | MRSA, Candida albicans | Surface topography | in vitro | S. aureus adhesion reduced; C. albicans aggregations reduced | 90 |
| Antibiotic impregnated microspheres | S. aureus | Tobramycin | in vivo | Total absence of infection; no effect on bone ingrowth | 99 |
| Covalently tethered vancomycin | S. epidermidis | Vancomycin | in vitro | Prevention of colonization and biofilm formation | 100 |
| Antiseptic dye coating | S. aureus | Gendine (chlorhexidine) | in vitro | Total prevention of bacterial adherence | 110 |
| Galvanic Cu deposition | S. aureus | Inactivate catabolic pathways | in vitro | Total clearing of adherent bacteria | 95 |
| HA-chitosan polyelectrolyte with RGD | S. aureus | Chitosan | in vitro | Adhesion reduced by 80% for 21 days | 108 |
| Antibiotic loaded hydrogel coating | S. aureus, S. epidermidis | Various antibiotics | in vitro | Inhibition of biofilm and planktonic growth | 104 |
| UV irradiation | S. aureus, S. epidermidis | Spontaneous wettability | in vitro | Bacteria adhere, not firmly attached | 85 |
| UV C irradiation | S. aureus, S. epidermidis | Increased ROS | in vitro | Bacteria killed for 60 min after UV treatment | 86 |
| Zn TiO2 | E. coli, S. aureus | ZnO mediated ROS | in vitro | Inhibition attachment | 115 |
| Zn-implanted Ti | E. coli, S. aureus | Zn ions | in vitro | Partial antibacterial effect; E. coli more inhibited than S. aureus | 116 |
| Alkali treatment | S. aureus, E. coli | Nanoroughness, increase local pH | in vitro | Bacteriostatic effect, reduced proliferation | 117 |
| Ag coating | Staphylococcus sp., Bacillus sp., Enterococcus sp., Corneybacterium | Ag ions | in vivo | Reduced infection rate | 97 |
| Material painting of N,N-dodecyl, methyl-PEI coating | S. aureus | Immobilized hydrophobic polycationic chains | both | Total absence of infection | 107 |
| Superhydrophobic TiO2 nanotube | S. aureus | Superhydrophobic surface | in vitro | Reduced adhesion | 118 |
| Mesoporous TiO2 coating | E. coli | Cephalothin controlled release | in vitro | All bacteria killed on contact | 119 |
| Photocatalytic TiO2 layer | S. aureus, S. epidermidis, P. aeruginosa | Increased ROS | in vitro | Antibacterial effect after 60 min UV treatment | 120 |
| Silk Sericin surface | S. aureus, S. epidermidis | Silk sericin | in vitro | Reduced adhesion | 121 |
| Ag-doped TiO2 nanotube | S. aureus | Ag ions | in vitro | Planktonic clearing, reduced adhesion | 91 |
Topographic Factors
Highly porous metal implants promote osseointegration,76 in part, by allowing diffusion of gases and nutrients, while providing attachment sites for bone forming host cells.77 However, pores are associated with increased surface roughness and higher surface areas, which may increase bacterial attachment.33,76–78 Topography-oriented antibacterial attachment strategies should consider the pore size and orientation of biomaterials,79 as well as micro- and macroporous features. For example, abiotic- and fibronectin-mediated adhesion are reduced by shear stress,80,81 such that the macroporous structure of some implant interfaces could shield pathogens from the natural flow of host body fluids, thereby promoting PJIs. Braem et al.82 found that implant surface roughness improves bacterial attachment, while surface hydrophilicity hinders it. Thus, engineering hydrodynamic porous implants may help retain the benefits of both improved osseointegration and resistance to bacterial adherence.
Macroscale Features
Modifications to implant surface topography can prevent bacteria from adhering, but are difficult to customize because of bacterial adhesin diversity. Anti-adhesive techniques tested in vitro have proven effective against aerial bacteria transmission or temporary breaches of surgical suite sterility.83 As an example, Ti naturally forms a thin titanium dioxide (TiO2) layer when exposed to air,84 and has an antibacterial effect from reactive oxygen species.85 An increase in reactive oxygen species activity may also be achieved by exposing materials to UV light,85,86 which kills both gram-positive and gram-negative bacteria. The TiO2 layer can also be thickened through chemical or electrolytic oxidation,87,88 allowing physical pliability of nanotopographic features and direct delivery of antibacterial agents.89–91 When used in combination with physical and chemical modifications to macroscale surface features, changes to the nanoscale topography should have a compounded resistance to PJIs.
Nanoscale Topography
Smaller scale features are also relevant to bacterial adhesion, as smooth surfaces can provide suitable substrates for many species. Indeed, Mitik-Dineva et al.92 experimented with glass surfaces: one with features >14 nm, and another that was 70% smoother, and showed 3× more bacterial adhesion on the smoother surface.92 To circumvent the challenge of modifying nanoscale surface features without causing cytotoxic changes,93 Lorenzetti et al.94 used TiO2 coatings to reduce bacterial adhesion by reducing available surface area.94 Similarly, Variola et al.90 modified Ti pores by oxidative “nanopatterning” and caused a dramatic decrease in the adhesion of both S. aureus and E. coli.90 Such nanoscale surface modifications to metal orthopaedic implants will continue to increase options for preventing bacterial adherence, particularly when used to leverage the inherent antimicrobial properties of some metals (and metal oxides).
