Abstract
Objective
Clinicians encounter patients who report experiencing hearing difficulty (HD) even when audiometric thresholds fall within normal limits. When there is no evidence of audiometric hearing loss, it generates debate over possible biomedical and psychosocial etiologies. It is possible that self-reported HDs relate to variables within and/or outside the scope of audiology. The purpose of this study is to identify how often, on a population basis, people with normal audiometric thresholds self-report HD and to identify factors associated with such HDs.
Design
This was a cross-sectional investigation of participants in the Beaver Dam Offspring Study. HD was defined as a self-reported HD on a four-item scale despite having pure-tone audiometric thresholds within normal limits (<20 dB HL0.5, 1, 2, 3, 4, 6, 8 kHz bilaterally, at each frequency). Distortion product otoacoustic emissions and word-recognition performance in quiet and with competing messages were also analyzed. In addition to hearing assessments, relevant factors such as sociodemographic and lifestyle factors, environmental exposures, medical history, health-related quality of life, and symptoms of neurological disorders were also examined as possible risk factors. The Center for Epidemiological Studies-Depression was used to probe symptoms associated with depression, and the Medical Outcomes Study Short-Form 36 mental score was used to quantify psychological stress and social and role disability due to emotional problems. The Visual Function Questionnaire-25 and contrast sensitivity test were used to query vision difficulties.
Results
Of the 2783 participants, 686 participants had normal audiometric thresholds. An additional grouping variable was created based on the available scores of HD (four self-report questions), which reduced the total dataset to n = 682 (age range, 21–67 years). The percentage of individuals with normal audiometric thresholds who self-reported HD was 12.0% (82 of 682). The prevalence in the entire cohort was therefore 2.9% (82 of 2783). Performance on audiological tests (distortion product otoacoustic emissions and word-recognition tests) did not differ between the group self-reporting HD and the group reporting no HD. A multivariable model controlling for age and sex identified the following risk factors for HD: lower incomes (odds ratio [OR] $50,000+ = 0.55, 95% confidence interval [CI] = 0.30–1.00), noise exposure through loud hobbies (OR = 1.48, 95% CI = 1.15–1.90), or firearms (OR = 2.07, 95% CI = 1.04–4.16). People reporting HD were more likely to have seen a doctor for hearing loss (OR = 12.93, 95% CI = 3.86–43.33) and report symptoms associated with depression (Center for Epidemiological Studies-Depression [OR = 2.39, 95% CI = 1.03–5.54]), vision difficulties (Visual Function Questionnaire-25 [OR = 0.93, 95% CI = 0.89–0.97]), and neuropathy (e.g., numbness, tingling, and loss of sensation [OR = 1.98, 95% CI = 1.14–3.44]).
Conclusions
The authors used a population approach to identify the prevalence and risk factors associated with self-reported HD among people who perform within normal limits on common clinical tests of auditory function. The percentage of individuals with normal audiometric thresholds who self-reported HD was 12.0%, resulting in an overall prevalence of 2.9%. Auditory and nonauditory risk factors were identified, therefore suggesting that future directions aimed at assessing, preventing, and managing these types of HDs might benefit from information outside the traditional scope of audiology.
Keywords: Aging, Auditory neuropathy, Central auditory processing disorder, Hidden hearing loss, King-Kopetzky syndrome, Obscure auditory dysfunction, Self-reported hearing loss, Temporal processing disorder
INTRODUCTION
The ability to communicate in everyday life relies on more than just sound audibility, and it is well documented that audiometric thresholds do not always reflect the communication difficulties experienced by individuals. Clinicians encounter patients who describe having hearing difficulty (HD), especially when in the presence of multiple talkers or competing noise, despite having audiometric thresholds that fall within or near-normal limits. Even though it has long been known that audiometric thresholds do not always reflect the suprathreshold HDs experienced by individuals (Gates et al. 1990; Jerger et al. 1990; Stach et al. 1990), the prevalence and sources of such HD remain poorly understood. The question “why do people without hearing loss complain of HDs?” is still being asked because these types of patient populations leave clinicians in the challenging position of managing a hearing problem that is difficult to define and thus difficult to treat (Jerger 2011). Moreover, when there is no evidence of measurable audiometric hearing loss, it generates debate over possible biomedical or psychosocial etiologies of such self-reported HD. It can also leave patients feeling dismissed by the audiologist or physician when no clear medical signs are found to support their symptoms (Pryce & Wainwright 2008).
Identifying underlying contributing factors to self-reported HD may also uncover potential opportunities for preventing such HDs. Historically, HD in the presence of normal audiometric thresholds has been associated with different types of auditory/audiological disorders, including central auditory processing disorders (Jerger et al. 1990), central presbycusis (Welsh et al. 1985), obscure auditory dysfunction (OAD) (Saunders & Haggard 1989), King-Kopetzky syndrome (KKS) (Hinchcliffe 1992), auditory disability with normal hearing (Stephens & Rendell 1988), idiopathic discriminatory dysfunction (Rappaport et al. 1993), hidden hearing loss (HHL) (Schaette & McAlpine 2011; Liberman et al. 2014), and auditory neuropathy (AN) (Starr et al. 1996). Deficits in auditory temporal processing (Rappaport et al. 1993; Schneider & Pichora-Fuller 2001) and age-related factors affecting neural synchrony (Willott 1996; Frisina 2001; Tremblay et al. 2003; for a review, see Billings et al. 2012) have also been queried.
Nonauditory contributions might also exist. These individuals could be sensitive to communication difficulties accrued from subclinical medical conditions, lifestyle factors, and even environmental exposures. For example, smoking habits and the risk of cardiovascular disease have been reported to contribute to hearing impairment (Cruickshanks et al. 2010; Nash et al. 2011, 2013; Zhan et al. 2011). Chemical exposures, most notably solvents and heavy metals, have as well (Fuente & McPherson 2006). Head/brain injuries (Fausti et al. 2009) can influence a person’s ability to hear in noise, and personality traits (e.g., neuroticism) can influence a person’s self-impression of hearing ability (Cox et al. 2007). Even cognition (e.g., processing speed and working memory capacity) has been shown to affect a person’s perceptual abilities (Craik 2007; Sommers et al. 2011). Therefore, HD may relate to a broad range of variables that fall within and outside the traditional scope of audiology.
Patients presenting with this type of profile are estimated to account for up to 10% of patients seen in Ear, Nose, and Throat and audiology clinics (Rappaport et al. 1993; Higson et al. 1994), but the actual prevalence numbers are not known. Estimates can vary depending on the audiometric criteria used, definitions of HD, and the age groups examined (Saunders & Haggard 1989; Zhao & Stephens 2006). Garstecki (1987), for example, stated as many as 29% of older adults who passed pure-tone hearing screenings self-reported having a hearing handicap. Gates et al. (1990), as part of the Framingham epidemiological study, cited 20.2% of the 683 people who self-reported having a hearing loss had a pure-tone average <26 dB HL and word-recognition scores in quiet >90% in the better ear. Chia et al. (2007) disclosed a prevalence of 51% of reported HD among subjects older than 49 years, even though only half of them actually had hearing loss as verified using audiometry. In a Finnish study involving adults ages 54 to 66 years, 60.3% reported finding it very difficult to follow a conversation if there is background noise (e.g., radio, TV, and children playing) despite having normal hearing—defined as an average HL better than 20 dB over the frequency range from 500 to 4000 Hz on the better ear (Hannula et al. 2011).
