Summary
Frontotemporal dementia (FTD) is a progressive neurologic syndrome with diverse clinical presentations and attendant underlying pathologies. Psychiatric prodrome, neuropsychiatric symptoms and language difficulties are common in FTD, but the diversity of presentation raises unique diagnostic challenges that can significantly impact patient care and counsel for caregivers regarding clinical status and prognosis. While neuropsychiatric symptom measures are helpful, more sensitive assessments delineating the specific behavioral and linguistic deficits accompanying FTD are needed. Comprehensive clinical assessment in combination with evaluation of language, socio-emotional functioning, cognition and neuroimaging aid in accurate and early diagnosis and treatment planning. In what follows, we review each of the FTD syndromes, highlight current research investigating the cognitive, behavioral and socio-emotional deficits observed with this disease, address common diagnostic challenges and summarize best practices associated with management of FTD.
Keywords: differential diagnosis, frontotemporal dementia, neuropsychology, primary progressive aphasia, socio-emotional functioning
Frontotemporal dementia (FTD) is the third most common dementia for individuals 65 years and older, and is the second most common form for individuals 65 years and younger [1–3]. FTD defines a heterogeneous group of clinical syndromes marked by the progressive, focal neurodegeneration of the frontal and anterior temporal lobes [4]. First described by Arnold Pick in 1892, FTD affects brain regions implicated in motivation, reward processing, personality, social cognition, attention, executive functioning and language. Currently, FTD incorporates three clinical subtypes. Behavioral variant FTD (bvFTD) accounts for about half of all FTD cases [2], and involves initial and progressive decline in social functioning and changes in personality. The behavioral variant is characterized by focal and prominent bilateral frontal atrophy, though some reports suggest more right-hemisphere involvement than left [5]. The remaining subtypes are classified as variants of primary progressive aphasia (PPA), and are marked by initial and prominent disturbance and decline of language functioning. Loss of semantic knowledge is associated with the semantic variant of primary progressive aphasia (svPPA), while agrammatism and motor–speech difficulties are associated with nonfluent variant of primary progressive aphasia (nfvPPA) [6,7]. The semantic variant is characterized by bilateral anterior temporal lobe atrophy, associated with language, compulsions and dysfunctions in emotional processing [8]. Most patients present initially with greater left hemisphere atrophy; however, approximately a quarter of cases present with initial right anterior temporal lobe atrophy, and is associated with a more behavioral presentation of symptoms, including: social awkwardness, loss of insight and difficulty with face recognition [9]. nfvPPA is accompanied by left inferior frontal and insular atrophy; expressive speech and syntax difficulties are characteristic of the disease early in its course.
This review summarizes the clinically relevant findings typically seen in each of the three FTD subtypes. Recent advances have shed more light on the specific cognitive, behavioral and socio-emotional deficits seen in these syndromes. This review underscores the importance of identification of these deficits to aid in early and accurate clinical diagnosis and management. We also discuss best practices for how to manage the challenges associated with FTD.
Epidemiology
Due to the diagnostic challenges associated with FTD, the true prevalence of this disease is likely underestimated. The number of psychiatric and neurological disorders that can resemble the spectrum of FTD symptomology may contribute to misclassification and underestimation. Recent epidemiological work estimates a range from 15 to 22/100,000 [10]. Independent studies investigating the prevalence of FTD in the UK estimated a prevalence of about 15 cases/100,000 in adults between 45 and 64 years of age, which was comparable to the prevalence of early-onset Alzheimer's disease [3].
Male predominance of bvFTD has been reported across a number of studies and centers. Of the PPA subtypes, male predominance has been reported in svPPA, with female predominance in nfvPPA [1–3]. Regarding progression, FTD is marked by shorter duration of survival and more rapid decline of cognition and function than Alzheimer's disease [11–13]. Average survival time of FTD ranges significantly depending on the subtype. The average survival time from diagnosis to death can be as brief as 3 years for bvFTD patients with motor neuron disease and up to 12 years in patients with svPPA [12].
Pathology, genetics & risk factors
Pathology
The nomenclature surrounding FTD has evolved over time. FTD encompasses the three clinical syndromes, while the term frontotemporal lobar degeneration (FTLD) specifies the underlying pathological aggregation of protein in the frontal and temporal lobes with microvacuolation, neuronal loss and astrocytic gliosis [14]. Immunohistochemical staining has provided clarity to the underlying pathology of most FTD syndromes. Approximately 40% of FTD cases are caused by abnormal accumulations of the microtubule-binding protein tau (FTLD-tau). Pick's disease is associated with three microtubule repeats (3-R), while four microtubule repeats (4-R) are associated with progressive supranuclear palsy (PSP) and cortical basal degeneration (CBD). 3- and 4-R Tauopathies are also strongly associated with nfvPPA [15,16]. More than half of FTD cases are tau negative and characterized by the abnormal accumulation of the 43 kDA TAR DNA-binding protein (TDP-43; FTLD-TDP) [15,16]. There are four subtypes of TDP-43 pathology: Types A, B, C and D, and each of these proteinopathies correlate with different FTD syndromes. Patients with progranulin (GRN) gene mutations have Type A, though both bvFTD and nfvPPA syndromes are present even without GRN mutation. Type B pathology is associated with FTD with motor neuron disease (FTD-ALS). The majority of patients with svPPA have type C pathology, and TDP-43 type D is also associated with FTD-ALS [17]. The majority of the remaining 10% of cases are associated with accumulation of the fused in sarcoma protein (FTLD-FUS) [18,19].
The ability to predict the underlying pathology in FTD syndromes in vivo has been a major focus of research. Within the PPA syndromes, nfvPPA is more often associated with FTLD-tau, and svPPA is almost always associated with FTLD-TDP [16,20]. Of our pathology confirmed cases at the University of California San Francisco Memory and Aging Center, 21 of 23 svPPA patients showed TDP-43 type C aggregation. In bvFTD, however, FTLD-TDP and FTLD-tau variants are equally as likely [16,21].
Genetics
Approximately 40% of FTD cases include a family history of dementia and approximately 10% of patients are autosomal dominant (affecting first-degree relatives across two generations) [22–24], with MAPT, GRN and C9ORF72 representing the most common genes responsible for autosomal dominant inheritance of FTD. Among FTD syndromes, svPPA is the least likely to be familial [23]. Mutations in the MAPT gene account for about 17% of autosomal dominant FTD in our center, though another series reported 32% of patients with both FTD and a positive family history [22]. MAPT mutation carriers tend to have more focal and symmetrical temporal lobe atrophy than other genetic forms [25]. Amyotrophic lateral sclerosis (ALS), bvFTD and FTD motor neuron disease are the most common syndromes associated with a hexanucleotide expansion in C9ORF72, with PPA subtypes being seen more infrequently [26]. Mutations in C9ORF72 account for 13–26% of familial FTD cases [27]. Patients with a C9ORF72 mutation often present with obsessive-compulsive behaviors, rituals and may also display psychotic features. A progression of this symptomology in younger patients may be indicative of early FTD [26,28]. Progranulin mutations account for about 8% of autosomal dominant forms of FTD, is characterized by asymmetrical cerebral atrophy and is strongly associated with bvFTD and nfvPPA [29].
Risk factors
More recent investigation into the roles of inflammation and the immune system have shown promise in identifying potential biomarkers involved in the pathogenesis and progression of neurodegenerative diseases [30]. Neuroinflammation may contribute to the underlying pathology of FTD syndromes [31], and recent studies examining peripheral levels of tumor necrosis factor suggest a role for early dysregulation of inflammation mediators in neurodegeneration associated with bvFTD [32,33]. Another recent study posits the presence of autoimmune disorders with increased vulnerability for FTD syndromes. This study found that rates of nonthyroid-spectrum autoimmune disorders were twice as common in patients with svPPA and in individuals with a mutation in the GRN gene [34]. Other factors that have shown promise as potential risk factors for the language presentations include diagnosis of learning disability in patients and first-degree relatives [35,36]. Miller et al. also suggest the possibility of a relationship between atypical brain hemispheric lateralization and FTLD-TAU, with an increased number of nonright-handedness in svPPA patients compared with the general population [35].
Behavioral variant FTD
Neurobehavior findings
bvFTD presents distinct diagnostic challenges due to the presence of behavioral symptoms, even at a very mild disease stage [37]. The hallmark symptoms of bvFTD include progressive changes in emotional regulation, conduct and personality, and are harbingers of the underlying dysfunction of the salience network, a neural network responsible for socio-emotional awareness, reward processing and motivation [38,39]. Typically, patients do not have insight into these changes; as such, family members and friends are critical in establishing the earliest symptoms and attendant progression of symptomology.