Intrinsic Properties of Metal
Some metal ions have intrinsic antibacterial properties. For example, Burghardt et al.95 found that copper (Cu) ions were effective at removing planktonic S. aureus and biofilms, but also killed mesenchymal stem cells.95 Alternatively, silver (Ag) has a broad range of activity that includes some antibiotic resistant bacteria.91,96 Although effective at preventing PJI, early experiments with Ag ions also encountered cytotoxicity-related complications.97 Of note, Ag-coated external fixation screws caused elevated levels of Ag in human serum,97 emphasizing the importance of controlled release. While manipulating AgNO3 concentrations, Zhao et al.91 used Ti nanotubes to deliver Ag nanoparticles and kill planktonic bacteria for 30 days. To address the potential trade-off between antibacterial activity and osseointegration, sol-gel methods were used to produce a hydroxyapatite-Ag coating, which increased antibacterial activity against S. aureus and S. epidermidis without detriment to bone growth.98 Although provocative, the inherent antimicrobial properties of metals need to be further investigated and optimized to avoid damage to host cells and tissues.
Localized Delivery Vehicles
In response to the widespread use of antibiotic-loaded bone cement, researchers have examined this method of administering antibiotics directly to the site of implantation.99–101 However, drug delivery is inefficient (e.g., only 20% release), temperature sensitive (exothermic reaction of curing cement), and limited to cemented implant systems.102 In contrast, cementless porous metal implants are suited to loading with antibiotic microspheres capable of controlled drug release. Ambrose et al.99 demonstrated this using a rabbit model; gentamicin-impregnated microspheres were loaded into porous implants and completely prevented S. aureus infection without negatively influencing osseointegration.99 This technique is efficacious and applicable to any implant material with pore diameters >20 μm. However, for implant components that are meant to be smooth (e.g., acetabular cup liners), microsphere delivery of antibiotics is not suitable. A possible alternative antibiotic strategy for smooth implants and other surfaces unfitting for localized delivery would be tethering them with antibiotics directly.
Antibiotic Tethering
Antibiotics can be immobilized and covalently tethered to an implant surface, thus remaining stable indefinitely at high concentrations.103 Antoci et al.100 examined the use of vancomycin tethered to Ti surfaces and revealed that exposed concentrations of S. epidermidis were much higher than would be encountered in situ, yet colonization was significantly reduced, even in the presence of serum proteins (e.g., fibronectin). Also promising was that after 8 weeks, antibiotic resistance was not detected. Although an exciting PJI-reduction strategy, this technique lacks effectiveness against E. coli (and likely other gram-negative bacteria),100 and is limited to stable membrane-disrupting agents. But as a key component of temporal mitigation strategies that may specifically reduce the potential for long-term hematogenous PJIs, the tethering of antibiotics holds great potential, particularly in combination with broad-acting hydrogels adept at controlled release.
Hydrogels
As a way to broaden the activity range of antimicrobial treatments, hydrogels have the capability of delivering combinations of multiple antibiotics to the implant site. The basic principle is to coat the entire implant in a quickly resorbable hydrogel that administers a local antibiotic cocktail, eliminating early bacterial colonization. The advantages of the procedure are: customizable antibiotic selection, maintained dosages above minimum inhibitory concentration, application to implants that are already in use, and resorption of the hydrogel without impediment to osseointegration.104 Although impregnation is effective, the overuse of antibiotics may further exacerbate antibiotic resistant PJIs (e.g., Enterobacteriaceae), as for the commonly used Carbapenem.21,105,106 Furthermore, polymicrobial PJIs are more difficult to treat using hydrogels because drug-resistance is conferred in situ from one species to another via horizontal gene transfer.105 A strategy that minimizes horizontal gene transfer would prevent the future generation of drug-resistance, while broad-spectrum antimicrobials will diminish the overall number of PJI occurrences.
Broad-Spectrum Antimicrobials
Considering the limitations of specific antibiotics, broad-spectrum antimicrobials such as N, N dodecyl, methyl-polyethylenimine (NNDMP), chitosan, and Gendine are becoming more relevant. Schaer et al.107 used in vitro models to demonstrate the inhibition of biofilms, and treated sheep with NNDMP-coated fracture plates to validate bone healing and antimicrobial activity after inoculation with S. aureus.107 Similarly, Chua et al.108 combined chitosan with arginine-glycine-aspartic acid to increase antimicrobial activity, promote osteoblast proliferation, and minimize detrimental effects on host bone.108 Coating implants with the aseptic dye Gendine also has broad range antimicrobial effects,109 and inhibits S. aureus adherence in vivo.110 Broad-spectrum antimicrobials are generally favorable because they are effective against both gram-positive and gram-negative bacteria, yet the mechanical delivery of superficial coating materials may require additional engineering to ensure adequate delivery to the implant-bone interface and localized retention within the site(s) of PJI establishment.