The purpose of this study was to ask the question: If audiometric thresholds fall within clinically defined normal limits, why do patients complain of having HD? Our aims were to determine how frequently this paradox occurs in the general population (prevalence) and to identify variables that are associated with self-reported HD. To accomplish this goal, we examined data acquired from participants of the Beaver Dam Offspring Study (BOSS), one of the few population studies to include in-depth audiometric testing as well as relevant nonauditory metrics such as sociodemographic and lifestyle factors, potentially ototoxic exposures, medical history and health care utilization, cognitive function, and other sensory/neurological information spanning a wide age range of adulthood.
MATERIALS AND METHODS
Participants
Participants were part of the BOSS, an ongoing study of sensory aging in the adult children (ages 21–84 years) of participants in the Epidemiology of Hearing Loss Study (Cruickshanks et al. 1998, 2010; Cruickshanks 2009; Nash et al. 2011). The offspring were examined between 2005 and 2008. The University of Wisconsin-Madison’s Health Sciences Institutional Review Board approved all methods, and all participants provided written informed consent.
Auditory Assessment
The hearing examination included pure-tone air- and bone-conduction audiometry as well as word recognition in quiet and in competing message (WRCM). All examiners were trained and certified in all study protocols. Consistent with guidelines of the American Speech-Language-Hearing Association (1987), audiometric testing was conducted in a sound-treated booth (Industrial Acoustics Company, New York, NY) using a GSI-61 clinical audiometer (Grason Stadler, Eden Prairie, MN). Headphones (TDH-50) were used for air-conduction testing, and insert earphones (EARtone 3A; Cabot Safety Corp., Indianapolis, IN) and masking were used when appropriate.
Air-conduction thresholds were determined for each ear at 0.5, 1, 2, 3, 4, 6, and 8 kHz. The cutoff for defining “normal” thresholds was <20 dB HL because it conservatively falls within many clinical protocols. The clinical audiometer was calibrated every 6 months according to the American National Standards Institute standards (ANSI 1989). Ambient noise levels were routinely measured throughout the study to ensure testing conditions remained within ANSI standards (1992). Tests of word recognition in quiet and word recognition with competing messages (WRCM) were conducted in a sound-treated booth using the Northwestern University Auditory Test Number 6 (NU6) (Wilson et al. 1976, 1990; Wiley et al. 1998). A 25-item word list was presented to the better ear at 36 dB HL above the individual’s threshold at 2 kHz (using a single female voice). In the event of thresholds being identical in both ears, the right ear was used. A competing message (single male talker) was then added at a level 8 dB HL below the speaker’s level in that same ear (Wiley et al. 1998). WRCM results were reported as percentage of words correct.
Cochlear function was estimated using distortion product otoacoustic emissions (DPOAEs) (Model GSI 60; Grason Stadler, Inc.). The two primary frequencies (f1 and f2) were presented at an f2/f1 ratio of 1.22. DPOAEs were tested in each ear with f2 varied from 1, 1.5, 2, 3, 4, 6, and 8 kHz. The level of f1 (L1) was 65 dB SPL and the level of f2 (L2) was 55 dB SPL. The DPOAE measurement protocol used a sampling rate of 32,000 Hz. If a single-frame noise level exceeded 30 dB SPL, then that frame was rejected; similarly, if L1 or L2 differed by more than ±5 dB from the specified protocol level, then that frame was rejected. The f1 and f2 primary tone pair presentation was rejected if any of the following conditions occurred: (1) the presentation time exceeded 16 sec or 1000 frames checked, (2) the noise level was exceeded for 250 frames or more, or (3) L1 or L2 were out of tolerance for 20 frames or more. DPOAEs were considered present for a signal-to-noise ratio of 3 dB or greater.
Trained interviewers also administered questionnaires about hearing health, including tinnitus, vertigo, and noise exposure. Participants were asked about the presence of tinnitus and/or vertigo. Examples include “In the past year, have you experienced any dizziness (not related to sudden changes in position)”? Tinnitus was defined as having had buzzing, ringing, or noise in the ears, which was either reported as moderate or severe or as causing problems getting to sleep. A positive history of occupational noise exposure was defined using self-report methods and included holding a full-time job that required speaking in a raised voice or louder to be heard, including military service. Examples of loud hobbies included woodworking, target shooting, and hunting.
Definition of HD
Participant interviews included questions from the Hearing Handicap Inventory for the Elderly-screening version as well as the Hearing Handicap Inventory for Adults-screening. For the purposes of this study, four questions reflecting the types of symptoms typically expressed by patients were identified.
Does a hearing problem cause you difficulty when in a restaurant with relatives or friends?
Does a hearing problem cause you difficulty hearing/understanding coworkers, clients, or customers?
Do you have difficulty understanding conversations when several people are talking?
How much does your hearing limit you from hearing when someone talks to you in a noisy, large group of people?
One point was given for every response of “A little”/“Sometimes.” Two points were given for a response of “Yes”/“A lot.” Zero points were given for a response of “No.” The score ranged from 0 to 8. A cut point of 4 was chosen to represent some degree of HD as that score could be achieved by answering a little/sometimes to all four questions or having some combination of responses with at least one “Yes.” A total score of ≥4 qualified the participant to fall in the “reported HD” group, while scores <4 resulted in an assignment to the “no reported HD” group.
Other Assessments
Trained interviewers also queried about relevant sociodemographic/lifestyle and exposures variables (e.g., income level, living conditions, smoking, alcohol intake, exercise/physical activity, and solvent/metal exposure). For example, a positive smoking history was defined as having smoked more than 100 cigarettes in his/her lifetime. Pack-years were calculated for smokers (number of cigarettes smoked per day divided by 20 then multiplied by the number of years smoked). A history of heavy drinking was defined as having ever in their life consumed four or more alcoholic beverages daily. Participants were considered physically active if they currently engaged in a regular activity long enough to work up a sweat at least once a week.
Items related to medical history, health-related quality of life, and other sensory or neurological dysfunction were also included (e.g., medications, head injuries, depression, cognition, vision, and neurological symptoms). Participants brought with them their medications and an inventory was recorded. The Center for Epidemiological Studies-Depression (CES-D) (Radloff 1977) was used to probe symptoms of depression such as restless sleep, poor appetite, and feeling lonely. It is a 20-item self-report depression scale for research in the general population, with a recommended cutoff score of 16. The Short-Form (36) Health Survey, consisting of 36 questions, was used to measure health-related quality of life including eight independent psychological (mental component) and physical health (physical component) dimensions (Ware et al. 1999). The Short-Form 36 Mental Component Scale was used to quantify psychological stress and social and role disability due to emotional problems. The Mini–Mental State Examination, a brief 30-point questionnaire test, was used to screen for cognitive impairment (Folstein et al. 1975), and the Trail Making Test was used to assess cognitive functions including visual attention and task switching (Arnett & Labovitz 1995). The Trail Making Test consists of two parts in which the subject is instructed to connect a set of 25 dots as fast as possible while still maintaining accuracy. Trails (part A) was used to examine cognitive processing speed, and trails B was used to assess executive functioning ability. In part A, the participant is instructed to draw lines to connect the numbers in ascending order—the circles are numbered 1 to 25. In part B, the circles include both numbers (1–13) and letters (A–L). The participant is asked to connect the circles in an ascending pattern, similar to part A, but with the added task of alternating between the numbers and letters (i.e., 1-A-2-B-3-C, etc.). The 9-Hole Pegboard Dexterity Test was used to assess each individual’s ability to coordinate the fingers and manipulate objects in a timely manner. This test consists of a square board with nine holes and a container with nine wooden pegs (Mathiowetz et al. 1985). It is a time-monitored test in which pegs are picked up from the container and put into the holes and then returned.