Two discrete and not mutually exclusive behavioral syndromes have been described: an apathetic type, characterized by decreased volition and motivation, isolating behaviors, loss of socio-emotional awareness and increased latency to pain response; and a disinhibited type characterized by hyperorality, preference for sweet foods, perseverative behaviors and motor stereotypies [40]. Increased disinhibition and impulsivity can lead to inappropriate remarks (e.g., sexually explicit comments), embarrassing social behavior, overspending, pathological gambling and more rarely, hyperreligiosity [41–43].
Increasingly, a subset of patients also presents with clinical features of bvFTD without a progressive neurodegenerative condition. These patients are considered to have bvFTD ‘phenocopy syndrome,’ presenting with the behavioral features characteristic of bvFTD, without progressing to dementia. The profile of these patients as reported by family members mimics that of bvFTD, though activities of daily living (ADL) appear less impaired [44,45]. Phenocopy cases also exhibit intact memory and socio-emotional functioning, and normal or only mild deficits on measures of executive functioning [46]. On imaging, phenocopy cases display minimal or no atrophy on MRI, and normal glucose metabolism on PET [47]. While the etiology of bvFTD phenocopy is still unknown, its resemblance to other neuropsychiatric conditions has led to the postulation that these cases may have either personality disorders, or autism spectrum disorders with subclinical symptomology for formal diagnosis [48].
Functional capacity
Impaired functional capacity in bvFTD is primarily a function of the behavioral deficits present early on. For example, when compared with patients with Alzheimer's disease (AD) matched on Mini-Mental State Examination (MMSE) score, bvFTD patients showed more functional impairment on the Clinical Dementia Rating scale (CDR) total score [49]. Patients with bvFTD are also more functionally impaired than AD patients on assessment of activities of daily living (ADL), and are often impaired in ADLs upon initial visit [11,13]. When compared with PPA subtypes, functional impairment in bvFTD is also typically more severe [50,51]. Little research exists related to driving and other areas of capacity in bvFTD. A recent review examining four articles investigating driving in bvFTD found that bvFTD drivers had more problems than control groups around issues of social cognition and behavior resulting in hit and run crashes, failure to stop at red lights and speeding [52].
Neurocognitive findings
One of the new diagnostic criteria for bvFTD is a pattern of cognitive performance showing relative sparing of memory and visuospatial functions with executive dysfunction present. However, there are conflicting reports concerning the extent to which episodic memory is spared [53]. Language also tends to be preserved initially. Executive dysfunction can be assessed using tasks of set-shifting (e.g., trail making), backward digit span, verbal and nonverbal fluency measures, inhibition (e.g., stroop task), as well as the Frontal Assessment and NIH EXAMINER batteries [54–56]. The EXAMINER is a recently developed executive function battery with strong psychometric properties, which incorporates item response theory to increase sensitivity. It is important to recognize that bvFTD patients may exhibit impaired performance for multiple reasons. Error monitoring is a crucial component of successful test performance. Impairments in error monitoring has been associated with right lateral prefrontal cortex atrophy [57], and is an area of neurodegeneration in bvFTD. Possin et al. (2012) also showed that bvFTD patients produce more repetition errors on tests of design fluency than other neurodegenerative disease patients [166].
Socio-emotional functioning
Deficits in emotional salience are part of the diagnostic criteria for bvFTD (e.g., loss of sympathy or empathy), though deficits in empathy are also seen in svPPA (see below) [58]. These deficits are partly explained by patients' inaccurate estimation of their ability to empathize [59], as well as impairment in detection and recognition of emotionally salient stimuli, particularly negative emotions [60, 61]. As the disease progresses, bvFTD patients exhibit stark changes in personality marked by declines in extraversion and warmth [37]. These deficits extend to impairments in social cognition and studies have demonstrated deficits in theory of mind (e.g., taking the point of view of someone else) [62], metacognition (e.g., awareness of one's own thought processes) [63], recognition of insincere communication [62] and moral reasoning [64]. Well-validated measures that track the progression of socio-emotional changes are needed given the role these symptoms play from the outset of the disease course [65].
Reward processing
Eating, financial decisions and social functioning are all impacted in bvFTD and these changes can be attributed to changes in reward processing. As mentioned above, the lack of warmth and isolating behaviors are suggestive of a lack of incentive for interpersonal connection [66]. Impulsive purchasing and overspending behavior is also common, and on reward-related decision-making gambling tasks (e.g., the Iowa gambling task) bvFTD patients choose options with high risk of monetary loss but with large possible gains [66–69]. Changes in eating behavior can also be understood within the construct of reward processing. Impaired satiety is seen in bvFTD patients across cultures [70], despite hormone profiles that would suggest decreased food consumption [71]. Increased preference for sweet food is also commonly observed [70].
Diagnostic criteria
The international consortium proposed new criteria from 2011 provide three levels of diagnostic certainty for a diagnosis of bvFTD: possible, probable and definite [72]. A summary of these criteria can be found in Box 1. Possible bvFTD requires a patient to have a progressive deterioration of behavior accompanied by three out of six core features (disinhibition, apathy, loss of sympathy/empathy, eating behavior changes, compulsive behaviors and an executive predominant pattern of dysfunction on cognitive testing). Additionally, functional decline and neuroimaging consistent with bvFTD are required for a probable bvFTD diagnosis. Neuroimaging findings consistent with probable bvFTD include frontal, or anterior temporal atrophy, or both, on CT or MRI, or frontal hypoperfusion or hypometabolism on single-photon emission computed tomography (SPECT) or PET [72,73]. Definite bvFTD requires presence of the clinical syndrome with genetic or pathological confirmation of FTLD.
Box 1. Summary of international consensus criteria for diagnosis and classification of behavioral variant frontotemporal dementia.
Neurodegenerative disease
- Must be present for any FTD clinical syndrome
- Shows progressive deterioration of behavior and/or cognition by observation or history
Possible bvFTD
- Three of the features (A–F) must be present; symptoms should occur repeatedly:
- A. Early (within first 3 years) behavioral disinhibition
- B. Early (within first 3 years) apathy or inertia
- C. Early (within first 3 years) loss of sympathy or empathy
- D. Early (within first 3 years) perseverative, stereotyped or compulsive/ritualistic behavior
- E. Hyperorality and dietary changes
- F. Neuropsychological profile: executive dysfunction with relative sparing of memory and visuospatial functions
Probable bvFTD
- All the following criteria must be present to meet diagnosis
- A. Meets criteria for possible bvFTD
- B. Significant functional decline
- C. Imaging results consistent with bvFTD (frontal and/or anterior temporal atrophy on CT or MRI or frontal hypoperfusion or hypometabolism on SPECT or PET)
Definite bvFTD
- Criteria A and either B or C must be present to meet diagnosis:
- A. Meets criteria for possible or probable bvFTD
- B. Histopathological evidence of FTLD on biopsy at post mortem
- C. Presence of a known pathogenic mutation
Exclusion criteria for bvFTD
- Criteria A and B for possible bvFTD must both be answered negatively; criterion C can be positive for possible bvFTD but must be negative for probable bvFTD:
- A. Pattern of deficits is better accounted for by other nondegenerative nervous system or medical disorders
- B. Behavioral disturbance is better accounted for by a psychiatric diagnosis
- C. Biomarkers strongly indicative of Alzheimer's disease or other neurodegenerative process
bvFTD: Behavioral-variant frontotemporal dementia; CT: Computerized tomography; FTD: Frontotemporal dementia; FTLD: Frontotemporal lobar degeneration; SPECT: Single-photon emission computed tomography.
Adapted with permission from [72].
Semantic variant primary progressive aphasia
Neurobehavioral findings
Between 20 and 25% of patients diagnosed with FTD have svPPA [2]. While language is fluent, it is characterized by loss of object knowledge, with impoverished content, semantic and paraphasic errors [74]. The initial and prominent symptom is loss of object knowledge [7]. Semantic loss begins with finer distinctions between things such as types of cars, then to differentiating between kinds of vehicles and ultimately loss of semantic knowledge of what vehicles are [75]. While svPPA typically presents with left greater than right anterior temporal atrophy, about 25% of cases present with initial right greater than left involvement [9]. In either case, the contralateral anterior temporal lobe becomes affected with disease progression [76]. As the disease progresses, cross-modal loss of person recognition is also frequently observed [77]. Behavioral changes are present initially in right predominant patients, including lack of interpersonal engagement, lack of empathy, compulsive behaviors (e.g., crossword puzzles) and increased rigidity expressed by strict schedules, restricted food preference and consumption and clock watching [78,79]. The prominence of behavioral symptoms early on may meet criteria for bvFTD, and apathy and disinhibition symptoms similar to bvFTD are visible 5–7 years after symptom onset [80]. It is noteworthy that some patients experience newfound interest and productivity in the verbal arts or visual arts and music as a result of asymmetric neurodegeneration of the anterior temporal lobes [81–83].
