Abstract
An isothermal in vitro DNA amplification method was developed based upon the following sequence of reaction events. Restriction enzyme cleavage and subsequent heat denaturation of a DNA sample generates two single-stranded target DNA fragments (T1 and T2). Present in excess are two DNA amplification primers (P1 and P2). The 3' end of P1 binds to the 3' end of T1, forming a duplex with 5' overhangs. Likewise, P2 binds to T2. The 5' overhangs of P1 and P2 contain a recognition sequence (5'-GTTGAC-3') for the restriction enzyme HincII. An exonuclease-deficient form of the large fragment of Escherichia coli DNA polymerase I (exo- Klenow polymerase) [Derbyshire, V., Freemont, P. S., Sanderson, M. R., Beese, L., Friedman, J. M., Joyce, C. M. & Steitz, T. A. (1988) Science 240, 199-201] extends the 3' ends of the duplexes using dGTP, dCTP, TTP, and deoxyadenosine 5'-[alpha-thio]triphosphate, which produces hemiphosphorothioate recognition sites on P1.T1 and P2.T2. HincII nicks the unprotected primer strands of the hemiphosphorothioate recognition sites, leaving intact the modified complementary strands. The exo- Klenow polymerase extends the 3' end at the nick on P1.T1 and displaces the downstream strand that is functionally equivalent to T2. Likewise, extension at the nick on P2.T2 results in displacement of a downstream strand functionally equivalent to T1. Nicking and polymerization/displacement steps cycle continuously on P1.T1 and P2.T2 because extension at a nick regenerates a nickable HincII recognition site. Target amplification is exponential because strands displaced from P1.T1 serve as targets for P2 and strands displaced from P2.T2 serve as targets for P1. A 10(6)-fold amplification of a genomic sequence from Mycobacterium tuberculosis or Mycobacterium bovis was achieved in 4 h at 37 degrees C.
Full text
PDF
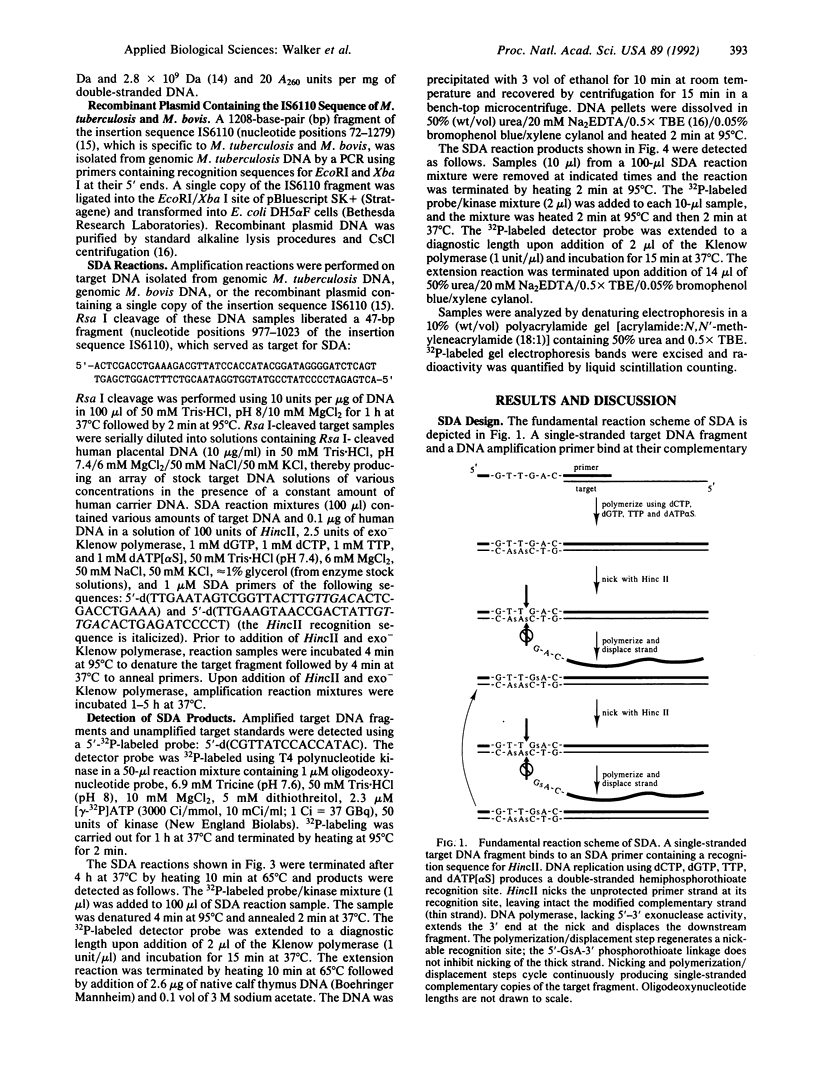

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barany F. Genetic disease detection and DNA amplification using cloned thermostable ligase. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):189–193. doi: 10.1073/pnas.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barringer K. J., Orgel L., Wahl G., Gingeras T. R. Blunt-end and single-strand ligations by Escherichia coli ligase: influence on an in vitro amplification scheme. Gene. 1990 Apr 30;89(1):117–122. doi: 10.1016/0378-1119(90)90213-b. [DOI] [PubMed] [Google Scholar]