Other sensory and neurological systems were considered as well. In the vision domain, a person can experience vision difficulties (VDs) even if they have 20/20 visual acuity. This difficulty can be related to a person’s contrast sensitivity. Contrast sensitivity is an important measure of visual function, especially in situations of low light, fog, or glare, when the contrast between objects and their background often is reduced. In our study, the Pelli-Robson chart was used to determine whether a person was able to distinguish between finer and finer increments of light versus dark (contrast). The Visual Function Questionnaire (VFQ-25) was also used to query symptoms of VDs including health and how vision may be impacting a person’s daily activities and quality of life (Mangione et al. 2001). Symptoms can include difficulty with near and distance vision activities, driving difficulties, limitations in social and role functioning, lack of independence due to vision, mental health symptoms caused by vision, peripheral and color vision, and eye pain. Finally, symptoms of neuropathy were probed (e.g., history of numbness or tingling in your hands or feet). These types of symptoms are nonspecific and are common, not only in peripheral neuropathy (e.g., diabetes) but also in other disorders such as multiple sclerosis, fibromyalgia, and inflammatory conditions (National Institutes of Health MedlinePlus 2015).
All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Logistic regression was used to estimate odds ratios (ORs) and examine risk factor associations. At the descriptive level, differences across HD status (yes/no) were tested using the Chi-square test or the independent group t test. Age–sex-adjusted models were run for each potential risk factor that made the cutoff of p = 0.25 at the descriptive level. Any risk factor that maintained a p value of 0.25 or less in the age–sex-adjusted models was included in a preliminary main effects model. This model was then further reduced by a manual backwards elimination approach that included only key risk factors with a p value <0.05. Excluded variables were then rechecked one at a time to assess the impact of including the variable on the effect sizes of the independent predictors. Variables that changed the estimate of effect size of at least one key independent predictor by 10% or more were retained in the final model. All plausible interactions with age as a continuous variable were individually assessed in the finalized model, and only those meeting the p value <0.05 (Wald test) were retained.
RESULTS
Percentage and Prevalence of People With Normal Audiograms Who Self-Report HD
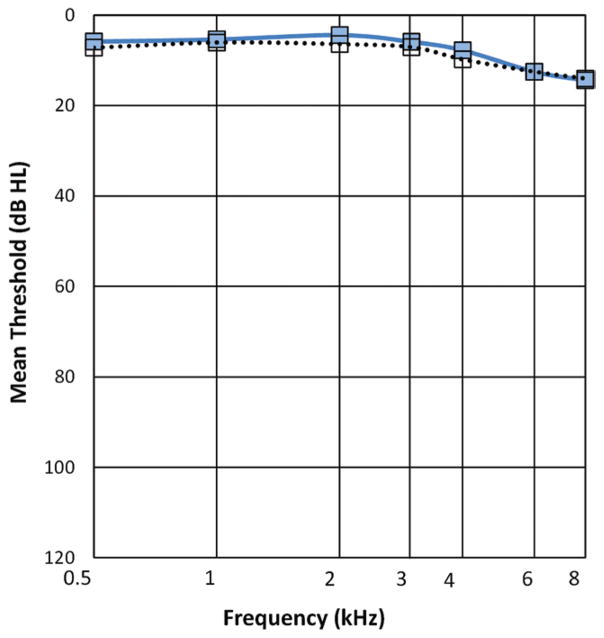
Of the 2783 BOSS participants with complete audiometric data, the number of participants with audiometric thresholds <20 dB HL bilaterally at each of the tested frequencies was 686 (24.6%). Four self-report questions were used to identify HD, and four participants did not have complete data (n = 682). Of the 682 participants whose audiometric thresholds fell within normal limits, 12.0% (82 of 682) self-reported having HD. Prevalence in the entire cohort was therefore 2.9% (82 of 2783). Mean audiometric thresholds for those with and without HD, but with normal hearing threshold levels, are shown in Figure 1. The distribution across age ranges is shown in Table 1.
Fig. 1.
Audiometric air-conduction thresholds (averaged across left and right ears) for people who self-report hearing difficulty □ and those who did not ■.
TABLE 1.
Distribution of people, across age ranges, with normal audiometric thresholds and self-report HD data (total = 682)
| Age Group | N Total | No HD | HD |
|---|---|---|---|
| 20–29 | 30 | 23 | 7 |
| 30–39 | 221 | 196 | 25 |
| 40–49 | 303 | 266 | 37 |
| 50–59 | 116 | 105 | 11 |
| 60–69 | 12 | 10 | 2 |
HD, hearing difficulty.
Auditory Factors Associated With Self-Reported HD and Normal Audiometric Thresholds
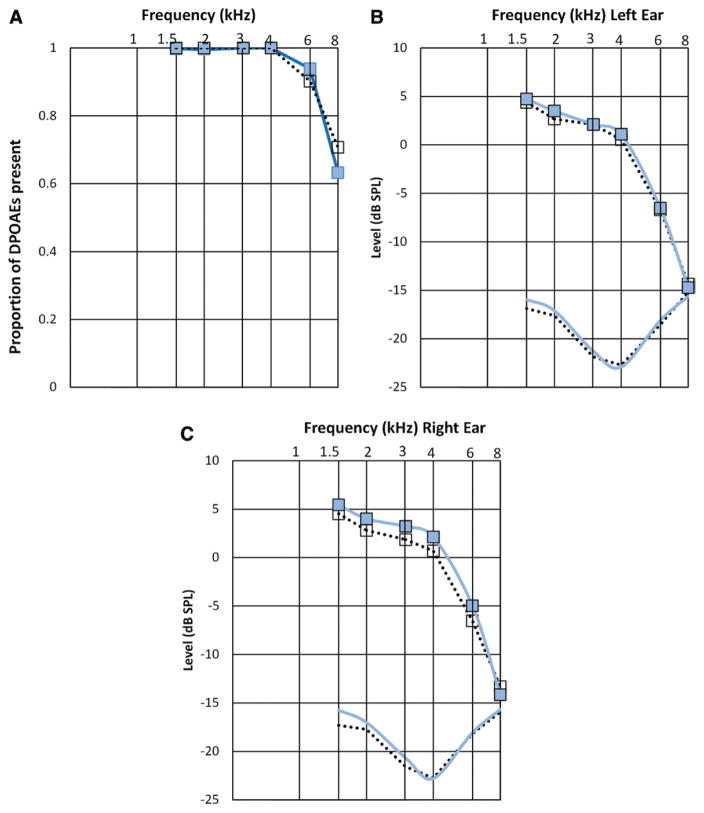
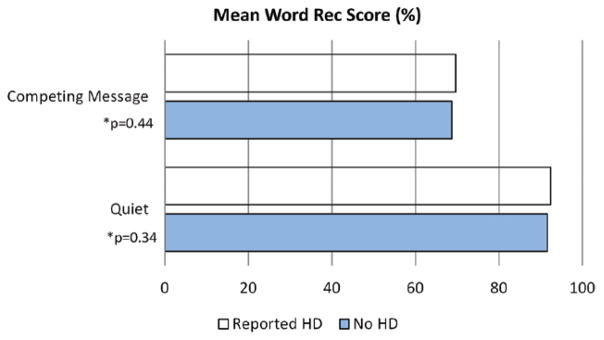
Audiologic testing did not differentiate the two groups. Audiometric thresholds were not significantly different for the group reporting HD and the non-HD group, neither were word-recognition scores in quiet and in competing noise nor were otoacoustic emissions (OAEs) (Figs. 1–3). Figure 3 shows the proportion of DPOAEs present by reported HD; approximately 64% of the participants without HD had measurable DPOAEs at 8000 Hz. Table 2 lists the percent with absent DPOAE responses in one or both ears by reported HD and frequency. There were no significant differences between the two groups. For example, there was a total of n = 598 participants with both 1.5 kHz data and reported HD data. Of those 598 participants, n = 73 reported HD; 2.7% of the n = 73 had some level (one or both ears) of absent OAE, and 4.8% of the remaining n = 525 participants who reported no HD had 1.5 kHz data and had some level (one or both ears) of absent OAE. Table 3 lists DPOAE data according to the signal-to-noise ratio and amplitude and shows a significant difference between groups at 3 kHz for the right ear (raw amplitude). Slight differences between the two groups at 4 kHz (right ear) are also observed, but not significant p = 0.06.
