Abstract
Testing the clinical efficacy of drugs that also have important side effects on locomotion needs to be properly designed in order to avoid erroneous identification of positive effects when the evaluation depends on motor-related tests. One such example is the evaluation of analgesic role of drugs that act on dopaminergic receptors, since the pain perception tests used in animal models are based on motor responses that can also be compromised by the same substances. The apparent analgesic effect obtained by modulation of the dopaminergic system is still a highly disputed topic. There is a lack of acceptance of this effect in both preclinical and clinical settings, despite several studies showing that D2/3 agonists induce antinociception. Some authors raised the hypothesis that this antinociceptive effect is enhanced by dopamine-related changes in voluntary initiation of movement. However, the extent to which D2/3 modulation changes locomotion at analgesic effective doses is still an unresolved question. In the present work, we performed a detailed dose-dependent analysis of the changes that D2/3 systemic modulation have on voluntary locomotor activity and response to four separate tests of both thermal and mechanical pain sensitivity in adult rats. Using systemic administration of the dopamine D2/3 receptor agonist quinpirole, and of the D2/3 antagonist raclopride, we found that modulation of D2/3 receptors impairs locomotion and exploratory activity in a dose-dependent manner across the entire range of tested dosages. None of the drugs were able to consistently diminish either thermal or mechanical pain perception when administered at lower concentrations; on the other hand, the larger concentrations of raclopride (0.5–1.0 mg/kg) strongly abolished pain responses, and also caused severe motor impairment. Our results show that administration of both agonists and antagonists of dopaminergic D2/3 receptors affects sensorimotor behaviors, with the effect over locomotion and exploratory activity being stronger than the observed effect over pain responses.
Keywords: dopamine, quinpirole, raclopride, pain
Introduction
Several clinical and preclinical studies have shown that pharmacological modulation of dopamine receptors has analgesic properties: levodopa administration in human beings was shown to alleviate the pain caused by several different conditions,1,2 while in animal models, the intrathecal administration of levodopa was shown to be particularly effective in pain relief.3,4 Pharmacological studies have shown that the analgesic effect of the dopaminergic system is specific to the activation of D2/3 receptors and not D1 receptors.5,6 More importantly, this analgesic effect was shown to be particularly effective when drugs were administered in the dorsolateral striatum, nucleus accumbens, or prefrontal cortex.7 The majority of studies suggest that the analgesic effect results from increased monoaminergic neurotransmission acting on the spinal cord,8–11 which is supported by the observation that spinal dopaminergic terminals are able to modulate nociceptive transmission12,13 and enhance the antinociceptive effect of other monoamines.14 On the other hand, several studies have also suggested that generalized dysfunction in forebrain dopaminergic condition is correlated with chronic pain severity15–17 and that the central effects of dopamine may override the peripheral role.18–20
However, it is also known that these dopaminergic drugs affect motor activity when administered at dosages that have analgesic effect,21 and many non-pain-related studies have shown that modulation of dopamine receptors at the same dosage affects both exploratory and goal-oriented movements.22,23 Since the majority of behavioral tests used to determine the analgesic efficacy of a substance depends on the timing of motor responses (eg, by observing if that drug increases or decreases the time to generate a pain-evoked withdrawal body movement, and attributing the difference in time to a change in painful threshold of sensitivity), special care is needed to assure that the analgesic effect is due to a reduction in pain perception and not caused by motor impairment blocking the withdrawal response. Given the important side effects of dopamine-related drugs on movement, it is important to detail the interaction between movement and analgesia in animal models of pain. Thus, the aim of the present study was to perform a detailed dose-dependent analysis of the effects that systemic administration of a D2/3 agonist and antagonist have on locomotor activity and in pain perception of both thermal and mechanical stimuli in adult rats.
