Abstract
Advances in genome sequencing and gene discovery have created opportunities to efficiently assess more genetic conditions than ever before. Given the large number of conditions that can be screened, the implementation of expanded carrier screening using genome sequencing will require practical methods of simplifying decisions about the conditions for which patients want to be screened. One method to simplify decision making is to generate a taxonomy based on expert judgment. However, expert perceptions of condition attributes used to classify these conditions may differ from those used by patients. To understand whether expert and patient perceptions differ, we asked women who had received preconception genetic carrier screening in the last 3 years to fill out a survey to rate the attributes (predictability, controllability, visibility, and severity) of several autosomal recessive or X-linked genetic conditions. These conditions were classified into one of five taxonomy categories developed by subject experts (significantly shortened lifespan, serious medical problems, mild medical problems, unpredictable medical outcomes, and adult-onset conditions). A total of 193 women provided 739 usable ratings across 20 conditions. The mean ratings and correlations demonstrated that participants made distinctions across both attributes and categories. Aggregated mean attribute ratings across categories demonstrated logical consistency between the key features of each attribute and category, although participants perceived little difference between the mild and serious categories. This study provides empirical evidence for the validity of our proposed taxonomy, which will simplify patient decisions for results they would like to receive from preconception carrier screening via genome sequencing.
Keywords: Gene carrier testing, genome sequencing, genetics, patient perceptions, genetic condition taxonomy
INTRODUCTION
Genetic carrier screening is routinely offered to average risk women for a small number of conditions, such as cystic fibrosis [American College of Medicine Genetics Board of [Directors 2004] and beta-thalassemia [Cao et al. 2002; Cousens et al. 2010], and offered in high-risk populations for conditions such as Tay-Sachs disease [Kaback 2001], Canavan disease, and familial dysautonomia. Rapid advances in genomic sequencing technologies may soon expand screening to a genome-wide scale and inform people about their carrier status for over 1000 autosomal recessive or X-linked conditions. The availability of commercial panels (e.g., Counsyl, Good Start, Pathway Genomics) that test for hundreds of known genetic variants or conditions is accelerating the debate on the utility of carrier screening on a wider scale, and sequencing for this purpose may become economically feasible. Even if assessing carrier status is not the primary purpose of sequencing, findings about carrier status can be made available.
The challenges and benefits of revising the current approach to carrier screening — by expanding carrier screening to include more conditions or by reporting carrier risks in the general population of individuals — must be thoughtfully considered. Currently, most carrier screening is targeted to high-risk individuals in response to the birth of an affected child to a near relative or to individuals with a high risk based on ancestry, and testing is focused on a small number of conditions. However, most genetic conditions are relatively rare, and the vast majority of people are not aware of them or have no first-hand experience with families affected by these conditions.
Thus, an important challenge of expanding carrier screening, in terms of the number of genetic conditions and types of populations, is how to communicate information about the test results of many conditions that could be part of the testing in a way that facilitates effective decision making by laboratories, health care providers, and patients alike. The American College of Medical Genetics guidelines on expanded carrier screening panels [Grody et al. 2013] suggests that patients should be able to opt in to receive results for conditions with mild or variable expression and to opt out of receiving results for high penetrance conditions. The genetic conditions may have technical names and their expression may be variable, making it difficult to describe their features to laypersons. For these reasons, it is challenging to consider how patients would decide what kinds of results they would want to receive from a significantly expanded carrier screening program.
Our team is conducting a trial of using genome sequencing for preconception carrier screening as part of the Clinical Sequencing Exploratory Research (CSER) program funded by the National Human Genome Research Institute of the National Institute of Health. Patients desire choice in terms of what results they would like to receive, but there are three major barriers to making these types of decisions: 1) the sheer number of conditions from which to choose, 2) most conditions are so rare that patients are not familiar with them, and 3) it is not feasible to describe each and every condition to patients in a way they would understand [Schneider et al. 2015]. To address the challenge of simplifying patient decisions about screening using genome or exome sequencing while preserving patient choice, we identified a range of genetic conditions and developed five taxonomy categories that might be meaningful to both patients and experts. This taxonomy was developed to provide a simple tool to allow patients to select among several categories of genetic conditions they would want to learn about as part of preconception reproductive planning. The taxonomy accomplishes this by reducing the number of decisions that would be made by asking a participant to choose from a small and manageable set of categories instead of among hundreds of conditions, and by describing the categories in terms that are familiar and understandable by patients.