Functional capacity
As would be expected, semantic loss and difficulty with voice and facial recognition can be devastating to one's ability to engage socially, whether through written correspondence, on the phone or face-to-face [6]. While these ADLS are often impaired initially, the typically slower rate of progression means that svPPA patients have preservation of basic ADLs for much longer when compared with bvFTD and nfvPPA patients [84,85]. Driving skills may remain intact until more moderate stages of the disease [86], and language-mediated activities may remain intact, despite a patient's inability to name items or communicate their intentions [86].
Neurocognitive findings
On formal testing svPPA patients show semantic knowledge loss [9,72], with relatively preserved episodic memory, particularly visual memory, visuospatial abilities and executive functions early on. Tests of confrontation naming, single-word comprehension, category fluency and word-picture matching are helpful in isolating the principal object knowledge loss deficit associated with svPPA [6,87]. Additionally, svPPA patients make regularization errors when asked to read aloud or engage in written dictation, where irregular words are read or spelled according to letter-sound rules [88,89].
Socio-emotional functioning
While early changes in personality and behavior are frequently present in svPPA patients [79,90], right predominant anterior temporal lobe dysfunction has been more strongly associated with loss of empathy, social awkwardness, loss of insight, occupational difficulties and face recognition problems [9,76,91]. Restricted diet and food fads may also be present. In comparison to bvFTD patients, right predominant svPPA patients are more rigid, and exhibit unique compulsions and eating disorders [6]. Right anterior temporal lobe dysfunction has also been implicated in theory of mind deficits [92]. In contrast, left predominant dysfunction is accompanied by more frank object knowledge loss.
Diagnostic criteria
With respect to svPPA and nfvPPA, recent international consortium criteria from 2011 also provide three levels of diagnostic certainty for a diagnosis of svPPA or nfvPPA: clinical diagnosis, neuroimaging-supported diagnosis and definite [7]. A summary of these criteria can be found in Box 2. Inclusion criteria for any of the three PPA variants requires the presence of three core features: language difficulty as the most prominent clinical feature, language difficulty as the cause of impaired daily living activities and the presence of aphasia as the most significant deficit at symptom onset and during the initial phase of the disease. Additionally, there can be no prominent initial behavioral disturbance, episodic or visual memory impairments or visuoperceptual difficulties, and the symptoms cannot be better accounted for by psychiatric or nondegenerative nervous system or medical disorders.
Box 2. Summary of international consensus criteria for clinical diagnosis and classification of primary progressive aphasia.
PPA
Most prominent clinical feature is a difficulty with language (object knowledge loss, word-finding difficulties, paraphasias, effortful speech and grammatical deficits)
Language deficits are the principal cause of impaired daily living activities
Aphasia should be the most prominent deficit at symptom onset and for the initial phases of the disease (prominent behavioral, memory and/or visuospatial deficits should not be present at the onset)
No other conditions better account for the language deficits present (i.e., nondegenerative or psychiatric conditions)
svPPA
Impaired confrontation naming and single-word comprehension, explained by semantic knowledge loss
At least three of the following additional features:
Impaired object knowledge, particularly for low-frequency items
Surface dyslexia or dysgraphia
Spared repetition
Spared speech production (grammar and motor speech)
nfvPPA
Either agrammatism or motor speech disorders with effortful, halting speech and inconsistent speech sound errors (apraxia of speech)
At least two of the following additional features:
Impaired comprehension of syntactically complex sentences
Spared single-word comprehension
Spared object knowledge
lvPPA
Impaired single-word retrieval in spontaneous speech (speech fluency interrupted by word finding pauses) and confrontational naming, and impaired repetition of sentences and phrases
At least three of the following additional features:
Speech (phonologic) errors in spontaneous speech and naming
Spared single-word comprehension and object knowledge
Spared motor speech
Absence of frank agrammatism
lvPPA: Logopenic variant primary progressive aphasia; nfvPPA: Nonfluent variant primary progressive aphasia; PPA: Primary progressive aphasia; svPPA: Semantic variant primary progressive aphasia.
Adapted with permission from [7].
Clinical diagnosis of svPPA requires both impaired confrontation naming, and single-word comprehension, with at least 3 out of 4 additional core features (impaired object knowledge, surface dyslexia or dysgraphia, spared repetition and spared speech production). In addition, neuroimaging consistent with svPPA is required for an imaging-supported diagnosis of the syndrome, and must show either predominant anterior temporal lobe atrophy, or predominant anterior temporal hypoperfusion or hypometabolism on SPECT or PET, or both. Definite svPPA requires presence of the clinical syndrome with genetic or pathological confirmation of FTLD.
Nonfluent variant primary progressive aphasia
Neurobehavioral findings
In keeping with PPA syndromes, language is the initial and prominent symptom marked by apraxia of speech (AOS) resulting in interrupted speech with pauses both within and between utterances [93]. Agrammatism and para-phasic errors can be present [7], and distortion of prosody (the pitch pattern used to provide emotional content and alert the listener of questions and emphasis) [94]. Difficulty with comprehension of syntactically complex sentences may also be present. As the disease progresses, the patients may become mute [95]. Acquisition of a speech sample is the best clinical measure to capture agrammatism and diminished rate of speech [96]. Aberrant behavior is not usually present in the initial presentation of nfvPPA, with neuropsychiatric inventories comparable to the logopenic variant of primary progressive aphasia (lvPPA) and AD [79], though may manifest as the disease progresses [97,98]. As in svPPA, some nfvPPA patients experience newfound interest and productivity in the verbal arts or visual arts and music as a result of asymmetric neurodegeneration of the anterior temporal lobes [83]. Seeley et al. has hypothesized the gradual loss of function in the language-dominant anterior temporal lobe leads to the release or remodeling of function in the nondominant hemisphere posterior structures [99].
Functional capacity
Similar to svPPA patients, the frank impairments in speech, writing and reading present early on in nfvPPA patients and significantly impact their ability to engage in interpersonal interactions [6]. These impairments can be severe by the second year into disease course [100]. In contrast, basic ADLs remain comparatively preserved for many years. As the disease progresses, patients may become mute and immobile, with more severe impairments in sentence comprehension and following of multistep instruction. Advanced stage of the disease has also been associated with features of bvFTD [101].
Neurocognitive findings
In conjunction with language difficulties, nfvPPA patients can exhibit executive dysfunction including: verbal fluency (e.g., letter fluency), working memory (e.g., digits backward) and set-shifting (e.g., Trail Making) [102]. Episodic memory and visuospatial functioning are relatively preserved in nfvPPA, but may deteriorate over the disease course [102], as well as in cases where nfvPPA is secondary to cortical basal degeneration (CBD) [103].
Socio-emotional functioning
nfvPPA patients exhibit fewer deficits in socio-emotional functioning than svPPA patients, though recent research has demonstrated that individuals with nfvPPA have selective deficits interpreting emotion from vocal prosody [104,105]. Couto et al. showed differential patterns of regional gray matter atrophy associated with social cognition deficits between bvFTD and nfvPPA patients, suggesting that socio-emotional function deficits in nfvPPA may be a consequence of more basic dysfunction of face and emotion recognition [106].
Diagnostic criteria
A clinical diagnosis of nfvPPA requires either agrammatism in language production or effortful, halting speech with inconsistent speech sound errors and distortions (AOS), along with two of the three remaining core features (impaired comprehension of syntactically complex sentences, spared single-word comprehension and spared object knowledge). An imaging-supported diagnosis of nfvPPA requires neuroimaging consistent with this syndrome, and must show either predominant left posterior fronto-insular atrophy on MRI, or predominant left posterior fronto-insular hypoperfusion or hypometabolism on SPECT or PET, or both [7]. See Figure 1 for representative MRI imaging of each of the FTD subtypes. Finally, definite nfvPPA requires presence of the clinical syndrome with genetic or pathological confirmation of FTLD.
Figure 1. T1-weighted structural MRI in patients with behavioral variant frontotemporal dementia, semantic variant primary progressive aphasia and nonfluent variant primary progressive aphasia.