Fig. 3.
Distortion product otoacoustic emission (DPOAE) data for people with □ and without ■ self-reported hearing difficulty. A, Proportion of DPOAEs present at each frequency tested. DPOAE amplitudes and noise floors (---- noise floor for hearing difficulty) as a function of frequency for (B) left and (C) right ears.
TABLE 2.
Percent with absent OAE responses in one or both ears by reported HD and frequency
| Frequency, kHz | Total N | Reported HD
|
p | |
|---|---|---|---|---|
| Yes, N (%) | No, N (%) | |||
| 1.5 | 598 | 2/73 (2.7%) | 25/525 (4.8%) | 0.76 |
| 2 | 645 | 3/79 (3.8%) | 34/566 (6.0%) | 0.61 |
| 3 | 673 | 0/82 (0.0%) | 8/591 (1.4%) | 0.61 |
| 4 | 675 | 1/82 (1.2%) | 6/593 (1.0%) | 0.60 |
| 6 | 676 | 23/82 (28.1%) | 150/594 (25.3%) | 0.59 |
| 8 | 674 | 63/82 (76.8%) | 460/592 (77.7%) | 0.86 |
HD, hearing difficulty; OAE, otoacoustic emission.
Shading indicates significant results.
TABLE 3.
DPOAE signal-to-noise ratio and amplitude data by HD status
| Frequency, kHz | Ear | Reported HD
|
p | |
|---|---|---|---|---|
| No | Yes | |||
| Mean raw amplitudes | ||||
| 1.5 | L | 4.74 | 4.39 | 0.67 |
| R | 5.44 | 4.51 | 0.23 | |
| 2 | L | 3.50 | 2.66 | 0.26 |
| R | 4.00 | 2.80 | 0.13 | |
| 3 | L | 2.11 | 2.11 | 1.00 |
| R | 3.21 | 1.84 | 0.04 | |
| 4 | L | 1.09 | 0.54 | 0.49 |
| R | 2.12 | 0.68 | 0.06 | |
| 6 | L | −6.53 | −6.71 | 0.85 |
| R | −4.97 | −6.54 | 0.12 | |
| 8 | L | −14.71 | −14.35 | 0.67 |
| R | −14.15 | −13.32 | 0.37 | |
| Mean signal-to-noise ratio | ||||
| 1.5 | L | 20.70 | 21.28 | 0.52 |
| R | 21.20 | 21.82 | 0.54 | |
| 2 | L | 20.60 | 20.26 | 0.73 |
| R | 21.02 | 20.53 | 0.63 | |
| 3 | L | 23.41 | 23.99 | 0.49 |
| R | 23.89 | 23.41 | 0.56 | |
| 4 | L | 24.00 | 23.26 | 0.38 |
| R | 24.92 | 23.44 | 0.07 | |
| 6 | L | 11.57 | 11.82 | 0.82 |
| R | 13.09 | 11.62 | 0.17 | |
| 8 | L | 0.92 | 0.82 | 0.91 |
DPOAE, distortion product otoacoustic emission; HD, hearing difficulty; L, left; R, right.
Shading indicates significant results.
Other Factors Associated With Self-Reported HD and Normal Audiometric Thresholds
Table 4 shows the demographic and descriptive characteristics between participants reporting HD and those who did not. There were no statistical differences in age (p = 0.72) or sex (p = 0.08). However, a higher proportion of people reporting HD also reported exposure to noise, solvents, and metals and, on average, had lower incomes and lower physical activity. People reporting HD took more medications, visited a doctor more often for hearing loss, and were more likely to complain of tinnitus, ear infections, sinus problems, and dizziness. They were also more likely to report poorer vision-related quality of life (VFQ-25), symptoms of depression (CES-D), and neuropathy-type symptoms (numbness/tingling/temporary loss of sensation). Slower response times, using the Pegboard Dexterity Test, were also demonstrated.
TABLE 4.