We used input from experts on our study team and from lay focus group participants to develop a taxonomy that categorized conditions based on their likely impact on affected offspring: significantly shortened lifespan, serious medical problems, mild medical problems, unpredictable medical outcomes, and adult-onset conditions [Korngeibel et al. (submitted as a companion paper to this journal)]. All five categories have some face validity, but none is supported by empirical data from patients’ mental models on how genetic conditions should be categorized or how the attributes or likely impacts of different genetic conditions affect patients’ perceptions of the risk for having them. Other attributes of genetic conditions could influence patients’ desire for certain aspects of preconception screening, including the types of impairments associated with the conditions (e.g., physical, sensory, cognitive), how noticeable the effects of the conditions would be, how expensive it would be to raise a child with a given condition, or how undesirable it would be to have a child affected by a given condition.
Any decision aid that truly facilitates patient decision-making about expanded carrier screening will reflect the attributes of conditions that drive a patient’s decisions about whether the results are worth knowing. Risk is a useful heuristic for understanding whether people want to act to avoid potential hazards. Risk appraisals involve both rational and affective processes [Slovic et al. 2005]. Expert assessment of risk in genetics relies heavily on quantitative characteristics, such as probabilities of occurrence, mortality estimates, and costs. In contrast layperson appraisals of risk in the context of illness and other social and technological hazards are often driven by affective characteristics of risk, such as the dread and uncertainty associated with the hazard and the perceived extent to which the risk can be controlled [Loewenstein et al. 2001; Shepperd et al. 2013; Slovic 1999; Slovic 2000; Slovic et al. 1979; Zikmund-Fisher et al. 2010]. The relative importance of these affective characteristics can vary across people and hazards. Thus, layperson perceptions of risk often differ from expert perceptions, and it is important to understand what these differences are. Three decades of research into perceptions of social and technological hazards have honed methods to illuminate legitimate, multi-faceted, qualitative and affective considerations about risk that are typically omitted from expert assessments [Kraus and Slovik 1988; Slovic 2000; Slovic et al. 1980]. Our primary objective in this study was to use such methods to evaluate the qualitative dimensions involved in patients’ assessments of the risks associated with a variety of genetic conditions. We expected the data generated by the study participants to provide empirical support for the validity of the expert-developed taxonomy as a decision-aid for preconception carrier screening that would greatly improve the ability of patients to make informed and satisfactory decisions about the results they would like to receive.
METHODS
Initial Selection of Characteristics of Genetic Conditions
We convened a panel with expertise in genetic counseling, medical genetics, risk perception, anthropology, biostatistics, and bioethics to select a manageable subsample of genetic conditions to be rated by survey participants from the long list of conditions that are potentially identifiable through genome sequencing. By their nature, genetic conditions are multidimensional and, because we wanted to ensure that our sample included variability across a spectrum of conditions, we decided to specify those characteristics, rate various conditions according to those characteristics, and then sample from those conditions such that all of the characteristics were adequately represented.
The expert panel developed a list of 13 characteristics upon which to rate genetic conditions (Table I). Next, the genetic counselors and physicians on the panel independently nominated genetic conditions as examples of each characteristic at various levels. For instance, Wilson disease, Tay-Sachs disease, and carnitine palmitoyltransferase 1A (CPT 1A) deficiency were deemed good examples, respectively, of genetic conditions resulting in a mild level of physical impairment, shortened lifespan, and maternal risk during pregnancy. No attempt was made to consider more than one characteristic at a time or to exhaustively characterize any one genetic condition. Rather, the goal was simply to identify a sample of conditions from across the spectrum of possibilities that could, collectively, represent exemplars of the various characteristics of conditions that we believed would drive layperson perceptions of risk and impact of genetic conditions. Nominations were reviewed by the entire expert panel. Wherever possible, conflicting judgments were resolved by discussion; where resolution was not possible, the condition was excluded. We selected 20 genetic conditions for inclusion in our investigation.
Table I.
Characteristics of Genetic Conditions Identified by Expert Panel
| Characteristic | Categories |
|---|---|
| Physical impairment | Mild; moderate; high |
| Cognitive impairment | Mild; moderate; high |
| Sensory impairment | Mild; moderate; high |
| Chronic pain | Mild; moderate-severe |
| Requirements of daily care | Low; moderate; high |
| Healthcare system interaction | Low; moderate; high |
| Effectiveness of treatment | Low; moderate; high |
| Care costs | Low; moderate; high |
| Lifespan | In years: 0–6; 7–20; 21–40; 41+; no effect |
| Infertility | Male; female |
| Age of onset | In years: 0–12; 13–21; 22+ |
| Maternal risk during pregnancy | Yes; no |
| Variability of expression | Yes; no |
Development of Taxonomy Categories
These 20 conditions were also categorized into the five categories used in our taxonomy: shortened lifespan, serious medical problems, mild medical problems, adult-onset conditions, and unpredictable medical problems (Table II). Our study used a return of results committee (RORC) — a panel of specialists in clinical and laboratory genetics, pediatrics, genetic counseling, and obstetrics; and were a separate group from the expert panel — to categorize individual conditions into the taxonomy. More information about the development of the categories and their definitions can be found in Korngeibel et al. (submitted as a companion paper to this journal).