Mechanical Considerations
Beyond considerations for anti-microbial properties and/or strategies that promote osseointegration, it is important to note that implant coatings used to achieve this (whether biological or inorganic) may need to withstand significant mechanical forces during surgical insertion. Long-term or short-term losses of common coatings (e.g.,111) caused by shear forces and/or physical abrasion of the implant could compromise the desired delivery of antibiotics or cell-based enhancements. It is to be anticipated that infection-protective surface layers on implants will not entirely delaminate during surgery or that solutions could be engineered to minimize loss of such coatings.
CONCLUSION
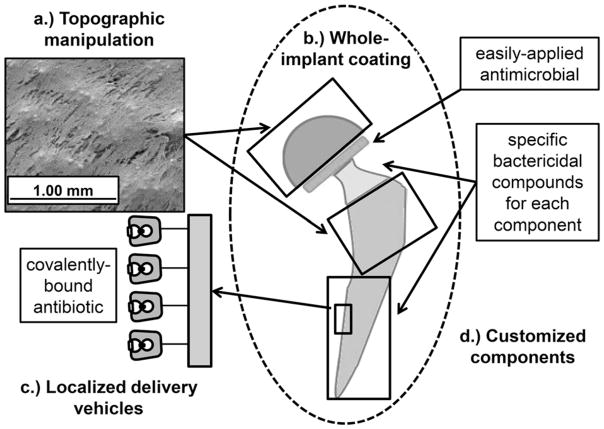
PJIs are not only costly, but potentially devastating to a patients’ joint function. The main challenges with treating PJIs are related to the taxonomic diversity of causative bacteria, and the increasing prevalence of polymicrobial and antibiotic-resistant bacteria. Suboptimal antibiotic delivery strategies can negatively affect patient host cells, reduce osseointegration, and compound the problem of diminished joint functionality. Nevertheless, some approaches have led to encouraging results, such as whole-implant coating with a generalized antibacterial. Antibiotic coating materials, which target a specific taxon of bacteria or implant component, will also continue to prove necessary. Ultimately, future strategies for preventing PJIs will require multifaceted and multidisciplinary approaches that leverage advanced physical and chemical techniques to both disrupt bacterial attachment, and kill bacteria by contact over short, intermediate and extended time scales (Fig. 1). Information obtained by ex vivo experimentation should be used to direct in vivo eradications of the most common and harmful bacteria species by exploiting their individual vulnerabilities. Customized, patient-based strategies that prevent PJIs will not only reduce the need for costly revision arthroplasty, but also will result in fewer patients that lose mobility.
Figure 1.
Conceptual schematic of a hip implant depicting multiple strategies that could be used in combination for the prevention of prosthetic joint infections. The strategies illustrated are: (a) topographic manipulations to improve osseointegration and minimize bacterial adhesion (e.g., scanning electron microscopy shows a porous implant surface covered by osteoblast-like mesenchymal cells), (b) whole implant coating with a generalized antibiotic (e.g., N,N-dodecyl methyl polyethylenimine), (c) localized antibiotic delivery vehicles (e.g., tethering implant surface with covalently bound antibacterial agents), and (d) customized components (e.g., specific antibiotic method designed for each implant component) to reduce susceptibility to PJIs.
Acknowledgments
Grant sponsor: National Institute of Arthritis and Musculoskeletal Skin Diseases; Grant numbers: R01 AR049069, F32 AR066508, T32 AR56950.
We thank our colleagues of the van Wijnen, Westendorf, Yaszemski, Bishop, Lerman and Dietz laboratories for stimulating discussions relevant to the topic of this review. Dr. Michael Stuart is acknowledged for administrative support of Matthew Getzlaf during training at Mayo Clinic. The Core facilities at Mayo Clinic continually provide support and assistance with various projects that help refine our research priorities. Additionally, the Reconstructive Research and Development group at Stryker Orthopedics, (particularly Brent Mitchell, Dale Swarts, and Mark Gruczynski) have provided useful feedback on currently available orthopaedic biomaterials. This work was supported by NIH grants from National Institute of Arthritis and Musculo-skeletal and Skin Diseases: R01 AR049069 (to AvW), F32 AR066508 (to AD), and T32 AR56950 (to EAL). Intramural funds were also provided by the Center for Regenerative Medicine at Mayo Clinic (to RT). We also appreciate the generous philanthropic support of William H. and Karen J. Eby, and the charitable foundation in their name. Mr. Robert Cohen serves as the Vice-President and General Manager of Stryker Research and Development. Outside the submitted work, Dr. David Lewallen reports personal fees and other from Stryker, Pipeline Biomedical, Zimmer, and Ketai Medical Devices. In addition, Dr. David Lewallen has patents on selected hip and knee implants with royalties paid by Zimmer, and is employed part time as the Medical Director for The American Joint Replacement registry. None of the other authors have competing interests to disclose. We thank the two anonymous reviewers who provided recommendations on how to improve this manuscript.