Descriptive statistics
| All, N = 686 | No Reported HD, N = 600 | Reported HD, N = 82 | Age-Sex Adjusted, p | |
|---|---|---|---|---|
| Continuous measures, mean (SD) | ||||
| Age, yrs | 42.5 (7.7) | 42.5 (7.5) | 42.1 (8.4) | 0.72 |
| No. loud hobbies (ever) | 1.4 (1.3) | 1.3 (1.2) | 1.8 (1.4) | <0.05 |
| No. medications taken | 1.9 (2.1) | 1.9 (2.0) | 2.3 (2.3) | <0.05 |
| MMSE, total score | 28.9 (1.2) | 29.0 (1.2) | 28.4 (1.6) | 0.14 |
| SF-36 mental component scale | 53.4 (7.5) | 53.8 (7.1) | 50.3 (9.5) | <0.05 |
| CES-D total score | 7.8 (6.7) | 7.4 (6.5) | 11.2 (7.1) | <0.05 |
| Pegs score (time, sec) | 66.0 (12.2) | 65.5 (11.5) | 69.2 (16.5) | <0.05 |
| Trails B–trails A (time, sec) | 32.1 (18.4) | 31.8 (17.3) | 34.2 (25.3) | 0.24 |
| VFQ-25 composite score | 93.5 (5.8) | 93.9 (5.8) | 90.9 (5.6) | <0.05 |
| Binary measures, N (%) | ||||
| Male | 208 (30.3%) | 176 (29.3%) | 32 (39.0%) | 0.08 |
| Gross income of 50K or more | 494 (73.5%) | 440 (75.0%) | 50 (61.7%) | <0.05 |
| No. people living in home (not counting self) | ||||
| None | 68 (9.9%) | 55 (9.2%) | 12 (14.6%) | Ref |
| One | 195 (28.4%) | 173 (28.8%) | 22 (26.8%) | 0.21 |
| Greater than one | 423 (61.7%) | 372 (62.0%) | 48 (58.5%) | 0.14 |
| Ever drank four + drinks/day | 86 (12.6%) | 69 (11.5%) | 17 (20.7%) | 0.05 |
| Regular exercise at least once/week | 471 (68.8%) | 420 (70.0%) | 49 (59.8%) | <0.05 |
| Current smoker | 111 (16.2%) | 92 (15.3%) | 19 (23.2%) | 0.10 |
| Ever served in military | 38 (5.6%) | 34 (5.7%) | 4 (4.9%) | 0.53 |
| Ever fired a gun (excludes military use) | 435 (63.4%) | 369 (61.5%) | 64 (78.1%) | <0.05 |
| Solvent/metal exposure at longest-held job | 83 (12.4%) | 66 (11.3%) | 16 (20.8%) | <0.05 |
| Occupational noise | 242 (35.3%) | 204 (34.0%) | 37 (45.7%) | 0.08 |
| Last time saw eye doctor | ||||
| Never | 21 (3.1%) | 20 (3.4%) | 1 (1.2%) | Ref |
| Past year/1–2 yrs ago | 488 (71.7%) | 419 (70.3%) | 65 (80.3%) | 0.21 |
| 3 or more years ago | 172 (25.3%) | 157 (26.3%) | 15 (18.5%) | 0.49 |
| Antidepressant medication | 112 (6.4%) | 92 (15.3%) | 19 (23.2%) | 0.05 |
| Sedative medication | 46 (6.7%) | 38 (6.3%) | 8 (9.8%) | 0.22 |
| Why saw doctor for hearing/ear problem? | ||||
| Never saw a doctor | 380 (56.7%) | 334 (57.0%) | 43 (53.8%) | Ref |
| Infection/middle ear symptom | 171 (25.5%) | 159 (27.1%) | 12 (15.0%) | 0.14 |
| Hearing loss | 18 (2.7%) | 8 (1.4%) | 10 (12.5%) | <0.05 |
| Wax, tinnitus, dizzy, other | 101 (15.1%) | 85 (14.5%) | 15 (18.8%) | 0.26 |
| History of ear surgery | 55 (8.1%) | 44 (7.4%) | 10 (12.2%) | 0.13 |
| Significant tinnitus | 39 (5.7%) | 30 (5.0%) | 9 (11.0%) | <0.05 |
| Two or more ear infections as adult | 144 (21.0%) | 118 (19.7%) | 25 (30.5%) | <0.05 |
| Sinus problem in the past week | 115 (16.8%) | 94 (15.8%) | 20 (24.4%) | <0.05 |
| Dizziness/balance problems in past year | 70 (10.2%) | 51 (8.5%) | 19 (23.2%) | <0.05 |
| History of head injury | 175 (25.5%) | 146 (24.3%) | 28 (34.2%) | 0.11 |
| Severe headaches/migraines past 3 mos | 145 (21.1%) | 122 (20.3%) | 23 (28.1%) | 0.07 |
| Impaired contrast sensitivity | 33 (4.8%) | 27 (4.5%) | 6 (7.4%) | 0.21 |
| Self-reported health | ||||
| Good or better | 642 (93.6%) | 569 (94.8%) | 69 (84.2%) | Ref |
| Fair/poor | 44 (6.4%) | 31 (5.2%) | 13 (15.9%) | <0.05 |
| CES-D total score of 16+ | 90 (13.2%) | 66 (11.1%) | 24 (29.3%) | <0.05 |
| Numbness/tingling/loss of temporary sensation | 217 (31.6%) | 178 (29.7%) | 38 (46.3%) | <0.05 |
CES-D, Center for Epidemiological Studies-Depression; HD, hearing difficulty; MMSE, Mini–Mental State Examination; SF-36, Short-Form 36; VFQ-25, Visual Function Questionnaire.
Shading indicates significant results.
After controlling for age and sex in the final multivariable model (Table 5), only some of these factors remained significant. People who reported HD were more likely to have loud hobbies (OR = 1.48, 95% confidence interval [CI] = 1.15–1.90) and have fired a gun sometime in their lives outside of military use (OR = 2.07, 95% CI = 1.04–4.16). They were also more likely to have seen a doctor for hearing loss (OR = 12.93, 95% CI = 3.86–43.33) and less likely to have seen a doctor for middle-ear symptoms/infections (OR = 0.36, 95% CI = 0.17–0.77). Mean CES-D (depression) scores fell within the normal range for both groups; however, people who reported HD were more likely to have a CES-D total score of 16 or greater (OR = 2.39, 95% CI = 1.03–5.54). Similarly, scores of visual function (VFQ-25) fell within the normal range for both groups and were not indicative of a vision disorder, but there was a lower likelihood of HD for every 1-unit increase in the VFQ-25 score (OR = 0.93, 95% CI = 0.89–0.97). Finally, symptoms of neuropathy (numbness/tingling/loss of sensation) (OR = 1.98, 95% CI=1.14–3.44) continued to be associated with self-reported HD. There were no significant interactions with age. To determine whether symptoms of neuropathy, VD, and HD might relate to diabetes, A1C blood glucose levels from the HD group were compared with the no HD group, and no significant differences were found (data not shown).
TABLE 5.
Multivariable model, retaining factors associated with hearing difficulty
| Factors and Their Association With Hearing Difficulty | Odds Ratio (95% CI) |
|---|---|
| Age (5 yrs) | 0.83 (0.69–1.00) |
| Sex (men vs. women) | 0.65 (0.32–1.33) |
| Gross income of 50K or more | 0.55 (0.30–1.00) |
| Solvent/metal exposure at longest-held job* | 1.78 (0.85–3.73) |
| No. loud hobbies (ever) | 1.48 (1.15–1.90) |
| Ever fired a gun (excludes military use) | 2.07 (1.04–4.16) |
| Why saw doctor for hearing/ear problem? | — |
| Never saw a doctor | Ref |
| Infection/middle ear symptom | 0.36 (0.17–0.77) |
| Hearing loss | 12.93 (3.86–43.33) |
| Wax, tinnitus, dizzy, other | 1.18 (0.57–2.44) |
| No. adult ear infections* | 1.41 (0.74–2.68) |
| SF-36 mental component scale* | 0.99 (0.95–1.03) |
| CES-D total score of 16+ | 2.39 (1.03–5.54) |
| Trails B–trails A (time, sec)* | 1.00 (0.98–1.01) |
| VFQ-25 composite score | 0.93 (0.89–0.97) |
| Numbness/tingling/loss of temporary sensation | 1.98 (1.14–3.44) |
Several auditory and nonauditory factors (shaded in gray) were significantly associated with hearing difficulty in the presence of normal audiometric data.
Not significant but retained in model as confounders.
CES-D, Center for Epidemiological Studies-Depression; CI, confidence interval; SF-36, Short-Form 36; VFQ-25, Visual Function Questionnaire-25.
Shading indicates significant results.
When the final model was re-evaluated using a cut point of 3, the results did not change. There were not enough individuals with scores of 5 or higher to perform a sensitivity analysis at this higher cut point. Effect sizes and statistical significance were similar for noise exposure because of loud hobbies, seeing a doctor, vision (VFQ-25), and symptoms of neuropathy. Effect sizes and statistical significance were lower and non-significant for noise exposure from firearms (OR = 1.12; 95% CI = 0.66–1.89) and depressive symptoms (CES-D) (OR = 1.57, 95% CI = 0.76–3.27).