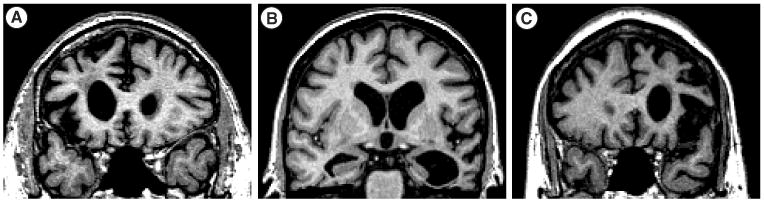
(A) Behavioural variant frontotemporal dementia (B) semantic variant frontotemporal dementia and (c) nonfluent variant primary progressive aphasia. Images are in radiological view (right hemisphere is on the left side of the image).
Syndromic overlap
Psychiatric syndromes
FTD can overlap in presentation with a number of psychiatric disorders, such as: late onset bipolar disorder, borderline personality disorder, late onset schizophrenia, obsessive-compulsive disorder and depression [107–109]. For example, in a study of 69 patients diagnosed with bvFTD, 51% received a prior psychiatric diagnosis. Younger age, higher education and a family history of psychiatric illness increase the likelihood of a prior psychiatric diagnosis. It is difficult to retrospectively invalidate the diagnosis of a psychiatric disorder, and it is possible that psychiatric conditions may represent a risk factor for bvFTD. Nevertheless, the preponderance of psychiatric diagnoses given to individuals ultimately diagnosed with bvFTD underscores the complexities of the disease course, and the overlap with many psychiatric conditions. In addition, genetic mutations have been implicated with psychiatric disease in FTD. The presence of the C9ORF72 mutation has been associated with a family history of psychiatric illness in both FTD and motor neuron disease [110,111].
Cortical basal degeneration & progressive supranuclear palsy
The clinical syndrome of FTD overlaps with several other FTLD and AD pathological syndromes [112–115]. The atypical parkinsonian spectrum disorders of PSP and CBD can both exhibit cognitive and behavioral changes characteristic of bvFTD (e.g., executive dysfunction, apathy and impulsivity). They may also show language impairment similar to that of nfvPPA. Extrapyramidal features are characteristic of PSP, along with supranuclear gaze restriction, constrained vertical gaze and slowed saccades. CBS presentation can include apraxia, cortical sensory deficits, rigidity and bradykinesia. While movement findings are typical in these syndromes, a behavioral syndrome without attendant movement disorder can be present with both PSP and CBS pathology.
Frontal variant Alzheimer's disease
While rare, an atypical frontal variant Alzheimer's disease (fvAD) can also present as a behavioral and dysexecutive syndrome [116–119]. When compared with typical AD patients, performance on tests of executive function are more impaired, and pathology confirmed cases of fvAD show marked plaque and neurofbrillary tangle deposition in the frontal cortex.
Motor neuron disease
The clinical syndrome and underlying pathology of FTD can also be present in motor neuron disease, including amyotrophic lateral sclerosis (ALS) [120–122]. ALS typically progresses faster than FTD with time-dependent survival at 5 and 10 years from diagnosis of 23.4 and 11.8%, respectively [123]. As many as half of ALS patients present with executive dysfunction [124,125], and about 15% of bvFTD patients eventually develop symptoms of ALS [122]. In cases of familial ALS, approximately a third are due to C9ORF72 expansion [126]. A distinct clinical profile is associated with C9ORF72 expansion carriers, including loss of empathy, disinhibition, apathy and psychotic behaviors, which can resemble aspects of the FTD clinical profile [126]. Research and assessment of patients with ALS provides the opportunity to better understand prodromal symptoms of bvFTD given their likelihood of developing the syndrome over their disease course.
Logopenic variant primary progressive aphasia
lvPPA represents a third PPA variant; however, this syndrome it is not classified as an FTD given the predominance of Alzheimer's pathology associated with this syndrome [127–129]. The clinical presentation of lvPPA includes minimal verbal output and intermittent disruptions in fluency, with relatively spared grammar and oral motor speech. On neuroimaging focal neurodegeneration is found in the left temporoparietal junction [7,67,130]. Diagnostic criteria for lvPPA include impaired single-word retrieval in spontaneous speech and confrontational naming, and impaired repetition of sentences and phrases. It is hypothesized that problems of short-term auditory phonological memory is a key cognitive deficit contributing to most language impairment in lvPPA, as verbal immediate recall is a core factor in sentence and phrase repetition, and comprehension of longer and grammatically complex sentences. Spontaneous speech (phonologic) errors are also common in lvPPA.
Differentiating lvPPA from svPPA and nfvPPA can be difficult, but comparison of the quality of spontaneous speech is a useful diagnostic technique. Specifically, lvPPA can be differentiated from svPPA by the absence of agrammatism and spared single-word comprehension and object knowledge, and it can differentiated from nfvPPA by the sparing of motor speech [131]. While both lvPPA and nfvPPA patients tend to present with slowed spontaneous speech and hesitations, there are distinct qualitative differences in speech presentation. Patients with lvPPA have been described as having frequent word-finding pauses with syntactically simple, though intact, utterances [132]. These patients tend to have pauses between words and often exhibit phonological paraphasias, but with motor capabilities intact. By contrast, patients with nfvPPA tend to exhibit stuttering, slurring or pausing within words due to oral motor articulation difficulties.
Pharmacological intervention
To date, no disease-modifying treatments exist that change the course of this neurodegenerative disease, and as such, the focus of treatment across subtypes is necessarily symptomatic. Behavioral symptoms are usually the focus of intervention, though executive dysfunction and working memory deficits can also be a treatment focus [133,134]. Treatment with antidepressants has fewer potential side effects than neuroleptics, and modest behavioral improvement has been shown with selective serotonin reuptake inhibitors [135,136] and trazodone [137]. The use of neuroleptics such as olanzapine has been shown to treat aggression, agitation and psychosis, with case studies supporting the efficacy of risperidone and aripiprazole [138–140]. While studies on anticholinesterase inhibitors in FTD have been limited, research to date leaves its status as a pharmacological treatment suspect. Treatment with Donepezil led to worsening neuropsychiatric symptoms without cognitive improvement [141]. Rivastigmine has been reported to improve neuropsychiatric symptoms, with concomitant gains in caregiver stress [142]. A trend toward efficacy of Galantamine has been reported in a subset of aphasic FTD and PPA patients, though this effect may have included lvPPA patients with underlying Alzheimer's pathology [143]. Large studies administering Memantine have shown no treatment gains [144,145]. Importantly, while no approved pharmacological treatments exist, advances in knowledge of the underlying molecular and genetic causes of FTD have clarified the foci of research for randomized control trials. Targets of clinical development for specific FTD therapies include progranulin replacement and immunomodulation in svPPA [31,146–148].
Caregivers
There are important implications for family caregivers of patients with FTD, as care and management of these patients can be demanding. Studies evaluating caregiver stress in FTD have found caregivers of FTD patients report higher levels of generalized distress burden and report feeling less competent than caregivers of AD patients [149–152]. One study found that caregivers of patients with svPPA and nfvPPA reported similar distress as caregivers of AD, while bvFTD caregivers reported the most distress [153]. In bvFTD, changes in behavior regardless of disease severity have been correlated with caregiver distress [151], and caregiver burden has been shown to increase across FTD syndromes as a result of the combination of disease progression, relationship changes and caregiver depression [153]. One study found that FTD caregiver report of depression accounted for 58% of the variance of generalized stress scores within the caregiver sample, suggesting a significant role for personal health in caregiver stress and coping [152]. A study comparing bvFTD with svPPA found that caregivers of bvFTD patients reported poorer sleep quality and more frequent use of sleep medications [154]. Apathy and its association with lowered daytime activity resulted in increased emotional distress for bvFTD caregivers [155].
Caregiver reporting of symptomology and treatment gains from therapeutic interventions is critically important in the management of care for persons suffering from FTD [134]. To that end, specialty nurse clinics educating caregivers about the best nonpharmacologic behavioral strategies, identification of triggers for problematic behaviors and increase coping skills have shown success in reducing caregiver stress and improving caregiver tools for the management of behavioral symptoms [156,157]. Five weekly, one-on-one sessions focused on building skills in mindfulness and positive appraisal improved positive affect and psychological outcomes for family caregivers of FTD patients [158]. Recent reviews have comprehensively outlined therapeutic recommendations for PPA patients and caregivers, addressing issues such as cognitive rehabilitation, speech therapy, meal preparation, telephone communication, driving, shopping, financial matters, written correspondence, medications, leisure activities and issues of constitution [84,159,160]. Resources such as The Association for Frontotemporal Degeneration [161], the Frontotemporal Dementia Research Group [162] and the Association for Frontotemporal Dementias [163] provide peer caregiver support and information for those caring for a loved one with FTD.