Footnotes
AUTHORS’ CONTRIBUTIONS
All listed authors approve of this manuscript and provided intellectual input. The primary literature search was completed by MAG, EAL, and DLJ. HMK, CAB, AD, RT, RCC, DGL and AvW consulted with the conceptual development of the narrative. Tables and Figures were assembled by MAG, DLJ and EAL. EAL, HMK, CAB, AD, RT, DGL and AvW edited the figures and final text. EAL responded to the reviewer’s comments.
References
- 1.Fehring TK, Odum S, Griffin WL, et al. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315–318. doi: 10.1097/00003086-200111000-00041. [DOI] [PubMed] [Google Scholar]
- 2.Nelson CL, McLaren AC, McLaren SG, et al. Is aseptic loosening truly aseptic? Clin Orthop Relat Res. 2005:25–30. doi: 10.1097/01.blo.0000175715.68624.3d. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi AV, Jr, Berend KR, Adams JB. Why knee replacements fail in 2013: patient, surgeon, or implant? Bone Joint J. 2014;96B:101–104. doi: 10.1302/0301-620X.96B11.34350. [DOI] [PubMed] [Google Scholar]
- 4.Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28:116–119. doi: 10.1016/j.arth.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 5.Philpott A, Weston-Simons JS, Grammatopoulos G, et al. Predictive outcomes of revision total hip replacement — a consecutive series of 1176 patients with a minimum 10-year follow-up. Maturitas. 2014;77:185–190. doi: 10.1016/j.maturitas.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Moojen DJ, Spijkers SN, Schot CS, et al. Identification of orthopaedic infections using broad-range polymerase chain reaction and reverse line blot hybridization. J Bone Joint Surg Am Vol. 2007;89:1298–1305. doi: 10.2106/JBJS.F.00822. [DOI] [PubMed] [Google Scholar]
- 7.Moojen DJ, van Hellemondt G, Vogely HC, et al. Incidence of low-grade infection in aseptic loosening of total hip arthroplasty. Acta Orthop. 2010;81:667–673. doi: 10.3109/17453674.2010.525201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribera A, Morata L, Moranas J, et al. Clinical and microbiological findings in prosthetic joint replacement due to aseptic loosening. J Infect. 2014;69:235–243. doi: 10.1016/j.jinf.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6:1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz SM, Lau E, Watson H, et al. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61–5. e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Bedair H, Goyal N, Dietz MJ, et al. A history of treated periprosthetic joint infection increases the risk of subsequent different site infection. Clin Orthop Relat Res. 2015;473:2300–2304. doi: 10.1007/s11999-015-4174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowry F. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 13.Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27:2331–2339. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerli WT, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 15.Benito N, Franco M, Coll P, et al. Etiology of surgical site infections after primary total joint arthroplasties. J Orthop Res. 2014;32:633–637. doi: 10.1002/jor.22581. [DOI] [PubMed] [Google Scholar]
- 16.Ghigo JM. Natural conjugative plasmids induce bacterial biofilm development. Nature. 2001;412:442–445. doi: 10.1038/35086581. [DOI] [PubMed] [Google Scholar]
- 17.Hausner M, Wuertz S. High rates of conjugation in bacterial biofilms as determined by quantitiative in situ analysis. Appl Environ Microbiol. 1999;65:3710–3713. doi: 10.1128/aem.65.8.3710-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donlan R. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunne WM. Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev. 2002;15:155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li YH, Lau PC, Lee JH, et al. Natural genetic transformation of Streptococcus mutans growing in biofilms. J Bacteriol. 2001;183:897–908. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Citorik RJ, Mimee M, Lu TK. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat Biotechnol. 2014;32:1141–1145. doi: 10.1038/nbt.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamagni T, Elgohari S, Harrington P. Trends in surgical site infections following orthopaedic surgery. Curr Opin Infect Dis. 2015;28:125–132. doi: 10.1097/QCO.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 23.Trampuz A, Zimmerli W. Antimicrobial agents in orthopaedic surgery: prophylaxis and treatment. Drugs. 2006;66:1089–1105. doi: 10.2165/00003495-200666080-00005. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi N, Procop GW, Krebs V, et al. Molecular identification of bacteria from aseptically loose implants. Clin Orthop Relat Res. 2008;466:1716–1725. doi: 10.1007/s11999-008-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunney M, Patrick S, Curran M, et al. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;37:3281–3290. doi: 10.1128/jcm.37.10.3281-3290.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Z, Borgwardt L, Hoiby N, et al. Prosthesis infections after orthopedic joint replacement: the possible role of bacterial biofilms. Orthop Rev. 2013;5:65–71. doi: 10.4081/or.2013.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salassa TE, Swiontkowski MF. Surgical attire and the operating room: role in infection prevention. J Bone Joint Surg Am Vol. 2014;96:1485–1492. doi: 10.2106/JBJS.M.01133. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Barrena E, Esteban J, Medel F, et al. Bacterial adherence to separated modular components in joint prosthesis: a clinical study. J Orthop Res. 2012;30:1634–1639. doi: 10.1002/jor.22114. [DOI] [PubMed] [Google Scholar]
