Abstract
Phyllodes tumor is attributed to a small fraction of primary tumors of the breast. Such tumors occur rarely in pregnancy and lactation. We report a case of a 25-year-old lactating mother presenting with a lump in the left breast. Core needle biopsy was opined as phyllodes tumor with lactational changes, and subsequent wide local excision confirmed the diagnosis of benign phyllodes tumor with lactational changes. The characteristic gross and microscopic findings of a well-circumscribed lesion with leaf-like fibroepithelial growth pattern and typical nonuniform or diffuse stromal proliferation with periductal accentuation even in the absence of mitotic figures can help clinch the diagnosis. Benign phyllodes is known for its recurrence and requires wide excision and close follow-up. It is vital to identify these lesions even on limited biopsies as therapeutic options differ. This case is presented for its rarity and the diagnostic challenge it poses in limited biopsy.
Keywords: benign phyllodes tumor, lactating breast, stromal proliferation
Introduction
Phyllodes tumor accounts for a minor proportion of all fibroepithelial tumors of the breast (2.5%). It is postulated that these tumors arise de novo from intralobular or periductal stroma or rarely in a background of a fibroadenoma. Usually, it occurs as a unilateral well-circumscribed mass and can grow rapidly. According to the World Health Organization guidelines, phyllodes tumor is graded according to a three-tier classification into benign, borderline, and malignant tumors. Phyllodes tumor is rarely encountered during pregnancy and lactation. Timely identification is essential for appropriate therapy and risk stratification.1–3
Case Findings