Materials and Methods
Animal model and ethical statement
Experiments were performed in Sprague Dawley adult male rats (weighing 275–320 g; Charles River Laboratories). The animals were housed in collective cages (two per box), and kept on a 12 hour light/dark cycle with ad libitum feeding and hydration regimen. All experimental procedures were performed at approximately the same time each day during the light portion of the cycle. All experiments were performed in accordance with the guidelines of the European Union directive (2010/63/CE) and with the Research and Ethical Issues of the International Association for the Study of Pain. The experimental protocols were approved by the local Ethical Committee for Animal Use and National DGAV Board (Lisbon, Portugal). All efforts were taken to minimize the number of animals used in this study.
Spared nerve injury
Rats were anesthetized with a ketamine/medetomidine mixture (75 and 0.5 mg/kg in saline, respectively, i.p.) and subjected to the spared nerve injury (SNI) model24 of neuropathic pain (SNI group, n = 10) or to a sham intervention (sham group, n = 10), involving the same extent of skin and muscle dissection. Briefly, SNI surgery consists of the ligation and transection of the tibial and common peroneal branches of the sciatic nerve while sparing the sural nerve. After surgery, rats were allowed to recover for 21 days before behavioral sessions began. During this recovery period, each animal was placed in the testing room daily to adapt to the laboratory environmental conditions.
Dopamine D2/3 receptor neuromodulators
Each experimental group of animals (sham or SNI) was split into two groups that received either the dopamine D2/D3 receptor agonist quinpirole hydrochloride (0.01, 0.05, 0.1, 0.5, and 1 mg/kg i.p.) or the dopamine D2/D3 antagonist raclopride tartrate (0.01, 0.05, 0.1, 0.5, and 1 mg/kg i.p.), injection volume of 1 mL/kg body weight. Both quinpirole and raclopride were purchased from Sigma-Aldrich, and the different drug concentrations were prepared in physiological saline solution under aseptic conditions. The saline solution (0.9% w/v NaCl) was the first tested administration, followed by ascending larger concentrations of quinpirole or raclopride, with a time-window interval of 48 hours between each drug dose injection; retesting of naïve exploration of the open field was performed 24 hours after each injection, to check the effect of drug washout over the motor activity. After each injection, animals remained in a holding cage for 30 minutes before initiating behavioral testing.
Nociceptive and motor activity testing
Before the initiation of the behavioral tests, each animal was subjected to a habituation period to the experimenter handling and exposed to the different testing apparatuses (2 daily sessions of 30 minutes during 5 consecutive days). During the habituation period, noxious stimuli were not delivered to the animals.
Locomotor activity performance
The dose-dependent effects on locomotor activity were evaluated using a squared open-field arena. The total dimension of the arena was 45 × 45 cm, with opaque walls 40 cm high; open-field arenas of this size are routinely used in rat studies since larger arenas are prone to more anxious behavior.25 The arena floor was divided into 25 squares of equal size. To measure locomotor activity, each animal was placed in the arena central square and the number of squares crossed by all four paws were quantified during five minutes. Additionally, the number of vertical exploratory movements performed by the animals was also quantified (rearings, standing on their rear limbs accompanied by sniffing behavior). After testing each rat, the open-field arena was cleaned with a 30% v/v ethanol solution.
Von Frey test
The sensory threshold for noxious stimulation was assessed by placing the animals in a small chamber with metal mesh floor and touching the plantar surface of the left hindpaw with Von Frey filaments (Somedic). The protocol used in this study has been described previously.26 A testing session for a particular rat began after habituation or as soon as the rat stopped exploring and appeared acclimatized to the testing environment.
Paw pressure test
A Randall-Selitto analgesiometer (Ugo Basile) was used according to described protocol.27 Briefly, a constant increasing pressure exerted by a blunt conical probe, controlled by a mechanical device, was applied to a small area of the surface of the rat hindpaw. Mechanical pressure was increased until vocalization or a withdrawal reflex threshold occurred. Reflex thresholds were expressed in grams. The measurements were repeated several times in order to obtain two consecutive values that did not differ more than 10%. To avoid injury, a cutoff value of 250 g was used.