Conclusion & future perspective
FTD is a common cause of early-onset dementia, and is often accompanied by psychiatric symptoms of depression, obsessions, compulsions, mania, as well as psychotic symptoms. In the case of svPPA or nfvPPA, initial and prominent language difficulties are present. The progressive deterioration of socio-emotional and language abilities – two uniquely human abilities – is devastating, particularly when onset of the disease is in late middle age. Awareness and recognition of the diversity of possible presentations associated with FTD can aid in early and accurate diagnosis.
The combination of comprehensive history, neuropsychological testing, measures of social cognition, as well as advances in neuroimaging modalities, all aid in earlier and more accurate diagnosis of FTD. The last decade has also seen a dramatic increase in our understanding of the distinct kinds of molecular changes responsible for the pathology involved in frontotemporal neurodegenerative processes; yet, accurate prediction of the underlying neuropathology responsible for FTD syndromes during life remains a challenge. The development of increasingly sensitive psychiatric and cognitive diagnostic measures and identification of salient biomarkers in the next decade may improve the predictive power of underlying neuropathology. Close longitudinal monitoring of patients may also provide additional data relating patterns of cognitive, behavioral and socioemotional functioning to underlying neuropathology.
Progressive monitoring of speech and language impairments has been developed for PPA syndromes [164]. Given the prominent socioemotional and behavioral symptoms associated with FTD syndromes throughout disease course, there is a great need to further develop clinical tests to operationalize decision-making impairments [165], methods for tracking progression of impairment [84] and ways to prospectively identify patients at greatest risk of harm in the presence of these impairments.
To date, no disease-modifying agents have been developed that effectively prevent, cure or slow the progression of FTD. While current pharmacological treatments focus on symptom management and support, we hope to see the development of pharmacological interventions in the next decade. Specifically, the creation of neuroprotective medications for those patients at risk for developing FTD may help delay the onset of disease. The characterization of distinct proteinopathies, including tau and TDP-43, has led to the development of clinical trials targeting these molecular abnormalities. While trials have yet to successfully report significant treatment gains, future trials hold out hope for remediating the effects of FTD disease processes.
Practice points.
Frontotemporal dementia is currently divided into three subtypes: behavioral variant frontotemporal dementia, semantic variant of primary progressive aphasia and nonfluent variant of primary progressive aphasia.
Diagnosis of patients with suspected frontotemporal dementia should include: detailed history from patient and informant; neurologic examination; neuropsychological evaluation; structural brain imaging with MRI and assessment of socioemotional functioning and speech and language.
Treatment is supportive and symptomatic with targeted pharmacologic interventions, and nonpharmacologic approaches, including: exercise, psychosocial therapy, physical therapy and speech therapy.
Referral for enrollment in research studies at specialized medical centers should be considered.
Acknowledgments
The authors express their appreciation and gratitude to the participants in research projects at the UCSF Memory and Aging Center and their families for their invaluable contributions to our understanding of neurodegenerative disease.
Footnotes
Financial & competing interests disclosure: The authors have no relevant affliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Brunnstrom H, Gustafson L, Passant U, et al. Prevalence of dementia subtypes: a 30-year retrospective survey of neuropathological reports. Arch Gerontol Geriatr. 2009;49(1):146–149. doi: 10.1016/j.archger.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62(6):925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- 3.Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 4.Pasquier F, Petit H. Frontotemporal dementia: its rediscovery. Eur Neurol. 1997;38(1):1–6. doi: 10.1159/000112894. [DOI] [PubMed] [Google Scholar]
- 5.Perry RJ, Graham A, Williams G, et al. Patterns of frontal lobe atrophy in frontotemporal dementia: a volumetric MRI study. Dement Geriatr Cogn Disord. 2006;22(4):278–287. doi: 10.1159/000095128. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24(5):375–398. doi: 10.2165/11533100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. Provides the new framework for the classification and diagnosis of primary progressive aphasia (PPA) subtypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rankin KP, Baldwin E, Pace-Savitsky C, et al. Self-awareness and personality change in dementia. J Neurol Neurosurg Psychiatry. 2005;76(5):632–639. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61(9):1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- 10.Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry. 2013;25(2):130–137. doi: 10.3109/09540261.2013.776523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mioshi E, Kipps CM, Dawson K, et al. Activities of daily living in frontotemporal dementia and Alzheimer disease. Neurology. 2007;68(24):2077–2084. doi: 10.1212/01.wnl.0000264897.13722.53. [DOI] [PubMed] [Google Scholar]
- 12.Roberson ED, Hesse JH, Rose KD, et al. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology. 2005;65(5):719–725. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]
- 13.Rascovsky K, Salmon DP, Lipton AM, et al. Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology. 2005;65(3):397–403. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- 14.Brun A. Frontal lobe degeneration of non-Alzheimer type. I. Neuropathology. Arch Gerontol Geriatr. 1987;6:193–208. doi: 10.1016/0167-4943(87)90021-5. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119(1):1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114(1):31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- 17•.Rohrer JD, Rosen HJ. Neuroimaging in frontotemporal dementia. Int Rev Psychiatry. 2013;25(2):221–229. doi: 10.3109/09540261.2013.778822. Provides a review of current techniques and findings in neuroimaging studies of frontotemporal dementia (FTD) subtypes. [DOI] [PubMed] [Google Scholar]
- 18.Mackenzie IR, Foti D, Woulfe J, et al. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008;131(Pt 5):1282–1293. doi: 10.1093/brain/awn061. [DOI] [PubMed] [Google Scholar]
- 19.Neumann M, Rademakers R, Roeber S, et al. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132(Pt 11):2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodges JR, Mitchell J, Dawson K, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;133(Pt 1):300–306. doi: 10.1093/brain/awp248. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, Shaw CL, Du Plessis D, et al. Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol. 2005;110(5):501–512. doi: 10.1007/s00401-005-1079-4. [DOI] [PubMed] [Google Scholar]
- 22.Rosso SM, Donker Kaat L, Baks T, et al. Frontotemporal dementia in the Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126(Pt 9):2016–2022. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- 23.Goldman JS, Farmer JM, Wood EM, et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology. 2005;65(11):1817–1819. doi: 10.1212/01.wnl.0000187068.92184.63. [DOI] [PubMed] [Google Scholar]
- 24.Rohrer JD, Guerreiro R, Vandrovcova J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73(18):1451–1456. doi: 10.1212/WNL.0b013e3181bf997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitwell JL, Jack CR, Jr, Boeve BF, et al. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology. 2009;72(9):813–820. doi: 10.1212/01.wnl.0000343851.46573.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snowden JS, Rollinson S, Thompson JC, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135(Pt 3):693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majounie E, Abramzon Y, Renton AE, et al. Repeat expansion in C9ORF72 in Alzheimer's disease. N Engl J Med. 2012;366(3):283–284. doi: 10.1056/NEJMc1113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sha SJ, Takada LT, Rankin KP, et al. Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology. 2012;79(10):1002–1011. doi: 10.1212/WNL.0b013e318268452e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 30.Bettcher BM, Kramer JH. Inflammation and clinical presentation in neurodegenerative disease: a volatile relationship. Neurocase. 2013;19(2):182–200. doi: 10.1080/13554794.2011.654227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boxer AL, Gold M, Huey E, et al. Frontotemporal degeneration, the next therapeutic frontier: molecules and animal models for frontotemporal degeneration drug development. Alzheimers Dement. 2013;9(2):176–188. doi: 10.1016/j.jalz.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BS, Jun IG, Kim SH, et al. Intrathecal gabapentin increases interleukin-10 expression and inhibits pro-inflammatory cytokine in a rat model of neuropathic pain. J Korean Med Sci. 2013;28(2):308–314. doi: 10.3346/jkms.2013.28.