Table II.
Description of taxonomy categories.
| Taxonomy Category | Description |
|---|---|
| Shortened Lifespan | Most children do not live past early childhood, even with medical interventions. |
| Serious Medical Problems | Most children will have medical problems that require regular medical visits, daily medications, carefully monitored diets, or surgeries; or will have serious problems with learning, vision, hearing or mobility. |
| Mild Medical Problems | Most children will have medical problems that require occasional extra medical visits, occasional medications, a slightly modified diet, or surgery; or will have mild problems with learning, vision, hearing, or mobility. |
| Unpredictable Medical Outcomes | The outcome is difficult to predict for many children, where some children will have more serious versions but others will have more mild versions or no problems at all. |
| Adult Onset Conditions | Few have any symptoms as children, but medical, behavioral, vision, or hearing problems may begin as adults. |
Genetic Condition Descriptions
The expert panel then developed descriptions for the 20 genetic conditions. We began with information available on the Counsyl website (www.counsyl.com) during the summer of 2013. As a first pass, we modified these descriptions by removing statements about probability (e.g., “…affects roughly 1 in 50,000…”) as well as ethnic group susceptibilities (e.g., “…the condition is particularly common among people of Finnish and Saudi Arabian descent”), because we did not want these elements to influence the judgments of survey participants (e.g., if the respondent does not have the ethnic background mentioned in the description, then they may be less likely to be invested in the description). We also removed mechanistic information about the condition (e.g., “the pancreas normally secretes insulin in response to rising blood sugar”) and, for the sake of parity, we ensured that each condition name appeared only once in its description. Following this step, the text describing each condition was organized by (a) the nature of the condition, (b) how the condition is treated, and (c) what someone with this condition might expect to happen over the life course. Then, each condition description was reviewed and edited by members of the expert panel, individually and collectively. Essential information was described consistently for each condition, and added to the description by genetic counselors if it was absent from the Counsyl description. The study team ensured that the exemplary characteristics of each condition were clearly communicated within the text for each condition. No formal or informal testing of these descriptions (e.g., language level or clinical comprehensiveness) was undertaken. A complete set of the condition descriptions used for this study can be found in the online supplemental materials.
Survey Instrument
We developed a web-based survey to evaluate laypersons’ perceptions regarding the perceived attributes of a genetic condition, their feelings about it, and the effect of knowledge of an elevated risk for having a child with that condition would have on their pregnancy plan. Adults with a near-term interest in undergoing carrier screening for reproductive planning were recruited for this survey. We collected data on participants’ age and education level. To limit respondent burden, five versions of the survey were prepared, each covering four of the 20 selected genetic conditions. The conditions were distributed across surveys to be balanced in the familiarity and severity of the conditions presented. The conditions included in each version of the survey, as well as the order in which the conditions were presented, are provided in the online supplemental materials.
During the online survey, the participant was presented with descriptions of four conditions, one at a time, after which they responded to 20 questions using a 7-point Likert scale ranging from strongly disagree (1) to strongly agree (7). The participant was asked to rate four attributes for each condition: controllability (for example, by treatment, diet, education, or avoiding things like cigarettes or exposure to the sun), predictability, visibility, and severity. These four attributes represent the critical dimensions that likely most influence patient perceptions of risk, and with the exception of visibility were also essential characteristics used by the RORC in classifying the conditions into the taxonomy. The remaining 16 items focused on how the respondent would think she would feel if she had a child with this condition (e.g., anxious, angry), her desire and responsibility to have knowledge regarding genetic risk, and intent to change plans for pregnancy based on a low or high increased likelihood of having a child with the condition. Finally, we asked the participant for an overall rating of the severity of the impact resulting from having a child with that condition and how well she understood the condition as described in the survey.
The survey questions were identical for each genetic condition. The text of the survey instrument is available in the online supplemental materials.
Survey Pilot Testing
The online survey was pilot tested in August 2013 by members of our study team and a convenience sample of adults unaffiliated with the study. We obtained and incorporated feedback about the survey instructions, condition descriptions, survey questions, online features of the survey instrument (e.g., navigation), and time required to complete the survey.
Data Collection
A total of 1,500 adult female Kaiser Permanente Northwest (KPNW) members (300 for each version of the survey) who had received preconception genetic testing in the preceding 3 years (generally for cystic fibrosis screening) were recruited for the study via e-mail. The five versions of the survey were randomly assigned to participants. Surveys were closed when 40 completed responses had been collected for a given version or after 5 days online, whichever came first. Most of the responses (>75%) were received within 48 hours of the survey being made available. Some versions had a slight over-quota because respondents were not turned away if they happened to be actively working on the questions when the 40th completed survey was submitted.
Participants who answered the questions for all four genetic conditions in their survey were compensated with a $10 gift card. The study was approved by the KPNW Institutional Review Board.
