Abstract
Sleep-disordered breathing (SDB) is a common comorbidity in a number of cardiovascular diseases, and mounting clinical evidence demonstrates that it has important implications in the long-term outcomes of patients with cardiovascular disease (CVD). While recognition among clinicians of the role of SDB in CVD is increasing, it too often remains neglected in the routine care of patients with CVD, and therefore remains widely undiagnosed and untreated. In this article, we provide an overview of SDB and its relationship to CVD, with the goal of helping cardiovascular clinicians better recognize and treat this important comorbidity in their patients. We will describe the two major types of SDB and discuss the pathophysiologic, diagnostic, and therapeutic considerations of SDB in patients with CVD.
Keywords: Sleep-disordered breathing, Obstructive sleep apnea, Central sleep apnea, Review
1. Introduction
Worldwide, the incidence of cardiovascular disease (CVD) continues to increase, and despite ongoing therapeutic advances, it continues to be associated with high rates of morbidity, hospitalization, and mortality.1, 2 In India alone, CVD is responsible for an estimated 20% of the nation's total annual deaths—a number that is likely to climb as the nation's rapid economic development and changing lifestyles fuel known risk factors for CVD, such as obesity, diabetes, dyslipidemia, and hypertension.3, 4
The onward march of CVD throughout the world clearly illustrates the need for new and innovative management strategies to further improve patient outcomes. One area under active investigation is the treatment of sleep-disordered breathing (SDB), which is now recognized as a common comorbidity in a number of CVDs. Mounting clinical evidence suggests that the presence of SDB may have important implications on the long-term outcomes of patients with CVD.
In this article, we provide an overview of SDB and its relationship to CVD, with the goal of helping cardiovascular clinicians better recognize and treat this important comorbidity in their patients. Below, we describe the two major types of SDB and discuss the pathophysiologic, diagnostic, and therapeutic considerations of SDB in patients with CVD.
2. Definitions
SDB is characterized by cycles of significant pauses in breathing followed by hypoxia and partial neurological arousals that disrupt sleep. An expanding body of research shows that repeated episodes of apnea, hypoxia, and arousal from sleep are associated with a number of pathophysiological effects that have important clinical consequences, including abnormal lipid and glucose metabolism, hypertension, stroke, and CVD.5
Terms commonly used to describe the abnormal breathing patterns associated with SDB include: apnea, the complete absence of breathing (≥10 s); hypopnea, an abnormally slow or especially shallow breathing; and hyperpnea, an abnormally rapid or deep breathing. The apnea-hypopnea index (AHI) is a commonly used clinical index that describes the severity of SDB. The AHI is defined as the average number of apnea and/or hypopnea episodes that occur during sleep expressed in events per hour. SDB severity is commonly defined as mild with an AHI ≥5 and <15, moderate with an AHI ≥15 and ≤30, or severe with an AHI >30.
3. Types, prevalence, and pathophysiology
SDB is classified into two types: obstructive sleep apnea (OSA) and central sleep apnea (CSA). Although it is not uncommon to see a mixture of both types in a patient with CVD (especially in patients with heart failure), one type usually predominates throughout the sleep period. OSA is caused by partial or complete upper airway collapse and obstruction during sleep. Each episode of airway obstruction is associated with decreased or absent air entry into the lungs and subsequent hypoxia despite ongoing respiratory effort. Airway obstruction is eventually terminated by an arousal from sleep. OSA is a relatively common sleep disorder worldwide, occurring in an estimated 3–8% of men and 1–5% of women.6 Although research into the prevalence of OSA in the Indian population is limited, one study found that 7.5% of middle-aged urban Indian men may have OSA.7 In otherwise healthy individuals, the presence of OSA is recognized as an important risk factor for the development of a number of CVDs, including hypertension,8, 9 coronary artery disease,10, 11 and stroke.12, 13 OSA is also a common comorbidity in patients with established CVD, occurring in an estimated 30% of hypertensive patients,14, 15 35% of heart failure patients,16, 17 and 30% of coronary artery disease patients.10, 18 In these individuals, OSA has been found to be an independent risk factor for the progression of CVD and adverse outcomes.5 In patients with heart failure, co-morbidities play an important role in the progression of cardiac dysfunction and overall patient status,19, 20, 21 and OSA, together with CSA, appear to be one of most prevalent co-morbidities in these patients.