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;3324(6028):478–48. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller ZA, Rankin KP, Graff-Radford NR, et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. J Neurol Neurosurg Psychiatry. 2013;84(9):956–962. doi: 10.1136/jnnp-2012-304644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller ZA, Mandelli ML, Rankin KP, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013;136(Pt 11):3461–3473. doi: 10.1093/brain/awt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogalski E, Johnson N, Weintraub S, et al. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol. 2008;65(2):244–248. doi: 10.1001/archneurol.2007.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sollberger M, Neuhaus J, Ketelle R, et al. Interpersonal traits change as a function of disease type and severity in degenerative brain diseases. J Neurol Neurosurg Psychiatry. 2011;82(7):732–739. doi: 10.1136/jnnp.2010.205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65(2):249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeley WW. Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr Opin Neurol. 2008;21(6):701–707. doi: 10.1097/WCO.0b013e3283168e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snowden JS, Bathgate D, Varma A, et al. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70(3):323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bozeat S, Gregory CA, Ralph MA, et al. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J Neurol Neurosurg Psychiatry. 2000;69:178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postiglione A, Milan G, Pappata S, et al. Fronto-temporal dementia presenting as Geschwind's syndrome. Neurocase. 2008;14(3):264–270. doi: 10.1080/13554790802269976. [DOI] [PubMed] [Google Scholar]
- 43.Manes FF, Torralva T, Roca M, et al. Frontotemporal dementia presenting as pathological gambling. Nat Rev Neurol. 2010;6(6):347–352. doi: 10.1038/nrneurol.2010.34. [DOI] [PubMed] [Google Scholar]
- 44.Hornberger M, Shelley BP, Kipps CM, et al. Can progressive and non-progressive behavioral variant frontotemporal dementia be distinguished at presentation? J Neurol Neurosurg Psychiatry. 2009;80(6):591–593. doi: 10.1136/jnnp.2008.163873. [DOI] [PubMed] [Google Scholar]
- 45.Davies RR, Kipps CM, Mitchell J, et al. Progression in frontotemporal dementia: identifying a benign behavioral variant by magnetic resonance imaging. Arch Neurol. 2006;63(11):1627–1631. doi: 10.1001/archneur.63.11.1627. [DOI] [PubMed] [Google Scholar]
- 46.Piguet O, Hornberger M, Mioshi E, et al. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10(2):162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- 47.Kipps CM, Hodges JR, Fryer TD, et al. Combined magnetic resonance imaging and positron emission tomography brain imaging in behavioural variant frontotemporal degeneration: refining the clinical phenotype. Brain. 2009;132(Pt9):2566–2578. doi: 10.1093/brain/awp077. [DOI] [PubMed] [Google Scholar]
- 48.Kipps CM, Hodges JR, Hornberger M. Nonprogressive behavioural frontotemporal dementia: recent developments and clinical implications of the ‘bvFTD phenocopy syndrome’. Curr Opin Neurol. 2010;23(6):628–632. doi: 10.1097/WCO.0b013e3283404309. [DOI] [PubMed] [Google Scholar]
- 49.Rosen HJ, Narvaez JM, Hallam B, et al. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alzheimer Dis Assoc Disord. 2004;18(4):202–207. [PubMed] [Google Scholar]
- 50.Wicklund AH, Johnson N, Rademaker A, et al. Profiles of decline in activities of daily living in non-Alzheimer dementia. Alzheimer Dis Assoc Disord. 2007;21(1):8–13. doi: 10.1097/WAD.0b013e3180324549. [DOI] [PubMed] [Google Scholar]
- 51.Rosen HJ, Allison SC, Schauer GF, et al. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(Pt 11):2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turk K, Dugan E. Research brief: a literature review of frontotemporal dementia and driving. Am J Alzheimers Dis Other Demen. 2014 doi: 10.1177/1533317513518656. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hornberger M, Piguet O, Graham AJ, et al. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology. 2010;74(6):472–479. doi: 10.1212/WNL.0b013e3181cef85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hornberger M, Piguet O, Kipps C, et al. Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology. 2008;71(19):1481–1488. doi: 10.1212/01.wnl.0000334299.72023.c8. [DOI] [PubMed] [Google Scholar]
- 55.Possin KL, Lamarre AK, Wood KA, et al. Ecological validity and neuroanatomical correlates of the NIH EXAMINER executive composite score. J Int Neuropsychol Soc. 2014;20(1):20–28. doi: 10.1017/S1355617713000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larner AJ. Can the Frontal Assessment Battery (FAB) help in the diagnosis of behavioural variant frontotemporal dementia? A pragmatic study. Int J Geriatr Psychiatry. 2013;28(1):106–107. doi: 10.1002/gps.3780. [DOI] [PubMed] [Google Scholar]
- 57.Possin KL, Brambati SM, Rosen HJ, et al. Rule violation errors are associated with right lateral prefrontal cortex atrophy in neurodegenerative disease. J Int Neuropsychol Soc. 2009;15(3):354–364. doi: 10.1017/S135561770909050X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rankin KP, Gorno-Tempini ML, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129(Pt 11):2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sollberger M, Rosen HJ, Shany-Ur T, et al. Neural substrates of socioemotional self-awareness in neurodegenerative disease. Brain Behav. 2014;4(2):201–214. doi: 10.1002/brb3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44(6):950–958. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Duque D, Black SE. Impaired recognition of negative facial emotions in patients with frontotemporal dementia. Neuropsychologia. 2005;43(11):1673–1687. doi: 10.1016/j.neuropsychologia.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Shany-Ur T, Poorzand P, Grossman SN, et al. Comprehension of insincere communication in neurodegenerative disease: lies, sarcasm, and theory of mind. Cortex. 2012;48(10):1329–1341. doi: 10.1016/j.cortex.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosen HJ, Alcantar O, Zakrzewski J, et al. Metacognition in the behavioral variant of frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2014;28(3):436–447. doi: 10.1037/neu0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiong W, Wilson SM, D'Esposito M, et al. The salience network causally influences default mode network activity during moral reasoning. Brain. 2013;136(Pt 6):1929–1941. doi: 10.1093/brain/awt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumfor F, Irish M, Leyton C, et al. Tracking the progression of social cognition in neurodegenerative disorders. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2013-307098. doi:10.1136jnnp-2013307098. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Perry DC, Sturm VE, Wood KA, et al. Divergent processing of monetary and social reward in behavioral variant frontotemporal dementia and Alzheimer disease. AlzheimerDis Assoc Disord. 2013 doi: 10.1097/WAD.0000000000000012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahman S, Sahakian BJ, Hodges JR, et al. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain. 1999;122(Pt 8):1469–1493. doi: 10.1093/brain/122.8.1469. [DOI] [PubMed] [Google Scholar]
- 68.Torralva T, Kipps CM, Hodges JR, et al. The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia. 2007;45(2):342–349. doi: 10.1016/j.neuropsychologia.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 69.Bechara A, Damasio AR, Damasio H, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 70.Shinagawa S, Ikeda M, Nestor PJ, et al. Characteristics of abnormal eating behaviours in frontotemporal lobar degeneration: a cross-cultural survey. J Neurol Neurosurg Psychiatry. 2009;80(12):1413–1414. doi: 10.1136/jnnp.2008.165332. [DOI] [PubMed] [Google Scholar]
- 71.Woolley JD, Khan BK, Natesan A, et al. Satiety-related hormonal dysregulation in behavioral variant frontotemporal dementia. Neurology. 2014;82(6):512–520. doi: 10.1212/WNL.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72••.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. Provides the new framework for the classification and diagnosis of behavioral variant FTD (bvFTD) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamarre AK, Rascovsky K, Bostrom A, et al. Interrater reliability of the new criteria for behavioral variant frontotemporal dementia. Neurology. 2013;80(21):1973–1977. doi: 10.1212/WNL.0b013e318293e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–346. doi: 10.1002/ana.10825. Provides comprehensive information regarding the cognitive deficits and underlying patterns of neurodegeneration for each of the PPA subtypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hodges JR, Patterson K, Oxbury S, et al. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 76.Seeley WW, Bauer AM, Miller BL, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64(8):1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127(Pt 4):860–872. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- 78.Henry ML, Wilson SM, Ogar JM, et al. Neuropsychological, behavioral, and anatomical evolution in right temporal variant frontotemporal dementia: a longitudinal and post-mortem single case analysis. Neurocase. 2014;20(1):100–109. doi: 10.1080/13554794.2012.732089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosen HJ, Allison SC, Ogar JM, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67(10):1752–1756. doi: 10.1212/01.wnl.0000247630.29222.34. [DOI] [PubMed] [Google Scholar]