DISCUSSION
For more than a quarter of a century, researchers have asked the question “why do people without hearing loss complain of HDs?” We approached this question from a population perspective to determine the prevalence of this type of HD as well as associated risk factors.
The percentage of individuals with self-reported HD and who had normal audiometric thresholds was 12.0%; the overall prevalence was 2.9%. Our percentages are similar to prior estimates of up to 10% (Saunders & Haggard 1989; Zhao & Stephens 2006) but lower than the 29% reported by Garstecki (1987) and the 20.2% reported by Gates et al. (1990). Reasons for the discrepancy are likely related to inclusion criteria. The latter two studies included mostly older people with clinically defined audiometric hearing loss and used different questions to define HD. Prevalence data are dependent on the definition of HD, the age group examined, and the selected audiometric threshold used to define normal limits.
In our study, participants ranged in age from 21 to 84 years. The mean age of the group reporting no HD (42.5 years) did not significantly differ from the HD group (42.1 years). One might have assumed that the group reporting HD might be older than the no HD group because older adults, with and without hearing loss, are known to have difficulty understanding speech in adverse listening environments (for a reviews, see Gordon-Salant 2005; Humes 2013). Such suprathreshold processing difficulties are often attributed to temporal processing disorders (Tremblay et al. 2003; Clinard et al. 2010; Clinard & Tremblay 2013). However, audiometric hearing loss increases dramatically with advancing age (Cruickshanks et al. 1998; Lee et al. 2005), and our exclusion of people with hearing loss likely affected the ability to observe an age effect. Despite this, our findings show that even younger- and middle-aged groups self-report having HD despite having normal audiometric thresholds, suggesting that something other than sound audibility is contributing to self-reported HD.
According to our multivariate model controlling for age and sex, variables associated with self-reported HD include low income, increased noise exposure, higher likelihood of depression (CES-D), and reduced vision function (VFQ-25). People reporting HD were also more likely to have seen a physician for hearing loss (and less so for ear infections) and report neuropathy-type symptoms (numbness/tingling/loss of temporary sensation). We discuss these findings in the following ways:
Noise Exposure
It is possible that people with normal audiograms who report having HD are experiencing noise-induced pathologies. People self-reporting HD were more likely to have seen a doctor for hearing loss but less likely to report seeing a doctor for ear infections or middle-ear conditions. These health utilization behaviors, along with increased reports of noise exposure, as well as a greater absence of DPOAE at higher frequencies, could suggest subclinical cochlear damage that is sufficient to result in reported HDs in real-world settings.
DPOAEs were analyzed because they have been shown to be sensitive to subclinical damage that is not always apparent from audiometric data (Bohne & Clark 1982; Kujawa & Liberman 2007; Lonsbury-Martin & Martin 2007; Job et al. 2009). Despite this, the presence or absence of DPOAEs did not differentiate the group that reported HD from the group that did not or did the majority of DPOAE amplitudes (with the exception of 3 kHz—right ear). Future studies might yield information that distinguishes the two groups from one another if ultra-high frequencies were tested (see Badri et al. 2011).
Auditory brainstem responses (ABR) might also shed light on these types of subclinical disorders. Recent studies show that wave I amplitude of the ABR is sensitive to age- and noise-related dysfunction involving inner hair cells, auditory nerves, or the synaptic transmission between them, even when audiometric and OAE data appear normal (Kujawa & Liberman 2009; Lin et al. 2011; Konrad-Martin et al. 2012; Sergeyenko et al. 2013; Stamper & Johnson 2015). Moreover, participants who described having tinnitus show significantly reduced wave I ABR amplitudes, and these results have been described within the context of HHL (Schaette & McAlpine 2011; for a review, see Plack 2014). In our study, the HD group was more likely to complain of tinnitus than the non-HD group, but this factor was not retained in the final multivariate model.
Neuropathy
The odds of reporting HD increased if a person reported symptoms of neuropathy (e.g., numbness, tingling, and loss of sensation) and VDs. VFQ-25 composite scores fell within normal limits for both groups, but people who reported limitations in social functioning related to vision, lack of independence due to vision, mental health symptoms caused by vision were more likely to report HD. Some of these subclinical symptoms of VD parallel that expressed in the auditory domain and possibly point to a multisensory problem. A recent study of the prevalence of dual sensory (hearing and vision) impairment in United Kingdom adults ages 40 to 69 years found a higher than expected prevalence of dual impairment, hinting at shared common causes for hearing and vision problems (Dawes et al. 2014).
The above-mentioned symptoms of neuropathy differ from those traditionally described as AN (Starr et al. 1996; Stein et al. 1996; for a review, see Hood & Morlet 2012). With an incidence estimated at 10%, AN is characterized by speech perception abilities that are often poorer than would be predicted from audiometric results (which may or may not fall within normal limits). Furthermore, OAEs are typically present as are cochlear microphonic recordings, but ABRs are usually irregular or absent. ABR testing is not included in the BOSS cohort test battery, so we are unable to compare the pattern of results associated with our HD group to those with diagnosed AN. However, enough similarities between our HD group and descriptions of neuropathy justify including ABR assessment in future studies.
Depression
Self-reported HD include was associated with a higher likelihood of depression (CES-D) and reduced vision function (VFQ-25). Linking CES-D scores to HD and VFQ-25 scores is speculative, but it is not unreasonable to think that reports of hearing and VDs, and their known social and mental health consequences, could be contributing to increased self-reports of depressive type symptoms (Gopinath et al. 2009). Then again a tendency toward depression could also result in a person being abnormally anxious about their health. Nonauditory contributions to HD, such as anxiety, depression, and hypochondriasis, have been examined in patients described as having OAD and KKS (Stephens & Rendell 1988; Yeoh 1997; Zhao & Stephens 2000; Pryce et al. 2010). Results of these studies suggest that they are multifaceted disorders with some degree of underlying psychological contribution (Stephens & Rendell 1988; Yeoh 1997), thus justifying the inclusion of psychological approaches when managing these patients (Zhao & Stephens 2000).
CONCLUSIONS
To summarize, we identified a portion of the population that self-reports having HD despite performing within normal limits on common audiological tests. HD in the presence of normal or near-normal audiometric thresholds has been described in terms of different auditory disorders (e.g., CAPD, CP, KKS, OAD, auditory disability with normal hearing, idiopathic discriminatory dysfunction, HHL, AN, and auditory temporal processing). These terms are used inconsistently in the literature to describe people with similar symptoms, making it difficult to compare and contrast our data to each diagnosis. Also, a limitation of this study is that the BOSS dataset does not include all of the necessary tests to differentiate each potential diagnosis (e.g., temporal processing). However, what can be said based on our population approach using BOSS data is auditory (e.g., noise exposure) and nonauditory risk factors were identified. If what we identified here are early pathological signs in a population that has experienced subclinical cochlear-neural damage possibly related to noise exposure, then the percentage of affected people is probably greater than what is described here because potential noise effects are likely to coexist in people with elevated audiometric thresholds as well. The same can be said for other potential contributors as well (e.g., neuropathy) because interactions are also possible. Still, nonauditory factors still need to be explained: greater likelihood of depression (CES-D), a higher likelihood of reduced vision function (VFQ-25), and symptoms of neuropathy (numbness/tingling/loss of temporary sensation). Thus, the sources of such HDs remain unclear, perhaps even falling outside of the scope of audiology.