A 25-year-old lactating mother (G2 P2) presented 8 months postpartum to the surgical outpatient department of our hospital on noticing a lump in her left breast associated with pain and difficulty in breastfeeding. She observed that the lump was progressively increasing in size, and she was facing difficulty in breastfeeding. There was no other significant medical or surgical history. The patient has given consent for publication of this report.
Clinical examination at our hospital indicated that the lump was present in the left breast located in the upper inner, lower inner, and central quadrant. Further workup included a core biopsy (Fig. 1A–C) that revealed a neoplastic lesion with leaf-like configuration lined by bilayered epithelial and myoepithelial cells and cellular fibrous stroma. Adjacent parenchyma demonstrated closely apposed acini lined by foamy cells harboring amorphous pale eosinophilic secretions within their lumina. The case was signed out as benign phyllodes tumor with lactational changes. The other investigations included routine hematological tests, liver function test, complete urine examination, blood urea, serum creatinine, random blood sugar, serum Thyroid stimulating hormone (TSH), and chest X-ray. These investigations were within normal limits.
Figure 1.
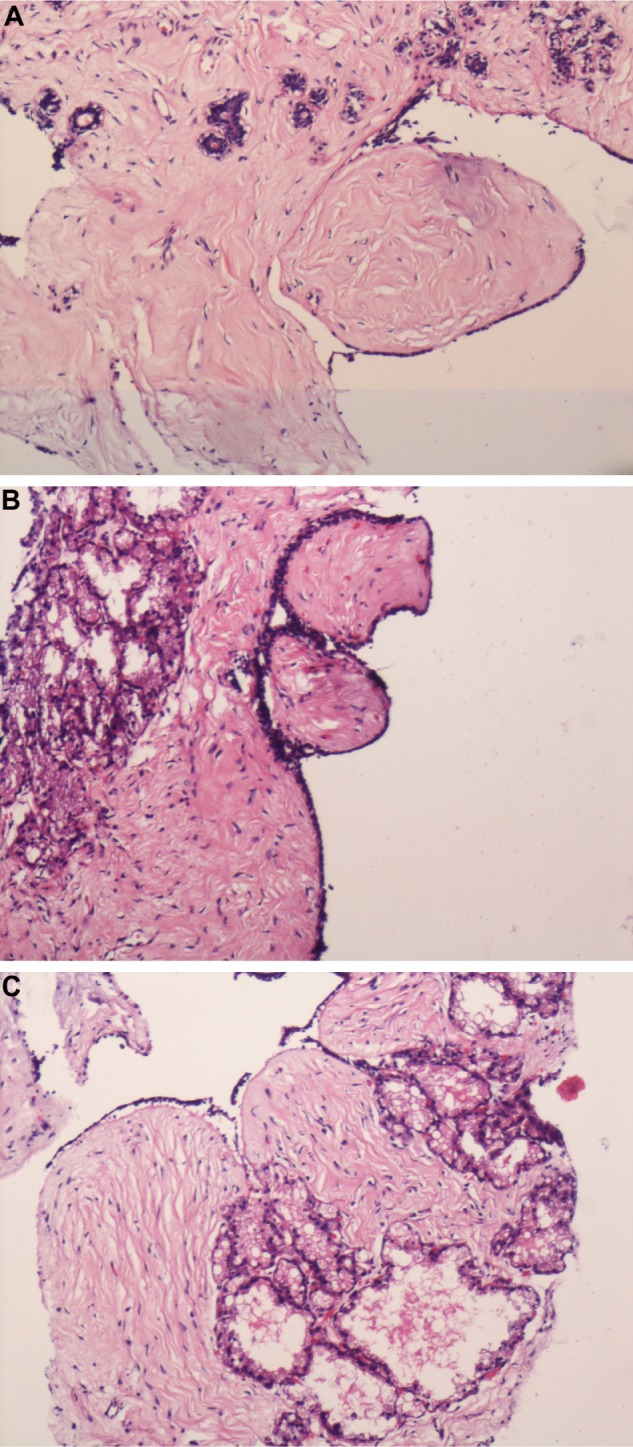
(A–C) Core needle biopsy. Depicting stromal prominence and closely packed acini lined by vacuolated cells characteristic of lactational changes with features suggestive of phyllodes tumor (100 ×).
Subsequently, the patient underwent wide local excision of the lump with a wide margin. The specimen consisted of a large grayish-white soft tissue mass measuring 10 cm × 9 cm × 4 cm covered on one aspect by a small ellipse of skin measuring 6 cm × 3 cm (Fig. 2). The cut surface showed a relatively circumscribed lesion with grayish-white solid and cystic areas exuding thick milky fluid.
Figure 2.
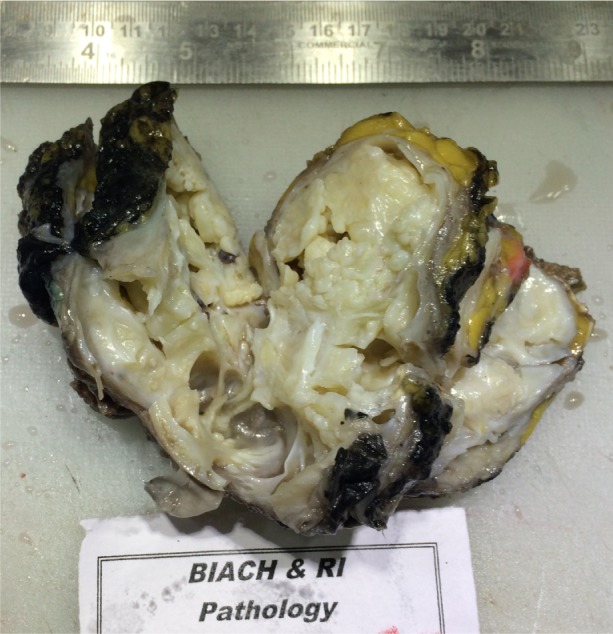
Gross photograph of the specimen demonstrating a grayish-white well-circumscribed mass with solid and cystic areas.