RESULTS
Data Cleaning
We obtained 812 condition ratings from survey participants. We excluded ratings if (1) responses to any of the 20 questions within a condition were missing (n=16), (2) there was little variance across the responses given all items within a condition (standard deviation <0.50; n=6), (3) the survey was filled out in <8 minutes, with the presumption that the condition description and items were not read fully (cutoff was based on pilot testing of the time required to go through the survey; n=37), and (4) the participant indicated that they did not understand the condition description by responding strongly disagree, disagree, slightly disagree, or neither agree or disagree to the first item (“The description of this condition was understandable”; n=14). The final sample sizes consisted of 193 participants and 739 condition ratings. Because demographic characteristics are collected on KPNW members, we were able to compare those participants included in the analysis to the entire sample that were sent survey invitations on age, ethnicity, and race to determine whether there was any response bias. There were no significant differences on age (mean difference=0.6 years, 95% CI [−0.2, 1.3] or race (χ2(1)=0.08, p=0.77). There were fewer Hispanic persons included in the analysis (3.9%) compared to the entire potential sample (9.6%), χ2(1)=4.41, p=0.04, but this difference was small and would not be significant if adjusted for multiple testing.
Demographic Characteristics
Seventy four (38%) respondents were between the ages of 30–34, 51 (26%) respondents were between 35–39 years, 29 (15%) respondents were between 40–44 years, 25 (13%) respondents were between 25–29 years and the remaining 14 (7%) chose not to answer or were adults outside the age range of 25–44 years. Eighty-four (44%) respondents reported having a graduate degree, 63 (33%) a bachelor’s degree, 34 (18%) some college or vocational school, and the remainder chose not to answer (4%) or had high school or GED (3%).
Descriptive Statistics
The means and standard deviations for each item by each of the 20 genetic conditions are presented in Table III. Respondents regarded Tay-Sachs disease (acute infantile form) and spinal muscular atrophy as the least controllable, highly predictable and visible, and the most severe conditions. Wilson disease was judged to be the most controllable, Gaucher disease type I the least predictable, alpha1-antitrypsin deficiency the least visible, and alkaptonuria the least severe. Of the four attributes, the controllable attribute had the most variability in ratings across participants.
Table III.
Genetic Condition Attribute Scores, by Taxonomy Category (n = 739 ratings)
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Controllable | Predictable | Visible | Severe | ||||||
|
| |||||||||
| M | SD | M | SD | M | SD | M | SD | ||
| Shortened lifespan | Spinal muscular atrophy | 1.76 | 1.84 | 5.21 | 1.56 | 6.47 | 1.36 | 6.71 | 1.34 |
| Tay-Sachs disease (acute infantile form) | 1.89 | 1.41 | 5.97 | 1.32 | 6.35 | 0.64 | 6.68 | 0.82 | |
|
| |||||||||
| Serious medical problems | Cohen syndrome | 3.73 | 1.26 | 4.78 | 1.07 | 6.22 | 0.82 | 5.05 | 1.06 |
| Bloom syndrome | 4.39 | 1.49 | 4.68 | 1.28 | 6.32 | 1.19 | 6.13 | 1.13 | |
| Duchenne muscular dystrophy | 2.72 | 1.60 | 4.79 | 1.71 | 6.18 | 1.48 | 6.41 | 1.01 | |
| Usher syndrome type 1F | 2.94 | 1.70 | 5.11 | 1.19 | 5.60 | 0.70 | 5.17 | 0.81 | |
| Phenylketonuria | 5.12 | 1.75 | 4.85 | 1.18 | 5.38 | 1.03 | 6.12 | 1.25 | |
| Wilson disease | 5.92 | 1.03 | 4.89 | 1.47 | 4.53 | 1.30 | 4.58 | 1.05 | |
| Galactosemia | 5.76 | 1.07 | 5.21 | 1.09 | 4.59 | 1.40 | 5.06 | 1.25 | |
| Cystic fibrosis | 4.12 | 1.19 | 4.53 | 0.93 | 3.82 | 0.56 | 5.79 | 1.38 | |
| Classic hemophilia type A | 4.75 | 0.84 | 4.83 | 1.12 | 4.05 | 1.46 | 6.00 | 1.40 | |
| CPT IA deficiency* | 5.86 | 0.68 | 5.39 | 1.02 | 3.47 | 1.59 | 5.06 | 1.49 | |
|
| |||||||||
| Mild medical problems | Hypohidrotic ectodermal dysplasia | 5.54 | 1.65 | 5.57 | 1.33 | 6.51 | 1.66 | 5.35 | 1.17 |
| Ataxia with vitamin E deficiency | 5.62 | 1.77 | 4.69 | 1.29 | 5.85 | 1.41 | 5.41 | 1.36 | |
| Achromotopsia | 3.85 | 1.33 | 5.10 | 1.65 | 4.90 | 1.57 | 4.69 | 1.53 | |
|
| |||||||||
| Unpredictable medical outcomes | Fanconi anemia C | 2.59 | 1.70 | 3.26 | 1.28 | 4.06 | 1.46 | 6.12 | 1.01 |
|
| |||||||||
| Adult-onset conditions | Alkaptonuria | 4.68 | 1.82 | 5.38 | 1.58 | 5.41 | 1.80 | 1.95 | 0.88 |
| Alpha1-antitrypsin deficiency | 5.50 | 1.33 | 4.33 | 1.59 | 3.28 | 0.99 | 4.78 | 1.41 | |
| Hereditary hemochromatosis | 5.63 | 1.17 | 4.50 | 1.61 | 3.95 | 0.60 | 2.65 | 0.65 | |
| Gaucher disease type I | 3.87 | 1.88 | 2.69 | 1.32 | 3.33 | 0.63 | 4.82 | 1.51 | |
In practice, CPT IA deficiency falls in the shortened lifespan category because it is potentially life threatening to the mother carrying a child with this condition. However, because of the potential impact to the mother, the Return of Results Committee considered it important that all participants receive this information.