- Bebenek K., Joyce C. M., Fitzgerald M. P., Kunkel T. A. The fidelity of DNA synthesis catalyzed by derivatives of Escherichia coli DNA polymerase I. J Biol Chem. 1990 Aug 15;265(23):13878–13887. [PubMed] [Google Scholar]
- Bradley S. G. Relationships among mycobacteria and nocardiae based upon deoxyribonucleic acid reassociation. J Bacteriol. 1973 Feb;113(2):645–651. doi: 10.1128/jb.113.2.645-651.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave M. D., Eisenach K. D., McDermott P. F., Bates J. H., Crawford J. T. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol Cell Probes. 1991 Feb;5(1):73–80. doi: 10.1016/0890-8508(91)90040-q. [DOI] [PubMed] [Google Scholar]
- Daniell H., Torres-Ruiz J. A., Inamdar A., McFadden B. A. Amplified expression of ribulose bisphosphate carboxylase/oxygenase in pBR322-transformants of Anacystis nidulans. Arch Microbiol. 1989;151(1):59–64. doi: 10.1007/BF00444670. [DOI] [PubMed] [Google Scholar]
- Derbyshire V., Freemont P. S., Sanderson M. R., Beese L., Friedman J. M., Joyce C. M., Steitz T. A. Genetic and crystallographic studies of the 3',5'-exonucleolytic site of DNA polymerase I. Science. 1988 Apr 8;240(4849):199–201. doi: 10.1126/science.2832946. [DOI] [PubMed] [Google Scholar]
- Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990 May;161(5):977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Erlich H. A., Gelfand D., Sninsky J. J. Recent advances in the polymerase chain reaction. Science. 1991 Jun 21;252(5013):1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- Guatelli J. C., Whitfield K. M., Kwoh D. Y., Barringer K. J., Richman D. D., Gingeras T. R. Isothermal, in vitro amplification of nucleic acids by a multienzyme reaction modeled after retroviral replication. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1874–1878. doi: 10.1073/pnas.87.5.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Eckstein F., Mildvan A. S., Koplitz R. M., Loeb L. A. Deoxynucleoside [1-thio]triphosphates prevent proofreading during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6734–6738. doi: 10.1073/pnas.78.11.