Microscopic findings were in concordance with the initial biopsy report. Multiple sections following extensive sampling, reviewed, exhibited slit-like ductal spaces lined by bilayered epithelial and myoepithelial cells with stromal hyperplasia (Fig. 3). There were foci demonstrating expansile stroma with periductal accentuation (Fig. 4A–C) and extensive areas of hyalinization. Adjacent breast parenchyma showed lobules and acini with scant intervening stroma. The luminal epithelial cells demonstrated secretory vacuoles (Fig. 5). No mitotic activity or necrosis was discerned. The resected margins were free of tumor. Immunohistochemical stains showed no expression of p53, CD117, CD10, and CD34. Proliferation index (Ki 67) was <1%. The final diagnosis rendered was benign phyllodes tumor of the breast with lactational changes.
Figure 3.
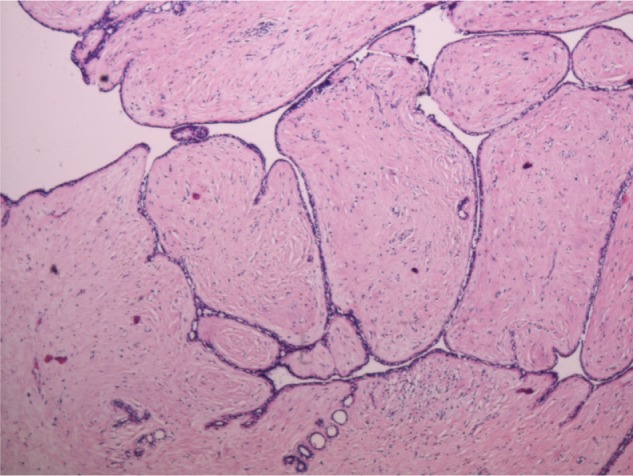
Benign phyllodes tumor with classic leaf-like fibroepithelial proliferation (40 ×).
Figure 4.
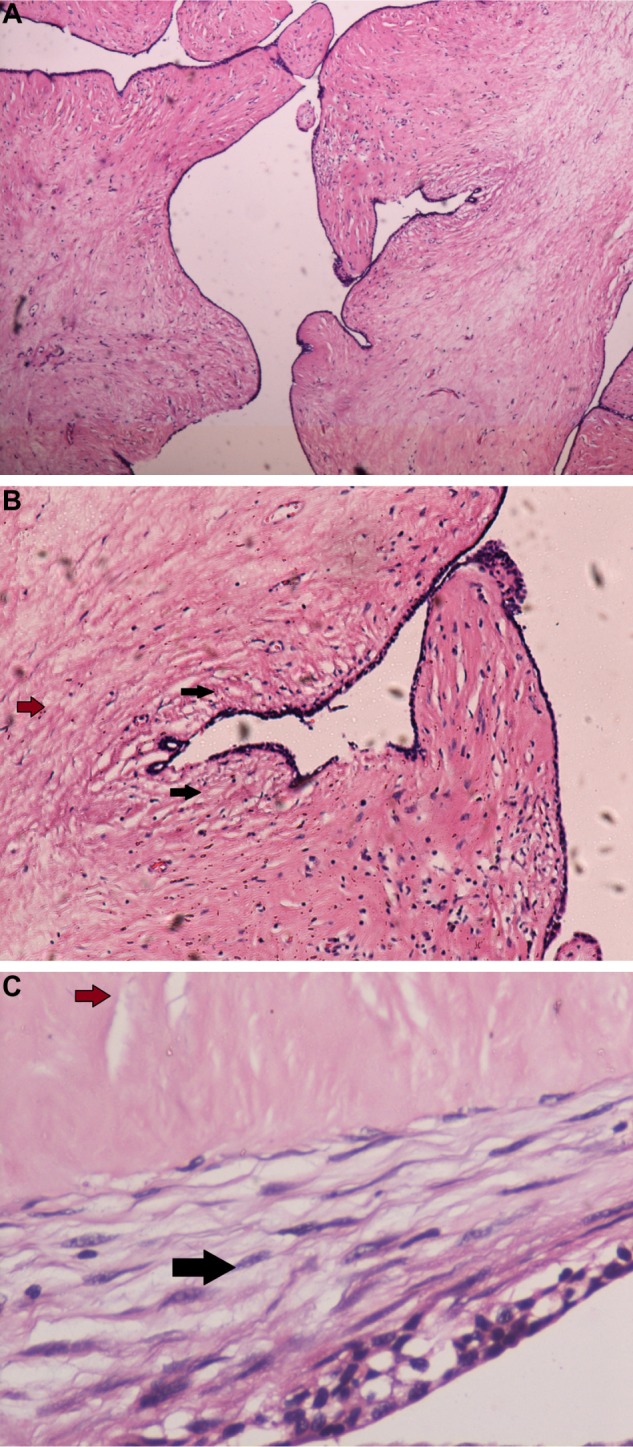
(A) Benign phyllodes tumor with characteristic expansile stroma (40 ×). (B) Black arrow depicts increased stromal cellularity in close proximity to the epithelium. Brown arrow depicts reduced stromal cellularity away from the epithelium (100 ×). (C) Another area of tumor (400 ×). Black arrow depicts increased stromal cellularity in close proximity to the epithelium. Brown arrow depicts reduced stromal cellularity away from the epithelium.