The correlations among the attribute ratings are presented in Table IV. The correlations ranged from extremely low (r=.01 for severity and predictability) to moderate (r=−.31 for severity and controllability). The small correlations suggest that respondents perceived genetic conditions as having distinct combinations of attributes (controllable, predictable, visible, and severe).
Table IV.
Means, standard deviations, and Pearson correlations among participants attribute ratings of condition characteristics
| Controllable | Predictable | Visible | Severe | |
|---|---|---|---|---|
| Controllable | 4.31 (1.96) | |||
| Predictable | .10* | 4.79 (1.52) | ||
| Visible | −.19* | .30* | 5.02 (1.66) | |
| Severe | −.31* | .01 | .22* | 5.22 (1.68) |
p<.01
Means and (standard deviations) are provided in the diagonal.
Primary Analysis
A profile plot of the five categories mapped over the four attributes of primary interest is presented in Figure 1. This plot does not depict any temporal relationship between the data points; rather, the graph is intended to show whether the attributes that survey respondents ascribe to the conditions in each of our taxonomy categories are similar or different. With the exception of the serious and mild categories, the profiles of the five genetic condition categories were distinctive. The mild and serious conditions had a similar profile, although the serious conditions were rated as slightly more uncontrollable, unpredictable, severe, and slightly less visible. Participants rated conditions in the shortened lifespan category as the most uncontrollable, visible, and severe and the least unpredictable. The sole condition in the unpredictable category, Fanconi anemia C, was rated the highest for being the most unpredictable and highly uncontrollable compared with the other categories. Finally, the conditions in the adult-onset category were rated as the least severe compared with conditions in the four other categories.
Figure 1. Profile Plot of mean aggregate participant ratings of the characteristics (controllability, predictability, severity, and visibility) for conditions in each taxonomy category.
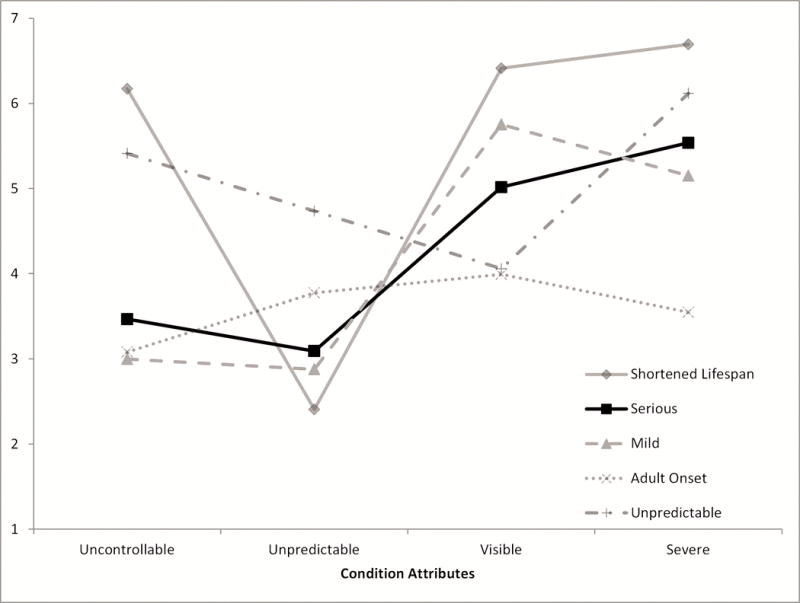
Conditions were categorized as described in Table III.
Figure 2 presents the same data represented in Figure 1 using individual bar charts, with the addition of 95% confidence intervals for the aggregated mean ratings. Based on the width of the confidence intervals, the adult onset category appeared to have the most variability in ratings. The five categories show clearly distinct patterns across the attributes, even allowing for the variation in responses. As noted previously, the observed patterns are most similar for the mild and severe categories.