A few additional limitations of this study should be noted: First, the study was cross-sectional in design, so HD and all potential associated factors were measured at the same time. Therefore, assessment of the temporal relationship between the factors and HD was not possible. Second, the group is predominately non-Hispanics, which may limit the generalizability of our findings to other populations (e.g., other racial groups, other geographic locations, etc.). Third, the strengths and limitations associated with self-report approaches to identifying subjects with HD using similar tools and cut points is acknowledged (American Speech-Language-Hearing Association 1989; Nondahl et al. 1998; Sindhusake et al. 2001). However, it is through self-report that patients describe their symptoms and history to clinicians.
We used a population approach to identify the prevalence and risk factors associated with self-reported HD among people who perform within normal limits on clinical tests of auditory function. The percentage of individuals with normal audiometric thresholds who self-reported HD was 12.0%, resulting in an overall prevalence of 2.9%. Auditory and nonauditory risk factors were identified. Therefore, future attempts at assessing, preventing, and managing these types of HDs might benefit from the consideration of both auditory and nonauditory factors.
Fig. 2.
There were no significant differences in word-recognition performance, between groups, in the quiet and competing message conditions. HD, hearing difficulty.
Acknowledgments
The authors thank the Virginia Merrill Bloedel Hearing Research Center for the traveling scholarship awarded to K.L.T. Laura Driesbach, PhD, is thanked for her comments on the otoacoustic emission data.
Funding was received from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders in the form of an Administrative Supplement award to K.J.C. (5R01AG021917). This project was supported by R01AG021917 (K.J.C.) from the National Institute on Aging, National Eye Institute, and National Institute on Deafness and Other Communication Disorders and an unrestricted grant from Research to Prevent Blindness. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
Portions of this article were presented at the 5th Aging and Speech Communication International and Interdisciplinary Conference, Bloomington, Indiana, 2013, as well as at the Association for Research in Otolaryngology Conference in 2015.
The authors declare no other conflict of interest.
References
- American National Standards Institute (ANSI) Specifications for Audiometers. ANSI S3.6-1989. New York, NY: American National Standards Institute; 1989. [Google Scholar]
- American National Standards Institute (ANSI) Specifications for Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. ANSI S3.1-1992. New York, NY: American National Standards Institute; 1992. [Google Scholar]
- American Speech-Language-Hearing Association. Guidelines for manual pure tone audiometry. ASHA. 1987;20:287–301. [Google Scholar]
- American Speech-Language-Hearing Association. Guidelines for the identification of hearing impairment/handicap in adult/elderly persons. ASHA. 1989;31:59–63. [Google Scholar]
- Arnett JA, Labovitz SS. Effect of physical layout in performance of the trail making test. Psychol Assess. 1995;7:220–221. [Google Scholar]
- Badri R, Siegel JH, Wright BA. Auditory filter shapes and high-frequency hearing in adults who have impaired speech in noise performance despite clinically normal audiograms. J Acoust Soc Am. 2011;129:852–863. doi: 10.1121/1.3523476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, Tremblay KL, Willott JW. The aging auditory system. In: Tremblay K, Burkard R, editors. Translational Perspectives in Auditory Neuroscience. San Diego, CA: Plural Publishing; 2012. pp. 62–83. [Google Scholar]
- Bohne B, Clark W. Growth of hearing loss and cochlear lesion with increasing duration of noise exposure. In: Hamernick RP, Henderson D, Salvi R, editors. New Perspectives on Noise-Induced Hearing Loss. New York, NY: Raven Press; 1982. pp. 283–301. [Google Scholar]
- Chia EM, Wang JJ, Rochtchina E, et al. Hearing impairment and health-related quality of life: The Blue Mountains Hearing Study. Ear Hear. 2007;28:187–195. doi: 10.1097/AUD.0b013e31803126b6. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL. Aging degrades the neural encoding of simple and complex sounds in the human brainstem. J Am Acad Audiol. 2013;24:590–599. doi: 10.3766/jaaa.24.7.7. quiz 643. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: Evidence from human frequency-following response recordings. Hear Res. 2010;264:48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RM, Alexander GC, Gray GA. Personality, hearing problems, and amplification characteristics: contributions to self-report hearing aid outcomes. Ear Hear. 2007;28:141–162. doi: 10.1097/AUD.0b013e31803126a4. [DOI] [PubMed] [Google Scholar]
- Craik FI. The role of cognition in age-related hearing loss. J Am Acad Audiol. 2007;18:539–547. doi: 10.3766/jaaa.18.7.2. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ. Population-based epidemiologic studies of aging: The contributions of a Wisconsin community. WMJ. 2009;108:271–272. [PMC free article] [PubMed] [Google Scholar]
- Cruickshanks KJ, Nondahl DM, Tweed TS, et al. Education, occupation, noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hear Res. 2010;264:3–9. doi: 10.1016/j.heares.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Dawes P, Dickinson C, Emsley R, et al. Vision impairment and dual sensory problems in middle age. Ophthalmic Physiol Opt. 2014;34:478–488. doi: 10.1111/opo.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti SA, Wilmington DJ, Gallun FJ, et al. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J Rehabil Res Dev. 2009;46:797–810. doi: 10.1682/jrrd.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Possible neurochemical and neuroanatomical bases of age-related hearing loss-presbycusis. Semin Hear. 2001;22:213–225. [Google Scholar]
- Fuente A, McPherson B. Organic solvents and hearing loss: The challenge for audiology. Int J Audiol. 2006;45:367–381. doi: 10.1080/14992020600753205. [DOI] [PubMed] [Google Scholar]
- Garstecki D. Self-perceived hearing difficulty in aging adults with acquired hearing loss. J Acad Rehabil Audiol. 1987;20:49–60. [Google Scholar]
- Gates GA, Cooper JC, Jr, Kannel WB, et al. Hearing in the elderly: The Framingham cohort, 1983–1985. Part I. Basic audiometric test results. Ear Hear. 1990;11:247–256. [PubMed] [Google Scholar]
- Gopinath B, Wang JJ, Schneider J, et al. Depressive symptoms in older adults with hearing impairments: The Blue Mountains Study. J Am Geriatr Soc. 2009;57:1306–1308. doi: 10.1111/j.1532-5415.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S. Hearing loss and aging: New research findings and clinical implications. J Rehabil Res Dev. 2005;42(4 Suppl 2):9–24. doi: 10.1682/jrrd.2005.01.0006. [DOI] [PubMed] [Google Scholar]
- Hannula S, Bloigu R, Majamaa K, et al. Self-reported hearing problems among older adults: Prevalence and comparison to measured hearing impairment. J Am Acad Audiol. 2011;22:550–559. doi: 10.3766/jaaa.22.8.7. [DOI] [PubMed] [Google Scholar]
- Higson JM, Haggard MP, Field DL. Validation of parameters for assessing obscure auditory dysfunction—Robustness of determinants of OAD status across samples and test methods. Br J Audiol. 1994;28:27–39. doi: 10.3109/03005369409077910. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe R. King-Kopetzky syndrome: An auditory stress disorder? J Audiol Med. 1992;1:89–98. [Google Scholar]