Figure 5.
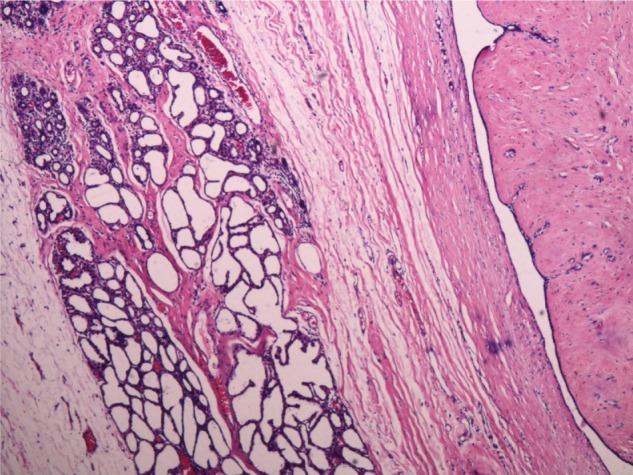
Benign phyllodes tumor with lactational changes demonstrating lobules of closely packed acini lined by vacuolated secretory epithelium with sparse intervening stroma (40 ×).
Discussion
Phyllodes tumor is attributed to a small fraction of primary tumors of the breast (~1%).1,2 Such tumors occur rarely in pregnancy and lactation. Previous studies indicated occurrence of phyllodes tumors during pregnancy or lactation as benign, borderline or malignant, and unilateral or bilateral in 14 cases.1,4–16 The findings of the present case of benign phyllodes tumor with lactational changes is concurrent with a case described previously by Likhitmaskul et al.1 in a 36-year-old pregnant woman who underwent mastectomy 3 weeks postpartum. Generally, phyllodes tumor presents as a relatively circumscribed painless mass. On an average, the size of the tumor measures 4–5 cm; however, large tumors >10 cm have been described. On transection, it appears as grayish-white to tan with slit-like spaces and bulging cut surface. Microscopic findings include fibroepithelial proliferation in a classic leaf-like pattern. The compressed ducts are lined by a bilayered epithelial and myoepithelial cells with expansile stroma and accentuation subjacent to the epithelium. Stromal overgrowth is characterized by the absence of ductal elements in at least one low power field and is predominantly demonstrated in tumors of increasing grade. The tumor can be graded based on the extent of stromal cellularity, nuclear pleomorphism, mitotic activity, stromal overgrowth, and well-defined or pushing margins.2
In view of tumor heterogeneity, the tumor has to be sampled extensively for accurate grading. Benign tumors demonstrate mild cellular stroma, well-defined tumor borders, lack of stromal atypia or mild atypia, and less than five mitotic figures per 10 high power fields with the absence of stromal overgrowth. Approximately 60%–75% of phyllodes tumors are reported as benign when compared to borderline and malignant phyllodes tumors. Borderline tumors have moderately cellular stroma, five to nine mitotic figures per 10 high power fields with mild-to-moderate atypia, whereas malignant tumors exhibit marked cellularity, atypia, brisk mitotic activity (>10/10 high power fields), infiltrative borders, and stromal overgrowth.2,3
Lactating breast demonstrates lobules of closely packed acini lined by vacuolated secretory epithelium with sparse intervening stroma and pale eosinophilic secretions within their lumina.