CSA is characterized by the temporary withdrawal of central neurological respiratory drive, resulting in the cessation of respiratory effort and airflow. Whereas OSA is common in the general population and is a comorbidity in a number of CVDs, CSA is more uniquely seen in patients with heart failure or following stroke.5 In these patients, CSA is typically accompanied by Cheyne-Stokes respiration. CSA with Cheyne-Stokes respiration is recognized by the simultaneous absence of air flow and respiratory effort (central apnea or hypopnea) followed by characteristic hyperventilation in a crescendo-decrescendo pattern. CSA in patients with heart failure has been particularly well-studied. Because of its unique relationship to heart failure and recent developments in this area, our discussion of CSA will be limited to its presence in heart failure. CSA is highly prevalent in heart failure, occurring in about 35% of cases.16, 22 Studies suggest that CSA is an independent risk factor for cardiac transplantation and death in patients with heart failure.23, 24
Underlying the development of CSA in heart failure is respiratory control system instability due to oscillation of the arterial blood carbon dioxide level (PaCO2) above and below the central threshold of ventilation termed the apneic threshold.25 A number of factors contribute to respiratory control system instability and predispose heart failure patients to fluctuations in PaCO2, including lung congestion-related J-receptor activation, hypersensitive central and peripheral carbon dioxide chemoreceptor gain, hypoxia-related arousals, as well as reduced cardiac output leading to prolonged circulation time.26 When the PaCO2 is periodically driven below the apneic threshold by an episode of hyperpnea—such as that which occurs with hyperventilation during arousal or changes in sleep stage—central neural outflow to the respiratory muscles is temporarily suppressed and central apnea ensues.25, 26
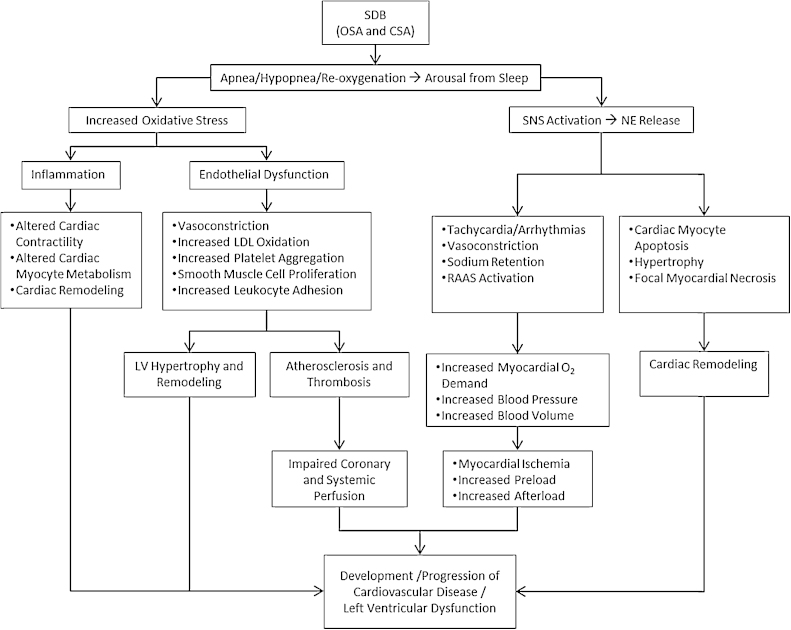
Despite differing mechanisms underlying the development of OSA and CSA, both disorders are characterized by repeated, prolonged apneas that result in hypoxia and partial neurological arousals that significantly disrupt sleep. These repeated episodes of apnea, hypoxia, and arousal trigger a number of neurohormonal, hemodynamic, metabolic, thrombotic, and inflammatory mechanisms that place patients with SDB at significantly greater risk for hypertension, myocardial ischemia, arrhythmias, and ventricular dysfunction (Fig. 1). Together, these effects ultimately contribute to the increased morbidity and mortality seen in patients with SDB.5, 26, 27
Fig. 1.