Tail-flick test
Tail-flick latency was tested with the Ugo Basile apparatus (model 37360). The rats were restrained in hard plastic tubes covered with a dark cloth, and after acclimatization, an infrared light was directed to three different points in the middle portion of the tail. A flick of the tail was considered as a sign of thermal nociception.28 The measurements were repeated three times using an interval of three minutes between them. Values are expressed as the mean of measurements. To avoid tissue damage, the cutoff was set at 10 seconds.
Paw flick test
To assess limb withdrawal latency to radiant heat, rats were placed on an elevated glass plate, and radiant heat (52°C) was applied from below unto the plantar surface (Plantar Test Apparatus; Ugo Basile). Paw withdrawal latency was measured automatically with a cutoff latency of 20 seconds. Three measurements spaced by one minute were performed in the left hindpaw, and the withdrawal latency of each animal was expressed as the mean value of measurements.29
Data analyses
All averaged values are given as the mean ± SEM. The Kolmogorov–Smirnov test (with Dallal–Wilkinson–Lilliefor corrected P-value) was used to validate the normal distribution of the datasets. Comparisons between experimental groups and drug doses are based on a repeated measures (RM) two-way analysis of variance (ANOVA) with post hoc Bonferroni test. Statistical significance was accepted at the level of 0.05. Statistical analyses were performed using the software Prism 6.0, GraphPad.
Results
Modulation of D2/3 receptors suppresses exploratory motor activity
Using separate groups of animals per drug (n = 5 adult male Sprague Dawley rats per group), we tested the effect that systemic administration of escalating doses of a D2/3 agonist and a D2/3 antagonist causes over exploratory movement in an open-field arena. The results revealed that almost all used doses of quinpirole and raclopride suppress locomotor activity of the rats when compared with the baseline saline administration (Fig. 1). Quinpirole injections caused a dose-dependent effect in the reduction of total distance traveled by the animals in the open-field arena (RM two-way ANOVA, group × drug dose: no interaction effect, F(5,40) = 1.50, P > 0.05; no group effect, F(1,8) = 0.0, P > 0.9; significant dose effect, F(5,40) = 15.43, P < 0.0001; Bonferroni post hoc tests of statistically significant differences for each drug dose are plotted in Fig. 1A). There was also a similar significant dose-dependent effect in the reduction of locomotion after raclopride administration (RM two-way ANOVA, group × drug dose: significant interaction effect, F(5,40) = 3.33, P < 0.5; no group effect, F(1,8) = 0.36, P > 0.5; significant dose effect, F(5,40) = 47.14, P < 0.0001; Bonferroni post hoc tests of statistically significant differences for each drug dose are plotted in Fig. 1B). Interestingly, statistical analysis revealed that quinpirole equally changes locomotion of both control and neuropathic rats at all tested dosages, while raclopride at the dose of 0.05 mg/kg has a distinct effect on each experimental group (Fig. 1B). We note that the repeated exposure of the animals to the same open-field arena may potentially lead to a progressive reduction in movement; however, we note that our protocol only analyzes the first 5 minutes of locomotion after entering the arena, and we specifically tested this effect by retesting all animals 24 hours after each drug administration and found that the results in these 5 naïve sessions were not statistically different from baseline locomotion.
Figure 1.
Locomotor activity. Effect of different doses of quinpirole (A, C) and raclopride (B, D) in distance traveled (A, B) and rearing activity (C, D) in the open-field arena. Black dots represent control animals (sham), and white dots represent animals with a peripheral nerve injury (SNI). Gray shaded area represents vehicle administration. Each point represents mean ± SEM. Statistical differences between saline injection and each drug concentration is separated per experimental group (RM two-way ANOVA followed by Bonferroni test: sham group, *P < 0.05, ***P < 0.001; SNI group, #P < 0.05, ##P < 0.01, ###P < 0.001).