The important differential diagnosis includes lactating adenoma, fibroadenoma, galactocele, and periductal stromal tumor.17–20 It is vital to distinguish between them as therapeutic options differ. Fibroadenomas usually have scant stromal cellularity and lack mitotic activity, whereas phyllodes tumor exhibits an increase in stromal cellularity, especially adjoining the epithelium at the epithelial stromal interface with low mitotic activity. Similarly, periductal stromal tumor has overlapping histological features similar to phyllodes tumor; however, the former is not circumscribed with spindle cell proliferation around open tubules and lacks the leaf-like growth pattern with compressed tubules typically seen in the latter.
Phyllodes tumor is rare in lactating breasts because the lactating breast normally demonstrates lobular and acinar hyperplasia to meet the physiological function and requirement during lactation. Therefore, it could be hypothesized that as stromal hyperplasia is not a feature of lactating breast, the probability of development of phyllodes tumor is low.
Age, ethnicity, and genetic factors may be contributing factors as it has been reported that Asian woman may present with phyllodes at a younger age group (second–third decades) when compared to the western population.2
Currently, there are no molecular markers of proven clinical benefit. A recent study by Yoshida et al.21 demonstrated that MED12 mutations were frequent among the phyllodes tumors of the breast, regardless of the tumor grade. On the contrary, few authors have shown that MED12 mutations are more common in fibroadenomas and benign phyllodes tumors than their malignant counterparts.22 There are studies indicating increased expression of CD117, p53, ki67, VEGF, and several other markers with increasing tumor grade.2 CD117 expression by stromal cells in phyllodes tumors has been shown to correlate with high grade, suggesting a possible role of CD117 being used as adjunctive marker of malignancy in these tumors. C-kit receptor-mediated tyrosine kinase involvement in the pathogenesis of phyllodes tumors has also been suggested by Tse et al.23 CD10 expression in stromal cells of phyllodes tumor is reported to correlate with increasing grade.24 There was no expression of CD10 in the stromal cells in this study. According to the current NCCN guidelines, conservative surgery with adequate resection-free margins is the treatment of choice. In cases of large tumors with doubtful margin status, simple mastectomy is undertaken.
The patient opted for breast conservation surgery with wide local excision. Generally, benign phyllodes tumor is associated with good prognosis; however, it is known for local recurrence (10%–17%) and requires wide excision and close follow-up.2,25 This patient at six months follow-up is doing well.
Table 1.