The pathophysiologic consequences of sleep-disordered breathing. Despite differing mechanisms underlying the development of obstructive sleep apnea and central sleep apnea, both disorders are characterized by repeated, prolonged apneas that result in hypoxia and partial neurological arousals that significantly disrupt sleep. These repeated episodes of apnea, hypoxia, and arousal trigger various neurohormonal, hemodynamic, metabolic, thrombotic, and inflammatory mechanisms that place patients with sleep-disordered breathing at significantly greater risk for hypertension, myocardial ischemia, arrhythmias, and ventricular dysfunction. Abbreviations: CSA, central sleep apnea; LDL, low density lipoprotein; NE, norepinephrine; OSA: obstructive sleep apnea; RAAS, renin-angiotensin-aldosterone system; SDB: sleep-disordered breathing; SNS, sympathetic nervous system.
4. Risk factors and clinical presentation
Risk factors associated with the development of OSA include obesity, male gender, advancing age, genetic predisposition to OSA, smoking and alcohol consumption, and craniofacial/pharyngeal anatomical anomalies.6 Obesity is a major risk factor in part because obese individuals often have peripharyngeal fatty deposits that contribute to pharyngeal obstruction. Pharyngeal anatomical anomalies are another major risk factor because they reduce the size of the posterior airway, which increases the chance of obstruction during sleep. The classic signs and symptoms of OSA are loud snoring, disrupted sleep, and daytime drowsiness. Other symptoms may include nocturnal gasping, restless legs, morning headaches, mood changes, and trouble concentrating.5, 27 On clinical examination, patients with OSA are typically middle-aged, overweight, and/or may have craniofacial or pharyngeal features (e.g., presence of redundant pharyngeal tissue, elongated soft palate, enlarged uvula, and/or small or receding jaw) that contribute to upper airway obstruction.5, 27 Additionally, they often have elevated daytime systolic blood pressure.5 In this regard, it should be noted that many patients with drug-resistant hypertension have undiagnosed OSA; thus, clinicians should maintain a high level of suspicion of OSA when encountering these patients.5
Risk factors for CSA are closely associated with those of heart failure, and include male gender, higher New York Heart Association class, lower left ventricular ejection fraction, waking hypocapnia, presence of atrial fibrillation, higher brain natriuretic peptide (BNP) levels, and frequent nocturnal ventricular arrhythmias.28, 29 Patients may be obese, but this is not as typical as in OSA. Signs and symptoms associated with CSA include insomnia, excessive daytime sleepiness, and/or fatigue.5 Sometimes a sleep partner may report witnessed apneas or the unusual breathing pattern of Cheyne-Stokes respiration. Patients may also report frequent awakenings, poor quality sleep, and/or shortness of breath.5 Paroxysmal nocturnal dyspnea may also be seen with CSA.5 However, since many of these findings are common to heart failure, the presence of CSA is often overlooked by patients and clinicians, and thus may lead to its under-diagnosis.
5. Diagnosis
The gold standard for diagnosing SDB is polysomnography, or the overnight sleep study, which is performed in a sleep laboratory. During polysomnography, various sensors are placed on the patient to monitor and record brain electrical activity, eye movements, heart rate and rhythm, breathing rate and rhythm, blood oxygen level, and chest, abdominal, and limb movements. The overnight sleep study is continuously supervised by a trained technician and scored by a sleep specialist.
The sleep study reports a number of key sleep variables, including the AHI. Based on current guidelines, the diagnosis of OSA is confirmed if the number of obstructive events (obstructive apneas, hypopneas + respiratory event related arousals) during the sleep study is greater than 15 events/hour, or greater than 5 events/hour in a patient who reports any of the following: unintentional sleep episodes during wakefulness; daytime sleepiness; non-refreshing sleep; fatigue; insomnia; waking up breath holding, gasping, or choking; or the bed partner describing loud snoring, breathing interruptions, or both during the patient's sleep.30 For CSA, the polysomnogram is diagnostic if respiratory monitoring demonstrates at least three consecutive cycles of crescendo-decrescendo change in breathing amplitude and one or both of the following: (1) five or more central sleep apneas or hypopneas per sleep hour and/or (2) cyclical crescendo-decrescendo breathing ≥10 consecutive minutes.31
An overnight sleep study performed in a sleep laboratory is a labor-intensive, time-consuming, and expensive test. Availability and timely access for testing are other potential concerns. There has, therefore, been increasing interest in the use of portable, home-based, overnight sleep monitors to diagnose SDB. Although many of these devices lack the sensitivity and/or specificity for diagnosing SDB, recent research suggests that Level III devices, which at a minimum record oxygen, nasal flow, and thoracic and abdominal movement, offer good diagnostic performance compared with polysomnography in patients with a high pretest probability of moderate to severe OSA and no unstable comorbidities.32 However, for patients suspected of having CSA, polysomnography remains the diagnostic standard.