- 29.Holinka J, Bauer L, Hirschl AM, et al. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res. 2011;29:617–622. doi: 10.1002/jor.21286. [DOI] [PubMed] [Google Scholar]
- 30.Lass R, Giurea A, Kubista B, et al. Bacterial adherence to different components of total hip prosthesis in patients with prosthetic joint infection. Int Orthop. 2014;38:1597–1602. doi: 10.1007/s00264-014-2358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thewes N, Loskill P, Jung P, et al. Hydrophobic interaction governs unspecific adhesion of staphylococci: a single cell force spectroscopy study. Beilstein J Nanotechnol. 2014;5:1501–1512. doi: 10.3762/bjnano.5.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao Y, Subramaniam PK, Tawfiq K, et al. Microbial biofouling: a mechanistic investigation. J Adhes Sci Technol. 2012;25:2155–2168. [Google Scholar]
- 33.Badihi Hauslich L, Sela MN, Steinberg D, et al. The adhesion of oral bacteria to modified titanium surfaces: role of plasma proteins and electrostatic forces. Clin Oral Implants Res. 2013;24:49–56. doi: 10.1111/j.1600-0501.2011.02364.x. [DOI] [PubMed] [Google Scholar]
- 34.van Oss CJ. Long-range and short-range mechanisms of hydrophobic attraction and hydrophilic repulsion in specific and aspecific interactions. J Mol Recognit. 2003;16:177–190. doi: 10.1002/jmr.618. [DOI] [PubMed] [Google Scholar]
- 35.Katiskogianni M, Missirlis Y. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. 2004;8:37–57. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- 36.Boland T, Latour R, Stutzenberger F. Molecular basis of bacterial adhesion. In: An YH, Friedman RJ, editors. Handbook of bacterial adhesion: Principles, methods, and applications. Totowa: Humana; 2000. pp. 29–41. [Google Scholar]
- 37.Hori K, Matsumoto S. Bacterial adhesion: from mechanism to control. Biochem Eng J. 2010;48:424–434. [Google Scholar]
- 38.Nasciment H, Silva L, Souza R, et al. Phenotypic and genotypic characteristics associated with biofilm formation in clinical isolates of atypical enteropathogenic Escherichia coli (aEPEC) strains. BMC Microbiol. 2014;14:184. doi: 10.1186/1471-2180-14-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratt L, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 40.O’Toole G, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 41.Barnhart MM, Chapman MR. Curli biogenesis and function. Annu Rev Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawar D, Rossman M, Chen J. Role of curli fimbriae in mediating the cells of enterohaemorrhagic Escherichia coli to attach to abiotic surfaces. J Appl Microbiol. 2005;99:418–425. doi: 10.1111/j.1365-2672.2005.02499.x. [DOI] [PubMed] [Google Scholar]
- 43.Vidal O, Longin R, Prigent-Combaret C, et al. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvment of a new ompR allele that increases curli expression. J Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prigent-Combaret C, Prensier G, Thuy Le Thi T, et al. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environ Microbiol. 2000;2:450–464. doi: 10.1046/j.1462-2920.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 45.Mauclaire L, Brombacher E, Bunger JD, et al. Factors controlling bacterial attachment and biofilm formation on medium-chain-length polyhydroxyalkanoates (mcl-PHAs) Colloids Surf B Biointerfaces. 2010;76:104–111. doi: 10.1016/j.colsurfb.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Beloin C, Houry A, Froment M, et al. A short-time scale colloidal system reveals early bacterial adhesion dynamics. PLoS Biol. 2008;6:e167. doi: 10.1371/journal.pbio.0060167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heilmann C, Hussain M, Peters G, et al. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 48.Gross M, Cramton SE, Gotz F, et al. Key role of teichoic acid net charge in staphylococcus aureus colonization of artificial surfaces. Infect Immun. 2001;69:3423–3426. doi: 10.1128/IAI.69.5.3423-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foster TJ, Geoghegan JA, Ganesh VK, et al. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Post V, Wahl P, Uckay I, et al. Phenotypic and genotypic characterisation of Staphylococcus aureus causing musculoskeletal infections. Int J Med Microbiol. 2014;304:565–576. doi: 10.1016/j.ijmm.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Luedicke C, Slickers P, Ehricht R, et al. Molecular fingerprinting of Staphylococcus aureus from bone and joint infections. Eur J Clin Microbiol Infect Dis. 2010;29:457–463. doi: 10.1007/s10096-010-0884-4. [DOI] [PubMed] [Google Scholar]