| SL. NO | CASE REPORT | FINAL DIAGNOSIS |
|---|---|---|
| 1 | Likhitmaskul T et al., Gland Surgery. 2015;4(4):339–343. | Giant benign phyllodes tumor with lactating changes, left breast |
| 2 | Way JC, Culham BA. Canadian Journal of Surgery. 1998; 41(5):407–409. | Benign phyllodes tumor, right breast |
| 3 | Aranda C et al., Ginecol Obstet Mex. 2005 Jul; 73(7):387–392. Spanish. | Benign phyllodes tumor, right breast |
| 4 | Carvalho Jr. Ary Wanderley de,et al., Gravidez e Tumor Filodes Bilateral: Uma Associação Rara. Rev. Bras. Ginecol. Obstet. [Internet]. 1999 Mar 21(2): 109–111. | Bilateral benign phyllodes tumor |
| 5 | Andreola, João, Andréa Damin et al.,Journal of the Senologic International Society [Online], 1.3 (2012). | Borderline phyllodes tumor bilateral breast. |
| 6 | Furuya S et al., Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association) Vol. 73 (2012) No 4, p 780–785. | Borderline phyllodes without lymphnode metastases, right breast |
| 7 | Karima Mrad,et al., Ann Diagn Pathol 4:370–372, 2000. | 1. Malignant cystosarcoma phyllodes, left breast 2. Benign cystosarcoma phyllodes, right breast (Bilateral synchronous cystosarcoma phyllodes) |
| 8 | Nejc D et al., Int J Gynecol Cancer. 2008 Jul–Aug; 18(4):856–9. Epub 2007 Sep 24. | Malignant cystosarcoma phyllodes, left breast |
| 9 | Blaker KM et al., Am Surg. 2010 Mar; 76(3):302–305. | Malignant phylloides tumor, right breast |
| 10 | Ray S,et al., Iranian Journal of Medical Sciences. 2011;36(4):315–317. | Malignant phylloides tumor right breast with coincidental nulliparous prolapse. |
| 11 | Pytel J et al., Przegląd Menopauzalny 2009;6: 331–333 (Polish). | Malignant phyllodes tumor, left breast |
| 12 | Simpson SA et al., images of a case. Breast J. 2007 Nov–Dec; 13(6):620–621. | Malignant phyllodes |
| 13 | Sharma JB,et al. Eur J Obstet Gynecol Reprod Biol. 2004 Aug 10;115(2):237–239. | Unilateral phylloides tumor with rapid growth during pregnancy |
| 14 | Abdelkrim SB et al., World Journal of Oncology, Vol. 1, No. 3, Jun 2010. | Single case of phyllodes tumor diagnosed during pregnancy. (No other details found) |
Footnotes
ACADEMIC EDITOR: Dama Laxminarayana, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1724 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: SSM, KVVNR. Analyzed the data: SSM, HGN. Wrote the first draft of the article: SSM, HGN. Contributed to the writing of the article: SSM, HGN. Agreed with the study results and conclusion: KVVNR, SSM, HGN. Jointly developed the structure and arguments for the article: SSM, HGN. Made critical revisions and approved the final version: KVVNR, SSM, HGN. All authors reviewed and approved the final article.
REFERENCES
- 1.Likhitmaskul T, Asanprakit W, Charoenthammaraksa S, et al. Giant benign phyllodes tumor with lactating changes in pregnancy: a case report. Gland Surg. 2015;4(4):339–43. doi: 10.3978/j.issn.2227-684X.2015.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th ed. Lyon: IARC Press; 2012. pp. 143–7. [Google Scholar]
- 3.Spitaleri G, Toesca A, Botteri E, et al. Breast phyllodes tumor: A review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol. 2013;88(2):427–36. doi: 10.1016/j.critrevonc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Way JC, Culham BA. Phyllodes tumour in pregnancy: a case report. Can J Surg. 1998;41(5):407–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Aranda C, Sotelo M, Torres A, Zárate M. Tumor phyllodes y embarazo. Reporte de un caso. Ginecol Obstet Mex. 2005;73(7):387–92. [PubMed] [Google Scholar]
- 6.Carvalho AW, Jr, Silva SM, Almeida PB, et al. Gravidez e Tumor Filodes Bilateral: Uma Associação Rara. Rev Bras Ginecol Obstet. 1999;21(2):109–11. [Google Scholar]
- 7.Andreola J, Damin A, Andreola J, et al. Giant borderline phyllodes breast tumor in pregnancy. J Senologic Int Society. 2012;1:3. [Google Scholar]