Finally, it is worth noting that several short patient questionnaires, such as the Epworth Sleepiness Scale and the Berlin Questionnaire, have been developed to help screen for OSA (but not CSA) in the general population. These questionnaires ask subjects to rate their likelihood of falling asleep in several common situations. In the general population, the scores from these questionnaires have been shown to correlate reasonably well with the presence and severity of OSA.33, 34 In the CVD population, however, their utility remains unclear. In heart failure patients, for example, they have not been found effective in screening for OSA.35 Their lack of sensitivity and specificity in the heart failure population is likely due to the questionnaires’ emphasis on subjective daytime sleepiness caused by chronically disrupted sleep—a finding that is significantly less common in patients with HF, and cardiac dysfunction in general.35 Thus, the use of sleepiness questionnaires to screen for SDB is probably best avoided in this population.
6. Treatment
6.1. OSA
A number of therapies are available to effectively treat OSA. Which therapy is chosen depends in large part on the results of the sleep study. For patients with mild OSA, weight loss for those who are obese and avoiding the supine sleeping position may successfully resolve OSA events. For moderate to severe cases of OSA, the gold standard treatment is continuous positive airway pressure (CPAP).30 CPAP therapy is delivered using a tight fitting nasal or facial mask through which constant positive air pressure is applied to maintain airway patency. OSA patients treated with CPAP often experience rapid and significant improvement in their AHI, nocturnal symptoms, daytime sleepiness, and fatigue.36, 37 Clinically, studies have shown that CPAP treatment favorably effects diurnal and nocturnal systolic and diastolic blood pressure38; improves serum total cholesterol and triglyceride levels39, 40; reduces serum levels of inflammatory markers, including C-reactive protein, tumor necrosis factor α, and interleukin 6, which contribute the atherosclerosis process41; and decreases the incidence of fatal and nonfatal cardiovascular events.42 In patients with drug-resistant hypertension and OSA, CPAP therapy has been shown to result in favorable reductions in both systolic and diastolic blood pressure.43 Other studies have shown that in patients with OSA and pre-existing coronary artery disease, treatment with CPAP protects against new cardiovascular events.44 In studies of heart failure patients with OSA, CPAP therapy has been shown to reduce nocturnal blood pressure and heart rate and improve left ventricular function.45, 46, 47 One small, nonrandomized trial reported that CPAP may reduce the risk of death and hospitalization among patients with heart failure and OSA.48
Successful treatment of OSA with CPAP requires that it be used consistently every night for a prescribed number of hours. Unfortunately, patient noncompliance with CPAP therapy is a common problem.49 Reasons for poor compliance include mask discomfort, nasal discharge or dryness, difficulty breathing against positive airway pressure, mask-related claustrophobia, and nighttime panic attacks. Many of these complaints can be addressed through modifications in therapy parameters. Nonetheless, many patients will still not tolerate long-term CPAP therapy. Beyond CPAP, a number of other therapies have been devised to treat OSA. They include custom-made oral appliances and tongue retaining devices that prevent pharyngeal obstruction during sleep; nasal devices worn over each nostril that create increased expiratory nasal resistance to maintain upper airway patency; various surgical procedures, such as uvulopalatopharyngoplasty, that enlarge the pharyngeal airway; oral negative pressure therapy, which draws the tongue and soft palate anteriorly using a mouthpiece connected to a suction mechanism; and hypoglossal nerve stimulation therapy, which stimulates key airway muscles to maintain upper airway patency.50 It is important to note, however, that none of these therapies have been found to be as effective as CPAP in reducing or eliminating OSA. Thus, these alternative therapies should be reserved for those patients who do not respond to or simply cannot tolerate CPAP therapy.
6.2. CSA
In contrast with OSA, the optimal treatment strategy for CSA is much less clear. What is known, however, it that once heart failure is clinically improved, CSA often improves as well.51, 52, 53 Therefore, optimizing treatment based on current heart failure medical management guidelines is of foremost importance.54, 55, 56, 57 Unfortunately, CSA often persists in many patients despite optimal heart failure therapy. This is especially true in patients with more advanced heart failure. Thus, for these patients, other targeted treatments for CSA must be considered.