- 80.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 81.Wu TQ, Miller ZA, Adhimoolam B, et al. Verbal creativity in semantic variant primary progressive aphasia. Neurocase. 2013 doi: 10.1080/13554794.2013.860179. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chatterjee A. The neuropsychology of visual artistic production. Neuropsychologia. 2004;42(11):1568–1583. doi: 10.1016/j.neuropsychologia.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 83.Miller BL, Cummings J, Mishkin F, et al. Emergence of artistic talent in frontotemporal dementia. Neurology. 1998;51(4):978–982. doi: 10.1212/wnl.51.4.978. [DOI] [PubMed] [Google Scholar]
- 84.Mioshi E, Hsieh S, Savage S, et al. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74(20):1591–1597. doi: 10.1212/WNL.0b013e3181e04070. [DOI] [PubMed] [Google Scholar]
- 85.Mioshi E, Hodges JR. Rate of change of functional abilities in frontotemporal dementia. Dement Geriatr Cogn Disord. 2009;28(5):419–426. doi: 10.1159/000255652. [DOI] [PubMed] [Google Scholar]
- 86•.O'connor C, Ahmed S, Mioshi E. Functional disability in primary progressive aphasia. Aphasiology. 2014;28:8–9. 1131–1149. Describes the patterns of functional disability associated with each of the PPA subtypes and provides recommendations for strategies addressing specific functional impairments. [Google Scholar]
- 87.Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer's disease. Neurology. 2000;54(12):2277–2284. doi: 10.1212/wnl.54.12.2277. [DOI] [PubMed] [Google Scholar]
- 88.Shim H, Hurley RS, Rogalski E, et al. Anatomic, clinical, and neuropsychological correlates of spelling errors in primary progressive aphasia. Neuropsychologia. 2012;50(8):1929–1935. doi: 10.1016/j.neuropsychologia.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kertesz A, Davidson W, Mccabe P. Primary progressive semantic aphasia: a case study. J Int Neuropsychol Soc. 1998;4(4):388–398. [PubMed] [Google Scholar]
- 90.Kertesz A, Jesso S, Harciarek M, et al. What is semantic dementia?: a cohort study of diagnostic features and clinical boundaries. Arch Neurol. 2010;(67):483–489. doi: 10.1001/archneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- 91.Mychack P, Kramer JH, Boone KB, et al. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56(11 Suppl 4):S11–15. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- 92.Irish M, Hodges JR, Piguet O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain. 2014;137(Pt 4):1241–1253. doi: 10.1093/brain/awu003. [DOI] [PubMed] [Google Scholar]
- 93.Knibb JA, Woollams AM, Hodges JR, et al. Making sense of progressive non-fluent aphasia: an analysis of conversational speech. Brain. 2009;132(Pt 10):2734–2746. doi: 10.1093/brain/awp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11(6):545–555. doi: 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gorno-Tempini ML, Ogar JM, Brambati SM, et al. Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology. 2006;67(10):1849–1851. doi: 10.1212/01.wnl.0000237038.55627.5b. [DOI] [PubMed] [Google Scholar]
- 96.Wilson SM, Henry ML, Besbris M, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(Pt 7):2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mesulam MM, Grossman M, Hillis A, et al. The core and halo of primary progressive aphasia and semantic dementia. Ann Neurol. 2003;54(Suppl 5):S11–14. doi: 10.1002/ana.10569. [DOI] [PubMed] [Google Scholar]
- 98.Le Rhun E, Richard F, Pasquier F. Natural history of primary progressive aphasia. Neurology. 2005;65(6):887–891. doi: 10.1212/01.wnl.0000175982.57472.84. [DOI] [PubMed] [Google Scholar]
- 99.Seeley WW, Matthews BR, Crawford RK, et al. Unraveling Bolero: progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;(131):39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- 100.Jang J, Cushing N, Clemson L, et al. Activities of daily living in progressive non-fluent aphasia, logopenic progressive aphasia and Alzheimer's disease. Dement Geriatr Cogn Disord. 2012;33(5):354–360. doi: 10.1159/000339670. [DOI] [PubMed] [Google Scholar]
- 101•.Harciarek M, Sitek EJ, Kertesz A. The patterns of progression in primary progressive aphasia: implications for assessment and management. Aphasiology. 2014;28:8–9. 964–980. Summarizes current knowledge regarding the assessment, course and management of PPA subtypes. [Google Scholar]
- 102.Libon DJ, Xie SX, Moore P, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68(5):369–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- 103.Murray R, Neumann M, Forman MS, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68(16):1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- 104.Rohrer JD, Sauter D, Scott S, et al. Receptive prosody in nonfluent primary progressive aphasias. Cortex. 2012;48(3):308–316. doi: 10.1016/j.cortex.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105•.Shany-Ur T, Rankin KP. Personality and social cognition in neurodegenerative disease. Curr Opin Neurol. 2011;24(6):550–555. doi: 10.1097/WCO.0b013e32834cd42a. Summarizes current knowledge of personality change and social cognition deficits in neurodegenerative disease, including each of the FTD subtypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Couto B, Manes F, Montanes P, et al. Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front Hum Neurosci. 2013;7:467. doi: 10.3389/fnhum.2013.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107•.Woolley JD, Khan BK, Murthy NK, et al. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72(2):126–133. doi: 10.4088/JCP.10m06382oli. Provides evidence that patients diagnosed with bvFTD are often previously diagnosed with psychiatric diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosness TA, Haugen PK, Passant U, et al. Frontotemporal dementia: a clinically complex diagnosis. Int J Geriatr Psychiatry. 2008;23(8):837–842. doi: 10.1002/gps.1992. [DOI] [PubMed] [Google Scholar]
- 109.Pose M, Cetkovich M, Gleichgerrcht E, et al. The overlap of symptomatic dimensions between frontotemporal dementia and several psychiatric disorders that appear in late adulthood. Int Rev Psychiatry. 2013;25(2):159–167. doi: 10.3109/09540261.2013.769939. [DOI] [PubMed] [Google Scholar]
- 110.Devenney E, Hornberger M, Irish M, et al. Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol. 2014;71(3):331–339. doi: 10.1001/jamaneurol.2013.6002. [DOI] [PubMed] [Google Scholar]
- 111.Byrne S, Heverin M, Elamin M, et al. Aggregation of neurologic and neuropsychiatric disease in amyotrophic lateral sclerosis kindreds: a population-based case-control cohort study of familial and sporadic amyotrophic lateral sclerosis. Ann Neurol. 2013;74(5):699–708. doi: 10.1002/ana.23969. [DOI] [PubMed] [Google Scholar]
- 112.Kertesz A, Mcmonagle P, Blair M, et al. The evolution and pathology of frontotemporal dementia. Brain. 2005;128(Pt 9):1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 113.Rankin KP, Mayo MC, Seeley WW, et al. Behavioral variant frontotemporal dementia with corticobasal degeneration pathology: phenotypic comparison to bvFTD with Pick's disease. J Mol Neurosci. 2011;45(3):594–608. doi: 10.1007/s12031-011-9615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70(2):327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chiu WZ, Papma JM, De Koning I, et al. Midcingulate involvement in progressive supranuclear palsy and tau positive frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2012;83(9):910–915. doi: 10.1136/jnnp-2011-302035. [DOI] [PubMed] [Google Scholar]
- 116.Taylor KI, Probst A, Miserez AR, et al. Clinical course of neuropathologically confirmed frontal-variant Alzheimer's disease. Nat Clin Pract Neurol. 2008;4(4):226–232. doi: 10.1038/ncpneuro0746. [DOI] [PubMed] [Google Scholar]
- 117.Woodward M, Jacova C, Black SE, et al. Differentiating the frontal variant of Alzheimer's disease. Int J Geriatr Psychiatry. 2010;25(7):732–738. doi: 10.1002/gps.2415. [DOI] [PubMed] [Google Scholar]
- 118.Blennerhassett R, Lillo P, Halliday GM, et al. Distribution of pathology in frontal variant Alzheimer's disease. J Alzheimers Dis. 2014;39(1):63–70. doi: 10.3233/JAD-131241. [DOI] [PubMed] [Google Scholar]
- 119.Johnson JK, Head E, Kim R, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56(10):1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 120.Lillo P, Mioshi E, Zoing MC, et al. How common are behavioural changes in amyotrophic lateral sclerosis? Amyotroph Lateral Scler. 2011;12(1):45–51. doi: 10.3109/17482968.2010.520718. [DOI] [PubMed] [Google Scholar]
- 121.Lomen-Hoerth C. Clinical phenomenology and neuroimaging correlates in ALS-FTD. J Mol Neurosci. 2011;45(3):656–662. doi: 10.1007/s12031-011-9636-x. [DOI] [PubMed] [Google Scholar]
- 122.Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59(7):1077–1079. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- 123.Pupillo E, Messina P, Logroscino G, et al. Long-term survival in amyotrophic lateral sclerosis: a population-based study. Ann Neurol. 2014;75(2):287–297. doi: 10.1002/ana.24096. [DOI] [PubMed] [Google Scholar]