We also quantified the number of rearings each rat performed during the open-field test, since this is a common indicator of exploratory activity of the animal.30 Injection of quinpirole caused a dose-dependent reduction in the total number of rearings (RM two-way ANOVA, group × drug dose: no interaction effect, F(5,40) = 2.04, P > 0.05; no group effect, F(1,8) = 1.24, P > 0.1; significant dose effect, F(5,40) = 27.18, P < 0.0001; Bonferroni post hoc tests of statistically significant differences for each drug dose are plotted in Fig. 1C). There was also a similar significant dose-dependent effect in the reduction of rearings after administration of raclopride (RM two-way ANOVA, group × drug dose: no interaction effect, F(5,40) = 1.35, P > 0.1; no group effect, F(1,8) = 2.19, P > 0.1; significant dose effect, F(5,40) = 24.65, P < 0.0001; Bonferroni post hoc tests of statistically significant differences for each drug dose are plotted in Fig. 1D).
SNI neuropathic model facilitates behavioral responses to noxious stimulation
The comparison of pain-related responses between sham and SNI animals at baseline conditions (ie, after saline administration) shows that the SNI model effectively induces allodynic and hyperalgesic conditions. After SNI, there was a significant reduction in somatosensory mechanical threshold of withdrawal to the Von Frey stimulation (sham: 66.20 ± 6.49, SNI: 9.50 ± 0.50; n = 10 per group; t(18) = 8.70, P < 0.001), a significant reduction in somatosensory mechanical threshold of withdrawal to paw pressure (sham: 115.0 ± 6.83, SNI: 55.50 ± 4.56; n = 10 per group; t(18) = 7.24, P > 0.001), and a significant reduction in painful threshold for thermic stimulation of the paw (sham: 15.97 ± 0.97, SNI: 11.29 ± 0.84; n = 10 per group; t(18) = 3.65, P > 0.01). We found no significant difference of pain threshold between sham and SNI animals in what concerns thermic stimulation of the tail, not directly affected by the lesion of the sciatic nerve (sham: 12.77 ± 1.19, SNI: 10.13 ± 0.62; n = 10 per group; t(18) = 1.97, P = 0.065).
Motor impairment affects both neuropathic and control animals
More importantly, our results show that the two drugs affect locomotion and exploration in a very similar way in both control and neuropathic animals. Paradoxically, there was also a significant increase in the number of rearings executed by control animals after administration of quinpirole at 1 mg/mL; this is in agreement with the small increase in the total traveled distance that was also observed in the control animals after administration of quinpirole at 1 mg/mL (Fig. 1A). The effect of quinpirole at 1 mg/mL violates its otherwise effect of motor suppression; other studies have also shown that pharmacological modulation of D2/3 receptors have a U-shaped efficacy curve in locomotion, in which large doses have opposite effects to those of small doses.31
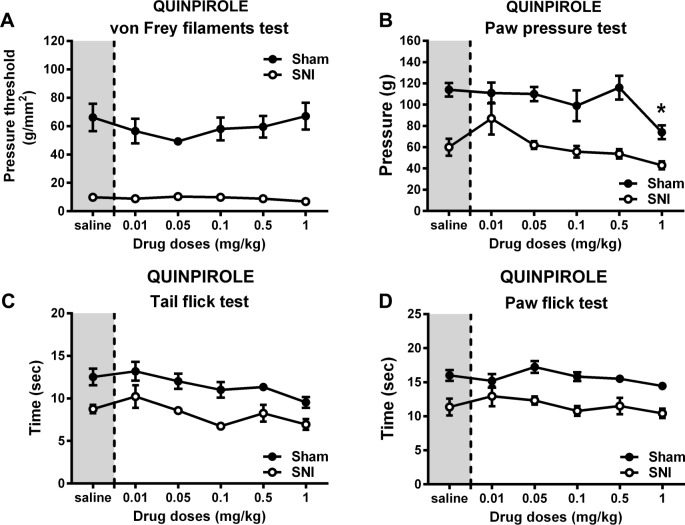
We observed no decrease in mechanical or thermal pain perception after the administration of quinpirole (Fig. 2). Only the smallest dose tested (0.01 mg/mL) showed a nonsignificant decrease in pain response in three of the sensory tests (Fig. 2B–D), while the 1 mg/mL dose caused enhanced response in the sham animals (Fig. 2B; sham, 1 mg/mL vs saline, Bonferroni, t(40) = 3.17, P > 0.01). Moreover, the dose-dependent effects caused by quinpirole were very similar between control and neuropathic animals.