Positive airway pressure (PAP) therapies, such as CPAP and adaptive pressure support servoventilation (ASV), have been the most widely prescribed and extensively investigated treatment for CSA in patients with heart failure.58 However, two large, randomized, controlled trials have failed to show improvement in morbidity and mortality with the use of PAP therapies for the treatment of CSA in heart failure. The first study, the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) Trial, randomly assigned optimally medically treated heart failure patients with CSA to receive either CPAP or no CPAP to determine whether the use of CPAP improved transplant-free survival.59 Overall, no mortality benefit was found; in fact, early divergence of survival curves favoring the non-CPAP group resulted in the early termination of the study. However, after a mean follow-up of two years, mortality was found to be similar in both groups. A post-hoc analysis of the CANPAP data suggested that insufficient reduction in the AHI, poor patient compliance with CPAP therapy, as well as the adverse effects PAP may have had on cardiopulmonary function (primarily due to PAP-induced increased intrathoracic pressure, which adversely affects both right and left ventricular preload and afterload) likely contributed to the disappointing results of the CANPAP study.60, 61 The second study, Treatment of Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure (SERVE-HF) Trial, evaluated the effect of treatment with ASV on morbidity and mortality in patients with chronic heart failure and CSA.62 ASV was designed to provide a more patient-tailored PAP therapy in order to reduce the adverse cardiovascular and pulmonary effects seen with CPAP treatment and to improve patient compliance with therapy. Recently reported results of the SERVE-HF Trial showed no significant difference between patients treated with ASV and those in the control group for the primary endpoints of time to all-cause mortality, life-saving cardiovascular intervention, or unplanned hospitalization for worsening heart failure. More importantly, there was a statistically significant 2.6% absolute increased annual risk of cardiovascular mortality for those randomized to ASV therapy compared to the control group.63, 64 However, two other large, randomized trials evaluating ASV in the treatment of SDB in HF—the Effect of Adaptive Servo Ventilation on Survival and Hospital Admissions In Heart Failure (ADVENT-HF) and Cardiovascular Improvements With Minute Ventilation-targeted ASV Therapy in Heart Failure (CAT-HF)—are ongoing, although CAT-HF will not be enrolling patients with an ejection fraction less than 45%.65, 66
Although PAP-based therapies have garnered much of the attention of researchers in recent years, a number of other therapies have also been proposed and studied. These therapies include nocturnal oxygen supplementation, atrial pacing, pharmacologic agents (e.g., theophylline and acetazolamide), and nocturnal carbon dioxide administration. Although small, preliminary trials have shown each to be of some benefit in reducing CSA and/or its symptoms, none have undergone large scale, prospective, randomized trials to establish their long-term safety and efficacy. Further research is needed to generate more confidence in their effectiveness and to justify their use for the chronic management of CSA in patients with heart failure.58
Recently, a new physiologic approach to the treatment of CSA has been introduced, and is currently completing follow-up in a randomized, controlled clinical trial. It is an implantable, lead-based device similar in appearance to a pacemaker that delivers unilateral transvenous phrenic nerve stimulation to cause diaphragmatic contraction similar to a normal breath. This diaphragmatic stimulation and subsequent contraction create negative intrathoracic pressure so that airflow is augmented and central apneas occurring during sleep are prevented. It is worth noting that this mechanism of action is very different than that of PAP therapy, which, by forcing air into the lungs under pressure, acts to increase intrathoracic pressure. Thus, with application of transvenous phrenic nerve stimulation therapy, cyclical periodic breathing and blood gas alterations are prevented. Additionally, as an implantable, lead-based device therapy, this system should avoid any patient compliance issues with treatment. Early studies using this therapy have shown significant improvement in major indices of CSA severity, although its effects on heart failure-related hospitalization, morbidity, and mortality remain unknown.67, 68 Active research with this system is currently ongoing. This includes a pivotal clinical trial to evaluate the chronic safety and efficacy of unilateral phrenic nerve stimulation using a fully implanted system. Results of this trial are expected to be reported in 2016.