- Hood LJ, Morlet T. Current issues in auditory neuropathy spectrum disorder. In: Tremblay KE, Burkard RF, editors. Translational Perspectives in Auditory Neuroscience. San Diego, CA: Plural Publishing; 2012. pp. 35–69. [Google Scholar]
- Humes LE. Understanding the speech-understanding problems of older adults. Am J Audiol. 2013;22:303–305. doi: 10.1044/1059-0889(2013/12-0066). [DOI] [PubMed] [Google Scholar]
- Jerger J. Why do people without hearing loss complain of hearing difficulties? J Am Acad Audiol. 2011;22:490–491. doi: 10.3766/jaaa.22.8.1. [DOI] [PubMed] [Google Scholar]
- Jerger J, Oliver TA, Pirozzolo F. Impact of central auditory processing disorder and cognitive deficit on the self-assessment of hearing handicap in the elderly. J Am Acad Audiol. 1990;1:75–80. [PubMed] [Google Scholar]
- Job A, Raynal M, Kossowski M, et al. Otoacoustic detection of risk of early hearing loss in ears with normal audiograms: A 3-year follow-up study. Hear Res. 2009;251:10–16. doi: 10.1016/j.heares.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, et al. Age-related changes in the auditory brainstem response. J Am Acad Audiol. 2012;23:18–35. doi: 10.3766/jaaa.23.1.3. quiz 74–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Matthews LJ, Dubno JR, et al. Longitudinal study of pure-tone thresholds in older persons. Ear Hear. 2005;26:1–11. doi: 10.1097/00003446-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Liberman LD, Maison SF. Efferent feedback slows cochlear aging. J Neurosci. 2014;34:4599–4607. doi: 10.1523/JNEUROSCI.4923-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, et al. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsbury-Martin B, Martin GK. Distortion-product otoacoustic emissions in populations with normal hearing sensitivity. In: Robinette MS, Glattke TJ, editors. Otoacoustic Emissions: Clinical Applications. New York, NY: Thieme; 2007. pp. 107–130. [Google Scholar]
- Mangione CM, Lee PP, Gutierrez PR, et al. National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Kashman N, Volland G, et al. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- Nash SD, Cruickshanks KJ, Huang GH, et al. Unmet hearing health care needs: The Beaver Dam Offspring Study. Am J Public Health. 2013;103:1134–1139. doi: 10.2105/AJPH.2012.301031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash SD, Cruickshanks KJ, Klein R, et al. The prevalence of hearing impairment and associated risk factors: The Beaver Dam Offspring Study. Arch Otolaryngol Head Neck Surg. 2011;137:432–439. doi: 10.1001/archoto.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health MedlinePlus Internet. Peripheral Neuropathy. 2015 Retrieved February 1, 2015, from http://www.nlm.nih.gov/medlineplus/ency/article/000593.htm.
- Nondahl DM, Cruickshanks KJ, Wiley TL, et al. Accuracy of self-reported hearing loss. Audiology. 1998;37:295–301. doi: 10.3109/00206099809072983. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Barker D, Prendergast G. Perceptual consequences of “hidden” hearing loss. Trends Hear. 2014;18 doi: 10.1177/2331216514550621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce H, Metcalfe C, Hall A, et al. Illness perceptions and hearing difficulties in King-Kopetzky syndrome: What determines help seeking? Int J Audiol. 2010;49:473–481. doi: 10.3109/14992021003627892. [DOI] [PubMed] [Google Scholar]
- Pryce H, Wainwright D. Help seeking for medically unexplained hearing difficulties: A qualitative study. Int J Ther Rehabil. 2008;15:1–7. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rappaport JM, Phillips DP, Gulliver JM. Disturbed speech intelligibility in noise despite a normal audiogram: A defect in temporal resolution? J Otolaryngol. 1993;22:447–453. [PubMed] [Google Scholar]
- Saunders GH, Haggard MP. The clinical assessment of obscure auditory dysfunction–1. Auditory and psychological factors. Ear Hear. 1989;10:200–208. doi: 10.1097/00003446-198906000-00011. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Age-related changes in temporal processing: Implications for speech perception. Semin Hear. 2001;22:227–239. [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, et al. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhusake D, Mitchell P, Smith W, et al. Validation of self-reported hearing loss. The Blue Mountains Hearing Study. Int J Epidemiol. 2001;30:1371–1378. doi: 10.1093/ije/30.6.1371. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Hale S, Myerson J, et al. Listening comprehension across the adult lifespan. Ear Hear. 2011;32:775–781. doi: 10.1097/AUD.0b013e3182234cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach BA, Spretnjak ML, Jerger J. The prevalence of central presbyacusis in a clinical population. J Am Acad Audiol. 1990;1:109–115. [PubMed] [Google Scholar]
- Stamper GC, Johnson TA. Auditory function in normal-hearing, noise-exposed human ears. Ear Hear. 2015;36:172–184. doi: 10.1097/AUD.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, et al. Auditory neuropathy. Brain. 1996;119(Pt 3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Stein LK, Tremblay K, Pasternak J, et al. Brainstem abnormalities in neonates with normal otoacoustic emissions. Semin Hear. 1996;17:197–213. [Google Scholar]
- Stephens S, Rendell R. Auditory disability with normal hearing. Quaderni di Audiologia. 1988;4:233–238. [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003;114:1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Ware JE., Jr . SF-36 health survey. In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 2. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1999. pp. 1227–1246. [Google Scholar]
- Welsh LW, Welsh JJ, Healy MP. Central presbycusis. Laryngoscope. 1985;95:128–136. doi: 10.1288/00005537-198502000-00002. [DOI] [PubMed] [Google Scholar]
- Wiley TL, Cruickshanks KJ, Nondahl DM, et al. Aging and word recognition in competing message. J Am Acad Audiol. 1998;9:191–198. [PubMed] [Google Scholar]
- Willott JF. Anatomic and physiologic aging: A behavioral neuroscience perspective. J Am Acad Audiol. 1996;7:141–151. [PubMed] [Google Scholar]
- Wilson RH, Coley KE, Haenel JL, et al. Northwestern University Auditory Test No. 6: Normative and comparative intelligibility functions. J Am Audiol Soc. 1976;1:221–228. [PubMed] [Google Scholar]
- Wilson RH, Zizz CA, Shanks JE, et al. Normative data in quiet, broadband noise, and competing message for Northwestern University Auditory Test No. 6 by a female speaker. J Speech Hear Disord. 1990;55:771–778. doi: 10.1044/jshd.5504.771. [DOI] [PubMed] [Google Scholar]
- Yeoh LH. Causes of hearing disorders. In: Stephens D, editor. Scott-Brown’s Otolaryngology. 6. Vol. 2. Oxford, United Kingdom: Butterworth-Heinemann; 1997. pp. 1–28. [Google Scholar]
- Zhan W, Cruickshanks KJ, Klein BE, et al. Modifiable determinants of hearing impairment in adults. Prev Med. 2011;53:338–342. doi: 10.1016/j.ypmed.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Stephens D. Subcategories of patients with King-Kopetzky syndrome. Br J Audiol. 2000;34:241–256. doi: 10.3109/03005364000000134. [DOI] [PubMed] [Google Scholar]
- Zhao F, Stephens D. Distortion product otoacoustic emissions in patients with King-Kopetzky syndrome. Int J Audiol. 2006;45:34–39. doi: 10.1080/02640410500243939. [DOI] [PubMed] [Google Scholar]