Figure 2.
Effect of quinpirole in pain-related behavior. Effect of different doses of quinpirole in normal (sham) and neuropathic rats (SNI), submitted to nociceptive tests of mechanical and thermal painful stimulation. Paw withdrawal thresholds of the left hind paw in the Von Frey test (A) and in paw pressure test (B). Thermal thresholds measured by radiant heat response applied to the tail (C) and to the hind paw (D). Gray shaded area represents vehicle administration. Each point represents mean ± SEM. Statistical differences between saline injection and each drug concentration is separated per experimental group (RM two-way ANOVA followed by Bonferroni test: sham group, *P < 0.05).
In contrast to the results observed after quinpirole administration, the i.p. injection of raclopride was able to decrease responses to painful stimulation, particularly at larger doses (Fig. 3). Injection of raclopride caused a dose-dependent increase in mechanical threshold in the Von Frey test (RM two-way ANOVA, group × drug dose: interaction effect, F(5,40) = 2.58, P < 0.05; dose effect, F(5,40) = 17.71, P < 0.001; sham, 1 mg/mL vs saline, Bonferroni, t(40) = 5.57, P < 0.001; SNI, 0.5 mg/mL vs saline, Bonferroni, t(40) = 3.11, P < 0.05; SNI, 1 mg/mL vs saline, Bonferroni, t(40) = 4.30, P < 0.001; Fig. 3A), in the tail-flick test (RM two-way ANOVA, group × drug dose: no interaction effect, F(5,40) = 1.84, P > 0.1; dose effect, F(5,40) = 6.98, P < 0.001; SNI, 1 mg/mL vs saline, Bonferroni, t(40) = 3.41, P < 0.01; Fig. 3C), and in the paw flick test (RM two-way ANOVA, group × drug dose: no interaction effect, F(5,40) = 1.05, P > 0.1; dose effect, F(5,40) = 3.35, P < 0.05; SNI, 0.5 mg/mL vs saline, Bonferroni, t(40) = 3.40, P < 0.01; Fig. 3D).
Figure 3.
Effect of raclopride in pain-related behavior. Effect of different doses of raclopride in normal (sham) and neuropathic rats (SNI), submitted to nociceptive tests of mechanical and thermal painful stimulation. Paw withdrawal thresholds of the left hind paw in the Von Frey test (A) and in paw pressure test (B). Thermal thresholds measured by radiant heat response applied to the tail (C) and to the hind paw (D). Gray shaded area represents vehicle administration. Each point represents mean ± SEM. Statistical differences between saline injection and each drug concentration is separated per experimental group (RM two-way ANOVA followed by Bonferroni test: sham group, ***P < 0.001; SNI group, #P < 0.05, ##P < 0.01, ###P < 0.001).
Discussion
It is widely known that systemic administration of D2/3 receptor agonists cause a strong depression on exploratory movement in a dose-dependent manner,32,33 in contrast to the usual increase in forward locomotion observed after administration of nonselective dopamine agonists.34 The same important effect of movement suppression was also confirmed by optogenetic stimulation of D2–expressing neurons in the dorsal striatum.35 The depression in locomotion is thought to be due to the inhibition of striatal dopamine release mediated by presynaptic D2/3 receptors.36 However, given the complex interactions that depend on receptor selectivity, pre- or postsynaptic activity, and the motivational component of the movement, it is difficult to predict the actual effect on movement that will result from a particular drug and dosage.37
In this study, we used more than one nociceptive test to assess the influence of dopamine receptors on pain perception: two tests evaluated sensitivity to mechanical painful stimulation (Von Frey filaments: withdrawal response to the awareness of a hidden stimulation at rising degrees of force; Randall–Selitto: withdrawal response to overt stimulation at rising degrees of pressure), while two tests evaluated sensitivity to thermal painful stimulation using an infrared light beam focused on either the tail or one hindpaw. In common, all tests are dependent on motor responses to mark sensitivity thresholds, and this is a known drawback in the assessment of analgesic drugs that may also alter voluntary muscle activation.21,22 The mechanical tests use increasing stimuli and are, therefore, capable of distinguishing allodynic vs hyperalgesic conditions. The four tests were performed in quick succession immediately following the 5-minute open-field test: total testing time for each animal was kept under 25 minutes.