7. Conclusion
SDB is a common comorbidity in a number of CVDs, and mounting clinical evidence demonstrates that it has important implications for the long-term outcomes of patients with CVD. While recognition among clinicians of the role of SDB in CVD is increasing, it remains too often neglected in the routine evaluation and management of patients with CVD, and thus it remains widely undiagnosed and untreated. This is unfortunate, since by simply asking a few additional questions about a patient's sleep habits and related symptoms at each office visit or hospital admission, patients in need of further diagnostic testing could be readily identified.
Polysomnography, or the overnight sleep study, remains the gold standard for diagnosing SDB. However, it is an expensive, labor-intensive, and time-consuming test, and availability and timely access for testing are common concerns. Portable, home-based, Level III devices offer good diagnostic performance compared with polysomnography in patients with a high pretest probability of moderate to severe OSA and no unstable comorbidities, and thus may be considered when polysomnography is unavailable. However, for patients suspected of having CSA, polysomnography remains the diagnostic standard.
A number of different treatment options are available to effectively treat OSA. Of these, CPAP is the primary treatment, as it has been shown to be highly effective in controlling symptoms, improving quality of life, and reducing the clinical consequences of OSA. In contrast, the optimal treatment of CSA in patients with heart failure remains unclear. When CSA is found in a patient with HF, optimizing medical therapy using currently published heart failure care guidelines is of foremost importance.54 In cases where CSA persists despite aggressive treatment of heart failure, PAP-based therapies have, until recently, been the treatment of choice. However, two large, randomized controlled studies of PAP therapy—CANPAP and SERVE-HF—have raised concerns about their safety and efficacy in patients with HF and low ejection fraction. Small, preliminary trials of other therapies, such as nocturnal oxygen supplementation, atrial pacing, pharmacologic agents (e.g., theophylline and acetazolamide), and nocturnal carbon dioxide administration, have shown them to offer some benefit in reducing CSA and/or its symptoms, but none as yet have undergone large-scale, prospective, randomized trials to establish their long-term safety and efficacy. Another therapy, transvenous stimulation of the phrenic nerve, may offer an important new way of treating CSA in patients with heart failure. Active research with this system is currently ongoing. Cardiovascular clinicians are encouraged to closely monitor the relevant literature to remain abreast of emerging options for treating this important comorbidity in the heart failure population.
Conflicts of interest
SDA and SvH are consultants to Respicardia, Inc. RG is an employee of Respicardia, Inc.
Acknowledgement
The authors wish to thank Janice Hoettels for her assistance with the preparation of this manuscript.
References
- 1.World Health Organization. Cardiovascular Diseases (CVDs) Fact Sheet 317. Available at: http://www.who.int/mediacentre/factsheets/fs317/en/; Accessed 26.09.15.
- 2.Ponikowski P., Anker S.D., Alhabib K.F. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 3.Bloom D.E., Cafiero-Fonseca E.T., Candeias V. Economics of non-communicable diseases in India: the costs and returns on investment of interventions to promote healthy living and prevent, treat, and manage NCDs. World Economic Forum; Harvard School of Public Health; 2014. Available at: http://www3.weforum.org/docs/WEF_EconomicNonCommunicableDiseasesIndia_Report_2014.pdf. Accessed 17.09.15. [Google Scholar]
- 4.Prabhakaran D., Yusuf S. Cardiovascular disease in India: lessons learnt and challenges ahead. Indian J Med Res. 2010;132:529–530. [PMC free article] [PubMed] [Google Scholar]
- 5.Somers V.K., White D.P., Amin R. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi N.M. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udwadia Z.F., Doshi A.V., Lonkar S.G. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004:169–173. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 8.Hla K.M., Young T.B., Bidwell T. Sleep apnea and hypertension. A population-based study. Ann Intern Med. 1994;120:382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Robinson G.V., Stradling J.R., Davies R.J.O. Sleep. 6: Obstructive sleep apnoea/hypopnea syndrome and hypertension. Thorax. 2004;59:1089–1094. doi: 10.1136/thx.2003.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peker Y., Kraiczi H., Hedner J. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–184. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 11.Peker Y., Carlson J., Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 12.Yaggi H.K., Concato J., Kernan W.N. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 13.Arzt M., Young T., Finn L. Association of sleep disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kales A., Bixler E.O., Cadieux R.J. Sleep apnoea in a hypertensive population. Lancet. 1984;2:1005–1008. doi: 10.1016/s0140-6736(84)91107-3. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher E.C., DeBehnke R.D., Lovoi M.S. Undiagnosed sleep apnea in patients with essential hypertension. Ann Int Med. 1985;103:190–195. doi: 10.7326/0003-4819-103-2-190. [DOI] [PubMed] [Google Scholar]