Figure 2. Bar charts of mean and 95% confidence intervals for aggregate participant ratings of the characteristics (controllability, predictability, severity, and visibility) for conditions in each taxonomy category.
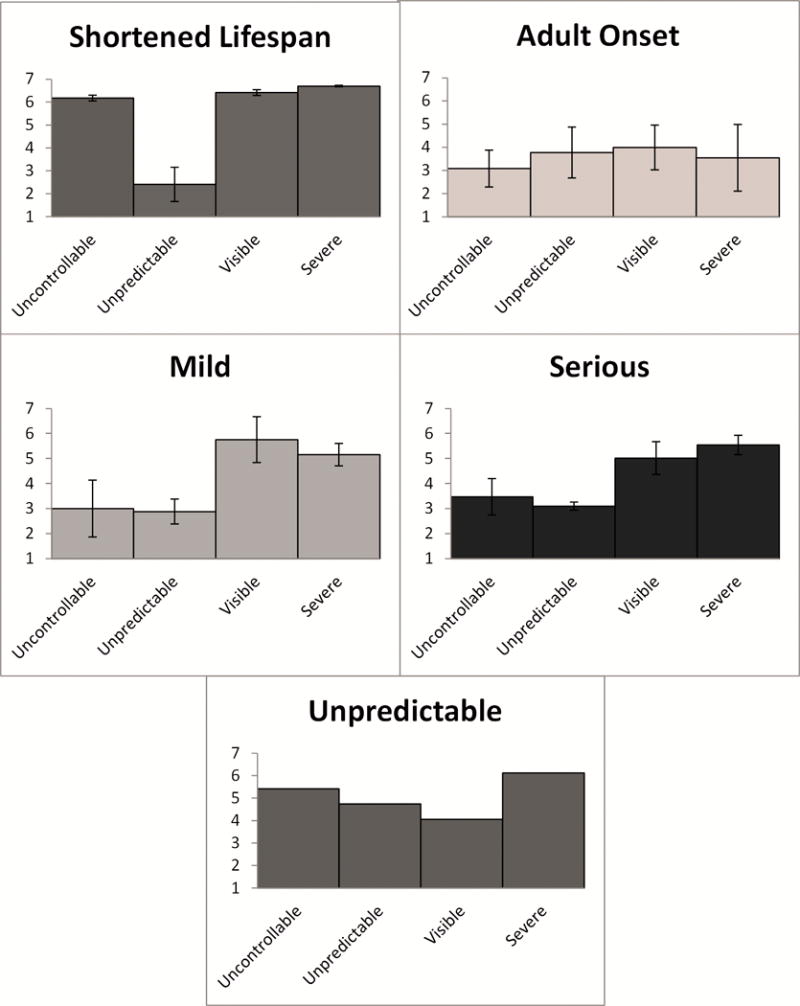
Conditions were categorized as described in Table III.
Note: Unpredictable category does not have 95% CI because it contains only one condition.
DISCUSSION
Our primary objective in this study was to empirically validate an expert-derived taxonomy as a decision-aid for preconception carrier screening using participant ratings of critical attributes involved in making assessments of overall risk. As a decision-aid, the taxonomy promotes patient autonomy by providing prospective parents with the means by which they can to make informed decisions about their carrier status by selecting categories of information they wish to receive. Overall, participants were able to differentiate among genetic conditions by key attributes (controllability, predictability, visibility, and severity), as evident by the low correlations among attributes and the differences in mean ratings for each condition. These results suggest that we presented complicated information to patients in a way that allowed them to make meaningful distinctions among the conditions. Furthermore, the five-category taxonomy developed by our experts to classify genetic conditions seemed to align well with participants’ attribute ratings. For example, the condition classified by the RORC in the variable expression category was also the condition rated by participants as the least predictable and controllable. The agreement in expert and lay perceptions lends confidence to the validity of our taxonomy for classifying genetic conditions.
Participants had difficulty in distinguishing between mild and serious medical problems. During its deliberations, the RORC itself had the most trouble with classifying conditions into these two categories. Thus, creating a break in the severity continuum between mild and serious is challenging because the appraisal of severity may be more complex than we anticipated; especially if many conditions fall on the boundary between what is considered mild and serious. If that is the case, we will need to refine the criteria for serious and mild conditions.
This preliminary but empirical validation of our taxonomy as a tool for distinguishing meaningful categories of genetic conditions from both lay and expert perspectives was developed from a deliberate, iterative process of negotiating meaningful categories that reflect how potential users of this decision aid (patients, clinicians, and laboratory personnel) construe the effects of various types of genetic conditions (see companion paper).