These results are in contrast to previous animal and human studies that suggest an antinociceptive effect of D1/D2/3 or D2/3 agonists.5 However, it must be noted that other studies have also shown paradoxical results of D2/3 activation, in which a short analgesia was followed by prolonged hyperalgesia, in a dose-dependent manner.38 Moreover, it must be noted that the most robust analgesic effects of D2/3 agonists are observed after either intrathecal or intracranial administration rather than after systemic administration.7,21,39 There are two important protocol differences between the present study and other comparable studies that tested the analgesic effect of pharmacological modulation of dopamine D2/3 receptors: first, we used a neuropathic model that has little inflammatory component, and the testing of pain responses began only 21 days after the onset of the nerve injury, while the majority of studies tested the analgesic effi-cacy after acute pain or during the early onset of pain models; and second, we tested pain responses only after 30 minutes after the drug administration, since it is known that quinpirole causes increase in locomotion for extended periods,22 while the majority of other studies tested the effect immediately following injection. This increase in voluntary activity is probably the cause for the increased responses to painful stimulation that is observed following the injection of the higher doses of quinpirole. Finally, the controversy may be resolved by better understanding the molecular difference between human and rodent tissues with different analgesic response from systemic analyses using next-generation sequencing technologies.40–42
However, it must be noted that the decrease in nocifensive responses induced by raclopride are most probably a result of the reduced movement of the animals, rather than a strict analgesic effect; in fact, both control and neuropathic animals showed parallel curves of diminished responsiveness to stimuli applied in the painful and nonpainful ranges (Fig. 3A). Our observation of diminished locomotion and exploration following higher doses of raclopride (Fig. 1B and D) is in good agreement with several studies that have demonstrated the cataleptic effect of D2/3 antagonists.36,43
Conclusions
Our present results highlight the importance of controlling for movement side effects, when performing pharmacologic studies of analgesic efficiency. The majority of tests for pain perception in preclinical models are based on nocifensive responses to stimuli, and drugs that diminish voluntary movement and body awareness may result in analgesia-like behavior. Our experiments suggest that modulation of D2/3 receptors does not have significant analgesic efficacy when administered at doses that do not interfere with general animal alertness. Thus, the present results raise the importance of careful design of pain perception testing in animal models, and the need to further distinct between restoration of normal behaviors and restoration of normal somatosensory perception.
Footnotes
ACADEMIC EDITOR: Edward Korzus, Deputy Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 949 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported by FEDER funds through the Operational Competitiveness Programme—COMPETE; by national funds through FCT—Fundação para a Ciência e a Tecnologia under the project PTDC/SAU-NEU-SCC/1516/201; PhD grant from FCT, SFRH/BD/70522/2010 to MD; and private funds through BIAL Foundation—Project 126/08. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: MD, VG. Analyzed the data: MD, HCC, CM, VG. Wrote the first draft of the manuscript: MD, HCC, VG. Contributed to the writing of the manuscript: MD, HCC, CM, VG. Agree with manuscript results and conclusions: MD, HCC, CM, VG. Jointly developed the structure and arguments for the paper: MD, VG. Made critical revisions and approved final version: MD, VG. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Dickey RP, Minton JP. Levodopa relief of bone pain from breast cancer. N Engl J Med. 1972;286(15):843. doi: 10.1056/NEJM197204132861518. [DOI] [PubMed] [Google Scholar]