- 16.Oldenburg O., Lamp B., Faber L. Sleep disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Javaheri S., Caref E.B., Chen E. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183:539–546. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 18.Schäfer H., Koehler U., Ewig S. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92:79–84. doi: 10.1159/000006952. [DOI] [PubMed] [Google Scholar]
- 19.von Haehling S., Anker S.D. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261–263. doi: 10.1007/s13539-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lainscak M., Anker S.D. Heart failure, chronic obstructive pulmonary disease, and asthma: numbers facts and challenges. ESC Heart Fail. 2015;2:103–107. doi: 10.1002/ehf2.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassanien M., Abdelhamid M., Ibrahim B. Clinical characteristics and management of hospitalized and ambulatory patients with heart failure – results from ESC Heart Failure long-term registry-Egyptian cohort. ESC Heart Fail. 2015;2:159–167. doi: 10.1002/ehf2.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanfranchi P., Somers V., Braghiroli A. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107:727–732. doi: 10.1161/01.cir.0000049641.11675.ee. [DOI] [PubMed] [Google Scholar]
- 23.Sin D.D., Logan A.G., Fitzgerald F.S. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102:61–66. doi: 10.1161/01.cir.102.1.61. [DOI] [PubMed] [Google Scholar]
- 24.Lanfranchi P.A., Braghiroli A., Bosimini E. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 25.Hanly P., Zuberi N., Gray R. Pathogenesis of Cheyne-Stokes respiration in patients with congestive heart failure: relationship to arterial pCO2. Chest. 1993;104:1079–1084. doi: 10.1378/chest.104.4.1079. [DOI] [PubMed] [Google Scholar]
- 26.Bradley T.D., Floras J.S. Sleep apnea in heart failure. Part II: central sleep apnea. Circulation. 2003;107:1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 27.Bradley T.D., Floras J.S. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107:1671–1678. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 28.Sin D., Fitzgerald F., Parker J.D. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 29.Calvin A.D., Somers V.K., van der Walt C. Relation of natriuretic peptide concentrations to central sleep apnea in patients with heart failure. Chest. 2011;140:1517–1523. doi: 10.1378/chest.10-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein L.J., Kristo D., Strollo P.J. Clinical guideline for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 31.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 32.El Shayeb M., Topfer L.A., Stafinski T. Diagnostic accuracy of level 3 portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: a systematic review and meta-analysis. CMAJ. 2014;186:E25–E51. doi: 10.1503/cmaj.130952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottlieb D.J., Whitney C.W., Bonekat W.H. Relation of sleepiness to respiratory disturbance index, the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–507. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 34.Punjabi N.M., Bandeen-Roche K., Young T. Predictors of objective sleep tendency in the general population. Sleep. 2003;26:678–683. doi: 10.1093/sleep/26.6.678. [DOI] [PubMed] [Google Scholar]
- 35.Arzt M., Young T., Finn L. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–1722. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 36.Engleman H.M., Martin S.E., Deary I.J. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343:572–575. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 37.Engleman H.M., Kingshott R.N., Wraith P.K. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:461–467. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 38.Montesi S., Edwards B., Malhotra A. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–596. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S., Agrawal S., Damodaran D. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–2286. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 40.Nadeem R., Singh M., Nida M. Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. Lancet. 2014;383:736–747. doi: 10.5664/jcsm.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baessler A., Nadeem R., Harvey M. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers – a meta-analysis. J Inflamm (Lond) 2013;10:13–16. doi: 10.1186/1476-9255-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marin J., Carrizo S., Vicente E. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 43.Iftikhar I., Valentine C., Bittencourt L. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens. 2014;32:2341–2350. doi: 10.1097/HJH.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milleron O., Pilliere R., Foucher A. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Tkacova R., Rankin F., Fitzgerald F.S. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98:2269–2277. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 46.Malone S., Liu P.P., Holloway R. Obstructive sleep apnea in patients with dilated cardiomyopathy: effects of continuous positive airway pressure. Lancet. 1991;338:1480–1484. doi: 10.1016/0140-6736(91)92299-h. [DOI] [PubMed] [Google Scholar]