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwoh D. Y., Davis G. R., Whitfield K. M., Chappelle H. L., DiMichele L. J., Gingeras T. R. Transcription-based amplification system and detection of amplified human immunodeficiency virus type 1 with a bead-based sandwich hybridization format. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1173–1177. doi: 10.1073/pnas.86.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwoh D. Y., Kwoh T. J. Target amplification systems in nucleic acid-based diagnostic approaches. Am Biotechnol Lab. 1990 Oct;8(13):14–25. [PubMed] [Google Scholar]
- Nakamaye K. L., Gish G., Eckstein F., Vosberg H. P. Direct sequencing of polymerase chain reaction amplified DNA fragments through the incorporation of deoxynucleoside alpha-thiotriphosphates. Nucleic Acids Res. 1988 Nov 11;16(21):9947–9959. doi: 10.1093/nar/16.21.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
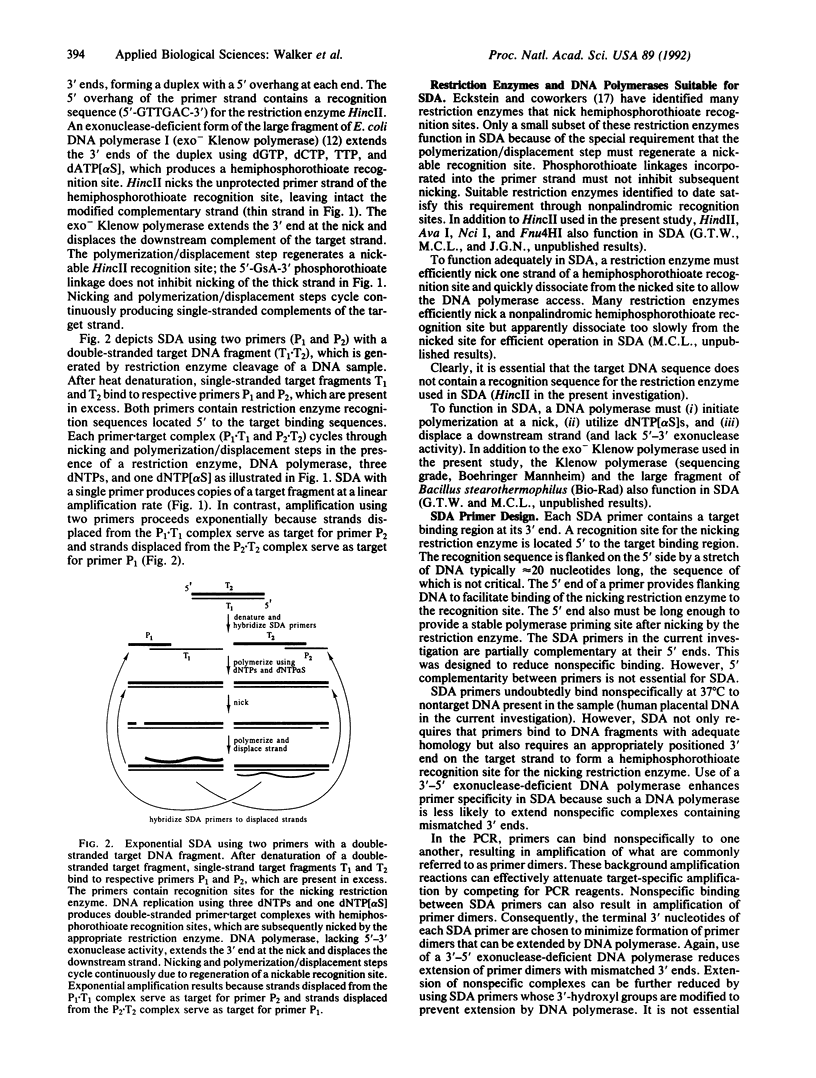
- Olsen D. B., Kotzorek G., Eckstein F. Investigation of the inhibitory role of phosphorothioate internucleotidic linkages on the catalytic activity of the restriction endonuclease EcoRV. Biochemistry. 1990 Oct 16;29(41):9546–9551. doi: 10.1021/bi00493a008. [DOI] [PubMed] [Google Scholar]
- Pierre C., Lecossier D., Boussougant Y., Bocart D., Joly V., Yeni P., Hance A. J. Use of a reamplification protocol improves sensitivity of detection of Mycobacterium tuberculosis in clinical samples by amplification of DNA. J Clin Microbiol. 1991 Apr;29(4):712–717. doi: 10.1128/jcm.29.4.712-717.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Thierry D., Cave M. D., Eisenach K. D., Crawford J. T., Bates J. H., Gicquel B., Guesdon J. L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990 Jan 11;18(1):188–188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. Y., Wallace R. B. The ligation amplification reaction (LAR)--amplification of specific DNA sequences using sequential rounds of template-dependent ligation. Genomics. 1989 May;4(4):560–569. doi: 10.1016/0888-7543(89)90280-2. [DOI] [PubMed] [Google Scholar]