Our taxonomy reflects the historical trajectory of the development of genetic testing and corresponds to the ongoing policy debates related to the expansion of the range of conditions for which genetic testing may be offered. Conditions that are significantly life shortening during childhood were the first targets of preconception and prenatal genetic testing in the 1970s. Over time, testing has expanded to a broader range of conditions that are medical involved but in whom children are expected to become adults. The policy controversies, in the context of prenatal testing and pediatric testing, focus on the expansion to conditions that are mild [Cox 2004; Silver and Warren 2006; Waalen and Beutler 2009], involve impairments such as those related to hearing or vision, do not manifest themselves until adulthood, or highly unpredictable in outcome. These uses are more controversial because the importance of such information is highly contingent on individual patient values [Wilfond and Goddard 2015]. Some people will want this information and others will not. Thus, our categorization of genetic conditions allows individuals to obtain information about the group of conditions that matter to them while avoiding information that would not be important to them. Offering choice for the results from genomic sequencing is also supported by a recent survey on the attitudes of genetics professionals, where 81% responded that patient preferences should guide the return of results [Yu et al. 2014]. Existing experience provides support for the broad categories that have been defined in this taxonomy.
Our current project is intended to provide prospective parents with additional information about their reproductive risks. However, carrier results may be secondary findings from clinical sequencing done for other reasons. The approach developed here could be applied to carrier results that are secondary findings. Additional empirical data about patient preferences in this context will be needed to assess the utility of this approach.
Limitations
The population we drew our sample from was predominantly of European Ancestry and all were insured, so the generalizability of the agreement between lay and expert ratings of genetic conditions may not hold in other populations. Nevertheless, KPNW members are generally representative of the Portland, Oregon area’s demographics and include about 20% of the area’s total population. Future work may include a more nationally representative sample and other ethnic and racial groups. Future studies should also evaluate men’s perceptions of genetic conditions.
Our sample was drawn from a population that represents the likely future users of carrier screening using genomic sequencing, as the study participants had recently received preconception carrier screening. Additional research should examine the perceptions of condition attributes and return of results effects of people affected by genetic conditions or their family members, as they may perceive attributes very differently than someone without knowledge of these conditions. Given the greater urgency and more limited options available in the prenatal context, it may be worthwhile to compare the perceptions for those undergoing preconception carrier screening versus prenatal carrier testing.
Only 20 of many possible genetic conditions were rated in this exercise, and some categories included more conditions than others. We suggest that future research include more conditions and ensure they are more representatively balanced across all categories. We could not do that in the current study because the development of the taxonomy occurred simultaneously with the development of the survey materials. Classification by experts of additional conditions into categories and the gathering of expert ratings on their attributes and comparing them to patient ratings may provide further insight into the perceptions of risk and impact that can drive decision-making about obtaining carrier screening results.
The descriptions of the conditions that were used in the survey were brief and were not meant to capture the nuances and breadth of each condition. However, we may need to develop more meaningful and accurate information in these descriptions. For instance, we were surprised by how high phenylketonuria was rated on severity by the study participants, and suspect that the condition was weighed too heavily towards the description of the untreated phenotype and did not adequately portray the experience of a treated individual. Also, despite our best efforts to write the descriptions as factual and descriptive to minimize bias in participant ratings, it is possible that the descriptions were still inherently or subliminally directive. For this reason, we include the full text given to participants in the online supplemental materials so that readers may evaluate the descriptions themselves.
A final limitation of our study was that these evaluations of selected condition descriptions were hypothetical in that participants were not going to be tested for the conditions that were rated. Our ongoing trial, which is part of the CSER consortium, is examining the patient experience with genome sequencing for preconception carrier screening. We will record patient preferences for the categories of results they choose to receive for carrier screening as well as their satisfaction and regret with these decisions immediately after receiving the results and 3 months later. We will also examine the behaviors and reactions to the information that is returned to participants from genome sequencing as well as the downstream effects of and utilization from learning this information. The trial will provide greater insight into actual participant experiences, and we will gain a better understanding of participant preferences and decisions around carrier screening. All participants in this CSER study will have recently received preconception carrier screening as part of their usual medical care and thus will have a near term interest in learning and using this information for reproductive decision-making.
Conclusion
Genetic conditions rated by participants are logically consistent with the classification of conditions made by an expert panel using a newly created taxonomy of autosomal recessive and X-linked conditions. The results provide empirical evidence for the utility of the taxonomy for patients in making informed decisions on what categories of conditions they want to learn about for reproductive decision-making. In developing a taxonomy and empirically validating it in other clinical contexts where patients face an array of genetic testing options, both expert and lay perceptions should be considered to account for potential differences in their perspectives.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Human Genome Research Institute (UM1HG007292; co-PIs: Wilfond, Goddard), with additional support from the Coordinating Center (U01HG007307; PI: Jarvik) and the NextMed Study (U01HG006375; PI: Jarvik) as part of the Clinical Sequencing Exploratory Research (CSER) consortium. The authors would like to thank the other members of the study team for their many useful insights and discussion about the study.