- 2.Ertas M, Sagduyu A, Arac N, Uludag B, Ertekin C. Use of levodopa to relieve pain from painful symmetrical diabetic polyneuropathy. Pain. 1998;75(2–3):257–259. doi: 10.1016/s0304-3959(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu T, Iwata S, Morioka H, Masuyama T, Fukuda T, Nomoto M. Antinociceptive mechanism of L-DOPA. Pain. 2004;110(1–2):246–249. doi: 10.1016/j.pain.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Cobacho N, De la Calle JL, Gonzalez-Escalada JR, Paino CL. Levodopa analgesia in experimental neuropathic pain. Brain Res Bull. 2010;83(6):304–309. doi: 10.1016/j.brainresbull.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Michael-Titus A, Bousselmame R, Costentin J. Stimulation of dopamine D2 receptors induces an analgesia involving an opioidergic but non enkephalinergic link. Eur J Pharmacol. 1990;187(2):201–207. doi: 10.1016/0014-2999(90)90007-s. [DOI] [PubMed] [Google Scholar]
- 6.Morgan MJ, Franklin KB. Dopamine receptor subtypes and formalin test analgesia. Pharmacol Biochem Behav. 1991;40(2):317–322. doi: 10.1016/0091-3057(91)90560-o. [DOI] [PubMed] [Google Scholar]
- 7.Magnusson JE, Fisher K. The involvement of dopamine in nociception: the role of D(1) and D(2) receptors in the dorsolateral striatum. Brain Res. 2000;855(2):260–266. doi: 10.1016/s0006-8993(99)02396-3. [DOI] [PubMed] [Google Scholar]
- 8.Mico JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27(7):348–354. doi: 10.1016/j.tips.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 10.Viisanen H, Ansah OB, Pertovaara A. The role of the dopamine D2 receptor in descending control of pain induced by motor cortex stimulation in the neuropathic rat. Brain Res Bull. 2012;89(3–4):133–143. doi: 10.1016/j.brainresbull.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163–168. doi: 10.1097/00000539-200207000-00029. table of contents. [DOI] [PubMed] [Google Scholar]
- 12.Tamae A, Nakatsuka T, Koga K, et al. Direct inhibition of substantia gelatinosa neurones in the rat spinal cord by activation of dopamine D2-like receptors. J Physiol. 2005;568(pt 1):243–253. doi: 10.1113/jphysiol.2005.091843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleetwood-Walker SM, Hope PJ, Mitchell R. Antinociceptive actions of descending dopaminergic tracts on cat and rat dorsal horn somatosensory neurones. J Physiol. 1988;399:335–348. doi: 10.1113/jphysiol.1988.sp017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munro G. Dopamine D(1) and D(2) receptor agonism enhances antinociception mediated by the serotonin and noradrenaline reuptake inhibitor duloxetine in the rat formalin test. Eur J Pharmacol. 2007;575(1–3):66–74. doi: 10.1016/j.ejphar.2007.07.062. [DOI] [PubMed] [Google Scholar]
- 15.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8(5):781–797. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- 16.Wood PB, Glabus MF, Simpson R, Patterson JC. Changes in gray matter density in fibromyalgia: correlation with dopamine metabolism. J Pain. 2009;10(6):609–618. doi: 10.1016/j.jpain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain. 2012;153(4):744–754. doi: 10.1016/j.pain.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor AM, Murphy NP, Evans CJ, Cahill CM. Correlation between ventral striatal catecholamine content and nociceptive thresholds in neuropathic mice. J Pain. 2014;15(8):878–885. doi: 10.1016/j.jpain.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardoso-Cruz H, Dourado M, Monteiro C, Matos MR, Galhardo V. Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury. J Neurosci. 2014;34(17):5861–5873. doi: 10.1523/JNEUROSCI.0021-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieb W, Ouachikh O, Durif F, Hafidi A. Nigrostriatal dopaminergic depletion produces orofacial static mechanical allodynia. Eur J Pain. 2015;20(2):196–205. doi: 10.1002/ejp.707. [DOI] [PubMed] [Google Scholar]
- 21.Cobacho N, de la Calle JL, Paino CL. Dopaminergic modulation of neuropathic pain: analgesia in rats by a D2-type receptor agonist. Brain Res Bull. 2014;106:62–71. doi: 10.1016/j.brainresbull.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Paino CL. Evaluation of activity patterns in quinpirole-treated rats. J Behav Brain Sci. 2014;4:291–296. [Google Scholar]