- 47.Kaneko Y., Floras J.S., Usui K. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 48.Kasai T., Narui K., Dohi T. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133:690–696. doi: 10.1378/chest.07-1901. [DOI] [PubMed] [Google Scholar]
- 49.American Thoracic Society Indications and standards for use of nasal continuous positive airway pressure (CPAP) in sleep apnea syndrome. Am J Respir Crit Care Med. 1994;150:1738–1745. doi: 10.1164/ajrccm.150.6.7952642. [DOI] [PubMed] [Google Scholar]
- 50.Freedman N. Improvements in current treatments and emerging therapies for adult obstructive sleep apnea. F1000Prime Rep. 2014;6:36. doi: 10.12703/P6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dark D.S., Pingleton S.K., Kerby G.R. Breathing pattern abnormalities and arterial oxygen desaturation during sleep in congestive heart failure syndrome: improvement following medical therapy. Chest. 1987;91:833–836. doi: 10.1378/chest.91.6.833. [DOI] [PubMed] [Google Scholar]
- 52.Walsh J.T., Andrews R., Starling R. Effects of captopril and oxygen on sleep apnoea in patients with mild to moderate congestive heart failure. Br Heart J. 1995;73:237–241. doi: 10.1136/hrt.73.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baylor P., Tayloe D., Owen D. Cardiac failure presenting as sleep apnea. Elimination of apnea following medical management of cardiac failure. Chest. 1988;94:1298–1299. doi: 10.1378/chest.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 54.McMurray J.J., Adamopoulos S., Anker S.D. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 55.Khawaja T., Chokshi A., Ji R. Ventricular assist device implantation improves skeletal muscle function, oxidative capacity, and growth hormone/insulin-like growth factor-1 axis signaling in patients with advanced heart failure. J Cachexia Sarcopenia Muscle. 2014;5:297–305. doi: 10.1007/s13539-014-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Habedank D., Meyer F.J., Hetzer R. Relation of respiratory muscle strength, cachexia and survival in severe chronic heart failure. J Cachexia Sarcopenia Muscle. 2013;4:277–285. doi: 10.1007/s13539-013-0109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Josiak K., Jankowska E.A., Piepoli M.F. Skeletal myopathy in patients with chronic heart failure: significance of anabolic-androgenic hormones. J Cachexia Sarcopenia Muscle. 2014;5:287–296. doi: 10.1007/s13539-014-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aurora R.N., Chowdhuri S., Ramar K. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35:17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bradley T.D., Logan A.G., Kimoff R.J. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 60.Arzt M., Floras J.S., Logan A.G. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 61.Javaheri S. CPAP should not be used for central sleep apnea in congestive heart failure patients. J Clin Sleep Med. 2006;2:399–402. [PubMed] [Google Scholar]
- 62.Cowie M.R., Woehrle H., Wegscheider K. Rationale and design of the SERVE-HF study: treatment of sleep-disordered breathing with predominant central sleep apnoea with adaptive servo-ventilation in patients with chronic heart failure. Eur J Heart Fail. 2013;15:937–943. doi: 10.1093/eurjhf/hft051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. doi:10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed]
- 64.Magalang UJ, Pack AI. Heart failure and sleep-disordered breathing—the plot thickens. N Engl J Med. doi:10.1056/NEJMe1510397. [DOI] [PubMed]
- 65.Effect of adaptive servo ventilation (ASV) on survival and hospital admissions in heart failure (ADVENT-HF). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01128816?term=ADVENT-HF&rank=1; Accessed 13.08.15.
- 66.Cardiovascular improvements with MV ASV therapy in heart failure (CAT-HF). Available at: https://clinicaltrials.gov/ct2/show/NCT01953874?term=CAT-HF&rank=1; Accessed 13.08.15.
- 67.Ponikowski P., Javaheri S., Michalkiewicz D. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J. 2012;33:889–894. doi: 10.1093/eurheartj/ehr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abraham W.T., Jagielski D., Oldenburg O. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail. 2015;3:360–369. doi: 10.1016/j.jchf.2014.12.013. [DOI] [PubMed] [Google Scholar]