- 8.Furuya S, Miura K, Aikawa T, Nakagomi H, Omori M, Oyama T. A case of phyllodes tumor of the breast grown rapidly during the last period of pregnancy. Nihon Rinsho Geka Gakkai Zasshi. 2012;73(4):780–5. [Google Scholar]
- 9.Mrad K, Driss M, Maalej M, Romdhane KB. Bilateral cystosarcoma phyllodes of the breast: A case report of malignant form with contralateral benign form. Ann Diagn Pathol. 2000;4:370–2. doi: 10.1053/adpa.2000.19375. [DOI] [PubMed] [Google Scholar]
- 10.Nejc D, Pasz-Walczak G, Piekarski J, et al. Astonishingly rapid growth of malignant cystosarcoma phyllodes tumor in a pregnant woman–a case report. Int J Gynecol Cancer. 2008;18(4):856–9. doi: 10.1111/j.1525-1438.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- 11.Blaker KM, Sahoo S, Schweichler MR, Chagpar AB. Malignant phylloides tumor in pregnancy. Am Surg. 2010;76(3):302–5. [PubMed] [Google Scholar]
- 12.Ray S, Basak S, Das S, Pal M, Konar H. Malignant phylloides tumor of breast in a pregnant woman with coincidental nulliparous vaginal prolapse. Iran J Med Sci. 2011;36(4):315–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Pytel J, Dedecjus M, Naze M, Stróżyk G, Brzeziński J. Malignant breast phyllodes tumor in pregnancy – a case report Meno-pause review. Prz Menopauzalny. 2009;6:331–3. [Google Scholar]
- 14.Simpson SA, Redstone J, Aziz MS, Bernik SF. Large malignant phyllodes tumor with rapid growth during pregnancy: images of a case. Breast J. 2007;13(6):620–1. doi: 10.1111/j.1524-4741.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharma JB, Wadhwa L, Malhotra M, Arora R, Singh S. A case of huge enlargement of cystosarcoma phylloides of breast in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2004;115(2):237–9. doi: 10.1016/j.ejogrb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Abdelkrim SB, Trabelsi A, Bouzrara M, et al. Phyllodes tumors of the breast: a review of 26 cases. World J Oncol. 2010;1(3):129–34. doi: 10.4021/wjon2010.06.220w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manipadam MT, Jacob A, Rajnikanth J. Giant lactating adenoma of the breast. J Surg Case Rep. 2010;2010(9):8. doi: 10.1093/jscr/2010.9.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah NM, Pandya NK, Dalal PR, Sharma RM, Joshi AS. Giant fibroadenoma: mimicking a phyllodes tumor. Gujarat Med J. 2009;64(2):96–8. [Google Scholar]
- 19.Lan Y, Zhu J, Liu J, Yang H, Jiang Y, Wei W. Periductal stromal sarcoma of the breast: A case report and review of the literature. Oncol Lett. 2014;8(3):1181–3. doi: 10.3892/ol.2014.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi S, Dialani V, Marotti J, Mehta TS, Slanetz PJ. Breast disease in the pregnant and lactating patient: radiological-pathological correlation. Insights Imaging. 2013;4(5):527–38. doi: 10.1007/s13244-012-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida M, Sekine S, Ogawa R, et al. Frequent MED12 mutations in phyllodes tumours of the breast. Br J Cancer. 2015;112:1703–8. doi: 10.1038/bjc.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfarr N, Kriegsmann M, Sinn P, et al. Distribution of MED12 mutations in fibroadenomas and phyllodes tumors of the breast – implications for tumor biology and pathological diagnosis: genes, chromosomes. Cancer. 2015;54(7):444–52. doi: 10.1002/gcc.22256. [DOI] [PubMed] [Google Scholar]
- 23.Tse GM, Putti TC, Lui PC, et al. Increased c-kit (CD117) expression in malignant mammary phyllodes tumors. Mod Pathol. 2004;17(7):827–31. doi: 10.1038/modpathol.3800125. [DOI] [PubMed] [Google Scholar]
- 24.Tse GMK, Tsang AKH, Putti TC, et al. Stromal CD10 expression in mammary fibroadenomas and phyllodes tumours. J Clin Pathol. 2005;58:185–9. doi: 10.1136/jcp.2004.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altıntoprak F, Kıvılcım T, Dilek ON. Therapeutic approaches to breast phyllodes tumor. Eur J Gen Med. 2014;1:7–10. [Google Scholar]