- 52.Brady RA, Mocca CP, Prabhakara R, et al. Evaluation of genetically inactivated alpha toxin for protection in multiple mouse models of staphylococcus aureus infection. PloS ONE. 2013;8:e63040. doi: 10.1371/journal.pone.0063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scherr TD, Hanke ML, Huang O, et al. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin ab and alpha-toxin. mBio. 2015;6:e01021–15. doi: 10.1128/mBio.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thammavongsa V, Kim HK, Missiakas D, et al. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. 2015;13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montanaro L, Speziale P, Campoccia D, et al. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol. 2011;6:1329–1349. doi: 10.2217/fmb.11.117. [DOI] [PubMed] [Google Scholar]
- 56.Vacheethasanee K, Temenoff J, Higashi J, et al. Bacterial surface properties of clinically isolated Staphylococcus epidermidis strains determin adhesion on polyethylene. J Biomed Mater Res. 1998;42:425–432. doi: 10.1002/(sici)1097-4636(19981205)42:3<425::aid-jbm12>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 57.Ponnuraj K, Bowden MG, Davis S, et al. A “dock, lock, and latch” structural model for a Staphylococcal adhesin binding to fibrinogen. Cell. 2003;115:217–228. doi: 10.1016/s0092-8674(03)00809-2. [DOI] [PubMed] [Google Scholar]
- 58.Burke FM, Di Poto A, Speziale P, et al. The A domain of fibronectin-binding protein B of Staphylococcus aureus contains a novel fibronectin binding site. FEBS J. 2011;278:2359–2371. doi: 10.1111/j.1742-4658.2011.08159.x. [DOI] [PubMed] [Google Scholar]
- 59.Ganesh VK, Barbu EM, Deivanayagam CC, et al. Structural and biochemical characterization of Staphylococcus aureus clumping factor B/ligand interactions. J Biol Chem. 2011;286:25963–25972. doi: 10.1074/jbc.M110.217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ganesh VK, Rivera JJ, Smeds E, et al. A structural model of the Staphylococcus aureus ClfA-fibrinogen interaction opens new avenues for the design of anti-staphylococcal therapeutics. PLoS Pathogens. 2008;4:e1000226. doi: 10.1371/journal.ppat.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nekhotiaeva N, Awasthi SK, Nielsen PE, et al. Inhibition of Staphylococcus aureus gene expression and growth using antisense peptide nucleic acids. Mol Ther. 2004;10:652–659. doi: 10.1016/j.ymthe.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Vazquez V, Liang X, Horndahl JK, et al. Fibrinogen is a ligand for the Staphylococcus aureus microbial surface components recognizing adhesive matrix molecules (MSCRAMM) bone sialoprotein-binding protein (Bbp) J Biol Chem. 2011;286:29797–29805. doi: 10.1074/jbc.M110.214981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zong Y, Xu Y, Liang X, et al. A ‘Collagen Hug’ model for Staphylococcus Aureus CNA binding to collagen. EMBO J. 2005;42:4224–4236. doi: 10.1038/sj.emboj.7600888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hansen EN, Zmistowski B, Parvizi J. Periprosthetic joint infection: what is on the horizon? Int J Artif Organs. 2012;35:935–950. doi: 10.5301/ijao.5000145. [DOI] [PubMed] [Google Scholar]
- 65.Peacock SJ, Day NP, Thomas MG, et al. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J Infect. 2000;41:23–31. doi: 10.1053/jinf.2000.0657. [DOI] [PubMed] [Google Scholar]
- 66.Arciola CRA, Campoccia YH, Donati D, et al. Etiology of implant orthopedic infections: a survey on 1027 clinical isolates. Int J Artif Organs. 2005;28:1091–1100. doi: 10.1177/039139880502801106. [DOI] [PubMed] [Google Scholar]
- 67.Bian Z, Brauner A, Li Y, et al. Expression of an cytokine activation by Escherichia coli curli fibers in human sepsis. J Infect Dis. 2000;181:602–612. doi: 10.1086/315233. [DOI] [PubMed] [Google Scholar]
- 68.Gophna U, Oelschlaeger T, Hacker J, et al. Role of fibronectin in curli-mediated internalization. FEMS Microbiol Lett. 2002;212:55–58. doi: 10.1111/j.1574-6968.2002.tb11244.x. [DOI] [PubMed] [Google Scholar]
- 69.Olsen A, Herwald H, Wikstrom M, et al. Identification of two protein-binding and functional regions of curli, a surface organelle and virulence determinant of Escherichia coli. J Biol Chem. 2002;277:34568–34572. doi: 10.1074/jbc.M206353200. [DOI] [PubMed] [Google Scholar]
- 70.Davis J, Freeze H. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta. 2001;1528:116–126. doi: 10.1016/s0304-4165(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 71.Prokuski L. Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg. 2008;16:283–293. doi: 10.5435/00124635-200805000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Strachan C. The prevention of orthopaedic implant and vascular graft infections. J Hosp Infect. 1995;30:54–63. doi: 10.1016/0195-6701(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 73.Brown NM, Cipriano CA, Moric M, et al. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27:27–30. doi: 10.1016/j.arth.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 74.Cheng MT, Chang MC, Wang ST, et al. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine. 2005;30:1689–1693. doi: 10.1097/01.brs.0000171907.60775.85. [DOI] [PubMed] [Google Scholar]