- 124.Lomen-Hoerth C, Murphy J, Langmore S, et al. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology. 2003;60(7):1094–1097. doi: 10.1212/01.wnl.0000055861.95202.8d. [DOI] [PubMed] [Google Scholar]
- 125.Murphy JM, Henry RG, Langmore S, et al. Continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Arch Neurol. 2007;64(4):530–534. doi: 10.1001/archneur.64.4.530. [DOI] [PubMed] [Google Scholar]
- 126.Devenney E, Hornberger M, Irish M, et al. Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol. 2014;71(3):31–39. doi: 10.1001/jamaneurol.2013.6002. [DOI] [PubMed] [Google Scholar]
- 127.Mesulam MM, Wieneke C, Thompson C, et al. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135(Pt 5):1537–1553. doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Harris JM, Gall C, Thompson JC, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013;81(21):1832–1839. doi: 10.1212/01.wnl.0000436070.28137.7b. [DOI] [PubMed] [Google Scholar]
- 129.Mesulam MM, Weintraub S, Rogalski EJ, et al. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137(Pt 4):1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rohrer JD, Caso F, Mahoney C, et al. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain Lang. 2013;127(2):121–126. doi: 10.1016/j.bandl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Teichmann M, Kas A, Boutet C, et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain. 2013;136(Pt 11):3474–3488. doi: 10.1093/brain/awt266. [DOI] [PubMed] [Google Scholar]
- 132••.Henry ML, Gorno-Tempini ML. The logopenic variant of primary progressive aphasia. Curr Opin Neurol. 2010;23(6):633–637. doi: 10.1097/WCO.0b013e32833fb93e. Summarizes current knowledge regarding the clinical symptoms, neuroanatomy, genetics and neuropathology associated with logopenic variant PPA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nardell M, Tampi RR. Pharmacological treatments for frontotemporal dementias: a systematic review of randomized controlled trials. Am J Alzheimers Dis Other Demen. 2014;29(2):123–132. doi: 10.1177/1533317513507375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jicha GA. Medical management of frontotemporal dementias: the importance of the caregiver in symptom assessment and guidance of treatment strategies. J Mol Neurosci. 2011;45(3):713–723. doi: 10.1007/s12031-011-9558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117–121. doi: 10.1159/000076343. [DOI] [PubMed] [Google Scholar]
- 136.Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms: a randomized, controlled, open 14-month study. Neurology. 2003;49(1):13–19. doi: 10.1159/000067021. [DOI] [PubMed] [Google Scholar]
- 137.Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355–359. doi: 10.1159/000077171. [DOI] [PubMed] [Google Scholar]
- 138.Moretti R, Torre P, Antonello R, et al. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer's disease and other dementias: a 24-month follow-up of 68 patients. Am J Alzheimers Dis Other Demen. 2003;18(4):205–214. doi: 10.1177/153331750301800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Curtis RC, Resch DS. Case of pick's central lobar atrophy with apparent stabilization of cognitive decline after treatment with risperidone. J Clin Psychopharmacol. 2000;20(3):384–385. doi: 10.1097/00004714-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 140.Fellgiebel A, Muller MJ, Hiemke C, et al. Clinical improvement in a case of frontotemporal dementia under aripiprazole treatment corresponds to partial recovery of disturbed frontal glucose metabolism. World J Biol Psychiatry. 2007;8(2):123–126. doi: 10.1080/15622970601016538. [DOI] [PubMed] [Google Scholar]
- 141.Mendez MF, Shapira JS, Mcmurtray A, et al. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15(1):84–87. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 142.Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931–937. doi: 10.2165/00002512-200421140-00003. [DOI] [PubMed] [Google Scholar]
- 143.Kertesz A, Morlog D, Light M, et al. Galantmine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord. 2008;25(2):178–185. doi: 10.1159/000113034. [DOI] [PubMed] [Google Scholar]
- 144.Vercelletto M, Boutoleau-Bretonniere C, Volteau C, et al. Memantine in behavioral variant frontotemporal dementia: negative results. J Alzheimers Dis. 2011;23(4):749–759. doi: 10.3233/JAD-2010-101632. [DOI] [PubMed] [Google Scholar]
- 145.Boxer AL, Knopman DS, Kaufer DI, et al. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebocontrolled trial. Lancet Neurol. 2013;12(2):149–156. doi: 10.1016/S1474-4422(12)70320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boxer AL, Gold M, Huey E, et al. The advantages of frontotemporal degeneration drug development (part 2 of frontotemporal degeneration: the next therapeutic frontier) Alzheimers Dement. 2013;9(2):189–198. doi: 10.1016/j.jalz.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Boxer AL, Boeve BF. Frontotemporal dementia treatment: current symptomatic therapies and implications of recent genetic, biochemical, and neuroimaging studies. Alzheimer Dis Assoc Disord. 2007;21(4):S79–S87. doi: 10.1097/WAD.0b013e31815c345e. [DOI] [PubMed] [Google Scholar]
- 148.Mendez MF. Frontotemporal dementia: therapeutic interventions. Front Neurol Neurosci. 2009;24:168–178. doi: 10.1159/000197896. [DOI] [PubMed] [Google Scholar]
- 149.De Vugt ME, Riedijk SR, Aalten P, et al. Impact of behavioural problems on spousal caregivers: a comparison between Alzheimer's disease and frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;22(1):35–41. doi: 10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- 150.Riedijk SR, De Vugt ME, Duivenvoorden HJ, et al. Caregiver burden, health-related quality of life and coping in dementia caregivers: a comparison of frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;22(5–6):405–412. doi: 10.1159/000095750. [DOI] [PubMed] [Google Scholar]
- 151.Boutoleau-Bretonniere C, Vercelletto M, Volteau C, et al. Zarit burden inventory and activities of daily living in the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Disord. 2008;25(3):272–277. doi: 10.1159/000117394. [DOI] [PubMed] [Google Scholar]
- 152.Mioshi E, Bristow M, Cook R, et al. Factors underlying caregiver stress in frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. 2009;27(1):76–81. doi: 10.1159/000193626. [DOI] [PubMed] [Google Scholar]
- 153.Mioshi E, Foxe D, Leslie F, et al. The impact of dementia severity on caregiver burden in frontotemporal dementia and Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27(1):68–73. doi: 10.1097/WAD.0b013e318247a0bc. [DOI] [PubMed] [Google Scholar]
- 154.Merrilees J, Hubbard E, Mastick J, et al. Sleep in persons with frontotemporal dementia and their family caregivers. Nurs Res. 2014;63(2):129–136. doi: 10.1097/NNR.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Merrilees J, Dowling GA, Hubbard E, et al. Characterization of apathy in persons with frontotemporal dementia and the impact on family caregivers. Alzheimer Dis Assoc Disord. 2013;27(1):62–67. doi: 10.1097/WAD.0b013e3182471c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barton C, Merrilees J, Ketelle R, et al. Implementation of advanced practice nurse clinic for management of behavioral symptoms in dementia: a dyadic intervention (innovative practice) Dementia (London) 2014;13(5):686–696. doi: 10.1177/1471301213519895. [DOI] [PubMed] [Google Scholar]
- 157.Merrilees J. A model for management of behavioral symptoms in frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2007;21(4):S64–69. doi: 10.1097/WAD.0b013e31815bf774. [DOI] [PubMed] [Google Scholar]
- 158.Dowling GA, Merrilees J, Mastick J, et al. Life enhancing activities for family caregivers of people with frontotemporal dementia. Alzheimer Dis Assoc Disord. 2013;28(2):175–181. doi: 10.1097/WAD.0b013e3182a6b905. [DOI] [PubMed] [Google Scholar]
- 159•.Kortte K, Rogalski E. Behavioral interventions for enhancing life participation in behavioral variant frontotemporal dementia and primary progressive aphasia. Int Rev Psychiatry. 2013;25(2):237–245. doi: 10.3109/09540261.2012.751017. Summarizes current knowledge and provides comprehensive recommendations for behavioral interventions in patients with FTD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Savage SA, Ballard KJ, Piguet O, et al. Bringing words back to mind: improving word production in semantic dementia. Cortex. 2013;49(7):1823–1832. doi: 10.1016/j.cortex.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 161.The Association for Frontotemporal Degeneration. www.theaftd.org.
- 162.Frontotemporal Dementia Research Group. www.ftdrg.org.
- 163.Association for Frontotemporal Dementias. www.ftd-picks.org.
- 164.Sapolsky D, Domoto-Reilly K, Negreira A, et al. Monitoring progression of primary progressive aphasia: current approaches and future directions. Neurodegener Dis Manag. 2011;1(1):43–55. [Google Scholar]
- 165.Perry DC, Kramer JH. Reward processing in neurodegenerative disease. Neurocase. 2014 doi: 10.1080/13554794.2013.873063. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Possin KL, Chester SK, Laluz V, et al. The frontal-anatomic specificity of design fluency repetitions and their diagnostic relevance for behavioral variant frontotemporal dementia. J Int Neuropsychol Soc. 2012;18(5):834–844. doi: 10.1017/S1355617712000604. [DOI] [PMC free article] [PubMed] [Google Scholar]