Footnotes
None of the authors has a conflict of interest to declare.
References
- American College Board of Directors. ACMG statement on direct-to-consumer genetic testing. Genet Med. 2004;6(1):60. doi: 10.1097/01.GIM.0000106164.59722.CE. [DOI] [PubMed] [Google Scholar]
- Cao A, Rosatelli MC, Monni G, Galanello R. Screening for thalassemia: a model of success. Obstet Gynecol Clin North Am. 2002;29(2):305–328. vi–vii. doi: 10.1016/s0889-8545(01)00006-7. [DOI] [PubMed] [Google Scholar]
- Cousens NE, Gaff CL, Metcalfe SA, Delatycki MB. Carrier screening for beta-thalassaemia: a review of international practice. Eur J Hum Genet. 2010;18(10):1077–1083. doi: 10.1038/ejhg.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DW. Prenatal diagnosis for alpha1-antitrypsin deficiency. Prenat Diagn. 2004;24(6):468–470. doi: 10.1002/pd.901. [DOI] [PubMed] [Google Scholar]
- Grody WW, Thompson BH, Gregg AR, Bean LH, Monaghan KG, Schneider A, Lebo RV. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15(6):482–483. doi: 10.1038/gim.2013.47. [DOI] [PubMed] [Google Scholar]
- Kaback MM. Screening and prevention in Tay-Sachs disease: origins, update, and impact. Adv Genet. 2001;44:253–265. doi: 10.1016/s0065-2660(01)44084-3. [DOI] [PubMed] [Google Scholar]
- Kraus N, Slovik P. Taxonomic analysis of perceived risk: Modeling individual and group perceptions within homogeneous hazard domains. Risk Anal. 1988;8(3):435. [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull. 2001;127(2):267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- Schneider JL, Goddard KA, Davis J, Wilfond B, Kauffman TL, Reiss JA, Gilmore M, Himes P, Lynch FL, Leo MC, McMullen C. “Is It Worth Knowing?” Focus Group Participants’ Perceived Utility of Genomic Preconception Carrier Screening. J Genet Couns. 2015 doi: 10.1007/s10897-015-9851-7. Epub 2015 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd JA, Lipkus IM, Sanderson SC, McBride CM, O’Neill SC, Docherty S. Testing different communication formats on responses to imagined risk of having versus missing the GSTM1 gene. J Health Commun. 2013;18(1):124–137. doi: 10.1080/10810730.2012.688245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RM, Warren JE. Preconception counseling for women with thrombophilia. Clin Obstet Gynecol. 2006;49(4):906–919. doi: 10.1097/01.grf.0000211959.53498.a4. [DOI] [PubMed] [Google Scholar]
- Slovic P. Trust, emotion, sex, politics, and science: surveying the risk-assessment battlefield. Risk Anal. 1999;19(4):689–701. doi: 10.1023/a:1007041821623. [DOI] [PubMed] [Google Scholar]
- Slovic P. The Perception of Risk. London: Earthscan Publications Ltd; 2000. [Google Scholar]
- Slovic P, Fischhoff B, Lichtenstein S. Rating the risks. Environment. 1979;21(3):14–20. 36–39. [Google Scholar]
- Slovic P, Fischhoff B, Lichtenstein S. Facts and Fears: Understanding Perceived Risk. In: Schwing RC, Albers WA Jr, editors. Societal Risk Assessment: How Safe is Safe Enough? New York: Plenum; 1980. [Google Scholar]
- Slovic P, Peters E, Finucane ML, Macgregor DG. Affect, risk, and decision making. Health Psychol. 2005;24(4 Suppl):S35–40. doi: 10.1037/0278-6133.24.4.S35. [DOI] [PubMed] [Google Scholar]
- Waalen J, Beutler E. Genetic screening for low-penetrance variants in protein-coding genes. Annu Rev Genomics Hum Genet. 2009;10:431–450. doi: 10.1146/annurev.genom.9.081307.164255. [DOI] [PubMed] [Google Scholar]
- Wilfond BS, Goddard KA. It’s complicated: criteria for policy decisions for the clinical integration of genome-scale sequencing for reproductive decision making. Mol Genet Genomic Med. 2015;3(4):239–242. doi: 10.1002/mgg3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JH, Harrell TM, Jamal SM, Tabor HK, Bamshad MJ. Attitudes of genetics professionals toward the return of incidental results from exome and whole-genome sequencing. Am J Hum Genet. 2014;95(1):77–84. doi: 10.1016/j.ajhg.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Risky feelings: why a 6% risk of cancer does not always feel like 6% Patient Educ Couns. 2010;81(Suppl):S87–93. doi: 10.1016/j.pec.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