- 75.Lewallen EA, Riester SM, Bonin CA, et al. Biological strategies for improved osseointegration and osteoinduction of porous metal orthopedic implants. Tissue Eng Part B Rev. 2015;21:218–230. doi: 10.1089/ten.teb.2014.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braem A, Van Mellaert L, Mattheys T, et al. Staphylococcal biofilm growth on smooth and porous titanium coatings for biomedical applications. J Biomed Mater Res Part A. 2013;102A:215–224. doi: 10.1002/jbm.a.34688. [DOI] [PubMed] [Google Scholar]
- 77.Otsuki B, Takemoto M, Fujibayashi S, et al. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials. 2006;27:5892–5900. doi: 10.1016/j.biomaterials.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 78.Wu Y, Zitelli JP, TenHuisen KS, et al. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials. 2011;32:951–960. doi: 10.1016/j.biomaterials.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Whitehead KA, Colligon J, Verran J. Retention of microbial cells in substratum surface features of micrometer and sub-micrometer dimensions. Colloids Surf B Biointerfaces. 2005;41:129–138. doi: 10.1016/j.colsurfb.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 80.Reddy K, Ross JM. Shear stress prevents fibronectin binding protein-mediated Staphylococcus aureus adhesion to resting endothelial cells. Infect Immun. 2001;69:3472–3475. doi: 10.1128/IAI.69.5.3472-3475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Subbiahdoss G, Kuijer R, Grijpma DW, et al. Microbial biofilm growth vs. tissue integration: “the race for the surface” experimentally studied. Acta biomaterialia. 2009;5:1399–1404. doi: 10.1016/j.actbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Braem A, De Cremer K, Delattin N, et al. Novel anti-infective implant substrates: controlled release of antibiofilm compounds from mesoporous silica-containing macroporous titanium. Colloids Surf B Biointerfaces. 2015;126:481–488. doi: 10.1016/j.colsurfb.2014.12.054. [DOI] [PubMed] [Google Scholar]
- 83.Gallo J, Holinka M, Moucha CS. Antibacterial surface treatment for orthopaedic implants. Int J Mol Sci. 2014;15:13849–13880. doi: 10.3390/ijms150813849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao H, Liu X. Activating titanium oxide coatings for orthopedic implants. Surf Coat Tech. 2013;233:57–64. [Google Scholar]
- 85.Gallardo-Moreno AM, Pacha-Olivenza MA, Saldana L, et al. In vitro biocompatibility and bacterial adhesion of physico-chemically modified Ti6Al4V surface by means of UV irradiation. Acta Biomater. 2009;5:181–192. doi: 10.1016/j.actbio.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 86.Gallardo-Moreno AM, Pacha-Olivenza MA, Fernandez-Claderon MC, et al. Bactericidal behaviour of Ti6Al4V surfaces after exposure to UV-C light. Biomaterials. 2010;31:5159–5168. doi: 10.1016/j.biomaterials.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Yi J-H, Bernard C, Variola F, et al. Characterization of a bioactive nanotextured surface created by controlled chemical oxidation of titanium. Surface Science. 2006;600:4613–4621. [Google Scholar]
- 88.Sowa M, Piotrowska M, Widziolek M, et al. Bioactivity of coatings formed on Ti-13Nb-13Zr alloy using plasma electrolytic oxidation. Mater Sci Eng C Mater Biol Appl. 2015;49:159–173. doi: 10.1016/j.msec.2014.12.073. [DOI] [PubMed] [Google Scholar]
- 89.Variola F, Brunski J, Orsini G, et al. Nanoscale surface modifications of medically relevant metals: state-of-the art and perspectives. Nanoscale. 2010;3:335–353. doi: 10.1039/c0nr00485e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Variola F, Zalzal SF, Leduc A, et al. Oxidative nanopatterning of titanium generates mesoporous surfaces with antimicrobial properties. Int J Nanomed. 2014;9:2319–2325. doi: 10.2147/IJN.S61333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao L, Wang H, Huo K, et al. Antibacterial nano-structured titania coating incorporated with silver nano-particles. Biomaterials. 2011;32:5706–5716. doi: 10.1016/j.biomaterials.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 92.Mitik-Dineva N, Wang J, Mocanasu RC, et al. Impact of nano-topography on bacterial attachment. Biotechnol J. 2008;3:536–544. doi: 10.1002/biot.200700244. [DOI] [PubMed] [Google Scholar]
- 93.Anselme K, Davidson P, Popa AM, et al. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010;6:3824–3846. doi: 10.1016/j.actbio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Lorenzetti M, Dogsa I, Stosicki T, et al. The influence of surface modification on bacterial adhesion to titanium-based substrates. ACS Appl Mater Interfaces. 2015;7:1644–1651. doi: 10.1021/am507148n. [DOI] [PubMed] [Google Scholar]