- 23.Stuchlik A, Rehakova L, Rambousek L, Svoboda J, Vales K. Manipulation of D2 receptors with quinpirole and sulpiride affects locomotor activity before spatial behavior of rats in an active place avoidance task. Neurosci Res. 2007;58(2):133–139. doi: 10.1016/j.neures.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 25.Belzung C. Measuring exploratory behavior. In: Crusio WE, Gerlai RT, editors. Handbook of Molecular Genetic Techniques for Brain and Behavior Research (Techniques in the Behavioral and Neural Sciences) Amsterdam: Elsevier; 1999. pp. 739–749. [Google Scholar]
- 26.Cardoso-Cruz H, Sousa M, Vieira J, Lima D, Galhardo V. Prefrontal cortex and mediodorsal thalamus reduced connectivity is associated with spatial working memory impairment in rats with inflammatory pain. Pain. 2013;154(11):2397–2406. doi: 10.1016/j.pain.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Leighton GE, Rodriguez RE, Hill RG, Hughes J. kappa-Opioid agonists produce antinociception after i.v. and i.c.v. but not intrathecal administration in the rat. Br J Pharmacol. 1988;93(3):553–560. doi: 10.1111/j.1476-5381.1988.tb10310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tjolsen A, Lund A, Berge OG, Hole K. An improved method for tail-flick testing with adjustment for tail-skin temperature. J Neurosci Methods. 1989;26(3):259–265. doi: 10.1016/0165-0270(89)90124-6. [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 30.Lever C, Burton S, O’Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17(1–2):111–133. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- 31.Li SM, Collins GT, Paul NM, et al. Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behav Pharmacol. 2010;21(3):171–181. doi: 10.1097/FBP.0b013e32833a5c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson DM, Ross SB, Larsson LG. Dopamine D-2 receptor agonist-induced behavioural depression: critical dependence upon postsynaptic dopamine D-1 function. A behavioural and biochemical study. Naunyn Schmiedebergs Arch Pharmacol. 1989;340(4):355–365. doi: 10.1007/BF00167035. [DOI] [PubMed] [Google Scholar]
- 33.Johansson P, Levin E, Gunne L, Ellison G. Opposite effects of a D1 and a D2 agonist on oral movements in rats. Eur J Pharmacol. 1987;134(1):83–88. doi: 10.1016/0014-2999(87)90134-8. [DOI] [PubMed] [Google Scholar]
- 34.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 35.Kravitz AV, Freeze BS, Parker PR, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol Ther. 1994;64:291–370. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 37.Keeler JF, Pretsell DO, Robbins TW. Functional implications of dopamine D1 vs. D2 receptors: a ‘prepare and select’ model of the striatal direct vs. indirect pathways. Neuroscience. 2014;282C:156–175. doi: 10.1016/j.neuroscience.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu T, Iwata S, Miyata A, Fukuda T, Nomoto M. Delayed L-DOPA-induced hyperalgesia. Pharmacol Biochem Behav. 2006;85(3):643–647. doi: 10.1016/j.pbb.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Gao X, Zhang Y, Wu G. Effects of dopaminergic agents on carrageenan hyperalgesia after intrathecal administration to rats. Eur J Pharmacol. 2001;418(1–2):73–77. doi: 10.1016/s0014-2999(01)00930-x. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011;364(12):1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y, Fuscoe JC, Zhao C, et al. A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat Commun. 2014;5:3230. doi: 10.1038/ncomms4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai B, Hales CM, Chen PC, et al. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013;110(41):16562–16567. doi: 10.1073/pnas.1310249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauber W. Impairments of movement initiation and execution induced by a blockade of dopamine D1 or D2 receptors are reversed by a blockade of N-methyl-d-aspartate receptors. Neuroscience. 1996;73(1):121–130. doi: 10.1016/0306-4522(96)00036-x. [DOI] [PubMed] [Google Scholar]