- 95.Burghardt I, Luthen F, Prinz C, et al. A dual function of copper in designing regenerative implants. Biomaterials. 2015;44:36–44. doi: 10.1016/j.biomaterials.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 96.Feng Q, Wu J, Chen G, et al. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 97.Masse A, Bosetti BA, Biasibetti A, et al. Prevention of pin track infection in external fixation with silver coated pins: clinical and microbiological results. J Appl Biomater. 2000;53:600–604. doi: 10.1002/1097-4636(200009)53:5<600::aid-jbm21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 98.Chen W, Oh S, Ong AP, et al. Antibacterial and osteogenic properties of silver-containing hydroxyapatite coatings produced using a sol gel process. J Biomed Mater Res Part A. 2007;82:899–906. doi: 10.1002/jbm.a.31197. [DOI] [PubMed] [Google Scholar]
- 99.Ambrose CG, Clyburn TA, Mika J, et al. Evaluation of antibiotic-impregnated microspheres for the prevention of implant-associated orthopaedic infections. J Bone Joint Surg Am volume. 2014;96:128–134. doi: 10.2106/JBJS.L.01750. [DOI] [PubMed] [Google Scholar]
- 100.Antoci V, Jr, Adams CS, Parvizi J, et al. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials. 2008;29:4684–4690. doi: 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64:1165–1176. doi: 10.1016/j.addr.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang BG, Myers DE, Wallace GG, et al. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int J Mol Sci. 2014;15:11878–11921. doi: 10.3390/ijms150711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shapiro IM, Hickok NJ, Parvizi J, et al. Molecular engineering of an orthopaedic implant: from bench to bedside. Eur Cell Mater. 2012;23:362–370. doi: 10.22203/ecm.v023a28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Drago L, Boot W, Dimas K, et al. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin Orthop Relat Res. 2014;472:3311–3323. doi: 10.1007/s11999-014-3558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peleg A, Hooper D. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18:263–272. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 107.Schaer TP, Stewart S, Hsu BB, et al. Hydrophobic polycationic coatings that inhibit biofilms and support bone healing during infection. Biomaterials. 2012;33:245–1254. doi: 10.1016/j.biomaterials.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 108.Chua PH, Neoh KG, Kang ET, et al. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials. 2008;29:1412–1421. doi: 10.1016/j.biomaterials.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 109.Chaiban G, Hanna H, Dvorak T, et al. A rapid method of impregnating endotracheal tubes and urinary catheters with gendine: a novel antiseptic agent. J Antimicrob Chemother. 2005;55:51–56. doi: 10.1093/jac/dkh499. [DOI] [PubMed] [Google Scholar]
- 110.Bahna P, Dvorak T, Hanna H, et al. Orthopaedic metal devices coated with a novel antiseptic dye for the prevention of bacterial infections. Int J Antimicrob Agents. 2007;29:593–596. doi: 10.1016/j.ijantimicag.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 111.Dunne CF, Gibbons J, FitzPatrick DP, et al. On the fate of particles liberated from hydroxyapatite coatings in vivo. Ir J Med Sci. 2015;184:125–133. doi: 10.1007/s11845-014-1243-8. [DOI] [PubMed] [Google Scholar]
- 112.Sharma D, Douglas J, Coulter C, et al. Microbiology of infected arthroplasty: implications for empiric perioperative antibiotics. J Orthop Surg. 2008;16:339–342. doi: 10.1177/230949900801600314. [DOI] [PubMed] [Google Scholar]
- 113.Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peel TN, Cheng AC, Buising KL, et al. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56:2386–2391. doi: 10.1128/AAC.06246-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu H, Zhang W, Qiao Y, et al. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012;8:904–915. doi: 10.1016/j.actbio.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 116.Jin G, Cao H, Qiao Y, et al. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf B Biointerfaces. 2014;117:158–165. doi: 10.1016/j.colsurfb.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 117.Li J, Wang G, Wang D, et al. Alkali-treated titanium selectively regulating biological behaviors of bacteria, cancer cells and mesenchymal stem cells. J Colloid Interface Sci. 2014;436:160–170. doi: 10.1016/j.jcis.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 118.Tang P, Zhang W, Wang Y, et al. Effect of super-hydrophobic surface of titanium on staphylococcus aureus adhesion. J Nanomater. 2011;2011:1–8. [Google Scholar]
- 119.Xia W, Grandfield K, Hoess A, et al. Mesoporous titanium dioxide coating for metallic implants. J Biomed Mater Res. 2012;100:82–93. doi: 10.1002/jbm.b.31925. [DOI] [PubMed] [Google Scholar]
- 120.Zaborowska M, Welch K, Branemark R, et al. Bacteria-material surface interactions: methodological development for the assessment of implant surface induced antibacterial effects. J Biomed Mater Res. 2015;103:179–187. doi: 10.1002/jbm.b.33179. [DOI] [PubMed] [Google Scholar]
- 121.Zhang F, Zhang Z, Zhu X, et al. Silk-functionalized titanium surfaces for enhancing osteoblast functions and reducing bacterial adhesion. Biomaterials. 2008;29:4751–4759. doi: 10.1016/j.biomaterials.2008.08.043. [DOI] [PubMed] [Google Scholar]